Abstract
Background
In a Phase 2 clinical trial, we aimed to determine the lutetium-177 [177Lu]-PSMA-617 activity and the clinical utility of levels of plasma androgen receptor (AR) gene in patients with heavily pretreated metastatic castration-resistant prostate cancer (mCRPC).
Methods
We determined AR copy number in pretreatment plasma samples. We used logistic regression to estimate the odds ratio (OR) and 95% confidence intervals (95% CIs) in order to evaluate the independent relevance of AR status and to evaluate patients with early progressive disease (PD) defined as treatment interruption occurring within 4 months after the start of 177Lu-PSMA-617.
Results
Twelve of the 15 (80%) with AR gene gain and 5 of the 25 (20%) patients with no gain of AR had early PD (p = 0.0002). The OR for patients without PSA response having AR gain was 3.69 (95% CI 0.83–16.36, p = 0.085). The OR for patients with early PD having AR gain was 16.00, (95% CI 3.23–79.27, p = 0.0007). Overall, median PFS and OS were 7.5 and 12.4 months, respectively. AR-gained had a significant shorter OS compared to AR-normal patients (7.4 vs 19.1 months, p = 0.020). No treatment interruptions due to adverse effects were reported.
Discussion
Plasma AR status helped to indicate mCRPC with early resistance to 177Lu-PSMA-617.
Trial registration
Subject terms: Predictive markers, Prostate cancer
Introduction
Prostate cancer is the second most common cancer among men worldwide and ranks sixth as leading cause of cancer death in men [1]. Standard care for patients with advanced disease is based on androgen deprivation therapy (ADT); however, prostate cancer will progress to become castration resistant despite castration levels of testosterone [2]. Androgen receptor (AR) aberrations represent the main mechanism associated with castration resistance, the main cause of death of patients with metastatic castration-resistant prostate cancer (mCRPC) [3–5]. Plasma AR detected by digital droplet PCR (ddPCR) identifies patients with mCRPC with worse outcome to life-prolonging novel anti-androgen drugs (NAD), while these patients may respond more favourably to taxanes as first-line treatment [6–9]. Thus, plasma AR status could be useful as biomarker for treatment selection.
Prostate-specific membrane antigen (PSMA) is a protease anchored to the epithelial prostate cell membrane and represents a promising target for diagnosis and theranostic therapy of mCRPC [10]. Higher grading score of prostate cancer is associated with increased level of PSMA expression and it makes these patients with mCRPC suitable for the theranostic approach [11, 12]. A PSMA radioligand therapy (PRLT) with a PSMA inhibitor (DKFZ-PSMA-617) was synthesised and labelled with 177Lutethium (177Lu). This theranostic agent, named 177Lu-PSMA-617, was initially administered in compassionate use programs in Germany to patients with mCRPC [13–16]. Recently, a first prospective Phase 2 study with 177Lu-PSMA-617 in 30 mCRPC patients reported favourable biochemical and radiological responses [17], confirmed in an expanded study with 50 patients.
AR has a mechanism by which ADT and other hormonal therapies synergise with radiation treatments [18, 19]. Recently, AR aberrations have been associated with sensitivity to ionising radiation in preclinical studies [20].
Preliminary results on dosimetry and safety of 177Lu-PSMA-617 along with polyglutamate parotid gland protector have been reported [21]. In the present study, we report the efficacy and side effect of RLT in a therapeutic schedule designed to identify the minimal effective dosage of 177Lu-PSMA-617 in heavily pretreated mCRPC patients who progressed after standard life-prolonging treatments. Moreover, we aimed to determine whether plasma AR status predicts early response or progression for mCRPC patients undergoing 177Lu-PSMA-617 RLT.
Patients and methods
Study design
The study is an investigator-initiated single-centre open-label Phase 2 study. The eligibility criteria included patients with histologically confirmed diagnosis of prostate adenocarcinoma without neuroendocrine differentiation. Patients should have mCRPC with progression following life-prolonging agents with instrumental and/or biochemical evidence of progressive disease (PD) according to Prostate Cancer Working Group-3 criteria [22]. Patients should also have Eastern Cooperative Oncology Group (ECOG) performance status ≤2; life expectancy ≥6 months; and adequate haematologic, liver, and renal function.
All patients had undergone imaging with 68Ga-PSMA-positron emission tomography (PET)/computed tomography (CT) scan as part of the screening assessments to confirm high PSMA expression. The Trial Management Group agreed to do a first analysis on 40 consecutive patients enrolled in the study with the primary aim to assess the activity and safety. The trial did not include 18F-fluorodeoxyglucose (FDG)-PET/CT in the pretreatment staging [17]. High PSMA expression was defined as activity in a site of metastatic disease significantly greater than the activity in the normal liver. Thus, standardised uptake value [SUV]max in tumour should be at least 1·5 times the SUVmax in the liver. Metastatic disease was quantified on the basis of maximum standardised uptake value (SUVmax), metabolic volume activity (MTV; i.e. volume of interest consisting of all spatially connected voxels within a fixed threshold of 40% of the SUVmax), and total lesion activity (TLA as the product of MTV and mean SUV) for each lesion at baseline PSMA-PET/CT. The study protocol was approved by the Institutional Review Board. It was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients had given written informed consent. This trial is registered with ClinicalTrials.gov identifier: NCT03454750.
Procedures
177Lu-PSMA-617 was administered with up to 4 cycles of treatment at 8–12 weekly intervals at a dosage ranging from 4.4 to 5.5 GBq per cycle. Patients <75 years unfit to be treated with docetaxel were treated with PRLT with 5.5 GBq/cycle. Patients previously treated with abiraterone/enzalutamide and docetaxel and/or aged >75 years were treated with PRLT with lower activities ranging from 3.7 to 4.4 GBq/cycle. The patients who responded clinically were given up to two more cycles. Activity was not modified based on weight, renal function, or the number of metastases. Patients were not permitted to have concurrent chemotherapy or second-generation anti-androgens. According to dosimetric estimation, a total cumulate activity up to 33 GBq was envisaged, respecting dose limitations for normal organs after repeated administrations of 177Lu PSMA-617 [13]. Regarding production of 177Lu-PSMA-617, the trial followed National Good Preparation (NBP) standards for pharmaceutical products, as per the Italian legislation requirements. Endocyte Inc. (West Lafayette, IN 47906, USA) kindly provided DOTA-PSMA-617 and we purchased 177Lu from AAA (LuMark®, Baarle-Nassau, Netherlands) or ITG (Endolucinbeta®, Isotope Technologies Garching GmbH, Garching, Germany). We performed the labelling procedure and quality control of 177Lu-DOTA-PSMA-617 compound according to NBP procedures at the Radiopharmacy Laboratory at the IRCCS Istituto Romagnolo per lo Studio dei Tumori (IRST) “Dino Amadori”, Meldola, Italy.
The patients were given the radiopharmaceutical in a dedicated room as a slow intravenous infusion over 15–30 min. To reduce off-target 68Ga-PSMA renal uptake in the kidneys, patients received prophylaxis with infusion of 250 mL mannitol 10% half an hour before the RLT and 250 mL after the RLT. To prevent off-target salivary gland uptake, patients were given two polyglutamate folate tablets half an hour before, during, and 4 h after RLT. During the infusion of RLT, ice packs were placed on the parotid glands to reduce uptake of the radioligand in salivary glands [14, 17, 21].
Molecular analyses
We assessed AR amplification in circulating plasma DNA by ddPCR. Peripheral blood samples were collected within 28 days of treatment initiation and plasma aliquots were stored at −80 °C. ddPCR assays were as described previously [9]. Germline evaluation by next-generation sequencing (NGS) techniques was done after sample collection and DNA extraction from the peripheral blood. Blood was stored at −80 °C until genomic DNA was extracted. DNA was purified by the Qama DNA Mini Kit (Qiagen, Hilden, Germany) and quantified using Qubit fluorometer (Thermo Fisher Scientific, Waltham, MA, USA) with the Qubit dsDNA BR Assay Kit. The NGS analysis was performed using an enrichment protocol (SOPHiA GENETICS®), which analyses 27 most clinically relevant genes associated with repair of DNA damage response (DDR), including BRCA1/2 and ATM. We performed DNA sequencing with the MiSeq Reagent Kit v3 600 cycles (Illumina©) on a MiSeq platform (Illumina©). All procedures were performed according to the manufacturer’s instructions. Data output files (fastaq) were imported into the Sophia DDM® Platform v5.5.0 (SOPHiA GENETICS®) for analysis.
Outcome
Baseline examinations for patients included anatomic and functional imaging with 68Ga-PSMA-PET/CT, radionuclide bone scan, contrast-enhanced CT within 8 weeks of treatment, and determination of prostate-specific antigen (PSA) within 7 days from therapy. We repeated safety reviews and blood tests 4 weeks after each cycle of RLT. Adverse events and assigned causality were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v.5.0 at each clinical examination.
The main objective of the study was the disease control rates defined as the percentage of patients who achieved complete or partial response and stable disease from therapy and safety according to CTCAE v.5.0. In particular, we evaluated the percentage of early PD defined as treatment interruption occurring within 4 months of the start of 177Lu-PSMA-617. Secondary objective was the biochemical response (PSA decline ≥50%), progression-free and overall survival (PFS/OS), and biological correlations. We previously reported dosimetry and biodistribution of 177Lu-PSMA-617 to tumours and normal tissues according to the above injection protocol [21].
Statistical analyses
We assessed association between categorical variables using chi-square or Fisher’s exact test. Patients who did not have tumour progression at the time of our analysis were censored at their last date of follow-up. We estimated PFS/OS using Kaplan-Meier estimates of survival. We used logistic regression to estimate the odds ratio (OR) and 95% confidence interval (CI) as we evaluated the independent relevance of AR status of patients without PSA response and of those with early PD. All p values were based on two-sided tests. p < 0.05 was considered to be statistically significant. We performed the statistical analyses with the use of SAS 9.4 software (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
Forty consecutive patients with PET/CT-68Ga-PSMA-positive mCRPC were enrolled in this study between April 2017 and February 2019. The age of the patients was median 72 years (range, 54–86). Baseline characteristics are summarised in Table 1. 177Lu-PSMA-617 was administered as second-line therapy for 2 (5%) patients, as third-line therapy for 10 (25%) patients, fourth-line therapy for 20 (50%) patients, and fifth-line therapy for 18 (20%) patients. Twenty-five (80.6%) patients earlier received docetaxel and 6 (19.4%) of the patients as first and second line, respectively. Twelve (38.7%) patients had previous AR-directed therapies and 19 (61.3%) were chemotherapy-naive and post-docetaxel patients, respectively.
Table 1.
Patient baseline characteristics.
| Variable | Overall N = 40 (%) |
|---|---|
| Median age (range) | 72 (54–86) |
| Median Gleason score at diagnosis, (IQR) | 8 (7–9) |
| Median alkaline phosphatase (U/L), (IQR) | 107 (70–241) |
| Median haemoglobin (g/L), (IQR) | 11.6 (10.8–12.4) |
| Median PSA (µg/L), (IQR) | 66.1 (21.5–125.2) |
| ECOG performance status | |
| 0 | 17 (42.5) |
| 1 | 19 (47.5) |
| 2 | 4 (10.0) |
| Previous treatment | |
| Radical prostatectomy or radiotherapy | 23 (57.5) |
| Abiraterone/enzalutamide | 31 (77.5) |
| Docetaxel | 31 (77.5) |
| Cabazitaxel | 25 (62.5) |
| Palliative-intent radiotherapy | 25 (62.5) |
| Site of disease on PSMA PET/CT | |
| Bone | 38 (95.0) |
| Nodal | 25 (62.5) |
| Visceral | 9 (22.5) |
| Pain at baseline | |
| No pain | 9 (22.5) |
| Mild | 14 (35.0) |
| Moderate | 14 (35.0) |
| Severe | 3 (7.5) |
| AR status | |
| AR normal | 25 (62.5) |
| AR amplified | 15 (37.5) |
Thirty (75%) patients received a low dosage of 177Lu-PSMA-617 ranging from 3.7 to 4.4 GBq/cycle. Ten (25%) patients received 5.5 GBq/cycle (Supplementary Fig. 1). Dose adjustments were undertaken based on the investigator’s clinical judgement according to age, comorbidities, number of previous therapies, and ECOG performance status. The median number of cycles was 3 (interquartile range (IQR) = 2–5). Median cumulative activity was 13.6 GBq (IQR = 8.8–22.0 GBq).
Fifteen (37.5%) patients had raised plasma AR. Patients with normal or raised levels of AR did not differ significantly regarding clinical characteristics (Supplementary Table 1).
Clinical outcome
The median follow-up was 15.5 months (range, 6–22). Twenty (50%) of the patients had a PSA decline ≥30% and 15 (37.5%) of the patients had a ≥50% PSA decline (Fig. 1).
Fig. 1. Association of plasma AR status with prostate-specific antigen (PSA) response.
Waterfall plots showing PSA declines by AR copy number status in castration-resistant prostate cancer patients treated with 177Lu-PSMA. Only two patients (one with AR gain) were not evaluable for PSA response due to rapid deterioration and death. Bars clipped at maximum 100%.
Seventeen (42.5%) of the patients had early PD, defined as treatment interruption for documented PD or rapid clinical deterioration with death occurring within 4 months of the start of 177Lu-PSMA-617. Five (20%) patients had PSMA-positive disease at the time of PD, relapse within approximately 7 months (range, 4–9) from the first radiological response (PSMA-negative disease).
The median PFS was 7.5 months (95% CI 4.8–10.5) with 60.0% (95% CI 43.2.3–73.2) PFS at 6 months. At the time of the present analysis, 22 (55%) patients had died. The median OS was 12.4 months (95% CI 7.4–20.3 months). The 12-month OS was 53.1% (95% CI 35.3–68.0).
Safety
Treatment with 177Lu-PSMA-617 was safe and well tolerated, with no treatment interruptions due to adverse effects. The most common adverse effects were mild grade 1 anaemia and fatigue, manageable with supportive measures. Six (15%) patients had grade 2 anaemia and 2 (5%) patients had grade 3 anaemia requiring blood transfusion. Nine (22.5%) patients had mild renal adverse effects (Supplementary Table 2).
Clinical impact of AR gene status
We observed a possible association between circulating AR status and pretreatment PSMA uptake more evident with MTV than TLA (Supplementary Table 3). Three of the 15 (20%) patients with amplified AR and 12 of the 25 (48%) with normal AR had PSA response (p = 0.080). Patients with AR gain were 2.4 times less likely to have a PSA response. The OR for patients without PSA response having AR gain was 3.69, 95% CI 0.83–16.36, p = 0.085. Figure 1 shows waterfall plots of PSA declines according to plasma AR status. Figure 2 shows a representative patient with durable response to the treatment.
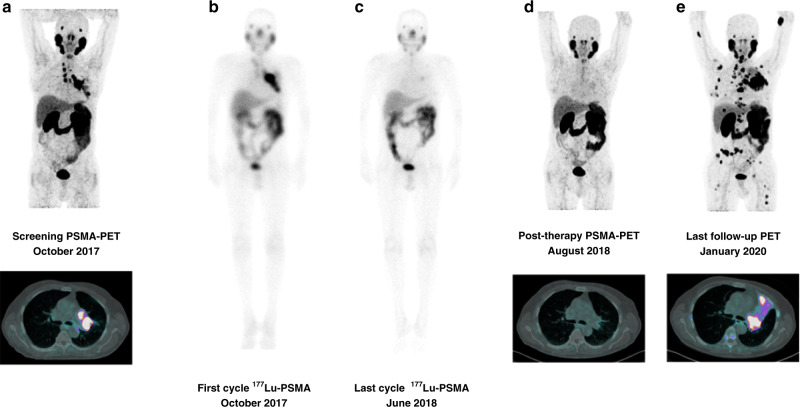
Fig. 2. A representative cases of durable activity of 177Lu-PSMA-617.
Seventy-one-year-old mCRPC patient with nodal and lung metastases and circulating AR normal, previously treated with docetaxel, abiraterone, and cabazitaxel. The patient received 4 cycles of 177Lu-PSMA-617 radioligand therapy (activity per cycle: 4.4 GBq every 6–8 weeks). October 2017, baseline PSA = 52.4 ng/mL; May 2018 post-177Lu-PSMA therapy PSA: 2.26 ng/mL. October 2018 disease relapse (nodes, lung, and bone). a Pre-therapy PSMA-PET/CT, b baseline 177Lu PSMA whole-body scan, c post-therapy 177Lu PSMA whole-body scan, d post-therapy PSMA-PET/CT, e PSMA-PET/CT at last follow-up.
Twelve of 15 (80%) patients with raised AR and 5 of 25 (20%) patients with normal AR had early PD (p = 0.0002). The OR for patients with early PD having raised AR was 16.00, 95% CI 3.23–79.27, p = 0.0007. Patients with raised AR gain had a median PFS of 4.7 months (95% CI 2.9–7.0 months), whereas patients with normal AR had a median PFS of 9.4 months (95% CI 6.9–11.5, p = 0.020) (Fig. 3a).
Fig. 3. Association between circulating AR status and clinical outcome.
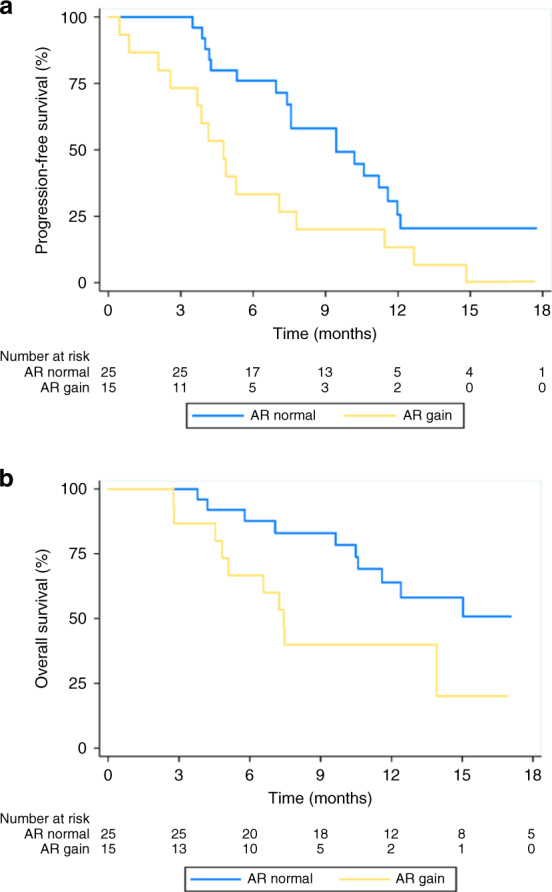
Kaplan–Meier estimate of overall survival (a) and progression-free survival (b) according to circulating AR status.
Patients with raised AR had median OS of 7.4 months (95% CI 4.5–10.3 months), whereas patients with normal AR had a median OS of 19.1 months (95% CI 10.6–20.3, p = 0.020, as shown in Fig. 3b). Figure 4 shows swimmers plot with individual outcomes and events.
Fig. 4. Swimmer plot of patient events.

Arrow indicates patients without PSA and radiological progression-free survival (PFS) up to cut-off date.
In this study, we also assessed, alterations in BRCA1, BRCA2, ATM, and PALB2 (Fig. 1). Two (10%) patients had germline mutations: one in ATM and one in BRCA2. The patient with germline ATM mutation had circulating raised AR and experienced early PD during 177Lu-PSMA-617 with OS of 9 months. The patient with BRCA2 mutation had circulating normal AR and presented a PSA response but early followed by PD with an OS of 5 months.
Discussion
In this prospective Phase 2 study, 37.5% of the patients had a PSA response to treatment with 177Lu-PSMA-617, a median PFS of 7.5 months, and a median OS of 12.4 months.
Our results are in line with those reported in a prospective single-arm Australian Phase 2 trial [17]. It reported that 17 of the 30 patients (57%) had a PSA decline, median PFS = 7.6 months, median OS = 13.5 months. Our trial was designed to evaluate the minimal effective activity of RLT, whereas the Australian trial was designed to optimise the effects of RLT excluding FDG-positive PSMA-negative disease [17]. This selection strategy was done in order to avoid enrolling cases with PSMA heterogeneity and neuroendocrine differentiation. Reports had indicated that a neuroendocrine phenotype may be more amenable to imaging by FDG rather than PSMA-targeting radioligands [23].
Overall, the 177Lu-PSMA-617 treatment was very well tolerated and even a low activity of 3.7 GBq/cycle was sufficient to produce objective response. The percentage of biochemical responses was <50%, but it should be underlined that 30 (75%) of these patients received a low dosage ranging from 3.7 to 4.4 GBq/cycle as they were at risk of side effects because of prior therapies received and older age. Although 6–7 GBq or higher dosage may produce better outcomes, the search for a minimal effective and non-toxic dosage is worth pursuing in the prospect of using RLT in combination with other treatments indicated at earlier disease stage.
In the past years, various retrospective studies and one prospective trial have assessed cumulative activity and safety of 177Lu-PSMA-617 for patients with mCRPC; however, in these mostly small trials, only a subgroup of recruited mCRPC patients seemed to derive a clear benefit [13–17, 24].
With established data of a prevalence of 30–40% of circulating AR aberrations in this group and unmet need of clinically relevant circulating biomarkers able to predict activity of radiometabolic treatments, interest in RLT arose in the hypothesis of increased 177Lu-PSMA-617 activity in a more selected group of patients with mCRPC according to circulating biomarker. The underlying biological mechanism by which AR amplification induces resistance to RLT remains to be defined. Yet we analysed the germinal DNA repair defects. The PROREPAIR-B study showed that DNA repair mutations had impact on mCRPC outcomes [25]. In addition, a recently reported series of 25 patients found that the patients with somatic BRCA2 mutation had potential better effect of 177Lu-PSMA-617 [26]. We analysed DDR genes, but we identified only two cases with germline mutations. However, larger studies analysing prostate cancer tissue are warranted because prostate cancers with defective DDR produce more PSMA [27]. Accordingly, such prostate cancers may respond better to PSMA-targeting treatments than prostate cancers without such defects.
The PSA decline to 177Lu-PSMA-617 in our study was comparable to that seen with cabazitaxel in the CARD trial [28]. This Phase 3 randomised trial assessed cabazitaxel vs NAD in patients already treated with NAD for <1 year. Cabazitaxel gave an OS benefit and was associated with a radiological response of 36.5% and a PSA decline ≥50% in 35.7% [28]. The Phase 2 TheraP trial randomised cabazitaxel 20 mg/m2 vs 177Lu-PSMA-617, and initial findings of the prospective randomised trial, TheraP, have been recently presented confirming the activity of 177Lu-PSMA-617 over that of cabazitaxel in mCRPC [29]. This trial is providing first comparative evidence of the efficacy of Lu-PSMA in pretreated mCRPC and also could provide an opportunity for post hoc analysis on biomarkers, considering that AR gain could be able to identify subgroup resistant to 177Lu-PSMA-617 according to the present results, but at least partially sensitive to full doses of taxanes [9, 30].
Interestingly, in view of sequential treatment strategy, the detection of relapse evidenced on 68Ga-PSMA-PET/CT observed in a few patients of our study underlines the possibility of use an alpha-emitting radioisotope 225Actinium in mCRPC progressing after 177Lu-PSMA-617 failure, due to its higher rates of double-strand DNA breaks in prostate cancer cells, with less tissue penetration and minimal bystander effects in PSMA-negative cells [31].
The safety and tolerability of 177Lu-PSMA-617 makes it amenable for combination therapy trials. Our results showing better outcome in patients with circulating normal AR support investigation of NAD in combination clinical trials with 177Lu-PSMA-617 in selected patients according to AR amplification.
The limitations of our study are the relatively small number of patients, the heterogeneous characteristics of patients, and the mono-institutional design. Moreover, because of the lack of tumour content assessment, detection of an AR-gained clone may be more likely at higher circulating tumour fraction that in itself is prognostic; this could bias the ability to ascertain the predictive value of plasma AR with 177Lu-PSMA-617. Lastly, a targeted NGS assay on tissue and/or circulating tumour DNA would be also required to more sensitively evaluate for somatic alterations of DDR genes. However, this study would first combine clinical and translational evidence on 177Lu-PSMA-617 therapy in the context of a hypothesis-generating research in advanced prostate cancer.
In summary,177Lu-PSMA-617 treatment at low dosage shows promising activity and exceptionally low toxicity amenable for combination therapy trials. Plasma AR gene amplification identifies mCRPC resistant to 177Lu-PSMA-617, suggesting potential better activity of 177Lu-PSMA-617 in earlier phases of prostate cancer. Prospective evaluation of making treatment decision based on plasma AR is warranted.
Supplementary information
Author contributions
Conception and design: UDG, GP. Development of methodology: UDG, MS, SS, MM, FF, DC, GP. Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): UDG, MS, SS, SN, MM, GG, FF, CC, VC, MC, VDI, DC, FM, FEvE, GA, GP. Analysis and interpretation of data (e.g. statistical analysis, biostatistics, computational analysis): SN, MM, GG, FF, CC, VDI. Writing, review, and/or revision of the manuscript: UDG, MS, SS, SN, MM, GG, FF, CC, VC, MC, VDI, DC, FM, FEvE, GA, GP. Administrative, technical, or material support (i.e. reporting or organising data, constructing databases): MM, FF. Study supervision: UDG, MS, SS, GP.
Funding information
This work was partially supported by Associazione Italiana per la Ricerca sul Cancro (AIRC) and Ricerca Finalizzata Italian Ministry of Health (no grant number applicable).
Data availability
All data generated or analysed during this study are included in this published article and its Supplementary information data.
Ethics approval and consent to participate
Written informed consent was obtained from each patient prior to entry into the study. The study was conducted in compliance with the principles of the Declaration of Helsinki and local ethical and legal requirements. The protocol and informed consent were approved by the Institutional Review Board of Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST), Meldola, Italy (NCT03454750).
Competing interests
UDG reports honoraria, consulting fees, or travel support from Merck, Bristol Myers Squibb, Janssen, Pfizer, Novartis, Astellas, Bayer, Sanofi, and Novartis and grant support from Merck and Amgen. VC reports honoraria, consulting fees, or travel support Bayer, Astellas, Janssen-Cilag, and Sanofi-Aventis. GA certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (e.g. employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: GA reports receiving commercial research grants from Janssen, Arno Therapeutics, and Innocrin Pharma; has received honoraria and/or travel support from the speakers’ bureaus of Janssen, Astellas, Sanofi-Aventis, and Roche/Ventana; has received travel support from Pfizer, Abbott Laboratories, Bayer Healthcare, and Essa Pharmaceuticals; has ownership interest (including patents) in The Institute of Cancer Research Rewards to Inventors; and is a consultant for/advisory board member of Janssen-Cilag, Veridex, Bayer Healthcare, Roche/Ventana, Astellas, Medivation, Pfizer, Novartis, Millennium Pharma, Abbott Laboratories, and Essa Pharma.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01508-5.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Attard G, Parker C, Eeles RA, Schroder F, Tomlins SA, Tannock I. Prostate cancer. Lancet. 2016;387:70–82. doi: 10.1016/S0140-6736(14)61947-4. [DOI] [PubMed] [Google Scholar]
- 3.Quigley DA, Dang HX, Zhao SG, Lloyd P, Aggarwal R, Alumkal JJ, et al. Genomic hallmarks and structural variation in metastatic prostate cancer. Cell. 2018;174:758–69. doi: 10.1016/j.cell.2018.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar A, Coleman I, Morrissey C, Zhang X, True LD, Gulati R, et al. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat Med. 2016;22:369–78. doi: 10.1038/nm.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mateo J, Seed G, Bertan C, Rescigno P, Dolling D, Figueiredo I, et al. Genomics of lethal prostate cancer at diagnosis and castration resistance. J Clin Invest. 2020;130:1743–51. doi: 10.1172/JCI132031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salvi S, Casadio V, Conteduca V, Burgio SL, Menna C, Bianchi E, et al. Circulating cell-free AR and CYP17A1 copy number variations may associate with outcome of metastatic castration-resistant prostate cancer patients treated with abiraterone. Br J Cancer. 2015;112:1717–24. doi: 10.1038/bjc.2015.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romanel A, Gasi Tandefelt D, Conteduca V, Jayaram A, Casiraghi N, Wetterskog D, et al. Plasma AR and abiraterone-resistant prostate cancer. Sci Transl Med. 2015;7:312re10. doi: 10.1126/scitranslmed.aac9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conteduca V, Wetterskog D, Sharabiani MTA, Grande E, Fernandez-Perez MP, Jayaram A, et al. Androgen receptor gene status in plasma DNA associates with worse outcome on enzalutamide or abiraterone for castration-resistant prostate cancer: a multi-institution correlative biomarker study. Ann Oncol. 2017;28:1508–16. doi: 10.1093/annonc/mdx155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conteduca V, Jayaram A, Romero-Laorden N, Wetterskog D, Salvi S, Gurioli G, et al. Plasma androgen receptor and docetaxel for metastatic castration-resistant prostate cancer. Eur Urol. 2019;75:368–73. doi: 10.1016/j.eururo.2018.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weineisen M, Schottelius M, Simecek J, Baum RP, Yildiz A, Beykan S, et al. 68Ga- and 177Lu-Labeled PSMA I&T: optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. J Nucl Med. 2015;56:1169–76. doi: 10.2967/jnumed.115.158550. [DOI] [PubMed] [Google Scholar]
- 11.Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer. 1998;82:2256–61. doi: 10.1002/(SICI)1097-0142(19980601)82:11<2256::AID-CNCR22>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 12.Bravaccini S, Puccetti M, Bocchini M, Ravaioli S, Celli M, Scarpi E, et al. PSMA expression: a potential ally for the pathologist in prostate cancer diagnosis. Sci Rep. 2018;8:4254. doi: 10.1038/s41598-018-22594-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schäfers M, Essler M, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017;58:85–90. doi: 10.2967/jnumed.116.183194. [DOI] [PubMed] [Google Scholar]
- 14.Ahmadzadehfar H, Wegen S, Yordanova A, Fimmers R, Kürpig S, Eppard E, et al. Overall survival and response pattern of castration-resistant metastatic prostate cancer to multiple cycles of radioligand therapy using [177Lu]Lu-PSMA-617. Eur J Nucl Med Mol Imaging. 2017;44:1448–54. doi: 10.1007/s00259-017-3716-2. [DOI] [PubMed] [Google Scholar]
- 15.Kratochwil C, Giesel FL, Stefanova M, Benešová M, Bronzel M, Afshar-Oromieh A, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with Lu-177 labeled PSMA-617. J Nucl Med. 2016;57:1170–6. doi: 10.2967/jnumed.115.171397. [DOI] [PubMed] [Google Scholar]
- 16.Kulkarni HR, Singh A, Schuchardt C, Niepsch K, Sayeg M, Leshch Y, et al. PSMA-based radioligand therapy for metastatic castration-resistant prostate cancer: the Bad Berka experience since 2013. J Nucl Med. 2016;57:97S–104S. doi: 10.2967/jnumed.115.170167. [DOI] [PubMed] [Google Scholar]
- 17.Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–33. doi: 10.1016/S1470-2045(18)30198-0. [DOI] [PubMed] [Google Scholar]
- 18.Goodwin JF, Schiewer MJ, Dean JL, Schrecengost RS, de Leeuw R, Han S, et al. A hormone-DNA repair circuit governs the response to genotoxic insult. Cancer Discov. 2013;3:1254–71. doi: 10.1158/2159-8290.CD-13-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polkinghorn WR, Parker JS, Lee MX, Kass EM, Spratt DE, Iaquinta PJ, et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 2013;3:1245–53. doi: 10.1158/2159-8290.CD-13-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin Y, Li R, Xu K, Ding S, Li J, Baek G, et al. Androgen receptor variants mediate DNA repair after prostate cancer irradiation. Cancer Res. 2017;77:4745–54. doi: 10.1158/0008-5472.CAN-17-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paganelli G, Sarnelli A, Severi S, Sansovini M, Belli ML, Monti M, et al. Dosimetry and safety of 177Lu PSMA-617 along with polyglutamate parotid glands protector: preliminary results in metastatic castration-resistant prostate cancer patients. Eur J Nucl Med Mol Imaging. 2020;47:3008–17. doi: 10.1007/s00259-020-04856-1. [DOI] [PubMed] [Google Scholar]
- 22.Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34:1402–18. doi: 10.1200/JCO.2015.64.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bakht MK, Lovnicki JM, Tubman J, Stringer KF, Chiaramonte J, Reynolds MR, et al. Differential expression of glucose transporters and hexokinases in prostate cancer with a neuroendocrine gene signature: a mechanistic perspective for FDG imaging of PSMA-suppressed tumors. J Nucl Med. 2020;61:904–10. doi: 10.2967/jnumed.119.231068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Eyben FE, Singh A, Zhang J, Nipsch K, Meyrick D, Lenzo N, et al. 177Lu-PSMA radioligand therapy of predominant lymph node metastatic prostate cancer. Oncotarget. 2019;10:2451–61. doi: 10.18632/oncotarget.26789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castro E, Romero-Laorden N, Del Pozo A, Lozano R, Medina A, Puente J, et al. PROREPAIR-B: a prospective cohort study of the impact of germline DNA repair mutations on the outcomes of patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2019;37:490–503. doi: 10.1200/JCO.18.00358. [DOI] [PubMed] [Google Scholar]
- 26.Conteduca V, Oromendia C, Vlachostergios PJ, Hackett A, Thomas C, Caseet A, et al. Clinical and molecular analysis of patients treated with prostate-specific membrane antigen (PSMA)-targeted radionuclide therapy. J Clin Oncol. 2019;37:272. doi: 10.1200/JCO.2019.37.7_suppl.272. [DOI] [Google Scholar]
- 27.Paschalis A, Sheehan B, Riisnaes R, Rodrigues DN, Gurel B, Bertan C, et al. Prostate-specific membrane antigen heterogeneity and DNA repair defects in prostate cancer. Eur Urol. 2019;76:469–78. doi: 10.1016/j.eururo.2019.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Wit R, de Bono J, Sternberg CN, Fizazi K, Tombal B, Wülfing C, et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med. 2019;381:2506518. doi: 10.1056/NEJMoa1911206. [DOI] [PubMed] [Google Scholar]
- 29.Hofman MS, Emmett L, Sandhu SK, Iravani A, Joshua AM, Goh JC, et al. TheraP: a randomised phase II trial of 177Lu-PSMA-617 (LuPSMA) theranostic versus cabazitaxel in metastatic castration resistant prostate cancer (mCRPC) progressing after docetaxel: Initial results (ANZUP protocol 1603) J Clin Oncol. 2020;38:5500. doi: 10.1200/JCO.2020.38.15_suppl.5500. [DOI] [Google Scholar]
- 30.Conteduca V, Castro E, Wetterskog D, Scarpi E, Jayaram A, Romero-Laorden N, et al. Plasma AR status and cabazitaxel in heavily treated metastatic castration-resistant prostate cancer. Eur J Cancer. 2019;116:158–68. doi: 10.1016/j.ejca.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 31.De Vincentis G, Gerritsen W, Gschwend JE, Hacker M, Lewington V, O’Sullivan JM, et al. Advances in targeted alpha therapy for prostate cancer. Ann Oncol. 2019;30:1728–39. doi: 10.1093/annonc/mdz270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and its Supplementary information data.