Abstract
There are no studies in literature on the effect of inoculant concentrations on plant growth promotion. Therefore, in the present study, two experiments were carried out, one under pot conditions and the other in the field with cotton crop, in order to verify the effect of Aspergillus and Bacillus concentrations on the biometric and nutritional parameters of plant and soil, in addition to yield. The pot experiment evaluated the effect of different concentrations, ranging from 1 × 104 to 1 × 1010 colony-forming units per milliliter (CFU mL–1) of microorganisms Bacillus velezensis (Bv188), Bacillus subtilis (Bs248), B. subtilis (Bs290), Aspergillus brasiliensis (F111), Aspergillus sydowii (F112), and Aspergillus sp. versicolor section (F113) on parameters plant growth promotion and physicochemical and microbiological of characteristics soil. Results indicated that the different parameters analyzed are influenced by the isolate and microbial concentrations in a different way and allowed the selection of four microorganisms (Bs248, Bv188, F112, and F113) and two concentrations (1 × 104 and 1 × 1010 CFU mL–1), which were evaluated in the field to determine their effect on yield. The results show that, regardless of isolate, inoculant concentrations promoted the same fiber and seed cotton yield. These results suggest that lower inoculant concentrations may be able to increase cotton yield, eliminating the need to use concentrated inoculants with high production cost.
Keywords: rhizobacteria, Aspergillus sydowii, Bacillus sp., yield, growth promoters, inoculants
Introduction
The use of plant-growth promoting microorganisms (PGPMs) has increased in the world as an alternative to the excessive application of mineral fertilizers that can contribute to soil degradation, emission of polluting gases into the atmosphere, and reduction of biodiversity in different ecosystems (Singh et al., 2016).
Inoculants are products that have in their composition live microorganisms capable of promoting plant development with different mechanisms or modes of action, such as production of phytohormones and siderophores, phosphate solubilization, and induction of resistance against abiotic and biotic stresses (Bhattacharyya and Jha, 2012; Malusá and Vassilev, 2014). PGPM application has been carried out in several agricultural cultures, and many studies have been developed to elucidate its mode of action in plants to meet the new requirements of industries in the sector and agricultural producers. The microorganisms most frequently used as inoculants are fungi of the genera Trichoderma, Purpureocillium, Metarhizium, Beauveria, and Aspergillus (Behie and Bidochka, 2014; Samson et al., 2014; Alori and Babalola, 2018; Baron et al., 2018, 2020; Ahmad et al., 2020), and bacteria of the genera Azospirillum, Azotobacter, Bacillus, Enterobacter, and Streptomyces (Kloepper et al., 1989; Okon and Labandera-Gonzalez, 1994; Glick et al., 1999; Tahmatsidou et al., 2006; Marulanda et al., 2009; Pedraza et al., 2010; Diaz et al., 2019).
Under field conditions, PGPMs are applied in the form of formulated products, which contain inerts and additives in addition to the active ingredient, which is the microorganism. The search for new inoculant formulations, which enhance plant development in order to reduce the use of mineral fertilizers, thus contributing to more sustainable agriculture, is increasing (Malusá and Vassilev, 2014; Bizos et al., 2020). These new formulations have included increasing the concentration of microorganisms to be applied in the field. However, despite the advance in the use of inoculants in agriculture, there are few studies that have evaluated the effect of inoculant concentration on plant growth promotion, particularly in cotton. Thus, this theme has become essential to define whether the increase in the concentration of microorganisms is an important aspect related to product efficiency or whether it is just an aspect of commercial advantage.
In this study, cotton was used because it is a crop that stands out for its high demand for mineral fertilizers and phytosanitary products to ensure good productivity, a situation that causes serious changes in the environment (Michereff and Barros, 2001; Carvalho and Barcellos, 2012).
The aim was to determine the effect of different concentrations of microorganisms Bacillus velezensis, Bacillus subtilis, Aspergillus brasiliensis, Aspergillus sydowii, and Aspergillus sp. (versicolor section) on the growth of cotton plants under pot conditions in greenhouse and field conditions.
Materials and Methods
Study Location
According to the Köppen and Geiger classification, the climate of the region corresponds to a tropical climate with dry season in the winter (Peel et al., 2007). The predominant soil at the site is classified as Red Eutrophic Latosol (Oxisol) with clayey texture (52% clay, 23% silt, and 24% total sand) (EMBRAPA, 2006).
Experiment 1: Determination of the Effect of Inoculation of Microorganisms at Different Concentrations in Greenhouse
Microorganisms and Inoculant Preparation
Microorganisms (bacteria and fungi) used in this study belong to the collection of the Laboratory of Soil Microbiology, UNESP, Campus of Jaboticabal (Table 1) and were selected for presenting growth-promoting characteristics such as phosphorus solubilization, biological nitrogen fixation, and indole acetic acid production (Baron et al., 2018; Diaz et al., 2019; Milani et al., 2019).
TABLE 1.
Description of microorganisms.
The microorganisms used in the study were pre-inoculated in Petri dishes containing nutrient agar for bacteria and potato dextrose agar for fungi. Incubation was carried out in BOD oven at 30°C for 24 h for bacteria and at 25°C for 7 days for fungi.
Each bacterial isolate was multiplied in Erlenmeyer flask containing 90 ml of sterile nutrient broth medium inoculated with isolates prepared on Petri dishes. Flasks were incubated at 30°C for 24 h under agitation at 150 rpm. Then, absorbance readings of each isolate were carried out in spectrophotometer at 600 nm to determine the optical density. In addition, 100 μl of each flask with the different isolates was seeded in Petri dishes containing nutrient agar for the determination and adjustment of cell concentrations (Kloepper et al., 1989).
For fungi, conidium suspension was prepared by scraping Petri dishes containing mycelium cultivated on potato dextrose agar for 7–10 days at 25°C. For scraping, 0.1% Tween 80 solution was used. Fungi suspensions obtained were filtered in sterile voile to remove excess mycelium. The determination of the conidium concentration of each fungus was performed by counting in Neubauer chamber. For all microorganisms (bacteria and fungi), concentrations of 1 × 104, 1 × 106, 1 × 108, and 1 × 1010 colony-forming units/ml (CFU mL–1) were standardized for bacteria and conidia ml for fungi.
Seed Inoculation
Cotton seeds were individually inoculated with microorganisms (bacteria or fungi) by immersion for 8 h at 25°C (Jaber and Enkerli, 2016). Immersion was carried out in the dark under agitation at 130 rpm. This procedure was performed for all microorganisms and concentrations. After the immersion period, cotton seeds were sown in pots containing previously sieved soil.
Cotton seedlings were inoculated three times from the beginning to the end of the experiment at 15-day intervals. In each inoculation, 10 ml of suspension containing the respective microorganism at concentrations of 1 × 104, 1 × 106, 1 × 108, and 1 × 1010 CFU mL–1 for bacteria and conidia ml for fungi was applied per pot. Inoculations were performed by applying the inoculum at the base and stem of plants using graduated micropipette (Kasvi monocanal premium black k1-1000 PB).
Experimental Design and Experiment Management
The experiment was carried out at the Horticulture Sector of the “Júlio de Mesquita Filho” São Paulo State University (UNESP), Campus of Jaboticabal, São Paulo, Brazil. The experiment was arranged in a randomized block design with 6 × 4 factorial arrangement + 1 additional treatment (control) with five replicates, totaling 125 pots. Microorganism factor sublevels were Bs248, Bs290, Bv188, F111, F112, and F113 (Table 1). Concentration factor sublevels were 1 × 104, 1 × 106, 1 × 108, and 1 × 1010 CFU or conidia ml–1. Pots of 5-L capacity were filled with sieved soil (particles smaller than 1 cm in diameter) and fertilized according to previously performed soil analysis (Table 2) and nutritional recommendations for pot experiments proposed by Malavolta et al. (1997) for cotton crop. Nitrogen (N: 3.33 g urea/pot), phosphorus (P: 5.5 g P2O5/pot), potassium (K: 1.66 g KCl/pot), calcium (Ca: 6.25 g super single/pot), magnesium (Mg: 0.5 g MgO/pot), sulfur (S: 3.125 g super single/pot), zinc (Zn: 0.125 g ZnSO4/pot), boron (B: 0.025 g H3BO3/pot), molybdenum (Mo: 0.002 g molybdate/pot), copper (Cu: 0.03 g CuSO4/pot), and manganese (Mn: 0.08 g MnSO4/pot) were added. All nutrients were mixed with the sieved soil 1 week before sowing. The moisture content of pots was kept around 70% of the field capacity with daily irrigations.
TABLE 2.
Analysis of soil used in greenhouse and field experiments.
| pH | OM | P | K | Ca | Mg | H + Al | S.B. | CEC | V |
| CaCl2 | g/dm3 | Mg/dm3 | ……………………mmolc/dm3………………… | % | |||||
| 6.9 | 10 | 23 | 0.7 | 79 | 13 | 11 | 93.4 | 104.2 | 90 |
OM, organic matter; S.B., Ca + Mg + Na + K; CEC, S.B. + H + Al; V%, (S.B./CEC) ∗ 100.
Five cotton seeds (Gossypium hirsutum–IMA7501 WS) were sown per pot; and 15 days after seedling emergence, thinning was performed, keeping one plant per pot. The experiment was carried out until the flowering of cotton plants, 70 days after emergence.
Evaluated Parameters
Shoot and Root Dry Matter
Plants were collected and separated into shoots and roots, washed in running water, and placed in paper bags for drying in oven with air circulation at 65°C until reaching constant weight. Root and shoot dry matter weight was determined using analytical scale.
Preparation of Soil Samples
Samples were separated into two subsamples of approximately 100 g each. A subsample was sieved and dried at room temperature for chemical analysis, and the other was kept in a refrigerator for microbiological analysis.
Counting Bacteria Present in the Soil
Ten grams of soil was placed in an Erlenmeyer flask containing 95 ml of 0.1% sodium pyrophosphate saline solution. All Erlenmeyer flasks were shaken for 1 h at 130 rpm, and the contents of flasks were used to prepare serial dilutions following methodology proposed by Wollum (1982). Aliquots of 100 μl of obtained dilutions were inoculated into Petri dishes containing nutrient agar medium or potato dextrose agar in triplicate. Plates were kept in BOD oven at 30°C for bacteria and 25°C for fungi. The number of CFU mL–1 was verified after 24, 48, and 72 h (Vieira and Nahas, 2000).
Counting of Endophytic Bacteria and Fungi
Plants were separated into leaves and roots and washed with running water. Samples containing 3 g of each vegetative tissue (leaves and roots) were submitted to superficial disinfection to eliminate epiphytic microorganisms. Each tissue (leaf or root) was sequentially immersed in 70% ethanol for 1 min, sodium hypochlorite solution (2.0–2.5% active Cl) for 4 min, and 70% ethanol for 30 s. Subsequently, tissues were washed three times with distilled water. Once washed and disinfected, tissues were macerated with 3 ml of sterile 0.85% saline solution with the aid of a flask and a pestle (de Araújo et al., 2002). The macerated material was used to prepare serial dilutions, and 100 μl of aliquots was seeded in Petri dishes containing tryptone soy agar (TSA) medium for bacterial isolation and potato dextrose agar for fungal isolation. Plates were grown in microbiological greenhouses at constant temperature of 30°C for 24 h for bacterial growth and at 25°C for 7 days in the case of fungal isolation (Caruso et al., 2000). Microorganism counts were performed in separate groups, fungi, and bacteria with their respective controls.
Determination of the Phosphorus Concentration in Plants and Soil
The determination of soluble soil phosphorus was carried out using the method proposed by Watanabe and Olsen (1965). For the determination of phosphorus in plants, phosphorus concentrations in roots and shoots were determined according to methodology proposed by Haag et al. (1975) and modified by Bezerra Neto and Barreto (2011).
Determination of the Total Nitrogen Concentration in Plants and Soil
The determination of the nitrogen concentration in shoots and roots was performed according to Haag et al. (1975) with sulfuric digestion of plant material to estimate the nitrogen concentration or dose associated with obtaining 90% of dry matter production. For the determination of total nitrogen in soil, the methodology proposed by Bremner and Mulvaney (1983) and modified by Wilke (2005) was used.
Microbial Respiratory Activity
The respiratory activity was determined by the method of quantification of released CO2 according to Jenkinson and Powlson (1976), using wide-mouth flasks with 100 g of soil (dry or wet). Inside flasks, two beakers (one containing 20 ml of NaOH, and the other 20 ml distilled water) were placed, were then sealed with plastic film, and incubated in the dark for 7 days. Microbial respiration was estimated from the amount of CO2 released from soil samples in a continuous air flow system free from CO2 and moisture. After incubation, the remaining NaOH was quantified by titration with HCl.
Microbial Biomass Carbon
Microbial biomass carbon was determined by the irradiation-extraction method (Islam and Weil, 1998; Mendonça and Matos, 2017), using microwave oven. After irradiation, samples were submitted to 0.5 mol/L of potassium sulfate extractor, and microbial biomass carbon was determined by oxidation with 0.066 mol/L of potassium dichromate followed by titration with 0.033 mol/L of ammonia ferrous sulfate (Brookes et al., 1982).
Statistical Analysis
Prior to analysis of variance, data normality (the Kolmogorov–Smirnov test) and homogeneity of variances (Levene’s test) were tested for each parameter evaluated. Data were transformed into (x + 0.5)1/2 to comply with assumptions of the analysis of variance. Comparisons of means were performed using Tukey’s test (α ≤ 0.05). Analyses were performed using the R 3.4.1 open software for Windows (R Core Team, 2020).
Experiment 2: Determination of the Effect of Inoculation of Microorganisms on Cotton Plants Under Field Conditions
Cotton Planting
The experiment was carried out at the Teaching, Research and Extension Farm (FEPE) – UNESP, Jaboticabal, São Paulo, during the off season (January–June 2020). The field soil was classified as Red Eutrophic Latosol (Oxisol) with clayey texture. Soil chemical analysis is detailed in Table 2.
Soil fertilization was performed once before sowing using the 8–28–16 of NPK + 0.5% Zn formula, with the amount of nitrogen 80% lower than the requirement to avoid masking the effect produced by microorganisms and their concentrations on cotton yield. Cotton was sown at spacing of 1 m between rows and 8–10 seeds per linear meter. The dimensions of the plot were 5 m in length by 5 m in width with useful area of 15 m2.
The microorganisms used in the experiment were selected based on results of experiment 1. Microorganisms Bs248, Bv188, F112, and F113 were tested at concentrations of 1 × 104 and 1 × 1010 CFU or conidia ml–1. The multiplication of these microorganisms was performed as previously described in experiment 1. Application was performed three times, every 15 days, using back sprayer with constant pressure. In this experiment, seeds were not inoculated, and the first application was carried out 7 days after the emergence of cotton seedlings.
Microorganisms were applied at dose of 1 L of suspension per hectare (ha). The amount of water used was 200 L/ha (500 ml per useful area of 15 m2). The control treatment was sprayed with water only. Cotton was manually harvested 151 days after seedling emergence. Seed cotton was harvested from plants of the useful plot (15 m2).
Experimental Design and Experiment Management
A randomized block design with 4 × 2 factorial arrangement + 1 additional treatment (control) with four replicates was used. Microorganism factor sublevels were Bs248, Bv188, F112, and F113. Concentration factor sublevels were 1 × 1010 and 1 × 104 CFU mL–1. Crop management was carried out considering commercial management for the region.
Evaluated Parameters
Parameters were evaluated by manual harvesting of plants in useful plots. The weight of seed cotton was measured using analytical scale. After drying in oven with air circulation at 65°C, seeds were manually separated from fibers and weighed on analytical scale. Fiber weight was obtained by the difference between the weight of the cotton harvested and the weight of the seed. Seed weight and fiber weight were estimated in kg/ha.
Data Analysis
Analyses were performed using the R software for Windows (R Core Team, 2020). The normality and homogeneity of variances were assessed using the Shapiro–Wilk test and Levene’s test (α ≤ 0.05), respectively. Treatments were analyzed using ANOVA, followed by Tukey’s test (α ≤ 0.05) to compare the mean of treatments.
Results and Discussion
Experiment 1: Determination of the Effect of Inoculation of Microorganisms at Different Concentrations in Greenhouse
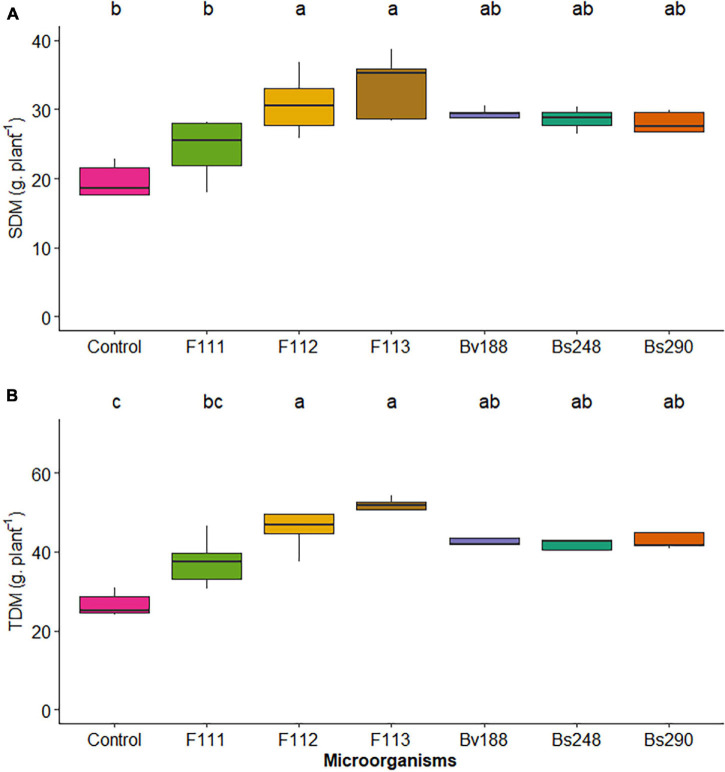
The results indicate that there was no interaction between microorganism factor and inoculant concentration for variables shoot, root, and total dry matter in cotton plants. This means that regardless of microorganism, the behavior was the same, given the different inoculant concentrations. Furthermore, there was no effect of the concentration factor on variables shoot, root and total dry matter, nitrogen content in root dry matter, phosphorus in shoot dry matter, and biomass carbon; however, there was a significant effect of the microorganism factor on variables shoot (Figure 1A) and total (Figure 1B) dry matter, highlighting fungi A. sydowii and Aspergillus sp. versicolor section, with values of 30.83 and 33.40 g/plant, respectively, for shoot dry matter, and 47.71 and 51.20 g/plant, respectively, for total dry matter, compared with control treatment, which was 23.40 g/plant for shoot dry matter and 30.04 g/plant for total dry matter.
FIGURE 1.
Boxplots (median and quartiles) of SDM (A) and TDM (B) in cotton inoculated with plant growth-promoting microorganisms. Different lowercase letters in the line indicate statistical difference between means (Tukey, p < 0.05). F111, Aspergillus brasiliensis; F112, Aspergillus sydowii; F113, Aspergillus sp.; Bv188, Bacillus velezensis strain Bv188; Bs248, Bacillus subtilis strain Bs248; Bs290, B. subtilis strain Bs290; Ctrl, control; SDM, shoot dry matter; TDM, total dry matter.
Plant–fungus associations are mainly established by two groups of fungi, mycorrhizal and endophytic fungi (Bonfante and Genre, 2010). Endophytic fungi are those capable of living endosymbiotically with plants without causing disease symptoms (Behie and Bidochka, 2014). They can act as plant growth promoters, increase germination rate, improve seedling establishment, and increase plant resistance to biotic and abiotic stresses, producing antimicrobial compounds, phytohormones, and other bioactive compounds. In addition, endophytic fungi are responsible for the acquisition of soil nutrients, including macronutrients such as phosphorus, nitrogen, potassium, and magnesium, and micronutrients such as zinc, iron, and copper (Behie and Bidochka, 2014; Rai et al., 2014; Khan et al., 2015).
Soil fungi are widely distributed and participate in ecological processes that influence plant growth and soil health. It is considered that the diversity of fungi that inhabit the soil and the rhizosphere can reach more than 200 species in a single soil (Vandenkoornhuyse et al., 2002).
Several Aspergillus species are commercially exploited due to their ability to produce and secrete many enzymes and metabolites, such as antibiotics and mycotoxins (Volke-Sepulveda et al., 2016). The ability of fungi of the genus Aspergillus to produce secondary metabolites is very important because they play a vital role in survival and adaptation in soil; in addition, they are involved in the degradation of a wide range of natural organic substrates, particularly plant materials (Goldman and Osmani, 2008).
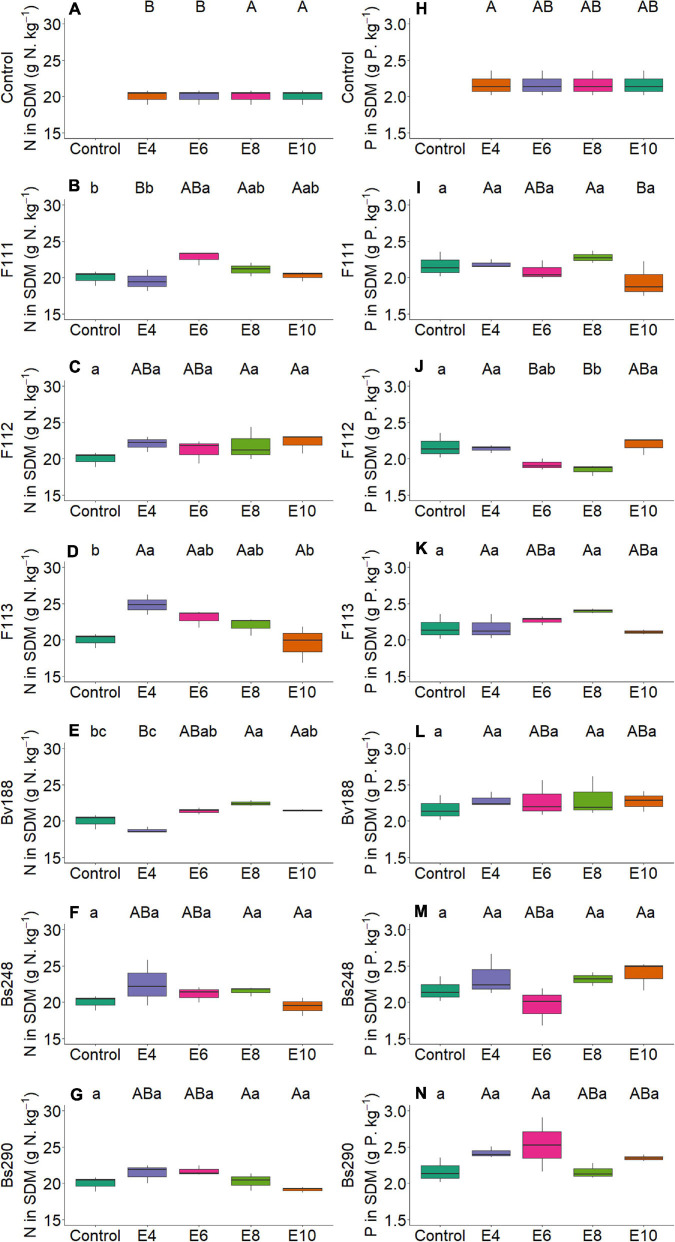
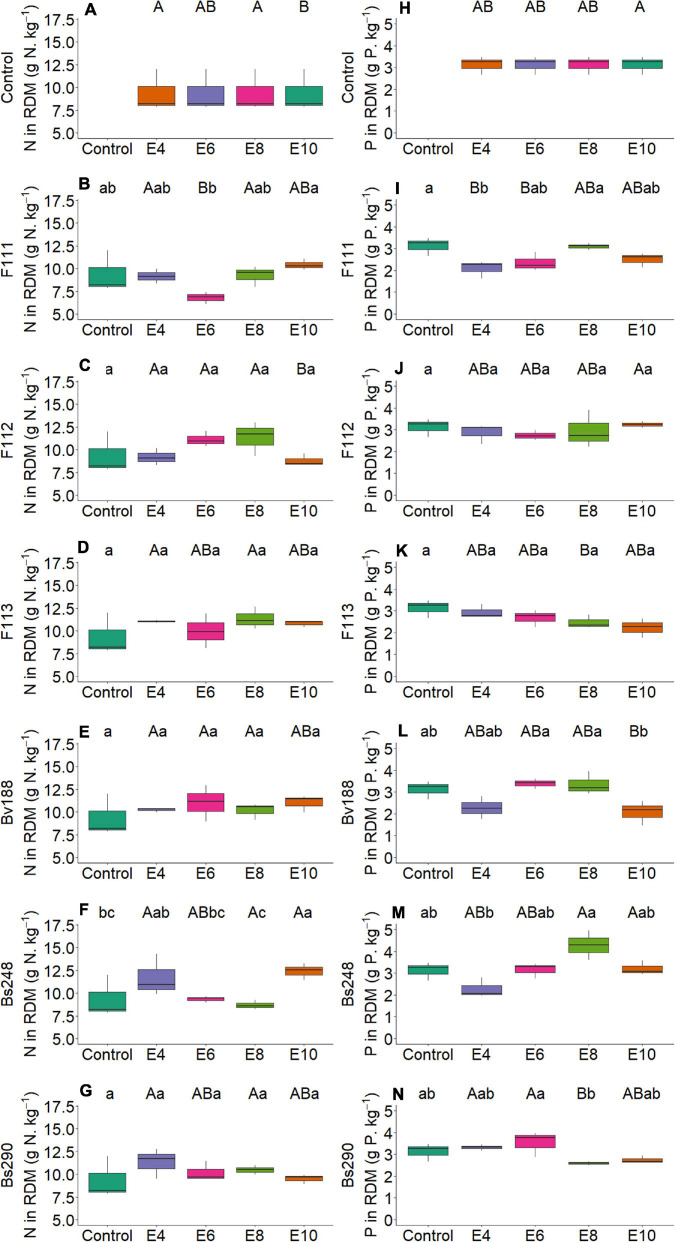
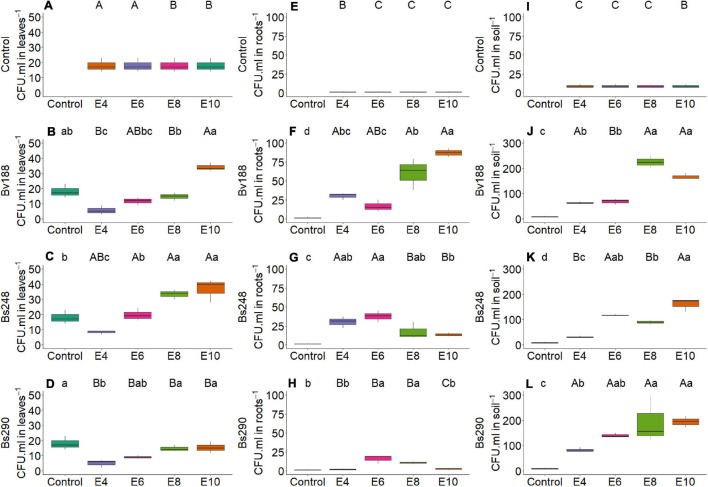
On the other hand, there was interaction between microorganism factor and inoculant concentration with variables nitrogen and phosphorus content in shoot (Figure 2) and root dry matter (Figure 3), soil phosphorus (Figure 4), soil nitrogen percentage (Figure 5), respiratory activity (Figure 6), colony-forming units in leaves (Figures 7, 8), and colony-forming units in roots and soil (Figure 9).
FIGURE 2.
Boxplots (median and quartiles) of nitrogen (A–G) and phosphorus (H–N) content in SDM in cotton inoculated with plant growth-promoting microorganisms. Different lowercase letters in a row and uppercase letters in a column indicate statistical difference between means (Tukey, p < 0.05). F111, Aspergillus brasiliensis; F112, Aspergillus sydowii; F113, Aspergillus sp.; Bv188, Bacillus velezensis strain Bv188; Bs248, Bacillus subtilis strain Bs248; Bs290, B. subtilis strain Bs290; E4, 1 × 104; E6, 1 × 106; E8, 1 × 108; E10, 1 × 1010 conidia or CFU mL−1; Ctrl, control; SDM, shoot dry matter.
FIGURE 3.
Boxplots (median and quartiles) of nitrogen (A–G) and phosphorus (H–N) content in RDM in cotton inoculated with plant growth-promoting microorganisms. Different lowercase letters in a row and uppercase letters in a column indicate statistical difference between means (Tukey, p < 0.05). F111, Aspergillus brasiliensis; F112, Aspergillus sydowii; F113, Aspergillus sp.; Bv188, Bacillus velezensis strain Bv188; Bs248, Bacillus subtilis strain Bs248; Bs290, B. subtilis strain Bs290; E4, 1 × 104; E6, 1 × 106; E8, 1 × 108; E10, 1 × 1010 conidia or CFU mL−1; Ctrl, control; RDM, root dry matter; CFU, colony-forming units.
FIGURE 4.
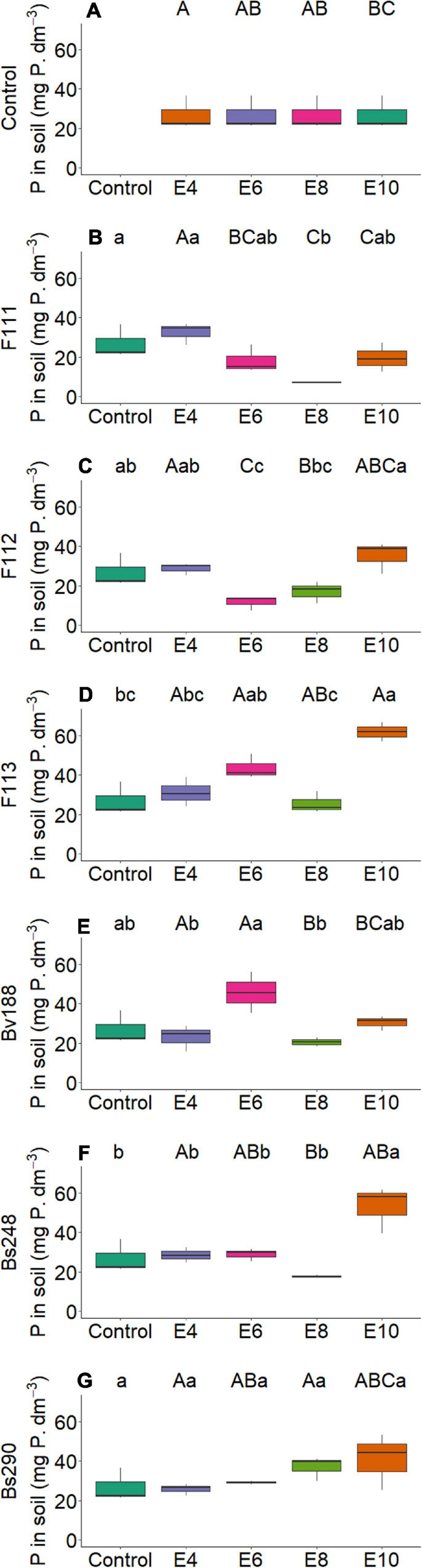
Boxplots (median and quartiles) of phosphorus in soil sown with cotton and inoculated with plant growth-promoting microorganisms: Control (A); F111 (B); F112 (C); F113 (D); Bv188 (E); Bs248 (F); and Bs290 (G). Different lowercase letters in row and uppercase letters in column indicate statistical difference between the means (Tukey, P < 0.05). Abbreviations: F111, Aspergillus brasiliensis; F112, A. sydowii; F113, Aspergillus sp.; Bv188, B. velezensis strain Bv188; Bs248, B. subtilis strain Bs248; Bs290, B. subtilis strain Bs290; E4, 1 × 104; E6, 1 × 106, E8, 1 × 108; E10, 1 × 1010 conidia or CFU/ml; Ctrl, Control; CFU, colony- forming units.
FIGURE 5.
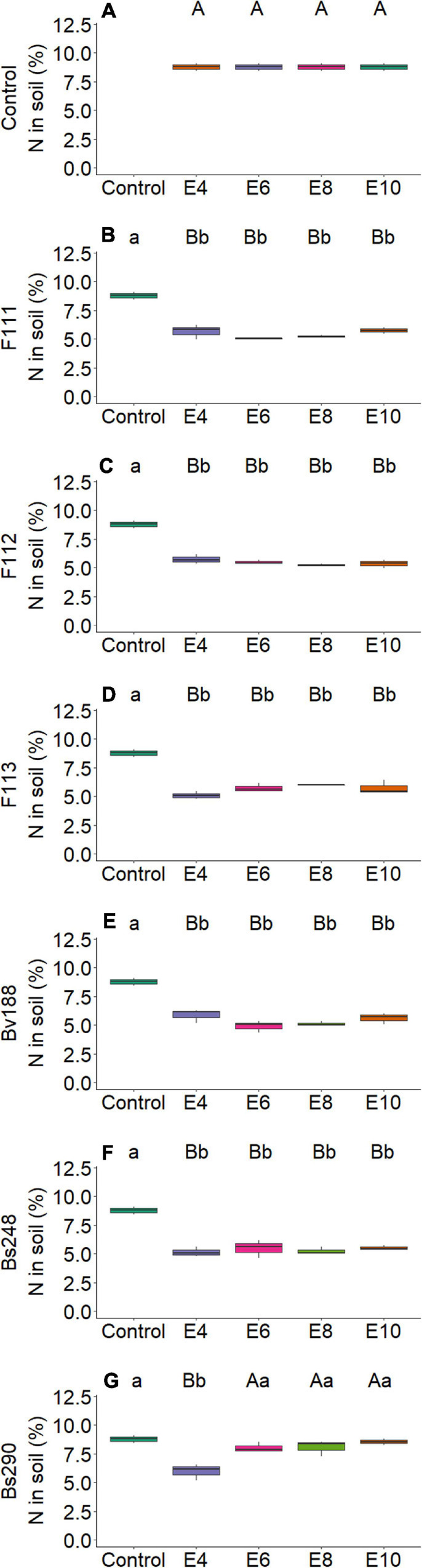
Boxplots (median and quartiles) of percentage of nitrogen in soil sown with cotton and inoculated with plant growth-promoting microorganisms: Control (A); F111 (B); F112 (C); F113 (D); Bv188 (E); Bs248 (F); and Bs290 (G). Different lowercase letters in row and uppercase letters in column indicate statistical difference between means (Tukey, P < 0.05). Abbreviations: F111, Aspergillus brasiliensis; F112, A. sydowii; F113, Aspergillus sp.; Bv188, B. velezensis strain Bv188; Bs248, B. subtilis strain Bs248; Bs290, B. subtilis strain Bs290; E4, 1 × 104; E6, 1 × 106; E8, 1 × 108; E10, 1 × 1010 conidia or CFU/ml; Ctrl, Control; CFU, colony- forming units.
FIGURE 6.
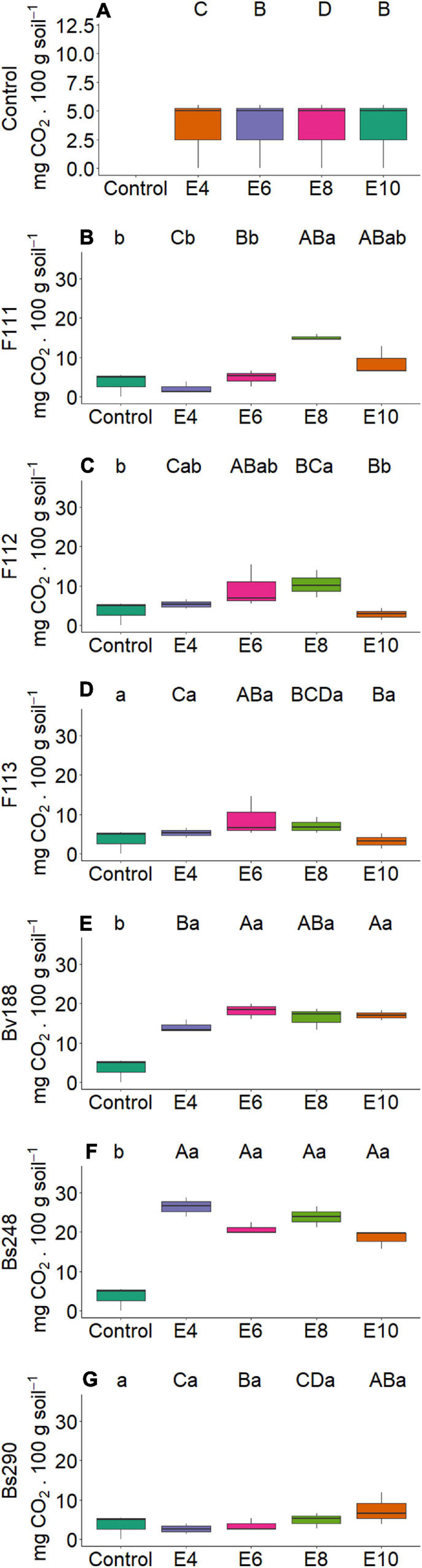
Boxplots (median and quartiles) of respiratory activity in soil sown with cotton and inoculated with plant growth-promoting microorganisms: Control (A); F111 (B); F112 (C); F113 (D); Bv188 (E); Bs248 (F); and Bs290 (G). Different lowercase letters in row and uppercase letters in column indicate statistical difference between means (Tukey, P < 0.05). Abbreviations: F111, Aspergillus brasiliensis; F112, A. sydowii; F113, Aspergillus sp.; Bv188, B. velezensis strain Bv188; Bs248, B. subtilis strain Bs248; Bs290, B. subtilis strain Bs290; E4, 1 × 104; E6, 1 × 106; E8, 1 × 108; E10, 1 × 1010 conidia or CFU/ml; Ctrl, Control; CFU, colony- forming units.
FIGURE 7.
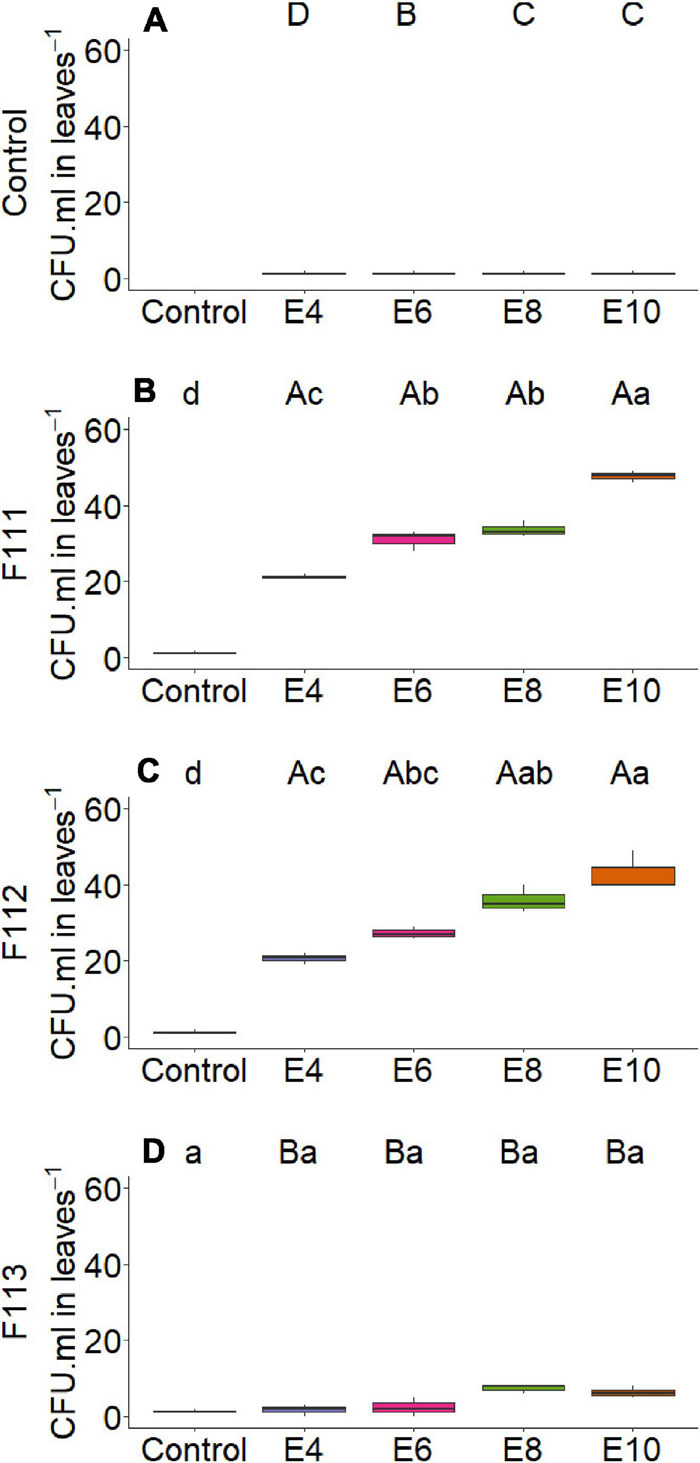
Boxplots (median and quartiles) of CFU in cotton leaves inoculated with (A) Aspergillus brasiliensis (B), Aspergillus sydowii (C), and Aspergillus sp. (D) in four concentrations. Different lowercase letters in a row and uppercase letters in a column indicate statistical difference between means (Tukey, p < 0.05). F111, Aspergillus brasiliensis; F112, A. sydowii; F113, Aspergillus sp.; E4, 1 × 104; E6, 1 × 106; E8, 1 × 108; E10, 1 × 1010 conidia or CFU mL−1; Ctrl, control; and CFU, colony-forming units.
FIGURE 8.
Boxplots (median and quartiles) of CFU in leaves (A–D), root (E–H), and soil (I–L) inoculated with plant growth-promoting microorganisms. Different lowercase letters in a row and uppercase letters in the vertical indicate statistical difference between means (Tukey, p < 0.05). Bv188, Bacillus velezensis strain Bv188; Bs248, Bacillus subtilis strain Bs248; Bs290, B. subtilis strain Bs290; E4, 1 × 104; E6, 1 × 106; E8, 1 × 108; E10, 1 × 1010 conidia or CFU mL−1; Ctrl, control; CFU, colony-forming units.
FIGURE 9.
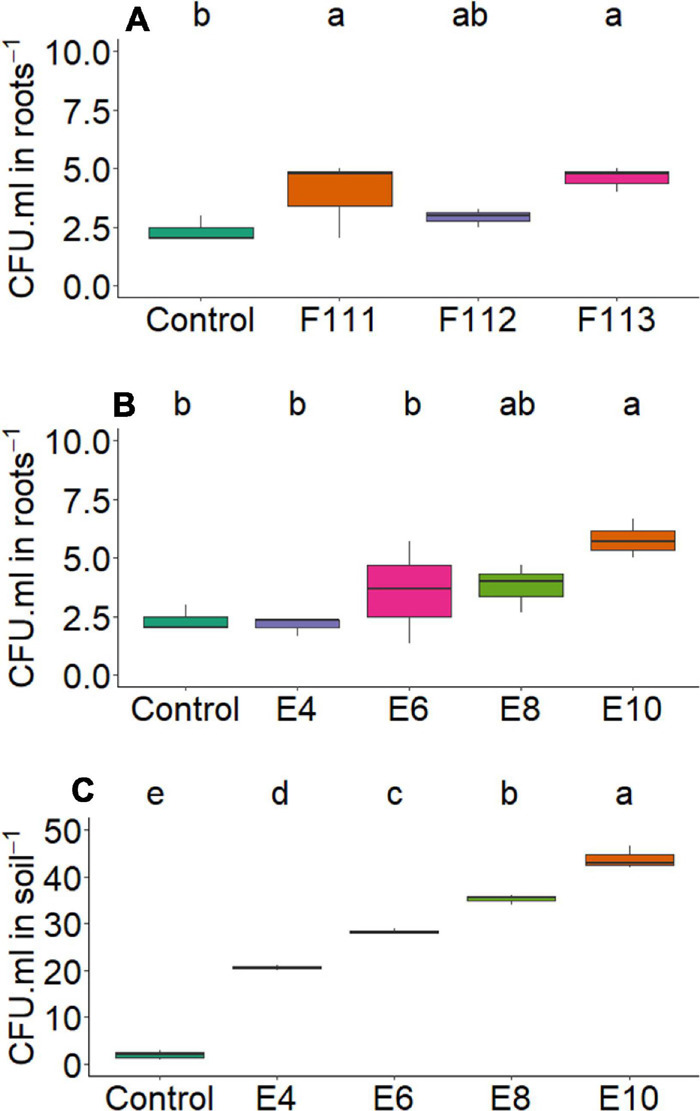
Boxplots (median and quartiles) of CFU in root (A,B) and soil (C) inoculated with Aspergillus brasiliensis, Aspergillus sydowii, and Aspergillus sp. Different lowercase letters in a row indicate statistical difference between means (Tukey, p < 0.05). F111, Aspergillus brasiliensis; F112, A. sydowii; F113, Aspergillus sp.; E4, 1 × 104; E6, 1 × 106; E8, 1 × 108; E10, 1 × 1010 conidia or CFU mL−1; Ctrl, control; CFU, colony-forming units.
For fungus A. brasiliensis, the unfolding of interactions indicates that inoculation in cotton plants at a concentration of 1 × 106 conidia ml–1 favored the increase in shoot nitrogen content (22.75 g N/kg; Figure 2B); root and soil phosphorus contents were lower at concentrations of 1 × 104 and 1 × 108 conidia ml–1, with values of 2.09 g P/kg and 7.10 mg P/dm3 soil, when compared with controls (3.13 g P/kg and 26.91 mg P/dm3 soil, respectively) (Figures 3I, 4B). Species of the genus Aspergillus, according to Souchie et al. (2006), Pacheco and Damasio (2013), and de Oliveira Mendes et al. (2014), highlight the phosphorus solubilization capacity and its potential for use as solubilizers for different sources of phosphorus in the soil. Schneider et al. (2010) reported the ability to synthesize organic acids and produce large amounts of citric acid, which is one of the main factors responsible for the solubilization of phosphorus in these fungi. The soil nitrogen percentage was lower than that of control at all inoculant concentrations (Figure 5B). These results suggest that A. brasiliensis can serve as hosts for nitrogen-fixing bacteria (endosymbionts) (Paul et al., 2020). These interactions may allow the plant to have absorbed nitrogen fixed and/or contained in the soil. The nitrogen-fixing property is absent in eukaryotes, but they circumvented this deficiency by associating with nitrogen-fixing bacteria (Kneip et al., 2007).
The soil respiratory activity reached the highest value (14.98 mg CO2/100 g soil) at a concentration of 1 × 108 conidia ml–1 compared with control, 3.50 mg CO2 (Figure 6B); and the number of colony-forming units in leaves was higher for all inoculant concentrations compared with control (Figure 7B). For values of colony-forming units in roots, although presenting no interaction, there was a significant effect of the microorganism factor, where A. brasiliensis stood out, with 3.92 CFU mL–1 (p < 0.039, Figure 9A); in addition, a positive correlation (p < 0.05) was observed between inoculant concentration and the number of colony-forming units in roots (Figure 10A). A. brasiliensis was isolated from the cotton plant, demonstrating that this fungus was probably able to colonize and enter the plant, showing its effects as an endophytic growth-promoting fungus on cotton. A. brasiliensis is described as a fast-growing and sporulating species, with characteristics closely related to Aspergillus niger (Varga et al., 2007); and A. sydowii is described as one of the fungi most commonly found in the soil (Raper and Fennell, 1965; Klich, 2002) and is used in industry for the production of enzymes such as β-glucosidase, α-galactosidase, cellulase, and xylanase (Tian et al., 2016).
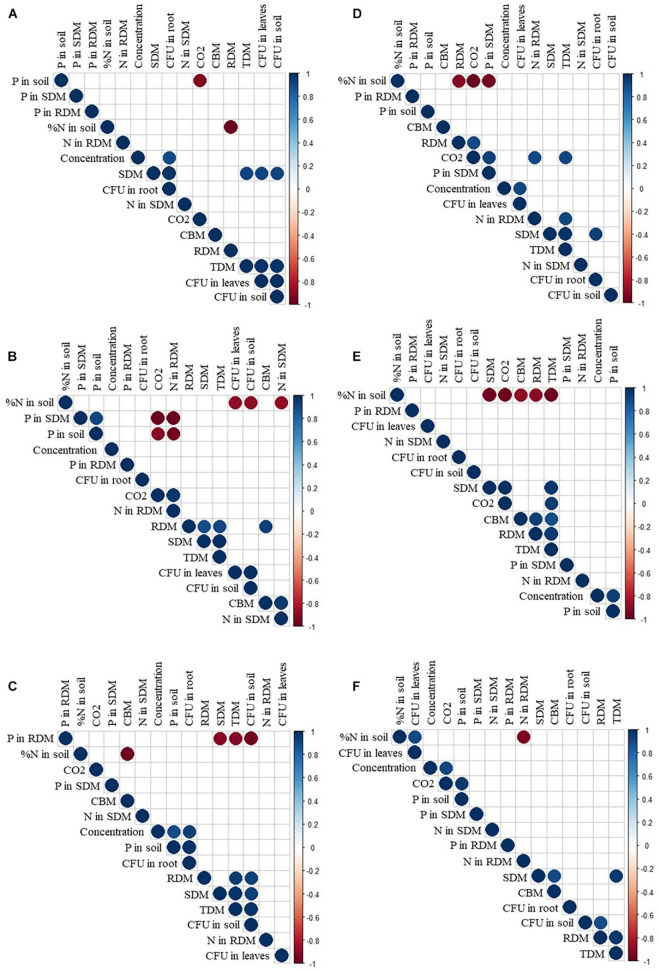
FIGURE 10.
Correlation of growth promotion variables and concentration of Aspergillus brasiliensis (A), Aspergillus sydowii (B), Aspergillus sp. (C), Bacillus velezensis (D), and Bacillus subtilis strain Bs248 (E) and Bs290 (F). P, phosphorus; N, nitrogen; SDM, shoot dry matter; RDM, root dry matter; TDM, total dry matter; CO2, respiratory activity; CBM, biomass carbon; and CFU, colony-forming units.
For A. sydowii, the unfolding of interactions indicates that the shoot phosphorus content presented lower value at a concentration of 1 × 108 conidia ml–1 (1.85 g P/kg, Figure 2J) when compared with control (2.17 g P/kg); the soil phosphorus content was lower with 11.68 mg P/dm3 at a concentration of 1 × 106 conidia ml–1, and control reached 26.91 mg P/dm3 (Figure 4C); the nitrogen percentage in soil inoculated with A. sydowii at all concentrations was lower than that of control (Figure 5C); the soil respiratory activity was higher (10.43 mg CO2/100 soil) with inoculation at a concentration of 1 × 108 conidia ml–1 compared with control, which was 3.5 mg CO2/100 soil (Figure 6C) and for colony-forming units in leaves, highlighting inoculation of A. sydowii at a concentration of 1 × 1010 conidia ml–1 with 43.00 CFU mL–1 compared with control, 1.33 CFU mL–1 (Figure 7C).
For Aspergillus sp. versicolor section, the interaction indicates that the highest nitrogen content in shoot dry matter was obtained at the lowest concentration of 1 × 104 conidia ml–1 (24.86 g N/kg; Figure 2D), when compared with control, 20.02 g N/kg; there was a positive correlation (p < 0.05, Figure 10C) between inoculum concentration and soluble phosphorus in soil, and the largest amount (62.00 mg P/dm3 soil) was obtained at a concentration of 1 × 1010 conidia ml–1 (Figure 4D) and control only 26.91 mg P/dm3 soil; and the soil nitrogen percentage was lower at all concentrations when compared with control (Figure 5D).
For colony-forming units in roots, there was a significant effect (p < 0.039, Figure 9A) of the microorganism factor, where Aspergillus sp. versicolor section stood out from control, with 4.58 CFU mL–1, and a positive correlation (p < 0.05) was observed between concentration and the number of colony-forming units in roots (Figure 10C). The greatest amount of CFU mL–1 in roots and soil was reached when plants were inoculated at maximum concentration (1 × 1010 conidia ml–1), regardless of fungus used (A. brasiliensis, A. sydowii, and Aspergillus sp. versicolor section) (Figures 9B,C).
For A. brasiliensis and A. sydowii, the increase in inoculum concentration had a positive effect on variable colony-forming units in leaves (Figures 7B,C); however, a concentration of 1 × 106 conidia ml–1 of A. brasiliensis proved to be appropriate to obtain higher shoot nitrogen contents (Figure 2B), and a concentration of 1 × 108 conidia ml–1 of A. brasiliensis or A. sydowii was suitable for higher respiratory activity values (Figures 6B,C).
The highest inoculant concentrations promoted the highest numbers of CFU mL–1 recovered from cotton roots and leaves. Endophytism promotes a more intimate interaction between a microorganism and a host, intensifying the benefits for both (Hardoim et al., 2008; Nadeem et al., 2014; Khan et al., 2015). Interestingly, treatments that presented a greater number of endophytic microorganisms did not necessarily promote greater plant development. Lobo et al. (2019) verified that the treatment that promoted a higher maize yield under field conditions, compared with control, also presented a lower number of recovered CFU mL–1. These results suggest that the growth-promoting effect probably depends more on the abilities of microorganisms and the interaction between microorganism and plant than on higher CFU mL–1 values.
According to results of the present study, the hypothesis that the highest A. brasiliensis and A. sydowii concentrations positively affect microorganism colonization can be confirmed. However, this greater colonization did not reflect in greater plant development. These results also show that A. brasiliensis and A. sydowii are fungi with endophytic capacity in cotton plants. This characteristic in both fungi is an advantage because the endophytic colonization of plant tissues allows the fungus to establish itself inside the organs for some time without causing apparent damage to the host (Petrini, 1991), in addition to protecting plants against eventual colonization and pathogen infection or pest infestation (Bulgarelli et al., 2013). Studies carried out in China have shown that A. niger P85 has the ability to solubilize phosphorus, produce indole acetic acid in maize plants, and increase available phosphorus in the soil (Yin et al., 2015); and in Brazil, similar studies have demonstrated the ability of A. sydowii and A. brasiliensis as phosphorus solubilizers in maize plants (Baron et al., 2018). A. brasiliensis and A. sydowii have great potential for use in other agricultural crops of great economic importance.
For Aspergillus sp. versicolor section, increasing inoculum concentration had a positive effect on soil phosphorus concentration and number of colony-forming units in roots (Figures 10B,C); however, a concentration of 1 × 104 conidia ml–1 was suitable for cotton plants to show the highest shoot nitrogen content (Figure 2D).
Aspergillus sp. versicolor section are accepted as distinct species based on molecular and phenotypic differences, are isolated from soil, and adapt to form part of the rhizospheric plant community (Zeljko et al., 2012). Aspergillus sp. versicolor section are fungi that are part of the microbial community of the rhizosphere of tea plants (Rahi et al., 2009). Similarly, in the present study, Aspergillus sp. versicolor section showed soil phosphorus solubilization capacity and root colonization. These characteristics are interesting in agriculture because inoculation with higher Aspergillus sp. versicolor section concentrations could decrease the need for use of mineral fertilizers in the field (Qiao et al., 2019; Caruso et al., 2020) as a consequence of the more efficient use of these fertilizers by plants. Some studies have shown that the association of this fungus with roots promotes abiotic stress tolerance and protection against pathogens (Singh et al., 2012; Begum et al., 2019; Rana et al., 2019).
For B. velezensis, the unfolding of interactions indicates that the nitrogen content in shoot dry matter of cotton plants was higher with 22.46 g N/kg at a concentration of 1 × 108 CFU mL–1 compared with control, 20.02 g N/kg (Figure 2E); the phosphorus content in the root dry matter and in the soil at all concentrations did not differ from that of control (Figures 3L, 4E); the soil nitrogen percentage was lower at all concentrations compared with that of control (Figure 5E); the respiratory activity was higher at all concentrations when compared with that of control (Figure 6E); the amount of colony-forming units in leaves, roots, and soil was higher at a concentration of 1 × 1010 CFU mL–1 (34.00, 93.67, and 163.33 CFU mL–1, respectively; Figures 8B,F,J); in addition, there was a positive correlation between concentration and colony-forming units in leaves (p < 0.05, Figure 10D).
For inoculation of B. subtilis Bs248, interaction indicates that the concentration of 1 × 1010 CFU mL–1 in cotton plants promoted the highest nitrogen content in the root dry matter (12.41 g N/kg) when compared with control (9.35 g N/kg) (Figure 3F); the phosphorus content in the root dry matter was not affected by concentration (Figure 3M); soil phosphorus at a concentration of 1 × 1010 CFU mL–1 was approximately double (53.15 mg P/dm3) that found at concentrations of 1 × 104, 1 × 106, and 1 × 108 CFU mL–1 and control (Figure 4F); in addition, there was a positive correlation between variable soil phosphorus and concentration (p < 0.05, Figure 10E); soil nitrogen percentage was lower, and the respiratory activity was higher when B. subtilis Bs248 was inoculated at any concentration (Figures 5F, 6F). The number of colony-forming units in leaves was higher when inoculum was applied at concentrations of 1 × 108 and 1 × 1010 CFU mL–1 (Figure 8C); the number of colony-forming units in roots was greater when inoculum was applied at a concentration of 1 × 106 CFU mL–1 (Figure 8G), and the number of colony-forming units in soil was greater at concentrations of 1 × 106 and 1 × 1010 CFU mL–1 (Figure 8K).
For B. subtilis Bs290, interaction indicates that the inoculation of cotton plants at a concentration of 1 × 104 CFU mL–1 had the lowest nitrogen percentage, 5.97%, when compared with control, which reached 8.77% (Figure 5G), and a smaller amount of colony-forming units in leaves with 5.00 CFU mL–1, when compared with control of 18.00 CFU mL–1 (Figure 8D); the number of colony-forming units in roots was higher, with 15.67 and 10.67 CFU mL–1, when the microorganism was inoculated at concentrations of 1 × 106 and 1 × 108 CFU mL–1, respectively (Figure 8H); and the number of colony-forming units in soil was higher, with 192.67 and 194.33 CFU mL–1, when inoculated at concentrations of 1 × 108 and 1 × 1010 CFU mL–1, respectively (Figure 8L). Additionally, a positive correlation was observed between concentration and respiratory activity (p < 0.05, Figure 10F).
Most Bacillus species are considered plant growth-promoting rhizobacteria and have the ability to colonize roots, improve nutrient availability, reduce abiotic stress, and produce a wide range of biologically active secondary metabolites that can inhibit the growth of pathogens (Ongena and Jacques, 2008; Lugtenberg and Kamilova, 2009; Bhattacharyya and Jha, 2012; Sivasakthi et al., 2014). The increase in inoculum concentration had a positive effect on variable colony-forming units in leaves for B. velezensis, soil phosphorus for B. subtilis Bs248, and a respiratory activity for B. subtilis Bs290.
Bacillus velezensis was previously grouped with B. subtilis and Bacillus amyloliquefaciens, and in recent years, several isolates of this bacterium have received attention due to their potential in disease control (Fan et al., 2017; Adeniji et al., 2019). Previous studies have determined that B. velezensis has the ability to produce indole acetic acid in pepper plants applied at a concentration of 1 × 108 CFU mL–1 (Zhang et al., 2019); in addition, it has been shown that metabolites produced have an antagonistic activity against bacterial and fungal pathogens under laboratory and greenhouse conditions in tomato crops (Cao et al., 2018). In the present study, B. velezensis showed the ability to colonize cotton leaves as the inoculum concentration increases. These results demonstrate that B. velezensis is an endophytic bacterium with capacity to promote growth through nitrogen content in shoot dry matter; in addition, results of colony-forming units in leaves suggest that B. velezensis has potential to inhibit the growth of pathogens in cotton plants.
On the other hand, studies have demonstrated the ability of B. subtilis to solubilize phosphate, produce indole acetic acid and siderophores, and increase dry weight in maize and sorghum (Aquino et al., 2019), okra, spinach, and tomato plants, in addition to presenting antagonistic action against Rhizoctonia solani (Adesemoye et al., 2009). Regarding colonization, studies carried out with cucumber and tomato plants inoculated with B. subtilis at concentrations of 105 and 106 CFU mL–1 of root were enough for the microorganism to be able to colonize and survive in the rhizosphere. Thus, in addition to protecting plants by suppressing Fusarium oxysporum from cucumber, B. subtilis had an antagonistic effect against Pseudomonas syringae after root colonization in tomato plants (Cao et al., 2011; Chen et al., 2013). In the present study, B. subtilis strains have shown a correlation between soil phosphorus content and respiratory activity. These results suggest that to improve phosphorus solubilization and respiration in the soil, it is necessary to increase inoculum concentration.
On the other hand, studies have shown that the long-term continuous use of inoculants influences the quantity and quality of microorganisms present in the soil rhizosphere, but this depends on conditions such as organic matter, availability of nutrients (such as phosphorus), and type of soil (Gnankambary et al., 2008; Angelina et al., 2020). Furthermore, it is important to consider that the composition of the soil community is largely influenced by environmental variability and the microbial community present in the soil (Xun et al., 2015).
As one of the most important and essential macronutrients in addition to nitrogen, phosphorus is important for plant development, but it is the nutrient element least mobile in plant and soil. Globally, P is extracted from geological sediments and added to agricultural soils in order to meet critical plant requirements for agronomic productivity. Phosphorus is present in soil in the organic and inorganic forms. The various inorganic forms of the element in the soil are salts with calcium, iron, and aluminum, while the organic forms come from decomposing vegetation and microbial residues. There is great diversity of plant microbiomes (epiphytic, endophytic, and rhizospheric) and soil microbiomes that have the ability to solubilize insoluble P and make it available for plants. The main solubilization mechanism of inorganic P is by the production of organic acids, which lower soil pH, or by the production of acids and alkaline phosphatases, which cause the mineralization of organic P. P-solubilizing and P-mobilizing microorganisms belong to all three domains: archaea, bacteria, and eukarya. Strains belonging to genera Arthrobacter, Bacillus, Burkholderia, Natrinema, Pseudomonas, Rhizobium, Serratia, and Aspergillus have been reported as efficient and potential P solubilizers. The use of P solubilizers, alone or in combination with another plant growth-promoting microbe as an ecological microbial consortium, could increase P uptake by plants, increasing their yields for agricultural and environmental sustainability (Kour et al., 2021). However, results have shown that for some treatments, phosphorus concentrations in soil and roots decreased. Factors such as mineral concentration, temperature, and availability of carbon and nitrogen (N) sources can affect the phosphorus solubilization potential of these microorganisms, and these results suggest that there was greater solubilization and absorption of phosphorus from the soil by plants and greater translocation to shoots.
For the field phase, A. sydowii was selected for presenting abilities to promote a positive effect on variables shoot and total dry matter, soil respiratory activity, and colony-forming units in leaves and roots; Aspergillus sp. versicolor section were selected for presenting the ability to promote positive effects on variables shoot and total dry matter, nitrogen content in shoot dry matter, colony-forming units in roots and soil phosphorus; B. velezensis (Bv188) was selected for presenting the ability and promoting positive effects on variables nitrogen content in shoot dry matter, respiratory activity, colony-forming units in leaves, roots, and soil; and B. subtilis 248 was selected for presenting the ability to promote positive effects on variables root nitrogen content, soil phosphorus, respiratory activity in soil, and colony-forming units in leaves, roots, and soil.
Experiment 2: Determination of the Effect of Inoculation of Microorganisms on Cotton Plants Under Field Conditions
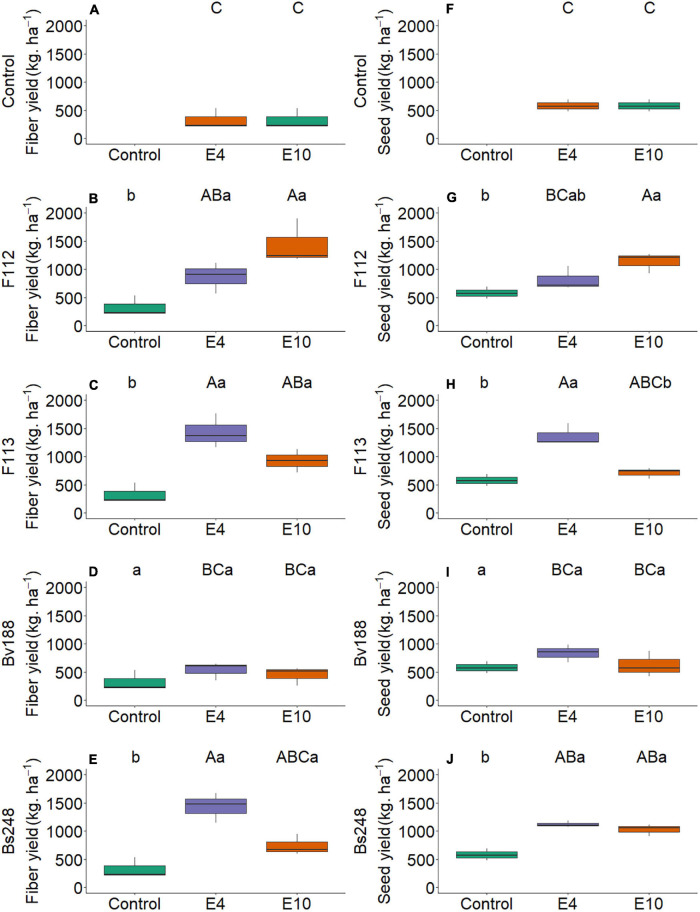
Regarding field yield, there was no interaction of concentration factor and microorganism factor on variables fiber yield (Figures 11A–E) and seed yield, except for Aspergillus sp. versicolor section (F113), which presented the lowest yield for a concentration of 1 × 1010 CFU mL–1 compared with a concentration of 1 × 104 CFU mL–1 (Figure 11H). Fiber yield in cotton plants inoculated with B. velezensis, B. subtilis 248, A. sydowii, and Aspergillus sp. versicolor section were superior to control, which had 326.94 kg/ha (Figures 11A–F). Inoculation of A. sydowii at a concentration of 1 × 1010 conidia ml–1 and Aspergillus sp. versicolor section at a concentration of 1 × 104 conidia ml–1 had the highest seed yield, with 1,131.14 and 1,364.96 kg/ha, respectively (Figures 11G,H). Inoculation with B. velezensis at a concentration of 1 × 104 and 1010 CFU mL–1 showed no differences when compared with that with control (Figure 11I). Inoculation with B. subtilis Bs248 showed no differences between concentrations of 1 × 104 and 1 × 1010 CFU mL–1, reaching values of 1,118.54 and 1,024.68, respectively (Figure 11J).
FIGURE 11.
Boxplots (median and quartiles) of fiber (A–E) and seed (F–J) cotton yield inoculated with plant growth-promoting microorganisms in two concentrations. Different lowercase letters in a row and uppercase letters in a column indicate statistical difference between means (Tukey, p < 0.05). F112, Aspergillus sydowii; F113, Aspergillus sp.; Bv188, Bacillus velezensis strain Bv188; Bs248, Bacillus subtilis strain Bs248; E4, 1 × 104; E10, 1 × 1010 conidia or CFU mL−1; Ctrl, control; and CFU, colony-forming units.
For A. sydowii and B. subtilis Bs248, the hypothesis that fiber and seed yield at concentrations of 1 × 104 or 1 × 1010 CFU mL−1 are similar is confirmed. Thus, the results of the present study demonstrate that there is no effect of concentration on cotton seed and fiber yield when inoculated with A. sydowii and B. subtilis Bs248 and that there is no effect of concentration on cotton seed yield when inoculated with Aspergillus sp. versicolor section.
Yield studies performed with A. sydowii and Aspergillus sp. versicolor section in cotton are scarce in scientific literature; for example, studies carried out on chickpea plants have shown the ability of fungi Aspergillus awamori and Penicillium citrinum inoculated at a concentration of 1 × 106 spores/ml to increase seed weight by approximately twice (Mittal et al., 2008). In addition, A. niger, Aspergillus fumigatus, and Penicillium pinophilum inoculated on wheat and fava beans at a concentration of 2 × 109 spores/ml–1 increased yield by 28.9–32.8% and 14.7–29.4%, respectively (Abdul Wahid and Mehana, 2000). Likewise, phosphorus uptake by both cultures increased due to inoculation with tested fungi. Other studies include arbuscular mycorrhizal fungi in maize plants using concentrations of 1 × 103 spores/ml where, in addition to increasing yield by 80%, these fungi are capable of inducing resistance against pathogenic A. niger strains (Molo et al., 2019).
For plant-growth promoting bacteria, Tripti et al. (2017) observed increase in the amount of fruits on tomato plants inoculated with Bacillus sp. strain A30 and Burkholderia sp. strain L2 at a concentration of 1010 CFU mL–1. Furthermore, inoculation with A. brasiliensis Ab-V5 and B. subtilis strain CCTB04 at a concentration of 1 × 108 CFU mL–1 positively affected corn yield by 39.5 and 29.1%, respectively (Pereira et al., 2020).
Microorganisms A. sydowii, Aspergillus sp. versicolor section, and B. subtilis Bs248 used at concentrations of 1 × 104 and 1 × 1010 conidia or CFU mL–1 in the field phase allow achieving similar results in cotton fiber and seed yield. These results show that lower inoculant concentrations could be used with no damage to plant growth efficiency promoted by the microbial isolate.
Conclusion
The parameters that were favored by the highest inoculant concentrations were soil respiratory activity, phosphorus in root dry matter, nitrogen in shoot dry matter, and number of colony-forming units in roots and leaves. Concentrations did not affect nitrogen in root dry matter, phosphorus in shoot dry matter, and microbial biomass carbon. However, other factors such as nitrogen and phosphorus contents in the soil, except for Aspergillus sp. versicolor section, were negatively affected with the highest inoculant concentrations. Interestingly, inoculant concentrations did not affect cotton fiber or seed yield.
The present study brings results that help in a better understanding of the effect of concentrations of fungi- and bacteria-based inoculants on the biometric parameters of plants, on microbial activities and soil fertility, on the nutritional status of plants, and on cotton crop productivity.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
PE thank the Coordination for the Improvement of Higher Education Personnel (CAPES) for the granting of scholarship.
References
- Abdul Wahid O. A., Mehana T. A. (2000). Impact of phosphate-solubilizing fungi on the yield and phosphorus-uptake by wheat and faba bean plants. Microbiol. Res. 155 221–227. 10.1016/S0944-5013(00)80036-1 [DOI] [PubMed] [Google Scholar]
- Adeniji A. A., Loots D. T., Babalola O. O. (2019). Bacillus velezensis: phylogeny, useful applications, and avenues for exploitation. Appl. Microbiol. Biotechnol. 103 3669–3682. 10.1007/s00253-019-09710-5 [DOI] [PubMed] [Google Scholar]
- Adesemoye A. O., Torbert H. A., Kloepper J. W. (2009). Plant growth-promoting rhizobacteria allow reduced application rates of chemical fertilizers. Microb. Ecol. 58 921–929. 10.1007/s00248-009-9531-y [DOI] [PubMed] [Google Scholar]
- Ahmad I., del Mar Jiménez-Gasco M., Luthe D. S., Shakeel S. N., Barbercheck M. E. (2020). Endophytic Metarhizium robertsii promotes maize growth, suppresses insect growth, and alters plant defense gene expression. Biol. Control 144:104167. 10.1016/j.biocontrol.2019.104167 [DOI] [Google Scholar]
- Alori E. T., Babalola O. O. (2018). Microbial inoculants for improving crop quality and human health in Africa. Front. Microbiol. 9:2213. 10.3389/fmicb.2018.02213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelina E., Papatheodorou E. M., Demirtzoglou T., Monokrousos N. (2020). Effects of Bacillus subtilis and Pseudomonas fluorescens inoculation on attributes of the lettuce (Lactuca sativa L.) soil rhizosphere microbial community: the role of the management system. Agronomy 10:1428. 10.3390/agronomy10091428 [DOI] [Google Scholar]
- Aquino J. P. A., Macedo F. B., Jr., Antunes J. E. L., Figueiredo M. V. B., Alcantara Neto F., Araujo A. S. F. (2019). Plant growth-promoting endophytic bacteria on maize and sorghum1. Pesqui. Agropecu. Trop. 49 56241–56241. 10.1590/1983-40632019v4956241 [DOI] [Google Scholar]
- Baron N. C., Costa N. T. A., Mochi D. A., Rigobelo E. C. (2018). First report of Aspergillus sydowii and Aspergillus brasiliensis as phosphorus solubilizers in maize. Ann. Microbiol. 68 863–870. 10.1007/s13213-018-1392-5 [DOI] [Google Scholar]
- Baron N. C., de Souza Pollo A., Rigobelo E. C. (2020). Purpureocillium lilacinum and Metarhizium marquandii as plant growth-promoting fungi. PeerJ 8:e9005. 10.7717/peerj.9005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum N., Qin C., Ahanger M. A., Raza S., Khan M. I., Ashraf M., et al. (2019). Role of arbuscular mycorrhizal fungi in plant growth regulation: implications in abiotic stress tolerance. Front. Plant Sci. 10:1068. 10.3389/fpls.2019.01068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behie S. W., Bidochka M. J. (2014). Nutrient transfer in plant–fungal symbioses. Trends Plant Sci. 19 734–740. 10.1016/j.tplants.2014.06.007 [DOI] [PubMed] [Google Scholar]
- Bezerra Neto E., Barreto L. P. (2011). Análises Químicas e Bioquímicas em Plantas. Recife: Editora Universitária da UFRPE. [Google Scholar]
- Bhattacharyya P. N., Jha D. K. (2012). Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J. Microbiol. Biotechnol. 28 1327–1350. 10.1007/s11274-011-0979-9 [DOI] [PubMed] [Google Scholar]
- Bizos G., Papatheodorou E. M., Chatzistathis T., Ntalli N., Aschonitis V. G., Monokrousos N. (2020). The role of microbial inoculants on plant protection, growth stimulation, and crop productivity of the olive tree (Olea europea L.). Plants 9:743. 10.3390/plants9060743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfante P., Genre A. (2010). Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat. Commun. 1:48. 10.1038/ncomms1046 [DOI] [PubMed] [Google Scholar]
- Bremner J. M., Mulvaney C. S. (1983). “Nitrogen-Total,” in Agronomy Monographs, 2ed Edn, ed. Page A. L. (Madison, WI: American Society of Agronomy, Soil Science Society of America; ), 595–624. 10.2134/agronmonogr9.2.2ed.c31 [DOI] [Google Scholar]
- Brookes P. C., Powlson D. S., Jenkinson D. S. (1982). Measurement of microbial biomass phosphorus in soil. Soil Biol. Biochem. 14 319–329. 10.1016/0038-0717(82)90001-3 [DOI] [Google Scholar]
- Bulgarelli D., Schlaeppi K., Spaepen S., van Themaat E. V. L., Schulze-Lefert P. (2013). Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 64 807–838. 10.1146/annurev-arplant-050312-120106 [DOI] [PubMed] [Google Scholar]
- Cao Y., Pi H., Chandrangsu P., Li Y., Wang Y., Zhou H., et al. (2018). Antagonism of two plant-growth promoting Bacillus velezensis isolates against Ralstonia solanacearum and Fusarium oxysporum. Sci. Rep. 8:4360. 10.1038/s41598-018-22782-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Zhang Z., Ling N., Yuan Y., Zheng X., Shen B., et al. (2011). Bacillus subtilis SQR 9 can control Fusarium wilt in cucumber by colonizing plant roots. Biol. Fertil. Soils 47 495–506. 10.1007/s00374-011-0556-2 [DOI] [Google Scholar]
- Caruso G., Golubkina N., Tallarita A., Abdelhamid M. T., Sekara A. (2020). Biodiversity, ecology, and secondary metabolites production of endophytic fungi associated with Amaryllidaceae crops. Agriculture 10:533. 10.3390/agriculture10110533 [DOI] [Google Scholar]
- Caruso M., Colombo A. L., Fedeli L., Pavesi A., Quaroni S., Saracchi M., et al. (2000). Isolation of endophytic fungi and actinomycetes taxane producers. Ann. Microbiol. 50 3–13. [Google Scholar]
- Carvalho N. L., Barcellos A. L. (2012). Adoção do manejo integrado de pragas baseado na percepção e educação ambiental. Rev. Eletrôn. Gestão Educ. Tecnol. Ambient. 5 749–766. 10.5902/223611704204 [DOI] [Google Scholar]
- Chen Y., Yan F., Chai Y., Liu H., Kolter R., Losick R., et al. (2013). Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environ. Microbiol. 15 848–864. 10.1111/j.1462-2920.2012.02860.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araújo W. L., de Souza Lima A. O., de Azevedo J. L., Marcon J. (2002). Manual: Isolamento de Microrganismos Endofíticos. Piracicaba: CALQ. [Google Scholar]
- de Oliveira Mendes G., Moreira de Freitas A. L., Liparini Pereira O., Ribeiro da Silva I., Bojkov Vassilev N., Dutra Costa M. (2014). Mechanisms of phosphate solubilization by fungal isolates when exposed to different P sources. Ann. Microbiol. 64 239–249. 10.1007/s13213-013-0656-3 [DOI] [Google Scholar]
- Diaz P. A. E., Baron N. C., Rigobelo E. C. (2019). Bacillus spp. As plant growth-promoting bacteria in cotton under greenhouse conditions. Aust. J. Crop Sci. 13 2003–2014. 10.21475/ajcs.19.13.12.p2003 [DOI] [Google Scholar]
- EMBRAPA (2006). Sistema Brasileiro de Classificação de Solos. Rio de Janeiro: Embrapa Solos. [Google Scholar]
- Fan B., Blom J., Klenk H. P., Borriss R. (2017). Bacillus amyloliquefaciens, Bacillus velezensis, and Bacillus siamensis form an “Operational Group B. amyloliquefaciens” within the B.subtilis species complex. Front. Microbiol. 8:22. 10.3389/fmicb.2017.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick B. R., Patten C. L., Holguin G., Penrose D. M. (1999). Biochemical and Genetic Mechanisms used by Plant Growth-Promoting Bacteria. London: Imperial College Press. [Google Scholar]
- Gnankambary Z., Ilstedt U., Nyberg G., Hien V., Malmer A. (2008). Nitrogen and phosphorus limitation of soil microbial respiration in two tropical agroforestry parklands in the south-Sudanese zone of Burkina Faso: the effects of tree canopy and fertilization. Soil Biol. Biochem. 40 350–359. 10.1016/j.soilbio.2007.08.015 [DOI] [Google Scholar]
- Goldman G. H., Osmani S. A. (2008). The Aspergilli Genomics, Medical aspects, Biotechnology, and Research Methods. Boca Raton, FL: CRC Press. [Google Scholar]
- Haag H. P., Sarruge J. R., de Oliveira G. D., Scoton L. C., Dechen A. R. (1975). Nutrição mineral do cajueiro (Anacardium occidentale L.): III – absorção de nutrientes - nota prévia. An. Esc. Super. Agric. Luiz Queiroz 32 197–204. 10.1590/S0071-12761975000100016 [DOI] [Google Scholar]
- Hardoim P. R., van Overbeek L. S., van Elsas J. D. (2008). Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 16 463–471. 10.1016/j.tim.2008.07.008 [DOI] [PubMed] [Google Scholar]
- Islam K. R., Weil R. R. (1998). Microwave irradiation of soil for routine measurement of microbial biomass carbon. Biol. Fertil. Soils 27 408–416. 10.1007/s003740050451 [DOI] [Google Scholar]
- Jaber L. R., Enkerli J. (2016). Effect of seed treatment duration on growth and colonization of Vicia faba by endophytic Beauveria bassiana and Metarhizium brunneum. Biol. Control 103 187–195. 10.1016/j.biocontrol.2016.09.008 [DOI] [Google Scholar]
- Jenkinson D. S., Powlson D. S. (1976). The effects of biocidal treatments on metabolism in soil—V. Soil Biol. Biochem. 8 209–213. 10.1016/0038-0717(76)90005-5 [DOI] [Google Scholar]
- Khan A. L., Hussain J., Al-Harrasi A., Al-Rawahi A., Lee I. J. (2015). Endophytic fungi: resource for gibberellins and crop abiotic stress resistance. Crit. Rev. Biotechnol. 35 62–74. 10.3109/07388551.2013.800018 [DOI] [PubMed] [Google Scholar]
- Klich M. A. (2002). Biogeography of Aspergillus species in soil and litter. Mycologia 94 21–27. 10.1080/15572536.2003.11833245 [DOI] [PubMed] [Google Scholar]
- Kloepper J. W., Lifshitz R., Zablotowicz R. M. (1989). Free-living bacterial inocula for enhancing crop productivity. Trends Biotechnol. 7 39–44. [Google Scholar]
- Kneip C., Lockhart P., Voss C., Maier U. G. (2007). Nitrogen fixation in eukaryotes – new models for symbiosis. BMC Evol. Biol. 7:55. 10.1186/1471-2148-7-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kour D., Rana K. L., Kaur T., Yadav N., Yadav A. N., Kumar M., et al. (2021). Biodiversity, current developments and potential biotechnological applications of phosphorus-solubilizing and -mobilizing microbes: a review. Pedosphere 31 43–75. 10.1016/S1002-0160(20)60057-1 [DOI] [Google Scholar]
- Lobo L. L. B., dos Santos R. M., Rigobelo E. C. (2019). Promotion of maize growth using endophytic bacteria under greenhouse and field conditions. Aust. J. Crop Sci. 13 2067–2074. 10.21475/ajcs.19.13.12.p2077 [DOI] [Google Scholar]
- Lugtenberg B., Kamilova F. (2009). Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 63 541–556. [DOI] [PubMed] [Google Scholar]
- Malavolta E., Vitti G. C., Oliveira S. A. (1997). Avaliação do Estado Nutricional das Plantas: Princípios e Aplicações, 2 Edn. ed. Associação Brasileira para Pesquisa da Potassa e do Fosfato. Available online at: https://www.infraestruturameioambiente.sp.gov.br/institutodebotanica/1997/01/avaliacao-do-estado-nutricional-das-plantas-principios-e-aplicacoes/ (accessed August 12, 2020). [Google Scholar]
- Malusá E., Vassilev N. (2014). A contribution to set a legal framework for biofertilisers. Appl. Microbiol. Biotechnol. 98 6599–6607. 10.1007/s00253-014-5828-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marulanda A., Barea J.-M., Azcón R. (2009). Stimulation of plant growth and drought tolerance by native microorganisms (AM Fungi and Bacteria) from dry environments: mechanisms related to bacterial effectiveness. J. Plant Growth Regul. 28 115–124. 10.1007/s00344-009-9079-6 [DOI] [Google Scholar]
- Mendonça E. D. S., Matos E. S. (2017). Matéria Orgânica do Solo: Métodos de Análises. ABEAS, 2nd Edn. Viçosa: Universidade Federal de Viçosa. [Google Scholar]
- Michereff S. J., Barros R. (2001). Proteção de Plantas na Agricultura Sustentável. Recife: UFRPE. [Google Scholar]
- Milani R., Santos R. M. D., Bentes L. L., Lazarovits G., Rigobelo E. C. (2019). Bacillus subtilis isolates with different abilities to promote plant growth in maize, cotton and soybean crops isolation and characterization of bacterial strains. Asian J. Microbiol. Biotechnol. Environ. Sci. 21 827–836. [Google Scholar]
- Mittal V., Singh O., Nayyar H., Kaur J., Tewari R. (2008). Stimulatory effect of phosphate-solubilizing fungal strains (Aspergillus awamori and Penicillium citrinum) on the yield of chickpea (Cicer arietinum L. cv. GPF2). Soil Biol. Biochem. 40 718–727. 10.1016/j.soilbio.2007.10.008 [DOI] [Google Scholar]
- Molo M. S., Heiniger R. W., Boerema L., Carbone I. (2019). Trial summary on the comparison of various non-aflatoxigenic strains of Aspergillus flavus on mycotoxin levels and yield in maize. Agron. J. 111 942–946. 10.2134/agronj2018.07.0473 [DOI] [Google Scholar]
- Nadeem S. M., Ahmad M., Zahir Z. A., Javaid A., Ashraf M. (2014). The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 32 429–448. 10.1016/j.biotechadv.2013.12.005 [DOI] [PubMed] [Google Scholar]
- Okon Y., Labandera-Gonzalez C. A. (1994). Agronomic applications of Azospirillum: an evaluation of 20 years worldwide field inoculation. Soil Biol. Biochem. 26 1591–1601. 10.1016/0038-0717(94)90311-5 [DOI] [Google Scholar]
- Ongena M., Jacques P. (2008). Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol. 16 115–125. 10.1016/j.tim.2007.12.009 [DOI] [PubMed] [Google Scholar]
- Pacheco S. M. V., Damasio F. (2013). Aplicação de microrganismos disponibilizadores de fosfato imobilizados em alginato de cálcio na agricultura. Rev. Eletrôn. Biol. 6:2. [Google Scholar]
- Paul K., Saha C., Nag M., Mandal D., Naiya H., Sen D., et al. (2020). A tripartite interaction among the basidiomycete Rhodotorula mucilaginosa, N2-fixing endobacteria, and rice improves plant nitrogen nutrition. Plant Cell 32 486–507. 10.1105/tpc.19.00385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedraza R. O., Teixeira K. R., Fernández Scavino A., García de Salamone I., Baca B. E., Azcón R., et al. (2010). Microorganismos que mejoran el crecimiento de las plantas y la calidad de los suelos. Revisión. Corpoica. Cienc. Tecnol. Agropecu. 11:155. [Google Scholar]
- Peel M. C., Finlayson B. L., McMahon T. A. (2007). Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 11 1633–1644. 10.5194/hess-11-1633-2007 [DOI] [Google Scholar]
- Pereira N. C. M., Galindo F. S., Gazola R. P. D., Dupas E., Rosa P. A. L., Mortinho E. S., et al. (2020). Corn yield and phosphorus use efficiency response to phosphorus rates associated with plant growth promoting bacteria. Front. Environ. Sci. 8:40. 10.3389/fenvs.2020.00040 [DOI] [Google Scholar]
- Petrini O. (1991). “Fungal endophytes of tree leaves,” in Microbial Ecology of Leaves, eds Andrews J. H., Hirano S. S. (New York, NY: Springer; ), 179–197. 10.1007/978-1-4612-3168-4_9 [DOI] [Google Scholar]
- Qiao Q., Zhang J., Ma C., Wang F., Chen Y., Zhang C., et al. (2019). Characterization and variation of the rhizosphere fungal community structure of cultivated tetraploid cotton. PLoS One 14:e0207903. 10.1371/journal.pone.0207903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2020). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Rahi P., Vyas P., Sharma S., Gulati A., Gulati A. (2009). Plant growth promoting potential of the fungus Discosia sp. FIHB 571 from tea rhizosphere tested on chickpea, maize and pea. Ind. J. Microbiol. 49 128–133. 10.1007/s12088-009-0026-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai M., Rathod D., Agarkar G., Dar M., Brestic M., Pastore G. M., et al. (2014). Fungal growth promotor endophytes: a pragmatic approach towards sustainable food and agriculture. Symbiosis 62 63–79. 10.1007/s13199-014-0273-3 [DOI] [Google Scholar]
- Rana K. L., Kour D., Yadav A. N. (2019). Endophytic microbiomes: biodiversity, ecological significance and biotechnological applications. Res. J. Biotechnol. 14 142–162. [Google Scholar]
- Raper K. B., Fennell D. I. (1965). The Genus Aspergillus. CIMMYT. Available online at: https://www.cabdirect.org/cabdirect/abstract/19662205082 (accessed August 12, 2020). [Google Scholar]
- Samson R. A., Visagie C. M., Houbraken J., Hong S.-B., Hubka V., Klaassen C. H. W., et al. (2014). Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 78 141–173. 10.1016/j.simyco.2014.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K. D., Van Straaten P., De Orduña R. M., Glasauer S., Trevors J., Fallow D., et al. (2010). Comparing phosphorus mobilization strategies using Aspergillus niger for the mineral dissolution of three phosphate rocks. J. Appl. Microbiol. 108 366–374. 10.1111/j.1365-2672.2009.04489.x [DOI] [PubMed] [Google Scholar]
- Singh V., Mawar R., Lodha S. (2012). Combined effects of biocontrol agents and soil amendments on soil microbial populations, plant growth and incidence of charcoal rot of cowpea and wilt of cumin. Phytopathol. Mediterr. 51 307–316. [Google Scholar]
- Singh Z., Kaur J., Kaur R., Hundal S. (2016). Toxic effects of organochlorine pesticides: a review. Am. J. Biosci. 4 11–18. 10.11648/j.ajbio.s.2016040301.13 [DOI] [Google Scholar]
- Sivasakthi S., Usharani G., Saranraj P. (2014). Biocontrol potentiality of plant growth promoting bacteria (PGPR) – Pseudomonas fluorescens and Bacillus subtilis: a review. Afr. J. Agric. Res. 9 1265–1277. 10.5897/AJAR2013.7914 [DOI] [Google Scholar]
- Souchie E. L., Saggin-Júnior O. J., Silva E. M. R., Campello E. F. C., Azcón R., Barea J. M. (2006). Communities of P-solubilizing bacteria, fungi and arbuscular mycorrhizal fungi in grass pasture and secondary forest of Paraty, RJ – Brazil. An. Acad. Bras. Ciênc. 78 183–193. 10.1590/S0001-37652006000100016 [DOI] [PubMed] [Google Scholar]
- Tahmatsidou V., O’Sullivan J., Cassells A. C., Voyiatzis D., Paroussi G. (2006). Comparison of AMF and PGPR inoculants for the suppression of Verticillium wilt of strawberry (Fragaria × ananassa cv. Selva). Appl. Soil Ecol. 32 316–324. 10.1016/j.apsoil.2005.07.008 [DOI] [Google Scholar]
- Tian J., Dong Q., Yu C., Zhao R., Wang J., Chen L. (2016). Biodegradation of the organophosphate trichlorfon and its major degradation products by a novel Aspergillus sydowii PA F-2. J. Agric. Food Chem. 64 4280–4287. 10.1021/acs.jafc.6b00909 [DOI] [PubMed] [Google Scholar]
- Tripti, Kumar A., Usmani Z., Kumar V., Anshumali (2017). Biochar and flyash inoculated with plant growth promoting rhizobacteria act as potential biofertilizer for luxuriant growth and yield of tomato plant. J. Environ. Manage. 190 20–27. 10.1016/j.jenvman.2016.11.060 [DOI] [PubMed] [Google Scholar]
- Vandenkoornhuyse P., Baldauf S. L., Leyval C., Straczek J., Young J. P. (2002). Extensive fungal diversity in plant roots. Science 295:2051. 10.1126/science.295.5562.2051 [DOI] [PubMed] [Google Scholar]
- Varga J., Kocsubé S., Tóth B., Frisvad J. C., Perrone G., Susca A., et al. (2007). Aspergillus brasiliensis sp. Nov., a biseriate black Aspergillus species with world-wide distribution. Int. J. Syst. Evol. Microbiol. 57 1925–1932. 10.1099/ijs.0.65021-0 [DOI] [PubMed] [Google Scholar]
- Vieira F. C. S., Nahas E. (2000). Quantification of total and sporulating bacteria in soils. Sci. Agric. 57 539–545. 10.1590/S0103-90162000000300026 [DOI] [Google Scholar]
- Volke-Sepulveda T., Salgado-Bautista D., Bergmann C., Wells L., Gutierrez-Sanchez G., Favela-Torres E. (2016). Secretomic insight into glucose metabolism of Aspergillus brasiliensis in solid-state fermentation. J. Proteome Res. 15 3856–3871. 10.1021/acs.jproteome.6b00663 [DOI] [PubMed] [Google Scholar]
- Watanabe F. S., Olsen S. R. (1965). Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from soil. Soil Sci. Soc. Am. J. 29 677–678. 10.2136/sssaj1965.03615995002900060025x [DOI] [Google Scholar]
- Wilke B. M. (2005). “Determination of chemical and physical soil properties,” in Monitoring and Assessing Soil Bioremediation, eds Margesin R., Schinner F. (Berlin: Springer; ), 47–95. 10.1007/3-540-28904-6_2 [DOI] [Google Scholar]
- Wollum A. G. (1982). “Cultural methods for soil microorganisms,” in Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, eds Page A. L., Miller R. H., Keeney D. R. (Madison, WI: American Society of Agronomy Monograph 9; ), 781–802. [Google Scholar]
- Xun F., Xie B., Liu S., Guo C. (2015). Effect of plant growth-promoting bacteria (PGPR) and arbuscular mycorrhizal fungi (AMF) inoculation on oats in saline-alkali soil contaminated by petroleum to enhance phytoremediation. Environ. Sci. Pollut. Res. 22 598–608. 10.1007/s11356-014-3396-4 [DOI] [PubMed] [Google Scholar]
- Yin Z., Shi F., Jiang H., Roberts D. P., Chen S., Fan B. (2015). Phosphate solubilization and promotion of maize growth by Penicillium oxalicum P4 and Aspergillus niger P85 in a calcareous soil. Can. J. Microbiol. 61 913–923. 10.1139/cjm-2015-0358 [DOI] [PubMed] [Google Scholar]
- Zeljko J., Peterson S. W., Horn B. W. (2012). Aspergillus section versicolores: nine new species and multilocus DNA sequence-based phylogeny. IMA Fungus 3 59–79. 10.5598/imafungus.2012.03.01.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. N., Wang D. C., Hu Q., Dai X. Q., Xie Y. S., Li Q., et al. (2019). Consortium of plant growth-promoting rhizobacteria strains suppresses sweet pepper disease by altering the rhizosphere microbiota. Front. Microbiol. 10:1668. 10.3389/fmicb.2019.01668 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.