Abstract
The receptors for interleukin-3 (IL-3) and granulocyte-macrophage colony-stimulating factor (GM-CSF) share a common β subunit, the distal cytoplasmic domain of which is essential for the promotion of cell survival by these two cytokines. Genes whose expression is specifically induced by signaling through the distal cytoplasmic domain of this receptor β subunit were screened by a subtraction cloning approach in derivatives of a mouse pro-B-cell line. One gene thus identified was shown to encode a protein highly homologous (with only 7 amino acid substitutions) to murine osteopontin (OPN), a secreted adhesion protein. Conditioned medium from cells expressing wild-type OPN, but not that from cells expressing a deletion mutant lacking residues 79 to 140, increased the viability of a non-OPN-producing cell line in the presence of human GM-CSF. Antibody blocking experiments revealed that OPN produced as a result of IL-3 or GM-CSF signaling was secreted into the medium and, through binding to its cell surface receptor, CD44, contributed to the survival-promoting activities of these two cytokines. Furthermore, coupling of the OPN-CD44 pathway to the survival response to IL-3 was also demonstrated in primary IL-3-dependent mouse bone marrow cells. These results thus show that induction of an extracellular adhesion protein and consequent activation of its cell surface receptor are important for the antiapoptotic activities of IL-3 and GM-CSF.
Both granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-3 (IL-3) belong to a family of cytokine growth factors that regulate the viability, differentiation, proliferation, and function of multipotential hematopoietic progenitors as well as of various other hematopoietic cells (1). On binding to their corresponding receptors, GM-CSF and IL-3, in most instances, trigger similar signaling events as a result of the fact that their receptors share a common β subunit. Signaling events mediated by this β subunit include tyrosine phosphorylation of various signaling proteins, such as the receptor β chain itself, JAK2, Shc, Vav, Fps, STAT5A, and STAT5B (4, 14, 19, 32, 35, 55); activation of phosphatidylinositol (PI) 3-kinase and the Ras-Raf-mitogen-activated protein (MAP) kinase pathway (10, 17, 27, 48, 50); and transcriptional activation of immediate-early genes such as c-jun, c-fos, c-myc, cis, and mcl-1 (8, 9, 63). Deletion analysis has revealed that the membrane-proximal domain of the receptor β subunit is important for the induction of the c-myc and cis genes as well as for the activation of JAK2 and STAT5 proteins (35, 41, 48, 63), whereas the membrane-distal domain is required for the induction of c-jun, c-fos, and mcl-1 as well as for the activation of PI 3-kinase and the Ras-Raf-MAP kinase cascade (8, 48). Activation of PI 3-kinase and the Ras-Raf-MAP kinase pathway is important for the antiapoptotic activities of GM-CSF and IL-3 (27, 56, 60).
We have previously shown that Mcl-1, a member of the Bcl-2 family of proteins, contributes to the maintenance of cell viability by GM-CSF (8). Analysis of murine IL-3-dependent Ba/F3 cells expressing the human GM-CSF receptor α chain in combination with various COOH-terminal truncation mutants of the receptor β chain revealed that the induction of Mcl-1 is dependent on the membrane-distal region of the β subunit between amino acids 573 and 755, a domain known to play an important role in the antiapoptotic activity of the activated receptor (8). Overexpression of Mcl-1 delayed, but did not prevent, apoptosis induced by cytokine withdrawal (8), suggesting that the distal region of the receptor β chain exerts additional effects that contribute to the antiapoptotic action of the receptor.
By use of a PCR-based subtraction cloning approach, we sought to identify additional genes whose expression is induced by the membrane-distal region of the β subunit of the GM-CSF and IL-3 receptors. One gene thus identified turned out to encode a protein highly homologous to murine osteopontin (OPN) (34), which we have designated BOPN (for Ba/F3-derived OPN). Furthermore, we provide evidence that, in response to stimulation with IL-3 or GM-CSF, OPN is induced and released into the medium of cultured cells and that, through binding to the cell surface receptor CD44, it contributes to the survival activities of these two cytokines.
MATERIALS AND METHODS
Cells and cell lines.
CHOP is a Chinese hamster ovary cell line stably transfected with the polyoma virus large T antigen (20) and was kindly provided by James W. Dennis (Mt. Sinai Hospital, Toronto, Canada). CHOP cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Ba/F3 is a murine IL-3-dependent pro-B-cell line and was maintained in RPMI 1640 supplemented with 10% FBS and 1% conditioned medium (CM) from WEHI 3B cells as a source of IL-3. The αβwt, αβ755, αβ573, and αβ453 derivatives of Ba/F3 have been described previously (8) and stably overexpress the human GM-CSF (hGM-CSF) receptor α chain in combination with either the wild-type β chain or a COOH-terminal truncation mutant of the β chain that terminates at residue 755, 573, or 453, respectively. Primary IL-3-dependent cells were isolated essentially as described by Rodriguez-Tarduchy et al. (44). Briefly, bone marrow was flushed from the femurs of BALB/c mice with RPMI 1640 and was then cultured for 48 h in the same medium containing 10% FBS and 10% CM from WEHI 3B cells. The cells remaining in suspension were then separated from the adherent population and maintained in medium containing 20 U of murine IL-3 (mIL-3) (R & D Systems, Minneapolis, Minn.)/ml for 10 to 14 days before analysis. Flow cytometric analysis (see below) confirmed that these primary cells expressed CD44 (data not shown). For all experiments described in the text, unless otherwise indicated, the recombinant mIL-3 and hGM-CSF (Sandoz Pharma Ltd., Basel, Switzerland) were used at concentrations of 10 U/ml and 1 ng/ml, respectively.
Subtraction cloning.
To clone genes that are activated by hGM-CSF in αβ755 cells but not in αβ573 cells, we isolated mRNA from the two cell lines after they had been stimulated with hGM-CSF (10 ng/ml) for 1, 3, 6, 12, or 18 h. The mRNAs isolated from each cell line at the different time points were pooled, and 2 μg of the pooled mixture was subjected to reverse transcription. The resulting cDNA derived from αβ573 and from αβ755 cells was used as the “driver” and “tester” cDNA, respectively, and those cDNA fragments present in the tester but not in the driver fraction were isolated by use of a PCR-Select cDNA Subtraction kit (Clontech). The cDNA fragments selected in this manner were then cloned and sequenced by standard methods.
Northern blot analysis.
Total RNA was isolated from cultured cells as previously described (8), and a portion (20 μg) was resolved on a 1% agarose-formaldehyde gel. The separated RNA molecules were transferred to a nitrocellulose filter, which was then subjected to sequential hybridization overnight at 42°C in a standard buffer containing 50% formamide with 32P-labeled probes specific for Bopn or the glyceraldehyde-3-phosphate dehydrogenase (G3PDH) gene. The blot was washed at 55°C once with 2× standard saline citrate containing 0.1% sodium dodecyl sulfate (SDS) and twice in 0.2× standard saline citrate containing 0.1% SDS and was then subjected to autoradiography.
Expression constructs.
For construction of the mammalian expression vector for hemagglutinin epitope (HA)-tagged BOPN (pcDNA3-BOPN-HA), the full-length BOPN cDNA was derived by reverse transcription and PCR amplification from mRNA that had been purified from hGM-CSF-treated αβ755 cells. PCR was performed with the primers 5′-GCGTCGACACCATGAGATTGGCAGTGATT-3′ (sense) and 5′-GCCTCGAGGTTGACCTCAGAAGATGA-3′ (antisense). The amplified cDNA fragments were digested with SalI and XhoI and were then cloned into the SalI site of the pJ3Ω vector (53) to generate a plasmid (pJ3Ω-BOPN-HA) in which a DNA sequence encoding the HA tag was fused in frame to the 3′ end of the BOPN cDNA. A DNA fragment spanning the BOPN-HA cDNA sequence was then released from pJ3Ω-BOPN-HA by digestion with SalI and BamHI, rendered blunt ended, and ligated into the EcoRV site of the pcDNA3 vector (Invitrogen). The resultant plasmid was further engineered to include a stop linker after the coding region for the HA tag, yielding the final construct pcDNA3-BOPN-HA for the synthesis of BOPN tagged at its COOH terminus with the HA sequence (LDMYPYDVPDYASRDP). The BOPN-HA fusion protein was synthesized from this plasmid in vivo, by transfection into mammalian cells, or in vitro, with the use of a TNT coupled reticulocyte lysate system (Promega).
The mouse OPN expression vector (pcDNA3-OPN-HA) was constructed in a manner similar to that for pcDNA3-BOPN-HA, with the exception that the mouse OPN cDNA was reverse transcribed from an mRNA isolated from a BALB/c mouse kidney. The sequence of this cDNA was confirmed to be identical to that of the mouse OPN reported in reference 34. The vector directed the synthesis of OPN fused at its COOH terminus with the HA tag (LDMYPYDVPDYASSPG).
The expression vector encoding the BOPNΔ79-140 mutant was constructed by isolating the KpnI-XmnI and EagI-BamHI fragments from the pcDNA3-BOPN-HA vector and ligating them, together with an XmnI-EagI adapter (annealed from the sense and antisense oligonucleotides 5′-TCTTCCAAGCAATTCCAATAAC-3′ and 5′-GGCCGTTATTGGAATTGCTTGGAAGA-3′, respectively), back into the KpnI and BamHI sites of pcDNA3-BOPN-HA. The BOPNΔ32-72 expression vector was constructed by isolating the KpnI-HindIII (the HindIII site was rendered blunt ended before digestion with KpnI) and XmnI-BamHI fragments of pcDNA3-BOPN-HA and ligating them back into KpnI- and BamHI-digested pcDNA3-BOPN-HA. The identities of all BOPN cDNA inserts (wild type and mutant) in these expression vectors were confirmed by direct sequencing.
Flow cytometric analysis of surface protein expression.
Cells were washed twice with phosphate-buffered saline and then incubated for 20 min with antibodies either to CD44 (clone IM7 or KM114; Pharmingen) or to integrin αv (clone H9.2B8; Pharmingen) in staining buffer (phosphate-buffered saline containing 0.1% NaN3 and 1% FBS). The cells incubated with antibodies to CD44 were washed twice with staining buffer and then incubated for 20 min with rabbit antibodies to rat immunoglobulin G (IgG) that had been charged with biotin-conjugated goat antibodies to rabbit IgG (Vector Laboratories) and phycoerythrin-conjugated streptavidin (Jackson ImmunoResearch Laboratories). After two washes with staining buffer, the cells were analyzed by flow cytometry with a Becton Dickinson FACScan. Cells incubated with antibodies to integrin αv were subjected to the same staining protocol, with the exception that the αv-positive cells were detected with fluorescein isothiocyanate-conjugated mouse antibodies to hamster IgG (Pharmingen). All incubations were performed on ice, and antibodies were used at the dilutions recommended by the manufacturers.
Immunoprecipitation and immunoblot analysis.
CM from CHOP cells transiently transfected with various OPN expression vectors was subjected to immunoblot analysis as previously described (8). In brief, 150 μg of CM protein was resolved by SDS-polyacrylamide gel electrophoresis on a 10% gel, transferred to a polyvinylidene difluoride membrane (Millipore), and probed with antibodies to HA (Boehringer Mannheim). Immune complexes were detected with horseradish peroxidase-conjugated goat antibodies to mouse IgG and an ECL (enhanced chemiluminescence) kit (Amersham). In some experiments, OPN in CM was first immunoprecipitated with rabbit antiserum to OPN (generated in response to the peptide antigen DPKSKEDDRYLKFRIS, corresponding to amino acids 268 to 283 of the mouse OPN) prior to immunoblot analysis.
Immunodepletion of OPN from CM.
Swollen protein A-Sepharose beads (Pharmacia) were washed with RPMI 1640 and incubated for 40 min at 4°C with the same medium containing bovine serum albumin (10 mg/ml). The beads were then washed twice with RPMI 1640 and incubated for 70 min at 4°C with either control antiserum (rabbit antiserum to Mcl-1) or rabbit antiserum to OPN. After a brief wash with RPMI 1640, the beads were incubated for 1 h at 4°C with CM. The resulting immune complexes were removed by centrifugation, the supernatant was transferred to a fresh tube, and the immunodepletion process was repeated two more times. The final OPN-depleted medium was then tested for its ability to stimulate the growth of αβ573 cells in the presence of hGM-CSF.
Transient transfection.
CHOP cells were transiently transfected with various OPN expression vectors by liposome-mediated gene transfer. In brief, vector DNA (12 μg) was gently mixed for 30 min with 25 μl of Lipofectamine (Gibco-BRL) to form the DNA-lipid complex, which was then added to 106 cells cultured in a volume of 10 ml that had been seeded 1 day earlier. After incubation for 4 h in serum-free medium, the transfected cells were incubated for 24 h in regular growth medium. The latter was then removed, filtered through a 0.2-μm-pore-size filter, and used as CM for the various assays as described.
Baculovirus expression of OPN.
To produce OPN with a baculovirus expression system, we used the BacVector-1000 DNA kit (Novagen). In brief, the mouse OPN cDNA fragment was subcloned into the BamHI site of the baculovirus transfer vector (pVL-1393), and the recombinant OPN-producing baculovirus was generated. Sf9 insect cells were then infected with the recombinant virus, and 3 days later, the culture supernatant was collected. Immunoblot analysis confirmed the presence of OPN in this supernatant (data not shown). The culture supernatant of Sf9 cells infected with the wild-type virus was used as a control. For all experiments described in the text involving the use of the recombinant OPN, unless otherwise indicated, the baculovirus-produced proteins were used.
Assay of [3H]thymidine incorporation.
αβ573 or primary IL-3-dependent cells were seeded at a density of 105 cells/ml in medium containing (or not) mIL-3, hGM-CSF, baculovirus-produced OPN, or control Sf9 cell supernatant, as indicated. After incubation for 24 h, 104 viable cells from each group were transferred to the wells of a 96-well culture plate in the same medium. The assay was initiated by the addition of 1 μCi of [3H]thymidine (Amersham) to each well and was terminated after 20 min (αβ573 cells) or 4 h (primary cells) by cell lysis. The incorporation of [3H]thymidine into DNA was then analyzed as previously described (62). All assays were performed in triplicate and repeated three times.
RESULTS
Activation of the opn gene by IL-3 and GM-CSF signaling pathways.
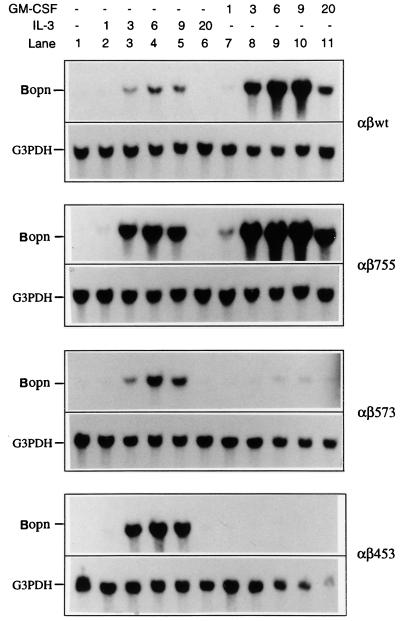
To detect additional genes that are specifically activated as a result of signaling by the membrane-distal region of the hGM-CSF receptor β chain and whose products might contribute to the prevention of apoptosis, we used four derivatives of the murine IL-3-dependent Ba/F3 cell line (αβwt, αβ755, αβ573, and αβ453) that we had previously established and characterized (8). These cells stably overexpress the hGM-CSF receptor α chain in combination with either the wild-type β chain (αβwt) or a β-chain mutant that terminates at residue 755, 573, or 453. Both αβwt and αβ755 cells are fully resistant to apoptosis in medium containing either mIL-3 or hGM-CSF, whereas αβ573 and αβ453 cells exhibit such resistance in medium supplemented with mIL-3 but not in medium containing hGM-CSF (8) (Table 1). We used a PCR-based subtraction cloning approach to detect genes that are specifically activated by hGM-CSF in αβ755 cells but not in αβ573 cells. One gene so detected, designated Bopn, was found to encode a protein highly homologous (with only 7 amino acid substitutions) to murine OPN (34), an acidic phosphoprotein that is secreted by osteoblasts, macrophages, cardiac fibroblasts, and many other cell types (12, 43). Of note, among the 7 different amino acid residues (Asp versus Asn at position 142, Tyr versus Asp at position 171, Tyr versus Asp at position 188, Ser versus Arg at position 224, Gly versus Glu at position 226, His versus Gln at position 232, and His versus Tyr at position 277), the substitutions at positions 142, 171, 188, 224, and 232 were found to be identical to those that appeared in one reported allele of the murine OPN (Eta-1b [37]). Northern analysis confirmed that expression of Bopn was induced by hGM-CSF (within 3 h) in αβwt and αβ755 cells but not in αβ573 and αβ453 cells (Fig. 1). The gene was also activated by mIL-3 in all four Ba/F3 derivatives, with kinetics similar to those apparent in αβwt and αβ755 cells treated with hGM-CSF. These results suggested that the induced expression of Bopn may play a role in the antiapoptotic activities of both IL-3 and GM-CSF.
TABLE 1.
Cell lines used in this study
| Cell line | Exogenously introduced hGM-CSF receptor subunits
|
Apoptosis in medium containing:
|
||
|---|---|---|---|---|
| α Chain | β Chaina | mIL-3 | hGM-CSFb | |
| Ba/F3 | None | None | No | Yes |
| αβwt | wt | wt (aa 1–881) | No | No |
| αβ755 | wt | mt (aa 1–755) | No | No |
| αβ573 | wt | mt (aa 1–573) | No | Yes |
| αβ453 | wt | mt (aa 1–453) | No | Yes |
wt, wild-type protein containing amino acid (aa) residues 1 to 881; mt, mutant protein containing amino acid residues 1 to 755.
From reference 8.
FIG. 1.
Activation of Bopn expression by IL-3 and GM-CSF. The indicated Ba/F3 derivatives were deprived of cytokine and then incubated with either hGM-CSF or mIL-3 for the indicated times (hours). Total RNA was then isolated, and a portion (20 μg) was subjected to Northern blot analysis with 32P-labeled probes specific for Bopn or the G3PDH gene.
Growth-stimulatory activity of OPN.
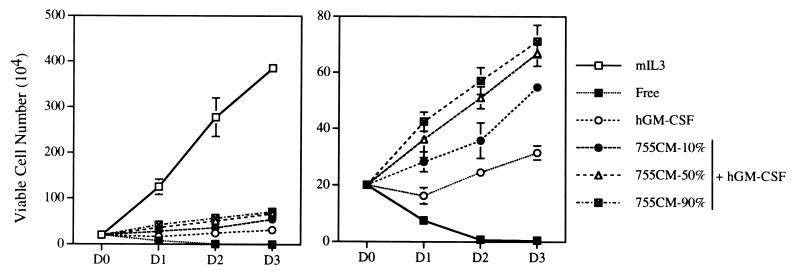
We therefore investigated whether OPN indeed contributes to the antiapoptotic activities of IL-3 and GM-CSF. Given that OPN is a secreted protein, we first examined whether CM from OPN-expressing cells (αβ755 cultured in the presence of hGM-CSF) would support the growth of cells that do not produce OPN (αβ573 cultured in the presence of hGM-CSF). We have previously shown that, in the presence of mIL-3, αβ573 cells both proliferate and are resistant to apoptosis; in contrast, in the presence of hGM-CSF, these cells exhibit a reduced proliferative response and are no longer resistant to apoptosis (8). Thus, whereas the number of viable cells decreased rapidly in cytokine-free medium and increased markedly in medium containing mIL-3, αβ573 cells showed a minimal proliferative response in medium supplemented with hGM-CSF (Fig. 2). However, addition of CM from hGM-CSF-treated αβ755 cells to the hGM-CSF-containing medium of αβ573 cells induced a reproducible, although relatively small, dose-dependent increase in the number of viable cells (Fig. 2).
FIG. 2.
Growth-stimulatory effect of OPN on αβ573 cells in the presence of hGM-CSF. Shown are growth curves of αβ573 cells in basal medium (Free) or in medium containing mIL-3, hGM-CSF, or hGM-CSF plus 10, 50, or 90% CM from αβ755 cells (755CM-10%, -50%, or -90%) grown in the presence of hGM-CSF. The right panel represents an expanded view of the curves in the left panel, with the omission of the growth curve for cells incubated in the presence of mIL-3. D0, the day that cells were seeded: D1, D2, and D3, 1 to 3 days, respectively, after initial seeding. Data are means ± standard deviations of results in duplicate wells from experiments that were repeated three times with similar results.
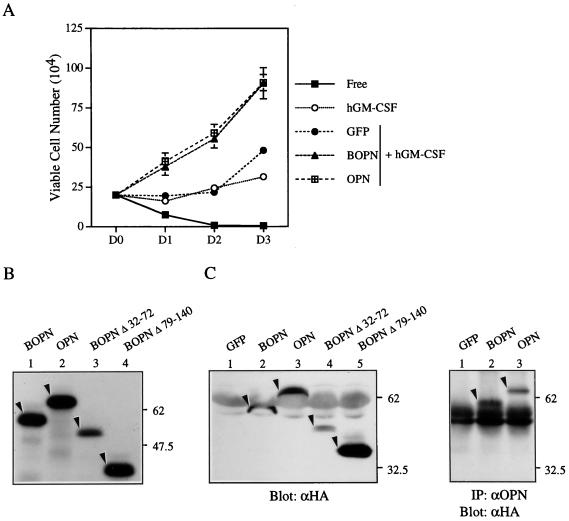
We next examined whether CM from a nonhematopoietic cell line, CHOP, that had been transiently transfected with an OPN expression vector would also stimulate the growth of αβ573 cells in the presence of hGM-CSF. Indeed, CM from CHOP cells transiently transfected with expression vectors encoding either HA-tagged BOPN (OPN encoded by the Bopn gene) or HA-tagged mouse OPN (OPN encoded by the mouse osteopontin gene as reported in reference 34) increased the number of viable αβ573 cells (Fig. 3A). In contrast, CM from CHOP cells transfected with a control vector encoding green fluorescent protein (GFP) did not stimulate cell proliferation. Immunoblot and immunoprecipitation-immunoblot analyses confirmed that CHOP cells transiently transfected with the expression vectors encoding the OPN constructs secreted into the culture medium HA-tagged molecules, that were similar in size to the corresponding proteins synthesized in vitro (Fig. 3B and C). The growth-stimulatory activities of CM containing BOPN or mouse OPN were highly similar, suggesting that the 7 amino acids that differ between the two proteins do not contribute substantially to this effect.
FIG. 3.
Recombinant OPN stimulates the growth of αβ573 cells in the presence of hGM-CSF. (A) Growth curves of αβ573 cells cultured in basal medium (Free) or in medium containing hGM-CSF alone or hGM-CSF plus CM (30%) from CHOP cells transiently transfected with a control (GFP), BOPN, or mouse OPN (OPN) expression vector. Data are means ± standard deviations of results in duplicate wells from experiments that were repeated three times with similar results. (B) The indicated full-length or mutant OPN proteins were synthesized and labeled with [35S]Met in vitro by use of the corresponding expression vectors and a reticulocyte lysate system and were then analyzed by SDS-polyacrylamide gel electrophoresis and autoradiography. The positions of molecular size standards (in kilodaltons) are indicated. (C) (Left) CM from CHOP cells transiently transfected with expression vectors encoding GFP or the indicated HA-tagged OPN proteins was subjected to immunoblot analysis with antibodies to HA (αHA). (Right) CM from CHOP cells expressing GFP or the indicated OPN proteins was subjected to immunoprecipitation (IP) with antibodies to OPN (αOPN), and the resulting immunoprecipitates were subjected to immunoblot analysis with antibodies to HA. Arrowheads in both panels indicate the corresponding HA-tagged OPN protein.
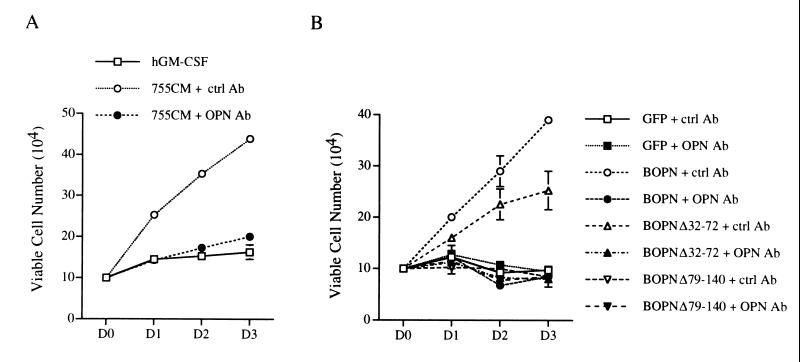
To confirm that the growth-stimulatory activities of CM from αβ755 cells and of CM from OPN-expressing CHOP cells were indeed due to OPN, we examined the effects on αβ573 cells of CM that had been immunodepleted of OPN by use of a specific rabbit antiserum. The growth-promoting effects of CM from hGM-CSF-treated αβ755 cells or from CHOP cells expressing BOPN were abolished by immunodepletion of OPN with the specific antiserum (Fig. 4); they were unaffected by mock immunodepletion with an irrelevant antiserum (rabbit antiserum to human Mcl-1). Furthermore, CM from CHOP cells expressing a BOPN mutant (Δ79-140) that lacks residues 79 to 140, the concentration of which in CM was ∼10 times that of the wild-type protein (Fig. 3C), did not stimulate the growth of αβ573 cells (Fig. 4B). Another BOPN mutant (Δ32-72) did stimulate the growth of αβ573 cells in a manner that was sensitive to specific immunodepletion (Fig. 4B); the extent of this effect was less than that observed for the wild-type protein, probably as a result of a reduced intrinsic activity or the reduced level of expression (Fig. 3C) of the mutant protein. Together, these results confirmed that the growth-stimulatory effect of OPN-containing CM was indeed due to the presence of OPN.
FIG. 4.
Effect of immunodepletion of OPN from CM on growth-stimulatory activity. (A) Growth curves of αβ573 cells in culture medium containing hGM-CSF as well as CM from hGM-CSF-treated αβ755 cells that had been subjected to immunodepletion with either control antibodies (755CM + ctrl Ab) or antibodies to OPN (755CM + OPN Ab) as described in Materials and Methods. (B) Growth curves of αβ573 cells in culture medium containing hGM-CSF as well as immunodepleted CM from CHOP cells expressing GFP or the indicated OPN proteins. Data in both panels are means ± standard deviations of results in duplicate wells from experiments that were repeated three times with similar results.
Role of CD44 in the growth-stimulatory effect of OPN.
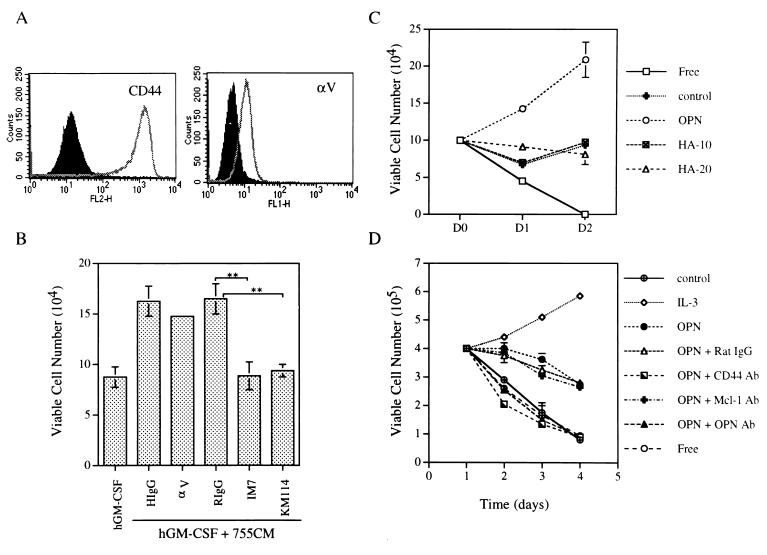
OPN binds to the cell surface receptor CD44 (61) as well as to integrins containing the αv subunit, αvβ1, αvβ3, and αvβ5 (22, 33, 46). To investigate which of these receptors might mediate the growth-stimulatory effect of OPN, we examined the growth response of αβ573 cells to OPN in the presence of neutralizing antibodies that block ligand binding to the αv subunit or to CD44. Flow cytometric analysis revealed that Ba/F3 cells and all derivatives used in the present study express both the integrin αv subunit and CD44 (Fig. 5A and data not shown). Whereas antibodies to the integrin αv subunit did not significantly inhibit the growth-promoting effect of OPN, antibodies to CD44 (clone IM7 or KM114) almost completely blocked the effect of OPN on the growth of αβ573 cells cultured in the presence of hGM-CSF (Fig. 5B). These results suggest that the growth-stimulatory effect of OPN is mediated predominantly, if not exclusively, through CD44. Given that hyaluronic acid is the principal ligand of CD44 (2), we then examined whether hyaluronic acid also stimulates the growth of αβ573 cells in the presence of hGM-CSF. However, unlike OPN, hyaluronic acid did not promote the growth of αβ573 cells (Fig. 5C).
FIG. 5.
Role of CD44 in mediating the growth-stimulatory activity of OPN. (A) Flow cytometric analysis of CD44 and integrin αv expression in αβ573 cells. Open peaks correspond to cells stained either with antibodies to CD44 (clone IM7; left panel) or with antibodies to the integrin αv subunit (right panel). Solid peaks represent cells stained with isotype-matched control antibodies. (B) Effects of antibodies to CD44 or to integrin αv on the growth-stimulatory effect of OPN. αβ573 cells were cultured for 48 h in medium containing hGM-CSF in the absence or presence of 30% CM from hGM-CSF-treated αβ755 cells (755CM) and antibodies to either CD44 (clone IM7 or KM114) or the αv subunit (clone H9.2B8); control incubations were also performed with hamster IgG (HIgG) or rat IgG (RIgG) as indicated. The number of viable cells in each group was then determined on the basis of trypan blue exclusion. ∗∗, P < 0.0001 by Student's t test. (C) Effect of hyaluronic acid on the growth of αβ573 cells. Growth curves were determined for αβ573 cells cultured in the presence of hGM-CSF and hyaluronic acid (10 or 20 ng/ml [HA-10 or HA-20, respectively]). For comparison, cells were also cultured in cytokine-free medium (Free) or in hGM-CSF-containing medium supplemented with 0.5% culture supernatant of Sf9 cells infected either with a baculovirus encoding mouse OPN or with the parent virus (control). (D) Growth curves of primary IL-3-dependent cells cultured in basal medium (Free) or in medium supplemented with either the control culture supernatant (control), mIL-3, or baculovirus-produced OPN alone or in combination with rat IgG (as a control) or antibodies (Ab) to CD44 (clone IM7), OPN, or Mcl-1. Data in panels B through D are means ± standard deviations of duplicates from experiments that were repeated three times with similar results.
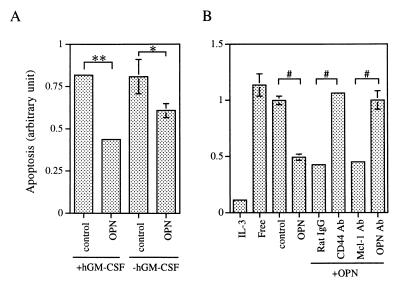
Next, we investigated whether the growth-stimulatory effect of OPN was unique to the Ba/F3 cell line. To address this issue, we performed a similar analysis with primary IL-3-dependent mouse bone marrow cells (see Materials and Methods). Northern blotting confirmed that the opn mRNA was induced within 3 h of treatment with IL-3 in these primary cells (data not shown). Addition of baculovirus-produced OPN to the cytokine-free medium prevented the rapid loss of cell viability, although it did not stimulate proliferation of these primary cells to the extent that IL-3 did (Fig. 5D). Furthermore, the protective effect of OPN on cell viability was abolished in the presence of antibodies to either OPN or CD44 but was unaffected by control antibodies (antibodies to Mcl-1 or rat IgG) (Fig. 5D). These results suggest that the growth-stimulatory effect of OPN was not a unique feature observed in the Ba/F3 cell line.
Relative effects of OPN on cell survival and cell mitogenesis.
The OPN-induced increase in the number of viable cells might reflect an effect on cell survival or on mitogenesis, or both. We therefore measured both the survival and, mitogenic responses of cells to OPN. As shown previously (8), αβ573 cells cultured in the presence of hGM-CSF underwent apoptosis to an extent similar to that observed in cytokine-free medium. However, under such conditions, the addition of baculovirus-produced OPN to the culture medium reduced the number of apoptotic cells by ∼50% (Fig. 6A). In the absence of hGM-CSF, OPN alone also inhibited apoptosis to a significant, although lesser, extent. Figure 6B shows that a similar result was observed in experiments using the primary IL-3-dependent cells and that this antiapoptotic effect of OPN was prevented in the presence of antibodies to CD44 or to OPN.
FIG. 6.
Effect of OPN on cell survival. αβ573 cells (A) or primary IL-3-dependent cells (B) were cultured for 24 h in the presence of the indicated agents, after which the number of apoptotic cells was quantified with an ELISA cell death detection kit (Boehringer Mannheim). The OPN proteins used here were produced by the baculovirus system. Control, culture supernatant of Sf9 cells infected with the wild-type virus. Data are means ± standard deviations of duplicates from experiments that were repeated three times with similar results. ∗, P < 0.01; #, P < 0.001; ∗∗, P < 0.0001.
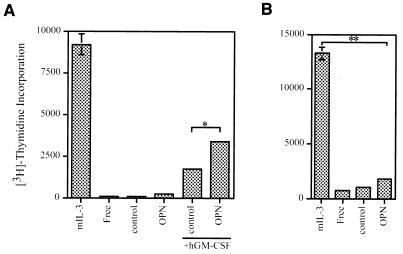
hGM-CSF induced a small mitogenic response in αβ573 cells (8) (Fig. 7A). Whereas these cells incorporated little [3H]thymidine in the presence of OPN alone, the combination of OPN and hGM-CSF induced a synergistic, although still moderate, mitogenic response (Fig. 7A). With the primary cells, only a marginal mitogenic response to OPN was observed (Fig. 7B). Together, these results suggest that prevention of apoptosis and stimulation of mitogenesis both contribute to the increase in the number of viable αβ573 cells induced by the combination of OPN and hGM-CSF. However, the OPN-induced increase in the number of viable primary cells is attributable largely to the prevention of apoptosis, with the stimulation of mitogenesis playing a smaller role.
FIG. 7.
Effect of OPN on cell mitogenesis. The effects of the indicated reagents or combinations of reagents on the incorporation of [3H]thymidine into αβ573 cells (A) or primary IL-3-dependent cells (B) were assayed as described in Materials and Methods. Data are means ± standard deviations of triplicates from an experiment that was repeated three times with similar results. ∗, P < 0.01; ∗∗, P < 0.0001.
Role of the OPN-CD44 pathway in the antiapoptotic activities of IL-3 and GM-CSF.
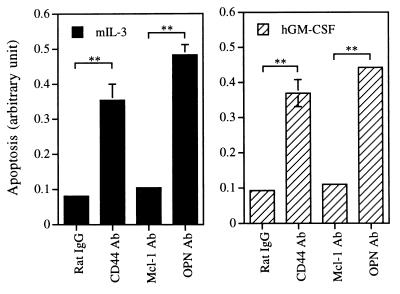
Given that OPN is induced not only by GM-CSF but also by IL-3, we next examined whether the antiapoptotic activities of these two cytokines are dependent on activation of the OPN-CD44 pathway. To address this issue, we examined whether antibodies that block the interaction between OPN and CD44 have any effect on the antiapoptotic activities of these two cytokines. Figure 8 shows that antibodies to either OPN or CD44, but not those to Mcl-1 or rat IgG, increased the number of apoptotic αβ755 cells in medium containing either mIL-3 or hGM-CSF. A nearly identical result was observed in the same type of experiment but with primary cells cultivated in medium containing mIL-3 (data not shown). Together, these results suggest that activation of the OPN-CD44 pathway plays an important role in the antiapoptotic activities of IL-3 and GM-CSF.
FIG. 8.
Effects of neutralizing antibodies (Ab) to OPN or to CD44 on the antiapoptotic activities of IL-3 and GM-CSF in αβ755 cells. Cells were cultured for 24 h in medium containing mIL-3 (left panel) or hGM-CSF (right panel) in the presence of control rat IgG or of antibodies to CD44 (clone IM7), OPN, or Mcl-1. The number of apoptotic cells was then quantified as described in the legend to Fig. 6. Data are means ± standard deviations of duplicates from an experiment that was repeated three times with similar results. ∗∗, P < 0.0001.
DISCUSSION
By use of a PCR-based subtraction cloning approach, we searched for additional antiapoptotic genes whose expression is induced by the membrane-distal region of the β subunit of the GM-CSF and IL-3 receptors. In this report, we demonstrated that one gene thus identified turned out to encode the OPN protein. Furthermore, we provide evidence that, in response to stimulation with IL-3 or GM-CSF, OPN is induced and released into the medium of cultured cells and that, through binding to the cell surface receptor CD44, it contributes to the survival activities of these two cytokines.
Since culture of IL-3-dependent cells in medium supplemented with OPN alone delayed, rather than completely prevented, apoptosis induced by cytokine deprivation, our result further suggests that other IL-3-activated signals are required for the full survival-promoting activity of this cytokine. These extra signals may include signals that lead to increased expression of two antiapoptotic proteins, Mcl-1 and Bcl-XL (8, 29, 39), and signals that lead to activation of the Akt kinase, which in turn phosphorylates and inactivates the proapoptotic molecule Bad (65). In view of the facts that activation of the Akt kinase is crucial to the survival activity of IL-3 (56, 60) and that Akt can modulate the activities of caspase-9 and some transcription factors known to regulate the cell death or cell survival pathways (6, 7, 13, 24, 38, 45, 60), it is likely that inactivation of caspase-9 and transcriptional regulation of other, yet-to-be-identified genes also contribute to the survival activity of IL-3. It remains to be determined how many IL-3-activated signals are required for the full survival activity of this cytokine.
OPN expression is increased in the blood of patients with metastatic disease (54). A few studies with an antisense approach have demonstrated that reduced production of OPN inhibits the tumorigenicity of transformed cell lines (5, 16, 57), and conversely, overexpression of OPN in benign cells has been shown to lead to increased metastasis (36). Using OPN-null mutant mice as a model system, Crawford et al. (11) recently demonstrated that OPN enhances the growth or survival of metastatic cells. However, the molecular mechanisms that underlie this activity of OPN remain unclear. As metastasis involves the migration of tumor cells from one location to a secondary site in vivo, these metastatic cells would face a situation that, in some sense, is similar to anoikis, which is induced in endothelial and epithelial cells by detachment from a substrate containing RGD sequence motifs or by growth in suspension (15, 31). OPN protects rat aorta-derived endothelial cells from apoptosis induced by serum withdrawal (49). Our present finding that OPN produced as a result of growth factor signaling is secreted into the medium, and through activation of another cell surface receptor exerts an antiapoptotic activity on the cultured cells, further helps us to understand why a large variety of malignant cells have evolved to produce an increased level of OPN and have a growth advantage in vitro and in vivo.
OPN contains a GRGDS amino acid sequence motif that mediates interaction with αvβ1, αvβ3, and αvβ5 integrins in a Ca2+-dependent manner (22, 33, 46). The interaction of OPN with these integrins is thought to contribute to various cellular processes, including cell attachment, spreading, and migration; vascular remodeling; and the regulation of mineralization, nitric oxide production, and tumor metastasis (12). OPN also interacts with the cell surface receptor CD44 (61), a protein that has been implicated in many cellular functions, including cell-cell and cell–extracellular-matrix interactions (2), extravasation of lymphocytes across the endothelium of blood vessels and their homing to peripheral organs (23, 40, 47, 59), tumor cell metastasis (18, 52, 58), and regulation of hematopoiesis and apoptosis (3, 21, 28, 51, 64, 66). OPN protects endothelial cells from serum withdrawal-induced apoptosis via interaction with integrin αvβ3 and activation of nuclear factor-B (NF-κB) (49). In contrast, we report here that the antiapoptotic activity of OPN in IL-3-dependent cells is mediated predominantly through interaction with the CD44 receptor but not with the αv-containing integrin. Furthermore, OPN failed to activate NF-κB in Ba/F3 cells (data not shown). These results suggest that the survival pathway activated by OPN in Ba/F3 cells, although still not clear, is likely to be distinct from that triggered in endothelial cells. Further analysis will be required to clarify this issue.
Hyaluronic acid is the principal ligand of CD44. However, in many cell types, CD44 does not bind hyaluronic acid (28). This variability in ligand binding specificity is mainly attributable to cell type-specific glycosylation of various CD44 isoforms generated as a result of alternative mRNA splicing (26). Unlike OPN, hyaluronic acid did not stimulate the growth of Ba/F3 cells, suggesting that the CD44 isoforms present in these cells either do not recognize hyaluronic acid or do not trigger a prominent biological response. Katagiri et al. (25) recently showed that only variant forms, not the standard form (CD44s), of CD44 allow cells to bind OPN. Our analysis of CD44 mRNA in Ba/F3 cells indicates that, in addition to CD44s, these cells express at least two other isoforms of CD44 (data not shown). It remains to be determined which isoform is responsible for the observed survival effect of OPN in the present study.
Although mice deficient in CD44 are developmentally normal, the egress of myeloid progenitors from bone marrow in these mice is defective (51). CD44 is implicated in tumor cell metastasis (18, 51, 52). Although the redistribution of hematopoietic progenitors and metastasis of tumor cells appear to be distinct processes, they both involve migration of cells from one location to another and are dependent on the expression of certain CD44 molecules. During such migration, the myeloid progenitors and metastatic cells might have to override an apoptosis signal triggered by the loss of their normal microenvironment. GM-CSF and IL-3 regulate the viability, differentiation, proliferation, and function of hematopoietic progenitors (1). Stimulation of OPN expression by IL-3 or GM-CSF and consequent activation of the CD44 signaling pathway may thus be one mechanism by which progenitor cells ensure their survival during their maturation and redistribution. Mice lacking OPN exhibit normal development (30, 42). However, it would be interesting to determine whether the distribution of hematopoietic progenitor cells in these mice is affected in a manner similar to that apparent in CD44-null mice.
ACKNOWLEDGMENTS
We thank James W. Dennis for providing CHOP cells.
This work was supported by grant NSC-88-2316-B-001-006-M46 from the National Science Council of Taiwan to H.-F.Y.-Y.
REFERENCES
- 1.Arai K, Lee F, Miyajima A, Arai N, Yokota T. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- 2.Aruffo A, Stamenkovic I, Melnick M, Underhill C B, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 3.Ayroldi E, Cannarile L, Migliorati G, Bartoli A, Nicoletti I, Riccardi C. CD44 (Pgp-1) inhibits CD3 and dexamethasone-induced apoptosis. Blood. 1995;86:2672–2678. [PubMed] [Google Scholar]
- 4.Azam M, Erdjument-Bromage H, Kreider B L, Xia M, Quelle F, Basu R, Saris C, Tempst P, Ihle J N, Schindler C. Interleukin-3 signals through multiple isoforms of Stat5. EMBO J. 1995;14:1402–1411. doi: 10.1002/j.1460-2075.1995.tb07126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behrend E I, Craig A M, Wilson S M, Denhardt D T, Chambers A F. Reduced malignancy of ras-transformed NIH3T3 cells expressing antisense osteopontin RNA. Cancer Res. 1994;54:832–837. [PubMed] [Google Scholar]
- 6.Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 7.Cardone M H, Roy N, Stennicke H R, Salvesen G S, Franke T F, Stanbridge E, Frisch S, Reed J C. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 8.Chao J-R, Wang J-M, Lee S-F, Peng H-W, Lin Y-H, Chou C-H, Li J-C, Huang H-M, Chou C-K, Kuo M-L, Yen J J-Y, Yang-Yen H-F. mcl-1 is an immediate-early gene activated by the granulocyte-macrophage colony-stimulating factor (GM-CSF) signaling pathway and is one component of the GM-CSF viability response. Mol Cell Biol. 1998;18:4883–4898. doi: 10.1128/mcb.18.8.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conscience J F, Verrier B, Martin G. Interleukin-3-dependent expression of the c-myc and c-fos proto-oncogenes in hemopoietic cell lines. EMBO J. 1986;5:317–323. doi: 10.1002/j.1460-2075.1986.tb04215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corey S, Eguinoa A, Puyana-Theall K, Bolen J B, Cantley L, Mollinedo F, Jackson T R, Hawkins P T, Stephens L R. Granulocyte macrophage-colony stimulating factor stimulates both association and activation of phosphoinositide 3-OH-kinase and src-related tyrosine kinase(s) in human myeloid derived cells. EMBO J. 1993;12:2681–2690. doi: 10.1002/j.1460-2075.1993.tb05929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crawford H C, Matrisian L M, Liaw L. Distinct roles of osteopontin in host defense activity and tumor survival during squamous cell carcinoma progression in vivo. Cancer Res. 1998;58:5206–5215. [PubMed] [Google Scholar]
- 12.Denhardt D V, Guo X. Osteopontin: a protein with diverse functions. FASEB J. 1993;7:1475–1482. [PubMed] [Google Scholar]
- 13.Du K, Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem. 1998;273:32377–32379. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- 14.Duronio V, Clark-Lewis I, Federsppiel B, Wieler J S, Schrader J W. Tyrosine phosphorylation of receptor beta subunits and common substrates in response to interleukin-3 and granulocyte-macrophage colony-stimulating factor. J Biol Chem. 1992;267:21856–21863. [PubMed] [Google Scholar]
- 15.Frisch S M, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner H A, Berse B, Senger D R. Specific reduction in osteopontin synthesis by antisense RNA inhibits the tumorigenicity of transformed Rat1 fibroblasts. Oncogene. 1994;9:2321–2326. [PubMed] [Google Scholar]
- 17.Gold M R, Duronio V, Saxena S P, Schrader J W, Aebersold R. Multiple cytokines activate phosphatidylinositol 3-kinase in hemopoietic cells. Association of the enzyme with various tyrosine-phosphorylated proteins. J Biol Chem. 1994;269:5403–5412. [PubMed] [Google Scholar]
- 18.Gunthert U, Hofmann M, Rudy W, Reber S, Zoller M, Haussmann I, Matzku S, Wenzel A, Ponta H, Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991;65:13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- 19.Hanazono Y, Chiba S, Sasaki K, Mano H, Miyajima A, Arai K, Yazaki Y, Hirai H. c-Fps/Fes protein-tyrosine kinase is implicated in a signaling pathway triggered by granulocyte-macrophage colony-stimulating factor and interleukin-3. EMBO J. 1993;12:1641–1646. doi: 10.1002/j.1460-2075.1993.tb05809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heffernan M, Dennis J W. Polyoma and hamster papovavirus large T antigen-mediated replication of expression shuttle vectors in Chinese hamster ovary cells. Nucleic Acids Res. 1991;19:85–92. doi: 10.1093/nar/19.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howie S E, Sommerfield A J, Gray E, Harrison D J. Peripheral T lymphocyte depletion by apoptosis after CD4 ligation in vivo: selective loss of CD44− and ‘activating’ memory T cells. Clin Exp Immunol. 1994;95:195–200. doi: 10.1111/j.1365-2249.1994.tb06036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu D D, Lin E C, Kovach N L, Hoyer J R, Smith J W. A biochemical characterization of the binding of osteopontin to integrins alpha v beta 1 and alpha v beta 5. J Biol Chem. 1995;270:26232–26238. doi: 10.1074/jbc.270.44.26232. [DOI] [PubMed] [Google Scholar]
- 23.Jalkanen S, Saari S, Kalimo H, Lammintausta K, Vainio E, Leino R, Duijvestijn A M, Kalimo K. Lymphocyte migration into the skin: the role of lympocyte homing receptor (CD44) and endothelial cell antigen (HECA-452) J Investig Dermatol. 1990;94:786–792. doi: 10.1111/1523-1747.ep12874646. [DOI] [PubMed] [Google Scholar]
- 24.Kane L P, Shapiro V S, Stokoe D, Weiss A. Induction of NF-κB by the Akt/PKB kinase. Curr Biol. 1999;9:601–604. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 25.Katagiri Y U, Sleeman J, Fujii H, Herrlich P, Hotta H, Tanaka K, Chikuma S, Yagita H, Okumura K, Murakami M, Saiki I, Chambers A F, Uede T. CD44 variants but not CD44s cooperate with beta1-containing integrins to permit cells to bind to osteopontin independently of arginine-glycine-aspartic acid, thereby stimulating cell motility and chemotaxis. Cancer Res. 1999;59:219–226. [PubMed] [Google Scholar]
- 26.Kincade P W, Zheng Z, Katoh S, Hanson L. The importance of cellular environment to function of the CD44 matrix receptor. Curr Opin Cell Biol. 1997;9:635–642. doi: 10.1016/s0955-0674(97)80116-0. [DOI] [PubMed] [Google Scholar]
- 27.Kinoshita T, Yokota T, Arai K, Miyajima A. Suppression of apoptotic death in hematopoietic cells by signalling through the IL-3/GM-CSF receptors. EMBO J. 1995;14:266–275. doi: 10.1002/j.1460-2075.1995.tb07000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lesley J, Hyman J R, Kincade P. CD44 and its interaction with extracellular matrix. Adv Immunol. 1993;54:271–335. doi: 10.1016/s0065-2776(08)60537-4. [DOI] [PubMed] [Google Scholar]
- 29.Leverrier Y, Thomas J, Perkins G R, Mangeney M, Collins M K L, Marvel J. In bone marrow derived Baf-3 cells, inhibition of apoptosis by IL-3 is mediated by two independent pathways. Oncogene. 1997;14:425–430. doi: 10.1038/sj.onc.1200845. [DOI] [PubMed] [Google Scholar]
- 30.Liaw L, Birk D E, Ballas C B, Whitsitt J S, Davidson J M, Hogan B L. Altered wound healing in mice lacking a functional osteopontin gene (spp1) J Clin Investig. 1998;101:1468–1478. doi: 10.1172/JCI1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meredith J E, Fazeli B, Schwartz M A. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993;4:953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyajima A, Mui A L-F, Ogorochi T, Sakamaki K. Receptors for granulocyte-macrophage colony-stimulating factor, interleukin-3 and interleukin-5. Blood. 1993;82:1960–1974. [PubMed] [Google Scholar]
- 33.Miyauchi A, Alvarez J, Greenfield E M, Teti A, Grano M, Colucci S, Zambonin-Zallone A, Ross F P, Teitelbaum S L, Cheresh D. Recognition of osteopontin and related peptides by an alpha v beta 3 integrin stimulates immediate cell signals in osteoclasts. J Biol Chem. 1991;266:20369–20374. [PubMed] [Google Scholar]
- 34.Miyazaki Y, Setoguchi M, Yoshida S, Higuchi Y, Akizuki S, Yamamoto S. The mouse osteopontin gene. Expression in monocytic lineages and complete nucleotide sequence. J Biol Chem. 1990;265:14432–14438. [PubMed] [Google Scholar]
- 35.Mui A L-F, Wakao H, O'Farrell A M, Harada N, Miyajima A. Interleukin-3, granulocyte-macrophage colony-stimulating factor and interleukin-5 transduce signals through two STAT5 homologues. EMBO J. 1995;14:1166–1175. doi: 10.1002/j.1460-2075.1995.tb07100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oates A J, Barraclough R, Rudland P S. The identification of osteopontin as a metastasis-related gene product in a rodent mammary tumour model. Oncogene. 1996;13:97–104. [PubMed] [Google Scholar]
- 37.Ono M, Yamamoto T, Nose M. Allelic difference in the nucleotide sequence of the Eta-1/Op gene transcript. Mol Immunol. 1995;32:447–448. doi: 10.1016/0161-5890(95)00053-h. [DOI] [PubMed] [Google Scholar]
- 38.Ozes O N, Mayo L D, Gustin J A, Pfeffer S R, Pfeffer L M, Donner D B. NF-κB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 39.Packham G, White E L, Eischen C M, Yang H, Parganas E, Ihle J N, Grillot D A, Zambetti G P, Nunez G, Cleveland J L. Selective regulation of Bcl-XL by a Jak kinase-dependent pathway is bypassed in murine hematopoietic malignancies. Genes Dev. 1998;12:2475–2487. doi: 10.1101/gad.12.16.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Picker L J, Terstappen L W, Rott L S, Streeter P R, Stein H, Butcher E C. Differential expression of homing-associated adhesion molecules by T cell subsets in man. J Immunol. 1990;145:3247–3255. [PubMed] [Google Scholar]
- 41.Quelle F W, Sato N, Witthuhn B A, Inhorn R C, Eder M, Miyajima A, Griffin J D, Ihle J N. JAK2 associates with the beta c chain of the receptor for granulocyte-macrophage colony-stimulating factor, and its activation requires the membrane-proximal region. Mol Cell Biol. 1994;14:4335–4341. doi: 10.1128/mcb.14.7.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rittling S R, Matsumoto H N, McKee M D, Nanci A, An X R, Novick K E, Kowalski A J, Noda M, Denhardt D T. Mice lacking osteopontin show normal development and bone structure but display altered osteoclast formation in vitro. J Bone Miner Res. 1998;13:1101–1111. doi: 10.1359/jbmr.1998.13.7.1101. [DOI] [PubMed] [Google Scholar]
- 43.Rodan G A. Osteopontin overview. Ann N Y Acad Sci. 1995;760:1–5. doi: 10.1111/j.1749-6632.1995.tb44614.x. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez-Tarduchy G, Collins M, Lopez-Rivas A. Regulation of apoptosis in interleukin 3-dependent hemopoietic cells by interleukin-3 and calcium ionophores. EMBO J. 1990;9:2997–3002. doi: 10.1002/j.1460-2075.1990.tb07492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romashkova J A, Makarov S S. NF-κB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 46.Ross F P, Chappel J, Alvarez J I, Sander D, Butler W T, Farach-Carson M C, Mintz K A, Robey P G, Teitelbaum S L, Cheresh D A. Interactions between the bone matrix proteins osteopontin and bone sialoprotein and the osteoclast integrin alpha v beta 3 potentiate bone resorption. J Biol Chem. 1993;268:9901–9907. [PubMed] [Google Scholar]
- 47.Salmi M, Jalkanen S. Regulation of lymphocyte traffic to mucosaassociated lymphatic tissues. Gastroenterol Clin N Am. 1991;20:495–510. [PubMed] [Google Scholar]
- 48.Sato N, Sakamaki K, Terada N, Arai K, Miyajima A. Signal transduction by the high-affinity GM-CSF receptor: two distinct cytoplasmic regions of the common beta subunit responsible for different signaling. EMBO J. 1993;12:4181–4189. doi: 10.1002/j.1460-2075.1993.tb06102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scatena M, Almeida M, Chaisson M L, Fausto N, Nicosia R F, Giachelli C M, Scatena M. NF-κB mediates αvβ3 integrin-induced endothelial cell survival. J Cell Biol. 1998;141:1083–1093. doi: 10.1083/jcb.141.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scheid M P, Lauener R W, Duronio V. Role of phosphatidylinositol 3-OH-kinase activity in the inhibition of apoptosis in haemopoietic cells: phosphatidylinositol 3-OH-kinase inhibitors reveal a difference in signalling between interleukin-3 and granulocyte-macrophage colony-stimulating factor. Biochem J. 1995;312:159–162. doi: 10.1042/bj3120159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmits R, Filmus J, Gerwin N, Senaldi G, Kiefer F, Kundig T, Wakeham A, Shahinian A, Catzavelos C, Rak J, Furlonger C, Zakarian A, Simard J J L, Ohashi P S, Paige C J, Gutierrez-Ramos J C, Mak T W. CD44 regulates hematopoietic progenitor distribution, granuloma formation, and tumorigenicity. Blood. 1997;90:2217–2233. [PubMed] [Google Scholar]
- 52.Seiter S, Arch R, Reber S, Komitowski D, Hofmann M, Ponta H, Herrlich P, Matzku S, Zoller M. Prevention of tumor metastasis formation by anti-variant CD44. J Exp Med. 1993;177:443–455. doi: 10.1084/jem.177.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sells M A, Chernoff J. Epitope-tag vectors for eukaryotic protein production. Gene. 1995;152:187–189. doi: 10.1016/0378-1119(94)00685-l. [DOI] [PubMed] [Google Scholar]
- 54.Senger D R, Perruzzi C A, Gracey C F, Papadopoulos A, Tenen D G. Secreted phosphoproteins associated with neoplastic transformation: close homology with plasma proteins cleaved during blood coagulation. Cancer Res. 1988;48:5770–5774. [PubMed] [Google Scholar]
- 55.Silvennoinen O, Witthuhn B, Quelle F W, Cleveland J L, Yi T, Ihle J N. Structure of the Jak2 protein tyrosine kinase and its role in IL-3 signal transduction. Proc Natl Acad Sci USA. 1993;90:8429–8433. doi: 10.1073/pnas.90.18.8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Songyang Z, Baltimore D, Cantley L C, Kaplan D R, Franke T F. Interleukin 3-dependent survival by the Akt protein kinase. Proc Natl Acad Sci USA. 1997;94:11345–11350. doi: 10.1073/pnas.94.21.11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su L, Mukherjee A B, Mukherjee B B. Expression of antisense osteopontin RNA inhibits tumor promoter-induced neoplastic transformation of mouse JB6 epidermal cells. Oncogene. 1995;10:2163–2169. [PubMed] [Google Scholar]
- 58.Sy M-S, Kuo Y-J, Stamenkovic I. Distinct effects of two CD44 isoforms on tumor growth in vivo. J Exp Med. 1991;174:859–866. doi: 10.1084/jem.174.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toyama-Sorimachi N, Miyake K, Miyasaka M. Activation of CD44 induces ICAM-1/LFA-1-independent, Ca2+, Mg(2+)-independent adhesion pathway in lymphocyte-endothelial cell interaction. Eur J Immunol. 1993;23:439–446. doi: 10.1002/eji.1830230221. [DOI] [PubMed] [Google Scholar]
- 60.Wang J-M, Chao J-R, Chen W, Kuo M-L, Yen J J-Y, Yang-Yen H-F. The anti-apoptotic gene mcl-l is up-regulated by the phosphatidylinositol 3-kinase/Akt signaling pathway through a transcription factor complex containing CREB. Mol Cell Biol. 1999;19:6195–6206. doi: 10.1128/mcb.19.9.6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weber G F, Ashkar S, Glimcher M J, Cantor H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1) Science. 1996;271:509–512. doi: 10.1126/science.271.5248.509. [DOI] [PubMed] [Google Scholar]
- 62.Yen J J-Y, Hsieh Y C, Yen C L, Chang C C, Lin S, Yang-Yen H-F. Restoring the apoptosis suppression response to IL-5 confers on erythroleukemic cells a phenotype of IL-5-dependent growth. J Immunol. 1995;154:2144–2152. [PubMed] [Google Scholar]
- 63.Yoshimura A, Ohkubo T, Kiguchi T, Jenkins N A, Gilbert D J, Copeland N G, Hara T, Miyajima A. A novel cytokine-inducible gene CIS encodes an SH2-containing protein that binds to tyrosine-phosphorylated interleukin 3 and erythropoietin receptors. EMBO J. 1995;14:2816–2826. doi: 10.1002/j.1460-2075.1995.tb07281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu Q, Toole B P, Stamenkovic I. Induction of apoptosis of metastatic mammary carcinoma cells in vivo by disruption of tumor cell surface CD44 function. J Exp Med. 1997;186:1985–1996. doi: 10.1084/jem.186.12.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zha J, Harada H, Yang E, Jockel J, Korsmeyer S J. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X. Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 66.Zhou T, Edwards III C K, Mountz J D. Prevention of age-related T cell apoptosis defect in CD2-fas-transgenic mice. J Exp Med. 1995;182:129–137. doi: 10.1084/jem.182.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]