Abstract
Ghrelin is a gastric-derived peptide hormone with demonstrated impact on alcohol intake and craving, but the reverse side of this bidirectional link, that is, the effects of alcohol on the ghrelin system, remains to be fully established. To further characterize this relationship, we examined (1) ghrelin levels via secondary analysis of human laboratory alcohol administration experiments with heavy-drinking participants; (2) expression of ghrelin, ghrelin receptor, and ghrelin-O-acyltransferase (GOAT) genes (GHRL, GHSR, and MBOAT4, respectively) in post-mortem brain tissue from individuals with alcohol use disorder (AUD) versus controls; (3) ghrelin levels in Ghsr knockout and wild-type rats following intraperitoneal (i.p.) alcohol administration; (4) effect of alcohol on ghrelin secretion from gastric mucosa cells ex vivo and GOAT enzymatic activity in vitro; and (5) ghrelin levels in rats following i.p. alcohol administration versus a calorically equivalent non-alcoholic sucrose solution. Acyl- and totalghrelin levels decreased following acute alcohol administration in humans, but AUD was not associated with changes in central expression of ghrelin system genes in post-mortem tissue. In rats, alcohol decreased acyl-ghrelin, but not des-acyl-ghrelin, in both Ghsr knockout and wild-type rats. No dose-dependent effects of alcohol were observed on acyl-ghrelin secretion from gastric mucosa cells or on GOAT acylation activity. Lastly, alcohol and sucrose produced distinct effects on ghrelin in rats despite equivalent caloric value. Our findings suggest that alcohol acutely decreases peripheral ghrelin concentrations in vivo, but not in proportion to alcohol’s caloric value or through direct interaction with ghrelin-secreting gastric mucosal cells, the ghrelin receptor, or the GOAT enzyme.
Keywords: acyl-ghrelin, alcohol, calorie, des-acyl-ghrelin, ghrelin, GOAT
1 |. INTRODUCTION
Alcohol use disorder (AUD) is a chronic relapsing disease characterized by consumption of alcohol to an extent that causes significant harm to the affected individual’s health and overall quality of life. According to the 2018 National Survey on Drug Use and Health, 5.8% of individuals aged 18 and older in the United States had AUD in the past year, and an estimated 88,000 annual deaths are alcohol related.1,2 Still, only three Food and Drug Administration (FDA)-approved medications are available for treatment of AUD, highlighting a significant need to develop novel pharmacotherapies for AUD. One such therapeutic strategy is based on the notion that harmful alcohol consumption can be alleviated by pharmacologically manipulating endocrine pathways that control both homeostatic and hedonic feeding, as well as stress-related pathways and reward processing.3–6 The orexigenic peptide ghrelin is one hormone that has been shown to play a role in alcohol-related behavior across numerous studies.7–9
Ghrelin is a 28 amino acid hormone secreted primarily from P/D1 cells (X/A-like cells in rodents) located in the oxyntic glands of the fundus portion of the stomach. Encoded by the ghrelin gene (GHRL), ghrelin is post-translationally formed by cleavage of the 117 amino acid preproghrelin into proghrelin, which can then be acylated at the serine-3 residue by the membrane-bound enzyme, ghrelin-O-acyltransferase (GOAT).10–13 Acylated proghrelin is then cleaved to form acyl-ghrelin—the endogenous ligand of the growth hormone secretagogue receptor 1a (GHSR1a). Acylation of ghrelin is essential for binding to GHSR1a, both centrally and peripherally, and mediates orexigenic effects.10,14,15 Much research over the past decade demonstrates that the ghrelin system has a complex biology due to several factors: (1) circulating acyl-ghrelin can be de-acylated by plasma esterases to des-acyl-ghrelin16; (2) GOAT can acylate ghrelin in target tissues, both in the central nervous system and the periphery17–19; (3) plasma anti-ghrelin immunoglobulin Gs (IgGs) may bind and protect ghrelin from degradation in circulation20; (4) des-acyl-ghrelin may have effects seemingly opposite to acyl-ghrelin through GHSR1a-independent mechanisms21; and (5) GHSR1a has high constitutive, ligand-independent activity.22,23 Moreover, an endogenous antagonist/inverse agonist for GHSR1a, known as liver-expressed antimicrobial peptide-2 (LEAP-2), was recently identified.24–26 These different components of the ghrelin system help regulate and balance acyl-ghrelin’s important effects on energy homeostasis to ensure survival of the organism.27
Central signaling of gastric-derived acyl-ghrelin occurs through activation of GHSR1a expressed on vagal afferent neurons in the stomach, as well as by acyl-ghrelin crossing the blood–brain barrier and binding to GHSR1a in the brain.28 Acyl-ghrelin’s central orexigenic signaling occurs directly through GHSR1a expressed on hypothalamic neuropeptide Y and agouti-related peptide-expressing neurons in the arcuate nucleus, and indirectly through activation of the lateral hypothalamus, hippocampus, amygdala, ventral tegmental area (VTA), and other regions.28 These brain regions communicate with origins and terminal regions of the mesolimbic dopamine system, which affects motivational behaviors, including hedonic feeding and drug and alcohol seeking. Indeed, administration of acyl-ghrelin into the brain’s reward circuitry increases extracellular dopamine via GHSR1a located in the mesolimbic pathway,29–36 and stimulates food intake.37,38 By communicating with these regions, the ghrelin system can regulate homeostatic and hedonic drives governing food-seeking behavior that seem to similarly affect alcohol-seeking behavior. In rodents, central or systemic ghrelin administration increases alcohol intake, whereas antagonism of GHSR1a and knockout (KO) of Ghrl or Ghsr decreases alcohol preference and consumption and blunts both conditioned place preference and dopamine release in the nucleus accumbens (NAc) induced by alcohol.39–44 Moreover, higher peripheral ghrelin concentrations are positively correlated with alcohol craving and risk of relapse in humans,41,45–49 and exogenous ghrelin administration increases cue-induced craving46 and intravenous self-administration of alcohol in heavy-drinking individuals with alcohol dependence.50 Collectively, these studies demonstrate a clear relationship between ghrelin and alcohol-related behaviors, wherein ghrelin appears to potentiate alcohol seeking and consumption, and partly regulate its reinforcing effects.
To obtain a better understanding of the crosstalk between ghrelin and alcohol, further research into how alcohol itself affects the ghrelin system is critically needed. To date, only a few studies have examined the effect of alcohol on peripheral ghrelin concentrations. In rodents, alcohol acutely decreased both acyl-ghrelin and total-ghrelin concentrations in plasma,51,52 and in humans, acute administration of alcohol decreased plasma ghrelin concentrations.53–58 Among individuals with AUD, abstainers had higher peripheral ghrelin concentrations compared to current drinkers.45,48,49,59–64 Ghrelin concentrations were significantly lower in individuals with AUD compared to matched, non-AUD controls.45 However, mean daily alcohol consumption over the past 12 months was positively correlated with plasma ghrelin concentrations among individuals without an AUD diagnosis.65 Collectively, these studies suggest that acute and chronic exposure to alcohol differentially affect the ghrelin system, with effects from chronic alcohol use likely reflecting compensatory mechanisms dependent on the extent and duration of alcohol use. Although the literature to date demonstrates an interplay between alcohol and ghrelin, it is unclear how these effects are occurring—whether through direct action on ghrelin-producing cells, modification of GOAT activity, and/or through other mechanism(s). Understanding the mechanism by which these effects are occurring is important for designing and understanding the clinical translation of inhibitors of the ghrelin system as a potential pharmacotherapy for AUD. The objective of the present body of work was therefore to further understand the effect of alcohol on the ghrelin system by first performing secondary analysis of data from alcohol administration in humans50,66,67 and conducting follow up experiments to probe for direct interactions between alcohol and the ghrelin system.
2 |. MATERIALS AND METHODS
2.1 |. Effects of alcohol on peripheral ghrelin levels in humans
To examine the effect of alcohol on endogenous ghrelin levels, we performed separate analyses of four human laboratory experiments conducted by our team at the National Institutes of Health (NIH) Clinical Center in Bethesda, Maryland. These experiments were originally performed as part of three placebo-controlled trials50,66,67 (ClinicalTrials.gov: NCT02039349, NCT01779024, NCT01751386) and included the administration of alcohol to non-treatment-seeking, heavy-drinking individuals, as well as measurement of plasma ghrelin levels. Here, we only included data from the placebo conditions of these experiments (Figure S1–4). Participants provided informed consent and were compensated for participating in each study. The eligibility criteria of each parent study can be found in appendices (Appendix S1A, S2A, S3A) and baseline characteristics of each sample analyzed here can be found in Table S1. We analyzed data separately for each of the following experiments: (1) oral alcohol self-administration (ASA),66 (2) oral fixed-dose alcohol administration,67 (3) intravenous (IV) ASA,50 and (4) IV fixed-dose alcohol administration.50 Detailed descriptions of these studies and their primary outcomes have been previously reported.50,66,67 Descriptions of standardized meals for each study can be found in the appendices (Appendix S1B, S2B, S3B). An overview of these experiments, including information about times of blood draws, meals, and alcohol administration can be found in Figure 1 and Table S2. Below we provide a brief description of each experiment.
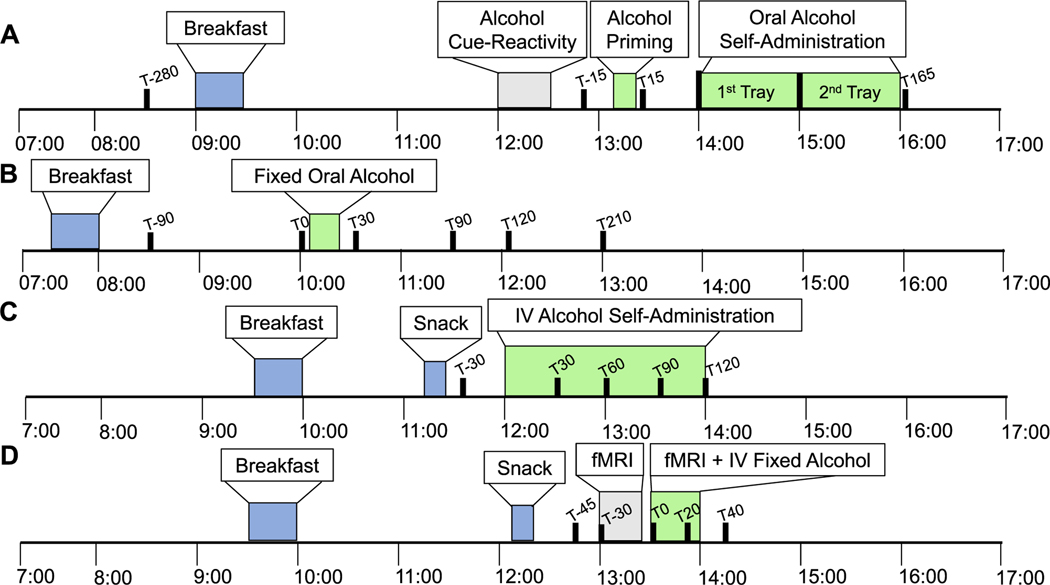
FIGURE 1.
Schematic overview of human laboratory alcohol administration experiments. Overview of human laboratory experiments depicting meals (blue), duration of alcohol intake (green), and blood draw times (black). Other study procedures not involving alcohol administration are also outlined (gray). (A) Oral priming and alcohol self-administration (ASA) experiment. Blood draw time points relative to 0 = alcohol administration at 13:15 are T-280, T-15, T15, and T165 min. (B) Oral fixed alcohol administration experiment. Blood draw time points relative to 0 = alcohol administration at 10:15 are T-90, T0, T30, T90, T120, and T210 min. (C) Intravenous alcohol self-administration experiment. Blood draw time points relative to 0 = alcohol administration at 12:00 are T-30, T30, T60, T90, and T120 min. (D) Intravenous fixed alcohol administration experiment. Blood draw time points relative to 0 = alcohol administration at 13:00 are T-45, T0, T20, T30, and T40 min
2.1.1 |. Oral alcohol self-administration
The main aim of the parent study was to test the role of baclofen on alcohol drinking, using a randomized, between-subjects, double-blind, placebo-controlled human laboratory design.66 Here, we analyzed data from the placebo group only. Participants received their assigned study medication (placebo only in this analysis) for approximately a week before returning to complete the experimental session. Participants were instructed to abstain from alcohol 24 h prior to the experiment (verified by breath alcohol concentration [BrAC] = 0 g/dl) and to take their first medication dose before arriving at the clinic. The experimental session consisted of alcohol cue reactivity followed by alcohol priming and ASA (for full details, see:66). Briefly, during alcohol priming, participants were provided with their preferred choice of alcohol and mixer (S1C, S2C). The amount of alcohol in the priming drink was calculated to raise each participant’s blood alcohol concentration (BAC) to 0.03 g/dl.68 Participants were asked to consume the entire drink within 5 min. The ASA session began 40 min after consumption of the priming drink. At the beginning of the ASA session, four mini-drinks were offered. Each mini-drink had half the amount of alcohol as the priming drink (BAC increase of 0.015 g/dl), and participants were allowed to drink as many of the mini-drinks as they chose, with the knowledge that they would receive $3 for each mini-drink not consumed. An additional four mini-drinks were offered 60 min after the beginning of the ASA session. The total ASA session lasted for 120 min, during which participants were monitored to not exceed a BrAC of 0.12 g/dl. Following completion of the ASA session, participants were escorted to an inpatient unit where they were monitored until BrAC reached 0 g/dl, and they were discharged the next morning.
2.1.2 |. Oral fixed-dose alcohol administration
The main aim of the parent study was to test the safety of a ghrelin receptor blocker (PF-5190457), co-administered with alcohol, using a Phase 1b, within-subjects, dose-escalating, single-blind, placebo-controlled human laboratory design.67 Here, we analyzed data from the placebo condition only. The alcohol administration experiment was held on the third day of an inpatient visit, after taking five doses of the study drug (placebo only in this analysis). A standardized alcoholic beverage (Smirnoff vodka, 40% alcohol by volume; 80% proof) was administered, and participants were instructed to drink the beverage within 15 min. Alcohol was provided as a mixed drink containing the participants’ choice from a list of common mixers (S1C). Alcohol administration was designed to bring each participant’s BAC to a target level of 0.06 g/dl.68
2.1.3 |. IV alcohol administration
The parent study under which both IV alcohol experiments were performed was a cross-over, randomized, double-blind, placebo-controlled study testing the effects of exogenous ghrelin administration and consisting of four experimental sessions: two IV ASA (one ghrelin, one placebo) sessions and two brain fMRI sessions (one ghrelin, one placebo). We analyzed data from the placebo sessions only. Participants were admitted to the NIAAA inpatient unit at the NIH Clinical Center on the evening before each experiment day. Before each experiment, an IV catheter was inserted into each arm (one for ghrelin/placebo and one for alcohol infusion/blood sampling). For the placebo conditions (which are the only ones considered in this analysis), saline solution was infused during the entire experiment. IV alcohol was given as 100% dehydrated alcohol diluted by saline to 6.0% (v/v).
IV ASA experiment
For the IV ASA experiment, participants were given the opportunity to press a button to receive IV alcohol infusions using a Computerized Alcohol Infusion System (CAIS) during a 120-min session. A progressive-ratio schedule for self-administration was applied, which required the participants to press the button an increasing number of times to receive the subsequent alcohol infusion. Incremental infusion rates were calculated individually to raise each participant’s BAC by 0.0075 g/dl within 2 min.68 BrAC measurements were taken every 15 min throughout the procedure and entered in the CAIS software for model-based algorithm adjustments and BAC prediction. Participants were not allowed to exceed a BrAC of 0.12 g/dl during the ASA session. For additional details, see the literature.50,69,70
IV fixed-dose alcohol administration experiment
The IV alcohol administration was conducted as part of a brain fMRI experiment in which subjects completed an alcohol–food incentive delay (AFID) task that exposed participants to food, alcohol, and neutral symbols. As part of this session, participants received an IV alcohol infusion calculated to raise each participant’s BAC linearly to 0.08 g/dl,68 within 20 min, and clamp the BAC at this target value until the end of the experiment. The total duration of the IV alcohol infusion was 35 min. For additional details, see the literature.50,69,70
2.1.4 |. Clinical blood collection, processing, and assay of ghrelin levels
For each experiment listed above, blood was collected at multiple time points throughout each experimental session to allow for repeated measures of plasma acyl- and total-ghrelin levels (Figure 1 and Table S2). The full technical details for blood collection, plasma extraction, and acyl- and total-ghrelin assays can be found in the appendices (S1D, S2D, S3C).
2.2 |. GHSR, GHRL, and MBOAT4 gene expression in human post-mortem brain tissue
Expression levels of the ghrelin receptor gene (GHSR), ghrelin gene (GHRL), and GOAT gene (MBOAT4) were analyzed in post-mortem brain samples from male subjects diagnosed with severe AUD (DSM-5) who also smoked, and controls who did not have a diagnosis of AUD. Human post-mortem brain tissue was obtained from the New South Wales Tissue Resource Centre (NSWBTRC) at the University of Sydney, Australia.71 GHSR, GHRL, and MBOAT4 RNA extraction, reverse transcription, and qPCR analysis were performed using procedures previously reported.72 mRNA was extracted from the five available brain regions, including amygdala, hippocampus, ventral tegmental area (VTA), nucleus accumbens (NAc), and prefrontal cortex (PFC; superior frontal Brodmann areas 8 and 9). Full technical details can be found in Appendix S4.
2.3 |. Effects of alcohol administration on peripheral ghrelin levels in Ghsr knockout and wild-type rats
Male wild-type (WT) and Ghsr knockout (KO; Wistar background) rats 18–20 weeks of age from date of birth were obtained from the Transgenic Breeding Facility at the National Institutes on Drug Abuse (NIDA) Intramural Research Program (IRP) (Baltimore, MD, USA). The development and characterization of the Ghsr KO rat has been previously described.73 All procedures in rats adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th edition) and were approved by the Institutional Animal Care and Use Committee of the National Institute on Drug Abuse Intramural Research Program. Animals were single-housed and maintained in temperature-controlled facilities on a 12 h/12 h light cycle with standard chow and water available ad libitum. At the time of the experiment, rats weighed 350–850 g. Rats from both genotype groups randomly received either ethanol (20% w/v) and saline: (1) Ghsr KO–alcohol (n = 8), (2) Ghsr KO–saline (n = 9), (3) WT–alcohol (n = 9), and (4) WT–saline (n = 8). Rats were given an intraperitoneal (i.p.) injection of either alcohol (1.5 g/kg, 7.5 ml/kg) or saline (7.5 ml/kg) 15 min before collection of trunk blood into EDTA coated tubes containing inhibitors appropriate for acyl-ghrelin/des-acyl-ghrelin measurement. The dose of 1.5 g/kg of alcohol in rats can be considered moderate as it typically reduces anxiety-like behaviors and produces moderate motor incoordination, but does not cause sedation (i.e., hypnosis).74 Full technical details of processing and assays can be found in Appendix S5.
2.4 |. The effect of alcohol on ghrelin secretion from gastric mucosal cells
Gastric mucosal cells were isolated and established from 8–12 week-old male C57BL/6 N mice, as reported previously,75,76 and then supplemented with sodium octanoate-bovine serum albumin (BSA) before they were treated with medium containing different ethanol concentrations. After incubation, mediums were assayed for acyl-ghrelin by ELISA. Full technical details can be found in Appendix S6.
2.5 |. Effect of alcohol on human GOAT ghrelin acylation activity
Assays were performed with 70 mg membrane protein from Sf9 cells expressing human GOAT (hGOAT), as determined by Bradford assay. Each ethanol concentration was tested by adding an ethanol stock to a mix of HEPES, membrane protein, and MAFP before initiating reactions with octanoyl-coA and GSSFLCAcDan peptide. Reactions were stopped after 1 hr with acetic acid, and the medium was analyzed using reverse-phase HPLC, as described previously.77,78 GOAT acylation activity was determined by substrate and product peak integration in the presence of either ethanol or water (vehicle). Percent activity for each reaction was calculated using Equations 1 and 2.79 Full technical details can be found in Appendix S7.
| (1) |
| (2) |
2.6 |. Effects of alcohol versus sucrose administration on peripheral ghrelin levels in rats
Male Wistar rats 18–20 weeks from date of birth were obtained from Charles River Laboratory (Wilmington, MA). Animals were single-housed and maintained in temperature-controlled facilities on a 12 h/12 h light cycle with standard chow and water available ad libitum. At the time of the experiment, rats weighed 400–700 g, and were randomized by weight to alcohol or sucrose groups. Prior to the experiment, rats were habituated to i.p. injections for 3 days by performing daily saline injections. On the day before the experiment, rats were also habituated to the testing room for 1 h. The following day, baseline measures for each rat were collected via tail bleed 6–7 h into the light cycle, at 15 and 60 min, following saline injection (7.5 ml/kg, i.p.). Rats were returned to their home cages in between injection and blood draws. Food and water remained accessible to the rats in the home cages. The following day, rats were divided into two groups and received either 20% w/v ethanol (1.5 g/kg, 7.5 ml/kg, i.p., 10.8 kcal/kg) or 35% w/v sucrose (2.8 g/kg, 8 ml/kg, i.p., 11.2 kcal/kg). Tail blood was again drawn at the 0, 15, and 60 min time points, and processed as described in Experiment 3.3 (Appendix S5).
2.7 |. Statistics
Human laboratory experiments:
outliers (defined as outside of ±1.5 interquartile range per hormone per time point) were removed. Data were analyzed using Linear Mixed Effects Models in SPSS 25 (IBM Corporation, Armonk, NY) and were evaluated for random effect of subject, main effect of time point, and covariates (age, gender, BMI, and race) on acyl- or total-ghrelin. Random effects were described with a scaled identity covariance structure. Covariates that were not significant in the initial run of each model were removed from the final model. Post hoc analyses were performed using pairwise comparisons of group means at each time point within an experiment, and Bonferroni correction was used to conservatively control for multiple comparisons. Human post-mortem experiment: Human post-mortem data were analyzed using Linear Mixed Effects Models in SPSS 25 and were evaluated for random effect of subject, fixed effect of group (AUD, Non-AUD), and covariates (post-mortem interval [PMI], age, brain weight, brain pH, BMI, and cigarette pack years) on fold change (2−ΔΔCt) mRNA expression in each brain region. Covariates that were not significant in the initial run of each model were removed from the final model. A variance components covariance structure was used to describe random effects. To conservatively control for multiple comparisons, Bonferroni correction was applied to correct for the number of brain regions tested (5 brain regions). Alcohol experiments with KO and WT rats: A two-way ANOVA was used to evaluate genotype (KO vs. WT), treatment (alcohol vs. saline), and genotype x treatment interaction main effects among saline- and alcohol-treated Ghsr KO and WT rats using GraphPad Prism 8 software (San Diego, CA, USA). Tukey’s multiple comparison test was used when appropriate. Gastric mucosal cell experiments: For ghrelin secretion studies in gastric mucosal cells, a one-way ANOVA followed by Dunnet’s test was used to analyze the effect of different concentrations of alcohol on acylghrelin secretion in Graph Pad Prism. Alcohol and sucrose experiment with rats: Lastly, two-way repeated measures ANOVA was used to evaluate treatment (alcohol vs. saline and sucrose vs. saline), time (0, 15, and 60 min), and treatment x time interaction main effects on acyl-ghrelin and des-acyl-ghrelin in rats using GraphPad Prism. Sidak’s multiple comparison test was used for post hoc analyses when appropriate. For all analyses, significance was set at P < 0.05.
2.8 |. Approvals
Human laboratory experiments were approved by the NIH Addictions Institutional Review Board, registered at ClinicalTrials.gov (NCT02039349, NCT01779024, NCT01751386), reviewed by the FDA if applicable under Investigational New Drug (IND) applications, and conducted in accordance with Declaration of Helsinki principles. All participants provided written informed consent before any protocol-specific research procedure took place. The human postmortem brain project was approved by the NIAAA Scientific Advisory Board and the NIH Office of Human Subjects Research Protections and was exempt from review by the NIH Institutional Review Board. Animal studies performed at the NIH IRP adhered to the National Research Council Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the NIDA IRP. All animal procedures and use of mice at UT Southwestern Medical Center (UTSW) were approved by the Institutional Animal Care and Use Committee of UTSW.
3 |. RESULTS
3.1 |. Plasma ghrelin levels are reduced after alcohol administration in humans
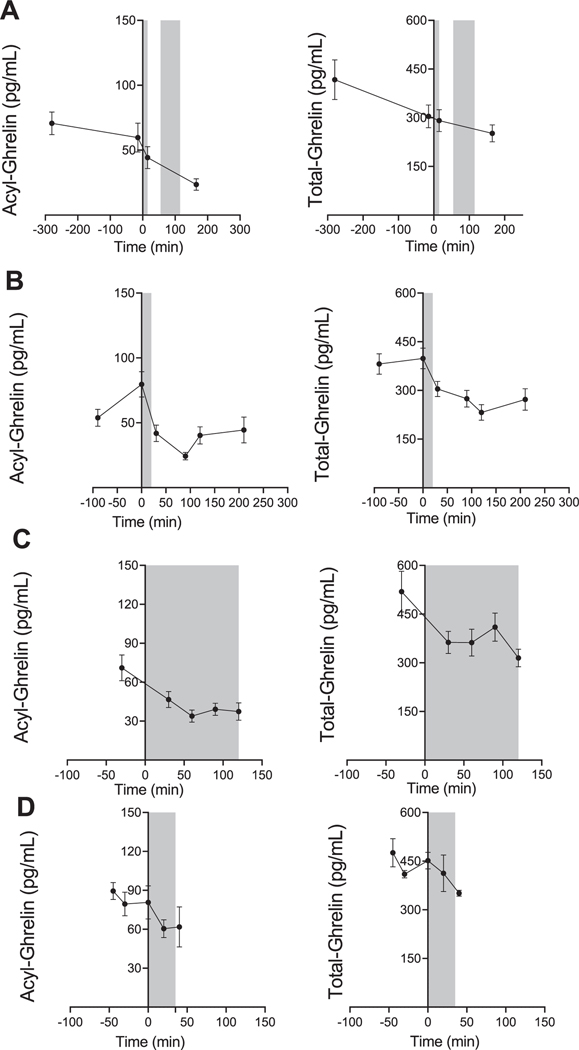
Overall, alcohol administration led to a reduction in ghrelin levels, regardless of the route of alcohol administration, within a time window ranging from 45–165 min post-alcohol administration. Using linear mixed effects modeling, we found that acyl-ghrelin [F(3, 27.5) = 6.6, P = 0.002] and total-ghrelin [F(3, 37.7) = 4.5, P = 0.009] were significantly reduced during the oral ASA session (Figure 2A). Moreover, there was a significant reduction in acyl-ghrelin [F(5, 53.8) = 10.5, P < 0.001] and total-ghrelin [F(5, 52) = 13.6, P < 0.001] during the oral fixed-dose alcohol administration session (Figure 2B). Analysis of peripheral ghrelin during the IV ASA session also revealed a significant reduction in both acyl-ghrelin [F(4,37.7) = 7.5, P < 0.001], and total-ghrelin [Covariate: Gender, F(4, 38.7) = 5.6, P = 0.001] (Figure 2C). Lastly, we observed a reduction in acyl-ghrelin [F (4,19) = 2.0, P = 0.134) and total-ghrelin [F(4,17.1) = 2.7, P = 0.067] during the IV fixed-dose alcohol administration session, but this change did not reach statistical significance (Figure 2D). Pairwise comparisons corrected for multiple testing were conducted between all time points for experiments where significant overall effects were found and are presented in Figure 2.
FIGURE 2.
Effect of alcohol administration on peripheral ghrelin levels in humans. Plasma ghrelin levels over the course of different alcohol administration experiments in participants with heavy drinking. Gray zones indicate time periods of alcohol administration. For all data, 0 min = beginning of alcohol administration session. Data are presented as mean ± SEM. (A) Oral alcohol self-administration analysis (N = 16); fixed effect of time (−280, −15, 15, 165) on acyl-ghrelin (left: P < 0.002) and total-ghrelin (right: P = 0.009); pairwise comparisons: Acyl-ghrelin (−280 vs. 165, −15 vs. 165; P < 0.05), total ghrelin (−280 vs. 165; P < 0.05). (B) Oral fixed-dose alcohol administration analysis (N = 12); fixed effect of time (−90, 0, 30, 90, 120, and 210 min) on acyl-ghrelin (left: P < 0.0001) and total-ghrelin (right: P < 0.0001); pairwise comparisons: Acyl-ghrelin (0 vs. −90; P < 0.05, 0 vs. 30, 90, 120, 210; P < 0.001), total-ghrelin (0 vs. −90, 30, 210; P < 0.05, 0 vs. 120, P < 0.001). (C) IV alcohol self-administration analysis (N = 11); fixed effect of time (−30, 30, 60, 90, and 120 min) on acyl-ghrelin (left: P < 0.0001), and total-ghrelin (right: P < 0.0001); pairwise comparisons: Acylghrelin (−30 vs. 30; P < 0.05, −30 vs. 60, 90, 120; P < 0.001), totalghrelin (−30 vs. 30, 60; P < 0.05, −30 vs. 120; P < 0.01). (D) IV fixed-dose alcohol administration analysis (N = 6) evaluated a fixed effect of time point (−45, 0, 20, 30, and 40 min) on acyl-ghrelin (left: P = NS), and total-ghrelin (right: P = NS). P values presented for pairwise comparisons are Bonferroni corrected
3.2 |. GHSR, GHRL, and MBOAT4 expression levels in select brain regions are not significantly altered by chronic alcohol consumption
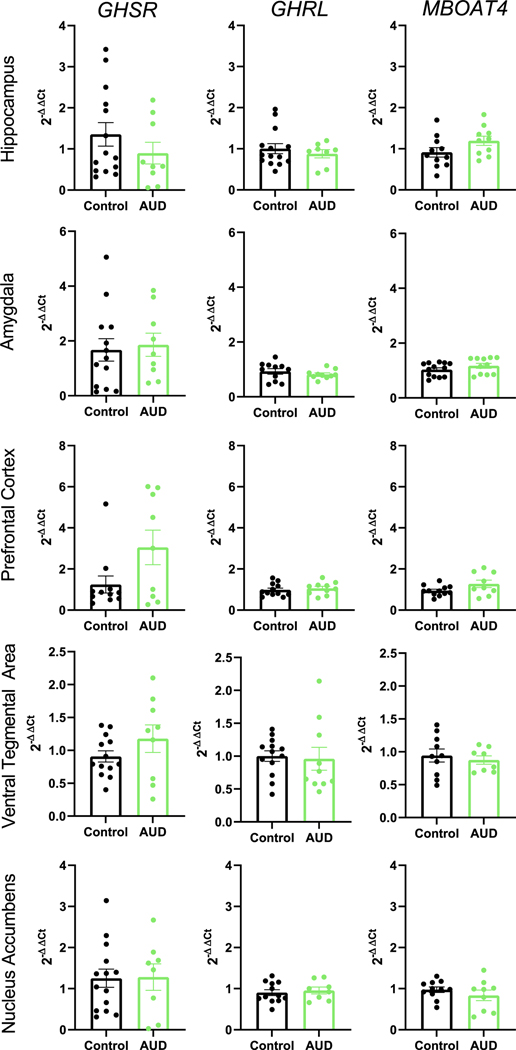
Fold changes of GHSR mRNA, GHRL mRNA, and MBOAT4 mRNA expression levels in the hippocampus, VTA, amygdala, PFC, and NAc were compared between smoking AUD individuals (N = 11) and controls (N = 15–16) (Table 1). Baseline characteristics of the sample are provided in Table S3; the differences between the two groups were controlled for in the analyses. Using linear mixed effects modeling, we found no significant effect of group (AUD vs. control) on GAPDH-corrected GHRL, GHSR, or MBOAT4 expression in any of these brain regions tested (Figure 3 and Table 1).
TABLE 1.
Comparison of GHRL, GHSR, and MBOAT4 expression changes in post-mortem brain tissue between AUD and controls
| AUD ΔCta | Control ΔCta | AUD 2−ΔΔCtb | Control 2−ΔΔCtb | Statistic | Adjusted P value | |
|---|---|---|---|---|---|---|
| GHRL | ||||||
| HIPP | 12.5 | 12.3 | 0.80 | 1.02 | F(1,17) = 5.311, P = 0.034 Brain pH, PMI, Agec | 0.17 |
| AMY | 10.3 | 10.2 | 0.85 | 0.93 | F(1,19) = 0.846, P = 0.369 | 1 |
| PFC | 12.9 | 13.0 | 1.04 | 0.98 | F (1,20) = 0.354, P = 0.559 | 1 |
| NA | 11.3 | 11.4 | 0.93 | 0.90 | F(1,18) = 0.218, P = 0.646 | 1 |
| VTA | 11.5 | 11.4 | 0.96 | 1.00 | F(1,19) = 3.900, P = 0.063 Pack yearsc | 0.315 |
| GHSR | ||||||
| HIPP | 10.1 | 9.1 | 0.90 | 1.35 | F(1,21) = 1.206, P = 0.284 | 1 |
| AMY | 12.6 | 13.1 | 1.77 | 1.67 | F(1,20) = 0.098, P = 0.758 | 1 |
| PFC | 14.4 | 15.5 | 2.95 | 1.24 | F(1,18) = 4.195, P = 0.055 | 0.275 |
| NA | 10.4 | 9.9 | 1.49 | 1.25 | F(1,18) = 1.126, P = 0.303 Brain pH, PMIc | 1 |
| VTA | 12.9 | 13.1 | 1.18 | 0.91 | F(1,20) = 1.829, P = 0.191 | 0.96 |
| MBOAT4 | ||||||
| HIPP | 14.3 | 14.8 | 0.91 | 1.19 | F(1,19) = 3.036, P = 0.098 | 0.49 |
| AMY | 11.7 | 11.5 | 1.17 | 1.03 | F(1,22) = 1.731, P = 0.202 | 1 |
| PFC | 13.0 | 13.4 | 1.28 | 0.94 | F(1,19) = 3.564, P = 0.074 | 0.37 |
| NA | 12 | 11.6 | 0.84 | 0.97 | F(1,15) = 4.693, P = 0.047 Pack years, BMIc | 0.235 |
| VTA | 15.2 | 15.3 | 0.88 | 0.94 | F(1,14) = 3.502, P = 0.082 Pack yearsc | 0.41 |
Abbreviations: AMY = amygdala, AUD = alcohol use disorder, GHRL = growth hormone receptor ligand, GHSR = growth hormone secretagogue receptor, HIPP = hippocampus, MBOAT4 = membrane-bound o-acyl-transferase 4, NAc = nucleus accumbens, PFC = prefrontal cortex, PMI = post-mortem interval, VTA = ventral tegmental area.
ΔCt = Cycle threshold (Ct) of gene of interest (MBOAT4, GHSR, or GHRL)—cycle threshold for GAPDH (housekeeping gene used as endogenous control).
2−ΔΔCt calculated based on ΔΔCt = ΔCt AUD −ΔCt controls.
Covariates in final model.
FIGURE 3.
Central post-mortem expression of GHSR, GHRL, and MBOAT4 in AUD individuals and controls. Fold expression of GHSR, GHRL, and MBOAT4 in five selected brain regions examined in post-mortem brain tissue from individuals with AUD and controls. Fold expression change is expressed as 2−ΔΔCt where ΔΔCt is the difference in ΔCt between AUD and control samples and ΔCt is the difference in cycle threshold (Ct) for the gene of interest—GADPH in the same sample. No regions are significantly different from each other after controlling for multiple comparisons. AMG = amygdala, AUD = alcohol use disorder, GHRL = growth hormone receptor ligand (ghrelin gene), GHSR = growth hormone secretagogue receptor (ghrelin receptor gene), HIPP = hippocampus, MBOAT4 = membrane-bound o-acyl transferase 4 (GOAT gene), NA = nucleus accumbens, PFC = prefrontal cortex, VTA = ventral tegmental area
3.3 |. Acyl-ghrelin levels are reduced by alcohol in rats, independent of ghrelin receptor knockout
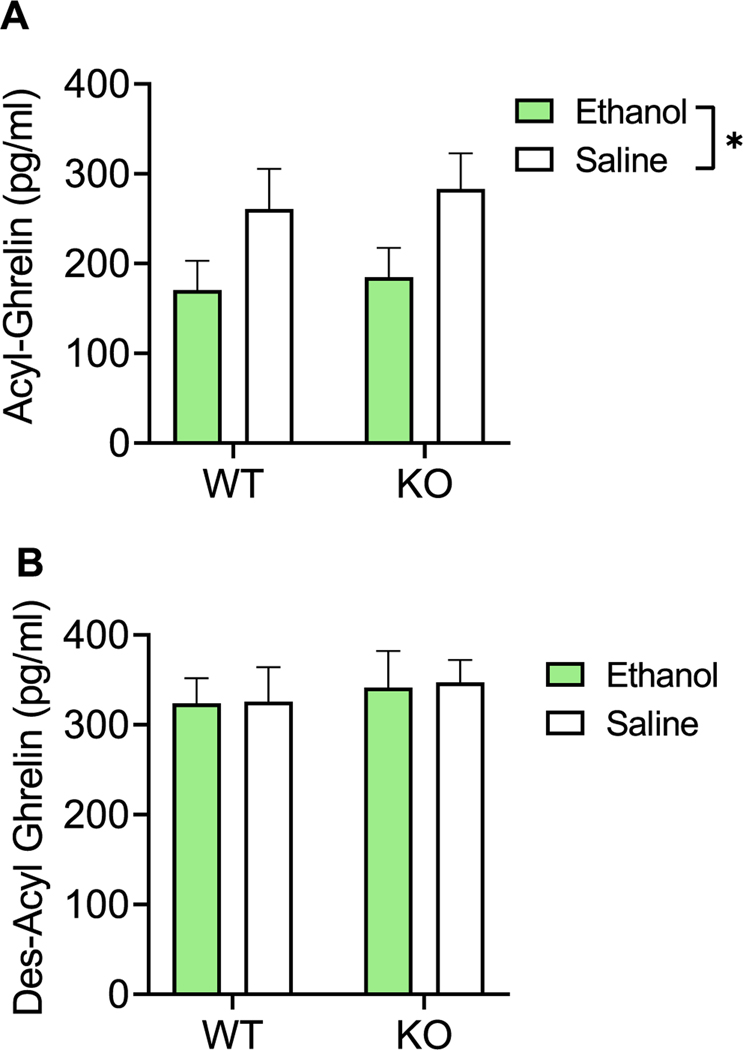
Rats given alcohol (n = 17, 625.3 g) or saline (n = 17, 619.5 g) had no differences in body weight (paired t test, P = 0.91) at the time of the experiment (Table S4). Using two-way ANOVA, we observed a significant main effect of treatment (alcohol vs. saline) on acyl-ghrelin levels [F(1,29) = 6.212, P = 0.019], where levels of acyl-ghrelin in alcohol-treated rats were reduced compared with saline-treated rats. There was no main effect of genotype (WT vs. KO) [F(1,29) = 0.2309, P = 0.63] or treatment × genotype interaction [F(1,29) = 0.01, P = 0.92] (Figure 4A). Moreover, there was no effect of treatment [F (1,29) = 0.013, P = 0.91], genotype [F(1,29) = 0.3245, P = 0.57], or treatment x genotype interaction [F(1,29) = 0.003, P = 0.95] on plasma des-acyl-ghrelin levels (Figure 4B).
FIGURE 4.
Effect of alcohol on peripheral ghrelin levels in Ghsr KO and WT rats. (A) Effect of alcohol (1.5 g/kg, i.p.) on plasma acyl-ghrelin levels in Ghsr KO and WT rats. Data represent change in ghrelin secretion with alcohol treatment versus saline. N = 8–9/group. Treatment effect: P < 0.019, genotype effect: P = NS, and interaction effect: P = NS. (B) Effect of alcohol (1.5 g/kg, i.p.) on plasma des-acyl-ghrelin levels in Ghsr KO and WT rats. Data represent change in ghrelin secretion with alcohol versus saline treatment. N = 8–9/group. Treatment effect: P = NS, genotype effect: P = NS, and interaction effect: P = NS. Data are presented as mean ± SEM. *P < 0.05
3.4 |. Ghrelin secretion from gastric mucosa cells is not altered by alcohol
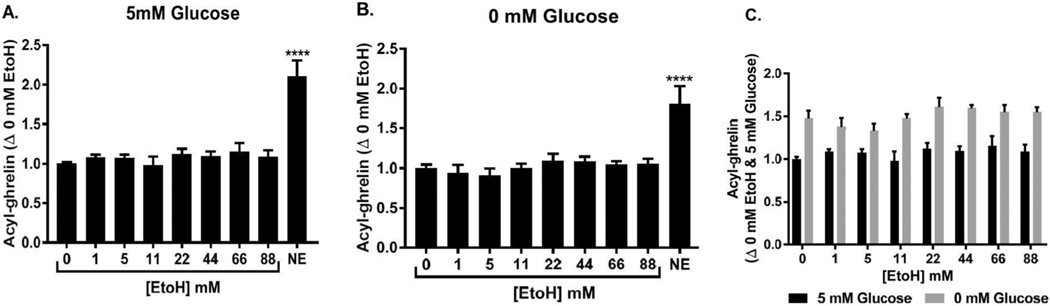
We evaluated ghrelin release from gastric mucosal cells in the presence of different concentrations of alcohol. We cultured cells in both 5 mM glucose environment and 0 mM glucose environments. A 5 mM glucose condition was used to study the effect of alcohol in settings simulating physiological blood glucose concentrations. A 0 mM glucose condition was used to study the effect of alcohol without any interference of glucose as an energy source, and is known to be associated with higher ghrelin secretion as compared to 5 mM glucose.80 Because it has been shown to stimulate ghrelin secretion from primary cultures of gastric mucosal cells, norepinrephine (10 μM) was used as a positive control for ghrelin secretion.76 As observed previously,80 absence of glucose (0 vs. 5 mM) increased acyl-ghrelin secretion from primary cultrues of gastric mucosal cells. However, alcohol did not change acyl-ghrelin secretion at any of the concentrations tested (Figure 5A–C).
FIGURE 5.
Effect of alcohol on ghrelin secretion from gastric mucosal cells. (A) Effect of increasing concentrations of alcohol on acyl-ghrelin secretion in mouse primary gastric mucosal cells incubated with medium containing 5 mM glucose. Data represent change in ghrelin secretion with alcohol (EtoH) treatment compared to untreated control (0 mM EtoH). N = 4 for each condition. ****P < 0.0001, one-way ANOVA followed by Dunnett’s test. (B) Effect of increasing concentrations of alcohol on acyl-ghrelin secretion in mouse primary gastric mucosal cells incubated with medium containing 0 mM glucose. Data represent change in ghrelin secretion with EtoH treatment compared to untreated control (0 mM ethanol). N = 4 for each condition. ***P < 0.0001, one-way ANOVA followed by Dunnett’s test. (C) Effect of increasing concentrations of alcohol on acyl-ghrelin secretion in mouse primary gastric mucosal cells incubated with medium containing either 5 or 0 mM glucose (for reference only). Data represent change in ghrelin secretion with treatment compared to untreated control (0 mM ethanol in 5 mM glucose). N = 4 for each condition
3.5 |. hGOAT acylation activity is not affected by alcohol
We assayed GOAT activity in vitro in increasing concentrations of ethanol, using membrane protein from Sf9 cells expressing hGOAT. Ethanol was tested at concentrations representing intracellular ethanol levels ranging from sub-intoxicating (1 mM) to grossly intoxicating, lethal (87 mM) doses.81,82 We found that ghrelin acylation by GOAT was not dose-dependently inhibited by ethanol over this physiologically relevant concentration range, with less than 20% inhibition observed at the highest concentration tested (Figure S5).
3.6 |. Plasma ghrelin levels in rats are differentially affected by alcohol and sucrose
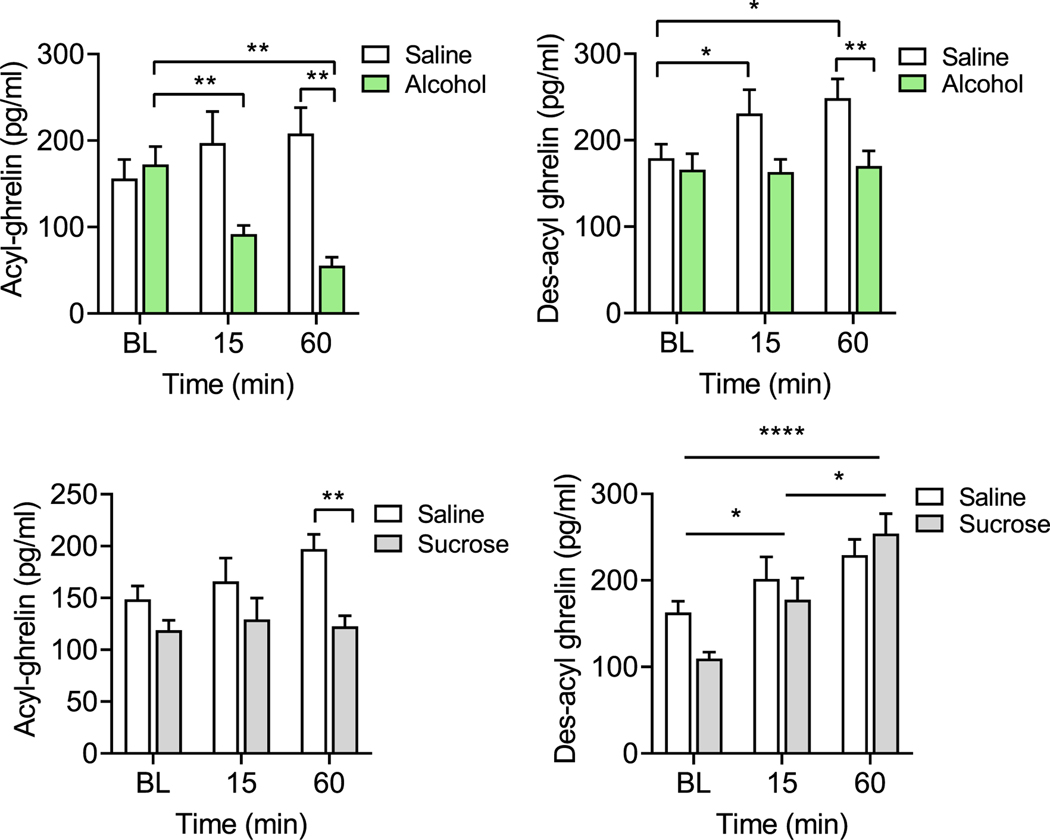
The body weights of both alcohol (n = 10, 596.3 g) and sucrose (n = 10, 600 g) treated rats were not significantly different at the time of the experiment (Student t test, P = 0.91). As for alcohol, we observed a significant main effect of treatment (alcohol vs. saline) on acyl-ghrelin levels [F(1, 18) = 7.83, P = 0.02], and a treatment × time interaction effect [F(2,36) = 18.09, P < 0.0001], but no main effect of time. Post hoc testing revealed a decrease in acyl-ghrelin levels following alcohol treatment compared to baseline and to saline (Figure 6A, left). For des-acyl-ghrelin, significant main effects of treatment [F(1,18) = 5.253, P = 0.034] and time [F(1.673, 30.12) = 3.799, P = 0.041], as well as treatment × time interaction [F(2, 36) = 3.301, P = 0.048] were observed. Post hoc testing revealed significant increase in des-acyl-ghrelin levels following saline treatment, but no changes following alcohol treatment (Figure 6A, right). There was also a significant main effect of treatment [F(1, 18) = 10.68, P = 0.0043], time [F(2, 36) = 59.03, P < 0.0001], and interaction [F(2, 36) = 50.02, P < 0.0001] on the acyl- to des-acyl-ghrelin ratio (AG:DAG ratio). Post hoc testing revealed significant reduction in AG:DAG ratio following alcohol treatment, but no changes following saline treatment (Figure S6).
FIGURE 6.
Change in peripheral ghrelin as a result of alcohol or sucrose. (A) Plasma acyl-ghrelin and des-acyl-ghrelin resulting from alcohol and saline at baseline (BL) and 15 and 60 min post-injection. Acyl-ghrelin (left): Two-way repeated measures (RM) ANOVA: Overall treatment (P < 0.05) and interaction (P < 0.0001) effect. Post hoc testing revealed a decrease in acyl-ghrelin levels at 15 min (P = 0.003) and 60 min (P = 0.004) following alcohol treatment, compared with the pre-treatment baseline. When compared to saline injections, acyl-ghrelin levels were significantly lower in alcohol-treated rats at 60 min post-treatment (P = 0.002). Des-acyl-ghrelin (right): Two-way RM ANOVA: Overall time (P < 0.05), treatment (P < 0.05), and interaction (P < 0.05). Post hoc testing revealed significant increases in des-acyl-ghrelin at 15 min (P = 0.047) and 60 min (P = 0.018) following saline treatment, whereas no changes in des-acyl-ghrelin levels were observed following alcohol treatment. Des-acyl-ghrelin levels following saline treatment were significantly higher compared to alcohol treatment at 60 min (P = 0.038). (B) Plasma acylghrelin and des-acyl-ghrelin resulting from sucrose and saline treatment at baseline and 15 and 60 min post-injection. Acyl-ghrelin (left): Two-way RM ANOVA: Overall treatment (P < 0.05) effect. Post hoc testing revealed significantly lower acyl-ghrelin levels at 60 min following sucrose treatment, compared to saline treatment (P = 0.002). Des-acyl-ghrelin (right): Two-way ANOVA: Overall time (P < 0.0001) effect. Post hoc testing revealed a significant increase in des-acyl-ghrelin at 15 min (P = 0.031) and 60 min (P < 0.001), compared to baseline, and at 60 min (P = 0.0190), compared to 15 min. Post hoc Sidak’s multiple comparison tests: *P < 0.05, ** P < 0.01, ***P < 0.001, ****P < 0.0001
As for sucrose, we observed an overall significant main effect of treatment (sucrose vs. saline) [F(1, 16) = 7.705, P = 0.01] on acyl-ghrelin levels, but no effect of time or interaction effect, indicating lower levels of acyl-ghrelin under sucrose treatment, compared to saline, regardless of time (Figure 6B, left). There was no effect of treatment or treatment × time interaction on des-acyl-ghrelin levels, but a significant effect of time [F(1.839, 29.43) = 20.11, P < 0.0001] was observed, with post hoc testing indicating an overall increase in des-acyl-ghrelin over time (Figure 6B, right). Lastly, we observed a significant main effect of treatment [F(1, 16) = 4.93, P = 0.041], time [F (1.966, 31.45) = 26.38, P < 0.0001], and treatment × time interaction [F (2, 32) = 19.18, P < 0.0001] on the AG:DAG ratio. Post hoc testing revealed significant reduction in AG:DAG ratio following sucrose treatment, but no, or less robust, changes following saline treatment (Figure S6).
4 |. DISCUSSION
The results presented herein demonstrate that alcohol administration acutely suppresses plasma acyl-ghrelin levels in humans and rats, while the effects on total-ghrelin (acyl-ghrelin + des-acyl-ghrelin + c-terminal ghrelin fragments) and des-acyl-ghrelin are more variable. We have additionally addressed several important questions regarding this effect by demonstrating that alcohol-induced suppression of acyl-ghrelin does not occur through direct interaction between alcohol and gastric mucosal cells or the GOAT enzyme, and by showing that alcohol does not suppress acyl-ghrelin in proportion to caloric load, as previously hypothesized.83 Moreover, we have further characterized the alcohol–ghrelin relationship by presenting preliminary results on the potential effect of long-term alcohol consumption on central ghrelin system gene expression in humans.
Our findings from analyzing four different human laboratory experiments demonstrate a consistent direction of an alcohol-induced change in ghrelin, where alcohol appears to decrease ghrelin despite each session employing a different duration, dose of alcohol, and type of alcoholic beverage. While a reduction in acyl- and total-ghrelin was observed during the fixed-dose administration of IV alcohol, this change did not reach statistical significance. Given that IV fixed alcohol administration only lasted 40 minutes while the other paradigms had time points available over a longer time period (120–165 min), it is likely that this smaller time window did not fully capture the extent of alcohol’s effect on ghrelin. For all experiments, the change in acyl-ghrelin was more robust and appeared to occur on a faster time scale than total-ghrelin. We observed a peak decrease in acyl-ghrelin at 165 minutes (−60% acyl-ghrelin from −15 min) during oral ASA, 90 min during oral fixed alcohol administration (−69% acyl-ghrelin from 0 min), and 60 min during IV ASA (−52% acyl-ghrelin from −30 min). For total-ghrelin, peak decreases occurred again at 120 min for oral fixed alcohol administration (−49% total-ghrelin from 0 min), at 120 min during IV ASA (−39% total-ghrelin from −30 min), and at 165 min during oral ASA (−17% total-ghrelin from −15 min). Pairwise comparisons revealed less significant decreases in total-ghrelin from time points just prior to alcohol administration only for IV ASA and oral fixed alcohol. For oral ASA, later time points may have revealed a larger change in total ghrelin from −15 minutes, given that alcohol was again provided at +40 and +80 minutes into the session. These data suggest that alcohol more potently affects acyl-ghrelin, and it is unclear whether the effects of alcohol on total-ghrelin are simply reflective of a decrease in acyl-ghrelin or also represent a change in des-acyl-ghrelin.
Our results are supported by data from previous publications. In humans, oral alcohol (0.55 g/kg), in comparison to a water beverage, has previously been found to significantly decrease acyl- and total-ghrelin levels in young, fasting, healthy males and females within an hour.54,55 In another study using heathy male participants, alcohol given orally with juice (0.6 g/kg) decreased total-ghrelin levels, in comparison to juice alone. This experiment found a significant decrease in total-ghrelin 15 minutes after alcohol administration that lasted for 120 min.56 Later, the effect of IV alcohol on ghrelin was examined by showing that continuous alcohol infusion (50 mg%) for 180 min significantly suppressed acyl-ghrelin levels in fed, male and female participants. Here, acyl-ghrelin was not significantly suppressed in comparison to baseline (0 min), but significantly blunted a fasting-induced rise in acyl-ghrelin levels observed under the placebo condition in healthy males and females.57 This was later expanded by data showing that both moderate (100 mg%) and low doses (40 mg%) of 1 h continuous IV alcohol infusion significantly decreased acyl, but not total-ghrelin, relative to a placebo condition, in fed, healthy male and female participants.58 While the present results are limited due to the secondary nature of the analysis and lack of a control for alcohol (i.e., placebo infusion/caloric beverage), they are strengthened by showing a consistent direction of effect with previous reports.
To address the limitation stated above, we followed up our findings from secondary analysis of human laboratory paradigms with a saline-controlled experiment in rats. Intraperitoneal injection of alcohol in Ghsr KO and WT rats had no acute effect on desacyl-ghrelin and only reduced acyl-ghrelin 15 min post-injection. Moreover, we found that this effect of alcohol on acyl-ghrelin occurred independently of the presence or absence of the ghrelin receptor, as we found no genotype or genotype interaction effect. While a direct interaction between alcohol and the ghrelin receptor does not likely underlie the effect of acute alcohol on reducing peripheral ghrelin, previous work has shown that rats with differing alcohol preference have differences in both GHSR1a expression levels in select brain regions and in alcohol-induced suppression of plasma ghrelin concentrations.51 Likewise, the ghrelin receptor is able to form heteromers with receptors for peptides known to regulate ghrelin secretion, such as the somatostatin receptor, oxytocin receptor, and dopamine receptors.84,85 This raises the possibility that ghrelin receptor heteromers could modulate downstream effects of alcohol-induced changes in heteromer ligands (i.e., dopamine, oxytocin, etc.) on ghrelin secretion. Our findings from our experiment with KO rats suggest that this is not the case. Still our data from this experiment add to the existing literature by demonstrating acute suppression of acyl-ghrelin, but not des-acyl-ghrelin, in rodents.
It has previously been hypothesized that ghrelin suppression induced by acute alcohol might be due to direct suppression of acyl-ghrelin secretion. Here we report that in vitro assay with the hGOAT enzyme, a ghrelin mimetic peptide, and octanoyl-CoA revealed no dose-dependent effects of alcohol on GOAT acylation activity within a physiologically relevant concentration range. These data indicate that alcohol does not mediate its effects on ghrelin secretion by allosteric modification or direct inhibition of GOAT acylation activity by interfering with GOAT substrate binding. Moreover, murine gastric mucosal cell secretion of acyl-ghrelin was unaffected by incubation with alcohol either alone or in the presence of glucose. Cells were also tested in the presence of glucose to eliminate the presence or absence of an energy source as a confounding variable in acyl-ghrelin secretion. As reported previously, absence of glucose increased ghrelin secretion,75,80 and alcohol had no effect on ghrelin in either condition. The concentrations of alcohol that were applied to the GOAT enzyme and murine gastric mucosal cells encompass BAC levels reached after alcohol administration in the human laboratory experiments (14–28 mM) and approximate those in rats (30–97 mM). It should be taken into consideration that we only evaluated secretion of acyl-ghrelin from gastric mucosal cells and did not evaluate any changes in des-acyl-ghrelin for these experiments. Taken together, our in vitro and ex vivo data point toward an indirect mechanism by which alcohol suppresses peripheral ghrelin levels.
Previous studies have suggested that post-prandial ghrelin suppression occurs in proportion to caloric load and that this may underlie alcohol-induced ghrelin suppression.83,86–88 Here, we show that acyl-ghrelin was significantly decreased as a result of i.p. alcohol administration in rats both 15 and 60 min following injection compared to baseline, whereas there was no significant change in des-acyl-ghrelin following alcohol treatment between each time point. Following sucrose treatment, there was no change in acyl-ghrelin relative to baseline, but des-acyl-ghrelin was significantly increased relative to baseline at 15 and 60 min. Interestingly, given these effects, alcohol and sucrose had similar effects on the AG:DAG ratio, where a decrease of acyl-ghrelin by alcohol, and an increase of des-acyl-ghrelin after sucrose both decreased the plasma AG:DAG ratio significantly at 15 and 60 min post-injection. Both acyl- and des-acyl-ghrelin increased over time among saline-treated controls. Relative to saline, acyl-ghrelin was decreased by alcohol, and the increase in des-acyl-ghrelin observed following saline treatment was blunted by alcohol. Sucrose treatment, however, blunted an increase in acyl-ghrelin compared to saline-treated controls and did not change the increase in des-acyl-ghrelin relative to saline. It is possible that the increase in acyl- and des-acyl-ghrelin following saline treatment represents a fasting-induced increase in ghrelin that is differentially affected by alcohol and sucrose. Given that calorically equivalent injections of alcohol and sucrose produced markedly different effects on acyl- and des-acyl-ghrelin, our data suggest that ghrelin is not suppressed in proportion to caloric value alone. This observation is supported by studies in humans demonstrating that administration of one type of macronutrient differentially affects ghrelin secretion when compared to a different macronutrient of equivalent caloric value.89,90 Moreover, acyl-ghrelin is not dose-dependently decreased by higher doses of IV alcohol (associated with higher caloric value).58 Our data also suggest that ghrelin acylation and ghrelin peptide secretion are regulated by separate mechanisms, given the markedly different effects of alcohol and sucrose on these different forms of ghrelin. Acyl-ghrelin plays an important role in relaying meal-related information,83 and it is likely that differences in post-prandial (or post-alcohol) acyl-ghrelin secretion are not simply reflective of calorie content, but represent a more complicated summation of the metabolic effects resulting from a meal or alcohol on energy homeostasis, which can vary according to macronutrients, meal status, and size of meal.
Lastly, we also show no change in central ghrelin, GOAT, or ghrelin receptor expression as a result of chronic exposure to alcohol in individuals with severe AUD (human post-mortem sample). More specifically, GHRL, GHSR, and MBOAT4 mRNA expression levels were not statistically different (after correcting for multiple comparisons) between post-mortem brain samples from individuals with AUD and non-AUD controls, suggesting that chronic alcohol exposure does not directly affect central ghrelin system expression in these brain regions. The results for GHSR are in disagreement with available preclinical literature, where alcohol preferring rats demonstrated increases in GHSR1a in the PFC, hippocampus, VTA, NAc, and amygdala in comparison to non-alcohol preferring rats depending on an alcohol access model used.51 However, it should also be considered that the expression of GHSR produces two transcripts, GHSR1a and GHSR1b, the latter of which heterodimerizes with and attenuates GHSR1a.91 Likewise, expression of GHRL can also produce products other than proghrelin.91 Therefore, these findings are not restricted to ghrelin and its receptor. Given that GHSR is not highly expressed in the hippocampus, amygdala, PFC, VTA, or NAc, and that our sample size was relatively small, we may not have been able to precisely capture any effect of long-term alcohol use on the ghrelin receptor that was statistically meaningful. Furthermore, the significance of central GHRL mRNA expression remains to be determined. While some GHRL mRNA levels are found in the brain, and central GHRL mRNA translation has been demonstrated in rodents, it remains to be demonstrated whether GHRL is centrally translated in humans, with the more significant sites of GHRL expression being the stomach and duodenum.92 Still, our results from human post-mortem samples provide preliminary data on the lack of effect of alcohol on central ghrelin system gene expression.
Overall, this set of results contributes to a better understanding of the complex interactions between alcohol and the ghrelin system. Nevertheless, these results should be interpreted in light of the study’s limitations. Data presented from human laboratory experiments are the result of secondary analyses and were therefore not designed a priori to evaluate the effect of alcohol on peripheral ghrelin (e.g., no control infusion/oral saline administration was performed to compare to alcohol). Additionally, the sample sizes from our human and post-mortem experiments are small. Our findings on the central expression of GHRL, GHSR, and MBOAT4 in humans should be further investigated in larger samples. We were unable to evaluate expression of these genes in the hypothalamus, a prominent site of central acyl-ghrelin action that might be more significantly altered by chronic exposure to alcohol. Moreover, while we have included BMI and pack years of cigarettes as covariates in our analysis of gene expression data, it should be noted that the majority of individuals with AUD were also smokers while controls were not, and that the control group had a relatively higher average BMI compared to the AUD group. Lastly, it should be noted that our results may only be generalized to males, given that the human laboratory experiment samples (all > 70% male) were largely male, and human post-mortem samples and animals, used in alcohol administration experiments and for gastric mucosal experiments, were all male. It is also important to note that all our studies were conducted in adult humans and adult rats. A recent study suggests that alcohol may actually increase peripheral ghrelin levels in adolescent rats,93 therefore posing the question whether alcohol may differently affect the ghrelin system during development, a critical question but outside the scope of this work.
We report here novel insights into the potential underlying mechanism(s) linking alcohol and the ghrelin system. Understanding both acute and long-term effects of alcohol on the ghrelin system is important to inform the use and clinical translation of ghrelin inhibitors as a potential pharmacotherapy for AUD. It is also important to examine a potential bidirectional interplay between alcohol use and ghrelin’s effects on alcohol consumption. Irrespective of how these effects occur, acute suppression of ghrelin by alcohol may produce compensatory changes whereby long-term alcohol use leading to an upregulation of ghrelin secretion over time. Acute increases in ghrelin secretion have been demonstrated to be a learned, anticipatory response that increases mesolimbic dopamine release in response to food and alcohol,94 and it is possible that long-term alcohol consumption results in an upregulation of this response. Indeed, increased levels of ghrelin have been demonstrated in abstinent participants with AUD.45,48,49,59,60,62–64 Our results suggest that an indirect mechanism underlies alcohol-induced suppression of acyl-ghrelin that is unique to alcohol. To date, models of acyl-ghrelin secretion have identified insulin, glucagon, long chain fatty acids, oxytocin, dopamine, norepinephrine, epinephrine, endocannabinoids, somatostatin, glutamate, and glucose as direct regulators of acyl-ghrelin secretion,75,95–101 and it is possible that alcohol may decrease acyl-ghrelin indirectly by affecting these targets. Another possibility is that alcohol-induced suppression of acyl-ghrelin results from alcohol’s marked acute inhibition of fatty acid β-oxidation.,102 as it has been suggested that ghrelin acylation can be supported by β-oxidation of long chain fatty acids to produce medium chain fatty acids able to act as GOAT substrates.97,100,103 Alternatively, alcohol may suppress ghrelin through its effects on vagal signaling.104,105 Further work should focus on identifying indirect mediators by which alcohol affects ghrelin.
In conclusion, our data collectively demonstrate that alcohol affects the ghrelin system by acutely decreasing acyl-ghrelin concentration in the circulation and by blunting fasting-induced increase of plasma des-acyl-ghrelin concentrations. This effect appears to occur independently of the ghrelin receptor, and without direct action on the GOAT enzyme or acyl-ghrelin secretion from gastric mucosal cells. Additionally, alcohol and sucrose in equivalent caloric amounts do not have the same effect on peripheral ghrelin, differentially affecting acyl- and des-acyl-ghrelin relative to baseline and saline-treated controls. Therefore, this study suggests that alcohol acutely suppresses ghrelin without directly interacting with the ghrelin system and not simply according to calorie content of alcohol. While further studies are needed to uncover this mechanism of alcohol-induced ghrelin suppression, our data provide new insight into how these effects occur.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the clinical and research staff involved in patient care, data collection/analysis, and technical support in the joint NIDA/NIAAA Clinical Psychoneuroendocrinology and Neuropsychopharmacology Section, in the NIAAA clinical program of the Division of Intramural Clinical and Biological Research (DICBR) (in particular the NIAAA Office of the Clinical Director and the NIAA Clinical Core Laboratory), at the NIH Clinical Center (Departments of Nursing, Nutrition, and Pharmacy), and in the Clinical Pharmacokinetics Research Laboratory at the University of Rhode Island. We would also like to thank Dr. Melanie Schwandt (Office of the Clinical Director, NIAAA) for data management. We would like to thank Dr. Vijay Ramchandani (Section on Human Psychopharmacology, NIAAA DICBR) and Dr. Reza Momenan (Clinical NeuroImaging Research Core, NIAAA DICBR) for their support in the execution of the parent studies from which these analyses stemmed. The authors would also like to express their gratitude to the participants who took part in these studies. We would like to thank the members of the Neurobiology of Addiction Section, the Transgenic Breeding Facility, and the Genetic Engineering and Viral Vector Core in the Intramural Research Program at NIDA/NIH involved in animal care and technical support. Finally, the authors would like to thank Ms. Donna Sheedy and Dr. Jillian Kril from the New South Wales Tissue Resource Centre (NSWBTRC) at the University of Sydney, Australia, for providing the human post-mortem brain tissue for this project. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The human laboratory studies were supported by the NIH intramural funding ZIA-DA-000635 and ZIA-AA000218 (Clinical Psychoneuroendocrinology and Neuropsychopharmacology Section—PI: LL), jointly supported by the NIDA Intramural Research Program and the NIAAA Division of Intramural Clinical and Biological Research. The rodent studies were supported by the NIDA IRP Neurobiology of Addiction Section (PI: GFK) and by the NIDA/NIAAA joint Clinical Psychoneuroendocrinology and Neuropsychopharmacology Section (PI: LL). The development of the Computerized Alcohol Infusion System (CAIS) software used in the IV ghrelin study was supported by Dr. Vijay Ramchandani’s Section on Human Psychopharmacology in the NIAAA Division of Intramural Clinical and Biological Research and by the NIAAA-funded Indiana Alcohol Research Center (AA007611). The PF-5190457 phase 1b study received additional funding from the National Center for Advancing Translational Sciences (NCATS), under an UH2/UH3 grant (TR000963—PIs: LL and FA). Pfizer kindly provided the PF-5190457 compound under the NCATS grant UH2/UH3-TR000963. Pfizer did not have any role in the study design, execution or interpretation of the results, and this publication does not necessarily represent the official views of Pfizer. The baclofen study received additional finding from the Brain and Behavior Research Foundation (BBRF; formerly NARSAD) grant number 17325 (PI: LL). Brain tissues were received from the New South Wales Brain Tissue Resource Centre (NSWBTRC) at the University of Sydney, which is supported by NIAAA under Award Number R28AA012725 and Neuroscience Research Australia. The GOAT enzyme activity studies were supported by NIGMS under grant R01GM134102 (PI: JLH). MF and RCNM were fellows of the Center for Compulsive Behaviors at NIH. BJT was additionally supported by NIH award DA048530. The ex vivo experiments in gastric mucosal cells were supported by a NIH extramural grant R01DK103884 (PI: JMZ).
Funding information
Brain and Behavior Research Foundation, Grant/Award Number: 17325; Human Psychopharmacology in the NIAAA Division of Intramural Clinical and Biological Research; National Center for Advancing Translational Sciences, Grant/Award Number: TR000963; NIGMS, Grant/Award Number: R01GM134102; NIAAA, Grant/Award Numbers: AA007611, R28AA012725; NIDA; NIH, Grant/Award Numbers: ZIA-DA-000635, ZIA-AA000218, DA048530, R01DK103884; NIDA/NIAAA joint Clinical Psychoneuroendocrinology and Neuropsychopharmacology Section; Neuroscience Research Australia; University of Sydney
Footnotes
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available upon reasonable request from the corresponding author.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
CONFLICT OF INTEREST
The authors declare that they have no competing conflicts of interest.
REFERENCES
- 1.SAMSHA. 2018 National Survey on Drug Use and Health (NSDUH). Table 5.4A—Alcohol use disorder in past year among persons aged 12 or older, by age group and demographic characteristics: numbers in thousands, 2017 and 2018. 2018; Available from: https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2018R2/NSDUHDetTabsSect5pe2018.htm#tab5-4a
- 2.(CDC), C.f.D.C.a.P. Alcohol and public health: Alcohol-Related Disease Impact (ARDI). Average for United States 2006–2010 alcohol-attributable deaths due to excessive alcohol use. [cited 2020 5/13/2020]; Available from: https://nccd.cdc.gov/DPH_ARDI/Default/Report.aspx?T=AAM%26P=f6d7eda7-036e-4553-9968-9b17ffad620e%26R=d7a9b303-48e9-4440-bf47-070a4827e1fd%26M=8E1C5233-5640-4EE8-9247-1ECA7DA325B9%26F=%26D
- 3.Engel JA, Jerlhag E. Role of appetite-regulating peptides in the pathophysiology of addiction: implications for pharmacotherapy. CNS Drugs. 2014;28(10):875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fletcher PC, Kenny PJ. Food addiction: a valid concept? Neuropsychopharmacology. 2018;43(13):2506–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R. Food and drug reward: overlapping circuits in human obesity and addiction. Curr Top Behav Neurosci. 2012;11:1–24. [DOI] [PubMed] [Google Scholar]
- 6.Witkiewitz K, Litten RZ, Leggio L. Advances in the science and treatment of alcohol use disorder. Sci Adv. 2019;5(9):eaax4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farokhnia M, Faulkner ML, Piacentino D, Lee MR, Leggio L. Ghrelin: from a gut hormone to a potential therapeutic target for alcohol use disorder. Physiol Behav. 2019;204:49–57. [DOI] [PubMed] [Google Scholar]
- 8.Koopmann A, Schuster R, Kiefer F. The impact of the appetite-regulating, orexigenic peptide ghrelin on alcohol use disorders: a systematic review of preclinical and clinical data. Biol Psychol. 2018;131: 14–30. [DOI] [PubMed] [Google Scholar]
- 9.Zallar LJ, Farokhnia M, Tunstall BJ, Vendruscolo LF, Leggio L. The role of the ghrelin system in drug addiction. Int Rev Neurobiol. 2017; 136:89–119. [DOI] [PubMed] [Google Scholar]
- 10.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. [DOI] [PubMed] [Google Scholar]
- 11.Ariyasu H, Takaya K, Tagami T, et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86 (10):4753–4758. [DOI] [PubMed] [Google Scholar]
- 12.Zhu X, Cao Y, Voodg K, Steiner DF. On the processing of proghrelin to ghrelin. J Biol Chem. 2006;281(50):38867–38870. [DOI] [PubMed] [Google Scholar]
- 13.Labarthe A, Fiquet O, Hassouna R, et al. Ghrelin-derived peptides: a link between appetite/reward, GH axis, and psychiatric disorders? Front Endocrinol (Lausanne). 2014;5:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kojima M, Kangawa K. Ghrelin: from gene to physiological function. Results Probl Cell Differ. 2010;50:185–205. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto M, Hosoda H, Kitajima Y, et al. Structure-activity relationship of ghrelin: pharmacological study of ghrelin peptides. Biochem Biophys Res Commun. 2001;287(1):142–146. [DOI] [PubMed] [Google Scholar]
- 16.De Vriese C, Gregoire F, Lema-Kisoka R, Waelbroeck M, Robberecht P, Delporte C. Ghrelin degradation by serum and tissue homogenates: identification of the cleavage sites. Endocrinology. 2004;145(11):4997–5005. [DOI] [PubMed] [Google Scholar]
- 17.Hopkins AL, Nelson TAS, Guschina IA, et al. Unacylated ghrelin promotes adipogenesis in rodent bone marrow via ghrelin O-acyl transferase and GHS-R1a activity: evidence for target cell-induced acylation. Sci Rep. 2017;7(1):45541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murtuza MI, Isokawa M. Endogenous ghrelin-O-acyltransferase (GOAT) acylates local ghrelin in the hippocampus. J Neurochem. 2018;144(1):58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim CT, Kola B, Grossman A, Korbonits M. The expression of ghrelin O-acyltransferase (GOAT) in human tissues. Endocr J. 2011;58(8): 707–710. [DOI] [PubMed] [Google Scholar]
- 20.Fetissov SO, Lucas N, Legrand R. Ghrelin-reactive immunoglobulins in conditions of altered appetite and energy balance. Front Endocrinol (Lausanne). 2017;8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delhanty PJ, Neggers SJ, van der Lely AJ. Des-acyl ghrelin: a metabolically active peptide. Endocr Dev. 2013;25:112–121. [DOI] [PubMed] [Google Scholar]
- 22.Holst B, Cygankiewicz A, Jensen TH, Ankersen M, Schwartz TW. High constitutive signaling of the ghrelin receptor—identification of a potent inverse agonist. Mol Endocrinol. 2003;17(11):2201–2210. [DOI] [PubMed] [Google Scholar]
- 23.Holst B, Schwartz TW. Constitutive ghrelin receptor activity as a signaling set-point in appetite regulation. Trends Pharmacol Sci. 2004; 25(3):113–117. [DOI] [PubMed] [Google Scholar]
- 24.Al-Massadi O, Müller T, Tschöp M, Diéguez C, Nogueiras R. Ghrelin and LEAP-2: rivals in energy metabolism. Trends Pharmacol Sci. 2018;39(8):685–694. [DOI] [PubMed] [Google Scholar]
- 25.Ge X, Yang H, Bednarek MA, et al. LEAP2 is an endogenous antagonist of the ghrelin receptor. Cell Metab. 2018;27(2):461–469.e6 [DOI] [PubMed] [Google Scholar]
- 26.Mani BK, Puzziferri N, He Z, et al. LEAP2 changes with body mass and food intake in humans and mice. J Clin Invest. 2019;129(9): 3909–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mani BK, Zigman JM. Ghrelin as a survival hormone. Trends Endocrinol Metab. 2017;28(12):843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanagi S, Sato T, Kangawa K, Nakazato M. The homeostatic force of ghrelin. Cell Metab. 2018;27(4):786–804. [DOI] [PubMed] [Google Scholar]
- 29.Abizaid A, Liu ZW, Andrews ZB, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116(12):3229–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jerlhag E. Systemic administration of ghrelin induces conditioned place preference and stimulates accumbal dopamine. Addict Biol. 2008;13(3–4):358–363. [DOI] [PubMed] [Google Scholar]
- 31.Jerlhag E, Egecioglu E, Dickson SL, Andersson M, Svensson L, Engel JA. Ghrelin stimulates locomotor activity and accumbal dopamine-overflow via central cholinergic systems in mice: implications for its involvement in brain reward. Addict Biol. 2006;11(1): 45–54. [DOI] [PubMed] [Google Scholar]
- 32.Jerlhag E, Egecioglu E, Dickson SL, Douhan A, Svensson L, Engel JA. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict Biol. 2007;12(1):6–16. [DOI] [PubMed] [Google Scholar]
- 33.Jerlhag E, Janson AC, Waters S, Engel JA. Concomitant release of ventral tegmental acetylcholine and accumbal dopamine by ghrelin in rats. PLoS One. 2012;7(11):e49557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang H, Betancourt L, Smith RG. Ghrelin amplifies dopamine signaling by cross talk involving formation of growth hormone secretagogue receptor/dopamine receptor subtype 1 heterodimers. Mol Endocrinol. 2006;20(8):1772–1785. [DOI] [PubMed] [Google Scholar]
- 35.Quarta D, di Francesco C, Melotto S, Mangiarini L, Heidbreder C, Hedou G. Systemic administration of ghrelin increases extracellular dopamine in the shell but not the core subdivision of the nucleus accumbens. Neurochem Int. 2009;54(2):89–94. [DOI] [PubMed] [Google Scholar]
- 36.Schellekens H, Dinan TG, Cryan JF. Taking two to tango: a role for ghrelin receptor heterodimerization in stress and reward. Front Neurosci. 2013;7:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dickson SL, Egecioglu E, Landgren S, Skibicka KP, Engel JA, Jerlhag E. The role of the central ghrelin system in reward from food and chemical drugs. Mol Cell Endocrinol. 2011;340(1):80–87. [DOI] [PubMed] [Google Scholar]
- 38.Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26(11): 2274–2279. [DOI] [PubMed] [Google Scholar]
- 39.Jerlhag E, Egecioglu E, Landgren S, et al. Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci U S a. 2009; 106(27):11318–11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bahi A, Tolle V, Fehrentz JA, et al. Ghrelin knockout mice show decreased voluntary alcohol consumption and reduced ethanol-induced conditioned place preference. Peptides. 2013;43:48–55. [DOI] [PubMed] [Google Scholar]
- 41.Panagopoulos VN, Ralevski E. The role of ghrelin in addiction: a review. Psychopharmacology (Berl). 2014;231(14):2725–2740. [DOI] [PubMed] [Google Scholar]
- 42.Kaur S, Ryabinin AE. Ghrelin receptor antagonism decreases alcohol consumption and activation of perioculomotor urocortin-containing neurons. Alcohol Clin Exp Res. 2010;34(9):1525–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jerlhag E, Landgren S, Egecioglu E, Dickson SL, Engel JA. The alcohol-induced locomotor stimulation and accumbal dopamine release is suppressed in ghrelin knockout mice. Alcohol. 2011;45(4): 341–347. [DOI] [PubMed] [Google Scholar]
- 44.Zallar LJ, Beurmann S, Tunstall BJ, et al. Ghrelin receptor deletion reduces binge-like alcohol drinking in rats. J Neuroendocrinol. 2019; 31(7):e12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Addolorato G, Capristo E, Leggio L, et al. Relationship between ghrelin levels, alcohol craving, and nutritional status in current alcoholic patients. Alcohol Clin Exp Res. 2006;30(11):1933–1937. [DOI] [PubMed] [Google Scholar]
- 46.Leggio L, Zywiak WH, Fricchione SR, et al. Intravenous ghrelin administration increases alcohol craving in alcohol-dependent heavy drinkers: a preliminary investigation. Biol Psychiatry. 2014;76(9): 734–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hillemacher T, Kraus T, Rauh J, et al. Role of appetite-regulating peptides in alcohol craving: an analysis in respect to subtypes and different consumption patterns in alcoholism. Alcohol Clin Exp Res. 2007;31(6):950–954. [DOI] [PubMed] [Google Scholar]
- 48.Koopmann A, von der Goltz C, Grosshans M, et al. The association of the appetitive peptide acetylated ghrelin with alcohol craving in early abstinent alcohol dependent individuals. Psychoneuroendocrinology. 2012;37(7):980–986. [DOI] [PubMed] [Google Scholar]
- 49.Leggio L, Ferrulli A, Cardone S, et al. Ghrelin system in alcohol-dependent subjects: role of plasma ghrelin levels in alcohol drinking and craving. Addict Biol. 2012;17(2):452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farokhnia M, Grodin EN, Lee MR, et al. Exogenous ghrelin administration increases alcohol self-administration and modulates brain functional activity in heavy-drinking alcohol-dependent individuals. Mol Psychiatry. 2018;23(10):2029–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Landgren S, Engel JA, Hyytiä P, Zetterberg H, Blennow K, Jerlhag E. Expression of the gene encoding the ghrelin receptor in rats selected for differential alcohol preference. Behav Brain Res. 2011;221(1): 182–188. [DOI] [PubMed] [Google Scholar]
- 52.Szulc M, Mikolajczak PL, Geppert B, Wachowiak R, Dyr W, Bobkiewicz-Kozlowska T. Ethanol affects acylated and total ghrelin levels in peripheral blood of alcohol-dependent rats. Addict Biol. 2013;18(4):689–701. [DOI] [PubMed] [Google Scholar]
- 53.Calissendorff J, Danielsson O, Brismar K, Röjdmark S. Alcohol ingestion does not affect serum levels of peptide YY but decreases both total and octanoylated ghrelin levels in healthy subjects. Metabolism. 2006;55(12):1625–1629. [DOI] [PubMed] [Google Scholar]
- 54.Calissendorff J, Danielsson O, Brismar K, Röjdmark S. Inhibitory effect of alcohol on ghrelin secretion in normal man. Eur J Endocrinol. 2005;152(5):743–747. [DOI] [PubMed] [Google Scholar]
- 55.Calissendorff J, Gustafsson T, Holst JJ, Brismar K, Röjdmark S. Alcohol intake and its effect on some appetite-regulating hormones in man: influence of gastroprotection with sucralfate. Endocr Res. 2012;37(3):154–162. [DOI] [PubMed] [Google Scholar]
- 56.Zimmermann US, Buchmann A, Steffin B, Dieterle C, Uhr M. Alcohol administration acutely inhibits ghrelin secretion in an experiment involving psychosocial stress. Addict Biol. 2007;12(1): 17–21. [DOI] [PubMed] [Google Scholar]
- 57.Leggio L, Schwandt ML, Oot EN, Dias AA, Ramchandani VA. Fasting-induced increase in plasma ghrelin is blunted by intravenous alcohol administration: a within-subject placebo-controlled study. Psychoneuroendocrinology. 2013;38(12):3085–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ralevski E, Horvath TL, Shanabrough M, Hayden R, Newcomb J, Petrakis I. Ghrelin is supressed by intravenous alcohol and is related to stimulant and sedative effects of alcohol. Alcohol Alcohol. 2017;52 (4):431–438. [DOI] [PubMed] [Google Scholar]
- 59.Kraus T, Schanze A, Gröschl M, et al. Ghrelin levels are increased in alcoholism. Alcohol Clin Exp Res. 2005;29(12):2154–2157. [DOI] [PubMed] [Google Scholar]
- 60.Kim DJ, Yoon SJ, Choi B, et al. Increased fasting plasma ghrelin levels during alcohol abstinence. Alcohol Alcohol. 2005;40(1): 76–79. [DOI] [PubMed] [Google Scholar]
- 61.Badaoui A, de Saeger C, Duchemin J, Gihousse D, de Timary P, Stärkel P. Alcohol dependence is associated with reduced plasma and fundic ghrelin levels. Eur J Clin Invest. 2008;38(6): 397–403. [DOI] [PubMed] [Google Scholar]
- 62.de Timary P, Cani PD, Duchemin J, et al. The loss of metabolic control on alcohol drinking in heavy drinking alcohol-dependent subjects. PLoS One. 2012;7(7):e38682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim JH, Kim SJ, Lee WY, et al. The effects of alcohol abstinence on BDNF, ghrelin, and leptin secretions in alcohol-dependent patients with glucose intolerance. Alcohol Clin Exp Res. 2013;37(Suppl 1): E52–E58. [DOI] [PubMed] [Google Scholar]
- 64.Wurst FM, Graf I, Ehrenthal HD, et al. Gender differences for ghrelin levels in alcohol-dependent patients and differences between alcoholics and healthy controls. Alcohol Clin Exp Res. 2007;31(12): 2006–2011. [DOI] [PubMed] [Google Scholar]
- 65.Wittekind DA, Kratzsch J, Mergl R, et al. Alcohol consumption is positively associated with fasting serum ghrelin in non-dependent adults: results from the population-based LIFE-Adult-Study. Psychoneuroendocrinology. 2018;97:143–148. [DOI] [PubMed] [Google Scholar]
- 66.Farokhnia M, Schwandt ML, Lee MR, et al. Biobehavioral effects of baclofen in anxious alcohol-dependent individuals: a randomized, double-blind, placebo-controlled, laboratory study. Transl Psychiatry. 2017;7(4):e1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee MR, Tapocik JD, Ghareeb M, et al. The novel ghrelin receptor inverse agonist PF-5190457 administered with alcohol: preclinical safety experiments and a phase 1b human laboratory study. Mol Psychiatry. 2018;25:461–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watson PE, Watson ID, Batt RD. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr. 1980;33(1):27–39. [DOI] [PubMed] [Google Scholar]
- 69.Ramchandani VA, Bolane J, Li TK, O’Connor S. A physiologically-based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcohol Clin Exp Res. 1999;23(4):617–623. [PubMed] [Google Scholar]
- 70.Zimmermann US, O’Connor S, Ramchandani VA. Modeling alcohol self-administration in the human laboratory. Curr Top Behav Neurosci. 2013;13:315–353. [DOI] [PubMed] [Google Scholar]
- 71.Sutherland GT, Sheedy D, Stevens J, et al. The NSW brain tissue resource centre: banking for alcohol and major neuropsychiatric disorders research. Alcohol. 2016;52:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee MR, Schwandt ML, Sankar V, Suchankova P, Sun H, Leggio L. Effect of alcohol use disorder on oxytocin peptide and receptor mRNA expression in human brain: a post-mortem case-control study. Psychoneuroendocrinology. 2017;85:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zallar LJ, Tunstall BJ, Richie CT, et al. Development and initial characterization of a novel ghrelin receptor CRISPR/Cas9 knockout wistar rat model. Int J Obes (Lond). 2019;43(2):344–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brendan J, Tunstall LFV. Krystal Allen–Worthington,, Chapter 26—rat models of alcohol use disorder. In: Suckow MA, Wilson RP, Foley PL, eds. The Laboratory Rat. American College of Laboratory Animal Medicine. Third ed.), H FC Editor(s). Academic Press; 2020: 967–986. [Google Scholar]
- 75.Sakata I, Park WM, Walker AK, et al. Glucose-mediated control of ghrelin release from primary cultures of gastric mucosal cells. Am J Physiol Endocrinol Metab. 2012;302(10):E1300-E1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mani BK, Osborne-Lawrence S, Vijayaraghavan P, Hepler C, Zigman JM. β1-Adrenergic receptor deficiency in ghrelin-expressing cells causes hypoglycemia in susceptible individuals. J Clin Invest. 2016;126(9):3467–3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Darling JE, Prybolsky EP, Sieburg M, Hougland JL. A fluorescent peptide substrate facilitates investigation of ghrelin recognition and acylation by ghrelin O-acyltransferase. Anal Biochem. 2013;437(1): 68–76. [DOI] [PubMed] [Google Scholar]
- 78.Sieburg MA, Cleverdon ER, Hougland JL. Biochemical assays for ghrelin acylation and inhibition of ghrelin O-acyltransferase. Methods Mol Biol. 2019;2009:227–241. [DOI] [PubMed] [Google Scholar]
- 79.Darling JE, Zhao F, Loftus RJ, Patton LM, Gibbs RA, Hougland JL. Structure–activity analysis of human ghrelin O-acyltransferase reveals chemical determinants of ghrelin selectivity and acyl group recognition. Biochemistry. 2015;54(4):1100–1110. [DOI] [PubMed] [Google Scholar]
- 80.Mani BK, Uchida A, Lee Y, et al. Hypoglycemic effect of combined ghrelin and glucagon receptor blockade. Diabetes. 2017;66(7):1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harrison NL, Skelly MJ, Grosserode EK, et al. Effects of acute alcohol on excitability in the CNS. Neuropharmacology. 2017;122:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S a. 2003; 100(4):2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cummings DE, Naleid AM, Figlewicz Lattemann DP. Ghrelin: a link between energy homeostasis and drug abuse? Addict Biol. 2007;12 (1):1–5. [DOI] [PubMed] [Google Scholar]
- 84.Hedegaard MA, Holst B. The Complex Signaling Pathways of the Ghrelin Receptor. Endocrinology. 2020;161(4):bqaa020. [DOI] [PubMed] [Google Scholar]
- 85.Wallace Fitzsimons SE, Chruscicka B, Druelle C, et al. A ghrelin receptor and oxytocin receptor heterocomplex impairs oxytocin mediated signalling. Neuropharmacology. 2019;152:90–101. [DOI] [PubMed] [Google Scholar]
- 86.Callahan HS, Cummings DE, Pepe MS, Breen PA, Matthys CC, Weigle DS. Postprandial suppression of plasma ghrelin level is proportional to ingested caloric load but does not predict intermeal interval in humans. J Clin Endocrinol Metab. 2004;89(3): 1319–1324. [DOI] [PubMed] [Google Scholar]
- 87.Le Roux CW, Patterson M, Vincent RP, Hunt C, Ghatei MA, Bloom SR. Postprandial plasma ghrelin is suppressed proportional to meal calorie content in normal-weight but not obese subjects. J Clin Endocrinol Metab. 2005;90(2):1068–1071. [DOI] [PubMed] [Google Scholar]
- 88.dit El Khoury DT, Obeid O, Azar ST, Hwalla N. Variations in postprandial ghrelin status following ingestion of high-carbohydrate, high-fat, and high-protein meals in males. Ann Nutr Metab. 2006;50 (3):260–269. [DOI] [PubMed] [Google Scholar]
- 89.Monteleone P, Bencivenga R, Longobardi N, Serritella C, Maj M. Differential responses of circulating ghrelin to high-fat or high-carbohydrate meal in healthy women. J Clin Endocrinol Metab. 2003; 88(11):5510–5514. [DOI] [PubMed] [Google Scholar]
- 90.Williams DL, Cummings DE. Regulation of ghrelin in physiologic and pathophysiologic states. J Nutr. 2005;135(5):1320–1325. [DOI] [PubMed] [Google Scholar]
- 91.Liu B, Garcia EA, Korbonits M. Genetic studies on the ghrelin, growth hormone secretagogue receptor (GHSR) and ghrelin O-acyl transferase (GOAT) genes. Peptides. 2011;32(11):2191–2207. [DOI] [PubMed] [Google Scholar]
- 92.Cabral A, Lopez Soto EJ, Epelbaum J, Perelló M. Is ghrelin synthesized in the central nervous system? Int J Mol Sci. 2017;18(3):638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Healey KL, Landin JD, Dubester K, et al. Effects of ethanol on plasma ghrelin levels in the rat during early and late adolescence. Alcohol. 2020;85:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Al Massadi O, Nogueiras R, Dieguez C, Girault JA. Ghrelin and food reward. Neuropharmacology. 2019;148:131–138. [DOI] [PubMed] [Google Scholar]
- 95.Gagnon J, Anini Y. Insulin and norepinephrine regulate ghrelin secretion from a rat primary stomach cell culture. Endocrinology. 2012; 153(8):3646–3656. [DOI] [PubMed] [Google Scholar]
- 96.Gagnon J, Anini Y. Glucagon stimulates ghrelin secretion through the activation of MAPK and EPAC and potentiates the effect of norepinephrine. Endocrinology. 2013;154(2):666–674. [DOI] [PubMed] [Google Scholar]
- 97.Godlewski G, Cinar R, Coffey NJ, et al. Targeting peripheral CB1 receptors reduces ethanol intake via a gut-brain axis. Cell Metab. 2019;29(6):1320–1333.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Iwakura H, Ariyasu H, Hosoda H, et al. Oxytocin and dopamine stimulate ghrelin secretion by the ghrelin-producing cell line, MGN3–1 in vitro. Endocrinology. 2011;152(7):2619–2625. [DOI] [PubMed] [Google Scholar]
- 99.Lu X, Zhao X, Feng J, et al. Postprandial inhibition of gastric ghrelin secretion by long-chain fatty acid through GPR120 in isolated gastric ghrelin cells and mice. Am J Physiol Gastrointest Liver Physiol. 2012;303(3):G367–G376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sakata I, Gong Z, Ikenoya C, Takemi S, Sakai T. The study of ghrelin secretion and acyl-modification using mice and ghrelinoma cell lines. Endocr J. 2017;64(Suppl):S27–S29. [DOI] [PubMed] [Google Scholar]
- 101.Zhao TJ, Sakata I, Li RL, et al. Ghrelin secretion stimulated by {beta} 1-adrenergic receptors in cultured ghrelinoma cells and in fasted mice. Proc Natl Acad Sci U S a. 2010;107(36):15868–15873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cederbaum AI. Alcohol metabolism. Clin Liver Dis. 2012;16(4): 667–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bando M, Iwakura H, Koyama H, et al. High incorporation of long-chain fatty acids contributes to the efficient production of acylated ghrelin in ghrelin-producing cells. FEBS Lett. 2016;590(7):992–1001. [DOI] [PubMed] [Google Scholar]
- 104.Nadareishvili K, Meskhishvili II, Kakhiani DD, Ormrtsadze GL, Khvedelidze MT, Chitanava ET. Effects of low ethanol doses on heart rhythm in rabbits. Bull Exp Biol Med. 2004;138(3):271–275. [DOI] [PubMed] [Google Scholar]
- 105.Ueno H, Nakazato M. Mechanistic relationship between the vagal afferent pathway, central nervous system and peripheral organs in appetite regulation. J Diabetes Investig. 2016;7(6):812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.