INTRODUCTION
Despite variable recommendations from professional societies, thyroid function testing has become ubiquitous in modern medicine.1,2 As a consequence, subtle abnormalities in serum thyroid stimulating hormone (TSH) levels have become a commonly encountered clinical scenario. Whereas overt thyroid dysfunction is defined by thyroid hormone levels [thyroxine (T4) and triiodothyronine (T3)] above or below the reference range in addition to an abnormal serum TSH, a significantly more common finding is a TSH level outside of the reference range with thyroid hormone levels within the reference range (Figure 1).3,4 Though purely a biochemical diagnosis, these thyroid function test abnormalities are collectively classified as subclinical thyroid disease.
Figure 1.
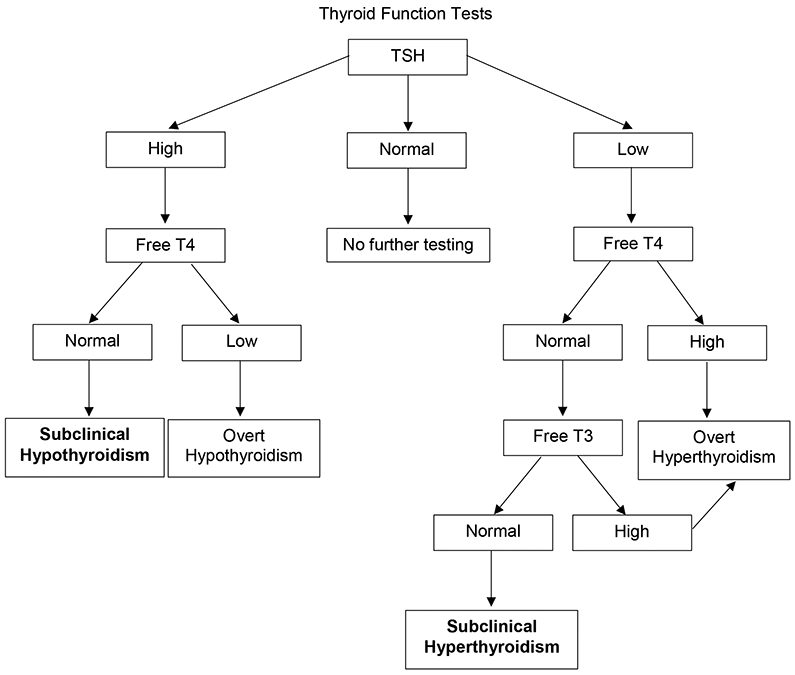
Suggested approach to interpretation of thyroid function testing. T4 = thyroxine. T3 = triiodothyronine. TSH = thyroid stimulating hormone.
Prior epidemiological studies suggest that the prevalence of subclinical hypothyroidism and subclinical hyperthyroidism in the general United States (U.S.) population are between 4.0-9.0% and 1.8-2.1%, respectively, with higher rates in women and older adults.5,6 While it is widely accepted that overt hypothyroidism and hyperthyroidism should be treated, significant uncertainty remains regarding the management of subclinical thyroid disease. Complementary to previous expert reviews 7-12 we will summarize the available evidence on the implications of subclinical thyroid disease and provide a practical guide for clinical decision-making and management.
DISCUSSION
OVERVIEW
Physiology of Subclinical Thyroid Disease
Thyroid hormone production and secretion are tightly regulated through an endocrine feedback loop in which hypothalamic thyroid releasing hormone (TRH) stimulates anterior pituitary production and release of TSH, which in turn acts directly on thyroidal TSH receptors.13 Prior work has demonstrated that intra-individual variations in T4 concentrations are much smaller than inter-individual variations, such that an individual’s normal range is generally narrower than the reference range for a population.14 This finding may explain the prevalence of subclinical disease.7,15
Changes in Thyroid Function with Age
There has been considerable debate regarding the most appropriate reference range for serum TSH.3,16 In general, a reference range is determined based on the values found in healthy, disease-free individuals in a population and encompasses two standard deviations on either side of the mean value. With modern immunometric assays, the upper limit of the range for TSH in most laboratories is considered to be between 4.0-5.0 mIU/L. Notably, TSH does not follow a normal distribution, and levels vary diurnally by up to approximately 50% of mean values.2,17 According to NHANES III, in a reference population defined as being disease-free without pregnancy, taking estrogen or androgen, without detectable thyroid antibodies and without laboratory evidence of hypothyroidism or hyperthyroidism, the range is narrower, with the upper limit of normal of serum TSH being 4.12 mIU/L.5
Additionally, a shift to higher TSH values has been demonstrated with increasing age, such that more than 14% of healthy individuals over the age of 80 years may have a TSH >4.5 mIU/L.18,19 Indeed, the 97.5% confidence interval for serum TSH in healthy persons older than 80 years increases to 7.5 mIU/L.19 This is particularly notable given prior studies suggesting that any increased cardiovascular risk associated with mild subclinical hypothyroidism may be attenuated in those over 80 years-old 20,21, and that there may be an association with improved survival in those over age 85 years with TSH values above the reference range.22 Taken together, these findings have prompted some experts to recommend age-specific TSH reference ranges.16,23
SUBCLINICAL HYPOTHYROIDISM
Evaluation
The etiologies of subclinical hypothyroidism and overt hypothyroidism are the same. The most common cause in the U.S. is autoimmune or lymphocytic thyroiditis, though the differential is extensive.7,15 Many authors have, arbitrarily, classified subclinical hypothyroidism as grade 1, or mild, if the initial TSH is <10 mIU/L and grade 2, or severe, if the initial TSH is ≥10 mIU/L. 8,11,15,24 Prior work suggests that in the vast majority of cases of subclinical hypothyroidism, the initial abnormal TSH is <10 mIU/L.25
The natural history appears to depend on the degree of initial TSH elevation. In a study by Somwaru et al., among adults aged 65 years and older with an initial TSH 4.5-7 mIU/L, 46% had normalization of TSH and only 1% showed progression to overt hypothyroidism over two years. Conversely, when initial TSH was >10 mIU/L, only 7% demonstrated normalization whereas 10% progressed to overt hypothyroidism over 2 years.25 Therefore, the finding of an abnormal TSH should lead to measurement of free thyroxine (free T4). If this value is within the normal range, repeat testing should be performed after an interval of 1-3 months, erring towards a shorter interval for those with grade 2 subclinical hypothyroidism.8,15 It is unclear whether detection of thyroid peroxidase antibodies (TPOAb) should change management; however, several studies demonstrated that progression to overt hypothyroidism is more likely when TPOAb are detected, and their presence provides confirmation of an autoimmune etiology (i.e., Hashimoto Disease).25-27
Implications
Cardiovascular Disease
Several prior studies evaluated the relationship between subclinical hypothyroidism and cardiovascular disease. Though there are limitations, older studies suggest that subclinical hypothyroidism may be associated with worsening of various measures of cardiac performance, including impaired left ventricular systolic and diastolic function.28,29 Also, while the relationship between overt hypothyroidism and hyperlipidemia is well-established, the impact of subclinical hypothyroidism on lipid metabolism is less certain.3,30 Some cross-sectional studies have suggested increases in total cholesterol and LDL 6,31, while others, including NHANES III, have not shown an association.32,33
Perhaps more clinically relevant is the potential increased risk of major cardiovascular events, heart failure, and death. Interestingly, several large cohort studies have found no association between subclinical hypothyroidism and cardiovascular disease.34,35 In the Cardiovascular Health Study, a cohort of adults aged 65 years and older followed longitudinally, there was no association between subclinical hypothyroidism and increased risk of coronary heart disease (CHD) events (hazard ratio [HR], 1.04; 95% confidence interval [CI], 0.86-1.26), heart failure (HR, 0.95; 95% CI, 0.78-1.15), or CHD mortality (HR, 1.06; 95% CI, 0.86-1.30), even for those with TSH >10 mIU/L.34 In a separate cohort of adults aged older than 85 years, despite combining subclinical and overt hypothyroidism, no association was found with cardiovascular or all-cause mortality.20 A recent cohort study of 9,020 adults utilized NHANES data to evaluate the association between subclinical hypothyroidism and overall mortality. In multivariable analysis, after adjusting for age, sex, race/ethnicity, education status, smoking cancer history and an estimate glomerular filtration rate, subclinical hypothyroidism was associated with an increased risk of all-cause mortality (HR, 1.90; 95% CI, 1.14-3.19). In mediation analyses, it was estimated that cardiovascular disease was responsible for 14.3% of the association between subclinical hypothyroidism and all-cause mortality.36 Even though this latter study yielded compelling results, risk of bias due to unmeasured confounders, such as obesity and diabetes, cannot be ruled out.
Furthermore, a meta-analysis evaluated the association between subclinical hypothyroidism and cardiovascular outcomes using individual participant data.37,38 In age- and sex-adjusted analyses, there was a non-significant trend towards increased CHD events, CHD mortality and total mortality. Notably, when stratified analyses were performed, a TSH >10 mIU/L was associated with significantly increased risk of heart failure (HR, 1.86; 95% CI, 1.27-2.72), CHD events (HR, 1.89; 95% CI, 1.28-2.80) and CHD mortality (HR, 1.58; 95% CI, 1.10-2.27). Additionally, a TSH of 7-9.9 mIU/L was associated with significantly increased risk of CHD mortality (HR, 1.42; 95% CI, 1.03-1.95). Age did not impact these findings.37,38 These findings suggest that grade 2 subclinical hypothyroidism may potentially be associated with increased risk of cardiovascular disease and even mortality.
Osteoporosis and Fractures
Multiple studies have evaluated the potential relationship between subclinical hypothyroidism and osteoporosis and fractures. Wirth et al. conducted a meta-analysis of high quality studies and found no relationship between subclinical hypothyroidism and hip (HR, 1.12; 95% C, 0.76-1.42) or non-spine (HR, 1.04; 95% CI, 0.76-1.42) fractures.39 An additional meta-analysis using individual participant data found no association between subclinical hypothyroidism and hip fractures (HR, 0.96; 95% CI, 0.83-1.10) or vertebral fractures (HR, 0.96; 95% CI, 0.59-1.55).40
Cognitive Decline and Dementia
The association between subclinical hypothyroidism and cognitive decline is controversial. A recent prospective cohort study evaluated adults aged 70-79 years over a median of 9 years using data from the Health, Aging, and Body Composition study. This study found no association between subclinical hypothyroidism and increased risk of dementia (HR, 0.91; 95% CI, 0.70-1.19).41 Several meta-analyses have also been performed and generally have found no association with increased risk of dementia.42,43 However, in stratified analysis from one such study, a significantly increased risk was seen in adults younger than 75 years (odds ratio [OR], 1.81; 95% CI 1.43-2.28).44 These findings suggest that, at least in older adults aged 75 and older, there is a lack of a consistent association between subclinical hypothyroidism and cognitive function.
Treatment of Subclinical Hypothyroidism: Evidence and Decision-Making
In general, large randomized controlled trials (RCTs) evaluating the effects of treatment of subclinical hypothyroidism on important clinical outcomes are lacking. As a result, it is unknown whether treatment reduces the apparent increased cardiovascular risk associated with subclinical hypothyroidism. A retrospective cohort study in the United Kingdom found that in patients with subclinical hypothyroidism aged 40-70 years, treatment with levothyroxine was associated with a lower risk of fatal and non-fatal ischemic heart disease events (HR, 0.61; 95% CI, 0.39-0.95) and all-cause mortality (HR, 0.36; 95% CI, 0.19-0.66), after controlling for age, sex, conventional cardiovascular risk factors and baseline serum TSH. There was no association with reduced risk of either outcome in those older than 70 years.45 These results should be interpreted with caution in view of the retrospective nature of this study and possible unmeasured confounders. Only adequately powered RCTs will be able to demonstrate whether treatment of subclinical hypothyroidism is beneficial in these patients in terms of mitigating cardiovascular risk. Contrary to this study, another retrospective cohort study in Sweden found no association between treatment and risk of myocardial infarction or cardiovascular death though there was an association with decreased all-cause mortality in those younger than 65 years of age (incidence rate ratio [IRR], 0.63; 95% CI, 0.40-0.99).46 Although underpowered for cardiovascular outcomes, a large RCT discussed further below found no decreased risk of fatal or non-fatal cardiovascular events in adults ≥65 years (HR, 0.89; 95% CI, 0.47-1.69).47
Multiple studies have examined the effect of thyroid hormone treatment on cognitive performance and quality of life measures.47-50 A small double-blind, randomized, placebo-controlled trial in adults ≥ 65 years of age evaluated the effects of thyroid hormone therapy on cognitive function over 12 months and found no significant change in any measure of cognitive performance.50 Stott et al. conducted a larger double-blind, randomized, placebo-controlled trial of low dose levothyroxine therapy in adults ≥ 65 years of age with persistent subclinical hypothyroidism.47 The primary outcome was change in Thyroid-Related Quality-of-Life Patient-Reported Outcome measure (ThyPRO) Hypothyroid Symptoms score and Tiredness score. Over 12 months, compared with placebo, there was no significant difference in either measure. An additional analysis combined data from this trial with a second, similar RCT of adults ≥80 years old. There was no improvement in either hypothyroid symptoms or fatigue scores.49 Also, a meta-analysis of RCTs found that treatment with thyroid hormone was not associated with improvement in general quality of life, thyroid-specific symptoms, depressive symptoms, fatigue, strength or BMI.48
Given a general lack of evidence that treatment of subclinical hypothyroidism ameliorates possible cardiovascular risk or improves quality of life, recommendations for treatment are based primarily on expert opinion. Most guidelines and expert reviews agree that treatment should be initiated in those younger than 65-70 years of age when the TSH is >10 mIU/L 2,7,11,24, however, recommendations are more variable for older individuals and when the TSH is between the upper limit of normal and 10 mIU/L. When the TSH is between the upper limit of normal and 10 mIU/L, the American Thyroid Association (ATA) recommends consideration of treatment in those that are symptomatic (e.g., with symptoms of fatigue, lack of energy, constipation), at high risk for progression (positive TPOAb), or at high cardiovascular risk.2 The European Thyroid Association (ETA) guidelines are similar, though they recommend against treatment in those over the age of 70 years.24 Some authors more strongly recommend treatment in those with TSH values between 7-9.9 mIU/L, particularly when younger than 65 years of age based on observational evidence of increased risk of CHD mortality.8,51 Notably, a clinical practice guideline published in response to the meta-analysis by Feller et al. recommends against treatment of subclinical hypothyroidism in the majority of adults with the exception of those with TSH >20 mIU/L and women pursuing pregnancy.52
If the decision is made to initiate treatment, levothyroxine (LT4) should be used.2,7,53 Because subclinical hypothyroidism is a state of sub-total thyroid hormone deficiency, many patients may not require a full, weight-based replacement dose (1.6 mcg/kg/day for the average adult), and starting with doses of 25-75 mcg/day should be considered, particularly in those over age 65 years or with risk factors for cardiovascular disease.2,53 Dose adjustment is made every 6-8 weeks based on serum TSH values, and the goal is a TSH within the reference range.24,53 However, it should be acknowledged that normal serum TSH ranges may be higher in the older adult population and it is reasonable to consider a target TSH of 4-6 mIU/L in those older than 70-80 years of age.53 Examples of subclinical hypothyroidism scenarios and decision making considerations in management are shown in Table 1.
Table 1.
Examples of subclinical hypothyroidism scenarios and decision making considerations
| Case Scenario | Decision Making Considerations |
|---|---|
| Case 1: A healthy 85-year-old man was screened for thyroid dysfunction. He is asymptomatic. Labs show TSH 6.1 mIU/L, normal free T4. | The TSH reference range shifts higher with aging and this result is within the age-adjusted reference range. Treatment is not recommended. Thyroid function tests should be repeated in 1-3 months. |
| Case 2: A 50-year-old woman with a history of type 1 diabetes underwent screening for thyroid dysfunction. Her TSH has increased from 4.5→7.5→10 mIU/L over the last year but free T4 remains normal. TPO Ab are positive. She feels well. | This patient most likely has underlying lymphocytic thyroiditis (Hashimoto’s thyroiditis). She has progressive subclinical hypothyroidism and has an increased risk of progressing to overt hypothyroidism due to the presence of TPO Ab. While data demonstrating that treatment reduces cardiovascular risk are lacking, treatment with levothyroxine should be strongly considered given her age, underlying disease and degree of TSH elevation. |
| Case 3: A 55-year-old woman with a history of hypertension, on lisinopril, was found to have a TSH of 7.5 mIU/L with repeat value of 7.8 mIU/L two months later. Free T4 is normal. She has worsening fatigue. | This is a relatively young individual with TSH values above the reference range, but below 10 mIU/L. She has symptoms that could relate to thyroid dysfunction, but are non-specific. While there is no evidence that treatment will improve her symptoms or cardiovascular risk, a trial of treatment is reasonable with reassessment of symptoms once TSH is normalized. An alternative approach is observation. |
SUBCLINICAL HYPERTHYROIDISM
Evaluation
As outlined in prior reviews, several clinical conditions and medications can lead to a low TSH, and should be considered prior to making a diagnosis of subclinical hyperthyroidism.8,11 In similar fashion to subclinical hypothyroidism, patients are stratified by TSH into grade 1 (TSH 0.1-0.4 mIU/L) and grade 2 (TSH <0.1 mIU/L) subclinical hyperthyroidism. Initial TSH is the best predictor of the clinical course; while overall rates of progression to overt hyperthyroidism vary by study, the risk is greater in those with initial TSH <0.1 mIU/L when compared with TSH 0.1-0.4 mIU/L.54-56 Repeat biochemical testing at an interval of 1-3 months is recommended to document persistence with a shorter interval favored for those with initial TSH <0.1 mIU/L.8,57 The most likely etiology depends on the patient’s age and iodine status; Graves’ disease is the most likely diagnosis in young individuals and in iodine-replete areas, while toxic multinodular goiter and toxic adenomas become more common in older adults and in areas of iodine deficiency.10,11 Evaluation depends on the clinical scenario. In a patient with classic signs of Graves’ disease, including a smooth, diffusely enlarged thyroid, thyroid bruit, and/or orbitopathy, no additional workup may be needed. Otherwise, measurement of thyrotropin receptor antibodies (TRAb) or thyroid stimulating immunoglobulin (TSI) provide high sensitivity and specificity for Graves’ disease. If negative, 24-hour radioactive iodine uptake provides quantification of iodine uptake and thyroid scintigraphy allows visualization of the uptake pattern. This is particularly helpful in differentiating between hyperfunctioning nodule(s) and Graves’ disease and can additionally identify subacute thyroiditis, which demonstrates very low iodine uptake.57 Thyroid ultrasound is primarily useful when thyroid scan suggests hyperfunctioning nodule(s) in order to further define size and extent of nodular disease, and for “cold nodules” to evaluate for biopsy.
Implications
Cardiovascular Disease
Both population-based studies and a meta-analysis have demonstrated an association between subclinical hyperthyroidism and increased risk of incident atrial fibrillation.58-62 It is uncertain whether this risk applies generally to those with subclinical hyperthyroidism or only to those with grade 2 subclinical hyperthyroidism. The largest population-based study to date, conducted in Denmark, found that the risk of atrial fibrillation progressively increased with decreasing TSH, though there was a statistically significant increased risk only for those subjects with TSH <0.1 mIU/L (HR, 1.41; 95% CI, 1.25-1.59).61 On the other hand, Cappola et al. found that in a population-based cohort of subjects aged 65 years and older, the increased risk remained in stratified analyses of those with TSH 0.1-0.44 mIU/L (HR, 1.85; 95% CI 1.14-3.00).58
Studies on the association between subclinical hyperthyroidism and cardiovascular disease are more heterogeneous. The Cardiovascular Health Study found no increased risk of cardiovascular events or death,58 while other population-based studies have demonstrated an association with increased risk of non-fatal cardiovascular events35,62 and cardiovascular mortality.63 A meta-analysis using individual participant data found that when adjusting for age and sex, subclinical hyperthyroidism was associated with an increased risk of CHD mortality (HR, 1.29; 95% CI, 1.02-1.62). In stratified analyses, however, this increased risk was seen only in those with TSH <0.1 mIU/L.59 Notably, when controlling for additional cardiovascular risk factors, in multivariable analyses, the association with increased cardiovascular death was no longer present.59 A meta-analysis using individual participant data found that subclinical hyperthyroidism was associated with significantly increased risk of heart failure events in those with TSH <0.1 mIU/L (HR, 1.94; 95% CI, 1.01-3.72).37 Lastly, a recent meta-analysis found no relationship with increased risk of stroke.64
Osteoporosis and Fractures
Due to effects of thyroid hormone on bone turnover, there has been significant concern about the possibility of increased risk of osteoporosis and fractures in patients with subclinical hyperthyroidism. Wirth et al. evaluated this relationship in a meta-analysis of prospective populated-based studies.39 Though limited by study heterogeneity, there was a trend toward increased risk of hip and non-spine fractures, however this did not reach statistical significance. A follow up meta-analysis published in 2015 used individual participant data and found that compared with euthyroid controls, subclinical hyperthyroidism was associated with an increased risk of hip fracture (HR, 1.36; 95% CI, 1.13-1.64), increased risk of any fracture (HR, 1.28; 95% CI, 1.06-1.53), and a trend toward increased risk of clinical vertebral fracture (HR, 1.51; 95% CI, 0.93-2.45). In stratified analyses, there was a significant trend toward increased hip fracture and any fracture with decreasing TSH.40
Cognitive Decline and Dementia
Two recent studies evaluated the possible association between subclinical hyperthyroidism and cognitive decline and dementia. Rieban et al. conducted a meta-analysis of prospective studies and found a positive association (relative risk [RR], 1.67; 95% CI, 1.04-2.69).43 Subsequently, the results of the Health, Aging, and Body Composition Study were published in 2017.41 This was a prospective cohort study of adults aged 70-89 years followed for 10 years. This study demonstrated a significantly increased risk of dementia in those with suppressed TSH (<0.1 mIU/L) (HR, 2.38; 95% CI, 1.13-5.04) but no association in those with mildly decreased TSH (0.10-0.44 mIU/L) (HR, 0.79; 95% CI, 0.45-1.38).
Treatment of Subclinical Hyperthyroidism: Evidence and Decision-Making
Ideally, decisions for initiating treatment in patients with subclinical hyperthyroidism should be based on high-quality evidence demonstrating reduced risk of one or more of the outcomes discussed above. Unfortunately, these data are generally lacking. In their absence, recommendations are based predominantly on expert opinion.
With subtle differences, recommendations for treatment of subclinical hyperthyroidism are generally consistent.8,11,57,65 The main considerations are the degree of TSH suppression, and the age and risk factors of the patient. When the TSH is persistently <0.1 mIU/L, treatment is strongly recommended for those 65 years and older and for those with or at high risk for complications including osteoporosis, arrhythmia and heart disease. Due to lack of robust data, for patients younger than age 65 without risk factors or symptoms and a persistent TSH <0.1 mIU/L, guidelines recommend that treatment can be considered at the physician’s discretion given possible improvement in symptoms and risk of progression. When TSH is persistently between 0.1 mIU/L and the lower limit of normal, treatment is generally favored for those 65 years and older and for those with risk factors. On the other hand, for asymptomatic individuals younger that 65 years old and a TSH between 0.1 mIU/L and the lower limit of normal, observation is generally appropriate. The specific treatment chosen (antithyroid medications, radioactive iodine or surgery) depends on the underlying etiology and patient preferences. Toxic multinodular goiter does not spontaneously remit and therefore many experts favor treatment with radioactive iodine or surgery,57,65 though it should be noted that thionamides can be used effectively. For those with Graves’ disease, a trial of thionamides is often appropriate, particularly in young patients. With this treatment, remission may occur in up to 40-50% of patients after 12-18 months.66 Alternative options for definitive management include radioactive iodine and thyroidectomy.57,66 Examples of subclinical hyperthyroidism scenarios and decision making considerations in management are shown in Table 2.
Table 2.
Examples of subclinical hyperthyroidism scenarios and decision making considerations
| Case Scenario | Decision Making Considerations |
|---|---|
| Case 1: A healthy 39-year-old woman has had TSH values between 0.3 and 0.4 mIU/L over the last year. Free T4 and Free T3 are normal. TRAb are negative and she has a normal 24-hour iodine uptake and scan. She is asymptomatic. | This is a young patient with a TSH slightly below the reference range (grade 1 subclinical hyperthyroidism) without an identified etiology. There is no evidence that treatment will be beneficial and observation is appropriate. |
| Case 2: A 75-year-old woman with a history of coronary artery disease, hypertension and hyperlipidemia is found to have a TSH of 0.05 mIU/L. Repeat TSH a month later is 0.08 mIU/L. Free T4 and Free T3 remain normal. She has palpable thyroid nodules and thyroid scintigraphy demonstrates toxic multinodular goiter. | This is an older, post-menopausal woman with known coronary artery disease. She has grade 2 subclinical hyperthyroidism. Given her age and risk factors, treatment is appropriate. Radioactive iodine is generally the preferred treatment, which would lead to hypothyroidism and the need for lifelong thyroid hormone replacement therapy. Anti-thyroid drugs can be used, but likely need to be continued indefinitely. Significant comorbidity (cardiovascular disease) weighs against the choice of surgery in this patient. |
| Case 3: A 59-year-old woman with osteopenia is found to have TSH values between 0.2 and 0.3 mIU/L over the last 6 months. Free T4 and Free T3 remain normal. TRAb is elevated. | This is a middle-aged woman with asymptomatic grade 1 subclinical hyperthyroidism. The etiology is likely Graves’ disease. Because she is post-menopausal and has low bone density, treatment should be considered though observation with close monitoring for progression is reasonable. |
SUMMARY
Subclinical thyroid disease is a frequently encountered clinical problem. While uncertainty remains, subclinical hypothyroidism appears to be associated with an increased risk of cardiovascular disease and death, particularly when TSH is >10 mIU/L. Subclinical hyperthyroidism has been shown to be associated with an increased risk of atrial fibrillation and osteoporosis. Additionally, there may be an increased risk of cardiovascular disease in those with TSH <0.1 mIU/L.
There is a lack of large, adequately powered randomized controlled trials evaluating the effects of treatment of subclinical thyroid disease on meaningful clinical outcomes. Treatment of subclinical hypothyroidism should generally be considered when TSH >10 mIU/L and possibly in younger individuals with TSH between the upper limit of normal and 10 mIU/L with convincing symptoms, positive TPOAb, or at high risk for cardiovascular disease. In subclinical hyperthyroidism, treatment decisions should be based on patient age, risk factors for cardiovascular disease and osteoporosis, and the degree of TSH suppression. Treatment is more strongly favored for those 65 years and older and for those with or at high risk for cardiovascular disease or osteoporosis. Future studies, including randomized prospective controlled trials, aimed at better understanding the potential benefits of treatment of subclinical thyroid disease would greatly add to our current understanding of this topic and improve our ability to make evidence-based recommendations to our patients.
Key Points:
Subclinical thyroid disease is defined by a serum thyroid stimulating hormone (TSH) either above or below the reference range with normal thyroxine (T4) and triiodothyronine (T3) concentrations.
Subclinical hypothyroidism may be associated with increased cardiovascular events and death, particularly when TSH is >10 mIU/L.
Though recommendations for treatment vary, there is limited evidence that treatment of subclinical hypothyroidism reduces the risk of cardiovascular events and no evidence that it improves quality of life or cognitive function.
Subclinical hyperthyroidism has been associated with increased risk of atrial fibrillation and osteoporosis in multiple studies.
Treatment of subclinical hyperthyroidism is generally recommended when the TSH is suppressed (<0.1 mIU/L) with variable recommendations when the TSH is between 0.1 mIU/L and the lower limit of normal.
Synopsis:
Subclinical thyroid disease is frequently encountered in clinic practice. While overt thyroid dysfunction has been associated with adverse clinical outcomes, uncertainty remains about the implications of subclinical thyroid disease. The available data suggests that subclinical hypothyroidism may be associated with increased risk of cardiovascular disease and death. Despite this finding, treatment with thyroid hormone has not been consistently demonstrated to reduce cardiovascular risk. Subclinical hyperthyroidism has been associated with increased risk of atrial fibrillation and osteoporosis, but the association with cardiovascular disease and death is uncertain. The decision to treat depends on the degree of TSH suppression and underlying comorbidities.
CLINICS CARE POINTS.
Many factors affect thyroid function testing, and a review of these should be undertaken when abnormal results are encountered.
Subclinical thyroid disease is defined by TSH values persistently outside of the reference range with normal levels of serum free T4 and free T3.
The TSH reference range shifts higher with advancing age, and this should be considered when interpreting results in older individuals.
Treatment decisions in subclinical thyroid disease should take into account the age and comorbidities of the individual, as well as the degree of the derangement in TSH.
Footnotes
Disclosure Statement: The authors have nothing to disclose. Dr. Papaleontiou is funded by K08 AG049684 from the National Institute on Aging.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Josh M. Evron, Division of Endocrinology and Metabolism, Department of Internal Medicine, University of North Carolina, Burnett-Womack, CB 7172, Chapel Hill, NC 27599.
Maria Papaleontiou, Division of Metabolism, Endocrinology and Diabetes, Department of Internal Medicine, University of Michigan, North Campus Research Complex, 2800 Plymouth Road, Bldg. 16, Rm 453S, Ann Arbor, MI 48109.
REFERENCES
- 1.LeFevre ML. Screening for thyroid dysfunction: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;162(9):641–650. [DOI] [PubMed] [Google Scholar]
- 2.Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22(12):1200–1235. [DOI] [PubMed] [Google Scholar]
- 3.Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29(1):76–131. [DOI] [PubMed] [Google Scholar]
- 4.Surks MI, Ortiz E, Daniels GH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 2004;291(2):228–238. [DOI] [PubMed] [Google Scholar]
- 5.Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489–499. [DOI] [PubMed] [Google Scholar]
- 6.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado Thyroid Disease Prevalence Study. Archives of Internal Medicine. 2000;160(4):526–534. [DOI] [PubMed] [Google Scholar]
- 7.Biondi B, Cappola AR, Cooper DS. Subclinical Hypothyroidism: A Review. JAMA. 2019;322(2):153–160. [DOI] [PubMed] [Google Scholar]
- 8.Biondi B, Cooper DS. Subclinical Hyperthyroidism. N Engl J Med. 2018;378(25):2411–2419. [DOI] [PubMed] [Google Scholar]
- 9.Peeters RP. Subclinical Hypothyroidism. N Engl J Med. 2017;377(14):1404. [DOI] [PubMed] [Google Scholar]
- 10.Carlé A, Andersen SL, Boelaert K, Laurberg P. MANAGEMENT OF ENDOCRINE DISEASE: Subclinical thyrotoxicosis: prevalence, causes and choice of therapy. European Journal of Endocrinology. 2017;176(6):R325. [DOI] [PubMed] [Google Scholar]
- 11.Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379(9821):1142–1154. [DOI] [PubMed] [Google Scholar]
- 12.Sue LY, Leung AM. Levothyroxine for the Treatment of Subclinical Hypothyroidism and Cardiovascular Disease. Frontiers in Endocrinology. 2020;11(824). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jameson JL, Mandel SJ, Weetman AP. Thyroid Gland Physiology and Testing. In: Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, eds. Harrison's Principles of Internal Medicine, 20e. New York, NY: McGraw-Hill Education; 2018. [Google Scholar]
- 14.Andersen S, Pedersen KM, Bruun NH, Laurberg P. Narrow Individual Variations in Serum T4 and T3 in Normal Subjects: A Clue to the Understanding of Subclinical Thyroid Disease. The Journal of Clinical Endocrinology & Metabolism. 2002;87(3):1068–1072. [DOI] [PubMed] [Google Scholar]
- 15.Peeters RP. Subclinical Hypothyroidism. N Engl J Med. 2017;376(26):2556–2565. [DOI] [PubMed] [Google Scholar]
- 16.Cappola AR. The Thyrotropin Reference Range Should Be Changed in Older Patients. JAMA. 2019. [DOI] [PubMed] [Google Scholar]
- 17.Caron PJ, Nieman LK, Rose SR, Nisula BC. Deficient nocturnal surge of thyrotropin in central hypothyroidism. J Clin Endocrinol Metab. 1986;62(5):960–964. [DOI] [PubMed] [Google Scholar]
- 18.Bremner AP, Feddema P, Leedman PJ, et al. Age-Related Changes in Thyroid Function: A Longitudinal Study of a Community-Based Cohort. The Journal of Clinical Endocrinology & Metabolism. 2012;97(5):1554–1562. [DOI] [PubMed] [Google Scholar]
- 19.Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92(12):4575–4582. [DOI] [PubMed] [Google Scholar]
- 20.Pearce SH, Razvi S, Yadegarfar ME, et al. Serum Thyroid Function, Mortality and Disability in Advanced Old Age: The Newcastle 85+ Study. J Clin Endocrinol Metab. 2016;101(11):4385–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waring AC, Arnold AM, Newman AB, Bùzková P, Hirsch C, Cappola AR. Longitudinal changes in thyroid function in the oldest old and survival: the cardiovascular health study all-stars study. J Clin Endocrinol Metab. 2012;97(11):3944–3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gussekloo J, van Exel E, de Craen AJ, Meinders AE, Frölich M, Westendorp RG. Thyroid status, disability and cognitive function, and survival in old age. JAMA. 2004;292(21):2591–2599. [DOI] [PubMed] [Google Scholar]
- 23.Baloch Z, Carayon P, Conte-Devolx B, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13(1):3–126. [DOI] [PubMed] [Google Scholar]
- 24.Pearce SH, Brabant G, Duntas LH, et al. 2013 ETA Guideline: Management of Subclinical Hypothyroidism. Eur Thyroid J. 2013;2(4):215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Somwaru LL, Rariy CM, Arnold AM, Cappola AR. The natural history of subclinical hypothyroidism in the elderly: the cardiovascular health study. J Clin Endocrinol Metab. 2012;97(6):1962–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Díez JJ, Iglesias P. Spontaneous subclinical hypothyroidism in patients older than 55 years: an analysis of natural course and risk factors for the development of overt thyroid failure. J Clin Endocrinol Metab. 2004;89(10):4890–4897. [DOI] [PubMed] [Google Scholar]
- 27.Huber G, Staub JJ, Meier C, et al. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab. 2002;87(7):3221–3226. [DOI] [PubMed] [Google Scholar]
- 28.Biondi B, Palmieri EA, Lombardi G, Fazio S. Subclinical hypothyroidism and cardiac function. Thyroid. 2002;12(6):505–510. [DOI] [PubMed] [Google Scholar]
- 29.Biondi B, Palmieri EA, Lombardi G, Fazio S. Effects of subclinical thyroid dysfunction on the heart. Ann Intern Med. 2002;137(11):904–914. [DOI] [PubMed] [Google Scholar]
- 30.Pearce EN. Update in lipid alterations in subclinical hypothyroidism. J Clin Endocrinol Metab. 2012;97(2):326–333. [DOI] [PubMed] [Google Scholar]
- 31.Kanaya AM, Harris F, Volpato S, Pérez-Stable EJ, Harris T, Bauer DC. Association Between Thyroid Dysfunction and Total Cholesterol Level in an Older Biracial Population: The Health, Aging and Body Composition Study. Archives of Internal Medicine. 2002;162(7):773–779. [DOI] [PubMed] [Google Scholar]
- 32.Hueston WJ, Pearson WS. Subclinical hypothyroidism and the risk of hypercholesterolemia. Ann Fam Med. 2004;2(4):351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vierhapper H, Nardi A, Grösser P, Raber W, Gessl A. Low-density lipoprotein cholesterol in subclinical hypothyroidism. Thyroid. 2000;10(11):981–984. [DOI] [PubMed] [Google Scholar]
- 34.Hyland KA, Arnold AM, Lee JS, Cappola AR. Persistent subclinical hypothyroidism and cardiovascular risk in the elderly: the cardiovascular health study. J Clin Endocrinol Metab. 2013;98(2):533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selmer C, Olesen JB, Hansen ML, et al. Subclinical and overt thyroid dysfunction and risk of all-cause mortality and cardiovascular events: a large population study. J Clin Endocrinol Metab. 2014;99(7):2372–2382. [DOI] [PubMed] [Google Scholar]
- 36.Inoue K, Ritz B, Brent GA, Ebrahimi R, Rhee CM, Leung AM. Association of Subclinical Hypothyroidism and Cardiovascular Disease With Mortality. JAMA Network Open. 2020;3(2):e1920745–e1920745. [DOI] [PubMed] [Google Scholar]
- 37.Gencer B, Collet TH, Virgini V, et al. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation. 2012;126(9):1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304(12):1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wirth CD, Blum MR, da Costa BR, et al. Subclinical thyroid dysfunction and the risk for fractures: a systematic review and meta-analysis. Ann Intern Med. 2014;161(3):189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blum MR, Bauer DC, Collet TH, et al. Subclinical thyroid dysfunction and fracture risk: a meta-analysis. JAMA. 2015;313(20):2055–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aubert CE, Bauer DC, da Costa BR, et al. The association between subclinical thyroid dysfunction and dementia: The Health, Aging and Body Composition (Health ABC) Study. Clin Endocrinol (Oxf). 2017;87(5):617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akintola AA, Jansen SW, van Bodegom D, et al. Subclinical hypothyroidism and cognitive function in people over 60 years: a systematic review and meta-analysis. Front Aging Neurosci. 2015;7:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rieben C, Segna D, da Costa BR, et al. Subclinical Thyroid Dysfunction and the Risk of Cognitive Decline: a Meta-Analysis of Prospective Cohort Studies. J Clin Endocrinol Metab. 2016;101(12):4945–4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pasqualetti G, Pagano G, Rengo G, Ferrara N, Monzani F. Subclinical Hypothyroidism and Cognitive Impairment: Systematic Review and Meta-Analysis. J Clin Endocrinol Metab. 2015;100(11):4240–4248. [DOI] [PubMed] [Google Scholar]
- 45.Razvi S, Weaver JU, Butler TJ, Pearce SH. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med. 2012;172(10):811–817. [DOI] [PubMed] [Google Scholar]
- 46.Andersen MN, Olsen AM, Madsen JC, et al. Levothyroxine Substitution in Patients with Subclinical Hypothyroidism and the Risk of Myocardial Infarction and Mortality. PLoS One. 2015;10(6):e0129793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stott DJ, Rodondi N, Kearney PM, et al. Thyroid Hormone Therapy for Older Adults with Subclinical Hypothyroidism. N Engl J Med. 2017;376(26):2534–2544. [DOI] [PubMed] [Google Scholar]
- 48.Feller M, Snel M, Moutzouri E, et al. Association of Thyroid Hormone Therapy With Quality of Life and Thyroid-Related Symptoms in Patients With Subclinical Hypothyroidism: A Systematic Review and Meta-analysis. JAMA. 2018;320(13):1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mooijaart SP, Du Puy RS, Stott DJ, et al. Association Between Levothyroxine Treatment and Thyroid-Related Symptoms Among Adults Aged 80 Years and Older With Subclinical Hypothyroidism. JAMA. 2019;322(20):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parle J, Roberts L, Wilson S, et al. A randomized controlled trial of the effect of thyroxine replacement on cognitive function in community-living elderly subjects with subclinical hypothyroidism: the Birmingham Elderly Thyroid study. J Clin Endocrinol Metab. 2010;95(8):3623–3632. [DOI] [PubMed] [Google Scholar]
- 51.Ross DS. Subclinical hypothyroidism in nonpregnant adults. Waltham, MA: UpToDate; 2020. [Google Scholar]
- 52.Bekkering GE, Agoritsas T, Lytvyn L, et al. Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline. BMJ. 2019;365:l2006. [DOI] [PubMed] [Google Scholar]
- 53.Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24(12):1670–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Das G, Ojewuyi TA, Baglioni P, Geen J, Premawardhana LD, Okosieme OE. Serum thyrotrophin at baseline predicts the natural course of subclinical hyperthyroidism. Clin Endocrinol (Oxf). 2012;77(1):146–151. [DOI] [PubMed] [Google Scholar]
- 55.Vadiveloo T, Donnan PT, Cochrane L, Leese GP. The Thyroid Epidemiology, Audit, and Research Study (TEARS): the natural history of endogenous subclinical hyperthyroidism. J Clin Endocrinol Metab. 2011;96(1):E1–8. [DOI] [PubMed] [Google Scholar]
- 56.Díez JJ, Iglesias P. An analysis of the natural course of subclinical hyperthyroidism. Am J Med Sci. 2009;337(4):225–232. [DOI] [PubMed] [Google Scholar]
- 57.Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid. 2016;26(10):1343–1421. [DOI] [PubMed] [Google Scholar]
- 58.Cappola AR, Fried LP, Arnold AM, et al. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295(9):1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collet TH, Gussekloo J, Bauer DC, et al. Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch Intern Med. 2012;172(10):799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gammage MD, Parle JV, Holder RL, et al. Association between serum free thyroxine concentration and atrial fibrillation. Arch Intern Med. 2007;167(9):928–934. [DOI] [PubMed] [Google Scholar]
- 61.Selmer C, Olesen JB, Hansen ML, et al. The spectrum of thyroid disease and risk of new onset atrial fibrillation: a large population cohort study. BMJ. 2012;345:e7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vadiveloo T, Donnan PT, Cochrane L, Leese GP. The Thyroid Epidemiology, Audit, and Research Study (TEARS): morbidity in patients with endogenous subclinical hyperthyroidism. J Clin Endocrinol Metab. 2011;96(5):1344–1351. [DOI] [PubMed] [Google Scholar]
- 63.Sgarbi JA, Matsumura LK, Kasamatsu TS, Ferreira SR, Maciel RMB. Subclinical thyroid dysfunctions are independent risk factors for mortality in a 7.5-year follow-up: the Japanese–Brazilian thyroid study. European Journal of Endocrinology. 2010;162(3):569. [DOI] [PubMed] [Google Scholar]
- 64.Chaker L, Baumgartner C, Ikram MA, et al. Subclinical thyroid dysfunction and the risk of stroke: a systematic review and meta-analysis. Eur J Epidemiol. 2014;29(11):791–800. [DOI] [PubMed] [Google Scholar]
- 65.Biondi B, Bartalena L, Cooper DS, Hegedüs L, Laurberg P, Kahaly GJ. The 2015 European Thyroid Association Guidelines on Diagnosis and Treatment of Endogenous Subclinical Hyperthyroidism. Eur Thyroid J. 2015;4(3):149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burch HB, Cooper DS. Management of Graves Disease: A Review. JAMA. 2015;314(23):2544–2554. [DOI] [PubMed] [Google Scholar]


