Abstract
Background
We have previously demonstrated S-1 is non-inferior to taxane with respect to overall survival as first-line chemotherapy for HER2-negative metastatic breast cancer. We aimed to confirm whether S-1 is also non-inferior to anthracycline-containing regimens in the same setting.
Methods
We conducted an open-label, non-inferiority, Phase 3 study. Individuals who had HER2-negative metastatic breast cancer, had received no chemotherapy for advanced disease and had endocrine therapy resistance, were randomly assigned to the anthracycline-containing regimens or S-1. The primary endpoint was overall survival. A pre-planned combined analysis of our two Phase 3 studies was also carried out.
Results
We enrolled 230 patients (anthracycline, n = 115; S-1, n = 115). Median overall survival was 30.1 months (95% CI 24.9–35.8) with the S-1 group and 33.7 months (95% CI 25.5–36.9) with the anthracycline group. The HR for the anthracycline group was 1.09 (95% CI 0.80–1.48). The combined analysis constituted 814 patients (395 assigned to standard treatment (anthracycline or taxane); 419 assigned to S-1). Median overall survival was 36.3 months in the standard treatment group and 32.7 months in the S-1 group. S-1 was non-inferior to standard treatment in terms of overall survival (HR 1.06 (95% CI 0.90–1.25); P non-inferiority = 0.0062).
Conclusions
S-1 could be considered a new treatment option for first-line chemotherapy for patients with HER2-negative metastatic breast cancer.
Clinical trial registration
The University Hospital Medical Information Network, Japan: UMIN000005449. This trial was registered on 15 April, 2011.
Subject terms: Breast cancer, Cancer therapy
Background
Although several therapies are available for patients with HER2-negative locally recurrent or metastatic breast cancer, no gold standard first-line treatment exists. However, anthracycline-containing regimens or taxane have been considered as standard treatment as first-line chemotherapy for this disease [1].
Orally administered drugs are generally more convenient than intravenous drugs [2]. S-1 and capecitabine are both oral fluorouracil derivatives and are widely used in Japan. S-1 is a combination drug, based on a biochemical modification of fluorouracil, containing tegafur, gimeracil, and oteracil in a molar ratio of 1:0·4:1 [3]. This combination enables the fluorouracil concentration to be increased while avoiding gastrointestinal toxicity.
We conducted a Phase 3 trial and reported that S-1 was non-inferior to taxane in terms of overall survival (OS), and it significantly improved health-related quality of life, when given as first-line chemotherapy for metastatic breast cancer (the SELECT BC trial) [4].
Next, we conducted a similar trial (SELECT BC-CONFIRM, UMIN000005449), which compared OS following anthracycline-containing regimens versus S-1 as first-line chemotherapy for HER2-negative metastatic breast cancer. SELECT BC-CONFIRM trial was designed to confirm the results of the SELECT BC trial and to perform a pre-planned combined analysis of two randomised trials.
Methods
Study design and patients
The SELECT BC-CONFIRM is Phase 3, randomised, open-label, parallel-group, non-inferiority studies carried out across 161 hospitals in Japan.
Eligible patients were females aged 20–75 years with histologically diagnosed HER2-negative and endocrine treatment-resistant breast cancer, with metastatic disease at presentation or recurrence after surgery, who had at least one assessable lesion, and an Eastern Cooperative Oncology Group performance status of 0 or 1. Previous chemotherapy was not allowed except preoperative or postoperative adjuvant use of taxane, anthracycline or oral fluorouracil before 24 weeks from enrollment.
Local investigators judged endocrine-resistant condition from the hormone therapy history of the patient. The main exclusion criteria were pregnant, breastfeeding, HER2-positive tumours, metastases requiring immediate treatment or life-threatening status, extensive liver metastases, lymphatic pulmonary metastases associated with subjective symptoms or pleural effusion, ascites or pericardial effusion requiring emergency treatment.
The study protocol was approved by the independent ethics committee of each study site and was performed in accordance with the Ethical Guidelines for Clinical Research of the Japanese Ministry of Health, Labor and Welfare, and the Declaration of Helsinki. All patients provided written informed consent. The study design and patients are the almost same as SELECT BC, as described previously [4].
Randomisation and masking
Eligible patients were randomly assigned (1:1) to receive either S-1 or anthracycline. The stratification factors were institution, presence or absence of liver metastasis, the sensitivity of oestrogen and progesterone receptor, treatment history of taxanes, oral fluorouracil or anthracycline, and the period from surgery to recurrence (<2 years, 2–5 years, ≥5 years, no surgery).
Treatment
Physicians could choose one regimen if the patients assigned to the anthracycline group as following: (a) doxorubicin 40–60 mg/m2 /cyclophosphamide 500 mg/m2 (with/without fluorouracil) at 3- to 4-week intervals; (b) epirubicin 60–100 mg/m2 /cyclophosphamide 500 mg/m2 (with/without fluorouracil) at 3–4-week intervals. S-1 was administered orally twice daily from day 1 to day 28, followed by 2 weeks of rest. The initial dose of S-1 was determined according to the patient’s body surface area at the time of registration: <1.25 m2, 80 mg/day; ≥1.25 to <1.5 m2, 100 mg/day; and ≥1.5 m2, 120 mg/day.
Patient continued treatment until she met one of the discontinuation criteria; tumour progression, unacceptable toxic effects, or completion of six courses (18 weeks in 3 weekly schedules or 24 weeks in 4 weekly schedule) in the standard regimen group or four courses (24 weeks) in the S-1 group. The patient could continue chemotherapy beyond six courses if the physician decided to need. Physician could treat another cytotoxic drug as second-line treatment after failure of the study intervention. The limited regimens stipulated in the protocol were allowed to treatment as second-line therapy. When treatment was resumed after a break it would be at a dose that was one level lower (Supplementary information, Criteria for suspending administration, dose reduction, and discontinuation, (9–12). In each treatment group, protocol treatment was discontinued if the patient with the minimum dose met any of the dose reduction criteria during treatment.
Tumour assessment based on RECIST criteria was experimented using radiographic or other imaging techniques after every three-treatment courses in the standard regimen group and every two-treatment courses in the S-1 group during protocol treatment. The patient received laboratory and physical assessments for peripheral blood and biochemistry at baseline and on day 1 of each treatment cycle. Physicians continuously monitored adverse events during protocol treatment. Health-related quality of life was assessed with the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core-30 (EORTC QLQ-C30) [5, 6] at baseline and every 2 months after the start of the protocol treatment. From the second year of treatment and thereafter until death, EuroQol 5 Dimension (EQ-5D) [7, 8] was also assessed every 6 months. We graded adverse events according to the Common Terminology Criteria for Adverse Events (version 3.0).
Study endpoints
The primary endpoint was overall survival, defined as the time from randomisation until death. Progression-free survival, time to treatment failure, safety and health-related quality of life were also assessed as secondary endpoints. We defined progression-free survival as the period from the date of randomisation to the earliest date of disease progression or death from any cause. In addition, we defined time to treatment failure as the period from the date of randomisation to the earlier date on which patients met progression-free survival definition or withdrawal of study treatment for any reason.
Statistical analysis
We designed the SELECT BC study and the subsequent SELECT BC-CONFIRM study to determine whether S-1 was non-inferior to standard treatment (taxane or anthracycline-containing regimens), using the threshold of hazard ratio (HR) of 1.333. The SELECT BC-CONFIRM study was designed based on the Bayesian posterior probability that does not exceed the threshold of 1.333; on the basis of the non-informative prior distribution, a posterior probability of 90% would require a total of 172 events (deaths). A patient accrual time of 2.5 years and a mean follow-up time of 4.5 years would require a total of 200 participants. A pooled analysis of SELECT BC and SELECT BC-CONFIRM was predefined if a posterior probability exceeded the threshold of 90% in the SELECT BC-CONFIRM study. We did Bayesian analysis of the log HR on the basis of the non-informative prior distribution.
The efficacy analysis groups comprised the full analysis set defined as all randomised participants those who treated at least one study drug and had available data after randomisation. For the safety analysis, we included all randomised participants who received ≧ 1 dose of study drug. Median time-to-event and HRs were assessed with the Kaplan–Meier method and Cox proportional hazards model, respectively, and the treatment group (S-1 group vs. standard treatment group) was the only covariate. For safety analysis, the proportions of adverse events of Grade 3 or higher in each group (Mantel test) between the taxane, anthracycline, and S-1 groups were compared and calculated P values.
We analysed the health-related quality of life using linear mixed-effect models adjusting for baseline score, assuming a compound symmetry structure for error term, to compare the average difference between groups, assumed to be common across 12 months.
We set the significance level as 0.05. We performed all statistical analyses using SAS software (version 9.4). The SELECT BC-CONFIRM is registered with the University Hospital Medical Information Network, Japan, number UMIN000005449.
Results
Patient population
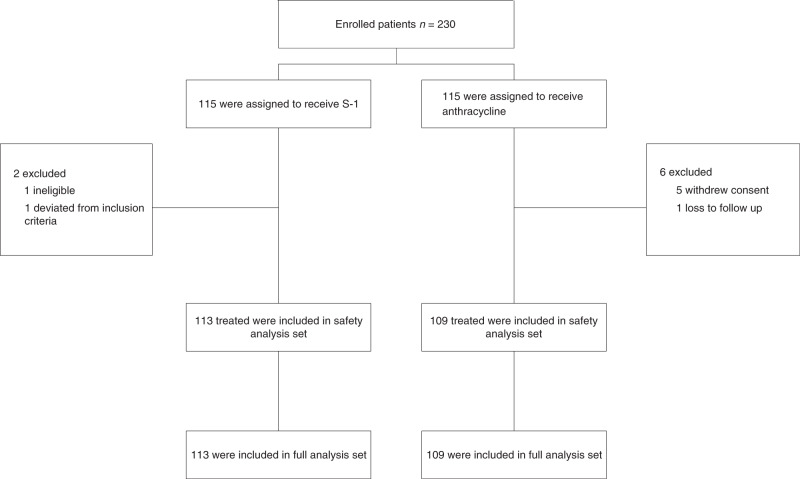
Between Jun 2011 and Dec 2013, we enrolled 230 patients in SELECT BC-CONFIRM: 115 were randomly assigned to the anthracycline group and 115 were to the S-1 group. One patient was ineligible for the efficacy analysis and seven patients did not start protocol treatment (Fig. 1). The baseline characteristics of patients are shown in Table 1.
Fig. 1.
Consort diagram for SELECT BC-CONFIRM.
Table 1.
Baseline characteristics
| SELECT BC-CONFIRM* | Combined analysis* | |||
|---|---|---|---|---|
| S-1 | Anthracycline | S-1 | Standard | |
| Characteristics | (n = 113) | (n = 109) | (n = 419) | (n = 395) |
| Median age (range) | 59.9 (31–75) | 61.0 (32–75) | 57.7 (29–75) | 58.2 (21–75) |
| Hormonal receptor status | ||||
| Positive | 92 (81.4) | 86 (78.9) | 315 (75.2) | 299 (75.7) |
| Negative | 21 (18.6) | 23 (21.1) | 90 (21.5) | 90 (22.8) |
| Liver metastasis | ||||
| Yes | 47 (41.6) | 46 (42.2) | 150 (35.8) | 142 (35.9) |
| No | 66 (58.4) | 63 (57.8) | 269 (64.2) | 253 (64.1) |
| HER2** | ||||
| Negative | 102 (90.3) | 97 (89.0) | 384 (91.6) | 361 (91.4) |
| Unknown | 11 (9.7) | 12 (11.0) | 35 (8.4) | 34 (8.6) |
| Components of (neo)adjuvant treatment | ||||
| Oral fluoropyrimidine | 12 (10.6) | 14 (12.8) | 47 (11.2) | 53 (13.4) |
| Taxane# | 31 (27.4) | 30 (27.5) | 111 (26.5) | 110 (27.8) |
| Endocrine therapy | 64 (56.6) | 65 (59.6) | 233 (55.6) | 235 (59.5) |
| Metastasis after surgery | ||||
| <2 yrs | 14 (12.4) | 16 (14.7) | 74 (17.7) | 73 (18.5) |
| ≥2 yrs, 5 < yrs | 30 (26.5) | 26 (23.9) | 133 (31.7) | 124 (31.4) |
| ≥5 yrs | 42 (37.2) | 42 (38.5) | 136 (32.5) | 128 (32.4) |
| Unknown | 0 (0.0) | 0 (0.0) | 2 (0.5) | 0 (0.0) |
| Without surgery | 27 (23.9) | 25 (22.9) | 74 (17.7) | 70 (17.7) |
*There were no significant differences between the groups in any of the characteristics listed in this table.
**HER2 denotes the human epidermal growth factor receptor.
#Taxane was docetaxel or paclitaxel.
Efficacy
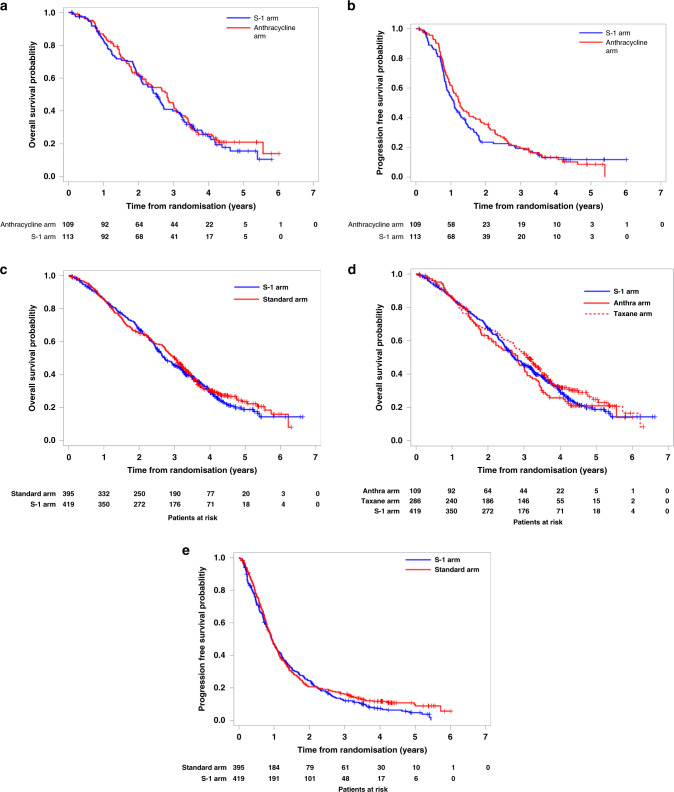
Median overall survival was 30.1 months (95% CI 24.9–35.8) with the S-1 group and 33.7 months (95% CI 25.5–36.9) with the anthracycline group (Fig. 2a). The median follow-up for the full analysis set was 28.9 months (IQR 15.4–42.4). The HR for the anthracycline group was 1.09 (95% CI 0.80–1.48), and so the estimated predictive posterior probability that the HR do not exceed the threshold 1.333 was 90.27%. Thus, the results of SELECT BC-CONFIRM appear to confirm the similar results were obtained, as we pre-planned. Progression-free survival was 15.2 months (95% CI 12.5–18.4) in the S-1 group and 13.1 months (95% CI 10.6–15.5) in the anthracycline group (Fig. 2b).
Fig. 2. Kaplan–Meier curves.
a Overall survival in SELECT BC-CONFIRM study. b Progression-free survival in SELECT BC-CONFIRM study. c Overall survival compared with standard arm. d Overall survival compared with anthracycline and taxane arm, respectively. e Progression-free survival compared with standard arm.
Combined analysis
A total of 848 patients were included in the combined analysis from SELECT BC and SELECT BC-CONFIRM trials (Supplementary Fig. 1). Baseline characteristics were similar in each group (Table 1). In the anthracycline group, 28 patients received doxorubicin and 81 received epirubicin. Of 814 patients in the full analysis set, 563 had died by the data cutoff date (2013/6/15 for SELECT BC, 2017/10/31 for SELECT BC-CONFIRM): 291 in the S-1 group and 272 in the standard treatment group.
Median OS was 32.7 months (95% CI 30.4–37.0) in the S-1 group and 36.3 months (32.9–38.8) in the standard treatment group. S-1 was non-inferior to standard treatment in terms of OS (HR 1.06 (95% CI 0.90–1.25); P non-inferiority = 0.0062; Fig. 2c, d). A sensitivity analysis of overall survival in the intention-to-treat population (n = 848) supported our primary findings (HR 1.05 [95% CI 0.89–1.24]; P non-inferiority = 0.0046). The assumption of proportionality of the hazards between the groups was confirmed by the log–log plot (Supplementary Fig. 2).
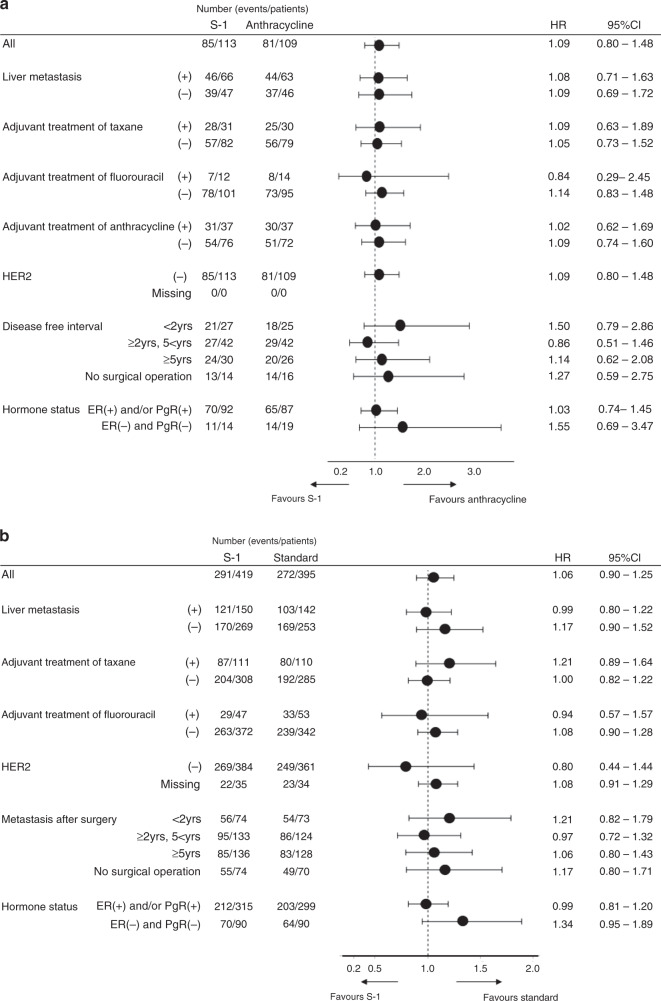
Median progression-free survival and time to treatment failure are shown in Fig. 2e and Supplementary Fig. 3. Exploratory subgroup analysis of overall survival is shown in Fig. 3a, b.
Fig. 3. A forest-plot analysis of overall survival.
a for the population of SELECT BC-CONFIRM study, b for combined analysis population.
The second-line and third-line treatments according to treatment groups are shown in Supplementary Table 1.
Toxicity
We assessed safety in 420 patients in the S-1 group and 399 in the standard treatment group (Table 2). The most common grade 3 or worse adverse events were neutropenia (29 (7%) of 420 patients in the S-1 group vs. 19 (5%) of 399 patients in the standard treatment group), febrile neutropenia (7 (2%) vs. 21 (5%)), fatigue (17 (4%) vs. 18 (5%)), and oedema (1 (<1%) vs. 12 (4%)). Dose reductions because of adverse events occurred in 46 (11.5%) of 420 patients in the S-1 group versus 61 (14.5%) of 399 patients in the standard treatment group. Treatments were discontinued because of adverse events in 24 (5.7%) patients in the S-1 group versus 26 (6.6%) patients in the standard treatment group. Treatment-related deaths were reported in two (1%) patients in the standard treatment group (one hypersensitivity reaction and one unknown). No treatment-related deaths were reported in the S-1 group.
Table 2.
Adverse events in the safety population.
| S-1 (n = 420) | Standard | CMH test* | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taxane (n = 290) | Anthracycline (n = 109) | ||||||||||||||||||
| Grade 1–2 | Grade 3 | Grade 4 | Grade 1–2 | Grade 3 | Grade 4 | Grade 1–2 | Grade 3 | Grade 4 | P value | ||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | ||
| Haematological | |||||||||||||||||||
| Leucopenia | 184 | 43.8% | 7 | 1.7% | 2 | 0.5% | 72 | 24.8% | 6 | 2.1% | 1 | 0.3% | 44 | 40.4% | 7 | 6.4% | 2 | 1.8% | <0.0001 |
| Neutropenia | 148 | 35.2% | 28 | 6.7% | 1 | 0.2% | 58 | 20.0% | 6 | 2.1% | 3 | 1.0% | 25 | 22.9% | 8 | 7.3% | 2 | 1.8% | <0.0001 |
| Thrombocytopenia | 143 | 34.0% | 3 | 0.7% | 2 | 0.5% | 13 | 4.5% | 0 | 0.0% | 0 | 0.0% | 8 | 7.3% | 1 | 0.9% | 0 | 0.0% | 0.0001 |
| Haemoglobin | 213 | 50.7% | 8 | 1.9% | 0 | 0.0% | 159 | 54.8% | 5 | 1.7% | 0 | 0.0% | 62 | 56.9% | 8 | 7.3% | 0 | 0.0% | <0.0001 |
| ALT | 188 | 44.8% | 2 | 0.5% | 0 | 0.0% | 70 | 24.1% | 1 | 0.3% | 1 | 0.3% | 33 | 30.3% | 0 | 0.0% | 1 | 0.9% | <0.0001 |
| Bilirubin | 177 | 42.1% | 2 | 0.5% | 0 | 0.0% | 11 | 3.8% | 1 | 0.3% | 0 | 0.0% | 2 | 1.8% | 2 | 1.8% | 0 | 0.0% | <0.0001 |
| Non-haematological | |||||||||||||||||||
| Fatigue | 166 | 39.5% | 17 | 4.0% | 0 | 0.0% | 141 | 48.6% | 12 | 4.1% | 0 | 0.0% | 59 | 54.1% | 6 | 5.5% | 0 | 0.0% | 0.0256 |
| Alopecia | 18 | 4.3% | 0 | 0.0% | 0 | 0.0% | 220 | 75.9% | 0 | 0.0% | 0 | 0.0% | 85 | 78.0% | 0 | 0.0% | 0 | 0.0% | <0.0001 |
| Oedema | 31 | 7.4% | 1 | 0.2% | 0 | 0.0% | 99 | 34.1% | 12 | 4.1% | 0 | 0.0% | 9 | 8.3% | 0 | 0.0% | 0 | 0.0% | <0.0001 |
| Motor neuropathy | 10 | 2.4% | 3 | 0.7% | 0 | 0.0% | 19 | 6.6% | 2 | 0.7% | 1 | 0.3% | 2 | 1.8% | 0 | 0.0% | 0 | 0.0% | 0.0327 |
| Sensory neuropathy | 42 | 10.0% | 2 | 0.5% | 0 | 0.0% | 134 | 46.2% | 8 | 2.8% | 1 | 0.3% | 15 | 13.8% | 0 | 0.0% | 0 | 0.0% | <0.0001 |
| Arthralgia | 31 | 7.4% | 1 | 0.2% | 0 | 0.0% | 62 | 21.4% | 0 | 0.0% | 0 | 0.0% | 7 | 6.4% | 1 | 0.9% | 0 | 0.0% | <0.0001 |
| Myalgia | 39 | 9.3% | 0 | 0.0% | 0 | 0.0% | 63 | 21.7% | 0 | 0.0% | 1 | 0.3% | 10 | 9.2% | 1 | 0.9% | 0 | 0.0% | <0.0001 |
| Allergy | 19 | 4.5% | 0 | 0.0% | 0 | 0.0% | 19 | 6.6% | 4 | 1.4% | 0 | 0.0% | 2 | 1.8% | 0 | 0.0% | 0 | 0.0% | 0.006 |
| Febrile neutropenia | 0 | 0.0% | 6 | 1.4% | 1 | 0.2% | 0 | 0.0% | 8 | 2.8% | 2 | 0.7% | 0 | 0.0% | 10 | 9.2% | 1 | 0.9% | 0.0002 |
| Fever | 29 | 6.9% | 0 | 0.0% | 0 | 0.0% | 28 | 9.7% | 1 | 0.3% | 0 | 0.0% | 23 | 21.1% | 1 | 0.9% | 0 | 0.0% | <0.0001 |
| Diarrhoea | 133 | 31.7% | 17 | 4.0% | 0 | 0.0% | 53 | 18.3% | 4 | 1.4% | 0 | 0.0% | 14 | 12.8% | 1 | 0.9% | 0 | 0.0% | <0.0001 |
| Mucositis | 109 | 26.0% | 4 | 1.0% | 0 | 0.0% | 45 | 15.5% | 0 | 0.0% | 0 | 0.0% | 41 | 37.6% | 2 | 1.8% | 0 | 0.0% | <0.0001 |
| Nausea | 154 | 36.7% | 6 | 1.4% | 0 | 0.0% | 63 | 21.7% | 3 | 1.0% | 0 | 0.0% | 60 | 55.0% | 3 | 2.8% | 0 | 0.0% | <0.0001 |
| Vomiting | 51 | 12.1% | 3 | 0.7% | 0 | 0.0% | 27 | 9.3% | 2 | 0.7% | 0 | 0.0% | 20 | 18.3% | 4 | 3.7% | 0 | 0.0% | 0.0012 |
| Anorexia | 162 | 38.6% | 16 | 3.8% | 0 | 0.0% | 84 | 29.0% | 4 | 1.4% | 0 | 0.0% | 60 | 55.0% | 2 | 1.8% | 0 | 0.0% | <0.0001 |
*Row mean score test of Cochran–Mantel–Haenszel (CMH) test.
Health-related outcome
There were no significant differences between the S-1 and anthracycline groups (3.2 (−2.3 to 8.7); P = 0.257; Fig. 4); this was also the case for other subscales (Supplementary Fig. 4). The difference between the S-1 and taxane groups were reported previously [4].
Fig. 4. Change from baseline of health-related quality of life (global health status).
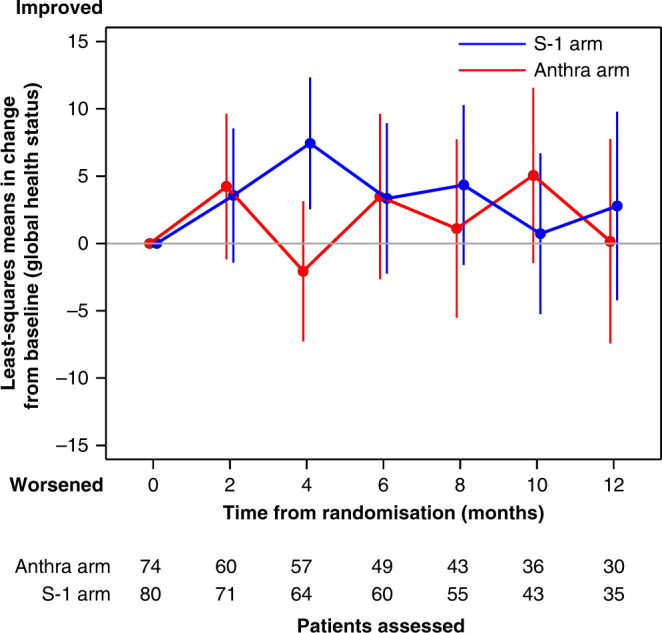
Mean changes from baseline scores in health-related quality of life compared with anthracycline or taxane arm.
Discussion
Our findings show that S-1 is non-inferior to the standard treatment group, anthracycline-containing regimens or taxane, with respect to overall survival and almost similar with regard to its effects on health-related quality of life as first-line chemotherapy for patients with HER2-negative metastatic breast cancer. There were no significant differences in progression-free survival or time to treatment failure between the treatment groups.
A reasonable interpretation of these data is indeed that S-1 is a reasonable treatment first-line option, being active in metastatic breast cancer and with the likelihood that many patients will receive an anthracycline or taxane subsequently so that sequence may not be especially important.
This study was designed to provide broad applicability to clinical practice. Our pragmatic approach allowed various administration methods and doses in the standard treatment group as long as they were within the ranges stipulated in advance. There were almost no clinical differences in the survival outcomes of patients receiving taxane or anthracycline; regardless of their administration intervals (Supplementary Fig. 5).
The TURANDOT trial [9, 10] showed that bevacizumab plus capecitabine is non-inferior to bevacizumab plus paclitaxel with respect to overall survival as a first-line treatment of HER2-negative metastatic breast cancer. The efficacy of an oral fluoropyrimidine monotherapy is not clear in this study. Although several studies of the position of oral fluoropyrimidine have been carried out [11–14], anthracycline or taxane-based regimens are usually considered as the standard therapy [1]. Several guidelines for metastatic breast cancer recommended anthracycline or taxanes as the first-line chemotherapy, and we used these treatments accordingly in practice [1, 15]. This is also the same situation in Japan [16]. However, better treatment is needed in terms of convenience of administration and in order to reduce the frequency of adverse events such as alopecia, peripheral neuropathy, oedema and cardiotoxicity. As one of the answers to these clinical issues, several trials reported the utility of capecitabine for front line chemotherapy with metastatic breast cancer [17–19]. Capecitabine showed equal or better effectiveness for paclitaxel or cyclophosphamide, methotrexate and fluorouracil (CMF) through the patients' number was limited. They also reported that capecitabine had a different safety profile compared with classical regimens, and it is well tolerable. In this way, although it has been suggested that oral fluorouracil may be able to solve the convenience and safety issues of classical regimens, we needed evidence from large clinical trials.
In the SELECT BC trial, we previously showed that S-1 was superior to taxane with respect to health-related quality of life. Although there were no significant differences between the S-1 and anthracycline groups in health-related quality of life, anthracycline was associated with a higher risk of cardiotoxicity due to cumulative dosing. So, S-1 can be a reasonable choice in terms of patient satisfaction comparing anthracycline or taxane.
As stated in “Methods”, we calculated the necessary sample size based on different methods for SELECT BC and SELECT BC-CONFIRM. In the latter study, the Bayesian posterior probability that did not exceed the threshold of 1.333 was 90.3%, which was higher than the predefined threshold of 90%. The HRs obtained in SELECT BC and SELECT BC-CONFIRM were 1.05 (95% CI 0.86–1.27) and 1.09 (95% CI 0.80–1.48), respectively. Because their point estimates were similar and the Bayesian posterior probability obtained in the latter study was higher than the predefined threshold, we conducted a combined analysis of the two studies. The analysis revealed an HR of 1.06 (95% CI 0.90–1.25, non-inferiority P = 0.0062). Though the P value must be interpreted with caution, we consider that the above HR and relatively precise 95% CI obtained from the combined analysis emphasise the non-inferiority of S-1 compared to standard treatment.
The upper limit of the non-inferiority margin was set at 1.333 by the executive committee of this study according to the medical judgement of a clinically appropriate in view of the convenience of S-1 administration. This margin is within such range in other trials [9, 10, 20].
Subgroup analysis of overall survival suggests that standard treatment was more effective than S-1 in patients with triple-negative breast cancer; however, such patients constituted only 20% of the full analysis set and therefore careful interpretation is needed.
We acknowledge there are several limitations in this study. First, the dose intensity of each chemotherapeutic agent in the anthracycline group varies in a certain range and heterogeneity of the control arm exists. The heterogeneity of the anthracycline group may mean patients in the control arm have received sub-optimal therapy whereas those in the S-1 group have received optimal/approved doses. Second, S-1 has not been widely used outside Japan but both S-1 and capecitabine are equally effective and well-tolerated treatments in patients with metastatic breast cancer due to data comparing 2 compounds directly [21].
However, our results provide much-needed evidence for the benefits of oral fluorouracil in the treatment of patients with metastatic breast cancer. This drug has been used in clinical practice on the basis of consensus among medical practitioners, but until our current study, the rationale for doing so has lacked sufficient supporting clinical evidence.
In summary, S-1 is non-inferior to taxane or anthracycline with respect to overall survival as a first-line treatment for HER2-negative metastatic breast cancer. S-1 can be considered as a new treatment option for this setting.
Supplementary information
Acknowledgements
We thank all the patients, their families and the investigators who participated in this study.
Author contributions
HM contributed to the design of the study. HM, YU, TW, YS, RN, TT and YH were members of the executive committee that oversaw the conduct of the study. HM, HA, TW, YP, MT, YS, RN, TT, TF and YH contributed substantially to patient recruitment. HM and YU contributed to data collection. HM contributed to data review. YU and TK contributed to data analysis. All authors contributed to data interpretation. HM contributed to the writing of the report. All authors contributed to review of the report and approved the final submitted version.
Funding information
This study was sponsored by the Comprehensive Support Project for Oncology Research (CSPOR) of the Public Health Research Foundation. The research fund was provided to CSPOR by Taiho Pharmaceutical Company Limited under the study contract. Taiho Pharmaceutical took no part in this study other than providing information relevant to the proper use of the study drug.
Data availability
All datasets used and analysed during this study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The studies were done in accordance with the Ethical Guidelines for Clinical Research of the Japanese Ministry of Health, Labor and Welfare, and the Declaration of Helsinki. All participants gave written informed consent. An independent ethics committee for each participating site approved the protocol and any modifications. A full list of the ethical committees can be found in the Supplementary Information.
Consent to publish
Not applicable.
Competing interests
HM has received personal fees from Daiichi Sankyo, Taiho, and Takeda; funding from the Japanese government, Pfizer, and Daiichi Sankyo, outside the submitted work. MT has received personal fees from Taiho, Eisai, Pfizer, Astra Zeneca, Kyowa Kirin, Nippon Kayaku and Eli Lilly. TT has received personal fees from Taiho, Chugai Kyowa Hakko Kirin, Eisai, Pfizer, Novartis, Astra Zeneca and Takeda, outside the submitted work. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01531-6.
References
- 1.Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, André F, et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4)dagger. Ann Oncol. 2018;29:1634–57. doi: 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sparreboom A, de Jonge MJ, Verweij J. The use of oral cytotoxic and cytostatic drugs in cancer treatment. Eur J Cancer. 2002;38:18–22. doi: 10.1016/S0959-8049(01)00322-7. [DOI] [PubMed] [Google Scholar]
- 3.Shirasaka T, Nakano K, Takechi T, Satake H, Uchida J, Fujioka A, et al. Antitumor activity of 1 M tegafur-0.4 M 5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against human colon carcinoma orthotopically implanted into nude rats. Cancer Res. 1996;56:2602–6. [PubMed] [Google Scholar]
- 4.Takashima T, Mukai H, Hara F, Matsubara N, Saito T, Takano T, et al. Taxanes versus S-1 as the first-line chemotherapy for metastatic breast cancer (SELECT BC): an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol. 2016;17:90–98. doi: 10.1016/S1470-2045(15)00411-8. [DOI] [PubMed] [Google Scholar]
- 5.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi K, Takeda F, Teramukai S, Gotoh I, Sakai H, Yoneda S, et al. A cross-validation of the European Organization for Research and Treatment of Cancer QLQ-C30 (EORTC QLQ-C30) for Japanese with lung cancer. Eur J Cancer. 1998;34:810–5. doi: 10.1016/S0959-8049(97)00395-X. [DOI] [PubMed] [Google Scholar]
- 7.EuroQol Group. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 8.Tsuchiya A, Ikeda S, Ikegami N, Nishimura S, Sakai I, Fukuda T, et al. Estimating an EQ-5D population value set: the case of Japan. Health Econ. 2002;11:341–53. doi: 10.1002/hec.673. [DOI] [PubMed] [Google Scholar]
- 9.Lang I, Brodowicz T, Ryvo L, Kahan Z, Greil R, Beslija S, et al. Bevacizumab plus paclitaxel versus bevacizumab plus capecitabine as first-line treatment for HER2-negative metastatic breast cancer: interim efficacy results of the randomised, open-label, non-inferiority, phase 3 TURANDOT trial. Lancet Oncol. 2013;14:125–33. doi: 10.1016/S1470-2045(12)70566-1. [DOI] [PubMed] [Google Scholar]
- 10.Zielinski C, Lang I, Inbar M, Kahán Z, Greil R, Beslija S, et al. Bevacizumab plus paclitaxel versus bevacizumab plus capecitabine as first-line treatment for HER2-negative metastatic breast cancer (TURANDOT): primary endpoint results of a randomised, open-label, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17:1230–9. doi: 10.1016/S1470-2045(16)30154-1. [DOI] [PubMed] [Google Scholar]
- 11.Welt A, Marschner N, Lerchenmueller C, Decker T, Steffens CC, Koehler A, et al. Capecitabine and bevacizumab with or without vinorelbine in first-line treatment of HER2/neu-negative metastatic or locally advanced breast cancer: final efficacy and safety data of the randomised, open-label superiority phase 3 CARIN trial. Breast Cancer Res Treat. 2016;156:97–107. doi: 10.1007/s10549-016-3727-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rochlitz C, Bigler M, von Moos R, Bernhard J, Matter-Walstra K, Wicki A, et al. SAKK 24/09: safety and tolerability of bevacizumab plus paclitaxel vs. bevacizumab plus metronomic cyclophosphamide and capecitabine as first-line therapy in patients with HER2-negative advanced stage breast cancer—a multicenter, randomized phase III trial. BMC Cancer. 2016;16:780. doi: 10.1186/s12885-016-2823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cinieri S, Chan A, Altundag K, Vandebroek A, Tubiana-Mathieu N, Barnadas A, et al. Final results of the randomized phase II NorCap-CA223 trial comparing first-line all-oral versus taxane-based chemotherapy for HER2-negative metastatic breast cancer. Clin Breast Cancer. 2017;17:91–99. doi: 10.1016/j.clbc.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Xu B, Yuan P, Ma F, Li Q, Zhang P, et al. Capecitabine combined with docetaxel versus vinorelbine followed by capecitabine maintenance medication for first-line treatment of patients with advanced breast cancer: phase 3 randomized trial. Cancer. 2015;121:3412–21. doi: 10.1002/cncr.29492. [DOI] [PubMed] [Google Scholar]
- 15.Partridge AH, Rumble RB, Carey LA, Come SE, Davidson NE, Leo AD, et al. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2–negative (or unknown) advanced breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2014;32:3307–29. doi: 10.1200/JCO.2014.56.7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimoi T, Nagai S, Yoshinami T, Takahashi M, Arioka H, Ishihara M, et al. The Japanese Breast Cancer Society Clinical Practice Guidelines for systemic treatment of breast cancer, 2018 edition. Breast Cancer. 2020;27:322–31. doi: 10.1007/s12282-020-01085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talbot DC, Moiseyenko V, Van Belle S, O’Reilly SM, Alba Conejo E, Ackland S, et al. Randomised, phase II trial comparing oral capecitabine (Xeloda) with paclitaxel in patients with metastatic/advanced breast cancer pretreated with anthracyclines. Br J Cancer. 2002;86:1367–72. doi: 10.1038/sj.bjc.6600261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stockler MR, Harvey VJ, Francis PA, Byrne MJ, Ackland SP, Fitzharris B, et al. Capecitabine versus classical cyclophosphamide, methotrexate, and fluorouracil as first-line chemotherapy for advanced breast cancer. J Clin Oncol. 2011;29:4498–504. doi: 10.1200/JCO.2010.33.9101. [DOI] [PubMed] [Google Scholar]
- 19.Oshaughnessy JA, Blum J, Moiseyenko V, Jones SE, Miles D, Bell D, et al. Randomized, open-label, phase II trial of oral capecitabine (Xeloda) vs. a reference arm of intravenous CMF (cyclophosphamide, methotrexate and 5-fluorouracil) as first-line therapy for advanced/metastatic breast cancer. Ann Oncol. 2001;12:1247–54. doi: 10.1023/A:1012281104865. [DOI] [PubMed] [Google Scholar]
- 20.Saad ED, Buyse M. Non-inferiority trials in breast and non-small cell lung cancer: choice of non-inferiority margins and other statistical aspects. Acta Oncol. 2012;51:890–6. doi: 10.3109/0284186X.2012.702924. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto D, Iwase S, Tsubota Y, Ariyoshi K, Kawaguchi T, Miyaji T, et al. Randomized study of orally administered fluorinated pyrimidines (capecitabine versus S-1) in women with metastatic or recurrent breast cancer: Japan Breast Cancer Research Network 05 Trial. Cancer Chemother Pharm. 2015;75:1183–9. doi: 10.1007/s00280-015-2738-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets used and analysed during this study are available from the corresponding author on reasonable request.