Abstract
A quality assessment method based on quantitative analysis of multi-components by single marker (QAMS) and fingerprint was constructed from 15 batches of dandelion (Taraxacum mongolicum), using multivariate chemometric methods (MCM). MCM were established by hierarchical cluster analysis (HCA) and factor analysis (FA). HCA was especially performed using the R language and SPSS 22.0 software. The relative correction factors of chlorogenic acid, caffeic acid, p-coumaric acid, luteolin and apigenin were calculated with cichoric acid as a reference, and their contents were determined. The differences between external standard method (ESM) and QAMS were compared. There was no significant difference (t-test, p > 0.05) in quantitative determination, proving the consistency of the two methods (QAMS and ESM). Dandelion material from Yuncheng, Shandong was used as a reference chromatogram. The fingerprints in 15 batches of dandelion were established by HPLC analysis. The similarity of the fingerprints in different batches of dandelion material was greater than or equal to 0.82. A total of 10 common peaks were identified. This strategy is simple, rapid and efficient in multiple component detection of dandelion. It is beneficial in simplifying dandelion's quality control processes and providing references to enhance quality control for other herbal medicines.
Keywords: dandelion, quality evaluation, quantitative analysis of multi-components by single marker, HPLC
1. Introduction
Dandelion (Taraxacum mongolicum Hand.-Mazz.) is a perennial plant in the Composite family. Its flowering period is from April to October [1]. Dandelion was distributed widely in many countries. There are more than 2000 kinds of varieties of dandelion; about 70 kinds of varieties are distributed in various provinces in China [2]. The edible value, medical value and nutritional value of dandelion have been highly appraised and affirmed in Compendium of Materia Medica and other ancient medical ceremonies [3]. The edible portion of dandelion reaches 84%; the leaves of dandelion, consumed as vegetable food, contains vitamin C, vitamin D, carotene and a lot of iron, calcium and other nutrients [4]. Dandelion has been reported to slow down the damage by the effects of oxygen [5], suppress or reduce inflammation [6], fight against cancer [7], resist high concentration of sugar in the blood [8], prevent or impair coagulation [9], soothe soreness [10] and reduce the pathological reaction caused by strong stimulation of the body [11].
Dandelion is rich in phenolic compounds and flavonoids compounds, which are known to promote health [12]. At present, HPLC and HPLC-MS have been used for qualitative and quantitative analysis of the main bioactive components of dandelion [13,14]. These external standard methods (ESM) rely on relative retention time, weak ultraviolet absorption, complex background interference and other shortcomings, which limit the application of these methods [15–17]. Above all, ESM was unable to concurrently determine multiple components in the target sample, resulting in a complicated process and low efficiency [18]. The quantitative analysis of multi-components by single marker (QAMS) only needs to select a reference in the sample. Establishing its relationship with other components in the sample can make the simultaneous determination of the content of multiple components become feasible [19]. This could reduce the time and cost spent in the quality control of herbaceous plant products and bring about ulteriorly improving the HPLC practicability [20,21]. Therefore, QAMS has extensive adhibition to regulate the quality of traditional Chinese medicine (TCM) [22], but it has not been reported in quality control of dandelion.
Recently, researchers used the chromatographic fingerprint to analyse the quality of TCM; it has been approved by many national drug administrations (FDA, SFDA, EMA) [23]. Chromatographic fingerprint method was used to identify substitutes and adulterants according to a limited number of characteristic peaks of genuine materials [24], but the characteristic fingerprint cannot give expression to the content of the active natural ingredients of dandelion. The whole information of dandelion is blurred by the characteristic fingerprint, and the multi-components of dandelion need to be determined. The combination of characteristic fingerprint and QAMS by multivariate chemometric methods (MCM) was used to compare the similarity of dandelion fingerprint. MCM were established by hierarchical cluster analysis (HCA) and factor analysis (FA) [25], and HCA was especially performed using the R language and SPSS 22.0 software.
2. Material and methods
2.1. Chemicals and materials
A total of dandelion samples (S1–S15) were collected from different Chinese provinces. Table 1 lists the detailed local information. Six standard controls (chlorogenic acid, caffeic acid, p-coumaric acid, cichoric acid, luteolin and apigenin) with purity greater than 98% were from Chengdu MUST Biotech Co., Ltd, [26]. The HPLC grade formic acid, acetonitrile and methanol were acquired from DIKMA Technologies (Beijing, China). Other chemicals used in the experiment were from Tianjin Tianli Reagents Co., Ltd (Tianjin, China).
Table 1.
The different geographical locations, similarities, score and comprehensive evaluation results of 15 batches of dandelion in China.
| no. | district | similarity | score | ranking |
|---|---|---|---|---|
| S1 | Baishan City, Jilin Province | 0.958 | 2.408 | 3 |
| S2 | Baishan City, Jilin Province | 0.982 | 0.956 | 13 |
| S3 | Bozhou City, Anhui Province | 0.952 | 1.519 | 9 |
| S4 | Bozhou City, Anhui Province | 0.951 | 2.500 | 2 |
| S5 | Changbai Mountain City, Jilin Province | 0.882 | 0.551 | 15 |
| S6 | Chengdu City, Sichuan Province | 0.919 | 1.797 | 6 |
| S7 | Hulun Buir City, Inner Mongolia Autonomous Region | 0.989 | 1.600 | 7 |
| S8 | Jinan City, Shandong Province | 0.963 | 1.533 | 8 |
| S9 | Kunming City, Yunnan Province | 0.975 | 1.011 | 11 |
| S10 | Laiyang City, Shandong Province | 0.832 | 0.971 | 12 |
| S11 | Laiyang City, Shandong Province | 0.829 | 0.676 | 14 |
| S12 | Lanxi County, Heilongjiang Province | 0.952 | 1.368 | 10 |
| S13 | Linyi City, Shandong Province | 0.961 | 2.223 | 4 |
| S14 | Nanjing City, Jiangsu Province | 0.984 | 2.647 | 1 |
| S15 | Yuncheng City, Shandong Province | 0.964 | 2.071 | 5 |
2.2. Instruments and chromatographic conditions
The analytical instrument was Agilent 1260 series HPLC device. Analytes were separated by Eco-silC18 column (5 µm, 250 × 4.6 mm). The HPLC system stood a flow rate of 0.8 ml min−1; the column temperature was settled as 35°C and the injection volume of the sample was set as 10 µl. The measurement wavelength was set at 254 nm. Mobile phase A was 0.2% phosphoric acid aqueous solution and B was acetonitrile. The elution gradient was 0–5 min, 20–27% B; 5–12 min, 27–32% B; 12–14 min, 32–34% B; 14–17 min, 34–37% B; 17–27 min, 37–45% B.
2.3. Preparation of sample solutions
One gram of dandelion powders was accurately weighed. It was soaked into 30 ml of 70% methanol–water solution, placed in a conical flask and ultrasonication (25°C, 250 W, 60 kHz) performed for 30 min. After the extract was fully mixed and shaken, the centrifugation was carried out at a fast speed of 10 000 r.p.m. The collected supernatant was filtered with a 0.45 µm filter membrane, and the obtained sample solution could be directly analysed by HPLC.
2.4. Preparation of standard solution
The chlorogenic acid, caffeic acid, p-coumaric acid, cichoric acid and luteolin standard references were weighed and dissolved into a standard solution of 1.0 mg ml−1 with methanol. The apigenin standard reference was weighed and dissolved into 0.5 mg ml−1 solution with methanol. The mixed standard solution was procured by blending 0.2 ml of the individual stock solutions. Except the concentration of apigenin was 0.083 mg ml−1, the other standard reference concentrations were 0.167 mg ml−1.
2.5. Computation of relative conversion factors
There are a variety of components in the sample. Among these components, which being stable, easy to obtain and separate from other components are selected as a single marker, so that a single marker can accurately determine other multiple components. And simultaneously cichoric acid is rich in dandelion [27], thus, it is suitable for the quality indicator of dandelion. Using cichoric acid as a single marker [28], the factor ratio of a single factor marker with other analytes is ƒsi using formula (2.1) [29]. The concentration of each other analyte (Ci) in the sample could be calculated according to formula (2.2) [30],
| 2.1 |
and
| 2.2 |
As is the peak area of cichoric acid and Ai is the peak area of other analytes. Cs is the concentration of cichoric acid and Ci is the concentration of other analytes (mg ml−1).
2.6. Statistical analysis
The data were analysed and evaluated by a similarity evaluation system for the chromatographic fingerprint of TCM (2012, China), which was recommended by SFDA [31]. The similarity among different chromatograms was quantified by calculating the correlative coefficient. The similarity between the samples was acquired by computing the correlation coefficients of different chromatograms. R language conducts HCA according to the similarity degree of each component among different samples. IBM SPSS Statistical 22.0 software (IBM, New York, USA) applies the square Euclidean distance computing of the content of each component in the sample to perform HCA. HCA based on R language and SPSS distinguish herbal species. In order to verify the feasibility of QAMS, the other five active components in dandelion samples were determined by applying cichoric acid as an internal reference.
3. Results and discussion
3.1. Screening of chromatographic conditions
The suitable extraction method and HPLC parameters were tested, and the optimal chromatographic fingerprint was finally obtained. We got the optimized extraction efficiency by three column temperatures (30°C, 35°C, 40°C), solid–liquid ratio (1 : 25, 1 : 30, 1 : 35 g ml−1), concentration of solvent (60%, 70%, 80%), extracting time (15, 30, 45 min). One gram of dandelion powder was soaked in 70% methanol–water ultrasonication for 30 min. It was simpler and more effective for the extraction of dandelion (table 2). Finally, the gradient solvent system consisted of 0.2% phosphoric acid in water (eluent A) and acetonitrile (eluent B) was at a column temperature of 35°C with a flow rate of 0.8 ml min−1; the detection wavelength was set at 254 nm. The above conditions were given the necessary best performance (reconstruction and separation) in a chromatographic fingerprint.
Table 2.
Horizontal table of orthogonal test factors.
| no. | solid–liquid ratio | concentration of solvent | extracting time | column temperature | total |
|---|---|---|---|---|---|
| 1 | 1 : 25 | 60% | 15 | 30 | 17.013 |
| 2 | 1 : 25 | 70% | 30 | 35 | 18.898 |
| 3 | 1 : 25 | 80% | 45 | 40 | 15.245 |
| 4 | 1 : 30 | 60% | 30 | 40 | 19.402 |
| 5 | 1 : 30 | 70% | 45 | 30 | 19.983 |
| 6 | 1 : 30 | 80% | 15 | 35 | 16.974 |
| 7 | 1 : 35 | 60% | 45 | 35 | 18.930 |
| 8 | 1 : 35 | 70% | 15 | 40 | 19.912 |
| 9 | 1 : 35 | 80% | 30 | 30 | 16.176 |
| k1 | 17.052 | 18.448 | 17.966 | 17.724 | |
| k2 | 18.786 | 19.597 | 18.159 | 18.267 | |
| k3 | 18.339 | 16.132 | 18.053 | 18.186 | |
| R | 1.734 | 3.465 | 0.193 | 0.543 |
Ki = the sum of the index values of the numbers in column 5 and ‘i’.
R = The difference between the maximum and the minimum of the average values of K1, K2… in column 5.
3.2. Method validation
3.2.1. Linearity
Six standard solutions (chlorogenic acid, caffeic acid, p-coumaric acid, cichoric acid, luteolin and apigenin) were diluted with methanol to six different concentrations. According to the relationship between the peak area (Y) and the concentration of each analyte (X), the partial least square method was used to draw the linear regression equation (Y = aX + b). The linear regression equation could be applied to QAMS analysis (table 3).
Table 3.
The regression data and linear range for six bioactive compounds analysed by HPLC (n = 6).
| standard solutions | regression equations | R2 | linear range (μg ml−1) |
|---|---|---|---|
| chlorogenic acid | y = 10.69x + 48.25 | R² = 0.9991 | 7.5–100.0 |
| caffeic acid | y = 35.03x − 56.98 | R² = 0.9991 | 2.5–163.0 |
| P-coumaric acid | y = 21.53x − 76.26 | R² = 0.9993 | 1.5–200.0 |
| cichoric acid | y = 29.75x + 23.08 | R² = 0.9990 | 75.0–525.0 |
| luteolin | y = 91.02x − 28.30 | R² = 0.9997 | 2.0–175.0 |
| apigenin | y = 22.08x + 49.15 | R² = 0.9996 | 1.6–87.50 |
3.2.2. Precision, stability, repeatability and accuracy
The precision was assessed by analytic judgement of the same solution of six standards (n = 6) within one day. The results showed that the relative standard deviations (RSDs) of chlorogenic acid, caffeic acid, p-coumaric acid, cichoric acid, luteolin and apigenin were 1.76%, 1.66%, 1.08%, 1.30%, 2.03% and 0.89% (n = 6), respectively. It indicated that the precision of the method was good.
The stabilities of the same sample solutions (S15) were analysed at 0, 2, 6, 8, 16 and 24 h after storage for one day (25°C). The RSD values for the stability tests of chlorogenic acid, caffeic acid, p-coumaric acid, cichoric acid, luteolin and apigenin were 1.23%, 1.40%, 1.35%, 2.45%, 2.21% and 1.32% (n = 6), respectively. It suggested that the method was steady within 24 h.
Take the same batch of samples (S15), according to the method of sample preparation, each inject 10 µl, respectively (n = 6). The results showed that the RSDs of chlorogenic acid, caffeic acid, p-coumaric acid, cichoric acid, luteolin and apigenin were 2.05%, 0.47%, 2.11%, 1.66%, 1.24% and 3.42%, (n = 6), respectively. It indicated that the reproducibility of the method was good.
Low, medium and high concentrations of the mixed standard were added into dandelion samples (S15), to determine the accuracy of the method. The average recovery of chlorogenic acid, caffeic acid, p-coumaric acid, cichoric acid, luteolin and apigenin were 99.82%, 100.25%, 99.89%, 101.24%, 103.33% and 100.10%, and the RSDs were 1.15%, 2.04%, 1.51%, 2.37%, 2.10% and 2.25%, respectively. It demonstrated the method was accurate (table 4).
Table 4.
RSD of precision, stability, repeatability and accuracy for determination of six components (n = 6).
| standard solutions | precision RSD (%) | stability RSD (%) | repeatability RSD (%) | accuracy |
|
|---|---|---|---|---|---|
| mean (%) | RSD (%) | ||||
| chlorogenic acid | 1.76 | 1.23 | 2.05 | 99.82 | 1.15 |
| caffeic acid | 1.66 | 1.40 | 0.47 | 100.25 | 2.04 |
| P-coumaric acid | 1.08 | 1.35 | 2.11 | 99.89 | 1.51 |
| cichoric acid | 1.30 | 2.45 | 1.66 | 101.24 | 2.37 |
| luteolin | 2.03 | 2.21 | 1.24 | 103.33 | 2.10 |
| apigenin | 0.89 | 1.32 | 3.42 | 100.10 | 2.25 |
3.3. The evaluation of quantitative analysis of multi-components by single marker and external standard method
In order to assess and validate QAMS feasibility for the determination of multi-compounds in dandelion, the contents of chlorogenic acid, caffeic acid, p-coumaric acid, cichoric acid, luteolin and apigenin in 15 batches of dandelion (S1–S15) were determined by ESM and QAMS, respectively. The relative conversion factors (RCF) (ƒsi) between the selected reference and other references in QAMS can be affected by a change in experimental conditions, such as flow rate, column temperature and standard concentration. Therefore, ƒsi affect the final analysis result. The RCF (ƒsi) is calculated by linear regression equation, which is relatively stable (table 5). Errors caused by instruments, reagents, experimental methods or environmental conditions in the course of an experiment are relative errors (REs). RE was built between QAMS and ESM to examine the deviations using formula (3.1).
| 3.1 |
The six compound contents in dandelion between two methods are shown in table 6. The changes of RE and RSDs were within the range of 5%, and there was no significant difference (t-test, p > 0.05) in quantitative determination proving the consistency of QAMS and ESM. It was observed that among these six components, the average contents of them were 0.7456, 0.4048, 0.2242, 9.1278, 0.0566 and 0.0490 mg g−1, respectively. QAMS can be applied in determinating the content of a variety of components in different laboratories.
Table 5.
The results of RCF (ƒsi).
| flow rate | column temperature | chlorogenic acid | caffeic acid | p-coumaric acid | luteolin | apigenin |
|---|---|---|---|---|---|---|
| 0.6 | 30 | 2.7026 | 0.8157 | 1.2796 | 0.3808 | 0.5750 |
| 0.6 | 35 | 2.7050 | 0.8162 | 1.2833 | 0.3862 | 0.5675 |
| 0.6 | 40 | 2.7036 | 0.8135 | 1.2842 | 0.3851 | 0.5759 |
| 0.8 | 30 | 2.7112 | 0.8198 | 1.2787 | 0.3823 | 0.5746 |
| 0.8 | 35 | 2.7033 | 0.8173 | 1.2820 | 0.3811 | 0.5802 |
| 0.8 | 40 | 2.6997 | 0.8187 | 1.2829 | 0.3833 | 0.5776 |
| 1 | 30 | 2.7028 | 0.8190 | 1.2814 | 0.3824 | 0.5754 |
| 1 | 35 | 2.7031 | 0.8211 | 1.2835 | 0.3835 | 0.5721 |
| 1 | 40 | 2.6981 | 0.8179 | 1.2830 | 0.3836 | 0.5760 |
| means | 2.7033 | 0.8177 | 1.2821 | 0.3831 | 0.5749 | |
| RSD% | 0.13 | 0.27 | 0.14 | 0.43 | 0.58 | |
Table 6.
The determination of six components in 15 batches of dandelion between QAMS and ESM (mg g−1). RE represents relative error. RSD represents relative standard deviation. p-values represent the paired t-test results.
| no. | cichoric acid | chlorogenic acid |
caffeic acid |
p-coumaric acid |
luteolin |
apigenin |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ESM | ESM | QAMS | RE% | RSD% | ESM | QAMS | RE% | RSD% | ESM | QAMS | RE% | RSD% | ESM | QAMS | RE% | RSD% | ESM | QAMS | RE% | RSD% | |
| S1 | 14.148 | 1.203 | 1.209 | 0.50 | 0.35 | 0.250 | 0.249 | −0.40 | 0.28 | 0.249 | 0.249 | 0.00 | 0.21 | 0.003 | 0.003 | 0.00 | 0.33 | 0.015 | 0.015 | 0.00 | 0.77 |
| S2 | 5.136 | 0.678 | 0.654 | −3.54 | 2.55 | 0.147 | 0.150 | 2.04 | 1.43 | 0.282 | 0.285 | 1.06 | 0.75 | 0.006 | 0.006 | 0.00 | 0.98 | 0.003 | 0.003 | 0.00 | 0.43 |
| S3 | 9.177 | 0.600 | 0.603 | 0.50 | 0.35 | 0.329 | 0.324 | −1.52 | 1.08 | 0.075 | 0.078 | 4.00 | 2.77 | 0.009 | 0.009 | 0.00 | 0.40 | 0.006 | 0.006 | 0.00 | 0.15 |
| S4 | 14.949 | 0.963 | 0.948 | −1.56 | 1.11 | 0.423 | 0.426 | 0.71 | 0.50 | 0.276 | 0.276 | 0.00 | 0.59 | 0.015 | 0.015 | 0.00 | 0.65 | 0.003 | 0.003 | 0.00 | 0.56 |
| S5 | 2.856 | 0.225 | 0.228 | 1.33 | 0.94 | 0.621 | 0.624 | 0.48 | 0.34 | 0.060 | 0.06 | 0.00 | 0.23 | 0.162 | 0.162 | 0.00 | 0.71 | 0.093 | 0.090 | −3.23 | 2.32 |
| S6 | 11.298 | 0.327 | 0.342 | 4.59 | 3.17 | 0.369 | 0.375 | 1.63 | 1.14 | 0.054 | 0.054 | 0.00 | 0.71 | 0.012 | 0.012 | 0.00 | 0.67 | 0.006 | 0.006 | 0.00 | 0.49 |
| S7 | 9.114 | 0.717 | 0.702 | −2.09 | 1.49 | 0.388 | 0.381 | −1.80 | 1.29 | 0.309 | 0.312 | 0.97 | 0.68 | 0.093 | 0.090 | −3.23 | 2.32 | 0.018 | 0.018 | 0.00 | 0.29 |
| S8 | 8.643 | 0.945 | 0.936 | −0.95 | 0.68 | 0.126 | 0.129 | 2.38 | 1.66 | 0.276 | 0.282 | 2.17 | 1.52 | 0.003 | 0.003 | 0.00 | 1.32 | 0.012 | 0.012 | 0.00 | 0.78 |
| S9 | 5.508 | 0.648 | 0.636 | −1.85 | 1.32 | 0.122 | 0.120 | −1.64 | 1.17 | 0.288 | 0.291 | 1.04 | 0.73 | 0.009 | 0.009 | 0.00 | 0.46 | 0.003 | 0.003 | 0.00 | 0.45 |
| S10 | 5.199 | 0.498 | 0.498 | 0.00 | 0.10 | 0.699 | 0.711 | 1.72 | 1.20 | 0.201 | 0.198 | −1.49 | 1.06 | 0.102 | 0.099 | −2.94 | 2.11 | 0.087 | 0.090 | 3.45 | 2.40 |
| S11 | 3.135 | 0.657 | 0.642 | −2.28 | 1.63 | 0.540 | 0.531 | −1.67 | 1.19 | 0.222 | 0.219 | −1.35 | 0.96 | 0.075 | 0.078 | 4.00 | 2.77 | 0.093 | 0.090 | −3.23 | 2.32 |
| S12 | 7.890 | 0.789 | 0.759 | −3.80 | 2.74 | 0.784 | 0.792 | 1.02 | 0.72 | 0.117 | 0.114 | −2.56 | 1.84 | 0.015 | 0.015 | 0.00 | 0.74 | 0.003 | 0.003 | 0.00 | 0.38 |
| S13 | 12.723 | 1.332 | 1.296 | −2.70 | 1.94 | 0.309 | 0.303 | −1.94 | 1.39 | 0.285 | 0.279 | −2.11 | 1.50 | 0.057 | 0.057 | 0.00 | 0.58 | 0.006 | 0.006 | 0.00 | 1.42 |
| S14 | 15.453 | 0.966 | 0.975 | 0.93 | 0.66 | 0.352 | 0.345 | −1.99 | 1.42 | 0.324 | 0.318 | −1.85 | 1.32 | 0.12 | 0.117 | −2.5 | 1.79 | 0.177 | 0.180 | 1.69 | 1.19 |
| S15 | 11.688 | 0.738 | 0.756 | 2.44 | 1.70 | 0.614 | 0.612 | −0.33 | 0.23 | 0.354 | 0.348 | −1.69 | 1.21 | 0.171 | 0.174 | 1.75 | 1.23 | 0.213 | 0.210 | −1.41 | 1.00 |
| max | 15.453 | 1.332 | 1.296 | 0.784 | 0.792 | 0.354 | 0.348 | 0.171 | 0.174 | 0.213 | 0.210 | ||||||||||
| min | 2.856 | 0.222 | 0.228 | 0.122 | 0.120 | 0.054 | 0.054 | 0.003 | 0.003 | 0.003 | 0.003 | ||||||||||
| means | 9.1278 | 0.7524 | 0.7456 | 0.4049 | 0.4048 | 0.2248 | 0.2242 | 0.0568 | 0.0566 | 0.0492 | 0.049 | ||||||||||
| correlation coefficient | 0.999** | 0.999** | 0.999** | 0.999** | 0.999** | ||||||||||||||||
| p-values | 0.126 | 0.967 | 0.550 | 0.670 | 0.678 | ||||||||||||||||
3.4. Quality evaluation of dandelion by fingerprint
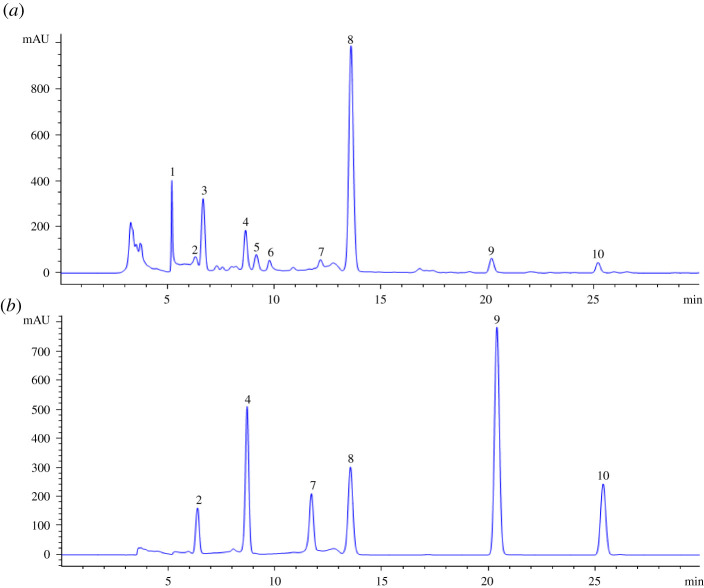
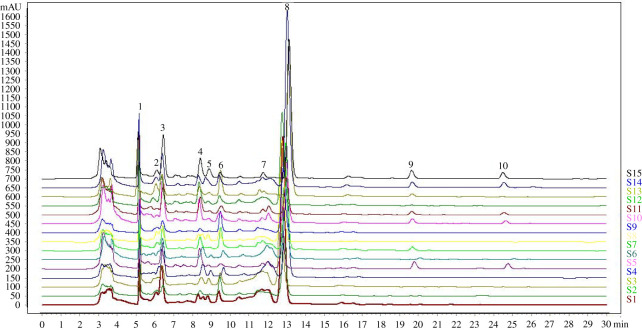
From each of 15 batches of dandelion treatment solution was taken 10 μl for HPLC determination, and generated characteristic chromatogram of the model with 10 common peaks using the similarity evaluation system for the chromatographic fingerprint of TCM (2012) (figure 1a). Six common peaks (chlorogenic acid, caffeic acid, p-coumaric acid, cichoric acid, luteolin and apigenin) were identified through retention time compared with the mixed standard reference. The chromatographic fingerprint of mixed standard reference is shown in figure 1b. In order to obtain an eminent fingerprint, the sample (S15) of good quality is screened as the reference chromatogram. HPLC characteristic fingerprints of 15 dandelion samples are shown in figure 2. The similarity of 15 batches of dandelion samples was evaluated (table 1). As a result, their similarity values calculated were greater than or equal to 0.82, which has a high degree of fit in different regions.
Figure 1.
HPLC fingerprint of dandelion and mixed standard. (a) the dandelion sample, (b) the mixed standards. 2: chlorogenic acid, 4: caffeic acid, 7: p-coumaric acid, 8: cichoric acid, 9: luteolin, 10: apigenin.
Figure 2.
HPLC characteristic fingerprints of 15 dandelion samples. 2: chlorogenic acid, 4: caffeic acid, 7: p-coumaric acid, 8: cichoric acid, 9: luteolin and 10: apigenin.
3.5. Hierarchical cluster analysis and factor analysis result
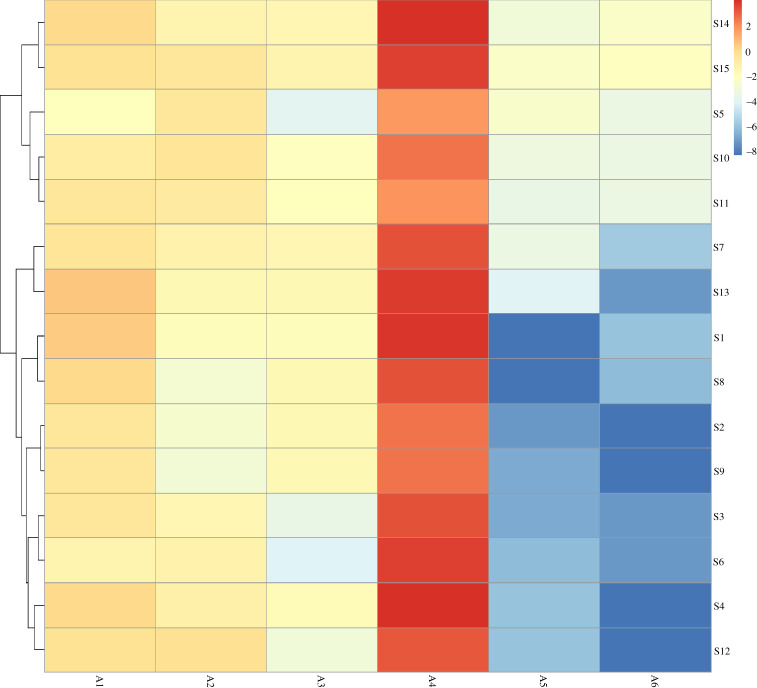
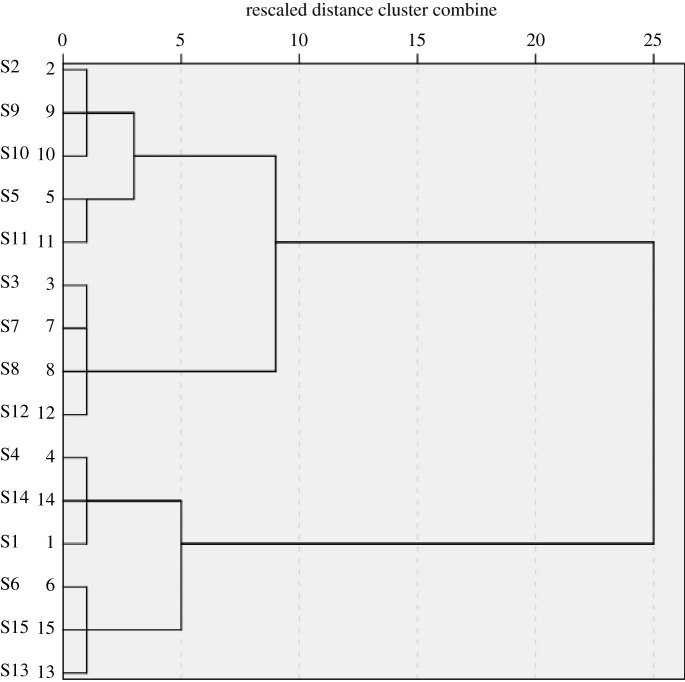
For the sake of highlighting the differences of a dandelion from different areas, 15 batches of dandelion collected from different areas were classified by HCA according to their similarities. Moreover, R language and SPSS software were used for HCA. The results are shown in figures 3 and 4.
Figure 3.
R language cluster analysis diagram of 15 dandelion samples. A1: chlorogenic acid, A2: caffeic acid, A3: p-coumaric acid, A4: cichoric acid, A5: luteolin and A6: apigenin.
Figure 4.
Dendrograms of HCA for the 15 tested samples of dandelion.
The R language heat map used the similarity degree of the contents of six active components in dandelion for HCA. The 15 batches of samples were mainly divided into two categories according to the similarity difference between luteolin and apigenin. S14, S15, S5, S10 and S11 were the mother category, and the rest of the batches were the second category. According to the similarity difference of cichoric acid content, the first group can be also divided into two categories, S14, S15, S5 as a group and S10, S11 as a group. The R language heat map refined the content difference of different components in different batches of dandelion. The contents of six active components in 15 selections of dandelion were taken as variables, and HCA was performed using SPSS 22.0 software, intergroup mean linking method and square Euclidean distance. When the square Euclidean distance was 5, it was divided into three groups: S2, S5, S9, S10, S11 as a group; S3, S7, S8, S12 as a group; S1, S4, S6, S13, S14, S15 as a group. The result corresponds to the FA ranking situation, and the batches with similar scores were classified into one group. From the two different HCA, it can be seen that the dandelion from the same province may not always be in the same category, which may be related to planting methods, harvesting methods, harvesting time and preliminary processing methods.
FA is to simplify the index through dimensionality reduction on the premise of keeping the original data information as much as possible. In this experiment, 10 common peak areas of 15 batches of samples were assessed by SPSS. The results are shown in table 7. The results of Kaiser–Meyer–Olkin (KMO) test and Bartlett test of sphericity show that KMO statistic is 0.542, Bartlett statistic of sphericity test is 45.487, and p-value is 0.000. It shows that the data have correlation and can be used for FA. FA was carried out after data conversion. The six factors were simplified into three main factors, and the load matrix of the rotated factors was obtained by orthogonal rotation with maximum variance. As can be seen from the table 7, the first three principal component eigenvalues are greater than 0.8, and the cumulative contribution rate of the difference is 89.283%. Therefore, multiple components of dandelion can be simplified into three principal components for analysis. The first major factor played a major role, and the contribution rate was 38.006%, which was mainly determined by luteolin and apigenin. The contribution rate of the second major factor was 27.720%, which was mainly determined by cichoric acid and chlorogenic acid. The contribution rate of the third major factor was 23.556%, which was mainly determined by p-coumaric acid and caffeic acid. According to the scoring coefficient of each factor after rotation, the scores of the first three main factors were calculated as F1, F2 and F3 (table 8). The comprehensive scoring model of dandelion quality, F = (38.006F1 + 27.720F2 + 23.556F3)/89.283, was established with the contribution rate of each major factor as the weight (table 1). The dandelion (S14) in Nanjing city, Jiangsu province, has the best quality due to the highest overall score. The overall score of dandelion in East China is higher, which is possible due to the superior natural environment conditions in this region. The terrain is mainly plain, monsoon climate and abundant water resources. Different growing environment, such as sunlight, soil and climatic conditions, havea great influence on the quality of dandelion.
Table 7.
Total variance explained. Extraction method: principal component analysis.
| component | initial eigenvalues |
extraction sums of squared loadings |
rotation sums of squared loadings |
||||||
|---|---|---|---|---|---|---|---|---|---|
| total | % of variance | cumulative % | total | % of variance | cumulative % | total | % of variance | cumulative % | |
| 1 | 2.717 | 45.282 | 45.282 | 2.717 | 45.282 | 45.282 | 2.280 | 38.006 | 38.006 |
| 2 | 1.836 | 30.596 | 75.878 | 1.836 | 30.596 | 75.878 | 1.663 | 27.720 | 65.727 |
| 3 | 0.804 | 13.405 | 89.283 | 0.804 | 13.405 | 89.283 | 1.413 | 23.556 | 89.283 |
| 4 | 0.414 | 6.898 | 96.181 | ||||||
| 5 | 0.168 | 2.803 | 98.984 | ||||||
| 6 | 0.061 | 1.016 | 100.000 | ||||||
Table 8.
FA results of dandelion. Extraction method: principal component analysis. Rotation method: varimax with Kaiser normalization.
| standard solutions | rotated component matrixa |
component score coefficient matrix |
||||
|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F1 | F2 | F3 | |
| chlorogenic acid | −0.176 | 0.806 | 0.428 | −0.006 | 0.453 | 0.070 |
| caffeic acid | 0.591 | −0.034 | −0.686 | 0.216 | 0.269 | −0.575 |
| p-coumaric acid | 0.192 | 0.338 | 0.869 | 0.171 | −0.062 | 0.685 |
| cichoric acid | −0.043 | 0.939 | 0.063 | 0.034 | 0.704 | −0.307 |
| luteolin | 0.977 | −0.097 | 0.004 | 0.441 | −0.026 | 0.115 |
| apigenin | 0.953 | −0.081 | −0.004 | 0.430 | −0.012 | 0.100 |
aRotation converged in four iterations.
4. Conclusion
In order to improve the quality assurance of dandelion on the basis of HPLC method, to overcome the shortage of the multi-component determination method, characteristic fingerprint combined with QAMS method was established. The similarity values of 15 selections of dandelion were calculated (greater than or equal to 0.82), which indicates that although dandelion is widely distributed, it still has a high degree of fit in different regions. The method is suitable for the determination of six active compounds in the dandelion sample. The correlation coefficient of dandelion content greater than 0.998 and RSD% less than 0.05 were determined by the single marker method and traditional ESM. HPLC-QAMS method can get as good results as ESM. The combination of fingerprint and QAMS via MCM (HCA, FA) was a comprehensive and efficient method for quality analysis and evaluation of dandelion.
Supplementary Material
Contributor Information
Chunjian Zhao, Email: zcj@nefu.edu.cn.
Yujie Fu, Email: yujie_fu@163.com.
Ethics
The study was approved by the College of Chemistry, Chemical Engineering and Resource Utilization, Northeast Forestry University.
Data accessibility
The data and sources of data used in the paper are included in this submission.
The data are provided in the electronic supplementary material [32].
Authors' contributions
C.L., Y.T., C.Z., S.L., T.W., B.Q. and Y.F. substantial contributions to conception and design; Y.T. acquisition of data; Y.T. and B.Q. analysis and interpretation of data; C.L. and C.Z. drafting the article or revising it critically for important intellectual content.
Competing interests
The authors have declared no conflict of interest.
Funding
This experiment and analysis were carried out at Key Laboratory of Forest Plant Ecology, Ministry of Education, Northeast Forestry University. This work was financially supported by the Fundamental Research Fund for Central Universities (grant no. 2572019CZ01), the National Natural Science Foundation (grant no. 31870609), the 111 Project of China (B20088) and Heilongjiang Touyan Innovation Team Program (Tree Genetics and Breeding Innovation Team).
References
- 1.Li F, Feng KL, Yang JC, He YS, Guo H, Wang SP, Gan RY, Wu DT. 2021. Polysaccharides from dandelion (Taraxacum mongolicum) leaves: insights into innovative drying techniques on their structural characteristics and biological activities. Int. J. Biol. Macromol. 167, 995–1005. ( 10.1016/j.ijbiomac.2020.11.054) [DOI] [PubMed] [Google Scholar]
- 2.Eggert M, Schiemann J, Thiele K. 2018. Yield performance of Russian dandelion transplants (Taraxacum koksaghyz L. Rodin) in flat bed and ridge cultivation with different planting densities. Eur. J. Agron. 93, 126–134. ( 10.1016/j.eja.2017.12.003) [DOI] [Google Scholar]
- 3.Biel W, Jaroszewska A, Lyson E, Telesinski A. 2017. The chemical composition and antioxidant properties of common dandelion leaves compared with sea buckthorn. Can. J. Plant. Sci. 97, 1165–1174. ( 10.1139/cjps-2016-0409) [DOI] [Google Scholar]
- 4.Yao SW, Xie SY, Jiang LZ, Li L. 2017. Effect of dandelion extract, sucrose and starter culture on the viscosity, water-holding capacity and pH of plain yogurt. Mljekarstvo 67, 305–311. ( 10.15567/mljekarstvo.2017.0408) [DOI] [Google Scholar]
- 5.Liu N, Song M, Wang NF, Wang Y, Wang RF, An XP, Qi JW. 2020. The effects of solid-state fermentation on the content, composition and in vitro antioxidant activity of flavonoids from dandelion. PLoS ONE 15, e0239076. ( 10.1371/journal.pone.0239076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xue YS, Zhang SM, Du M, Zhu MJ. 2017. Dandelion extract suppresses reactive oxidative species and inflammasome in intestinal epithelial cells. J. Funct. Foods 29, 10–18. ( 10.1016/j.jff.2016.11.032) [DOI] [Google Scholar]
- 7.Ovadje P, Ammar S, Guerrero JA, Arnason JT, Pandey S. 2016. Dandelion root extract affects colorectal cancer proliferation and survival through the activation of multiple death signalling pathways. Oncotarget 7, 73 080–73 100. ( 10.18632/oncotarget.11485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Y, Wu P, Ying J, Dong Z, Chen XD. 2021. Mechanistic study on inhibition of porcine pancreatic alpha-amylase using the flavonoids from dandelion. Food Chem. 344, 128610. ( 10.1016/j.foodchem.2020.128610) [DOI] [PubMed] [Google Scholar]
- 9.Lis B, Jedrejek D, Stochmal A, Olas B. 2018. Assessment of effects of phenolic fractions from leaves and petals of dandelion in selected components of hemostasis. Food Res. Int. 107, 605–612. ( 10.1016/j.foodres.2018.03.012) [DOI] [PubMed] [Google Scholar]
- 10.Schutz K, Carle R, Schieber A. 2006. Taraxacum—a review on its phytochemical and pharmacological profile. J. Ethnopharmacol. 107, 313–323. ( 10.1016/j.jep.2006.07.021) [DOI] [PubMed] [Google Scholar]
- 11.Yang Y, Ying GX, Wu SS, Wu FT, Chen Z. 2020. In vitro inhibition effects of hepatitis B virus by dandelion and taraxasterol. Infect. Agents Cancer 15, 44. ( 10.1186/s13027-020-00309-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grauso L, Emrick S, de Falco B, Lanzotti V, Bonanomi G. 2019. Common dandelion: a review of its botanical, phytochemical and pharmacological profiles. Phytochem. Rev. 18, 1115–1132. ( 10.1007/s11101-019-09622-2) [DOI] [Google Scholar]
- 13.Schutz K, Kammerer DR, Carle R, Schieber A. 2005. Characterization of phenolic acids and flavonoids in dandelion (Taraxacum officinale WEB. ex WIGG.) root and herb by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 19, 179–186. ( 10.1002/rcm.1767) [DOI] [PubMed] [Google Scholar]
- 14.Zu YG, Li CY, Fu YJ, Zhao CJ. 2006. Simultaneous determination of catechin, rutin, quercetin kaempferol and isorhamnetin in the extract of sea buckthorn (Hippophae rhamnoides L.) leaves by RP-HPLC with DAD. J. Pharmaceut. Biomed. 41, 714–719. ( 10.1016/j.jpba.2005.04.052) [DOI] [PubMed] [Google Scholar]
- 15.Liang Y, Zhu XC, Wu TB, Zhao MP, Liu HW. 2012. Rapid and sensitive detection of auxins and flavonoids in plant samples by high-performance liquid chromatography coupled with tandem mass spectrometry. J. Sep. Sci. 35, 2559–2566. ( 10.1002/jssc.201200418) [DOI] [PubMed] [Google Scholar]
- 16.Li JK, He XY, Li MY, Zhao W, Liu L, Kong XH. 2015. Chemical fingerprint and quantitative analysis for quality control of polyphenols extracted from pomegranate peel by HPLC. Food Chem. 176, 7–11. ( 10.1016/j.foodchem.2014.12.040) [DOI] [PubMed] [Google Scholar]
- 17.Meng XY, et al. 2018. Development of the HPLC-ELSD method for the determination of phytochelatins and glutathione in Perilla frutescens under cadmium stress conditions. R. Soc. Open Sci. 5, 171659. ( 10.1098/rsos.171659) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ben Ahmed Z, Yousfi M, Viaene J, Dejaegher B, Demeyer K, Mangelings D, Vander Heyden Y. 2016. Antioxidant activities of Pistacia atlantica extracts modeled as a function of chromatographic fingerprints in order to identify antioxidant markers. Micro. Chem. J. 128, 208–217. ( 10.1016/j.microc.2016.04.023) [DOI] [Google Scholar]
- 19.Wang CQ, Jia XH, Zhu S, Komatsu K, Wang X, Cai SQ. 2015. A systematic study on the influencing parameters and improvement of quantitative analysis of multi-component with single marker method using notoginseng as research subject. Talanta 134, 587–595. ( 10.1016/j.talanta.2014.11.028) [DOI] [PubMed] [Google Scholar]
- 20.Li YH, Zhang YM, Zhang ZJ, Hu YP, Cui XM, Xiong Y. 2019. Quality evaluation of Gastrodia elata tubers based on HPLC fingerprint analyses and quantitative analysis of multi-components by single marker. Molecules 24, 8. ( 10.3390/molecules24081521) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui LL, Zhang YY, Shao W, Gao DM. 2016. Analysis of the HPLC fingerprint and QAMS from Pyrrosia species. Ind. Crop. Prod. 85, 29–37. ( 10.1016/j.indcrop.2016.02.043) [DOI] [Google Scholar]
- 22.Wang N, Li ZY, Zheng XL, Li Q, Yang X, Xu H. 2018. Quality assessment of Kumu injection, a traditional Chinese medicine preparation, using HPLC combined with chemometric methods and qualitative and quantitative analysis of multiple alkaloids by single marker. Molecules 23, 4. ( 10.3390/molecules23040856) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao XX, Sun LL, Li D, You GJ, Wang M, Ren XL. 2018. Quality evaluation of phellodendri chinensis cortex by fingerprint-chemical pattern recognition. Molecules 23, 9. ( 10.3390/molecules23092307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong F, Liang YZ, Xie PS, Chau FT. 2003. Information theory applied to chromatographic fingerprint of herbal medicine for quality control. J. Chromatogr. A 1002, 25–40. ( 10.1016/S0021-9673(03)00648-4) [DOI] [PubMed] [Google Scholar]
- 25.Huang Y, Wang T, Yin G, Wang J, Jiang K, Tu J. 2020. High-performance liquid chromatography-based fingerprint analysis with chemical pattern recognition for evaluation of Mahonia bealei (Fort.) Carr. J. Sep. Sci. 43, 3625–3635. ( 10.1002/jssc.201901219) [DOI] [PubMed] [Google Scholar]
- 26.Hu C, Kitts DD. 2004. Luteolin and luteolin-7-O-glucoside from dandelion flower suppress iNOS and COX-2 in RAW264.7 cells. Mol. Cell. Biochem. 265, 107–113. ( 10.1023/B:MCBI.0000044364.73144.fe) [DOI] [PubMed] [Google Scholar]
- 27.Lis B, Jedrejek D, Moldoch J, Stochmal A, Olas B. 2019. The anti-oxidative and hemostasis-related multifunctionality of L-chicoric acid, the main component of dandelion: an in vitro study of its cellular safety, antioxidant and anti-platelet properties, and effect on coagulation. J. Funct. Foods 62, 103524. ( 10.1016/j.jff.2019.103524) [DOI] [Google Scholar]
- 28.Gao XY, Jiang Y, Lu JQ, Tu PF. 2009. One single standard substance for the determination of multiple anthraquinone derivatives in rhubarb using high-performance liquid chromatography-diode array detection. J. Chromatogr. A 1216, 2118–2123. ( 10.1016/j.chroma.2008.11.104) [DOI] [PubMed] [Google Scholar]
- 29.Hou JJ, et al. 2011. Ruggedness and robustness of conversion factors in method of simultaneous determination of multi-components with single reference standard. J. Chromatogr. A 1218, 5618–5627. ( 10.1016/j.chroma.2011.06.058) [DOI] [PubMed] [Google Scholar]
- 30.Huang J, Yin L, Dong L, Quan HF, Chen R, Hua SY, Ma JH, Guo DY, Fu XY. 2018. Quality evaluation for Radix Astragali based on fingerprint, indicative components selection and QAMS. Biomed. Chromatogr. 32, e4343. ( 10.1002/bmc.4343) [DOI] [PubMed] [Google Scholar]
- 31.SFDA. 2000. Technical requirements for studying fingerprint of traditional Chinese medicine injections (Draft); drug administration Bureau of China: Beijing. China.
- 32.Li C, Tian Y, Zhao C, Li S, Wang T, Qiao B, Fu Y. 2021. Application of fingerprint combined with quantitative analysis and multivariate chemometric methods in quality evaluation of dandelion (Taraxacum mongolicum). Figshare. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Li C, Tian Y, Zhao C, Li S, Wang T, Qiao B, Fu Y. 2021. Application of fingerprint combined with quantitative analysis and multivariate chemometric methods in quality evaluation of dandelion (Taraxacum mongolicum). Figshare. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data and sources of data used in the paper are included in this submission.
The data are provided in the electronic supplementary material [32].