Abstract
Introduction
Live biotherapeutic products (LBPs), or therapeutic microbes, are an emerging therapeutic modality for prevention and treatment of gastrointestinal diseases. Since LBPs are living, they are uniquely sensitive to external stresses (e.g., oxygen, acid) encountered during manufacturing, storage, and delivery. Here, we systematically evaluate how polymer and crosslinker concentration affects the performance of an encapsulated LBP toward developing a comprehensive framework for the characterization and optimization of LBP delivery systems.
Methods
We encapsulate a model LBP, Lactobacillus casei ATCC 393, in calcium chloride (CaCl2)-crosslinked alginate beads, and evaluate how alginate and CaCl2 concentrations influence LBP formulation performance, including: (i) encapsulation efficiency, (ii) shrinkage upon drying, (iii) survival upon lyophilization, (iv) acid resistance, (v) release, and (vi) metabolite secretion. Approaches from microbiology (e.g., colony forming unit enumeration), materials science (e.g., scanning electron microscopy), and pharmaceutical sciences (e.g., release assays) are employed.
Results
LBP-encapsulating alginate beads were systematically evaluated as a function of alginate and CaCl2 concentrations. Specifically: (i) encapsulation efficiency of all formulations was >50%, (ii) all alginate beads shrunk (after lyophilization) and recovered (after rehydration) similarly, (iii) at 10% alginate concentration, lower CaCl2 concentration decreased survival upon lyophilization, (iv) 10% alginate improved acid resistance, (v) sustained release was enabled by increasing alginate and CaCl2 concentrations, and (vi) encapsulation did not impair secretion of l-lactate as compared to free LBP.
Conclusions
This research demonstrates that polymer content and crosslinking extent modulate the performance of polymer-based LBP delivery systems, motivating research into the optimization of material properties for LBP delivery systems.
Keywords: Drug delivery, Polymer encapsulation, Probiotics, Alginate, Live biotherapeutic products
Introduction
Live biotherapeutic products (LBPs), or therapeutic microbes, include transplanted microbiota, feces-derived spores, rationally designed consortia, and engineered microbes that produce drugs locally.46,49,50 LBPs are currently investigated in various clinical trials for the treatment of pathogenic infections, inflammatory diseases, and metabolic disorders46; recently, positive results from clinical trials of LBPs have been reported for treatment of recurrent Clostridioides difficile infections.14 Uniquely, LBPs have the potential to: (i) serve as alternatives to antibiotics by circumventing current limitations of antibiotic therapies, such as displacing pathogens without risks of introducing antibiotic resistance,35 or (ii) continuously produce biologics in situ39 with engineered molecular machinery and potentially reduce the high dosing frequency required by biologics.3 As such, LBPs have received considerable interest as a next-generation therapeutic.
Unlike other therapeutic modalities, LBPs are alive and face unique challenges in their development, formulation, manufacturing, and delivery. In particular, they are limited by: (i) manufacturing conditions that expose LBPs to oxygen,43 heat,9 and desiccation36; (ii) storage requirements such as ultra-low temperatures (e.g., -80 °C)25; (iii) delivery challenges such as acid and bile insults.9 Toward addressing these issues and accelerating clinical translation of LBPs, efforts focus on developing a variety of delivery systems for LBPs.28 One common approach to formulate LBPs is through polymer encapsulation.28 Studies have reported encapsulation of LBPs in poly(vinyl alcohol),38 alginate,7 chitosan,11 and gelatin.2 The bulk polymeric matrix provides physical barriers to protect payloads from environmental stressors (e.g., oxygen, acid, bile) while providing additional functions, such as sustained release.38 Another encapsulation strategy is to microscopically coat polymers onto individual microbes.4,13,21 For instance, alternating alginate and chitosan layers have been used to decorate microbes to improve acid resistance and mucoadhesion.4 While these examples describe promising benefits of polymer encapsulation toward formulating LBPs,8 few studies have elucidated the quantitative relationship between material content (e.g., polymer and crosslinker concentrations in hydrogels) and LBP formulation performance (e.g., encapsulation efficiency, storage, acid resistance, release profiles). The motivation for investigating these relationships stems from a wide body of literature detailing the effects that material content can have on the formulation, delivery, and efficacy of other therapeutic modalities.23 For example, encapsulated mammalian cells exhibited distinct cell viability, and in vivo outcomes in polymeric matrices with varying concentrations of polymer,37 and crosslinker.30 Tuning material content can vary the porosity, swelling, and degradation time of delivery systems, thus potentially modulating LBP encapsulation, stability, release profiles, and metabolism. Bridging this knowledge gap will improve understanding of LBP-material interactions and facilitate rational design of LBP delivery systems for different applications.
Here, we encapsulated a model LBP, Lactobacillus casei ATCC 393 (L. casei ATCC 393) in calcium chloride (CaCl2)-crosslinked alginate beads with different polymer and crosslinker concentrations. Alginate is a naturally-derived polysaccharide material comprising glucuronic and mannuronic units, and generally recognized as safe (GRAS) 6; as such, CaCl2 crosslinked alginate has been a widely studied encapsulation material for LBPs 22. L. casei ATCC 393 is a probiotic with preclinical evidence to mitigate diseases such as colon inflammation,29,45 and produced through batch culture in this work. With a broad range of alginate (2%, 5%, and 10% wt vol−1) and CaCl2 (0.1 M, 0.5 M, and 1 M) concentrations (Table 1), we systematically evaluated the effects of polymer and crosslinker content on aspects of LBP formulation and performance, including: (i) encapsulation efficiency, (ii) shrinkage upon drying, (iii) survival upon lyophilization, (iv) acid resistance, (v) release, and (vi) secretion of l-lactate, a major metabolite of L. casei ATCC 393. Approaches from microbiology (e.g., colony forming unit enumeration), materials science (e.g., scanning electron microscopy), and pharmaceutical sciences (e.g., release assays) are employed toward offering a comprehensive framework to characterize LBP formulations. The results highlight that controlling polymer and crosslinker content modulates LBP formulations, thereby motivating research to optimize material properties of LBP delivery systems.
Table 1.
Formulations with different concentrations of alginate and CaCl2
| Formulation # | F1 | F2 | F3 | F4 | F5 |
|---|---|---|---|---|---|
| Alginate (wt vol−1) | 2% | 5% | 10% | 10% | 10% |
| CaCl2 | 1 M | 1 M | 1 M | 0.5 M | 0.1 M |
Results and Discussion
Fabrication and Characterization of Alginate Beads
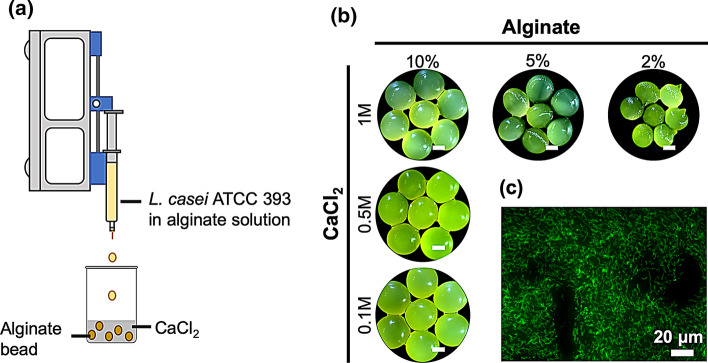
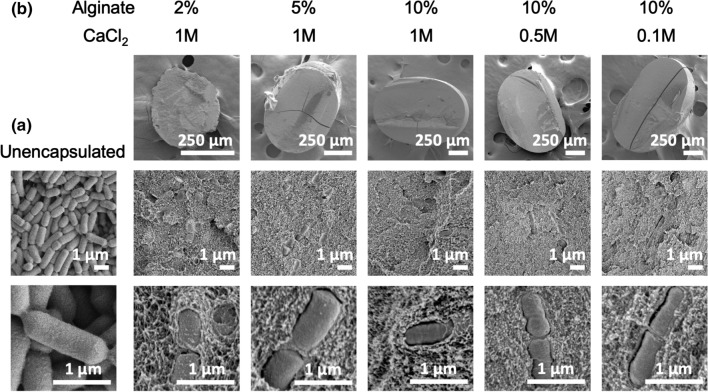
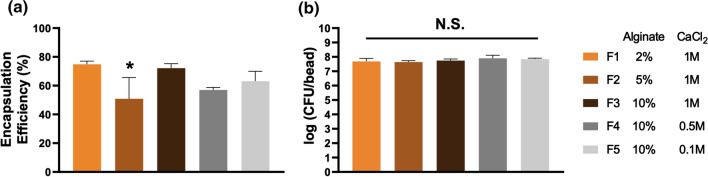
One common approach to fabricate crosslinked alginate is to introduce solubilized alginate into a solution of CaCl2 crosslinker.26 Here, we applied this technique to encapsulate L. casei ATCC 393 in alginate beads. Solubilized alginate with suspended L. casei ATCC 393 were loaded into syringes and added dropwise into CaCl2 solution by a syringe pump (Fig. 1a). To comprehensively capture the effects of polymer and crosslinker content on LBP formulations, we investigated: (i) 2%, 5%, and 10% wt vol−1 alginate at 1 M CaCl2, and (ii) 0.1 M, 0.5 M, and 1 M CaCl2 at 10% wt vol−1 alginate collectively. These broad ranges of alginate and CaCl2 concentrations have been described to influence aspects of alginate beads including material morphology, degradation, and substance diffusion,33,48 thus potentially leading to distinct performance of LBP-encapsulating formulations. Fluorescein was conjugated onto the surface of L. casei ATCC 393 through NHS-mediated reactions to visualize encapsulation. L. casei ATCC 393 was successfully encapsulated into alginate beads as demonstrated by brightfield imaging of the macroscopic alginate beads (Fig. 1b) and fluorescence imaging of microscopic cross-sections (Fig. 1c). Beads with 10% alginate concentration appeared more spherical in shape as compared to counterparts fabricated with lower alginate concentrations (Fig. 1b). Detailed morphology of unencapsulated L. casei ATCC 393 (Fig. 2a) and L. casei ATCC 393-encapsulating alginate beads (Fig. 2b) were examined through scanning electron microscopy (SEM). L. casei ATCC 393-encapsulating alginate beads were dehydrated through treatment of gradient ethanol solutions followed by vacuum drying. Beads at 2% alginate exhibited irregular shape and looser polymer matrices with identifiable pores as compared to counterparts at higher alginate concentrations (Fig. 2b), indicating alginate concentrations regulated matrix porosity. L. casei ATCC 393 was individually embedded in all groups of alginate beads with clear boundaries between microbes and the encapsulating matrix (Fig. 2b), suggesting that the encapsulation did not involve strong interactions between cell walls and crosslinked alginate. The encapsulation efficiency (EE) across all the groups was above 50% (Fig. 3a). While the EE of 5% alginate group was significantly different from 2% and 10% alginate groups at 1 M CaCl2, there were no monotonic correlations between EE and concentrations of alginate or CaCl2 on a per-bead basis. Notably, colony forming unit (CFU) loading of L. casei ATCC 393 across all groups was statistically insignificant when starting with the same L. casei ATCC 393 concentration in the alginate suspensions (Fig. 3b). These results highlighted that alginate and CaCl2 concentrations did not affect loading of L. casei ATCC 393.
Figure 1.
Fabrication and characterization of alginate beads. (a) Schematic of alginate bead fabrication. (b) Brightfield images of all groups of alginate beads encapsulating fluorescein-labeled L. casei ATCC 393. Scale bar = 1 mm. (c) Fluorescence imaging of the cross-section of an alginate bead (5% alginate, 1 M CaCl2) encapsulating fluorescein-labeled L. casei ATCC 393.
Figure 2.
SEM images of L. casei ATCC 393-encapsulating alginate beads. (a) Unencapsulated L. casei ATCC 393. (b) L. casei ATCC 393 encapsulated in alginate beads with different alginate and CaCl2 concentrations.
Figure 3.
Encapsulation efficiency and CFU loading of L. casei ATCC 393 in alginate beads. (a) Encapsulation efficiency and (b) CFU loading of L. casei ATCC 393 in alginate beads with different alginate and CaCl2 concentrations. Five alginate beads were analyzed in each of the three replicates (n = 3). Each error bar represents standard deviation. Statistical analysis was conducted using one-way ANOVA followed by post hoc Tukey’s HSD test for pairwise comparison (statistical significance defined at p < 0.05). *: significantly different from F1 and F3. N.S.: not significant.
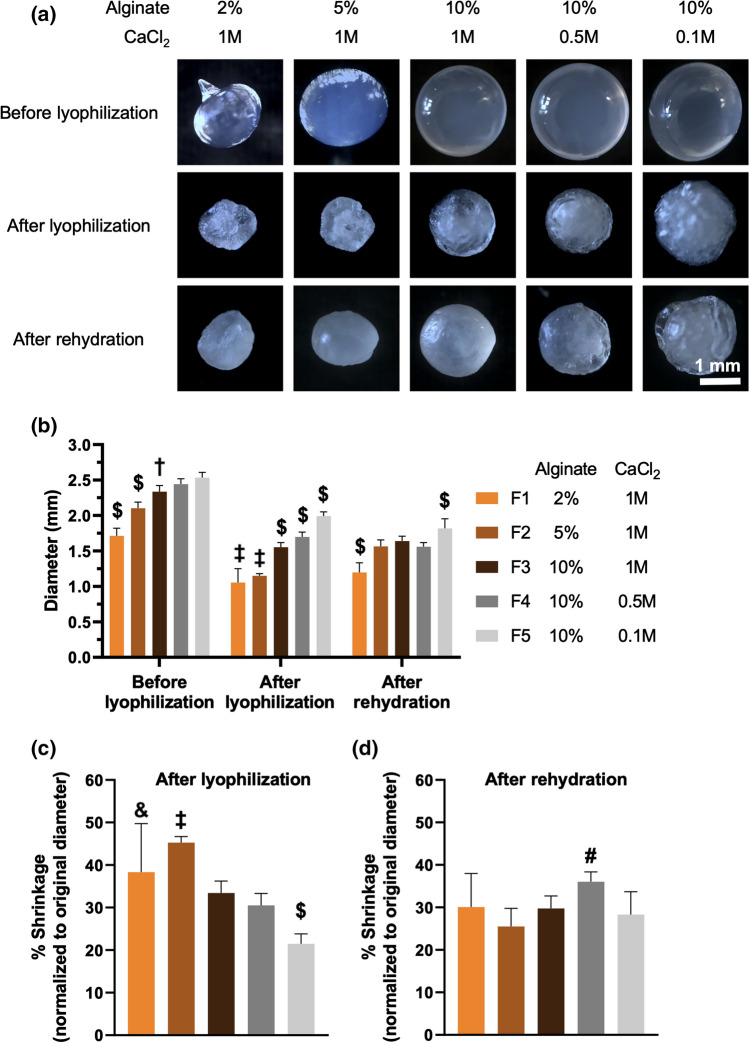
Desiccation of formulations is a commoly used approach for long term preservation of microbes.36 Among existing drying techniques to remove water in the formulations,36 lyophilization is widely used to dry and formulate LBPs, including several investigational products in clinical trials46; as such, we have investigated how polymer and crosslinker content influences the lyophilization process. Lyophilization removes water in the LBP formulations through systematic freezing and drying steps. These processes have been reported to drastically impact morphology of hydrogels, including size and shape, which reflects their structural features and dictates performance upon rehydration such as swelling.15,17 As such, we examined morphological changes of L. casei ATCC 393-free alginate beads through lyophilization and rehydration. All groups of alginate beads exhibited shrunk size, irregular shape, and wrinkled texture after lyophilization (Fig. 4a). After fabrication, and prior to lyophilization, higher alginate concentrations led to formation of beads with larger size (Fig. 4b). The alginate-dependent size differences were mostly dictated by the size of droplets, which was governed by the liquid viscosity, surface tension, and gravity.26 While varying CaCl2 concentrations led to minimal differences in bead size at 10% alginate before lyophilization (Fig. 4a and 4b), these differences were more pronounced after lyophilization. Specifically, after lyophilization, smaller bead size demonstrated a trend with higher CaCl2 concentrations, at the same alginate concentrations, during formulation (Fig. 4b). This could be explained by higher CaCl2 concentrations providing more ion-crosslinked points in the matrix, eliciting stronger structural constraints toward bead shrinkage after water removal. Overall, alginate beads maintained structural integrity (Fig. 4a) and exhibited < 50% shrinkage of their original diameter after lyophilization (Fig. 4c), indicating these alginate beads possessed rigid structures. As lyophilized hydrogels may rehydrate upon administration into physiological fluids, we sought to evaluate the rehydration of lyophilized alginate beads. We incubated all groups of lyophilized alginate beads in pure water at room temperature, and observed rehydration and swelling after 30 min incubation (Fig. 4a). All rehydrated alginate beads were at least > 60% of their original, pre-lyophilized, diameter (Fig. 4d).
Figure 4.
Size analysis of fresh, lyophilized, and rehydrated alginate beads. (a) Representative brightfield images of alginate beads before lyophilization, after lyophilization, and after rehydration in water for 30 min at room temperature. (b) Diameter of alginate beads throughout the lyophilization and rehydration process. (c) Shrinkage, relative to the original diameter, of alginate beads after lyophilization. (d) Shrinkage, relative to the original diameter, of alginate beads after rehydration. In (b–d), n = 10 beads. Each error bar represents standard deviation. Statistical analysis was conducted using one-way ANOVA followed by post hoc Tukey’s HSD test for pairwise comparison (statistical significance defined at p < 0.05). $: significantly different from all other groups. †: significantly different from F1, F2, and F5. ‡: significantly different from F3, F4, and F5. &: significantly different from F4 and F5. #: significantly different from F2 and F5.
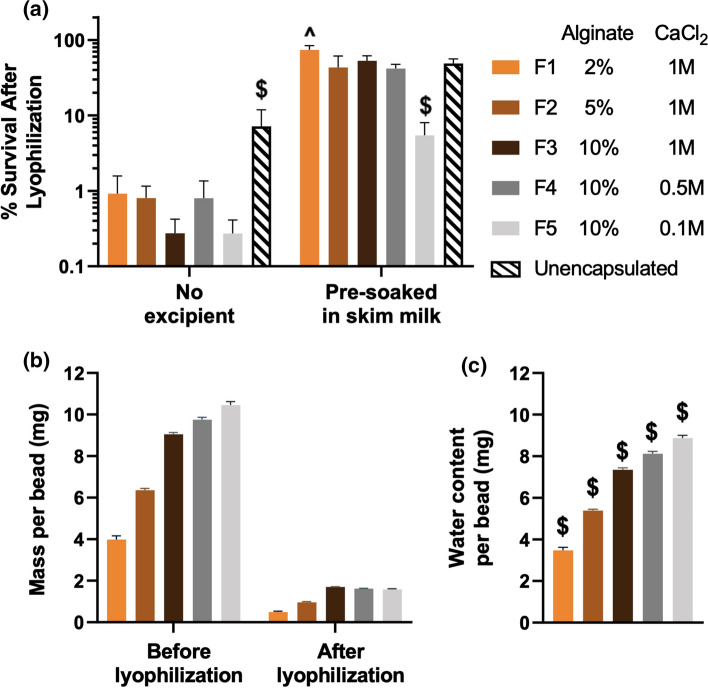
Lyophilization involves freezing and drying steps that can lead to microbe damage and subsequent viability loss of LBPs.41 As such, lyoprotectants such as sugars, amino acids and proteins are often incorporated into the LBP formulations to mitigate these stresses.36 To investigate how encapsulation with different alginate and CaCl2 concentrations influenced LBP survival through lyophilization, we used skim milk as a model lyoprotectant. Skim milk contains lactose and whey proteins, and has been widely used to preserve LBP viability during lyophilization by stabilizing microbe structures.5L. casei ATCC 393-encapsulating alginate beads were pre-soaked in skim milk for 30 min at room temperature and subsequently removed from skim milk for lyophilization. Encapsulated L. casei ATCC 393 in most alginate bead formulations exhibited similar survival as compared to the unencapsulated control in the presence of skim milk (Fig. 5a), suggesting that crosslinked alginate allowed diffusion of skim milk into the polymer matrices and subsequent interaction of L. casei ATCC 393 for improving survival. Notably, beads formulated at the lowest CaCl2 concentration exhibited decreased survival during lyophilization at 10% alginate (Fig. 5a). Towards explaining this, we analyzed water content of L. casei ATCC 393-encapsulating, skim milk-soaked, alginate beads by calculating weight loss before and after lyophilization on a per-bead basis (Fig. 5b and 5c). Higher water content in alginate beads may both increase ice formation and extend exposure to stresses during the water removal process, thus resulting in more microbe death during the lyophilization process.1,27,31,32 We observed that alginate beads with decreased CaCl2 concentrations contained higher water content (Fig. 5c), which was weakly correlated to lower survival of L. casei ATCC 393 after lyophilization (Fig. 5a). The higher water content in alginate beads with lower CaCl2 concentrations at 10% alginate was due to the increasing swelling capacity in the presence of lower crosslinking density and thus less matrix constraints.18 While the relationship between water content of alginate beads and the survival of encapsulated L. casei ATCC 393 were not linear and require additional studies, this could potentially be explained by other confounding formulation differences, such as excipient distribution and material properties (e.g., stiffness) of alginate beads, which may play a role during lyophilization. Interestingly, unencapsulated L. casei ATCC 393 exhibited higher survival compared to encapsulated groups in the absence of lyoprotectants, indicating that CaCl2-crosslinked alginate alone did not improve survival upon lyophilization (Fig. 5a). Combined together, these results highlight that polymer encapsulation is compatible with lyophilization approaches for formulating LBPs, while critical evaluation of polymer and crosslinker concentrations is needed to optimize morphology and LBP storage for developing polymer-based delivery systems.
Figure 5.
Viability and water content of lyophilized L. casei ATCC 393-encapsulated alginate beads. (a) Survival of encapsulated and unencapsulated L. casei ATCC 393 after lyophilization. Five representative beads were analyzed in each of the replicates (n = 3). (b) Mass of L. casei ATCC 393, skim milk-soaked, alginate beads before and after lyophilization. (c) Water content in L. casei ATCC 393, skim milk-soaked alginate beads. In (b) and (c), fifteen representative alginate beads were analyzed in each of the replicates (n = 3). Each error bar represents standard deviation. Statistical analysis was conducted using one-way ANOVA followed by post hoc Tukey’s HSD test for pairwise comparison (statistical significance defined at p < 0.05). $: significantly different from all other groups. ^: significantly different from F2, F4, and F5.
Resistance to Simulated Gastrointestinal Challenges
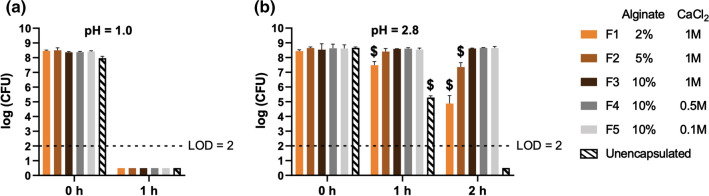
As a major targeted site for LBP delivery are the intestines,46 the upstream acidic environment in the stomach represents a considerable challenge that can decrease LBP viability and subsequently prevent their intestinal colonization.8,9 Viability loss during gastric passage will lead to reduced exposure of LBP in the intestines, and suboptimal quantities of LBP has been attributed to compromised therapeutic outcomes in the clinic.34 As such, it is critical to assess acid resistance of polymer-based systems toward LBP application in oral delivery. To mimic acid challenges, L. casei ATCC 393-encapsulating alginate beads with different alginate and CaCl2 concentrations were subjected to simulated gastric fluid (SGF) at pH = 1.0 and 2.8 at 37 °C for up to 2 h. No viable L. casei ATCC 393 were detected for both the encapsulated and unencapsulated groups after incubation at pH = 1.0 for 1 h (Fig. 6a). However, all the encapsulated groups exhibited higher viability of L. casei ATCC 393 at 1 h and 2 h when incubated at pH = 2.8 compared to the unencapsulated control (Fig. 6b). Importantly, we observed that: (i) beads with 10% alginate demonstrated the highest protection against acid challenge at 2 h, regardless of CaCl2 concentration, and (ii) lower alginate concentration decreased survival during acid challenge (Fig. 6b). These results could be explained by the denser polymer networks of the alginate beads that higher alginate concentrations provide, thereby likely reducing proton diffusion into the polymeric matrix. As a wide body of literature report complex macroscopic structures to improve acid resistance of delivery systems,10,16,24 these findings highlight that simply tuning polymer concentration offers a facile approach to addressing acid challenges. The viability of encapsulated L. casei ATCC 393 remained comparable by changing the CaCl2 concentration in the range of 0.1 M and 1 M at the fixed alginate concentration, suggesting the crosslinker concentrations did not result in detectable differences in acid resistance at 10% alginate.
Figure 6.
Acid challenge of L. casei ATCC 393 in simulated gastric fluid (SGF). (a) Viability of L. casei ATCC 393 at pH = 1.0. (b) Viability of L. casei ATCC 393 at pH = 2.8. The unencapsulated group was prepared by directly suspending L. casei ATCC 393 in 1 mL of either solution. Five representative alginate beads were analyzed in each of the replicates (n = 3). Each error bar represents standard deviation. At each time point, statistical analysis was conducted using one-way ANOVA followed by post hoc Tukey’s HSD test for pairwise comparison (statistical significance defined at p < 0.05). $: significantly different from all other groups. LOD: limit of detection.
In vitro Release
While no compelling evidence has yet to correlate LBP release profiles to in vivo therapeutic outcomes, we have previously demonstrated that slower release from an LBP depot resulted in a 1.5-fold increase of surface area coverage on ex vivo porcine intestines, as compared to bolus delivery given the same transit time.38 Increases in surface area coverage of LBPs can potentially provide two distinct advantages in vivo: (i) increased interactions between LBPs and targeted surfaces (e.g., mucus, epithelium) may improve adhesion and subsequent colonization opportunities for LBPs; (ii) enhanced systemic absorption of LBP-secreted therapeutics through epithelial barriers. As such, there is a need to understand the effects of polymer and crosslinker concentrations on LBP release toward optimizing release profiles of LBP formulations. Alginate beads were incubated in simulated intestinal fluid (SIF) as a model release condition. The phosphate ions in the SIF can competitively bind with calcium ions in crosslinked alginate and accelerate bead dissolution. We found that higher alginate concentrations prolonged release of L. casei ATCC 393 from the alginate beads, leading to 3 distinct profiles with 75% cumulative release at 2 h, 4 h, and 6 h (Fig. 7). Similarly, we observed higher CaCl2 concentrations also prolonged release (Fig. 7). Notably, F2, F3, and F4 exhibited biphasic release profiles (initial slow release followed by rapid release), implying dominance of bulk erosion during bead dissolution. The distinct release properties of LBP delivery systems may be useful in controlling LBP distribution at sites of interest in vivo. While many approaches exist to manipulate LBP release, such as modulating material choice and geometry,38 these results highlighted that varying polymer and crosslinker concentrations is also capable of tuning LBP release profiles, thus offering new insights toward achieving controlled release of LBPs. Future efforts need to focus on elucidating how different release profiles impact LBP performance in vivo, which will pave the way for translating these polymer-encapsulated LBP delivery systems to clinical application.
Figure 7.
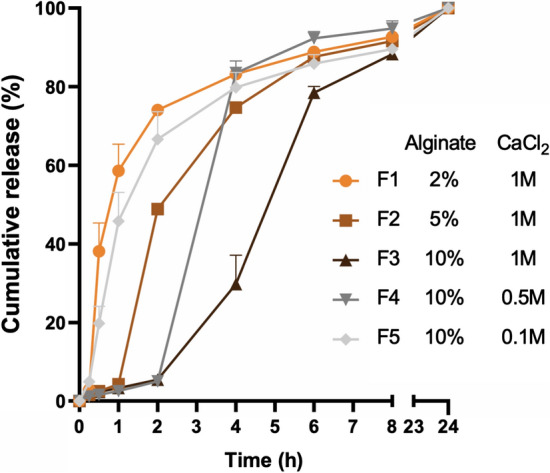
Tunable release of L. casei ATCC 393 from alginate beads in simulated intestinal fluid at 37 °C. Five representative alginate beads were analyzed in each of the replicates (n = 3). Each error bar represents standard deviation.
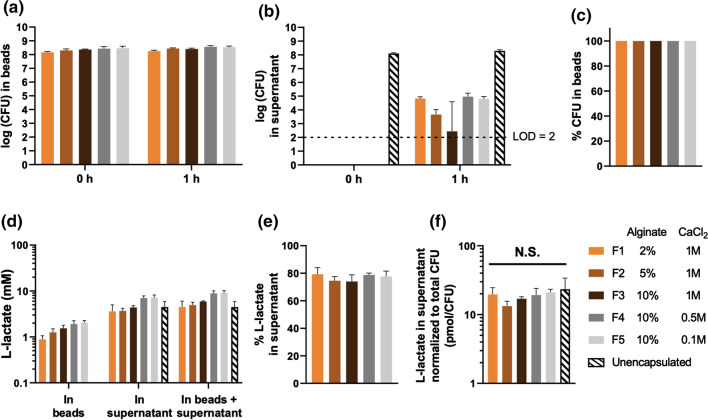
While polymer encapsulation enables acid protection and controlled release, it may also impact critical substance exchange for encapsulated LBPs, such as nutrient uptake and metabolite secretion. Notably, metabolites are widely recognized as important mediators of LBPs that modulate microbiome and host physiology.42l-lactate, one major metabolite of L. casei ATCC 393, has been reported to render anti-inflammatory effects in mammalian intestines.19 As such, we sought to quantify the effects of polymer encapsulation on l-lactate secretion of encapsulated L. casei ATCC 393. We immersed approximately 108 CFU encapsulated L. casei ATCC 393 in DeMan-Rogosa-Sharpe (MRS), a standard growth media for lactobacilli, at 37 °C for 1 h (Fig. 8a). To rule out the confounding l-lactate secreted by free L. casei ATCC 393 that were released from the alginate beads during incubation, we also examined viability of L. casei ATCC 393 in the supernatant (Fig. 8b). Quantitatively, nearly 100% of viable L. casei ATCC 393 remained encapsulated in the alginate beads at 1 h (Fig. 8c), implying that the l-lactate secreted by free L. casei ATCC 393 was negligible. We then quantified the l-lactate content in the alginate beads and supernatant (Fig. 8d). Over 70% of total l-lactate was distributed in the supernatant across all groups (Fig. 8e), suggesting crosslinked alginate allowed diffusion of metabolites into the external microenvironment. To account for the variance of CFU when comparing secreted l-lactate content across different groups, l-lactate in the supernatant was normalized to the total CFU in the corresponding group. No significant difference of l-lactate secretion was observed across groups of encapsulated and unencapsulated L. casei ATCC 393 (Figure 8f), highlighting that key metabolic activities, and secretion of metabolites, were not influenced by polymer and crosslinker concentrations. These results indicated that encapsulated L. casei ATCC 393 retained the capability of modulating the surrounding environment through metabolite secretion. Considering that l-lactate is a small molecule, this study will inspire future work on biologic-secreting LBPs, as biologics are larger in size and thus their release into the microenvironment from microbial hosts within alginate beads will be more likely to be impacted by polymer matrices as compared to small molecules. In addition, emerging efforts use encapsulation strategies to achieve biocontainment (preventing escape into external environment and avoiding safety concerns) of genetically engineered microbes while still allowing substance diffusion to support functions of these microbes.44 As such, our study will motivate evaluation of material content as an important parameter in designing delivery systems that aim to accomplish biocontainment and secretion-related functions of encapsulated microbes simultaneously.
Figure 8.
l-lactate secretion from L. casei ATCC 393-encapsulating alginate beads. (a) Viability of L. casei ATCC 393 in the alginate beads. (b) Viability of L. casei ATCC 393 in the supernatant. (c) Percentage of viable L. casei ATCC 393 in the alginate beads as compared to the summation of CFU in the alginate beads and supernatant at 1 h. (d) l-lactate content in the alginate beads and supernatant at 1 h. (e) Percentage of l-lactate in the supernatant as compared to the summation of l-lactate in the alginate beads and supernatant. (f) l-lactate content in the supernatant normalized to the summation of CFU in the alginate beads and supernatant. Five representative alginate beads were analyzed in each of the replicates (n = 3). Statistical analysis was conducted using one-way ANOVA (statistical significance defined at p < 0.05). N.S.: not significant. LOD: limit of detection.
Conclusion
In summary, by tuning alginate and CaCl2 concentrations, we demonstrate that polymer and crosslinker content modulates performance of polymer-based LBP delivery systems (Table 2). While alginate has been used for encapsulating probiotics preclinically for decades,10 alginate-encapsulated LBPs are not currently approved for clinical use; existing LBPs in clinical trials are typically suspensions or lyophilized powders.46 Although alginate delivery systems have been widely studied and even advanced into the clinic for other living therapeutic modalities, for example alginate encapsulation of mammalian cells (e.g., islet beta-cells) for transplantation,40 systematic characterization of alginate-encapsulated LBPs has yet to be performed. Importantly, unlike widely studied alginate delivery systems for mammalian cell transplantation into subcutaneous tissues, encapsulated LBPs are predominately designed for oral delivery.28 As such, compared to challenges (e.g., immunological response, fibrosis) facing the subcutaneous administered alginate systems for mammalian cell transplantation,12 the unique microenvironment and distinct delivery requirements of the GI tract may lead to LBP-specific opportunities for clinical translation of alginate systems.20 Therefore, evaluating and describing the effects of alginate and crosslinker content on encapsulated LBPs may support the eventual rational design of clinically relevant LBP formulations in future applications. Additionally, considering ongoing research of other polymer-based LBP formulations (e.g., gelatin)2 and strategies (e.g., microbial surface modification),4,47 efforts to provide a comprehensive framework toward characterizing performance of LBP formulations may motivate future research into how material properties influence encapsulated LBPs in different formulations.
Table 2.
Effects of alginate and CaCl2 concentrations on performance of L. casei ATCC 393-encapsulating alginate beads
| Alginate (2%, 5%, and 10% wt vol−1) | CaCl2 (0.1 M, 0.5 M, and 1 M) | |
|---|---|---|
| Size | Higher alginate concentrations led to larger size | Lower CaCl2 concentrations led to larger size |
| Shape | Higher alginate concentrations led to more spherical shape | Comparably spherical |
| Encapsulation efficiency | > 50%, comparable across all groups evaluated | |
| CFU Loading | Comparable across all groups evaluated | |
| Post-lyophilization/rehydration morphology | Comparable across all groups evaluated: (i) < 50% shrinkage of original diameter post lyophilization, (ii) > 60% of original diameter post rehydration at 30 min, (iii) irregular shape post lyophilization and rehydration | |
| Survival upon lyophilization | Comparable | Lower CaCl2 concentrations decreased LBP survival |
| Acid resistance | (i) comparable at pH = 1.0, (ii) higher alginate concentrations improved LBP survival at pH = 2.8 | Comparable |
| Release | Higher alginate concentrations slowed release | Higher CaCl2 concentrations slowed release |
| l-lactate secretion | Comparable across all groups evaluated | |
Materials and Methods
Materials
Sodium alginate and ethanol were purchased from Sigma-Aldrich (Missouri, USA). Calcium chloride (CaCl2), sodium hydroxide, sucrose, glutaraldehyde, phosphate-buffered saline (PBS), acetone, and dimethyl sulfoxide (DMSO) were purchased from Fisher Scientific (Massachusetts, USA). Lactobacillus casei ATCC 393 (L. casei ATCC 393), NHS-fluorescein, and DeMan-Rogosa-Sharpe (MRS) broth were purchased from Thermo Scientific (California, USA). MRS agar and skim milk powder were purchased from Becton, Dickinson and Company (New Jersey, USA). Simulated gastric fluid, enzyme-free simulated intestinal fluid (SIF), LB broth, and agar were purchased from VWR (Pennsylvania, USA).
Methods
Bacteria Growth and Quantification
L. casei ATCC 393 was inoculated from glycerol stocks into autoclaved MRS broth and incubated overnight statically at 37 °C. Before use, bacteria were collected via centrifugation at 4000 rpm for 10 min at room temperature and washed once in sterile water. OD600 values were determined by GENESYS 30 visible spectrophotometer (Thermo Scientific, California, USA) with bacteria-free media subtracted. To quantify bacterial viability, aqueous samples were serially diluted, and drop-plated (10 L) on MRS agar. Colony forming units (CFUs) were enumerated after incubation of agar plates at 37 °C for 48–60 h. To obtain each replicate of per-bead viability, five alginate beads were first homogenized in 1 mL sodium citrate (0.055 M); viability in the homogenized suspension was quantified as described above and divided by five as the per-bead viability.
Fabrication of Alginate Beads
L. casei ATCC 393 (0.3–1 × 1010 CFU/mL, final concentration) was added into alginate solutions (2%, 5%, and 10% wt vol−1) followed by vortexing to obtain homogenous suspensions. Then the suspensions were introduced dropwise into 0.1 M, 0.5 M or 1 M CaCl2 solutions (stirred at 60 rpm) at the speed of 10 mL/h through a 27-gauge needle, powered by a syringe pump (Fisher Scientific, Massachusetts, USA). After hardening in the CaCl2 solutions for 20 min, alginate beads were collected with cell strainers (40 µm mesh filters) and briefly washed in sterile water once before further experiments. To encapsulate fluorescein-labeled L. casei ATCC 393 in the alginate beads, bacteria were first incubated in 1 mg/mL NHS-fluorescein in DMSO-PBS (Vol(DMSO): Vol(PBS) = 1:9) solution at room temperature for 1 h. Then the bacteria were pelleted through centrifugation and washed in PBS three times to remove free NHS-fluorescein before addition into alginate solutions.
Characterization of Alginate Beads
For fluorescence imaging, alginate beads (5% wt vol−1 alginate, 1 M CaCl2) encapsulating fluorescein-labeled L. casei ATCC 393 were incubated in 10% vol vol−1 formaldehyde overnight at room temperature and then dehydrated in 30% wt vol−1 sucrose solution for 24 h. Next, alginate beads were frozen in optimal cutting temperature compound (Sakura Tissue-Tek) on dry ice and stored at -80 °C overnight. Samples were sliced into pieces with 3 µm in thickness and imaged with a fluorescence microscope. To obtain scanning electron microscopy (SEM) images, alginate beads were first fixed in glutaraldehyde (2.5% vol vol−1) for 1 h at room temperature followed by treatment with each of the gradient ethanol solutions (50%, 70%, 90%, and 100% vol vol−1 in water) for 10 min at room temperature. Then alginate beads were mounted onto a SEM stub with adhesive carbon tape and stored in a vacuum desiccator before imaging. Alginate beads were sectioned by a razor blade to expose cross-sections. Plain L. casei ATCC 393 was prepared by resuspending glutaraldehyde-treated and dehydrated L. casei ATCC 393 in acetone and loading an aliquot on a stub until complete solvent evaporation.
Encapsulation Efficiency (EE)
0.5 mL (V) alginate solution containing fluorescein-labeled L. casei ATCC 393 was used for bead fabrication as described above and the number (N) of alginate beads fabricated with the 0.5 mL liquid was recorded. Then five alginate beads were incubated in 1 mL (V1) sodium citrate (0.055 M) for 15 min followed by manual homogenization. The bacterial concentration in the homogenized sodium citrate solution (C1) and the original bacterial concentration in alginate solution (C) were indicated by the fluorescence signal on a plate reader at excitation/emission = 487 nm/528 nm, respectively. EE = (C1V1N)/(5CV) × 100%.
Lyophilization
For morphology analysis, L. casei ATCC 393-free alginate beads were snap frozen in liquid nitrogen and then dried on a benchtop lyophilizer overnight. Lyophilized alginate beads were rehydrated in water for 30 min at room temperature. Diameter of alginate beads, calculated as the average of three measurements in orthogonal directions, was measured by a digital caliper (World Precision Instruments, Florida, USA). To evaluate survival upon lyophilization, L. casei ATCC 393-encapsulating alginate beads were immersed in skim milk (12% wt vol−1) for 30 min at room temperature, then separated from skim milk, and lyophilized as described above. Alginate beads without incubation in skim milk were lyophilized for comparison. Unencapsulated L. casei ATCC 393 were directly lyophilized in water or skim milk (12% wt vol−1). L. casei ATCC 393 in pre-lyophilized formulations were considered as 100% viability to calculate post-lyophilization survival. Water content of L. casei ATCC 393-encapsulating, skim milk-soaked alginate beads was calculated as the weight loss before and after lyophilization on a per-bead basis.
Acid Challenge
Five alginate beads of each group were incubated in 1 mL SGF (pH = 1.0 or pH = 2.8, pH adjusted with 1 M sodium hydroxide) at 37 °C. At indicated time points, alginate beads were removed from SGF and the viability was quantified as described above.
Controlled Release
Five alginate beads encapsulating fluorescein-labeled L. casei ATCC 393 were incubated in 5 mL filter-sterilized SIF at 37 °C under rotation. At indicated time points, the release media containing alginate solids was filtered through a cell strainer (40 µm) and the collected solids in the strainer were transferred into 5 mL fresh SIF for continuous release study. 200 µL of the filtrate was read on a plate reader (excitation/emission = 487 nm/528 nm) to quantify bacterial release.
l-Lactate Measurement
Five alginate beads of each group were incubated in 1 mL MRS at 37 °C. At indicated time points, MRS was removed and centrifuged at 8000 rpm for 1 min to pellet down released bacteria. l-lactate in the supernatant was quantified with an EnzyChrom™ Lactate Assay Kit (BioAssay Systems, California, USA) according to the instruction manual. To quantify the l-lactate within alginate beads, alginate beads were isolated and homogenized in 1 mL sodium citrate (0.055 M). Then the suspension was pelleted at 8000 rpm for 1 min and l-lactate in the supernatant was quantified as described above. Unencapsulated L. casei ATCC 393 in MRS was used as control.
Statistical Analysis
Experiments were run in triplicate and data were presented as mean ± SD unless otherwise noted. Parametric one-way ANOVA and post hoc Tukey’s HSD test were used to evaluate significant differences, as indicated in relevant figure legends. α = 0.05. P values less than 0.05 were considered significantly different. All statistical analysis was performed in Prism (version 8.4.3, Graphpad Software, LLC).
Acknowledgments
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R35GM137898. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
KQ., Y.H., and A.C.A. declare that they have no conflict of interest.
Ethical Approval
No animal studies were carried out by the authors for this article. No human studies were carried out by the authors for this article.
Footnotes
Dr. Aaron C. Anselmo is an Assistant Professor in the Division of Pharmacoengineering and Molecular Pharmaceutics, in the Eshelman School of Pharmacy, at the University of North Carolina at Chapel Hill. Dr. Anselmo’s research program focuses on developing delivery systems for live biotherapeutic products (LBPs) (e.g., therapeutic microbes) to improve their colonization, control interactions with the host, and optimize the pharmacokinetic profile of their metabolites or secreted therapeutics. Dr. Anselmo has co-authored over 45 papers in journals such as Science Translational Medicine, Nature Nanotechnology, Science, Nature Reviews Drug Discovery, Nature Biomedical Engineering, PNAS, Advanced Materials, and ACS Nano. Dr. Anselmo has recently been recognized with awards such as the AIChE 35 Under 35 award, Pharmaceutics Young Investigator Award, and the Young Innovator Award in Nanobiotechnology. Dr. Anselmo’s research is supported by multiple grants from the NIH, including an NIH NIGMS R35 Maximizing Investigators’ Research Award for Early Stage Investigators. Dr. Anselmo completed his bachelor’s degree in chemical engineering at Rensselaer Polytechnic Institute, his Ph.D. in chemical engineering as an NSF GRFP Fellow at the University of California at Santa Barbara working with Professor Samir Mitragotri, and his postdoc at the Massachusetts Institute of Technology working with Professor Robert Langer.
This article is part of the 2021 CMBE Young Innovators special issue.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Acker J, McGann L. Membrane damage occurs during the formation of intracellular ice. Cryo Lett. 2001;22:241–254. [PubMed] [Google Scholar]
- 2.Annan N, Borza A, Hansen LT. Encapsulation in alginate-coated gelatin microspheres improves survival of the probiotic Bifidobacterium adolescentis 15703T during exposure to simulated gastro-intestinal conditions. Food Res. Int. 2008;41:184–193. doi: 10.1016/j.foodres.2007.11.001. [DOI] [Google Scholar]
- 3.Anselmo AC, Gokarn Y, Mitragotri S. Non-invasive delivery strategies for biologics. Nat. Rev. Drug Discov. 2019;18:19–40. doi: 10.1038/nrd.2018.183. [DOI] [PubMed] [Google Scholar]
- 4.Anselmo AC, McHugh KJ, Webster J, Langer R, Jaklenec A. Layer-by-layer encapsulation of probiotics for delivery to the microbiome. Adv. Mater. 2016;28:9486–9490. doi: 10.1002/adma.201603270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carvalho AS, Silva J, Ho P, Teixeira P, Malcata FX, Gibbs P. Relevant factors for the preparation of freeze-dried lactic acid bacteria. Int. Dairy J. 2004;14:835–847. doi: 10.1016/j.idairyj.2004.02.001. [DOI] [Google Scholar]
- 6.Cattelan G, Guerrero Gerbolés A, Foresti R, Pramstaller PP, Rossini A, Miragoli M, Caffarra Malvezzi C. Alginate formulations: current developments in the race for hydrogel-based cardiac regeneration. Front. Bioeng. Biotechnol. 2020;8:414. doi: 10.3389/fbioe.2020.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng Q, Liu L, Xie M, Li H, Ma D, Xue W. A colon-targeted oral probiotics delivery system using an enzyme-triggered fuse-like microcapsule. Adv. Healthc. Mater. 2021;10(8):2001953. doi: 10.1002/adhm.202001953. [DOI] [PubMed] [Google Scholar]
- 8.Cook MT, Tzortzis G, Charalampopoulos D, Khutoryanskiy VV. Microencapsulation of probiotics for gastrointestinal delivery. J. Control. Release. 2012;162:56–67. doi: 10.1016/j.jconrel.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Ding W, Shah N. Acid, bile, and heat tolerance of free and microencapsulated probiotic bacteria. J. Food Sci. 2007;72:M446–M450. doi: 10.1111/j.1750-3841.2007.00565.x. [DOI] [PubMed] [Google Scholar]
- 10.Dong QY, Chen MY, Xin Y, Qin XY, Cheng Z, Shi LE, Tang ZX. Alginate-based and protein-based materials for probiotics encapsulation: a review. Int. J. Food Sci. Technol. 2013;48:1339–1351. doi: 10.1111/ijfs.12078. [DOI] [Google Scholar]
- 11.Falco CY, Falkman P, Risbo J, Cárdenas M, Medronho B. Chitosan-dextran sulfate hydrogels as a potential carrier for probiotics. Carbohydr. Polym. 2017;172:175–183. doi: 10.1016/j.carbpol.2017.04.047. [DOI] [PubMed] [Google Scholar]
- 12.Farina M, Alexander JF, Thekkedath U, Ferrari M, Grattoni A. Cell encapsulation: overcoming barriers in cell transplantation in diabetes and beyond. Adv. Drug Del. Rev. 2019;139:92–115. doi: 10.1016/j.addr.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 13.Feng P, Cao Z, Wang X, Li J, Liu J. On-Demand Bacterial Reactivation by Restraining within a Triggerable Nanocoating. Adv. Mater. 2020;32:2002406. doi: 10.1002/adma.202002406. [DOI] [PubMed] [Google Scholar]
- 14.Garber K. First microbiome-based drug clears phase III, in clinical trial turnaround. Nat. Rev. Drug Discov. 2020;19:655–656. doi: 10.1038/d41573-020-00163-4. [DOI] [PubMed] [Google Scholar]
- 15.Guziewicz N, Best A, Perez-Ramirez B, Kaplan DL. Lyophilized silk fibroin hydrogels for the sustained local delivery of therapeutic monoclonal antibodies. Biomaterials. 2011;32:2642–2650. doi: 10.1016/j.biomaterials.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hébrard G, Hoffart V, Beyssac E, Cardot J-M, Alric M, Subirade M. Coated whey protein/alginate microparticles as oral controlled delivery systems for probiotic yeast. J. Microencapsul. 2010;27:292–302. doi: 10.3109/02652040903134529. [DOI] [PubMed] [Google Scholar]
- 17.Heimbuck AM, Priddy-Arrington TR, Sawyer BJ, Caldorera-Moore ME. Effects of post-processing methods on chitosan-genipin hydrogel properties. Mater. Sci. Eng. C. 2019;98:612–618. doi: 10.1016/j.msec.2018.12.119. [DOI] [PubMed] [Google Scholar]
- 18.Hong W, Zhao X, Zhou J, Suo Z. A theory of coupled diffusion and large deformation in polymeric gels. J. Mech. Phys. Solids. 2008;56:1779–1793. doi: 10.1016/j.jmps.2007.11.010. [DOI] [Google Scholar]
- 19.Iraporda C, Romanin DE, Bengoa AA, Errea AJ, Cayet D, Foligné B, Sirard J-C, Garrote GL, Abraham AG, Rumbo M. Local treatment with lactate prevents intestinal inflammation in the TNBS-induced colitis model. Front. Immunol. 2016;7:651. doi: 10.3389/fimmu.2016.00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jimenez M, Langer R, Traverso G. Microbial therapeutics: new opportunities for drug delivery. J. Exp. Med. 2019;216:1005. doi: 10.1084/jem.20190609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jonas AM, Glinel K, Behrens A, Anselmo AC, Langer RS, Jaklenec A. Controlling the growth of Staphylococcus epidermidis by layer-by-layer encapsulation. ACS Appl. Mater. Interfaces. 2018;10:16250–16259. doi: 10.1021/acsami.8b01988. [DOI] [PubMed] [Google Scholar]
- 22.Kailasapathy K. Microencapsulation of probiotic bacteria: technology and potential applications. Curr. Issues Intest. Microbiol. 2002;3:39–48. [PubMed] [Google Scholar]
- 23.Kamath KR, Park K. Biodegradable hydrogels in drug delivery. Adv. Drug Del. Rev. 1993;11:59–84. doi: 10.1016/0169-409X(93)90027-2. [DOI] [Google Scholar]
- 24.Krasaekoopt W, Bhandari B, Deeth H. The influence of coating materials on some properties of alginate beads and survivability of microencapsulated probiotic bacteria. Int. Dairy J. 2004;14:737–743. doi: 10.1016/j.idairyj.2004.01.004. [DOI] [Google Scholar]
- 25.Kurtz CB, Millet YA, Puurunen MK, Perreault M, Charbonneau MR, Isabella VM, Kotula JW, Antipov E, Dagon Y, Denney WS. An engineered E. coli Nissle improves hyperammonemia and survival in mice and shows dose-dependent exposure in healthy humans. Sci. Transl. Med. 2019;11(475):7975. doi: 10.1126/scitranslmed.aau7975. [DOI] [PubMed] [Google Scholar]
- 26.Lee BB, Ravindra P, Chan ES. Size and shape of calcium alginate beads produced by extrusion dripping. Chem. Eng. Technol. 2013;36:1627–1642. [Google Scholar]
- 27.Leslie SB, Israeli E, Lighthart B, Crowe JH, Crowe LM. Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl. Environ. Microbiol. 1995;61:3592–3597. doi: 10.1128/aem.61.10.3592-3597.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li S, Jiang W, Zheng C, Shao D, Liu Y, Huang S, Han J, Ding J, Tao Y, Li M. Oral delivery of bacteria: basic principles and biomedical applications. J. Control. Release. 2020;327:801–833. doi: 10.1016/j.jconrel.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Llopis M, Antolin M, Carol M, Borruel N, Casellas F, Martinez C, Espín-Basany E, Guarner F, Malagelada JR. Lactobacillus casei downregulates commensals’ inflammatory signals in Crohn’s disease mucosa. Inflamm. Bowel Dis. 2009;15:275–283. doi: 10.1002/ibd.20736. [DOI] [PubMed] [Google Scholar]
- 30.Lueckgen A, Garske DS, Ellinghaus A, Mooney DJ, Duda GN, Cipitria A. Enzymatically-degradable alginate hydrogels promote cell spreading and in vivo tissue infiltration. Biomaterials. 2019;217:119294. doi: 10.1016/j.biomaterials.2019.119294. [DOI] [PubMed] [Google Scholar]
- 31.Mazur P. Physical factors implicated in the death of microorganisms at subzero temperatures. Ann. N. Y. Acad. Sci. 1960;85:610–629. doi: 10.1111/j.1749-6632.1960.tb49986.x. [DOI] [PubMed] [Google Scholar]
- 32.Mazur P, Leibo SP, Chu EHY. A two-factor hypothesis of freezing injury: evidence from Chinese hamster tissue-culture cells. Exp. Cell Res. 1972;71:345–355. doi: 10.1016/0014-4827(72)90303-5. [DOI] [PubMed] [Google Scholar]
- 33.McEntee MKE, Bhatia SK, Tao L, Roberts SC, Bhatia SR. Tunable transport of glucose through ionically-crosslinked alginate gels: effect of alginate and calcium concentration. J. Appl. Polym. Sci. 2008;107:2956–2962. doi: 10.1002/app.27478. [DOI] [Google Scholar]
- 34.McGovern BH, Ford CB, Henn MR, Pardi DS, Khanna S, Hohmann EL, O’Brien EJ, Desjardins CA, Bernardo P, Wortman JR. SER-109, an investigational microbiome drug to reduce recurrence after Clostridioides difficile infection: lessons learned from a phase 2 trial. Dis: Clin. Infect; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mimee M, Citorik RJ, Lu TK. Microbiome therapeutics—advances and challenges. Adv. Drug Del. Rev. 2016;105:44–54. doi: 10.1016/j.addr.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgan CA, Herman N, White P, Vesey G. Preservation of micro-organisms by drying; a review. J. Microbiol. Methods. 2006;66:183–193. doi: 10.1016/j.mimet.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 37.Park JS, Woo DG, Sun BK, Chung H-M, Im SJ, Choi YM, Park K, Huh KM, Park K-H. In vitro and in vivo test of PEG/PCL-based hydrogel scaffold for cell delivery application. J. Control. Release. 2007;124:51–59. doi: 10.1016/j.jconrel.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 38.Qiu K, Young I, Woodburn BM, Huang Y, Anselmo AC. Polymeric films for the encapsulation, storage, and tunable release of therapeutic microbes. Adv. Healthc. Mater. 2020;9:1901643. doi: 10.1002/adhm.201901643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, Fiers W, Remaut E. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289:1352–1355. doi: 10.1126/science.289.5483.1352. [DOI] [PubMed] [Google Scholar]
- 40.Strand BL, Coron AE, Skjak-Braek G. Current and future perspectives on alginate encapsulated pancreatic islet. Stem Cells Transl. Med. 2017;6:1053–1058. doi: 10.1002/sctm.16-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strasser S, Neureiter M, Geppl M, Braun R, Danner H. Influence of lyophilization, fluidized bed drying, addition of protectants, and storage on the viability of lactic acid bacteria. J. Appl. Microbiol. 2009;107:167–177. doi: 10.1111/j.1365-2672.2009.04192.x. [DOI] [PubMed] [Google Scholar]
- 42.Suez J, Elinav E. The path towards microbiome-based metabolite treatment. Nat. Microbiol. 2017;2:17075. doi: 10.1038/nmicrobiol.2017.75. [DOI] [PubMed] [Google Scholar]
- 43.Talwalkar A, Kailasapathy K. The role of oxygen in the viability of probiotic bacteria with reference L. acidophilus and Bifidobacterium spp. Curr. Issues Intest. Microbiol. 2004;5:1–8. [PubMed] [Google Scholar]
- 44.Tang T-C, Tham E, Liu X, Yehl K, Rovner AJ, Yuk H, Isaacs FJ, Zhao X, Lu TK. Tough hydrogel-based biocontainment of engineered organisms for continuous, self-powered sensing and computation. BioRxiv. 2020 doi: 10.1101/2020.02.11.941120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tien M-T, Girardin SE, Regnault B, Le Bourhis L, Dillies M-A, Coppée J-Y, Bourdet-Sicard R, Sansonetti PJ, Pédron T. Anti-inflammatory effect of Lactobacillus casei on Shigella-infected human intestinal epithelial cells. J. Immunol. 2006;176:1228–1237. doi: 10.4049/jimmunol.176.2.1228. [DOI] [PubMed] [Google Scholar]
- 46.Vargason AM, Anselmo AC. Clinical translation of microbe-based therapies: current clinical landscape and preclinical outlook. Bioeng. Transl. Med. 2018;3:124–137. doi: 10.1002/btm2.10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vargason AM, Santhosh S, Anselmo AC. Surface modifications for improved delivery and function of therapeutic bacteria. Small. 2020;16:2001705. doi: 10.1002/smll.202001705. [DOI] [PubMed] [Google Scholar]
- 48.Voo W-P, Ooi C-W, Islam A, Tey B-T, Chan E-S. Calcium alginate hydrogel beads with high stiffness and extended dissolution behaviour. Eur. Polym. J. 2016;75:343–353. doi: 10.1016/j.eurpolymj.2015.12.029. [DOI] [Google Scholar]
- 49.Wang LL-W, Janes ME, Kumbhojkar N, Kapate N, Clegg JR, Prakash S, Heavey MK, Zhao Z, Anselmo AC, Mitragotri S. Cell therapies in the clinic. Bioeng. Transl. Med. 2021 doi: 10.1002/btm2.10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Young VB. Therapeutic manipulation of the microbiota: past, present, and considerations for the future. Clin. Microbiol. Infect. 2016;22:905–909. doi: 10.1016/j.cmi.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]