Abstract
Background
Novel immunotherapies targeting cancer-associated truncated O-glycans Tn (GalNAcα-Ser/Thr) and STn (Neu5Acα2–6GalNacα-Ser/Thr) are promising strategies for cancer treatment. However, no comprehensive, antibody-based mapping of truncated O-glycans in tumours exist to guide drug development.
Methods
We used monoclonal antibodies to map the expression of truncated O-glycans in >700 tissue cores representing healthy and tumour tissues originating from breast, colon, lung, pancreas, skin, CNS and mesenchymal tissue. Patient-derived xenografts were used to evaluate Tn expression upon tumour engraftment.
Results
The Tn-antigen was highly expressed in breast (57%, n = 64), colorectal (51%, n = 140) and pancreatic (53%, n = 108) tumours, while STn was mainly observed in colorectal (80%, n = 140) and pancreatic (56%, n = 108) tumours. We observed no truncated O-glycans in mesenchymal tumours (n = 32) and low expression of Tn (5%, n = 87) and STn (1%, n = 75) in CNS tumours. No Tn-antigen was found in normal tissue (n = 124) while STn was occasionally observed in healthy gastrointestinal tissue. Surface expression of Tn-antigen was identified across several cancers. Tn and STn expression decreased with tumour grade, but not with cancer stage. Numerous xenografts maintained Tn expression.
Conclusions
Surface expression of truncated O-glycans is limited to cancers of epithelial origin, making Tn and STn attractive immunological targets in the treatment of human carcinomas.
Subject terms: Glycosylation, Glycosylation, Tumour biomarkers, Cancer imaging, Glycobiology
Background
Novel immunological therapies such as Chimeric Antigen Receptor (CAR) T cells, Bi-specific T-cell Engagers (BiTEs), and antibody–drug conjugates (ADCs) are promising strategies for cancer treatment. Nevertheless, treatment of solid tumours, as well as severe toxic reactions, are still substantial challenges [1, 2]. In order to improve therapy, identification of tumour–antigens that are both highly and specifically expressed in tumours is needed. Much research is currently focused on targeting aberrant protein expression arising from mutations occurring in the tumour genome [3]. However, pursuing the tumour glycome provides a complimentary and attractive route for cancer-antigen discovery and therapy by enabling simultaneous, highly specific targeting of tumour-associated glycans and proteins [4, 5].
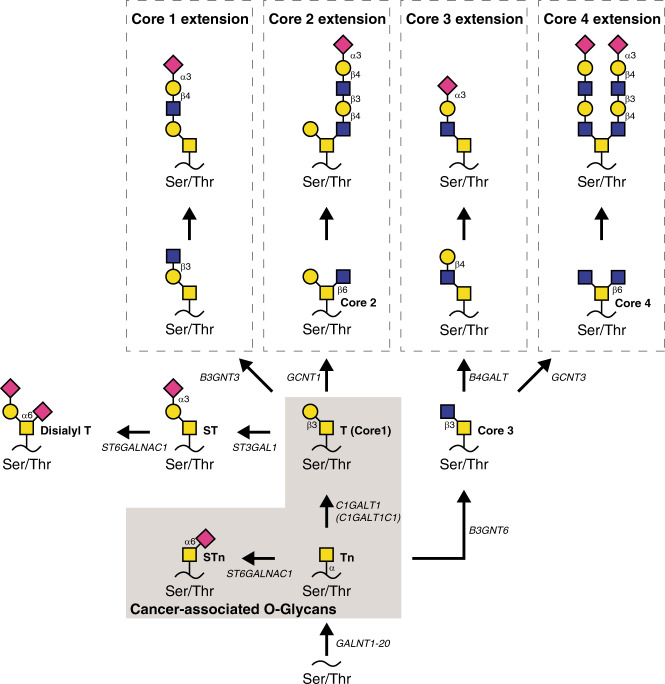
Glycosylation of proteins is one of the most abundant and diverse post-translational modifications and aberrant glycosylation is a common feature of cancer cells [6–8]. Notably, most FDA-approved biomarkers in cancer diagnostics detected by antibodies are either glycans or glycoproteins [9]. The two most common classes of glycans found on proteins are N-linked glycans, which are attached to asparagine residues, and GalNAc-type O-linked glycans, often attached to serine and threonine or, occasionally, tyrosine residues (from here on referred to as “O-glycans”) [10]. O-glycosylation is known to occur at ~85% of surface presented or secreted proteins [11, 12] and alterations in O-glycosylation is associated with malignancy [13]. In healthy human tissue, O-glycosylation is initiated when GalNAc (N-acetylgalactosamine) residues are attached to a polypeptide, which is followed by further elongation, branching and capping to form elaborate structures (Fig. 1). However, in many human cancers, incomplete O-glycosylation results in surface expression of truncated O-glycans that usually reside as intermediates in the secretory pathway [6, 14–17]. These include the Tn-antigen (Thomsen-nouveau; GalNAcα-Ser/Thr), its sialylated version the STn-antigen (sialyl-Tn; Neu5Acα2–6GalNAcα-Ser/Thr) and the elongated T-antigen (Thomsen-Friedenreich; Galβ1-3GalNAcα-Ser/Thr).
Fig. 1. O-glycan biosynthesis and structure.
Overview of the biosynthesis of core 1, 2, 3 and 4 O-GalNAc glycans (adapted from Varki et al. [15]). Black backbones depict proteins, black lines depict glycosidic bonds with the type of bond assigned. Arrows illustrate enzymatic elongation of glycan structures catalysed by assigned transferases. Structures highlighted in the grey box are the cancer-associated glycans mapped in this study. The graphic depiction is in accordance with the Symbol Nomenclature for the Graphical Representation of Glycans [80]. Yellow square = GalNAc. Pink square = Sialic acid (Neu5Ac). Blue square = Glucose. Yellow circle = Galactose. Tn-antigen = Thomsen-nouveau antigen, STn-antigen = sialyl-Tn antigen, T-antigen = Thomsen-Friedenreich antigen, Ser/Thr = serine/threonine.
As early as 1969, researchers identified the Tn-antigen on the surface of tumour cells using the Helix pomatia lectin (HPL) [18]. Seminal work was done by Springer et al. in the 70s and 80s, establishing the Tn-antigen as a pan-cancer marker in human cancers [13]. In parallel, the STn-antigen attracted much attention, especially in the 80s and 90s, with an STn-targeted anticancer vaccine entering Phase III clinical trials [19]. The STn-based vaccine was well tolerated in patients, but the Phase III clinical trial failed to demonstrate improved median time to disease progression, possibly due to the fact that patients had not been evaluated for STn expression prior to enrolment [20–22]. In contrast, the T-antigen, although expressed on many cancer cells, is not regarded as a cancer-specific antigen due to its expression in healthy tissue, e.g. hematopoietic stem cells and skin keratinocytes, and has attracted less attention as a therapeutic target [6, 19, 23].
The relevance of truncated O-glycans as targets for treatment is underscored by the fact that expression of truncated O-glycans contribute to several hallmarks of cancer and correlates with clinical outcome [6, 24, 25]. Expression of truncated O-glycans directly induces oncogenic features such as a change in differentiation, increased invasion and impaired adhesion in skin organoids [23, 26, 27] and intestinal cancers in murine models [28, 29]. In addition, elongation of the Tn-antigen confers resistance to the immunological killing of tumour cells [30]. Furthermore, truncated O-glycans have been found in the early stages of tumorigenesis, e.g. in polyps, pre-malignant lesions and cancer-adjacent tissue [31, 32]. In a clinical setting, investigators have found that the expression of truncated glycans correlates with poor clinical outcomes amongst patients with urinary bladder carcinomas and colorectal cancer [33, 34]. Similarly, Tn expression has been found to correlate positively with higher pathological tumour-node metastasis (pTNM) stages, shortened disease-free intervals and positive lymph node status in breast cancer patients [35, 36], while changes in O-glycosylation of mucins correlate with the progression of pancreatic cancer and influence oncogenic properties in pancreatic tumour cells [37, 38]. In addition, levels of STn in carcinomas or sera of patients with gastric, colorectal, and ovarian cancer correlates with poor prognosis while autoantibodies against aberrantly glycosylated mucins have been found in colorectal cancer patients [39–42]. Taken together, these findings suggest that truncated O-glycans induce oncogenic features, contribute to tumour severity, and affect tumour-immune interactions—and thus, escaping potential treatments targeting truncated O-glycans may come with a severe fitness cost for cancer cells.
Despite being identified as tumour markers almost half a century ago, no FDA-approved drugs target truncated O-glycans. Even so, the emergence of novel immunological therapies aiming at the tumour glycome is predicted to change the status quo and enable us to harness the therapeutic potential of cancer-associated truncated O-glycans. However, this requires a revisited and updated characterisation of the expression of truncated O-glycans in human cancers. The literature on Tn, STn and cancer is vast [6, 19]. Still, many studies do not directly address the subcellular location of the Tn, STn or T-antigens on the cell surface vis-à-vis solely as intracellular, biosynthetic intermediates in the secretory pathway, or the heterogeneity of the antigen expression within single tumours. Furthermore, mapping of truncated O-glycans has been hampered by methodological challenges, such as distinguishing between the blood type antigens (blood type a; GalNAc-α) and the Tn epitope (i.e. GalNAc attached to a protein) when using lectins such as HPL—a challenge that well-executed, antibody-based strategies potentially resolves [43]. In this paper, we provide a reference mapping of Tn, STn and T-expression in a range of human cancers of epithelial and non-epithelial origin using well-characterised monoclonal antibodies for the guidance of modern, therapeutic strategies targeting truncated O-glycans in cancers.
Methods
Tumour microarrays
Tumour microarrays were acquired through US Biomax, Inc., USA, and a total of 769 tumour cores were probed for expression of truncated O-glycans. The following microarrays were used in this study: BC0813b, CO1501, LC806, PA2081c, Hski-C072PT-01, GL803c, GL151, T291b, T261a and FDA999v. Patient specifications (age and sex), as well as tumour grade, stage, organ of origin, pathological diagnosis, and tissue type (adjacent, inflammation, malignant, metastasis or normal), were included in the dataset based on information from the US Biomax Inc. website (https://www.biomax.us/). Individual tumour cores that were lost during staining procedures were excluded from the analyses and n-numbers used in results indicate the number of remaining samples reliably stained.
Immunohistochemistry of tumour microarrays
Tumour microarrays were deparaffinated and heat-induced epitope retrieval was conducted using Tris-EDTA buffer (pH = 9). The microarrays were stained for immunohistochemistry (IHC) using the monoclonal murine antibodies 5F4 (HB-Tn, anti-Tn, IgM), 3F1 (HB-STn, anti-STn, IgG1), or 3C9 (HB-T, anti-T, IgM) at 4 °C overnight. All three antibodies were produced from hybridoma cells in our own laboratory and have been thoroughly characterised [30, 44–49]. Staining was performed using the EnVision System-HRP (cat# K400111-2, Agilent, Glostrup, DK) according to the manufacturer’s instructions. Samples were mounted with Pertex (Sigma-Aldrich, Søborg, DK) using the manufacturer’s instructions and scanned using Axio Scan Z1. Each core was manually evaluated by at least two individuals independently, including a trained pathologist. The final scoring was achieved by averaging the scores, and cores with large discrepancies were re-evaluated. The intensity of staining was graded from 0–4 and the percentage of the tumour stained was noted (excluding non-tumour tissue). Images scoring 3 or 4, with ≥10% of the tumour being positive, and with membranous accentuation, were annotated as positive for truncated O-glycan surface expression. Images scoring 0–2, with <10% of the tumour staining positive, or with only cytosolic staining, were annotated as negative.
Confocal microscopy and co-localisation analysis
Sections were deparaffinized and heat-induced epitope retrieval was conducted using Tris-EDTA buffer (pH = 9). Indirect immunofluorescence was performed using biotinylated Vicia Villosa Lectin (VVA; cat# B-1235-2, Vector Laboratories, Burlingame, CA, USA) and polyclonal rabbit anti-Desmocollin-2 antibody (DSC2; cat# 13876-1-AP, Proteintech, Manchester, UK) followed by secondary streptavidin Alexa Fluor 488-conjugate (cat# S11223, ThermoFischer Scientific) and Alexa Fluor 594-conjugated goat anti-rabbit IgG (H + L) antibody (cat# A-11012, ThermoFischer Scientific). Nuclei were stained with DAPI and sections were mounted with ProLong Gold Antifade Mountant (ThermoFischer Scientific). Confocal images were captured on a Zeiss LSM 710 using a 405-nm diode laser for the DAPI channel, a 488-nm argon laser for the Alexa Fluor 488 channel and a 561-nm solid-state laser for the Alexa Fluor 594 channel. Exposure was set for the optimal signal-to-noise ratio for each image. Confocal images were used for co-localisation analysis using the ImageJ addon Coloc 2. The background was subtracted from the individual channels, and the Pearson correlation coefficient, Costes’ P value, and Mander’s co-localisation coefficients tM1 and tM2 values were determined using the Costes’ automatic threshold algorithm. Costes’ P value was calculated using 200 randomisations [50–52]. Costes’ P value >0.95 was considered significant.
Patient-derived xenograft models
Studies using a low-passage Patient-Derived Xenograft (PDX) model for various primary tumours were carried out at Champions Oncology. Briefly, patient-derived tumour cells derived from endometrial, non-small-cell lung carcinoma (NSCLC), bladder, hepatocellular carcinoma, pancreatic, gastric, ovarian, uterine, breast, colorectal, cholangiocarcinoma (CCA), head and neck, sarcoma and oesophageal cancers were expanded in nu/nu mice. When tumour size fell within the range of 150–300 mm3, tumours were fixed in neutral buffered formalin, embedded in paraffin, and processed for IHC using the 5F4 antibody, as described above. The histochemical score (H-score) was calculated for each PDX core using the QuPath software [53]. The diameters of each core were automatically set to 1.2 mm. Cells were detected using the following parameters: pixel size 1 µm, background radius = 10 µm, sigma = 1.5 µm, minimum area = 10 µm2, maximum area = 400 µm2, threshold = 0.1, maximum background intensity = 2, and cell expansion = 15 µm. Cancer cells, stromal cells and immune cells were detected by training the object classifier. The staining intensity was determined by the mean intensity and was classified as either high intensity (>75th percentile), medium intensity (50th−75th percentile), low intensity (25th–50th percentile), and negative (<25th percentile). H-scores were reported between 0–300, with 300 being homogenous, high-intensity stain. The cellular surface stain was scored manually. Cores were scored positive only if ≥10% of the core stained medium or high and if they were annotated for surface staining.
Statistics, image analysis and code availability
Statistical analysis was conducted in R and the source code is included in the supplementary materials [54]. Briefly, odds ratios and confidence intervals were calculated using logistic regression. Correlation tests were performed by calculating the Pearson correlation coefficient and its associated P value. The Wilcoxon rank-sum test was used to test for significant differences in H-scores, percent positivity and staining intensity of PDX cores annotated with surface staining or non-surface staining. Two-tailed P values <0.05 were regarded as statistically significant.
Results
Tumours of epithelial origin express high levels of truncated O-glycans
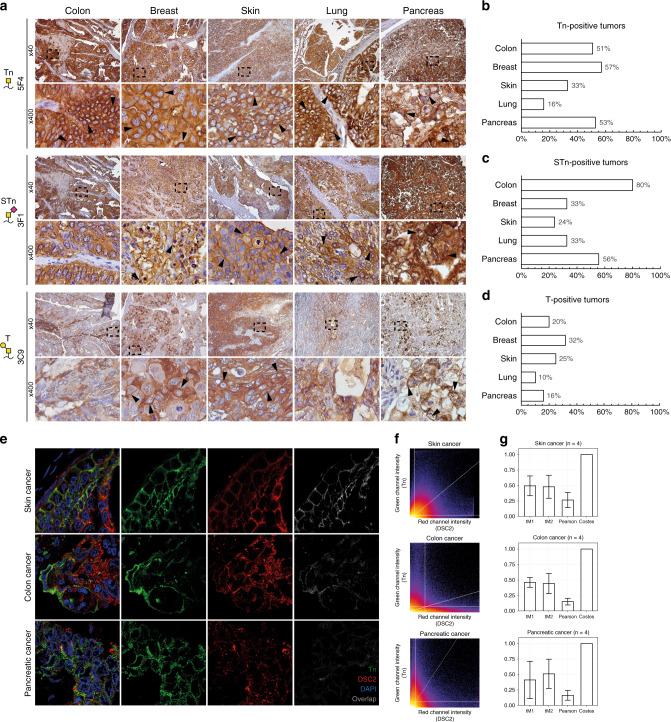
We scored all IHC stained cores as either positive (medium or high staining intensity, with ≥10% positivity, and with membranous accentuation) or negative (weak staining intensity, <10% positivity, no surface staining, or pure cytosolic staining). Both the Tn-antigen (GalNAcα-Ser/Thr) and the STn-antigen (Neu5Acα2–6GalNAcα-Ser/Thr) showed high levels of surface expression in a large fraction of the tumours derived from epithelial tissue of the breast, colon, lung, skin and pancreas (Table 1 and Fig. 2a–c). In contrast, lower surface expression levels of the T-antigen was observed (Table 1 and Fig. 2a, d). More specifically, the Tn-antigen was detected in 57% of all breast cancer cores, while the STn-antigen was detected in 33%; of which the majority were invasive ductal carcinomas. In colorectal adenocarcinomas, we found surface expression of the Tn-antigen in 51% of the cases, while STn was seen in 80% of the cases. In pancreatic cancer, we found 53% Tn-positive and 56% STn-positive cases, with an almost even distribution among the examined subtypes. The Tn-antigen was identified in only 16% of all lung cancers, while STn was detected in 33% of lung cancers. Surface-localised Tn-antigen was detected in 33% of all skin tumours from both squamous and basal carcinomas, while STn was detected in 24% of the skin tumours. Reactivity against truncated O-glycans was also prominent in the cytosol of all tumour types, often showing supranuclear staining indicative of Golgi localisation, whereas nuclear staining was not observed (Fig. 2a, e). Using confocal microscopy, we confirmed co-localisation of the Tn-antigen and the cell surface marker desmocollin-2 in a subset of tumours from skin (n = 4), colon (n = 4) and pancreatic (n = 4) cancers (Fig. 2e–g). Co-localisation analysis revealed a weak but significant correlation between desmocollin-2 and Tn-antigen staining with an average Pearson correlation coefficient of 0.27 (SD = 0.12) for skin cancer, 0.15 (SD = 0.05) for colon cancer and 0.16 (SD = 0.08) for pancreatic cancer, all with Costes’ P values of 1. Calculation of Manders’ co-localisation coefficients showed that, on average, 49% (SD = 16%), 46% (SD = 8%) or 41% (SD = 30%) of all desmocollin-2 signal above threshold co-localised with Tn-antigen for skin, colon and pancreatic cancers, respectively, while 48% (SD = 18%), 44% (SD = 16%), or 51% (SD = 24%) of all Tn-antigen signal above threshold co-localised with desmocollin-2 (Fig. 2g).
Table 1.
Truncated O-glycans in human cancer.
| Positive/Total (% positive) | |||||||
|---|---|---|---|---|---|---|---|
| Tn | STn | T | |||||
| Breast cancer, total | 35/64 | (57%) | 22/68 | (33%) | 18/57 | (32%) | |
| Invasive ductal carcinoma | 32/55 | (58%) | 22/59 | (37%) | 15/48 | (31%) | |
| Medullary carcinoma | 3/9 | (33%) | 0/9 | (0%) | 3/9 | (33%) | |
| Colorectal cancer, total | 71/140 | (51%) | 112/140 | (80%) | 26/128 | (20%) | |
| Adenocarcinoma | 69/136 | (51%) | 110/135 | (81%) | 26/124 | (21%) | |
| Signet-ring cell carcinoma | 2/4 | (50%) | 2/4 | (50%) | 0/4 | (0%) | |
| Lung cancer, total | 13/80 | (16%) | 26/79 | (33%) | 8/79 | (10%) | |
| Adenocarcinoma | 2/17 | (12%) | 8/17 | (47%) | 1/16 | (6%) | |
| Bronchioloalveolar carcinoma | 1/9 | (11%) | 1/9 | (11%) | 1/9 | (11%) | |
| Large cell undifferentiated carcinoma | 2/8 | (25%) | 2/8 | (25%) | 1/8 | (13%) | |
| Papillary adenocarcinoma | 3/9 | (33%) | 6/9 | (67%) | 2/9 | (22%) | |
| Squamous cell carcinoma | 5/31 | (16%) | 10/31 | (32%) | 3/31 | (10%) | |
| Small cell carcinoma | 0/5 | (0%) | 0/4 | (0%) | 0/5 | (0%) | |
| Skin cancer, total | 13/39 | (33%) | 9/37 | (24%) | 6/24 | (25%) | |
| Squamous cell carcinoma | 4/18 | (22%) | 9/18 | (50%) | 4/15 | (27%) | |
| Basal cell carcinoma | 7/15 | (47%) | 0/15 | (0%) | 2/13 | (15%) | |
| Pancreatic cancer, total | 57/108 | (53%) | 60/108 | (56%) | 17/108 | (16%) | |
| Adenocarcinoma | 7/12 | (58%) | 8/12 | (67%) | 2/12 | (19%) | |
| Adenosquamous carcinoma | 5/6 | (83%) | 5/6 | (83%) | 0/6 | (0%) | |
| Duct adenocarcinoma | 36/54 | (67%) | 41/54 | (76%) | 10/54 | (19%) | |
| Islet cell tumour | 0/20 | (0%) | 0/20 | (0%) | 2/20 | (10%) | |
| Mucinous adenocarcinoma | 2/2 | (100%) | 2/2 | (100%) | 1/2 | (50%) | |
| Neuroendocrine tumour | 1/2 | (50%) | 0/2 | (0%) | 0/2 | (0%) | |
| Undifferentiated adenocarcinoma | 1/2 | (50%) | 0/2 | (0%) | 0/2 | (0%) | |
| Osteosarcoma, total | 0/8 | (0%) | N/D | 0/8 | (0%) | ||
| Chondroblastic | 0/4 | (0%) | 0/4 | (0%) | |||
| Osteoblastic | 0/4 | (0%) | 0/4 | (0%) | |||
| Bone marrow cancers | 0/8 | (0%) | 0/8 | (0%) | N/D | ||
| Plasma cell myeloma | 0/8 | (0%) | 0/8 | (0%) | |||
| Tumours of the CNS, total | 4/87 | (5%) | 1/75 | (1%) | 17/75 | (23%) | |
| Glioblastoma | 4/40 | (10%) | 0/40 | (0%) | 6/40 | (15%) | |
| Ependymoma | 0/16 | (0%) | 0/4 | (0%) | 2/4 | (50%) | |
| Astrocytoma | 0/15 | (0%) | 0/15 | (0%) | 6/15 | (40%) | |
| Oligoastrocytoma | 0/4 | (0%) | 0/4 | (0%) | 1/4 | (25%) | |
| Oligodendroglioma | 0/9 | (0%) | 1/9 | (11%) | 2/9 | (22%) | |
| Medulloblastoma | 0/3 | (0%) | 0/3 | (0%) | 0/3 | (0%) | |
Tn Thomsen-nouveau antigen, GalNAcα-Ser/Thr, STn sialyl-Tn antigen, Neu5Acα2–6GalNAcα -Ser/Thr. T Thomsen-Friedenreich antigen, Galβ1-3GalNAcα-Ser/Thr. N/D not determined, i.e. samples were not available for analysis.
The number of tumour cores classified as positive for Tn-, STn- or T-expression defined as (a) strong staining, (b) membranous accentuation and (c) >10 % of the tumour being strongly stained using IHC (before /) relative to the total number of tumour cores sampled (after /). The percentage of positive tumour cores is given in brackets. Bold values are aggregated results from all tumor core of a specific tumor type (e.g. “Breast cancer”) whereas results from tumor subclasses (e.g. “Medullary carcinoma”) are not bold.
Fig. 2. Truncated O-glycans are surface exposed in tumours of epithelial origin.
a Representative micrographic pictures at ×40 magnification and ×400 magnification (black box) of five different epithelial tumour samples IHC stained using monoclonal antibodies against the Tn- (5F4), STn- (3F1) and T-antigens (3C9). Arrows highlight membranous accentuation. b–d Graphic illustrations of the percentage of each stained type of cancer staining positive for the given glycan structure. e Representative confocal images of skin, colon and pancreatic tumours stained for the Tn-antigen using the VVA lectin (green) and desmocollin-2 (DSC2, red). Nuclear staining was done using DAPI. From left to right: combined channel image, green channel image (Tn), red channel image (DSC2) and the grayscale image showing only co-localised pixels following co-localisation analysis. f Representative fluorograms, dot plots showing the green and red intensity of each individual pixel of the image. Thresholds for the green and red channels are indicated as horizontal and vertical lines, respectively, and a trendline for the correlation has been drawn as a diagonal line. g Barplot representing mean ± SD of calculated Manders’ co-localisation coefficients (tM1 and tM2), Pearson correlation coefficients, Costes’ P values from confocal images of skin (n = 4), colon (n = 4) and pancreatic (n = 4) tumours.
None or few tumours originating from the CNS or mesenchymal tissue express truncated O-glycans
When probing expression of truncated O-glycans in tumours of the CNS or mesenchymal tissue, little surface expression was observed (Table 1 and Supplementary Fig. 1). Importantly, we observed surface expression of the Tn-antigen in a subset of glioblastoma cancers (10%), while STn expression was only detected in a single oligodendroglioma case. T-expression was in general more predominant in tumours of the CNS and was observed in 23% of the samples. We found no expression of truncated O-glycans in cancers of mesenchymal origin.
Tn is not expressed in healthy, human tissue, whereas STn and T are occasionally detected
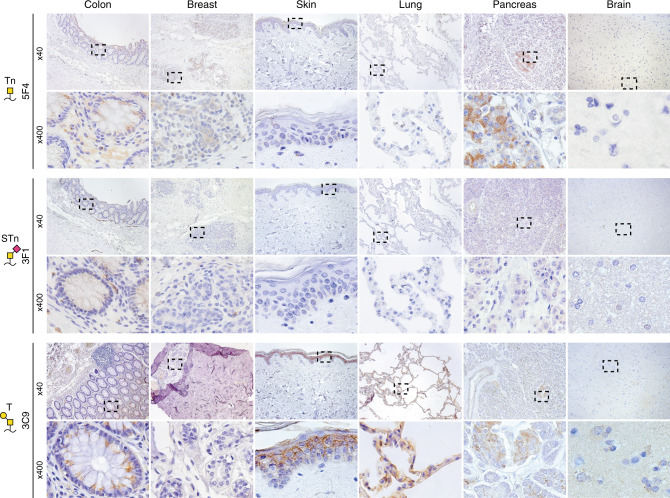
To probe the expression of truncated O-glycans outside a cancer context, we analysed the expression of Tn-antigen, STn-antigen and T-antigen in normal tissue, tumour-adjacent tissue and inflamed tissue (Fig. 3 and Supplementary Table 1). We did not find Tn surface expression in any healthy, normal tissues and only found Tn surface expression in a single sample of tumour-adjacent breast tissue. Surface STn expression was observed in tissue from healthy gastrointestinal tracts, with 31% of colon samples being STn-positive and reactivity observed in oesophageal, laryngeal and tonsillar tissue. Importantly, STn surface expression was only observed in suprabasal cells of oesophageal and laryngeal tissues, while STn expression in the basal cells was confined to the intracellular compartment. Likewise, STn expression in the healthy colon was localised to the apical cell membrane and was not found in the basal membrane of the cells. The T-antigen was surface exposed in normal skin and pancreatic tissue and in tumour-adjacent tissue in the breast and pancreas, with minute detection in healthy laryngeal and colon tissue.
Fig. 3. Truncated O-glycans are rarely surface exposed in healthy tissue.
Representative micrographic pictures at ×40 magnification and ×400 magnification (black box) of healthy tissue IHC stained using monoclonal antibodies against the Tn- (5F4), STn- (3F1) and T-antigens (3C9).
Truncated O-glycans are heterogeneously expressed within single tumours
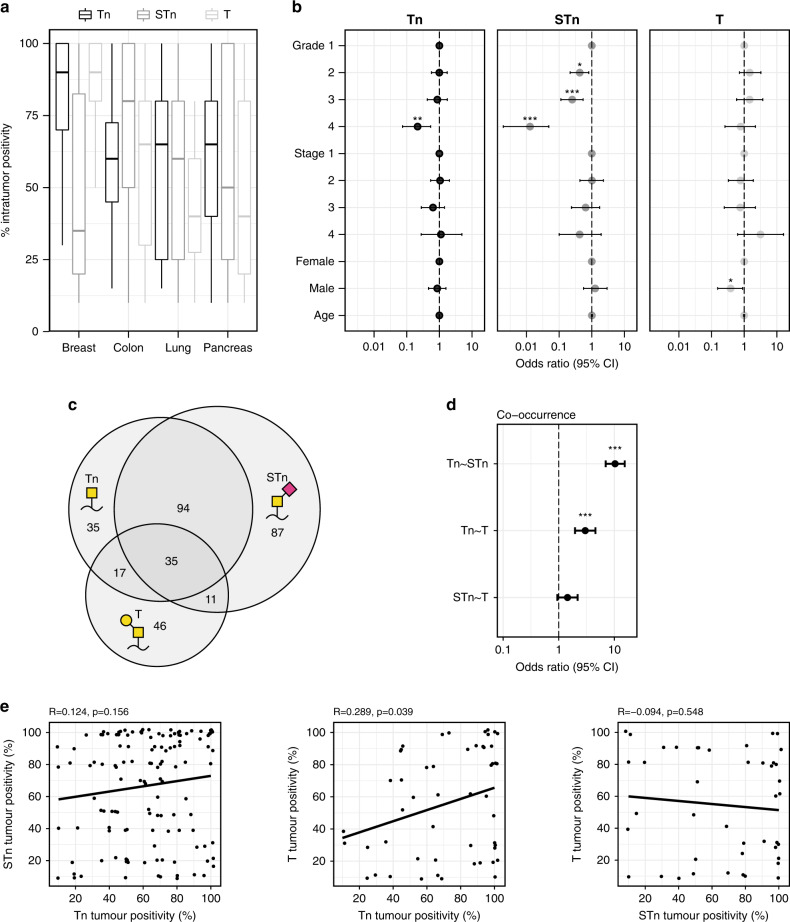
A substantial level of intratumor heterogeneity was observed within Tn, STn and T-positive tumours of the breast, colon, lung, pancreas and skin, ranging from a small subset of positive cells to the entire tumour core showing surface expression of truncated O-glycans (Figs. 4a and 2a). Across Tn-positive tumours, breast tumours showed the most consistently tumour-wide Tn expression, with an average of 84% of the cancer cells being positive. Likewise, the T-antigen was expressed in a high percentage of cancer cells in T-positive breast tumours, averaging at 77%, whereas the STn-antigen was expressed, on average, by just 46% of the cancer cells of STn-positive breast tumours. A high proportion of positive cancer cells was seen in STn-positive colon tumours, with an average of 73% of the cancer cells being STn positive. In lung and pancreatic tumours, Tn-positive tumours showed similar average proportions of cancer cells carrying Tn-antigen (60% in pancreatic and 56% in lung tumours), while the proportion of positive cancer cells in T-positive tumours were relatively low for both tumour types.
Fig. 4. Statistical analysis of expression of truncated O-glycans.
a Boxplots showing the distribution of the percentage of cancer cells expressing truncated O-glycans within positive tumours. b Odds ratios and 95% confidence intervals for the expression of the Tn-, STn- or T-antigen. For cancer grade, odds ratios were calculated compared to grade 1 cancers and were controlled for sex and age. For the cancer stage, odds ratios were calculated compared to stage 1 and were controlled for sex and age. For sex, odds ratios were calculated comparing male to female and were controlled for the grade, stage and age. For age, odds ratios were controlled for the grade, stage, and sex. c Venn diagram showing the number of tumour sections staining positive for respectively the Tn-, STn- and T-antigen, either alone or in combination. The number of tumour sections within the given sections of the diagram are depicted. d Odds ratios and 95% confidence intervals for the co-occurrence of Tn-, STn- and T-antigens. e Scatterplots representing the percentage of cancer cells within tumours positive for Tn-, STn- and T-antigen. The line represents trendline calculated for Pearson correlation coefficient. *P < 0.05, **P < 0.01, ***P < 0.001.
Truncated O-glycans are expressed in all stages of cancer development, but decrease with tumour grade
Cancers with truncated O-glycans were found throughout different tumour grades and stages (Fig. 4b). By calculating odds ratios comparing the odds for expression of truncated O-glycans in cancer grades 2, 3 and 4 to grade 1, we found a statistically significant lower odds ratio for Tn surface expression in grade 4 cancers (OR = 0.22, 95% CI =0.07–0.54, P value = 0.002), indicating that Tn surface expression may decrease in high-grade cancer. Furthermore, we found a gradual reduction in the odds ratio for STn surface expression with increasing cancer grades (grade 2 OR = 0.43, 95% CI =0.22–0.81, P value = 0.01; grade 3 OR = 0.25, 95% CI =0.11–0.54, P value <0.001; grade 4 OR = 0.01, 95% CI =0.002–0.05, P value <0.001). However, we did not observe any significant association between tumour grade and surface expression of T-structures. Likewise, no significant association was observed in this dataset between tumour stage and surface expression of truncated O-glycans. When controlled for grade, stage and sex, no statistically significant association was seen between age and surface expression of truncated O-glycans. Neither Tn nor STn expression was associated with gender when controlled for the grade, stage and age, but we did find a statistically significant lower odds ratio for the surface expression of the T-antigen in males compared to females (OR = 0.38, 95% CI =0.15–0.89, P value = 0.03).
The Tn- and STn-antigens often co-occur in the same tumours
Out of the 769 tissue cores from both healthy and cancerous tissues that were analysed in this work, 674 cores were stained for expression of the Tn-, STn- and T-antigen in parallel to test for co-occurrence of these truncated O-glycans. Of these, a total of 325 cores were stained positively for at least one of these three antigens (Fig. 4c). Of the 181 Tn-positive tumour cores, 129 (71%) also stained positive for STn-antigen, whereas only 52 (29%) stained positive for T-antigen. For the 227 STn-positive tumour cores, 129 (57%) also express Tn-antigen whereas only 46 (20%) showed simultaneous T-expression. In the 109 T-positive tumour cores, Tn-antigen and STn-antigen were observed in 52 (48%) and 46 (42%) of the same cores, respectively. Of all the stained tissue cores, 35 stained positive for the Tn-, STn- and T-antigens simultaneously. We found a statistically significant association between Tn- and STn-antigen expression (OR = 10.3, 95% CI =7–15.37, P value <0.001) and between Tn- and T-antigen expression (OR = 2.99, 95% CI =1.96–4.57, P value <0.001), but not STn- and T-antigen expression (Fig. 4d). We next tested the linear correlation between the percentage of positive cancer cells for Tn-, STn- and T-antigens within each of the analysed tumours and found a significant correlation between the percentage of cells positive for the Tn- and T-antigens (R = 0.289, P value = 0.039) (Fig. 4e).
Patient-derived xenografts express truncated O-glycans after engraftment and tumour growth
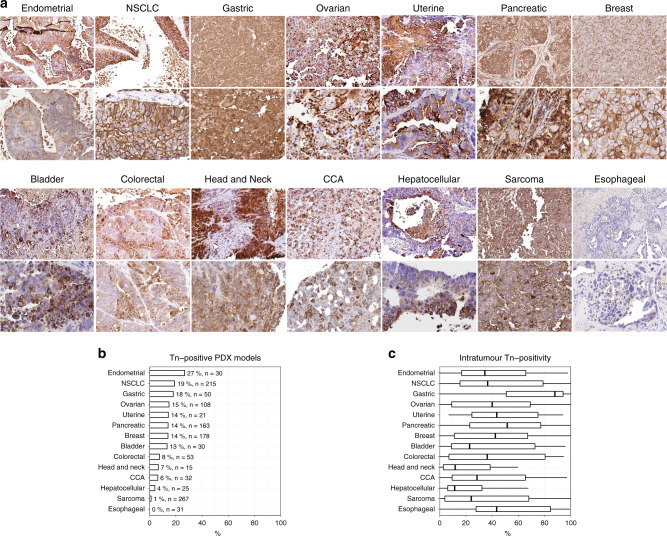
To investigate whether truncated O-glycans are expressed after grafting of tumour cells in a new microenvironment, we examined PDX models with implanted tumour cells from patients into nu/nu nude mice (Fig. 5). We probed Tn expression in xenografts of 14 different types of human tumours with the 5F4 antibody and evaluated the level of membrane staining (Fig. 5a). Tn expression (>10% medium-high intensity, surface staining) was observed in multiple xenografts from 13 out of 14 types of cancer, with a varying proportion of the xenografts staining positive within each cancer type (Fig. 5b, c). The tumours with the highest percentage of Tn-positive xenografts were endometrial cancers (30%, n = 30) followed by NSCLC (19%, n = 215), gastric cancers (18%, n = 50), ovarian cancers (15%, n = 108), uterine cancers (14%, n = 21), pancreatic cancers (14%, n = 163), breast cancers (14%, n = 178), bladder cancers (13%, n = 30), colorectal cancers (8%, n = 53), head and neck cancers (7%, n = 15), cholangiocarcinomas (CCA; 6%, n = 32), hepatocellular carcinomas (4%, n = 25), and sarcomas (1%, n = 267). No surface-localised Tn expression was observed in oesophageal cancers (n = 31). The intratumor staining patterns were heterogenous, with some tumours showing surface staining of individual cancer cells in a diffuse pattern (e.g., colorectal cancer), others showing a patched staining pattern (e.g., head and neck cancer) and some cancers showing near total Tn surface expression (e.g. NSCLC) (Fig. 5a, c).
Fig. 5. Tn is conserved on the cell surface after xenografting of tumour cells.
a Representative micrographic pictures at ×40 magnification and ×400 magnification of PDX samples after resection and IHC stained using a monoclonal antibody against Tn-antigen (5F4). b Quantification of the percentage of PDX models of each type of cancer that presented surface expression of Tn-antigen. c Boxplots showing the distribution of the percentage of cancer cells expressing truncated O-glycans with xenograft cores. NSCLC non-small-cell lung carcinoma, CCA cholangiocarcinoma.
Discussion
Despite improving clinical outcomes and representing conceptual innovations, immunotherapeutic strategies are currently hampered by a lack of efficacy against solid tumours and severe side effects. For example, patients undergoing therapy with CAR T cells experience cytokine release syndrome in 40–80% of cases and up to 50% experience neurological side effects ranging from transient aphasia to cerebral oedema and death [2]. Some toxic reactions are directly associated with damage to healthy cells expressing targeted antigens [55, 56]. Targeting the cancer-associated truncated O-glycans provides an attractive route to improve immunotherapies, but requires robust knowledge of the expression level, heterogeneity and subcellular location of O-glycans in different types of cancer.
Our study provides a reference mapping of truncated O-glycans in a range of human tumours derived from both epithelial and non-epithelial tissues using well-characterised monoclonal antibodies and more than 700 tumour cores. We found high expression levels of Tn- and STn-antigen in human cancers derived from epithelial tissue, such as tumours of the breast, colon, lung, pancreas, and skin (Fig. 2a–d and Table 1). These findings are in agreement with the literature, where cancers of epithelial origin in several studies have been identified as expressing truncated O-glycans [13, 31, 35, 57]. Of note, we found distinct variation in the expression of truncated O-glycans between different cancer types from the same organ. In breast cancer, ductal carcinomas often expressed both Tn- and STn-antigen, with lesser reactivity observed in medullary carcinomas (Table 1). Regarding lung cancer subtypes, adenocarcinomas and squamous cell carcinomas often showed STn expression, but rarely Tn-expression, whereas pancreatic tumours almost exclusively showed expression of truncated O-glycans in adenocarcinomas and never in islet cell tumours (Table 1). These findings could be due to different tissue or cell-specific mechanisms driving cancer development and further studies correlating molecular subtypes of cancers to the expression of truncated O-glycans would be of interest. In contrast to epithelial tumours, we found only limited expression of truncated O-glycans on the cell surface in the vast majority of cancers derived from the CNS, except for a subset (10%) of glioblastomas positive for the Tn-antigen (Table 1 and Supplementary Fig. S1). Similarly, our analysis of cancers derived from mesenchymal tissues suggests that the expression of truncated O-glycans in cancer is a phenomenon largely restricted to cancers of epithelial origin, and more specifically to adenocarcinomas and squamous cell carcinomas (Table 1 and Supplementary Fig. S1).
From an immunotherapeutic standpoint, the presence of truncated glycans on the surface of cancer cells is paramount for efficient targeting. Because of this, we included clear surface accentuation as a criterium marking a tumour core positive for Tn-, STn- or T-antigen in our immunohistochemical analysis. In order to provide further evidence of true surface localisation of these glycan structures in cancer cells, we conducted a co-localisation analysis between the Tn-antigen and the surface adhesion protein desmocollin-2 using confocal microscopy. We found variable, but significant, co-localisation between the two channels across multiple cancers (Fig. 2e–g). In addition, we found a low Pearson correlation coefficient, despite a clear membrane localisation of the Tn-staining in several tumour cores. Most likely, this discrepancy occurs when cancer cells with evident surface expression also have a high intracellular accumulation of Tn, which results in low tM2 coefficients. In addition, we observed that cancer cells with surface expression of the Tn-antigen tend to lose expression of desmocollin-2, which is consistent with our previous finding that Tn expression correlates with decreased cell–cell interactions [23]. We can therefore conclude that the Tn-antigen is localised to the plasma membrane in cancer cells, but the choice of desmocollin-2 as a surface marker is clearly suboptimal. For future endeavours, alternative methods, such as proximity ligation assays, may be more reliable to quantify the amount of truncated O-glycan structures localised to the plasma membrane. Indeed, the proximity ligation assay has previously been used to successfully detect the co-localisation of truncated Tn, STn and T glycans to the MUC16 and MUC1 mucins of ovarian cancers [58].
In our analysis, we set a threshold of ≥10% tumour positivity before a tumour core is annotated as positive. However, it should be emphasised that a large proportion of the examined tumours have ≥75% Tn surface positivity (20%, 42%, 5% and 19% of colon, breast, lung and pancreas tumours, respectively). The same is the case for STn, where we find 50% and 19%, respectively, of colon and pancreas tumours to have ≥75% STn positivity. Our findings are based on tumour microarrays (TMAs) and clinicopathological findings in TMAs have been shown to correlate well with findings in classical, pathological samples [59, 60]. Furthermore, we show that expression of Tn and STn is reduced with increasing tumour grade. This could indicate that truncation of O-glycans occurs at an early stage in the dedifferentiation of cancer cells, which is in agreement with studies finding that truncated of O-glycans drive early tumour development [28, 29] and that expression levels of T-synthase increase with tumour grade [61]. It could be hypothesised that truncated of O-glycans might provide proliferative or immune-shielding advantages to the tumour that is not needed when mutations accumulate and dedifferentiation drives additional growth.
Importantly, we did not identify surface expression of the Tn-antigen in 30 different healthy human tissues (Supplementary Table S1). We found noteworthy expression of STn-antigen in the healthy gastrointestinal tract and T-expression in pancreas and skin samples, but in all cases found the staining to be either intracellular or localised to suprabasal cells of the laryngeal and oesophageal epithelium or to the apical membrane of colon cells (Fig. 3). The interrogation of truncated O-glycans expression in healthy human tissue is important for any translational endeavour. Previous studies have found occasional reactivity with antibodies and lectins towards Tn-antigen in normal, colonic mucosa [57]. In addition, Tn-antigen has been found in human pancreatic acinar cells when using the VVA lectin [62]. However, lectins show lower specificity as compared to antibodies and furthermore, the samples were derived from tumour-adjacent tissue of patients undergoing surgery for pancreatic cancer, and thus samples were likely to be of pre-malignant character. A recent study has furthermore identified STn-antigen in the gastric epithelia of canines [63], while deacetylation has been found to expose STn-antigen on human colonic epithelia, but neither gastric nor pancreatic tissue [64]. However, the relevance of these findings is questionable in a clinical setting due to low expression levels, the use of non-specific lectins, acetyl masking of epitopes in human tissue, or insecure localisation of epitopes to the extra- versus intracellular milieu. Hence, there is continued interest and need to target short O-glycans possible combined with cancer-associated proteins in therapeutic strategies.
Interestingly, we found expression of Tn-antigen in 13 out of 14 tumour types in our PDX models (Fig. 5), indicating that cells carrying truncated O-glycans are maintained upon engraftment. Similar to the pattern observed in primary tumours, we observed a heterogenous staining pattern in the xenografts, with varying degrees of staining intensity and coverage of the graft. This heterogenous expression pattern implies that cells carrying truncated O-glycans might have a distinct function in tumorigenesis. Previously, we observed that induction of truncated O-glycans promotes invasion in a human organotypic model, possibly explaining the remaining Tn-expression upon engraftment and tumour growth [23]. With the use of xenografts as a model for metastasis and tumour growth, this could suggest that tumour cells carrying truncated O-glycans play a role in the seeding of cancer cells in a secondary microenvironment. However, comparing the percentage of PDX models with surface expression of the Tn-antigen to that of primary tumours, there seems to be a decreased expression of the Tn-antigen in xenograft tissues. One possibility is that this discrepancy could be induced by the culturing of the cancer cells prior to xenografting them into the mice, as cells that express Tn-antigen has been shown to have decreased adherence [23, 26]. In addition, it is very likely that the lack of an immunological selective pressure in the nude mice decreases the Tn expression, as it has been shown that Tn-antigen may be directly involved in immunosuppression through interaction with MGL [65, 66].
Furthermore, we found that most individual tumours show intratumor heterogeneity in Tn, STn and T-expression patterns (Fig. 4a). Many tumours show only partial Tn, STn or T positivity, suggesting that only a subset of tumour cells carry truncated O-glycans at a given point of time in tumour development. These findings are in agreement with previous studies that report a heterogenous expression pattern of e.g. the STn-antigen in gastric and colonic cancer [67]. In concert with the poor clinical prognosis of Tn or STn-positive tumours and the oncogenic features induces by O-glycans truncation, these findings might suggest that truncation of O-glycans occur in a crucial subpopulation of tumour cells, potentially driving cancer growth.
We found a significant association between the presence of different truncated O-glycans within the same tumour (Fig. 4c, d). Interestingly, even though Tn-antigen expression was found to be a predictor of both STn- and T-antigen expression, we only found a significant but weak correlation between the percentage of cancer cells positive for both the Tn- and T-antigens. In addition, we did not find a significant association between the STn- and T-antigens either. These findings indicate that while the Tn-, STn- and T-antigens will likely co-occur in some tumours that express truncated O-glycans, the tumour predominantly expresses one specific structure. This is in agreement with the apparent lack of a universal mechanism for induction of truncated O-glycan expression in cancers [16]. Loss-of-function mutations in the chaperone Cosmc/C1GALT1C1 (required for the proper functioning of the enzyme T -Synthase/C1GALT1) disrupt elongation from the initial GalNAc-residues and result in expression of Tn and STn (see Fig. 1) [68–70]. Studies have found that the Cosmc promotor is hypermethylated in a subset of Tn-positive pancreatic tumours [23], while other findings indicate no downregulation of neither Cosmc nor T-Synthase in Tn-positive colorectal cancers [31]. Meanwhile, pre-malignant lesions and tumours of the gastrointestinal tract are known to express the STn-antigen, a process in which the ST6GalNAc-I enzyme has been shown to be a key player [71]. As such, it can be speculated that the affinity of tumours towards specific truncated glycan structures arise due to competition of the Tn-antigen as the substrate for multiple elongation pathways. Our findings support this hypothesis, as we observed a significant association between Tn-antigen and respectively STn- and T-antigen, both of which are direct elongations of the Tn-antigen, but not between STn-antigen and T-antigen that are mutually exclusive elongations of Tn-antigen. Other findings suggest that pH regulation [72, 73] and re-localisation of glycosyltransferases in the secretory pathway [74, 75] contribute to truncation of O-glycans, but these findings are primarily ex vivo. Changes in the expression pattern of glycosyltransferases have also been found to influence the expression of truncated O-glycans, and upregulation of certain ppGalNAc-Ts induce oncogenic features [76–78], while mutations in BRAF have also been correlated to the expression of Tn [79]. None of these hypotheses has yet to—individually or combined—explain more than a limited subset of cases or provide any models that translate into clinical practice. In this study, we find that 29% of Tn-positive tumours also express T-antigen, suggesting that the surface expression of Tn-antigen does not result from a global obliteration of Tn-elongation in all cancers. Understanding truncation of O-glycans universally—or in the context of individual cancers—is important for the assessment of truncated O-glycans as potential targets for anticancer treatments.
In conclusion, our study provides a reference mapping of truncated O-glycan expression in human cancers including tumours of the breast, colon, lung, pancreas, and skin. We report that cancers of epithelial origin display high expression levels of Tn- and STn-antigen, with either the entire tumour or a subset of tumour cells carrying truncated O-glycans. In addition, we conducted a co-localisation analysis of confocal microscopy images to successfully verify surface localisation of the Tn-antigen in epithelial cancer cells. However, we found little surface expression of Tn- and STn-antigen in tumours of the CNS and no detectable truncated O-glycans in tumours of mesenchymal tissue. We found no surface expression of Tn-antigen when surveying 30 different types of healthy, human tissue and limited expression of STn- and T-antigen. These findings underscore the importance of considering truncated O-glycans as potential targets for the treatment of epithelial cancers in the era of immunological, antigen-targeted therapies.
Supplementary information
R_script_statistics_mapping_truncated_o_glycans
Supplementary Dataset for R Script - Primary
Supplementary Dataset for R Script - PDX
Acknowledgements
We thank laboratory technician Karin Uch Hansen for excellent technical assistance. We are also deeply thankful to Professor Jesper Reibel (jrei@sund.ku.dk) for an extraordinary job evaluating the immunohistochemistry of human cancers and healthy tissues.
Author contributions
TBR, MKMA, SD and HHW conceived and designed the study. TBR drafted the manuscript with MKMA and HHW. Experiments were performed by MKMA, SD, TBR, AG, JS, ET, JWP and ADH, while SD, TBR, JS, MKMA and HHW analysed the data with assistance from ET and AG. Statistical analysis was conducted by TBR and MKMA. All authors read and approved the manuscript.
Funding information
This work was supported by the European Commission (GlycoSkin H2020-ERC), The Friis Foundation, The Michelsen Foundation and the Neye Foundation. TBR has received funds from the University of Copenhagen Faculty of Health and Medical Sciences and The Danish Cancer Society. JWP has received funds from The Danish Cancer Society. The founders did not have any role in the acquisition, analysis of or decision to publish the results.
Data availability
All data included in the study are available upon request to the corresponding author.
Competing interests
HHW owns stocks and is a consultant for and co-founder of EbuMab, ApS. and GO-Therapeutics, Inc. JWP, HHW and MKMA are co-inventors of a novel antibody targeting truncated O-glycans. The remaining authors declare no competing interests. AG, JS and ET are employed by GO-Therapeutics, Inc.
Ethics approval and consent to participate
TMA material was obtained from US Biomax, Inc. The company informs that all human tissues are collected under HIPPA approved protocols, with full information of the donor and with their consent. All standard medical care has been followed, and protection of the donors’ privacy has been ensured.
Consent to publish
TMA material from US Biomax, Inc. is collected with full information of the donor and consent to use tumour material for research purposes, including publication of results.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Troels Boldt Rømer, Mikkel Koed Møller Aasted.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01530-7.
References
- 1.Martinez M, Moon EK. CAR T cells for solid tumors: new strategies for finding, infiltrating, and surviving in the tumor microenvironment. Front Immunol. 2019;10:128. [DOI] [PMC free article] [PubMed]
- 2.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321–30. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yarchoan M, Johnson BA, Lutz ER, Laheru DA, Jaffee EM. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer. 2017;17:209–22. doi: 10.1038/nrc.2016.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steentoft C, Migliorini D, King TR, Mandel U, June CH, Posey AD. Glycan-directed CAR-T cells. Glycobiology. 2018;28:656–69. doi: 10.1093/glycob/cwy008. [DOI] [PubMed] [Google Scholar]
- 5.Scott E, Elliott DJ, Munkley J. Tumour associated glycans: a route to boost immunotherapy? Clin Chim Acta Int J Clin Chem. 2020;502:167–73. doi: 10.1016/j.cca.2019.12.015. [DOI] [PubMed] [Google Scholar]
- 6.Stowell SR, Ju T, Cummings RD. Protein glycosylation in cancer. Annu Rev Pathol. 2015;10:473–510. doi: 10.1146/annurev-pathol-012414-040438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: diversity, synthesis and function. Nat Rev Mol Cell Biol. 2012;13:448–62. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kudelka MR, Ju T, Heimburg-Molinaro J, Cummings RD. Simple sugars to complex disease-mucin-type O-glycans in cancer. Adv Cancer Res. 2015;126:53–135. doi: 10.1016/bs.acr.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer. 2005;5:845–56. doi: 10.1038/nrc1739. [DOI] [PubMed] [Google Scholar]
- 10.Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, et al. editors. Essentials of glycobiology. 3rd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2015. p. 99–125. [PubMed]
- 11.Steentoft C, Vakhrushev SY, Joshi HJ, Kong Y, Vester-Christensen MB, Schjoldager KT, et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013;32:1478–88. [DOI] [PMC free article] [PubMed]
- 12.King SL, Joshi HJ, Schjoldager KT, Halim A, Madsen TD, Dziegiel MH, et al. Characterizing the O-glycosylation landscape of human plasma, platelets, and endothelial cells. Blood Adv. 2017;1:429–42. doi: 10.1182/bloodadvances.2016002121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Springer GF. T and Tn, general carcinoma autoantigens. Science. 1984;224:1198–206. doi: 10.1126/science.6729450. [DOI] [PubMed] [Google Scholar]
- 14.Chia J, Goh G, Bard F. Short O-GalNAc glycans: regulation and role in tumor development and clinical perspectives. Biochim Biophys Acta. 2016;1860:1623–39. doi: 10.1016/j.bbagen.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Brockhausen I, Stanley P. O-GalNAc glycans. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, et al., editors. Essentials of Glycobiology. 3rd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2015. p.113–125.
- 16.Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15:540–55. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- 17.Cervoni GE, Cheng JJ, Stackhouse KA, Heimburg-Molinaro J, Cummings RD. O-glycan recognition and function in mice and human cancers. Biochem J. 2020;477:1541–64. doi: 10.1042/BCJ20180103. [DOI] [PubMed] [Google Scholar]
- 18.Prokop O, Uhlenbruck G. N-acetyl-D-galactosamine in tumor cell membranes: demonstration by means of Helix agglutinins. Med Welt. 1969;46:2515–9. [PubMed] [Google Scholar]
- 19.Julien S, Videira PA, Delannoy P. Sialyl-tn in cancer: (how) did we miss the target? Biomolecules. 2012;2:435–66. doi: 10.3390/biom2040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miles D, Roché H, Martin M, Perren TJ, Cameron DA, Glaspy J, et al. Phase III multicenter clinical trial of the sialyl-TN (STn)-keyhole limpet hemocyanin (KLH) vaccine for metastatic breast cancer. Oncologist. 2011;16:1092–100. doi: 10.1634/theoncologist.2010-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacLean GD, Reddish MA, Koganty RR, Longenecker BM. Antibodies against mucin-associated sialyl-Tn epitopes correlate with survival of metastatic adenocarcinoma patients undergoing active specific immunotherapy with synthetic STn vaccine. J Immunother Emphas Tumor Immunol. 1996;19:59–68. doi: 10.1097/00002371-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Miles DW, Towlson KE, Graham R, Reddish M, Longenecker BM, Taylor-Papadimitriou J, et al. A randomised phase II study of sialyl-Tn and DETOX-B adjuvant with or without cyclophosphamide pretreatment for the active specific immunotherapy of breast cancer. Br J Cancer. 1996;74:1292–6. doi: 10.1038/bjc.1996.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radhakrishnan P, Dabelsteen S, Madsen FB, Francavilla C, Kopp KL, Steentoft C, et al. Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proc Natl Acad Sci USA. 2014;111:E4066–4075. doi: 10.1073/pnas.1406619111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ju T, Otto VI, Cummings RD. The Tn antigen-structural simplicity and biological complexity. Angew Chem Int Ed Engl. 2011;50:1770–91. doi: 10.1002/anie.201002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ju T, Wang Y, Aryal RP, Lehoux SD, Ding X, Kudelka MR, et al. Tn and sialyl-Tn antigens, aberrant O-glycomics as human disease markers. Proteom Clin Appl. 2013;7:618–31. doi: 10.1002/prca.201300024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dabelsteen S, Pallesen EMH, Marinova IN, Nielsen MI, Adamopoulou M, Rømer TB, et al. Essential functions of glycans in human epithelia dissected by a CRISPR-Cas9-engineered human organotypic skin model. Dev Cell. 2020;54:669–7. doi: 10.1016/j.devcel.2020.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bagdonaite I, Pallesen EM, Ye Z, Vakhrushev SY, Marinova IN, Nielsen MI, et al. O‐glycan initiation directs distinct biological pathways and controls epithelial differentiation. EMBO Rep. 2020; 4;21. [DOI] [PMC free article] [PubMed]
- 28.Bergstrom K, Liu X, Zhao Y, Gao N, Wu Q, Song K, et al. Defective intestinal mucin-type O-glycosylation causes spontaneous colitis-associated cancer in mice. Gastroenterology. 2016;151:152–.e11. doi: 10.1053/j.gastro.2016.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao N, Bergstrom K, Fu J, Xie B, Chen W, Xia L. Loss of intestinal O-glycans promotes spontaneous duodenal tumors. Am J Physiol Gastrointest Liver Physiol. 2016;311:G74–83. doi: 10.1152/ajpgi.00060.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madsen CB, Lavrsen K, Steentoft C, Vester-Christensen MB, Clausen H, Wandall HH, et al. Glycan elongation beyond the mucin associated Tn antigen protects tumor cells from immune-mediated killing. PLoS ONE. 2013;8:e72413. doi: 10.1371/journal.pone.0072413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun X, Ju T, Cummings RD. Differential expression of Cosmc, T-synthase and mucins in Tn-positive colorectal cancers. BMC Cancer. 2018;16;18. [DOI] [PMC free article] [PubMed]
- 32.Itzkowitz SH, Bloom EJ, Lau TS, Kim YS. Mucin associated Tn and sialosyl-Tn antigen expression in colorectal polyps. Gut. 1992;33:518–23. doi: 10.1136/gut.33.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coon JS, Weinstein RS, Summers JL. Blood group precursor T-antigen expression in human urinary bladder carcinoma. Am J Clin Pathol. 1982;77:692–9. doi: 10.1093/ajcp/77.6.692. [DOI] [PubMed] [Google Scholar]
- 34.Oshikiri T, Miyamoto M, Morita T, Fujita M, Miyasaka Y, Senmaru N, et al. Tumor-associated antigen recognized by the 22-1-1 monoclonal antibody encourages colorectal cancer progression under the scanty CD8+ T cells. Clin Cancer Res J Am Assoc Cancer Res. 2006;12:411–6. doi: 10.1158/1078-0432.CCR-05-1257. [DOI] [PubMed] [Google Scholar]
- 35.Desai PR, Immunoreactive T. and Tn antigens in malignancy: role in carcinoma diagnosis, prognosis, and immunotherapy. Transfus Med Rev. 2000;14:312–25. doi: 10.1053/tmrv.2000.16229. [DOI] [PubMed] [Google Scholar]
- 36.Brooks SA, Leathem AJ. Prediction of lymph node involvement in breast cancer by detection of altered glycosylation in the primary tumour. Lancet Lond Engl. 1991;338:71–4. doi: 10.1016/0140-6736(91)90071-V. [DOI] [PubMed] [Google Scholar]
- 37.Remmers N, Anderson JM, Linde EM, DiMaio DJ, Lazenby AJ, Wandall HH, et al. Aberrant expression of mucin core proteins and O-linked glycans associated with progression of pancreatic cancer. Clin Cancer Res. 2013;19:1981–93. doi: 10.1158/1078-0432.CCR-12-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hofmann BT, Schlüter L, Lange P, Mercanoglu B, Ewald F, Fölster A, et al. COSMC knockdown mediated aberrant O-glycosylation promotes oncogenic properties in pancreatic cancer. Mol Cancer. 2015;29:14. [DOI] [PMC free article] [PubMed]
- 39.Itzkowitz SH, Bloom EJ, Kokal WA, Modin G, Hakomori S, Kim YSSialosyl-Tn. A novel mucin antigen associated with prognosis in colorectal cancer patients. Cancer. 1990;66:1960–6. doi: 10.1002/1097-0142(19901101)66:9<1960::AID-CNCR2820660919>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 40.Werther JL, Tatematsu M, Klein R, Kurihara M, Kumagai K, Llorens P, et al. Sialosyl-Tn antigen as a marker of gastric cancer progression: an international study. Int J Cancer. 1996;69:193–9. doi: 10.1002/(SICI)1097-0215(19960621)69:3<193::AID-IJC8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi H, Terao T, Kawashima Y. Serum sialyl Tn as an independent predictor of poor prognosis in patients with epithelial ovarian cancer. J Clin Oncol. 1992;10:95–101. doi: 10.1200/JCO.1992.10.1.95. [DOI] [PubMed] [Google Scholar]
- 42.Pedersen JW, Blixt O, Bennett EP, Tarp MA, Dar I, Mandel U, et al. Seromic profiling of colorectal cancer patients with novel glycopeptide microarray. Int J Cancer. 2011;128:1860–71. doi: 10.1002/ijc.25778. [DOI] [PubMed] [Google Scholar]
- 43.Matsumoto Y, Kudelka MR, Hanes MS, Lehoux S, Dutta S, Jones MB, et al. Identification of Tn antigen O-GalNAc-expressing glycoproteins in human carcinomas using novel anti-Tn recombinant antibodies. Glycobiology. 2020;30:282–300. doi: 10.1093/glycob/cwz095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mandel U, Petersen OW, Sørensen H, Vedtofte P, Hakomori S, Clausen H, et al. Simple mucin-type carbohydrates in oral stratified squamous and salivary gland epithelia. J Invest Dermatol. 1991;97:713–21. doi: 10.1111/1523-1747.ep12484064. [DOI] [PubMed] [Google Scholar]
- 45.Thurnher M, Clausen H, Sharon N, Berger EG. Use of O-glycosylation-defective human lymphoid cell lines and flow cytometry to delineate the specificity of Moluccella laevis lectin and monoclonal antibody 5F4 for the Tn antigen (GalNAc alpha 1-O-Ser/Thr) Immunol Lett. 1993;36:239–43. doi: 10.1016/0165-2478(93)90095-J. [DOI] [PubMed] [Google Scholar]
- 46.Böhm CM, Mulder MC, Zennadi R, Notter M, Schmitt-Gräff A, Finn OJ, et al. Carbohydrate recognition on MUC1-expressing targets enhances cytotoxicity of a T cell subpopulation. Scand J Immunol. 1997;46:27–34. doi: 10.1046/j.1365-3083.1996.d01-91.x. [DOI] [PubMed] [Google Scholar]
- 47.Sørensen AL, Reis CA, Tarp MA, Mandel U, Ramachandran K, Sankaranarayanan V, et al. Chemoenzymatically synthesized multimeric Tn/STn MUC1 glycopeptides elicit cancer-specific anti-MUC1 antibody responses and override tolerance. Glycobiology. 2006;16:96–107. doi: 10.1093/glycob/cwj044. [DOI] [PubMed] [Google Scholar]
- 48.Clausen H, Stroud M, Parker J, Springer G, Hakomori S. Monoclonal antibodies directed to the blood group A associated structure, galactosyl-A: specificity and relation to the Thomsen-Friedenreich antigen. Mol Immunol. 1988;25:199–204. doi: 10.1016/0161-5890(88)90068-5. [DOI] [PubMed] [Google Scholar]
- 49.David L, Nesland JM, Clausen H, Carneiro F, Sobrinho-Simões M. Simple mucin-type carbohydrate antigens (Tn, sialosyl-Tn and T) in gastric mucosa, carcinomas and metastases. APMIS Suppl. 1992;27:162–72. [PubMed] [Google Scholar]
- 50.Manders EM, Stap J, Brakenhoff GJ, van Driel R, Aten JA. Dynamics of three-dimensional replication patterns during the S-phase, analysed by double labelling of DNA and confocal microscopy. J Cell Sci. 1992;103:857–62. doi: 10.1242/jcs.103.3.857. [DOI] [PubMed] [Google Scholar]
- 51.Manders EMM, Verbeek FJ, Aten JA. Measurement of co-localization of objects in dual-colour confocal images. J Microsc. 1993;169:375–82. doi: 10.1111/j.1365-2818.1993.tb03313.x. [DOI] [PubMed] [Google Scholar]
- 52.Costes SV, Daelemans D, Cho EH, Dobbin Z, Pavlakis G, Lockett S. Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys J. 2004;86:3993–4003. doi: 10.1529/biophysj.103.038422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, et al. QuPath: open source software for digital pathology image analysis. Sci Rep. 2017;7:16878. doi: 10.1038/s41598-017-17204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2018. Available from: https://www.R-project.org/.
- 55.Lamers CH, Sleijfer S, van Steenbergen S, van Elzakker P, van Krimpen B, Groot C, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther J Am Soc Gene Ther. 2013;21:904–12. doi: 10.1038/mt.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther J Am Soc Gene Ther. 2010;18:843–51. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Itzkowitz SH, Yuan M, Montgomery CK, Kjeldsen T, Takahashi HK, Bigbee WL, et al. Expression of Tn, sialosyl-Tn, and T antigens in human colon cancer. Cancer Res. 1989;49:197–204. [PubMed] [Google Scholar]
- 58.Ricardo S, Marcos-Silva L, Pereira D, Pinto R, Almeida R, Söderberg O, et al. Detection of glyco-mucin profiles improves specificity of MUC16 and MUC1 biomarkers in ovarian serous tumours. Mol Oncol. 2015;9:503–12. doi: 10.1016/j.molonc.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmidt LH, Biesterfeld S, Kümmel A, Faldum A, Sebastian M, Taube C, et al. Tissue microarrays are reliable tools for the clinicopathological characterization of lung cancer tissue. Anticancer Res. 2009;29:201–9. [PubMed] [Google Scholar]
- 60.Camp RL, Neumeister V, Rimm DL. A decade of tissue microarrays: progress in the discovery and validation of cancer biomarkers. J Clin Oncol J Am Soc Clin Oncol. 2008;26:5630–7. doi: 10.1200/JCO.2008.17.3567. [DOI] [PubMed] [Google Scholar]
- 61.Chou C-H, Huang M-J, Chen C-H, Shyu M-K, Huang J, Hung J-S, et al. Up-regulation of C1GALT1 promotes breast cancer cell growth through MUC1-C signaling pathway. Oncotarget. 2015;6:6123–35. doi: 10.18632/oncotarget.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Itzkowitz S, Kjeldsen T, Friera A, Hakomori S, Yang US, Kim YS. Expression of Tn, sialosyl Tn, and T antigens in human pancreas. Gastroenterology. 1991;100:1691–700. doi: 10.1016/0016-5085(91)90671-7. [DOI] [PubMed] [Google Scholar]
- 63.Flores AR, Lemos I, Rema A, Taulescu M, Seixas F, Reis CA, et al. Tn and Sialyl-Tn antigens in canine gastric tissues. Vet Comp Oncol. 2020;18:615–25. [DOI] [PubMed]
- 64.Ogata S, Ho I, Chen A, Dubois D, Maklansky J, Singhal A, et al. Tumor-associated sialylated antigens are constitutively expressed in normal human colonic mucosa. Cancer Res. 1995;55:1869–74. [PubMed] [Google Scholar]
- 65.Cornelissen LAM, Blanas A, Zaal A, van der Horst JC, Kruijssen LJW, O’Toole T, et al. Tn antigen expression contributes to an immune suppressive microenvironment and drives tumor growth in colorectal cancer. Front Oncol. 2020;10:1622. doi: 10.3389/fonc.2020.01622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mathiesen CBK, Carlsson MC, Brand S, Möller SR, Idorn M, Straten P Thor, et al. Genetically engineered cell factories produce glycoengineered vaccines that target antigen-presenting cells and reduce antigen-specific T-cell reactivity. J Allergy Clin Immunol. 2018;142:1983–7. doi: 10.1016/j.jaci.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 67.Nakasaki H, Mitomi T, Noto T, Ogoshi K, Hanaue H, Tanaka Y, et al. Mosaicism in the expression of tumor-associated carbohydrate antigens in human colonic and gastric cancers. Cancer Res. 1989;49:3662–9. [PubMed] [Google Scholar]
- 68.Ju T, Cummings RD. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 beta 3-galactosyltransferase. Proc Natl Acad Sci USA. 2002;99:16613–8. doi: 10.1073/pnas.262438199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Y, Ju T, Ding X, Xia B, Wang W, Xia L, et al. Cosmc is an essential chaperone for correct protein O-glycosylation. Proc Natl Acad Sci USA. 2010;107:9228–33. doi: 10.1073/pnas.0914004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sewell R, Bäckström M, Dalziel M, Gschmeissner S, Karlsson H, Noll T, et al. The ST6GalNAc-I sialyltransferase localizes throughout the Golgi and is responsible for the synthesis of the tumor-associated sialyl-Tn O-glycan in human breast cancer. J Biol Chem. 2006;281:3586–94. doi: 10.1074/jbc.M511826200. [DOI] [PubMed] [Google Scholar]
- 71.Marcos NT, Bennett EP, Gomes J, Magalhaes A, Gomes C, David L, et al. ST6GalNAc-I controls expression of sialyl-Tn antigen in gastrointestinal tissues. Front Biosci Elite Ed. 2011;3:1443–55. doi: 10.2741/e345. [DOI] [PubMed] [Google Scholar]
- 72.Hassinen A, Pujol FM, Kokkonen N, Pieters C, Kihlström M, Korhonen K, et al. Functional organization of Golgi N- and O-glycosylation pathways involves pH-dependent complex formation that is impaired in cancer cells. J Biol Chem. 2011;286:38329–40. doi: 10.1074/jbc.M111.277681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Axelsson MA, Karlsson NG, Steel DM, Ouwendijk J, Nilsson T, Hansson GC. Neutralization of pH in the Golgi apparatus causes redistribution of glycosyltransferases and changes in the O-glycosylation of mucins. Glycobiology. 2001;11:633–44. doi: 10.1093/glycob/11.8.633. [DOI] [PubMed] [Google Scholar]
- 74.Gill DJ, Tham KM, Chia J, Wang SC, Steentoft C, Clausen H, et al. Initiation of GalNAc-type O-glycosylation in the endoplasmic reticulum promotes cancer cell invasiveness. Proc Natl Acad Sci USA. 2013;110:E3152–3161. doi: 10.1073/pnas.1305269110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nguyen AT, Chia J, Ros M, Hui KM, Saltel F, Bard F. Organelle specific O-glycosylation drives MMP14 activation, tumor growth, and metastasis. Cancer Cell. 2017;32:639–.e6. doi: 10.1016/j.ccell.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 76.Brockhausen I. Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions. EMBO Rep. 2006;7:599–604. doi: 10.1038/sj.embor.7400705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lavrsen K, Dabelsteen S, Vakhrushev SY, Levann AMR, Haue AD, Dylander A, et al. De novo expression of human polypeptide N-acetylgalactosaminyltransferase 6 (GalNAc-T6) in colon adenocarcinoma inhibits the differentiation of colonic epithelium. J Biol Chem. 2018;293:1298–314. doi: 10.1074/jbc.M117.812826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burchell JM, Beatson R, Graham R, Taylor-Papadimitriou J, Tajadura-Ortega V. O-linked mucin-type glycosylation in breast cancer. Biochem Soc Trans. 2018;46:779–88. doi: 10.1042/BST20170483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lenos K, Goos JACM, Vuist IM, den Uil SH, Delis-van Diemen PM, Belt EJTh, et al. MGL ligand expression is correlated to BRAF mutation and associated with poor survival of stage III colon cancer patients. Oncotarget. 2015;6:26278–90. doi: 10.18632/oncotarget.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Varki A, Cummings RD, Aebi M, Packer NH, Seeberger PH, Esko JD, et al. Symbol nomenclature for graphical representations of glycans. Glycobiology. 2015;25:1323–4. doi: 10.1093/glycob/cwv091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
R_script_statistics_mapping_truncated_o_glycans
Supplementary Dataset for R Script - Primary
Supplementary Dataset for R Script - PDX
Data Availability Statement
All data included in the study are available upon request to the corresponding author.