Abstract
Late-life depression (LLD) is a particularly debilitating illness. Older adults suffering from depression commonly experience poor outcomes in response to antidepressant treatments, medical comorbidities, and declines in daily functioning. This review aims to further our understanding of the brain network dysfunctions underlying LLD that contribute to disrupted cognitive and affective processes and corresponding clinical manifestations. We provide an overview of a network model of LLD that integrates the salience network, the default mode network (DMN) and the executive control network (ECN). We discuss the brain-based structural and functional mechanisms of LLD with an emphasis on their link to clinical subtypes that often fail to respond to available treatments. Understanding the brain networks that underlie these disrupted processes can inform the development of targeted interventions for LLD. We propose behavioral, cognitive, or computational approaches to identifying novel, personalized interventions that may more effectively target the key cognitive and affective symptoms of LLD.
Keywords: Aging, Depression, Functional Connectivity, White Matter, Apathy, Executive Function
1. Introduction
Depression is the leading cause of disability worldwide.1,2 Late-life depression (LLD) is particularly prone to poor outcomes, including failure to remit despite adequate treatments with antidepressant medications and/or psychotherapy, exacerbation of medical comorbidities, and diminished daily functioning.3,4 One promising approach to improving the efficacy of interventions for LLD is to elucidate the functional and structural neuroanatomy corresponding to clinical subtypes of depression that are common in LLD and respond poorly to standard antidepressant approaches. A better understanding of the brain network dysfunctions underlying LLD can inform the development of targeted interventions for these poor responders.
In this review, we present the results of studies that have examined the brain-based mechanisms of LLD with an emphasis on some of the common clinical subtypes – namely, depression with executive dysfunction, negative cognitive bias, and apathy – that frequently do not remit with antidepressant medications. We focus primarily on functional and structural neuroimaging studies that include detailed behavioral and/or cognitive assessments and/or take advantage of recent advances in MRI sequences or computational approaches. Next, we present two examples of how neuroimaging can inform novel treatment approaches - physical exercise interventions and cognitive training - that may optimize functioning of networks underlying these common clinical syndromes in LLD.
2. Network Model of Late-Life Depression
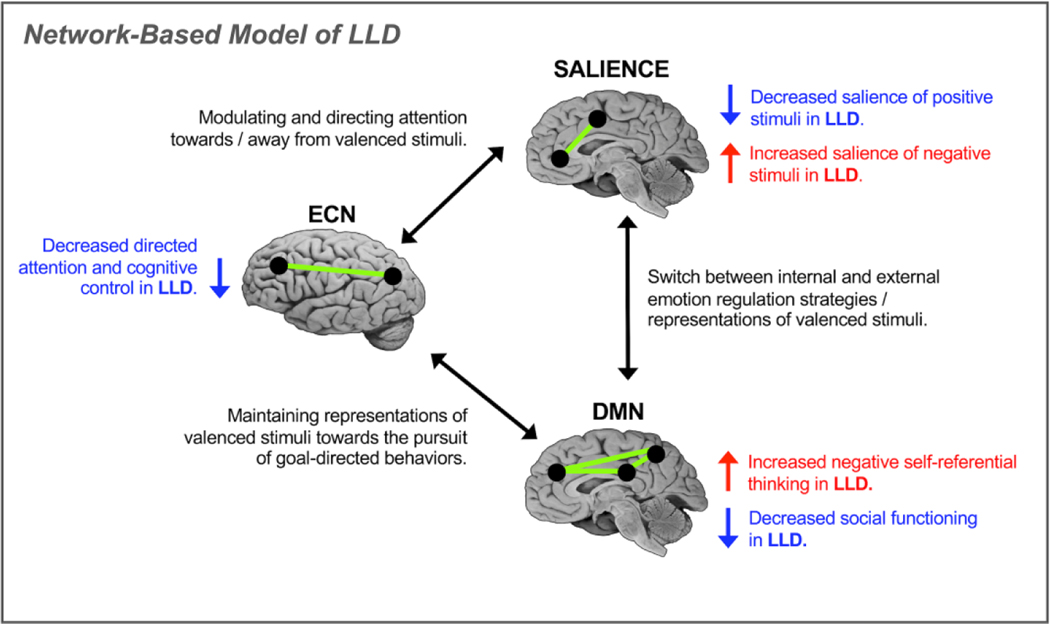
Our conceptualization of LLD is based on the premise that major depression in older adults is dependent both on disrupted intrinsic functional and structural connectivity within key networks – the salience network, the default mode network (DMN), and the executive control network (ECN) – as well as large-scale interactions between these brain networks. In this model (Figure 1), the salience network (dorsal anterior cingulate cortex (dACC) and anterior insula), the DMN, (medial PFC, posterior cingulate cortex, and precuneus), and the ECN (dACC, dorsolateral prefrontal cortex (DLPFC), and posterior parietal cortices) are involved in implementing attentional and regulatory processes that are core to both the implicit cognitive and affective biases common in LLD and to the maladaptive cognitive strategies implicated in the abnormal experience and regulation of emotional responses in depression.
Figure 1:
Proposed network model of late-life depression (LLD). The model accounts for intrinsic connectivity within and extrinsic connectivity between the salience network, the default mode network (DMN) and the executive control network (ECN). Network-level disruptions in connectivity give rise to disrupted cognitive processes of LLD related to disrupted pursuits of goal-directed behaviors and maladaptive representations of affectively valenced stimuli.
2.1. Salience Network
The salience network is critical for prioritizing stimuli.5–7Salience is the process by which attention is directed towards or away from stimuli; more salient stimuli capture attention.8–10 and stimuli with affective meaning (valence) have greater salience.11 In addition the salience network works in concert with the ECN to allocate attentional resources to accomplish goal-directed behaviors while ignoring goal-irrelevant information.5,6 In aging, the salience network, particularly the insula, shows decreased intrinsic connectivity as well as decreased connectivity with other networks and with the locus coeruleus.12–14 For individuals suffering from depression, the salience network is biased toward selectively attending to negative stimuli. This bias toward negative information is related to the reduced extrinsic functional connectivity between the salience network and the ECN, along with increased functional connectivity of the DMN.13,15,16
2.2. Default Mode Network (DMN)
The DMN plays a key role in the individual’s understanding of their place in the world.17 Self-referential thinking, or making sense of one’s place in the internal and external environment, is necessary for effective daily functioning,18including social aspects of daily functioning that are commonly impaired in LLD. The ability to reflect on past experiences and apply them to current and future experiences also depends on the DMN and facilitates goal-directed behaviors 19,20. In normal aging, intrinsic functional connectivity of the DMN appears to be decreased in older adults relative to in young adults.21 In individuals suffering from depression, biases in processing stimuli (e.g., interpreting neutral stimuli as negative, assigning greater salience to negative internal states and external stimuli) contribute to negative representations of the past and future, as well as negative self-referential thoughts, guilty rumination, depressive ideation and reduced well-being.22–25
2.3. Executive Control Network (ECN)
The ECN supports the flexible maintenance of goal-directed behaviors in the face of changing internal and environmental demands.26,27 More specifically, control processes are “managed” by the ECN, a regulatory system that modulates the operation of other cognitive and emotional systems to enable the individual to function efficiently. This network is comprised of the dACC as well as the DLPFC and posterior parietal regions.26 The dACC contributes to control processes by detecting conditions that signal the demand for increased cognitive control, which contributes to the engagement of the DLPFC. Parietal regions work in concert with the dACC and DLPFC to engage cognitive resources in response to the changing. Both intrinsic and extrinsic connectivity of the ECN is quite susceptible to disruption in aging,28 which may contribute to the comorbid cognitive symptoms and poor mood outcomes often observed in LLD. Thus, disruption of the ECN likely contributes to both the cognitive and affective symptoms of LLD.
2.4. Pathophysiology of LLD
Multiple etiologic mechanisms contribute to network disturbances and lead to the clinical expression of LLD. Genetic variation, age- and disease-related processes have been implicated in the pathogenesis of LLD; including vascular disease, abnormal neurotrophin levels, transcriptomic variation, and dysregulation in endocrinologic and immunologic systems.29 In particular, cerebrovascular comorbidities, including small vessel ischemic changes, are common in LLD.30,31 Cardiovascular risk factors (e.g., diabetes, hypertension, atherosclerosis, hyperlipidemia) increase risk of LLD onset. Further, vascular changes including endothelial dysfunction and increased intima media thickness are more pronounced among depressed older adults.32–34 Such vascular pathology compromises cerebral blood flow by reducing blood flow velocities and decreasing vasomotor reactivity, with LLD participants showing perfusion deficits in frontal, temporal, and subcortical areas.31,35,36 Additional pathogenic pathways include abnormalities in inflammatory signaling, which exacerbates vascular burden31 and promotes excitotoxicity and oxidative damage.30 Disruptions in neural homeostasis and accelerated cellular aging,29 perhaps indexed by telomere length,37 may also contribute to the development and course of LLD.
3. The Relationship of White Matter Abnormalities to Network Disturbances in LLD
White matter abnormalities are common in aging and pronounced in LLD. They may contribute to the clinical manifestation of depression by disrupting connections among key regions regulating mood and cognitive processes. LLD is associated with greater severity of microvascular lesions, including white matter hyperintensities (WMH), a radiologic hallmark of small vessel disease.38 Greater WMH burden predicts incidence of depression in older adulthood, persistence of symptoms, and non-remission following antidepressant treatment.39–42 Along with global burden, LLD is associated with greater severity of WMH in specific white matter tracts including the cingulum bundle, uncinate fasciculus, and superior longitudinal fasciculus.43–45 By providing dense structural connections between key nodes of the salience network, the DMN and the ECN, these fiber bundles support executive functions, salience detection, and self-referential thinking.
LLD is also characterized by compromised microstructural integrity in normal-appearing white matter, as assessed by diffusion-tensor imaging.46–51 Relative to controls, reduced white matter integrity among depressed older adults is observed across a distributed set of cerebral networks,46,51 though lower integrity of the uncinate fasciculus, cingulum, and frontal association fibers is most consistently reported.52,53
Compromised white matter tracts within the salience network, the DMN and the ECN may contribute to functional network abnormalities and mediate the expression of depression symptomatology. WMH in non-depressed older adults have been associated with decreased structural connectivity of white matter tracts,54 and alterations in functional activation and resting state connectivity.55 In depressed older adults, WMH severity predicted greater activation of the anterior cingulate during an affective-reactivity task (i.e., presentation of fearful faces), suggesting an association between WMH and heighted sensitivity of the dACC to negative stimuli.56 Greater WMH burden in LLD has also been liked to altered connectivity within the DMN,57 suppressed activation of the DLPFC during a cognitive control task, and reduced connectivity of the DLPFC with task-relevant brain regions including the middle frontal gyrus and supramarginal gyrus.58 Moreover, when compared to healthy controls, depressed older adults exhibit reductions in functional as well as structural connectivity between the PCC/precuneus and the dACC of the ECN, which, in turn, predicts poorer executive function and working memory performance.59 These data collectively suggest that white matter abnormalities in LLD are associated with alterations in neural recruitment and connectivity in networks that subserve cognitive and emotional processing. In addition, as outlined below, microvascular lesions and disruptions in microstructural integrity may predispose to specific clinical phenotypes of LLD and predict response to antidepressant treatment.
4. Network-Level Disruptions Contribute to Clinical Manifestations of LLD
4.1. Depression with Executive Dysfunction
Executive dysfunction, which is present in approximately 40% of older individuals with LLD,60 appears to reflect inefficient functioning within the ECN,28,61–64 as well as the ECN’s interactions with the salience network and the DMN. These network-level dysfunctions give rise to behavioral manifestations of executive dysfunction that are common in patients with LLD and executive dysfunction, or depression-executive dysfunction (DED) syndrome. These behavioral disruptions include difficulty inhibiting attention towards task-irrelevant stimuli and a diminished ability to accomplish goal directed actions.60 Further, DED syndrome is characterized by disability, declines in quality of life, and poor antidepressant response.65–67Longitudinal study of intact aging adults suggests that declines in executive function and decreased white matter integrity both attenuate the often observed age-related increase in positive mood and may contribute to difficulties with emotion regulation that make older adults susceptible to depression68. Further, in those who do suffer from LLD, impairments in executive functions often persist even after remission of depression.69 Thus, as with other LLD subtypes, understanding specific patterns of abnormal network interactions in DED can help to inform novel treatment approaches.
Converging evidence shows reduced functional connectivity between the dACC and spatially distal brain regions in LLD. For example, Respino et al.28 observed that resting state functional connectivity is decreased between a seed in the dACC and posterior regions of the bilateral precuneus, consistent with other observations of decreased connectivity among the salience network and the posterior ECN and DMN in aging.70–72 This decoupling between the precuneus and the dorsal ACC is particularly important due to the putative role of the precuneus in executive functions supported by the DMN that are often disrupted in LLD, including self-referential thinking,73 task-switching, working memory, and cognitive flexibility.74
Relying on a novel approach for the analyses of resting state fMRI data termed regional homogeneity, which leverages the synchronization of the fMRI time series to provide a measure of local network connectivity, Respino et al.28 reported greater regional homogeneity of the dACC in depressed older adults relative to age-matched healthy controls. Further, within the subjects with LLD, greater regional homogeneity of the dACC was associated with better cognitive flexibility and working memory. The observed link between dACC regional homogeneity and executive functions in LLD highlights the role of the dACC as an important hub that supports efficient connectivity among networks central to LLD, whereas disrupted connectivity with this region may contribute to the expression of LLD with executive dysfunction.75,76
There is a relationship between measures of connectivity at the structural level, particularly increased WMH, and executive dysfunction in LLD both cross-sectionally77,78 and longitudinally.79 In a prospective study of 64 older adults, a dose-response relationship between WMH and cognition was detected. Specifically, greater baseline WMH severity was associated with greater memory and executive function deficits in those with LLD, but not in controls.79 Recent studies have also investigated the impact of WMH in older adults on connectivity at the network level, investigating how network disruptions contribute to poor executive functions. Among non-depressed older adults, WMH are associated with altered functional connectivity in the ECN80 and in the DMN.81
In LLD, WMH may preferentially affect specific white matter fibers tracts, such as the superior longitudinal fasciculus and the uncinate.44 WMH in these fiber tracts are tied to executive dysfunction44 and disrupted reward learning.82 Further, decreased structural connectivity of the anterior and posterior cingulate, middle frontal cortex, supramarginal gyrus, and thalamus is associated with poorer executive function, as measured by performance on a task requiring set-shifting and cognitive inhibition,63 supporting a relationship between WMH and regionally-specific structural alterations in connectivity. Thus strategically located microvascular lesions and corresponding disruptions in structural connectivity may predict the nature and intensity of cognitive symptoms and contribute to a profile of executive deficits in LLD.
4.2. Negative Cognitive Bias
Preferential processing of negative stimuli in depression reflects a discounting of positive, rewarding information,83,84 giving rise to depressive ideation, sadness, and anhedonia.85,86 Individuals with LLD display a range of mood-congruent negative processing biases, illustrated by slower reaction times and less accuracy on tasks using positively-valenced stimuli, a heightened sensitivity to negative feedback, and a tendency to interpret ambiguous stimuli (e.g., neutral faces) as negative compared to healthy controls. These biases are believed to contribute directly to depression, where individuals have a persistently negative view of oneself, the world, and the future.87 Moreover, these abnormalities in processing of emotional information predict recurrence of mood episodes.88 Affective processing in MDD89 is characterized by deficits in attentional disengagement from negatively-valenced stimuli and impaired cognitive control when processing negative information.90
In individuals suffering from depression, negative cognitive biases in processing of both internal and external stimuli contribute to negative self-referential thoughts.91–95 Negative self-referential thoughts produce a range of cognitive (guilt, rumination, and self-criticism) and affective (e.g., feelings of worthlessness and sadness) symptoms of depression22–25 and are associated with resistance to traditional antidepressant treatments.96,97 Alterations in network connectivity both within and between the salience network, the DMN, and the ECN have been tied to negative cognitive biases, including negative self-referential thinking. For example, negative self-referential thinking in depression is associated with abnormal task-based activation of the anterior nodes of the DMN.98–100 Dominance of the DMN relative to the “task-positive” network engaged during executive control is associated with symptoms of rumination during the depressed state.101 Moreover, following treatment with antidepressant medication, normalization of hyperconnectivity has been observed in the posterior, but not anterior DMN.102
In addition to the role of abnormalities in functional connectivity, abnormal white matter integrity in the salience network and the DMN of older depressed adults interferes with the shifting of attention away from negative thoughts about one’s self.103,104 Further, aging related white matter microstructural abnormalities in the dACC and in the uncinate are associated with residual negative self-referential thinking following treatment with an SSRI.105 These findings suggest that disrupted connections among the salience network, the DMN and the ECN may interfere with the maintenance of one’s self-representation, leading to persistent negative self-referential thoughts.106–108
4.3. Apathy
One-third to one-half of LLD patients suffer from apathy109 a persistent and disabling disorder of motivation characterized by reduced goal-directed behavior, emotional blunting, and cognitive disturbances.110,111 Apathy of LLD predicts chronicity of depression, compromised quality of life, and high caregiver burden.86,112–116 Remission rates with pharmacotherapy are especially low in LLD with prominent apathy;117 in some cases, selective serotonin reuptake inhibitors (SSRIs) even may result in worsening of apathy.118–121
Behavioral and cognitive disturbances in apathy of LLD include impairments in the generation and execution of strategies that guide goal-directed behavior, attentional set-shifting, and working memory.10,121,122 Abnormalities in brain networks subserving these processes, including the salience network and the ECN, and their reciprocal interactions with reward structures (e.g., nucleus accumbens) may underlie apathy of LLD. Through dense reciprocal connections, the SN interacts with structures of the reward network to signal anticipatory reward and intrinsic motivation, key processes that trigger goal-directed action123 that are disrupted in apathy.10 Structural abnormalities in the salience network are common biological substrates of apathy.124,125 Apathetic, depressed older adults exhibit focal atrophy in the dACC of the salience network,126,127 and our group has shown that structural abnormalities in frontolimbic white matter and the insula of the salience network predict persistence of apathetic states in LLD.128 Impaired integration of signals among these networks has also been associated with the presence of apathy in LLD.129 Depressed older adults with apathy exhibit diminished intrinsic connectivity of the salience network and alterations in functional connectivity between the salience network and key nodes of the ECN and anterior portion of the DMN.130,131
In addition to the functions noted earlier, the ECN serves to translate intention into action,132 by supporting planning, action generation, and selective and sustained attention132,133 processes that are commonly disrupted in apathy.10 Not surprisingly, then, compromise of the ECN, and its large-scale network connections, may contribute to the development of apathy in LLD. Relative to non-apathetic depressed older adults, depressed older adults with comorbid apathy demonstrate altered connectivity between structures of the ECN and the salience network (e.g., insula).128 Moreover, the ECN interacts with structures of the reward network to guide reward-based decision making. Reduced rsFC between regions of the ECN and the reward network is associated with slowed decision speed and greater effort sensitivity,134 factors that contribute to reduced action initiation in apathy.135 In addition, depressed adults with comorbid apathy exhibit microstructural abnormalities in fiber bundles connecting hub nodes within these networks, which may hinder the integration of reward-related signals.126 Taken together, these studies indicate that a core set of brain networks (salience, DMN, ECN) interact to support different phases of motivated behavior, and the apathy syndrome may arise in LLD from disturbances in connectivity among these key circuits. In other words, apathy within the context of depression (emotional indifference, unwillingness to exert effort to perform goal-directed behaviors) may be a result, at least in part, of disruption in the networks important for salience detection and goal-directed behavior.
5. The Use of Computational Models to Understand Specific Behavioral and Network Disturbances in LLD
There is a high degree of variability in network-level changes and associated maladaptive behaviors and cognitive processes that arise due to the combined effects of aging and depression. As a result, there are multiple potential combinations of motivational and mood disturbances that contribute to poor course of illness and treatment response in LLD, reflecting underlying network pathology. Understanding these individual differences at the network level can inform targeted, personalized interventions. In the following section we review how specific network changes may point at such focused treatment strategies.
5.1. Computational Approaches to Identifying Networks Implicated in LLD
Computational modeling approaches provide precise, quantifiable parameters of maladaptive behaviors in aging and MDD, particularly deficits emerging from negative cognitive biases, including associative learning and value representation.136–140 These models may be applied to address the combined effects of aging and depressive symptomatology on resulting motivational and mood disturbances that are common in LLD. Thus far, computational models in LLD have been particularly useful in accounting for changes in goal-directed behaviors, decision-making, and motivation in depressed older adults.141,142 For example, Dombrovski et al.143 used a reinforcement learning modeling approach and observed that depressed older adults with a history of suicide attempts were less likely to effectively use positive vs. negative feedback to learn stimulus-response contingencies. These findings suggest that deficits in experiential learning and optimal choice selection may contribute to depressive symptoms and suicidality in a subgroup of older adults who possess an inability to consider alternative options in the context of a negative view of the world.
A promising application of computational approaches in the study of late-life mood disorders is to select and optimize model parameters corresponding to behavioral performance (e.g., learning rate, prediction error) to understand individual differences in the disrupted cognitive processes of LLD. The application of individualized modeling approaches can enhance our understanding of individual developmental trajectories in aging and depression, disrupted networks and behaviors that may be risk factors for psychopathology late in life, and personalized treatment targets in individuals with late-life depression.
These modeling approaches have been applied to characterize behavioral and network disruptions that correspond to specific clinical symptom profiles of depression, which may be used to identify more effective personalized treatments for depression. Reinforcement learning models of the pursuit of positive, rewarding outcomes of behavior have been widely shown to demonstrate deficits in motivation and effort expenditure that give rise to motivational disturbances in depression, including apathy and anhedonia.139,144,145 The patterns of behaviors identified by these reinforcement learning models are associated with reduced activation in response to regions of the salience network (dACC and anterior insula) and prefrontal regions of the ECN (e.g., DLPFC).146,147
Computational modeling has been less widely explored in older adults with depression, but may be useful for identifying individual differences in the clinical manifestations of LLD at the network level to identify individuals who are at high risk for relapse following treatment148 and would benefit from treatments that target motivational disturbances, such as the interventions utilizing cognitive remediation or physical activity described below.
5.2. Advanced Modeling of White Matter Abnormalities and LLD Pathogenesis
Modern quantitative techniques have the potential to characterize the pathophysiology of white matter abnormalities in LLD and assess their impact on cognitive and affective circuitry. Graph-theoretical approaches can be applied to diffusion imaging to model the network topology of white matter tracts.149 By quantifying multiple properties of the structural connectome, including estimates of network efficiency and integration, these metrics may provide greater insight into the morphologic substrates of brain network dysfunction in LLD. In a sample of adults with small vessel disease, those with comorbid depression showed impaired edge connections in select subnetworks including corticolimbic fibers, commissural fibers, and frontoparietal pathways, compared to controls.150 Similarly, depressed older adults with comorbid cognitive weaknesses showed greater disruptions in network properties (lower connective strength) within corticostriatal and ECN systems compared to cognitively intact depressed older adults,151 highlighting select structural network characteristics associated with the presentation of cognitive deficits in LLD.
Modern DTI-derived parameters have also been established to probe microstructural properties with greater sensitivity and specificity. For instance, peak-width of skeletonized mean diffusivity (PSMD) is a novel, DTI-derived metric of small vessel disease that provides a global estimate of diffuse white matter dysfunction. A recent study found that a PSMD outperformed conventional SVD (WMH) and diffusion markers in predicting cognitive performance and dysregulation of executive function behaviors in participants with LLD.152 Another DTI-derived metric, free water diffusion, models extracellular abnormalities in the white matter compartment, and has recently been applied to interrogate diffusion properties in schizophrenia and Alzheimer’s disease, though it has not yet been evaluated in LLD. Finally, dynamic contrast-enhanced MRI is a novel technique for quantifying blood-brain barrier permeability in vivo.153,154 While this approach has not yet been used in LLD, it may be leveraged to characterize the contribution of blood brain barrier dysfunction to structural and functional network abnormalities in LLD. Applying these modern approaches to identify pathophysiologic processes contributing to white matter abnormalities in LLD may reveal novel mechanistic pathways for targeted intervention.
5.3. The Use of Statistical Classifiers to Identify Subtypes of Depression
A particular application of computational approaches to further our understanding of depression and treatment response focuses on identification of brain-based subtypes of psychiatric disorders. Neuroimaging-based computational approaches have been developed to guide personalized approaches to targeted treatment selection, relying on individual-level predictions to determine whether a given treatment is likely to succeed. A recent computational development in quantifying individual differences for personalized treatments is based on the classification of subtypes of depression based on unbiased computational methods with the goal of identifying statistical classifiers that can be applied at the level of the individual.van Waarde et al.155 used resting state fMRI and trained a support vector machine (SVM) classifier on individual patients’ pre-treatment scans to predict the outcome of ECT in individuals suffering from TRD.
A study by Drysdale et al.156 used a canonical correlation and hierarchical clustering approach to identify subtypes of depression based on unique combinations of network-level resting-state functional connectivity and clinical symptoms of MDD. With this approach, they identified distinct biotypes of depression that varied in degree of anhedonia and anxiety (i.e., the clinical features most highly associated with observed patterns of functional connectivity in the depressed sample). Further, the biotypes differed in their response to treatment with TMS, suggesting that connectivity-based biotype classification may be an indicator of potential for treatment response and provide actionable information about the optimal treatment option at the individual level. In another study, Dunlop et al.157 used baseline resting state functional connectivity (RSFC) between the subcallosal cingulate cortex and other brain areas to predict differential outcome to cognitive behavioral therapy or pharmacotherapy in individuals with MDD. They found that RSFC patterns differentially predicted response to the two treatments, with negative summed RSFC associated with remission after pharmacotherapy and positive summed connectivity associated with remission after psychotherapy.
These studies all highlight the application of rigorous computational approaches to facilitate precision psychiatry by classifying complex patterns of neural, behavioral, and clinical features of depression. By targeting specific deficits, this may increase the likelihood of symptomatic response.158,159 These computational approaches may also be extended to treatment prediction in late-life depression, with preliminary evidence for modeling approaches that may account for age-related changes in network connectivity and cognition that contribute to accelerated brain aging and negative cognitive biases in LLD.160
6. Neuroscience-Informed Interventions for LLD
As we have noted, many individuals treated with antidepressant medications or psychotherapy do not achieve adequate response, even after multiple treatment trials.3,4,161Even when standard treatments improve mood symptoms, some individuals, especially older adults, are left with persistent executive dysfunction,65,97,162,163 negative self-referential thinking or persistent motivational disturbances that are associated with disability,65,97,163 and increased risk of depression recurrence.30,65,69,97,163 Clearly, additional treatment strategies are needed to improve outcomes among these patients. In particular, nonpharmacological interventions targeting putative network mechanisms of LLD, some of which, may harness neural plasticity in the aging brain, offer promising treatment alternatives.
6.1. Physical Activity Interventions
Physical activity is a promising therapeutic strategy for LLD, demonstrating a capacity to target symptoms with poor treatment response rates and provoke connectivity changes in distinct neural networks. Structured physical activity training significantly reduces depressive symptoms in mid- and late-life,164,165 yielding effect sizes moderate to large in magnitude. When paired with antidepressant medication, physical activity may also maximize treatment outcomes. In a large-scale RCT of 121 depressed older adults, 81% of participants achieved remission following 24 weeks of sertraline combined with aerobic exercise training, compared to 45% in the sertraline-only condition.166,167
Physical activity may also attenuate symptoms of LLD inadequately addressed by traditional antidepressants, including executive dysfunction, apathy, and disability. Physical activity effectively improves cognitive performance in older adulthood, producing the greatest gains in executive function,168,169 the domain most impacted in LLD. In older adults with major depression, medication combined with aerobic exercise training produced significantly greater improvements in general cognition, visuospatial/executive functions, and disability than medication alone.170 Apathy symptoms are reduced following physical activity training in nursing home residents171 and individuals with schizophrenia,172 and future studies are needed to characterize its therapeutic potential for the treatment of apathy in LLD. Taken together, physical activity is a low-cost and accessible strategy to reduce geriatric depression with the potential to attenuate the most treatment-resistant symptoms of LLD.
Physical activity may generate improvements in mood and cognitive function by selectively remediating neural circuits disrupted in LLD. In community-dwelling older adults, 24 weeks of aerobic exercise training altered resting state connectivity within the DMN,173 as well as inter-network connectivity between key nodes of the DMN and the ECN.174 In a recent RCT, 12 months of moderate-intensity walking increased connectivity in the salience and dorsal attention networks.175 Exercise-induced changes in DMN connectivity have been shown to mediate the relationship between exercise and improvements in executive functioning.176 Along with modulating functional network dynamics, physical activity produces volumetric increases in the prefrontal cortex and hippocampus,177–179 key structures that exhibit morphologic changes in LLD180 and have been linked to poor antidepressant treatment response rates.181,182 Physical activity stimulates several cellular processes that may have restorative or neuroprotective effects on neural network abnormalities in LLD, leading to improved mood and improvement in cognitive function. Physical activity promotes neurogenesis, upregulates neurotrophic factors, and suppresses pro-inflammatory signaling and oxidative damage183 – directly targeting cellular and molecular pathways implicated in depression pathogenesis.
Vascular health is also improved by physical activity, which increases cerebral blood flow and angiogenesis184 and thus may be a particularly promising treatment for depressed older adults with pronounced vascular pathology. Future trials selectively targeting distinct symptom dimensions or profiles will advance our understanding of the clinical phenotypes of LLD that may benefit most from physical activity.
6.2. Cognitive Remediation for LLD
Network dysfunction in aging and depression can be targeted using cognitive interventions that are designed to improve functions in the networks implicated in LLD through repetitive stimulation. For example, we conducted a proof-of-concept randomized clinical trial of a video game-like intervention designed to target the ECN by improving age-related deficits in multitasking. Individuals over the age of 60 with a current major depressive episode were randomized to problem solving therapy (PST) adapted to treat depression with executive dysfunction or the digital multitasking intervention. The two intervention groups showed a similar improvement in depression, whereas those randomized to the video game-like condition demonstrated significant improvements in working memory and sustained attention.185 Further, relative to subjects completing PST, subjects completing the multitasking intervention showed greater improvement in self-referential thinking.
Cognitive training may alleviate both depression and executive dysfunction by targeting engagement of the ECN, as well as the salience network and the DMN. A cognitive intervention designed to improve reasoning and problem-solving in older adults found increased cerebral blood flow in regions of the ECN post-training.186Though few studies have examined the underlying neural mechanisms tied to improved mood symptoms in LLD. 187,188, a group-based metacognitive intervention for older adults targeting executive functioning strategies was associated with increased resting state functional connectivity within the ECN, as well as increased anticorrelation between the ECN and DMN.189 Further, in a recent single-arm study of middle-aged and older adults with LLD, four weeks of a digital cognitive control intervention effectively engaged the ECN (increased ECN connectivity), and generated improvements in mood, executive function performance, and self-reported dysexecutive behaviors.190 These results suggest that the engagement of the ECN through repeated cognitive stimulation may be a central mechanism for rescuing dysfunctional brain networks that contributes to both the mood and executive dysfunction in many suffering from depression.
Cognitive training in LLD with apathy has been less widely studied. However, in patients suffering from schizophrenia, targeted cognitive training reduced negative symptoms, including motivational disturbances, and improved associated cognitive and functional deficits.191 Thus, a similar approach that selectively targets cognitive processes and neural networks disrupted in apathy of LLD may prove to be efficacious. In older adults with major depression, four weeks of a novel mobile DCT program improved working memory and sustained attention, key processes disrupted in apathy.190 Moreover, in the cognitive training study referenced above, training-related changes in functional connectivity of the ECN and DMN were associated with reduced apathy post-intervention.190 These data provide the first evidence that selective cognitive training of attentional and cognitive control deficits may rescue neural circuits disrupted in apathy and contribute to mood and cognitive improvements.
7. Conclusions
While standard approaches to LLD treatment are efficacious in alleviating the mood symptoms of many suffering from LLD, many individuals are left with residual cognitive symptoms and are prone to relapse. Understanding networks that contribute to common clinical subtypes of LLD can inform the development and application of alternative treatment approaches by optimizing the functioning of aspects of the aging brain. Two such approaches that demonstrate preliminary evidence of efficacy, at least in a subset of older individuals with depression, are digital cognitive training and physical exercise interventions. While we are not advocating that these types of interventions replace traditional antidepressant treatments for LLD, these may be reasonable alternatives for depressed older adults who either don’t respond well or can’t tolerate traditional antidepressants. Further, given the difficulty many older adults have in accessing expert care for depression using neuroscience to inform and test interventions that are not only efficacious but scalable may prove to be quite beneficial.
Highlights.
Brain aging may contribute to poor antidepressant response of late-life depression.
Disrupted brain networks contribute to common subtypes of late-life depression.
Novel interventions may rescue brain networks central to late-life depression.
Acknowledgments
Funding Sources
This work was supported by the National Institute of Mental Health: T32 MH019132 (PI: Alexopoulos) & K01 MH118480 (PI: Victoria)
Footnotes
Declaration of interests
☒The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry. 2005;62(10):1097–1106. doi: 10.1001/archpsyc.62.10.1097 [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen H-U. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21(3):169–184. doi: 10.1002/mpr.1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rush AJ. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009;60(11):1439–1445. doi: 10.1176/ps.2009.60.11.1439 [DOI] [PubMed] [Google Scholar]
- 4.Insel TR, Wang PS. The STAR*D trial: revealing the need for better treatments. Psychiatr Serv. 2009;60(11):1466–1467. doi: 10.1176/ps.2009.60.11.1466 [DOI] [PubMed] [Google Scholar]
- 5.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goulden N, Khusnulina A, Davis NJ, et al. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. Neuroimage. 2014;99:180–190. doi: 10.1016/j.neuroimage.2014.05.052 [DOI] [PubMed] [Google Scholar]
- 7.. Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yantis S, Hillstrom AP. Stimulus-driven attentional capture: Evidence from equiluminant visual objects. J Exp Psychol Hum Percept Perform. 1994;20(1):95–107. doi: 10.1037/0096-1523.20.1.95 [DOI] [PubMed] [Google Scholar]
- 9.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- 10.Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16(1):55–61. doi: 10.1038/nrn3857 [DOI] [PubMed] [Google Scholar]
- 11.Vuilleumier P. How brains beware: Neural mechanisms of emotional attention. Trends Cogn Sci. 2005;9(12):585–594. doi: 10.1016/j.tics.2005.10.011 [DOI] [PubMed] [Google Scholar]
- 12.He X, Qin W, Liu Y, et al. Abnormal salience network in normal aging and in amnestic mild cognitive impairment and Alzheimer’s disease. Hum Brain Mapp. 2014;35(7):3446–3464. doi: 10.1002/hbm.22414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mather M. The Affective Neuroscience of Aging. Ssrn. Published online 2016. doi: 10.1146/annurev-psych-122414-033540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bachman SL, Dahl MJ, Werkle-Bergner M, et al. Locus coeruleus MRI contrast is associated with cortical thickness in older adults. Neurobiol Aging. 2021;100:72–82. doi: 10.1016/j.neurobiolaging.2020.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Etkin A, Büchel C, Gross JJ. The neural bases of emotion regulation. Nat Rev Neurosci. 2015;16(11):693–700. doi: 10.1038/nrn4044 [DOI] [PubMed] [Google Scholar]
- 16.Samanez-Larkin GR, Knutson B. Decision making in the ageing brain: Changes in affective and motivational circuits. Nat Rev Neurosci. 2015;16(5):278–289. doi: 10.1038/nrn3917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raichle ME. A paradigm shift in functional brain imaging. J Neurosci. 2009;29(41):12729–12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- 19.Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain-A meta-analysis of imaging studies on the self. Neuroimage. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002 [DOI] [PubMed] [Google Scholar]
- 20.Huang H, Movellan J, Paulus MP, Harlé KM. The influence of depression on cognitive control: Disambiguating approach and avoidance tendencies. PLoS One. 2015;10(11):1–13. doi: 10.1371/journal.pone.0143714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saverino C, Grigg O, Churchill NW, Grady CL. Age differences in the default network at rest and the relation to self-referential processing. Soc Cogn Affect Neurosci. 2015;10(2):231–239. doi: 10.1093/scan/nsu046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koster EHW, De Lissnyder E, Derakshan N, De Raedt R. Understanding depressive rumination from a cognitive science perspective: The impaired disengagement hypothesis. Clin Psychol Rev. 2011;31(1):138–145. doi: 10.1016/j.cpr.2010.08.005 [DOI] [PubMed] [Google Scholar]
- 23.Mennin DS, Fresco DM. What, me worry and ruminate about dsm-5 and rdoc? The importance of targeting negative self-referential processing. Clin Psychol Sci Pract. 2013;20(3):258–267. doi: 10.1111/cpsp.12038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olatunji BO, Naragon-Gainey K, Wolitzky-Taylor KB. Specificity of rumination in anxiety and depression: A multimodal meta-analysis. Clin Psychol Sci Pract. 2013;20(3):225–257. doi: 10.1111/cpsp.12037 [DOI] [Google Scholar]
- 25.Stuhrmann A, Dohm K, Kugel H, et al. Mood-congruent amygdala responses to subliminally presented facial expressions in major depression: Associations with anhedonia. J Psychiatry Neurosci. 2013;38(4):249–258. doi: 10.1503/jpn.120060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12(2):241–268. doi: 10.3758/s13415-011-0083-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage. Published online 2007. doi: 10.1016/j.neuroimage.2007.03.071 [DOI] [PubMed] [Google Scholar]
- 28.Respino M, Hoptman MJ, Victoria LW, et al. Cognitive Control Network Homogeneity and Executive Functions in Late-Life Depression. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5(2). doi: 10.1016/j.bpsc.2019.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andreescu C, Ajilore O, Aizenstein HJ, et al. Disruption of Neural Homeostasis as a Model of Relapse and Recurrence in Late-Life Depression. Am J Geriatr psychiatry Off J Am Assoc Geriatr Psychiatry. 2019;27(12):1316–1330. doi: 10.1016/j.jagp.2019.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexopoulos GS. Mechanisms and treatment of late-life depression. Transl Psychiatry. 2019;9(1):188. doi: 10.1038/s41398-019-0514-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18(9):963–974. doi: 10.1038/mp.2013.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tiemeier H, Breteler MMB, van Popele NM, Hofman A, Witteman JCM. Late-life depression is associated with arterial stiffness: a population-based study. J Am Geriatr Soc. 2003;51(8):1105–1110. doi: 10.1046/j.1532-5415.2003.51359.x [DOI] [PubMed] [Google Scholar]
- 33.van Agtmaal MJM, Houben AJHM, Pouwer F, Stehouwer CDA, Schram MT. Association of Microvascular Dysfunction With Late-Life Depression: A Systematic Review and Meta-analysis. JAMA psychiatry. 2017;74(7):729–739. doi: 10.1001/jamapsychiatry.2017.0984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen D-D, Chen AF. CuZn superoxide dismutase deficiency: culprit of accelerated vascular aging process. Published online 2006. [DOI] [PubMed] [Google Scholar]
- 35.Direk N, Koudstaal PJ, Hofman A, Ikram MA, Hoogendijk WJ, Tiemeier H. Cerebral hemodynamics and incident depression: the Rotterdam Study. Biol Psychiatry. 2012;72(4):318–323. doi: 10.1016/j.biopsych.2012.01.019 [DOI] [PubMed] [Google Scholar]
- 36.Jaywant A, DelPonte L, Kanellopoulos D, O’Dell MW, Gunning FM. The Structural and Functional Neuroanatomy of Post-Stroke Depression and Executive Dysfunction: A Review of Neuroimaging Findings and Implications for Treatment. J Geriatr Psychiatry Neurol. Published online October 2020:891988720968270. doi: 10.1177/0891988720968270 [DOI] [PubMed] [Google Scholar]
- 37.Gillis JC, Chang S-C, Wang W, et al. The relation of telomere length at midlife to subsequent 20-year depression trajectories among women. Depress Anxiety. 2019;36(6):565–575. doi: 10.1002/da.22892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salo KI, Scharfen J, Wilden ID, Schubotz RI, Holling H. Confining the concept of vascular depression to late-onset depression: a meta-analysis of MRI-defined hyperintensity burden in major depressive disorder and bipolar disorder. Front Psychol. 2019;10:1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gunning-Dixon FM, Walton M, Cheng J, et al. MRI signal hyperintensities and treatment remission of geriatric depression. J Affect Disord. 2010;126(3):395–401. doi: 10.1016/j.jad.2010.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park JH, Lee SB, Lee JJ, et al. Epidemiology of MRI-defined vascular depression: A longitudinal, community-based study in Korean elders. J Affect Disord. 2015;180:200–206. doi: 10.1016/j.jad.2015.04.008 [DOI] [PubMed] [Google Scholar]
- 41.van Sloten TT, Sigurdsson S, van Buchem MA, et al. Cerebral Small Vessel Disease and Association With Higher Incidence of Depressive Symptoms in a General Elderly Population: The AGES-Reykjavik Study. Am J Psychiatry. 2015;172(6):570–578. doi: 10.1176/appi.ajp.2014.14050578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tully PJ, Debette S, Mazoyer B, Tzourio C. White matter lesions are associated with specific depressive symptom trajectories among incident depression and dementia populations: three-city Dijon MRI study. Am J Geriatr Psychiatry. 2017;25(12):1311–1321. [DOI] [PubMed] [Google Scholar]
- 43.Dalby RB, Chakravarty MM, Ahdidan J, et al. Localization of white-matter lesions and effect of vascular risk factors in late-onset major depression. Psychol Med. 2010;40(8):1389–1399. doi: 10.1017/S0033291709991656 [DOI] [PubMed] [Google Scholar]
- 44.Sheline YI, Price JL, Vaishnavi SN, et al. Regional white matter hyperintensity burden in automated segmentation distinguishes late-life depressed subjects from comparison subjects matched for vascular risk factors. Am J Psychiatry. 2008;165(4):524–532. doi: 10.1176/appi.ajp.2007.07010175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor WD, Zhao Z, Ashley-Koch A, et al. Fiber tract-specific white matter lesion severity Findings in late-life depression and by AGTR1 A1166C genotype. Hum Brain Mapp. 2013;34(2):295–303. doi: 10.1002/hbm.21445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Uden IWM, Tuladhar AM, de Laat KF, et al. White matter integrity and depressive symptoms in cerebral small vessel disease: the RUN DMC study. Am J Geriatr Psychiatry. 2015;23(5):525–535. [DOI] [PubMed] [Google Scholar]
- 47.Charlton RA, Lamar M, Zhang A, Yang S, Ajilore O, Kumar A. White-matter tract integrity in late-life depression: associations with severity and cognition. Psychol Med. 2014;44(7):1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Emsell L, Adamson C, De Winter F-L, et al. Corpus callosum macro and microstructure in late-life depression. J Affect Disord. 2017;222:63–70. doi: 10.1016/j.jad.2017.06.063 [DOI] [PubMed] [Google Scholar]
- 49.Guo W, Liu F, Xun G, et al. Disrupted white matter integrity in first-episode, drug-naive, late-onset depression. J Affect Disord. 2014;163:70–75. [DOI] [PubMed] [Google Scholar]
- 50.Mettenburg JM, Benzinger TL, Shimony JS, Snyder AZ, Sheline YI. Diminished performance on neuropsychological testing in late life depression is correlated with microstructural white matter abnormalities. Neuroimage. 2012;60(4):2182–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reppermund S, Zhuang L, Wen W, et al. White matter integrity and late-life depression in community-dwelling individuals: diffusion tensor imaging study using tract-based spatial statistics. Br J Psychiatry. 2014;205(4):315–320. [DOI] [PubMed] [Google Scholar]
- 52.Wen M, Steffens DC, Chen M, Zainal NH. Diffusion tensor imaging studies in late-life depression: systematic review and meta-analysis. Int J Geriatr Psychiatry. 2014;29(12):1173–1184. [DOI] [PubMed] [Google Scholar]
- 53.Shen X, Adams MJ, Ritakari TE, Cox SR, McIntosh AM, Whalley HC. White matter microstructure and its relation to longitudinal measures of depressive symptoms in mid- and late life. Biol Psychiatry. 2019;86(10):759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seiler S, Fletcher E, Hassan-Ali K, et al. Cerebral tract integrity relates to white matter hyperintensities, cortex volume, and cognition. Neurobiol Aging. 2018;72:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Langen CD, Cremers LGM, de Groot M, et al. Disconnection due to white matter hyperintensities is associated with lower cognitive scores. Neuroimage. 2018;183:745–756. [DOI] [PubMed] [Google Scholar]
- 56.Aizenstein HJ, Andreescu C, Edelman KL, et al. fMRI correlates of white matter hyperintensities in late-life depression. Am J Psychiatry. 2011;168(10):1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu M, Andreescu C, Butters MA, Tamburo R, Reynolds III CF, Aizenstein H. Default-mode network connectivity and white matter burden in late-life depression. Psychiatry Res Neuroimaging. 2011;194(1):39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mayda AB V, Westphal A, Carter CS, DeCarli C. Late life cognitive control deficits are accentuated by white matter disease burden. Brain. 2011;134(6):1673–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yin Y, He X, Xu M, et al. Structural and functional connectivity of default mode network underlying the cognitive impairment in late-onset depression. Sci Rep. 2016;6(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alexopoulos GS, Kiosses DN, Klimstra S, Kalayam B, Bruce ML. Clinical presentation of the “depression-executive dysfunction syndrome” of late life. Am J Geriatr Psychiatry. Published online 2002. doi: 10.1097/00019442-200201000-00012 [DOI] [PubMed] [Google Scholar]
- 61.Gandelman JA, Albert K, Boyd BD, et al. Intrinsic Functional Network Connectivity Is Associated With Clinical Symptoms and Cognition in Late-Life Depression. Biol Psychiatry Cogn Neurosci Neuroimaging. Published online 2019. doi: 10.1016/j.bpsc.2018.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord. Published online 2012. doi: 10.1016/j.jad.2011.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Respino M, Jaywant A, Kuceyeski A, et al. The impact of white matter hyperintensities on the structural connectome in late-life depression: Relationship to executive functions. NeuroImage Clin. 2019;23:101852. doi: 10.1016/j.nicl.2019.101852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tadayonnejad R, Ajilore O. Brain Network Dysfunction in Late-Life Depression: A Literature Review. doi: 10.1177/0891988713516539 [DOI] [PubMed] [Google Scholar]
- 65.Alexopoulos GS, Kiosses DN, Heo M, Murphy CF, Shanmugham B, Gunning-Dixon F. Executive dysfunction and the course of geriatric depression. Biol Psychiatry. Published online 2005. doi: 10.1016/j.biopsych.2005.04.024 [DOI] [PubMed] [Google Scholar]
- 66.. Potter GG, Kittinger JD, Wagner HR, Steffens DC, Krishnan KRR. Prefrontal neuropsychological predictors of treatment remission in late-life depression. Neuropsychopharmacology. Published online 2004. doi: 10.1038/sj.npp.1300551 [DOI] [PubMed] [Google Scholar]
- 67.. Manning KJ, Alexopoulos GS, Banerjee S, et al. Executive functioning complaints and escitalopram treatment response in late-life depression. Am J Geriatr Psychiatry. Published online 2015. doi: 10.1016/j.jagp.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cotter DL, Walters SM, Fonseca C, et al. Aging and Positive Mood: Longitudinal Neurobiological and Cognitive Correlates. Am J Geriatr psychiatry Off J Am Assoc Geriatr Psychiatry. 2020;28(9):946–956. doi: 10.1016/j.jagp.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: A systematic review and meta-analysis. Psychol Med. Published online 2014. doi: 10.1017/S0033291713002535 [DOI] [PubMed] [Google Scholar]
- 70.La Corte V, Sperduti M, Malherbe C, et al. Cognitive Decline and Reorganization of Functional Connectivity in Healthy Aging: The Pivotal Role of the Salience Network in the Prediction of Age and Cognitive Performances. Front Aging Neurosci. 2016;8:204. doi: 10.3389/fnagi.2016.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li R, Yin S, Zhu X, et al. Linking Inter-Individual Variability in Functional Brain Connectivity to Cognitive Ability in Elderly Individuals. Front Aging Neurosci. 2017;9:385. doi: 10.3389/fnagi.2017.00385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage. 2009;46(2):530–541. doi: 10.1016/j.neuroimage.2009.01.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004 [DOI] [PubMed] [Google Scholar]
- 74.Piguet C, Cojan Y, Sterpenich V, Desseilles M, Bertschy G, Vuilleumier P. Alterations in neural systems mediating cognitive flexibility and inhibition in mood disorders. Hum Brain Mapp. 2016;37(4):1335–1348. doi: 10.1002/hbm.23104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang L, Potter GG, Krishnan RKR, Dolcos F, Smith GS, Steffens DC. Neural correlates associated with cognitive decline in late-life depression. Am J Geriatr Psychiatry. Published online 2012. doi: 10.1097/JGP.0b013e31823e2cc7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior Cingulate Conflict Monitoring and Adjustments in Control. Science (80- ). Published online 2004. doi: 10.1126/science.1089910 [DOI] [PubMed] [Google Scholar]
- 77.Vasudev A, Saxby BK, O’Brien JT, et al. Relationship between cognition, magnetic resonance white matter hyperintensities, and cardiovascular autonomic changes in late-life depression. Am J Geriatr Psychiatry. 2012;20(8):691–699. doi: 10.1097/JGP.0b013e31824c0435 [DOI] [PubMed] [Google Scholar]
- 78.Lesser IM, Boone KB, Mehringer CM, Wohl MA, Miller BL, Berman NG. Cognition and white matter hyperintensities in older depressed patients. Am J Psychiatry. 1996;153(10):1280–1287. doi: 10.1176/ajp.153.10.1280 [DOI] [PubMed] [Google Scholar]
- 79.Köhler S, Thomas AJ, Lloyd A, Barber R, Almeida OP, O’Brien JT. White matter hyperintensities, cortisol levels, brain atrophy and continuing cognitive deficits in late-life depression. Br J Psychiatry. 2010;196(2):143–149. doi: 10.1192/bjp.bp.109.071399 [DOI] [PubMed] [Google Scholar]
- 80.Lockhart SN, Luck SJ, Geng J, et al. White matter hyperintensities among older adults are associated with futile increase in frontal activation and functional connectivity during spatial search. PLoS One. 2015;10(3). doi: 10.1371/journal.pone.0122445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu M, Andreescu C, Butters MA, Tamburo R, Reynolds CF, Aizenstein H. Default-mode network connectivity and white matter burden in late-life depression. Psychiatry Res - Neuroimaging. 2011;194(1):39–46. doi: 10.1016/j.pscychresns.2011.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dombrovski AY, Szanto K, Clark L, et al. Corticostriatothalamic reward prediction error signals and executive control in late-life depression. Psychol Med. 2015;45(7):1413–1424. doi: 10.1017/S0033291714002517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fresco DM, Heimberg RG, Abramowitz A, Bertram TL. The effect pf a negative mood priming challenge on dysfunctional attitudes, explanatory style, and explanatory flexibility. Br J Clin Psychol. 2006;45(2):167–183. doi: 10.1348/014466505X35137 [DOI] [PubMed] [Google Scholar]
- 84.Hallion LS, Ruscio AM. A Meta-Analysis of the Effect of Cognitive Bias Modification on Anxiety and Depression. Psychol Bull. 2011;137(6):940–958. doi: 10.1037/a0024355 [DOI] [PubMed] [Google Scholar]
- 85.Strigo IA, Simmons AN, Matthews SC, Craig AD (Bud), Paulus MP. Association of Major Depressive Disorder With Altered Functional Brain Response During Anticipation and Processing of Heat Pain. Arch Gen Psychiatry. 2008;65(11):1275. doi: 10.1001/archpsyc.65.11.1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yuen GS, Gunning-Dixon FM, Hoptman MJ, et al. The salience network in the apathy of late-life depression. Int J Geriatr Psychiatry. 2014;29(11):1116–1124. doi: 10.1002/gps.4171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Disner SG, Beevers CG, Haigh EAP, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12(8):467–477. doi: 10.1038/nrn3027 [DOI] [PubMed] [Google Scholar]
- 88.Ruhe HG, Mocking RJT, Figueroa CA, et al. Emotional Biases and Recurrence in Major Depressive Disorder. Results of 2.5 Years Follow-Up of Drug-Free Cohort Vulnerable for Recurrence. Front psychiatry. 2019;10:145. doi: 10.3389/fpsyt.2019.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Foland-Ross LC, Gotlib IH. Cognitive and neural aspects of information processing in major depressive disorder: an integrative perspective. Front Psychol. 2012;3:489. doi: 10.3389/fpsyg.2012.00489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Keller AS, Leikauf JE, Holt-Gosselin B, Staveland BR, Williams LM. Paying attention to attention in depression. Transl Psychiatry. 2019;9(1):279. doi: 10.1038/s41398-019-06161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Beck AT, Brown G, Steer RA, Eidelson JI, Riskind JH. Differentiating Anxiety and Depression: A Test of the Cognitive Content-Specificity Hypothesis. J Abnorm Psychol. 1987;96(3):179–183. doi: 10.1037/0021-843X.96.3.179 [DOI] [PubMed] [Google Scholar]
- 92.Bradley BP, Mogg K, Millar N, White J. Selective processing of negative information: effects of clinical anxiety, concurent depression, and awareness. J Abnorm Psychol. 1995;104(3):532–536. doi: 10.1037/0021-843x.104.3.532 [DOI] [PubMed] [Google Scholar]
- 93.Groenewold NA, Opmeer EM, de Jonge P, Aleman A, Costafreda SG. Emotional valence modulates brain functional abnormalities in depression: Evidence from a meta-analysis of fMRI studies. Neurosci Biobehav Rev. 2013;37(2):152–163. doi: 10.1016/j.neubiorev.2012.11.015 [DOI] [PubMed] [Google Scholar]
- 94.Harmer CJ, O’Sullivan U, Favaron E, et al. Effect of acute antidepressant administration on negative affective bias in depressed patients. Am J Psychiatry. 2009;166(10):1178–1184. doi: 10.1176/appi.ajp.2009.09020149 [DOI] [PubMed] [Google Scholar]
- 95.Hilimire MR, Mayberg HS, Holtzheimer PE, et al. Effects of subcallosal cingulate deep brain stimulation on negative self-bias in patients with treatment-resistant depression. Brain Stimul. 2015;8(2):185–191. doi: 10.1016/j.brs.2014.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jones NP, Siegle GJ, Thase ME. Effects of rumination and initial severity on remission to cognitive therapy for depression. Cognit Ther Res. 2008;32(4):591–604. doi: 10.1007/s10608-008-9191-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McIntyre RS, Cha DS, Soczynska JK, et al. Cognitive deficits and functional outcomes in major depressive disorder: Determinants, substrates, and treatment interventions. Depress Anxiety. 2013;30(6):515–527. doi: 10.1002/da.22063 [DOI] [PubMed] [Google Scholar]
- 98.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72(6):603–611. doi: 10.1001/jamapsychiatry.2015.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Langenecker SA, Jacobs RH, Passarotti AM. Current Neural and Behavioral Dimensional Constructs Across Mood Disorders. Curr Behav Neurosci Reports. 2014;1(3):144–153. doi: 10.1007/s40473-014-0018-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: Implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70(4):327–333. doi: 10.1016/j.biopsych.2011.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.An J, Wang L, Li K, et al. Differential effects of antidepressant treatment on long-range and short-range functional connectivity strength in patients with major depressive disorder. Sci Rep. 2017;7(1):10214. doi: 10.1038/s41598-017-10575-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rizk MM, Rubin-Falcone H, Keilp J, et al. White matter correlates of impaired attention control in major depressive disorder and healthy volunteers. J Affect Disord. 2017;222(June):103–111. doi: 10.1016/j.jad.2017.06.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Taylor WD, Boyd B, McQuoid DR, Kudra K, Saleh A, MacFall JR. Widespread white matter but focal gray matter alterations in depressed individuals with thoughts of death. Prog Neuro-Psychopharmacology Biol Psychiatry. 2015;62:22–28. doi: 10.1016/j.pnpbp.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Victoria LW, Alexopoulos GS, Ilieva IP, et al. White matter abnormalities predict residual negative self-referential thinking following treatment of late-life depression with escitalopram: A preliminary study. J Affect Disord. 2018;243(September 2018):62–69. doi: 10.1016/j.jad.2018.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.LeMoult J, Kircanski K, Prasad G, Gotlib IH. Negative Self-Referential Processing Predicts the Recurrence of Major Depressive Episodes. Clin Psychol Sci. 2017;5(1):174–181. doi: 10.1177/2167702616654898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Renner F, Siep N, Lobbestael J, Arntz A, Peeters FPML, Huibers MJH. Neural correlates of self-referential processing and implicit self-associations in chronic depression. J Affect Disord. 2015;186:40–47. doi: 10.1016/j.jad.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 108.Schmaling KB, Dimidjian S, Katon W, Sullivan M. Response Styles Among Patients With Minor Depression and Dysthymia in Primary Care. J Abnorm Psychol. 2002;111(2):350–356. doi: 10.1037//0021-843X.111.2.350 [DOI] [PubMed] [Google Scholar]
- 109.Pimontel MA, Kanellopoulos D, Gunning FM. Neuroanatomical Abnormalities in Older Depressed Adults With Apathy: A Systematic Review. J Geriatr Psychiatry Neurol. 2020;33(5):289–303. doi: 10.1177/0891988719882100 [DOI] [PubMed] [Google Scholar]
- 110.Radakovic R, Abrahams S. Multidimensional apathy: evidence from neurodegenerative disease. Curr Opin Behav Sci. 2018;22:42–49. [Google Scholar]
- 111.Robert P, Lanctôt KL, Agüera-Ortiz L, et al. Is it time to revise the diagnostic criteria for apathy in brain disorders? The 2018 international consensus group. Eur Psychiatry. 2018;54:71–76. doi: 10.1016/j.eurpsy.2018.07.008 [DOI] [PubMed] [Google Scholar]
- 112.Ayers E, Shapiro M, Holtzer R, Barzilai N, Milman S, Verghese J. Symptoms of Apathy Independently Predict Incident Frailty and Disability in Community-Dwelling Older Adults. J Clin Psychiatry. 2017;78(5):e529–e536. doi: 10.4088/JCP.15m10113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yuen GS, Bhutani S, Lucas BJ, et al. Apathy in late-life depression: common, persistent, and disabling. Am J Geriatr psychiatry Off J Am Assoc Geriatr Psychiatry. 2015;23(5):488–494. doi: 10.1016/j.jagp.2014.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Funes CM, Lavretsky H, Ercoli L, St Cyr N, Siddarth P. Apathy Mediates Cognitive Difficulties in Geriatric Depression. Am J Geriatr psychiatry Off J Am Assoc Geriatr Psychiatry. 2018;26(1):100–106. doi: 10.1016/j.jagp.2017.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ismail Z, Smith EE, Geda Y, et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: Provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement. 2016;12(2):195–202. doi: 10.1016/j.jalz.2015.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ceïde ME, Warhit A, Ayers EI, Kennedy G, Verghese J. Apathy and the Risk of Predementia Syndromes in Community-Dwelling Older Adults. J Gerontol B Psychol Sci Soc Sci. 2020;75(7):1443–1450. doi: 10.1093/geronb/gbaa063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lavretsky H, Reinlieb M, St Cyr N, Siddarth P, Ercoli LM, Senturk D. Citalopram, methylphenidate, or their combination in geriatric depression: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2015;172(6):561–569. doi: 10.1176/appi.ajp.2014.14070889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Volicer L, Frijters DHM, van der Steen JT. Apathy and weight loss in nursing home residents: longitudinal study. J Am Med Dir Assoc. 2013;14(6):417–420. doi: 10.1016/j.jamda.2012.12.004 [DOI] [PubMed] [Google Scholar]
- 119.Lanctôt KL, Agüera-Ortiz L, Brodaty H, et al. Apathy associated with neurocognitive disorders: Recent progress and future directions. Alzheimers Dement. 2017;13(1):84–100. doi: 10.1016/j.jalz.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 120.Le Heron C, Apps MAJ, Husain M. The anatomy of apathy: A neurocognitive framework for amotivated behaviour. Neuropsychologia. 2018;118(Pt B):54–67. doi: 10.1016/j.neuropsychologia.2017.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kawagoe T, Onoda K, Yamaguchi S. Apathy and Executive Function in Healthy Elderly-Resting State fMRI Study. Front Aging Neurosci. 2017;9:124. doi: 10.3389/fnagi.2017.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Husain M, Roiser JP. Neuroscience of apathy and anhedonia: A transdiagnostic approach. Nat Rev Neurosci. 2018;19(8):470–484. doi: 10.1038/s41583-018-0029-9 [DOI] [PubMed] [Google Scholar]
- 124.Le Heron C, Holroyd CB, Salamone J, Husain M. Brain mechanisms underlying apathy. J Neurol Neurosurg Psychiatry. 2019;90(3):302–312. doi: 10.1136/jnnp-2018-318265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lavretsky H, Ballmaier M, Pham D, Toga A, Kumar A. Neuroanatomical characteristics of geriatric apathy and depression: a magnetic resonance imaging study. Am J Geriatr psychiatry Off J Am Assoc Geriatr Psychiatry. 2007;15(5):386–394. doi: 10.1097/JGP.0b013e3180325a16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tay J, Tuladhar AM, Hollocks MJ, et al. Apathy is associated with large-scale white matter network disruption in small vessel disease. Neurology. 2019;92(11):E1157–E1167. doi: 10.1212/WNL.0000000000007095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Raimo S, Santangelo G, D’Iorio A, Trojano L, Grossi D. Neural correlates of apathy in patients with neurodegenerative disorders: an activation likelihood estimation (ALE) meta-analysis. Brain Imaging Behav. 2019;13(6):1815–1834. doi: 10.1007/s11682-018-9959-0 [DOI] [PubMed] [Google Scholar]
- 128.Alexopoulos GS, Hoptman MJ, Yuen G, et al. Functional connectivity in apathy of late-life depression: A preliminary study. J Affect Disord. 2013;149(1–3):398–405. doi: 10.1016/j.jad.2012.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Breukelaar IA, Antees C, Grieve SM, et al. Cognitive control network anatomy correlates with neurocognitive behavior: A longitudinal study. Hum Brain Mapp. 2017;38(2):631–643. doi: 10.1002/hbm.23401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ventura J, Subotnik KL, Gretchen-Doorly D, et al. Cognitive remediation can improve negative symptoms and social functioning in first-episode schizophrenia: A randomized controlled trial. Schizophr Res. 2019;203:24–31. doi: 10.1016/j.schres.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.De Marco M, Meneghello F, Duzzi D, Rigon J, Pilosio C, Venneri A. Cognitive stimulation of the default-mode network modulates functional connectivity in healthy aging. Brain Res Bull. 2016;121:26–41. doi: 10.1016/j.brainresbull.2015.12.001 [DOI] [PubMed] [Google Scholar]
- 132.Shen K-K, Welton T, Lyon M, et al. Structural core of the executive control network: A high angular resolution diffusion MRI study. Hum Brain Mapp. 2020;41(5):1226–1236. doi: 10.1002/hbm.24870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bissonette GB, Roesch MR. Neurophysiology of Reward-Guided Behavior: Correlates Related to Predictions, Value, Motivation, Errors, Attention, and Action. Curr Top Behav Neurosci. 2016;27:199–230. doi: 10.1007/7854_2015_382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huskey R, Craighead B, Miller MB, Weber R. Does intrinsic reward motivate cognitive control? a naturalistic-fMRI study based on the synchronization theory of flow. Cogn Affect Behav Neurosci. 2018;18(5):902–924. doi: 10.3758/s13415-018-0612-6 [DOI] [PubMed] [Google Scholar]
- 135.Le Heron C, Manohar S, Plant O, et al. Dysfunctional effort-based decision-making underlies apathy in genetic cerebral small vessel disease. Brain. 2018;141(11):3193–3210. doi: 10.1093/brain/awy257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Etkin A, Patenaude B, Song YJC, et al. A cognitive-emotional biomarker for predicting remission with antidepressant medications: a report from the iSPOT-D trial. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2015;40(6):1332–1342. doi: 10.1038/npp.2014.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Corlett PR, Fletcher PC. Computational psychiatry: a Rosetta Stone linking the brain to mental illness. The Lancet Psychiatry. 2014;1(5):399–402. [DOI] [PubMed] [Google Scholar]
- 138.Costa VD, Tran VL, Turchi J, Averbeck BB. Reversal Learning and Dopamine: A Bayesian Perspective. J Neurosci. 2015;35(6):2407–2416. doi: 10.1523/JNEUROSCI.1989-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cooper JA, Arulpragasam AR, Treadway MT. Anhedonia in depression: biological mechanisms and computational models. Curr Opin Behav Sci. 2018;22:128–135. doi: 10.1016/j.cobeha.2018.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nassar MR, Gold JI. A Healthy Fear of the Unknown: Perspectives on the Interpretation of Parameter Fits from Computational Models in Neuroscience. PLoS Comput Biol. 2013;9(4):1–6. doi: 10.1371/journal.pcbi.1003015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Daw ND, Doya K. The computational neurobiology of learning and reward. Curr Opin Neurobiol. 2006;16(2):199–204. doi: 10.1016/j.conb.2006.03.006 [DOI] [PubMed] [Google Scholar]
- 142.Bornstein AM, Khaw MW, Shohamy D, Daw ND. Reminders of past choices bias decisions for reward in humans. Nat Commun. 2017;8(May 2015):1–9. doi: 10.1038/ncomms15958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dombrovski AY, Hallquist MN, Brown VM, Wilson J, Szanto K. Value-Based Choice, Contingency Learning, and Suicidal Behavior in Mid- and Late-Life Depression. Biol Psychiatry. 2019;85(6):506–516. doi: 10.1016/j.biopsych.2018.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Treadway MT, Zald DH. Parsing Anhedonia: Translational Models of Reward-Processing Deficits in Psychopathology. Curr Dir Psychol Sci. 2013;22(3):244–249. doi: 10.1177/0963721412474460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Frey A-L, McCabe C. Impaired social learning predicts reduced real-life motivation in individuals with depression: A computational fMRI study. J Affect Disord. 2020;263:698–706. doi: 10.1016/j.jad.2019.11.049 [DOI] [PubMed] [Google Scholar]
- 146.Chong TT-J, Apps M, Giehl K, Sillence A, Grima LL, Husain M. Neurocomputational mechanisms underlying subjective valuation of effort costs. PLoS Biol. 2017;15(2):e1002598. doi: 10.1371/journal.pbio.1002598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bonnelle V, Manohar S, Behrens T, Husain M. Individual Differences in Premotor Brain Systems Underlie Behavioral Apathy. Cereb Cortex. 2016;26(2):807–819. doi: 10.1093/cercor/bhv247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Berwian IM, Wenzel JG, Collins AGE, et al. Computational Mechanisms of Effort and Reward Decisions in Patients With Depression and Their Association With Relapse After Antidepressant Discontinuation. JAMA psychiatry. 2020;77(5):513–522. doi: 10.1001/jamapsychiatry.2019.4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ter Telgte A, van Leijsen EMC, Wiegertjes K, Klijn CJM, Tuladhar AM, de Leeuw F-E. Cerebral small vessel disease: from a focal to a global perspective. Nat Rev Neurol. 2018;14(7):387–398. [DOI] [PubMed] [Google Scholar]
- 150.Xie X, Shi Y, Zhang J. Structural network connectivity impairment and depressive symptoms in cerebral small vessel disease. J Affect Disord. 2017;220:8–14. [DOI] [PubMed] [Google Scholar]
- 151.Mai N, Zhong X, Chen B, et al. Weight Rich-Club Analysis in the White Matter Network of Late-Life Depression with Memory Deficits. Front Aging Neurosci. 2017;9:279. doi: 10.3389/fnagi.2017.00279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Oberlin LE, Respino M, Victoria L, et al. Late-life depression accentuates cognitive weaknesses in older adults with small vessel disease. Neuropsychopharmacology. Published online 2021. doi: 10.1038/s41386-021-00973-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ivanidze J, Mackay M, Hoang A, et al. Dynamic Contrast-Enhanced MRI Reveals Unique Blood-Brain Barrier Permeability Characteristics in the Hippocampus in the Normal Brain. AJNR Am J Neuroradiol. 2019;40(3):408–411. doi: 10.3174/ajnr.A5962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Varatharaj A, Liljeroth M, Darekar A, Larsson HBW, Galea I, Cramer SP. Blood-brain barrier permeability measured using dynamic contrast-enhanced magnetic resonance imaging: a validation study. J Physiol. 2019;597(3):699–709. doi: 10.1113/JP276887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.van Waarde JA, Scholte HS, van Oudheusden LJB, Verwey B, Denys D, van Wingen GA. A functional MRI marker may predict the outcome of electroconvulsive therapy in severe and treatment-resistant depression. Mol Psychiatry. 2015;20(5):609–614. doi: 10.1038/mp.2014.78 [DOI] [PubMed] [Google Scholar]
- 156.Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2016;23(1):28–38. doi: 10.1038/nm.4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dunlop BW, Rajendra JK, Craighead WE, et al. Functional Connectivity of the Subcallosal Cingulate Cortex And Differential Outcomes to Treatment With Cognitive-Behavioral Therapy or Antidepressant Medication for Major Depressive Disorder. Am J Psychiatry. 2017;174(6):533–545. doi: 10.1176/appi.ajp.2016.16050518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fernandes BS, Williams LM, Steiner J, Leboyer M, Carvalho AF, Berk M. The new field of “precision psychiatry”. BMC Med. 2017;15(1):80. doi: 10.1186/s12916-017-0849-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Godlewska BR, Harmer CJ. Cognitive neuropsychological theory of antidepressant action: a modern-day approach to depression and its treatment. Psychopharmacology (Berl). Published online January 2020. doi: 10.1007/s00213-019-05448-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dunlop K, Victoria LW, Downar J, Gunning FM, Liston C. Accelerated brain aging predicts impulsivity and symptom severity in depression. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2021;46(5):911–919. doi: 10.1038/s41386-021-00967-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cuijpers P, Sijbrandij M, Koole SL, Andersson G, Beekman AT, Reynolds CF 3rd. The efficacy of psychotherapy and pharmacotherapy in treating depressive and anxiety disorders: a meta-analysis of direct comparisons. World Psychiatry. 2013;12(2):137–148. doi: 10.1002/wps.20038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zuckerman H, Pan Z, Park C, et al. Recognition and Treatment of Cognitive Dysfunction in Major Depressive Disorder. Front psychiatry. 2018;9:655. doi: 10.3389/fpsyt.2018.00655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kiosses DN, Alexopoulos GS. IADL functions, cognitive deficits, and severity of depression: a preliminary study. Am J Geriatr psychiatry Off J Am Assoc Geriatr Psychiatry. 2005;13(3):244–249. doi: 10.1176/appi.ajgp.13.3.244 [DOI] [PubMed] [Google Scholar]
- 164.Cooney G, Dwan K, Mead G. Exercise for depression. JAMA. 2014;311(23):2432–2433. [DOI] [PubMed] [Google Scholar]
- 165.Schuch FB, Vancampfort D, Firth J, et al. Physical Activity and Incident Depression: A Meta-Analysis of Prospective Cohort Studies. Am J Psychiatry. 2018;175(7):631–648. doi: 10.1176/appi.ajp.2018.17111194 [DOI] [PubMed] [Google Scholar]
- 166.Belvederi Murri M, Amore M, Menchetti M, et al. Physical exercise for late-life major depression. Br J Psychiatry. 2015;207(3):235–242. doi: 10.1192/bjp.bp.114.150516 [DOI] [PubMed] [Google Scholar]
- 167.Murri MB, Ekkekakis P, Menchetti M, et al. Physical exercise for late-life depression: Effects on symptom dimensions and time course. J Affect Disord. 2018;230:65–70. doi: 10.1016/j.jad.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 168.Colcombe SJ, Erickson KI, Raz N, et al. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58(2):176–180. doi: 10.1093/gerona/58.2.m176 [DOI] [PubMed] [Google Scholar]
- 169.Wang C-H. The cognitive gains of exercise. Nat Hum Behav. 2020;4(6):565–566. doi: 10.1038/s41562-020-0856-3 [DOI] [PubMed] [Google Scholar]
- 170.Neviani F, Belvederi Murri M, Mussi C, et al. Physical exercise for late life depression: effects on cognition and disability. Int psychogeriatrics. 2017;29(7):1105–1112. doi: 10.1017/S1041610217000576 [DOI] [PubMed] [Google Scholar]
- 171.Telenius EW, Engedal K, Bergland A. Effect of a high-intensity exercise program on physical function and mental health in nursing home residents with dementia: an assessor blinded randomized controlled trial. PLoS One. 2015;10(5):e0126102. doi: 10.1371/journal.pone.0126102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Vogel JS, van der Gaag M, Slofstra C, Knegtering H, Bruins J, Castelein S. The effect of mind-body and aerobic exercise on negative symptoms in schizophrenia: A meta-analysis. Psychiatry Res. 2019;279:295–305. doi: 10.1016/j.psychres.2019.03.012 [DOI] [PubMed] [Google Scholar]
- 173.Flodin P, Jonasson LS, Riklund K, Nyberg L, Boraxbekk CJ. Does Aerobic Exercise Influence Intrinsic Brain Activity? An Aerobic Exercise Intervention among Healthy Old Adults. Front Aging Neurosci. 2017;9:267. doi: 10.3389/fnagi.2017.00267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Prehn K, Lesemann A, Krey G, et al. Using resting-state fMRI to assess the effect of aerobic exercise on functional connectivity of the DLPFC in older overweight adults. Brain Cogn. 2019;131:34–44. doi: 10.1016/j.bandc.2017.08.006 [DOI] [PubMed] [Google Scholar]
- 175.Voss MW, Sutterer M, Weng TB, et al. Nutritional supplementation boosts aerobic exercise effects on functional brain systems. J Appl Physiol. 2019;126(1):77–87. doi: 10.1152/japplphysiol.00917.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Voss MW, Prakash RS, Erickson KI, et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2010;2. doi: 10.3389/fnagi.2010.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gujral S, Aizenstein H, Reynolds CF 3rd, et al. Exercise for Depression: A Feasibility Trial Exploring Neural Mechanisms. Am J Geriatr psychiatry Off J Am Assoc Geriatr Psychiatry. 2019;27(6):611–616. doi: 10.1016/j.jagp.2019.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Stillman CM, Donofry SD, Erickson KI. Exercise, fitness and the aging brain: A review of functional connectivity in aging. Arch Psychol. 2019;3(4). [Google Scholar]
- 180.Gray JP, Müller VI, Eickhoff SB, Fox PT. Multimodal Abnormalities of Brain Structure and Function in Major Depressive Disorder: A Meta-Analysis of Neuroimaging Studies. Am J Psychiatry. 2020;177(5):422–434. doi: 10.1176/appi.ajp.2019.19050560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gunning FM, Cheng J, Murphy CF, et al. Anterior cingulate cortical volumes and treatment remission of geriatric depression. Int J Geriatr Psychiatry. 2009;24(8):829–836. doi: 10.1002/gps.2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hsieh M-H, McQuoid DR, Levy RM, Payne ME, MacFall JR, Steffens DC. Hippocampal volume and antidepressant response in geriatric depression. Int J Geriatr Psychiatry. 2002;17(6):519–525. doi: 10.1002/gps.611 [DOI] [PubMed] [Google Scholar]
- 183.Stillman CM, Cohen J, Lehman ME, Erickson KI. Mediators of Physical Activity on Neurocognitive Function: A Review at Multiple Levels of Analysis. Front Hum Neurosci. 2016;10:626. doi: 10.3389/fnhum.2016.00626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Morland C, Andersson KA, Haugen ØP, et al. Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat Commun. 2017;8:15557. doi: 10.1038/ncomms15557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Anguera JA, Gunning FM, Areán PA. Improving late life depression and cognitive control through the use of therapeutic video game technology: A proof-of-concept randomized trial. Depress Anxiety. 2017;34(6):508–517. doi: 10.1002/da.22588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chapman SB, Aslan S, Spence JS, et al. Neural mechanisms of brain plasticity with complex cognitive training in healthy seniors. Cereb Cortex. 2015;25(2):396–405. doi: 10.1093/cercor/bht234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kim SM, Kim H-J, Hwang HC, et al. The Effects of a Serious Game on Depressive Symptoms and Anxiety in Breast Cancer Patients with Depression: A Pilot Study Using Functional Magnetic Resonance Imaging. Games Health J. 2018;7(6):409–417. doi: 10.1089/g4h.2017.0183 [DOI] [PubMed] [Google Scholar]
- 188.Williams LM, Pines A, Goldstein-Piekarski AN, et al. The ENGAGE study: Integrating neuroimaging, virtual reality and smartphone sensing to understand self-regulation for managing depression and obesity in a precision medicine model. Behav Res Ther. 2018;101:58–70. doi: 10.1016/j.brat.2017.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cao W, Cao X, Hou C, et al. Effects of cognitive training on resting-state functional connectivity of default mode, salience, and central executive networks. Front Aging Neurosci. 2016;8(APR):1–11. doi: 10.3389/fnagi.2016.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gunning FM, Anguera JA, Victoria LW, Areán PA. A Digital Intervention Targeting Cognitive Control Network Dysfunction in Middle Age and Older Adults with Major Depression. Under Rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ventura J, Subotnik KL, Gretchen-Doorly D, et al. Cognitive remediation can improve negative symptoms and social functioning in first-episode schizophrenia: A randomized controlled trial. Schizophr Res. 2019;203:24–31. doi: 10.1016/j.schres.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]