Key Points
Question
Do intra-articular platelet-rich plasma injections improve ankle symptoms and function in patients with ankle osteoarthritis?
Findings
In this randomized clinical trial that included 100 patients, treatment with 2 intra-articular platelet-rich plasma injections vs placebo injections with saline resulted in a mean change in the American Orthopaedic Foot and Ankle Society score (range, 0-100; higher scores indicate less pain and better function) of 10 vs 11 points over 26 weeks; the between-group difference was not statistically significant.
Meaning
These findings do not support the use of platelet-rich plasma injections for patients with ankle osteoarthritis.
Abstract
Importance
Approximately 3.4% of adults have ankle (tibiotalar) osteoarthritis and, among younger patients, ankle osteoarthritis is more common than knee and hip osteoarthritis. Few effective nonsurgical interventions exist, but platelet-rich plasma (PRP) injections are widely used, with some evidence of efficacy in knee osteoarthritis.
Objective
To determine the effect of PRP injections on symptoms and function in patients with ankle osteoarthritis.
Design, Setting, and Participants
A multicenter, block-randomized, double-blinded, placebo-controlled clinical trial performed at 6 sites in the Netherlands that included 100 patients with pain greater than 40 on a visual analog scale (range, 0-100) and tibiotalar joint space narrowing. Enrollment began on August 24, 2018, and follow-up was completed on December 3, 2020.
Interventions
Patients were randomly assigned (1:1) to receive 2 ultrasonography-guided intra-articular injections of either PRP (n = 48) or placebo (saline; n = 52).
Main Outcomes and Measures
The primary outcome was the validated American Orthopaedic Foot and Ankle Society score (range, 0-100; higher scores indicate less pain and better function; minimal clinically important difference, 12 points) over 26 weeks.
Results
Among 100 randomized patients (mean age, 56 years; 45 [45%] women), no patients were lost to follow-up for the primary outcome. Compared with baseline values, the mean American Orthopaedic Foot and Ankle Society score improved by 10 points in the PRP group (from 63 to 73 points [95% CI, 6-14]; P < .001) and 11 points in the placebo group (from 64 to 75 points [95% CI, 7-15]; P < .001). The adjusted between-group difference over 26 weeks was −1 ([95% CI, –6 to 3]; P = .56). One serious adverse event was reported in the placebo group, which was unrelated to the intervention; there were 13 other adverse events in the PRP group and 8 in the placebo group.
Conclusions and Relevance
Among patients with ankle osteoarthritis, intra-articular PRP injections, compared with placebo injections, did not significantly improve ankle symptoms and function over 26 weeks. The results of this study do not support the use of PRP injections for ankle osteoarthritis.
Trial Registration
Netherlands Trial Register: NTR7261
This randomized clinical trial assessed the efficacy of platelet-rich plasma injections, compared with placebo, in ankle (tibiotalar) osteoarthritis.
Introduction
Osteoarthritis affects an estimated 250 million people worldwide and is associated with pain and disability, especially in the lower extremities.1 In 2018, ankle (tibiotalar) osteoarthritis was estimated in a UK-based study to affect approximately 3.4% of adults.2 Younger active patients with ankle osteoarthritis have reduced quality of life, comparable to people with hip osteoarthritis, kidney failure, and congestive heart failure.3,4 However, effective nonsurgical interventions are not available for ankle osteoarthritis.5,6
Platelet-rich plasma (PRP) injections are used increasingly to manage osteoarthritis.5,7,8,9 PRP is derived from autologous blood using a centrifugation system to facilitate growth factor release from the α-granules found in platelets.10,11 These growth factors modulate the intra-articular environment, potentially facilitating an anti-inflammatory, anabolic, and analgesic effect.10,11 The global commercial market for PRP is projected to more than double, from an estimated $190 million in 2019 to $400 million in 2024 and an estimated $1.2 billion by 2028.8,9
PRP injections for knee osteoarthritis have been investigated in 21 randomized clinical trials.7 Meta-analyses report some benefits for PRP injections in knee osteoarthritis.7
Investigation of PRP for ankle osteoarthritis is limited to 4 small case series, and all have reported statistically significant improvements in symptoms and function.5,12 Randomized clinical trials comparing PRP with placebo in patients with ankle osteoarthritis have not been performed. The Platelet-Rich Plasma Injections for the Management of Ankle Osteoarthritis (PRIMA) randomized clinical trial assessed the efficacy of PRP injections in ankle (tibiotalar) osteoarthritis.
Methods
Study Design
This study was a multicenter, stratified, block-randomized, double-blind, placebo-controlled trial performed in 6 centers (2 university medical centers, 2 teaching hospitals, 1 general hospital, and 1 private specialist clinic) in the Netherlands. A detailed description of the study design has been published.13 The initial and revised protocol and statistical analysis plan are shown in Supplement 1.13 The study protocol and all amendments were approved by the local medical ethics review committee of Amsterdam UMC (Amsterdam, The Netherlands). Written informed consent was obtained from all participants. The study was monitored by the clinical research unit of the Amsterdam UMC (Amsterdam, The Netherlands). The study protocol was amended and approved by the local medical ethics review committee on May 5, 2020, after the start of enrollment but before any results were available. Due to the COVID-19 pandemic, some patients were unable to receive their second study injection. Therefore, based on recent literature recommendations for studies affected by the COVID-19 pandemic, the participation of these patients in this study was discontinued and new patients were enrolled (COVID-19 lockdown–related protocol amendment).14 Due to this COVID-19 amendment, only the patients who were able to receive 2 injections were included and were analyzed according to their randomization group.
Study Participants
Patients with ankle osteoarthritis were informed of the study at orthopedic and sports medicine outpatient clinics at the 6 centers. Patients were eligible if they were 18 years or older, had a score of at least 40 for ankle osteoarthritis pain severity on a visual analog scale (VAS; range, 0-100; higher scores indicate more severe pain) during daily activities, and had radiographic imaging (anteroposterior and lateral view) indicating at least grade 2 tibiotalar osteoarthritis on the van Dijk classification.15 Patients were excluded if they received injection therapy for ankle osteoarthritis in the past 6 months, declined either therapy, had signs of concomitant osteoarthritis of 1 or more other major joints of the lower extremities that impaired their daily activity level, or underwent a previous ankle operation for osteoarthritis or osteochondral defects less than 1 year before randomization (not including surgery for an ankle fracture in the past). Further details on baseline measurements, including radiological variables, are provided in eTables 1 and 2 in Supplement 2 and the published protocol.13
Randomization and Blinding
Patients were randomized to receive PRP vs placebo (saline) via intra-articular injections (Figure 1). A Good Clinical Practice–approved data management system (Castor EDC) was used to perform computer-generated block randomization stratified by center using variable block sizes of 2, 4, and 6 in a 1:1 ratio. Physicians referred potentially eligible patients. The coordinating research physician determined eligibility based on inclusion and exclusion criteria, obtained written informed consent, and enrolled patients in the study. The coordinating research physician initiated randomization in the data management system but remained blinded to the allocated intervention. To ensure blinding of the intervention and concealment of randomization, the coordinating research physician prepared a syringe with PRP and a syringe with placebo (isotonic saline: 0.9% sodium chloride). Only the independent research assistants had access to the randomization result in the data management system. These research assistants covered study syringes with a specially manufactured thick plastic covering sheath to conceal the appearance of the study intervention and temperature of the syringe. After the intra-articular injection, the syringe covered by the sheath (containing either the remnants of the PRP or saline) was handed back to the independent research assistant, who disposed of the syringe in effort to maintain blinding of the patient, treating physician, and coordinating researcher. The success of blinding was assessed by asking patients just after the injections what treatment they thought they had received.
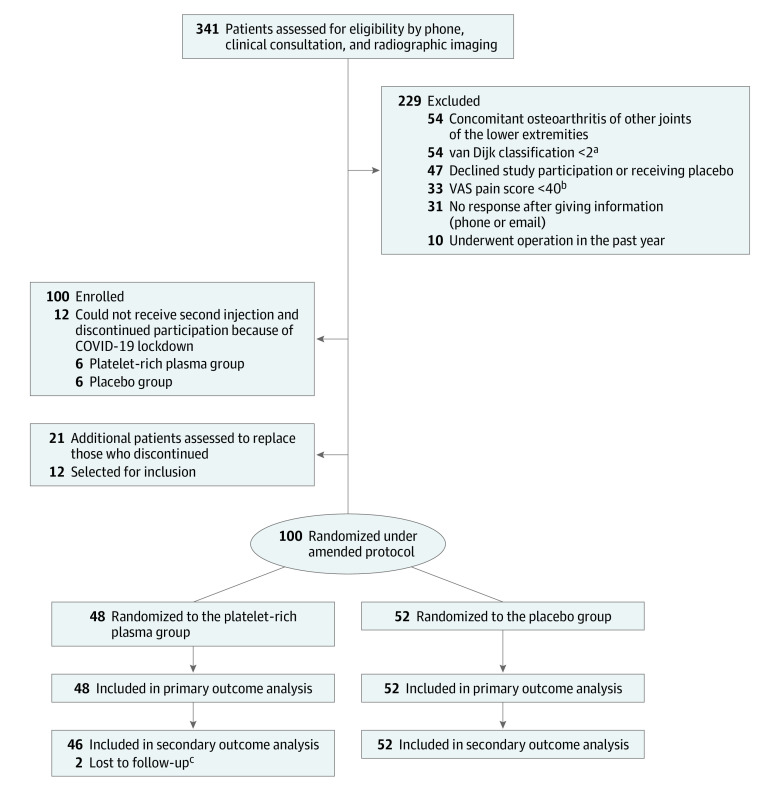
Figure 1. Patient Flow in a Study of the Effect of Platelet-Rich Plasma Injections vs Placebo on Ankle Symptoms and Function in Patients With Ankle Osteoarthritis.
avan Dijk classification: 0 indicates normal joint or subchondral sclerosis; 1, osteophytes without joint space narrowing; 2, joint space narrowing with or without osteophytes; 3, (sub)total disappearance or deformation of the joint space.
bVisual analog scale (VAS) score ranges from 0 to 100, higher scores indicate more severe pain.
cPrior to each consult the questionnaires were checked for completeness. A reminder was then sent by email in the event of incompleteness. Two patients did not complete the secondary outcome questionnaires at 26 weeks because they felt it was too time consuming.
Procedures
Patients received 2 intra-articular injections 6 weeks apart. An optimal PRP formulation has not been identified. Therefore, PRP (leukocyte poor) was prepared using a widely used and commercially available system (Arthrex double syringe PRP system, Arthrex Medizinische Instrumente GmbH) used previously in other studies.16,17,18 One syringe of 15 mL of autologous blood was collected from the cubital vein at inclusion and 6 weeks later. After blood collection, the syringe was centrifuged for 5 minutes and the injection was administered within 30 minutes after venipuncture to prevent blood clot formation. No additional substances (calcium, thrombin, or citrate) were added to the PRP solution. For each procedure, 2 mL of PRP or placebo was injected into the affected ankle joint under ultrasonography guidance using sterile technique. The anteromedial needle placement was located medially from the tendon of the tibialis anterior, lateral to the medial malleolus, and at the level of the ankle joint line. The anterolateral needle placement was located just lateral to the peroneus tertius tendon, medial to the lateral malleolus, and at the level of the ankle joint line. Local anesthetic was not used. After the injection, patients were advised to avoid heavy or repetitive stress to the ankle joint for 48 hours. Patients were instructed to avoid co-interventions and nonsteroidal anti-inflammatory drugs (NSAIDs) 24 hours prior to the intervention and, if possible, up to 1 year after the first injection. Both PRP and NSAIDs potentially affect the inflammatory cascade and may interact with and reduce the efficacy of PRP.10 Throughout the study, co-interventions, such as NSAIDs or intra-articular injections, used by patients were registered. All participants received lifestyle and exercise counseling for osteoarthritis at enrollment, consistent with standard care for patients not undergoing surgical treatment (Supplement 2).
Outcomes
The primary outcome was the American Orthopaedic Foot and Ankle Society (AOFAS) score over 26 weeks of follow-up.13 The AOFAS is a validated scale for ankle osteoarthritis (range, 0-100 points; higher scores indicate less pain and better function) that measures 3 subdomains (pain [40 points; 1 item], function [50 points; 7 items], and alignment [10 points; 1 item]) totaling 9 items.19,20 The AOFAS is translated and validated in Dutch. The AOFAS was administered at baseline, 6-week follow-up, and 26-week follow-up by the coordinating research physician, who traveled to all sites for all patients. Secondary outcome measures were assessed at baseline and at 6-, 12-, and 26-week follow-up. Secondary outcomes were total AOFAS score at 6 weeks (other time points than the primary outcome)19; the AOFAS pain subscale score (range, 0-40 points; lower scores indicate more pain; minimal clinically important difference [MCID] unknown for ankle osteoarthritis)19; the Foot and Ankle Outcome Score (5 scales: pain [MCID, 15], symptoms [MCID, 7], quality of life [MCID, 18], activity of daily living [MCID, 23], and sport and recreation [MCID, 21]; all scales range from 0 to 100 points; higher scores indicate fewer symptoms)21; the Ankle Osteoarthritis Scale, measuring pain and disability (range, 0-100 points; higher scores indicate more symptoms; MCID, 28 points)20,22; pain during activities of daily living, measured on a visual analog scale (range, 0-100; higher scores indicate more pain; MCID unknown for ankle osteoarthritis)23; the Ankle Activity Score (scored according to a chart based on the performable activity level; range, 0-10 points; higher scores indicate higher ankle stress activities; MCID unknown for ankle osteoarthritis)24; self-reported patient satisfaction (4 categories: excellent, good, fair, poor); the 36-Item Short Form Health Survey (measuring health-related quality of life; range, 0-100 points; higher scores indicate better quality of life; MCID unknown for ankle osteoarthritis)20; the Global Attainment Scaling (based on achievement related to predetermined goals in agreement with the patient; higher scores indicate more achievement; score of −2 to 3 indicate decline from baseline; MCID unknown for ankle osteoarthritis)25; and the 3-Level EuroQol 5-Dimension tool (measuring the generic quality of life across 5 dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression expressed using a summary index of 0-1, with 0 indicating death and 1 indicating full health, and a health visual analog scale ranging from 0 to 100, with 0 indicating the worst health imaginable and 100 indicating the best; MCID unknown for ankle osteoarthritis).26 All adverse events reported spontaneously by the patient or observed by the investigator or their staff were recorded.
Sample Size
The study was designed to have statistical power to detect a MCID of 12 points on the primary outcome of AOFAS score (range, 0-100) over 26 weeks.27,28,29 There is no official agreement on the MCID for the AOFAS score regarding ankle osteoarthritis. In knee and hip osteoarthritis with comparable disease specific patient-reported outcome measures, a 10% to 15% change of the used scale was reported as minimal clinically important difference.27,28,29 Our predefined MCID of 12% is located within this range.27,28,29
With a 2-sided significance level of 5%, 90% power, a dropout rate of 10%, and an expected SD of 16.3, a total of 50 patients per group were needed (100 in total).13
Statistical Analysis
To test for the effect of treatment on the between-group difference in the primary outcome, we used a general linear model for repeated measures. Changes from baseline to all follow-up time points were included in the model. Adjustments were made for those baseline variables that were associated with the primary outcome, with P < .10, using a multivariable analysis (general linear model repeated measures) with stepwise backward elimination. To test for the effect of treatment on between-group differences in the secondary outcomes, we used a general linear model for repeated measures. Changes from baseline to all follow-up time points were included in the model.
Patients were analyzed according to their randomization group. The efficacy results that include patients whose participation was discontinued due to the COVID-19 lockdown were analyzed in a sensitivity analysis.
For missing data, single imputation by last observation carried forward was planned if missing data occurred within 10 weeks of the last observation. Multiple imputation was planned if there were more than 10% missing items on a scale. Little’s missing completely at random test was used to allow an assumption that the missing data were missing at random. A sensitivity analysis was planned if more than 5% of data were missing.
The data were interpreted according to a blinded data interpretation plan.30 The principal investigator, coordinating research physician, and co-investigators interpreted the blinded statistical results until a consensus was reached (Supplement 2). Patients (none of whom were randomized into the trial) attended this meeting and were given opportunity to interpret the results from a patient perspective. Once study investigators and patients agreed on result interpretation, an independent investigator assessed interpretation of the blinded results.30 Following the written interpretation of the independent investigator, data were unblinded and no changes were made to the interpretation (Supplement 2). Statistical analyses were performed using IBM SPSS, version 26, for Windows. A 2-sided P ≤ .05 was considered statistically significant. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory.
Post Hoc Analysis
To test the robustness of study findings, we performed a post hoc mixed-effects model analysis for the primary and secondary outcomes adjusting for the enrolling centers (as random effects) to account for biases introduced by greater similarities of patients within sites than among sites.
Results
Enrollment began August 24, 2018, and the last patient completed the 26-week follow-up on December 3, 2020. In total, 320 patients were assessed for eligibility, of whom 100 (31%) were enrolled in the trial. The initial protocol included randomization of 100 patients (49 in the PRP group and 51 in the placebo group). Due to the COVID-19 lockdown, 12 participants could not receive their planned second injection and were excluded from the trial, and were replaced by 12 new randomized participants. A total of 21 additional patients were evaluated to identify the 12 (57%) who were randomized in the trial to replace the 12 participants who discontinued during the COVID-19 lockdown. Of the 100 included participants, 48 were randomized to receive PRP and 52 were randomized to receive placebo (Figure 1). There were no missing data for the primary outcome. Two patients did not complete the secondary outcome questionnaires at 26 weeks. Baseline characteristics are presented in Table 1. The study population had a mean (SD) age of 55.6 (13.8) years, 45 participants were women (45%), and the population had a mean (SD) body mass index of 26.7 (3.8).
Table 1. Baseline Characteristics of the Study Population.
| Characteristic | No. (%) | |
|---|---|---|
| PRP group (n = 48) | Placebo group (n = 52) | |
| Men | 26 (54) | 29 (56) |
| Women | 22 (46) | 23 (44) |
| Age, mean (SD), y | 54.8 (13.3) | 56.4 (14.4) |
| Weight, mean (SD), kg | 86.5 (15.3) | 82.6 (14.4) |
| Height, mean (SD), m | 1.77 (0.09) | 1.78 (0.10) |
| Body mass index, mean (SD) | 27.5 (4.2) | 26.0 (3.3) |
| Left laterality | 25 (52) | 27 (52) |
| Duration of ankle OA symptoms, median (IQR), y | 5 (2 to 8) | 8 (3 to 14) |
| Frequency of playing sports | ||
| <1 time per week | 19 (40) | 25 (48) |
| 1-2 times per week | 21 (44) | 16 (31) |
| 3-4 times per week | 6 (13) | 6 (12) |
| >5 times per week | 2 (4) | 5 (10) |
| Recreational exercise (not competitive) | 48 (100) | 52 (100) |
| Previously sustained ankle trauma | 47 (98) | 52 (100) |
| Anterior drawer test present | 14 (29) | 10 (19) |
| Ankle ROM, median (IQR), degrees | 55 (46 to 62) | 55 (41 to 65) |
| Weighted radiographs, No. (%) | 39 (81) | 38 (73) |
| Radiological ankle OA van Dijk15 classificationa | ||
| Grade 2 (joint space narrowing with or without osteophytes) | 29 (60) | 40 (77) |
| Grade 3 ([sub]total disappearance or deformation of the joint space) | 19 (40) | 12 (23) |
| Radiological ankle OA according to Kellgren-Lawrenceb | ||
| Grade 3 (moderate diminution of joint space) | 29 (60) | 40 (77) |
| Grade 4 (joint space greatly impaired, subchondral sclerosis) | 19 (40) | 12 (23) |
| Radiological ankle OA according to Takakurac | ||
| Stage 1 (signs of subchondral sclerosis or osteophyte formation) | 24 (50) | 31 (60) |
| Stage 2 (tibiotalar tilt with varus alignment, no subchondral bone contact) | 6 (13) | 10 (19) |
| Stage 3 (tibiotalar tilt with varus alignment, subchondral bone contact) | 9 (19) | 6 (12) |
| Stage 4 (global tibiotalar joint space narrowing with complete contact) | 9 (19) | 5 (10) |
| Radiological medial distal tibial angle, median (IQR), degreesd | 90.0 (87.5 to 92.0) | 90.6 (87.5 to 91.6) |
| Radiological talar tilt, median (IQR), degreese | −0.25 (−3.4 to 2.0) | −0.15 (−1.9 to 1.7) |
| Baseline AOFAS score, mean (SD)f | 63 (13) | 64 (16) |
Abbreviations: OA, osteoarthritis; PRP, platelet-rich plasma; ROM, range of motion (calculated as plantar flexion + dorsal flexion).
van Dijk classification: 0 indicates normal joint or subchondral sclerosis; 1, osteophytes without joint space narrowing; 2, joint space narrowing with or without osteophytes; 3, (sub)total disappearance or deformation of the joint space.
Takakura classification: 0 indicates no tibiotalar tilt, no signs of arthritis; 1, no tibiotalar tilt, signs of subchondral sclerosis, or osteophyte formation; 2, tibiotalar tilt with varus alignment, no subchondral bone contact; 3, tibiotalar tilt with varus alignment, subchondral bone contact; 4, global tibiotalar joint space narrowing with complete contact.
Kellgren-Lawrence classification: 1 indicates minute osteophyte of doubtful significance; 2, definite osteophyte, joint space unimpaired; 3, moderate diminution of joint space; 4, joint space greatly impaired, subchondral sclerosis.
The medial distal tibial angle is the angle between the center of the tibia shaft and the tibia plafond; <90° is a valgus angle and >90° is a varus angle.
Radiological talar tilt = (tibiotalar angle) – (medial distal tibial angle). The tibiotalar angle is the angle between the center of the tibia shaft and the talar dome. All negative values indicate a varus alignment, while positive values indicate a valgus alignment.
American Orthopaedic Foot and Ankle Society (AOFAS) score ranges from 0 to 100 (higher scores indicate less pain and better function); no clinical cutoff scores are available, but as an indication, patients with end-stage ankle OA undergoing an ankle arthrodesis or arthroplasty were reported to have a mean AOFAS score of 36 to 43.
Primary Outcome
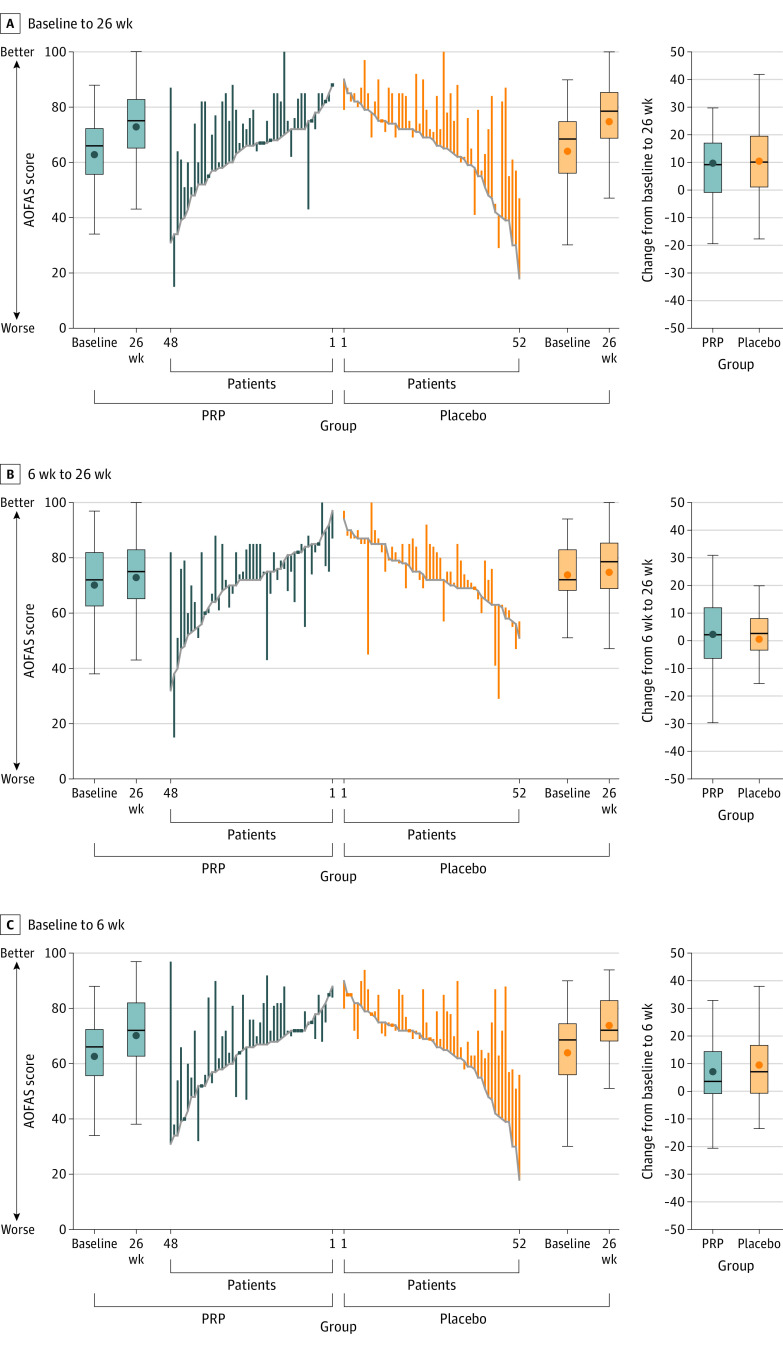
The mean (SD) baseline AOFAS scores were 63 (13) in the PRP group and 64 (16) in the placebo group. Between baseline and 26-week follow-up, the mean AOFAS score improved by 10 points (95% CI, 6-14) in the PRP group compared with 11 points (95% CI, 7-15) in the placebo group (Table 2; Figure 2 and Figure 3). The following 2 baseline variables were associated with the primary outcome, with P <.10: duration of symptoms of ankle osteoarthritis (in years) and radiological talar tilt (in degrees) (eTable 16 in Supplement 2). The adjusted between-group difference of PRP vs placebo for AOFAS improvement over 26 weeks was −1 point ([95% CI, –6 to 3]; P = .56). The unadjusted between-group difference of the primary outcome is presented in Supplement 2. The sensitivity analysis of all 112 randomized patients showed an adjusted between-group difference of PRP vs placebo for AOFAS improvement at 26 weeks of −2 points ([95% CI, –8 to 3]; P = .40) (Supplement 1 and eTable 3 in Supplement 2). In a post hoc sensitivity analysis, there was no statistically significant between-group difference of PRP vs placebo for AOFAS change over 26 weeks (–2 points [95% CI, –5 to 1]; P = .16) (eTable 4 in Supplement 2).
Table 2. Primary and Secondary Outcomes Over 26 Weeks.
| Outcome | Median (IQR) | ||
|---|---|---|---|
| PRP group (n = 48) | Placebo group (n = 52) | Mean difference (95% CI)a | |
| Primary outcome over 26 wk | |||
| AOFAS score, mean (SD)b | 73 (14) [n = 48] | 75 (14) [n = 52] | –1 (–6 to 3) |
| Secondary outcomes over 26 wkc | |||
| AOFAS pain subscale scored | 30 (20 to 30) | 30 (20 to 30) | 0 (–2 to 2) |
| Foot and Ankle Outcome Scoree | |||
| Pain | 67 (52 to 78) [n = 46] | 71 (50 to 88) | –2 (–8 to 4) |
| Symptoms, mean (SD) | 54 (18) [n = 46] | 55 (21) | –2 (–8 to 4) |
| Activity of daily living | 82 (69 to 93) [n = 46] | 84 (71 to 97) | –1 (–7 to 6) |
| Quality of life | 31 (25 to 50) [n = 46] | 38 (25 to 55) | –1 (–7 to 6) |
| Sport and recreation | 40 (20 to 51) [n = 46] | 40 (25 to 60) | –1 (–9 to 8) |
| AOSf | 26 (12 to 36) [n = 46] | 23 (8 to 41) | 1 (–6 to 8) |
| VASg | 40 (24 to 50) [n = 46] | 44 (19 to 65) | 3 (–5 to 10) |
| AASh | 4.0 (2.8 to 5.0) [n = 46] | 4.0 (1.3 to 5.0) | 0.1 (–0.7 to 0.9) |
| SF-36, mean (SD)i | |||
| Mental component summary scorej | 42 (6) [n = 46] | 43 (6) | 0 (–2 to 2) |
| Physical component summary scorej | 47 (7) [n = 46] | 47 (8) | –1 (–3 to 2) |
| GASk | –1 (–2 to 0) [n = 46] | –1 (–2 to 0) | 0.0 (–0.3 to 0.3) |
| EQ-5D-3Ll | |||
| EQ-5D-3L summary index | 0.8 (0.7 to 0.8) [n = 46] | 0.8 (0.8 to 0.8) | 0 (–0.1 to 0.0) |
| EQ-5D-3L health VAS | 80 (68 to 89) [n = 46] | 80 (71 to 86) | –3 (–9 to 2) |
Abbreviation: PRP, platelet-rich plasma.
General linear repeated measures model including all time points up to 26 weeks.
American Orthopaedic Foot and Ankle Society (AOFAS) score ranges from 0-100 points; higher scores indicate less pain and better function; adjusted for duration of clinical symptoms of ankle osteoarthritis and radiological talar tilt. See footnote of Table 1 for examples of this score.
Secondary outcomes are unadjusted.
AOFAS pain subscale ranges from 0-40 points; higher scores indicate more pain. No clinical cutoff or indication is available.
Foot and Ankle Outcome Score consists of 5 scales (pain, symptoms, quality of life, activity of daily living, and sport and recreation); range, 0-100 points; higher scores indicate less pain and better function and quality of life. No clinical cutoff scores are available, but as an indication, end-stage ankle OA patients undergoing an ankle arthrodesis or arthroplasty were reported to have preoperative mean scores of 26 (pain), 32 (symptoms), 37 (quality of life), 17 (activity of daily living), and 13 points (sport and recreation).
Ankle Osteoarthritis Score (AOS) measures pain and disability; range, 0-100 points; higher scores indicate more symptoms. No clinical cutoff scores are available, but as an indication, patients with end-stage ankle OA undergoing an ankle arthrodesis or arthroplasty were reported to have a preoperative mean score of 58 to 62 points.20,22
Pain during activities of daily living measured on a visual analog scale (VAS); range, 0-100, with higher scores indicating more pain. No clinical cutoff scores are available, but as an indication, patients with end-stage ankle OA undergoing an ankle arthrodesis or arthroplasty were reported to have a preoperative mean score of 60 points.23
Ankle Activity Score (AAS) measures performable activity level; range, 0-10 points; higher scores indicate higher ankle stress activities. No clinical cutoff or indication is available.
36-Item Short Form Health Survey (SF-36) measures health-related quality of life using 8 subscales that can be summarized into a mental and a physical component summary score; range 0-100 points; higher scores indicate higher quality of life. No clinical cutoff scores are available, but as an indication, surgically treated patients with end-stage ankle OA were reported to have preoperative mean scores of 30 (physical)20,22 and 51 (mental) points.22 The reference value for the general population is 50 points.
Adjusted for the Dutch population.
Global Attainment Scaling (GAS) is based on achievement related to predetermined goals in agreement with the patient; range, −2 to 3, with lower scores indicating decline from baseline and higher scores indicating achieving more than the predefined goals. No clinical cutoff or indication is available.
The 3-Level EuroQol 5-Dimension (EQ-5D-3L) tool measures the generic quality of life across 5 dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) using a summary index (0-1, with 0 indicating death and 1 indicating full health) and a health VAS (range, 0-100; 0 indicates worst health imaginable and 100 indicates best health imaginable). No clinical cutoff or indication is available for the EQ-5D-3L summary index. For the EQ-5D-3L VAS, 64 points has been reported for surgically treated patients with end-stage ankle OA.
Figure 2. Changes in the American Orthopaedic Foot and Ankle Society (AOFAS) Score in a Study of the Effect of Platelet-Rich Plasma (PRP) Injections vs Placebo on Ankle Symptoms and Function in Patients With Ankle Osteoarthritis.
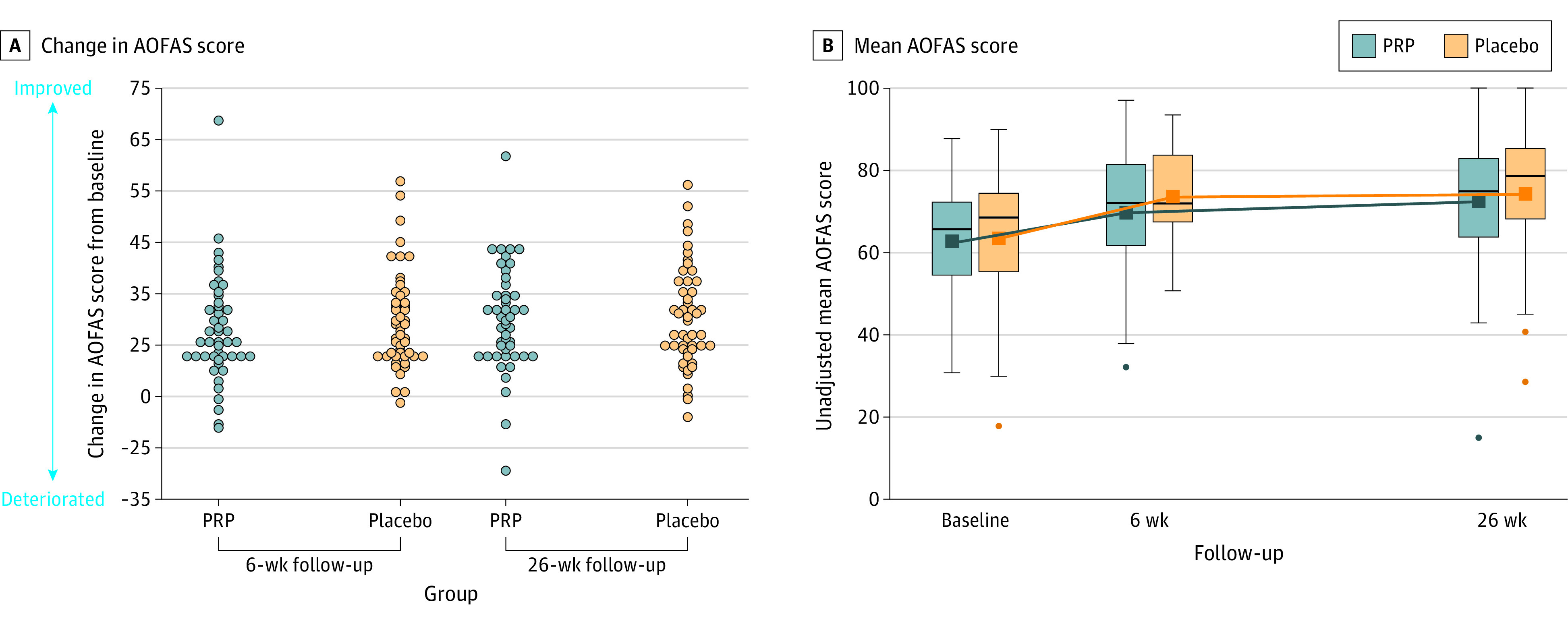
See Table 1 footnotes for scale definitions. The mean difference between the PRP and the placebo group over 26 weeks was −1 (95% CI, −6 to 3). The boxes show the median and IQR, with the bottom and top indicating the 25th and 75th percentiles, respectively. The upper whisker extends from the top of the box to the largest value no further than 1.5 times the IQR. The bottom whiskers extend from the bottom of the boxes to the smallest value no further than 1.5 times the IQR. Dots indicate outliers outside the whisker range.
Figure 3. Change in American Orthopaedic Foot and Ankle Society (AOFAS) Scores for Each Participant in a Study of the Effect of Platelet-Rich Plasma (PRP) Injections vs Placebo on Ankle Symptoms and Function in Patients With Ankle Osteoarthritis.
See Table 1 footnotes for scale definitions. Changes from baseline or from 6 weeks to 26 weeks or 6 weeks are represented by the vertical lines. Upward and downward lines indicate improvement and deterioration, respectively. The horizontal lines in the boxplots from bottom to top show the 25th, 50th (median), and 75th percentiles. The dot in the boxplot indicates the mean. The whiskers indicate the highest and lowest values.
Secondary Outcomes
No statistically significant between-group differences were found for any secondary outcomes at 6, 12, or 26 weeks (Figure 4 and eTable 4-13 in Supplement 2). No statistically significant between-group differences were found in the post hoc sensitivity analysis for any secondary outcomes at 6, 12, or 26 weeks (eTables 4-13 and eFigures 1-3 in Supplement 2).
Figure 4. Secondary Outcome Measures in a Study of the Effect of Platelet-Rich Plasma (PRP) Injections vs Placebo on Ankle Symptoms and Function in Patients With Ankle Osteoarthritis.
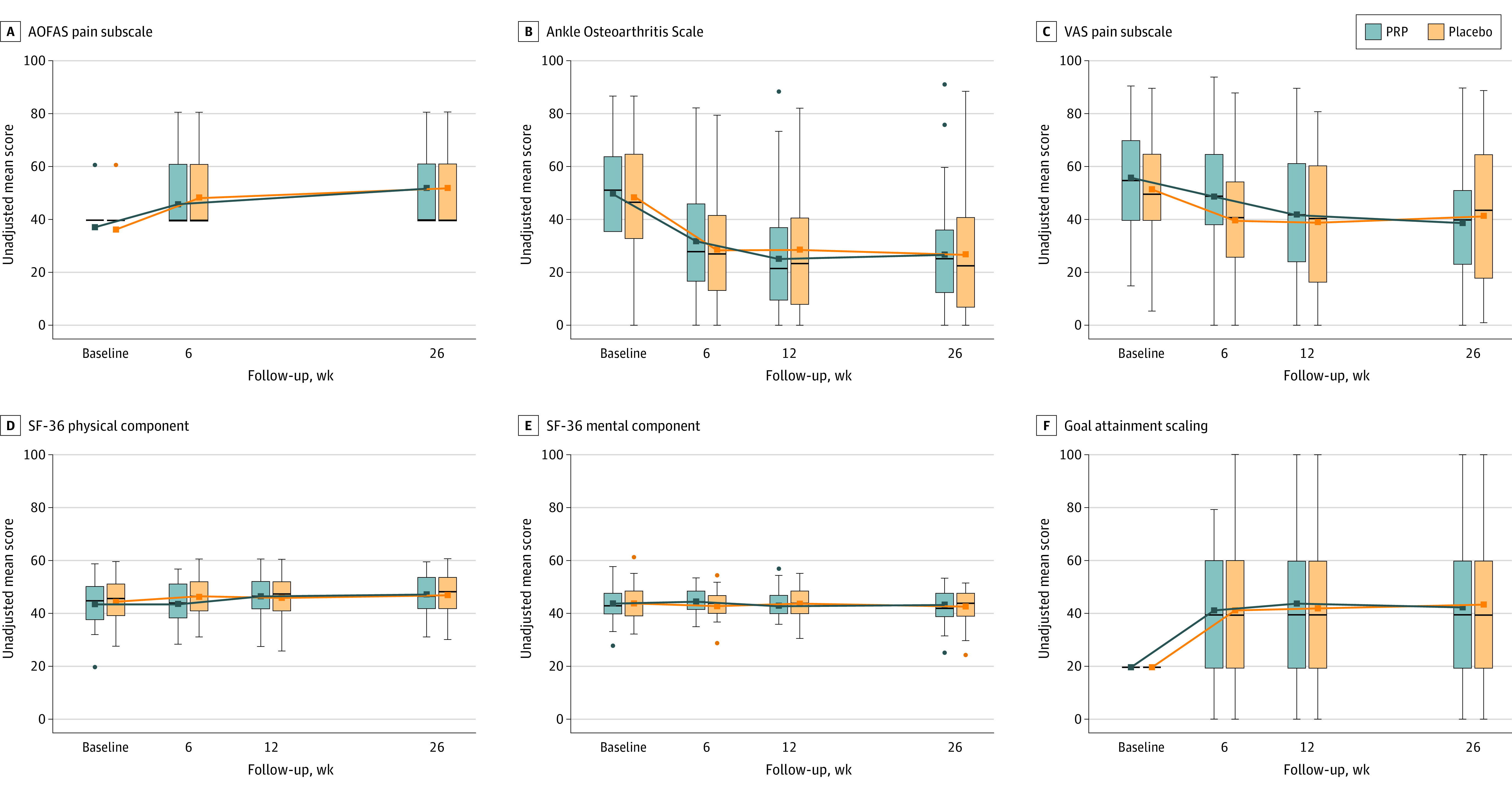
See Table 2 footnotes for scale definitions. The other secondary outcome measures can be found in Supplement 2 (eTables 4-13 and eFigures 1-3). The boxes show the median and IQR of the data, with the bottom and top indicating the 25th and 75th percentiles, respectively. The upper whisker extends from the top of the box to the largest value no further than 1.5 times the IQR. The bottom whiskers extend from the bottom of the boxes to the smallest value no further than 1.5 times the IQR. Dots indicate outliers outside the whisker range.
Adverse Events
One serious adverse event was reported and deemed unrelated to the injection intervention. It consisted of a transient ischemic attack in the placebo-group three weeks after the first injection. No other patients reported any symptoms of infection or intra-articular hematoma caused by the injection of PRP or saline. There were 13 other adverse events in the PRP group and 8 in the placebo group (eTable 15 in Supplement 2).
Success of Blinding
After the first (baseline) injection 33 patients (69%) in the PRP group and 36 (69%) in the placebo group thought they had received the PRP injection. After the second injection at 6 weeks, 29 (60%) of the PRP group and 36 (69%) of the placebo group thought they had received the PRP injection.
Discussion
In this double-blind, randomized, multicentered, placebo-controlled clinical trial involving patients with ankle (tibiotalar) osteoarthritis, intra-articular PRP injections, compared with saline placebo injection, did not significantly improve the primary outcome that assessed pain, function, and alignment over 26 weeks or any other secondary outcome measures. The likelihood of clinically relevant benefit is small, because the minimum clinically important difference was outside the 95% CI of the primary outcome.
Previous evidence for PRP injections in ankle osteoarthritis was limited to 4 small case series with methodological flaws.5,12 Two retrospective case series of 5 and 20 patients reported an improvement of 21% and 67% on the VAS. Two prospective case series, of 20 and 44 patients, reported an improvement of 29% and 59% on the VAS at 6 months.5,12
In knee osteoarthritis, 14 of the 21 randomized clinical trials of PRP showed methodological limitations, including moderate to high risk of bias and small sample sizes.7 Four of these trials were placebo-controlled, and all reported beneficial results for PRP.16,31,32,33 The pooled results in a recent meta-analysis of the total Western Ontario and McMaster Universities Osteoarthritis Index (range, 0-100) of 125 patients show a weighted mean difference for the placebo group of 21 points (95% CI, 15-27), suggesting a clinically relevant benefit.7 Results reported here for ankle osteoarthritis were not consistent with these potentially beneficial effects in knee osteoarthritis.
The improvement within the placebo (saline) group observed in this study was consistent with other placebo studies.34,35 Clinical efficacy of saline is unlikely considering the low injection volume (2 mL) and previous sham-controlled studies in knee osteoarthritis that showed no difference between saline joint irrigation (1-10 L) and sham intervention.36,37
Strengths of this study included the placebo-controlled double-blind study design, absence of any loss to follow-up for the primary outcome, and performance of all primary outcome measurements by coordinating single research physician. The nationwide recruitment in 6 centers (2 university medical centers, 2 teaching hospitals, 1 general hospital and 1 private specialist clinic) enhances the generalizability of the results.
Limitations
This study has several limitations. First, the generalizability of results to other platelet-rich blood products may be limited. Alternative platelet-rich blood interventions differ in dose, timing, and number of injections and in composition of platelets and leukocytes. However, the product administered in this trial was also used as in several other osteoarthritis trials and the concentration of the platelet rich plasma was comparable to that used in these prior trials.16,38,39 Second, analysis of the composition of PRP in this study was not conducted.13 However, the composition of this specific system has been analyzed previously,40 including in a previous randomized clinical trial.18 PRP analysis is typically not performed in clinical practice prior to injection. Third, magnetic resonance imaging, sensitive for detecting potential structural cartilage changes and degree of inflammation in joints was not a secondary outcome due to financial constraints. Fourth, there was no control for differences in physical therapy between the two groups.
Conclusions
Among patients with ankle osteoarthritis, intra-articular platelet-rich plasma injections, compared with placebo injections, did not significantly improve ankle symptoms and function over 26 weeks. The results of this study do not support the use of PRP injections for ankle osteoarthritis.
Trial protocol and statistical analysis plan
eMethods and eResults
Nonauthor collaborators
Data sharing statement
References
- 1.Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745-1759. doi: 10.1016/S0140-6736(19)30417-9 [DOI] [PubMed] [Google Scholar]
- 2.Murray C, Marshall M, Rathod T, Bowen CJ, Menz HB, Roddy E. Population prevalence and distribution of ankle pain and symptomatic radiographic ankle osteoarthritis in community dwelling older adults: a systematic review and cross-sectional study. PLoS One. 2018;13(4):e0193662. doi: 10.1371/journal.pone.0193662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glazebrook M, Daniels T, Younger A, et al. Comparison of health-related quality of life between patients with end-stage ankle and hip arthrosis. J Bone Joint Surg Am. 2008;90(3):499-505. doi: 10.2106/JBJS.F.01299 [DOI] [PubMed] [Google Scholar]
- 4.Saltzman CL, Zimmerman MB, O’Rourke M, Brown TD, Buckwalter JA, Johnston R. Impact of comorbidities on the measurement of health in patients with ankle osteoarthritis. J Bone Joint Surg Am. 2006;88(11):2366-2372. doi: 10.2106/JBJS.F.00295 [DOI] [PubMed] [Google Scholar]
- 5.Vannabouathong C, Del Fabbro G, Sales B, et al. Intra-articular injections in the treatment of symptoms from ankle arthritis: a systematic review. Foot Ankle Int. 2018;39(10):1141-1150. doi: 10.1177/1071100718779375 [DOI] [PubMed] [Google Scholar]
- 6.Witteveen AGH, Hofstad CJ, Kerkhoffs GMMJ. Hyaluronic acid and other conservative treatment options for osteoarthritis of the ankle. Cochrane Database Syst Rev. 2015;(10):CD010643. doi: 10.1002/14651858.CD010643.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong Y, Zhang B, Yang Q, Zhu J, Sun X. The effects of platelet-rich plasma injection in knee and hip osteoarthritis: a meta-analysis of randomized controlled trials. Clin Rheumatol. 2021;40(1):263-277. doi: 10.1007/s10067-020-05185-2 [DOI] [PubMed] [Google Scholar]
- 8.Global platelet rich plasma (PRP) market 2019 by manufacturers, regions, type and application, forecast to 2024. Published February 22, 2019. Accessed September 27, 2021. https://www.industryresearch.biz/global-platelet-rich-plasma-prp-market-13877695
- 9.Platelet rich plasma market size, share & trends analysis report by type, by application (orthopedics, sports medicine, cosmetic surgery), by end-use (hospitals, clinics), by region, and segment forecasts, 2021-2028. Grand View Research. Published February 2021. Accessed September 30, 2021. https://www.grandviewresearch.com/industry-analysis/platelet-rich-plasma-prp-market
- 10.Filardo G, Kon E, Roffi A, Di Matteo B, Merli ML, Marcacci M. Platelet-rich plasma: why intra-articular? a systematic review of preclinical studies and clinical evidence on PRP for joint degeneration. Knee Surg Sports Traumatol Arthrosc. 2015;23(9):2459-2474. doi: 10.1007/s00167-013-2743-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu Y, Yuan M, Meng HY, et al. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: a review. Osteoarthritis Cartilage. 2013;21(11):1627-1637. doi: 10.1016/j.joca.2013.07.017 [DOI] [PubMed] [Google Scholar]
- 12.Sun SF, Hsu CW, Lin GC, et al. Efficacy and safety of a single intra-articular injection of platelet-rich plasma on pain and physical function in patients with ankle osteoarthritis: a prospective study. J Foot Ankle Surg. 2021;60(4):676-682. doi: 10.1053/j.jfas.2020.12.003 [DOI] [PubMed] [Google Scholar]
- 13.Paget L, Bierma-Zeinstra S, Goedegebuure S, et al. Platelet-rich plasma injection management for ankle osteoarthritis study (PRIMA): protocol of a Dutch multicentre, stratified, block-randomised, double-blind, placebo-controlled trial. BMJ Open. 2019;9(10):e030961. doi: 10.1136/bmjopen-2019-030961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDermott MM, Newman AB. Preserving clinical trial integrity during the coronavirus pandemic. JAMA. 2020;323(21):2135-2136. doi: 10.1001/jama.2020.4689 [DOI] [PubMed] [Google Scholar]
- 15.van Dijk CN, Verhagen RA, Tol JL. Arthroscopy for problems after ankle fracture. J Bone Joint Surg Br. 1997;79(2):280-284. doi: 10.1302/0301-620X.79B2.0790280 [DOI] [PubMed] [Google Scholar]
- 16.Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis: an FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44(4):884-891. doi: 10.1177/0363546515624678 [DOI] [PubMed] [Google Scholar]
- 17.Cole BJ, Karas V, Hussey K, Pilz K, Fortier LA. Hyaluronic acid versus platelet-rich plasma: a prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2017;45(2):339-346. doi: 10.1177/0363546516665809 [DOI] [PubMed] [Google Scholar]
- 18.Reurink G, Goudswaard GJ, Moen MH, et al. ; Dutch Hamstring Injection Therapy (HIT) Study Investigators . Platelet-rich plasma injections in acute muscle injury. N Engl J Med. 2014;370(26):2546-2547. doi: 10.1056/NEJMc1402340 [DOI] [PubMed] [Google Scholar]
- 19.de Boer AS, Tjioe RJC, Van der Sijde F, et al. ; AOFAS Study Group . The American Orthopaedic Foot and Ankle Society Ankle-Hindfoot Scale: translation and validation of the Dutch language version for ankle fractures. BMJ Open. 2017;7(8):e017040. doi: 10.1136/bmjopen-2017-017040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madeley NJ, Wing KJ, Topliss C, Penner MJ, Glazebrook MA, Younger ASE. Responsiveness and validity of the SF-36, ankle osteoarthritis scale, AOFAS ankle hindfoot score, and foot function index in end stage ankle arthritis. Foot Ankle Int. 2012;33(1):57-63. doi: 10.3113/FAI.2012.0057 [DOI] [PubMed] [Google Scholar]
- 21.Sierevelt IN, Beimers L, van Bergen CJA, Haverkamp D, Terwee CB, Kerkhoffs GMMJ. Validation of the Dutch language version of the Foot and Ankle Outcome Score. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2413-2419. doi: 10.1007/s00167-014-3017-2 [DOI] [PubMed] [Google Scholar]
- 22.Glazebrook M, Burgesson BN, Younger AS, Daniels TR. Clinical outcome results of total ankle replacement and ankle arthrodesis: a pilot randomised controlled trial. Foot Ankle Surg. 2021;27(3):326-331. doi: 10.1016/j.fas.2020.10.005 [DOI] [PubMed] [Google Scholar]
- 23.Suo H, Fu L, Liang H, Wang Z, Men J, Feng W. End-stage ankle arthritis treated by ankle arthrodesis with screw fixation through the transfibular approach: a retrospective analysis. Orthop Surg. 2020;12(4):1108-1119. doi: 10.1111/os.12707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halasi T, Kynsburg A, Tállay A, Berkes I. Development of a new activity score for the evaluation of ankle instability. Am J Sports Med. 2004;32(4):899-908. doi: 10.1177/0363546503262181 [DOI] [PubMed] [Google Scholar]
- 25.Turner-Stokes L. Goal attainment scaling (GAS) in rehabilitation: a practical guide. Clin Rehabil. 2009;23(4):362-370. doi: 10.1177/0269215508101742 [DOI] [PubMed] [Google Scholar]
- 26.Lamers LM, Stalmeier PFM, McDonnell J, Krabbe PFM, van Busschbach JJ. Measuring the quality of life in economic evaluations: the Dutch EQ-5D tariff. Ned Tijdschr Geneeskd. 2005;149(28):1574-1578. [PubMed] [Google Scholar]
- 27.Pham T, van der Heijde D, Altman RD, et al. OMERACT-OARSI initiative: Osteoarthritis research society international set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis Cartilage. 2004;12(5):389-399. doi: 10.1016/j.joca.2004.02.001 [DOI] [PubMed] [Google Scholar]
- 28.de Vos RJ, Weir A, van Schie HTM, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144-149. doi: 10.1001/jama.2009.1986 [DOI] [PubMed] [Google Scholar]
- 29.Rozendaal RM, Koes BW, van Osch GJVM, et al. Effect of glucosamine sulfate on hip osteoarthritis: a randomized trial. Ann Intern Med. 2008;148(4):268-277. doi: 10.7326/0003-4819-148-4-200802190-00005 [DOI] [PubMed] [Google Scholar]
- 30.Järvinen TLN, Sihvonen R, Bhandari M, et al. Blinded interpretation of study results can feasibly and effectively diminish interpretation bias. J Clin Epidemiol. 2014;67(7):769-772. doi: 10.1016/j.jclinepi.2013.11.011 [DOI] [PubMed] [Google Scholar]
- 31.Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356-364. doi: 10.1177/0363546512471299 [DOI] [PubMed] [Google Scholar]
- 32.Wu YT, Hsu KC, Li TY, Chang CK, Chen LC. Effects of platelet-rich plasma on pain and muscle strength in patients with knee osteoarthritis. Am J Phys Med Rehabil. 2018;97(4):248-254. doi: 10.1097/PHM.0000000000000874 [DOI] [PubMed] [Google Scholar]
- 33.Görmeli G, Görmeli CA, Ataoglu B, Çolak C, Aslantürk O, Ertem K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017;25(3):958-965. doi: 10.1007/s00167-015-3705-6 [DOI] [PubMed] [Google Scholar]
- 34.Saltzman BM, Leroux T, Meyer MA, et al. The therapeutic effect of intra-articular normal saline injections for knee osteoarthritis: a meta-analysis of evidence level 1 studies. Am J Sports Med. 2017;45(11):2647-2653. doi: 10.1177/0363546516680607 [DOI] [PubMed] [Google Scholar]
- 35.Leopoldino AO, Machado GC, Ferreira PH, et al. Paracetamol versus placebo for knee and hip osteoarthritis. Cochrane Database Syst Rev. 2019;2(2):CD013273. doi: 10.1002/14651858.CD013273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moseley JB, O’Malley K, Petersen NJ, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347(2):81-88. doi: 10.1056/NEJMoa013259 [DOI] [PubMed] [Google Scholar]
- 37.Bradley JD, Heilman DK, Katz BP, Gsell P, Wallick JE, Brandt KD. Tidal irrigation as treatment for knee osteoarthritis: a sham-controlled, randomized, double-blinded evaluation. Arthritis Rheum. 2002;46(1):100-108. doi: [DOI] [PubMed] [Google Scholar]
- 38.Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39(2):266-271. doi: 10.1177/0363546510387517 [DOI] [PubMed] [Google Scholar]
- 39.Angthong C, Khadsongkram A, Angthong W. Outcomes and quality of life after platelet-rich plasma therapy in patients with recalcitrant hindfoot and ankle diseases: a preliminary report of 12 patients. J Foot Ankle Surg. 2013;52(4):475-480. doi: 10.1053/j.jfas.2013.04.005 [DOI] [PubMed] [Google Scholar]
- 40.Mariani E, Pulsatelli L. Platelet concentrates in musculoskeletal medicine. Int J Mol Sci. 2020;21(4):1328. doi: 10.3390/ijms21041328 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol and statistical analysis plan
eMethods and eResults
Nonauthor collaborators
Data sharing statement