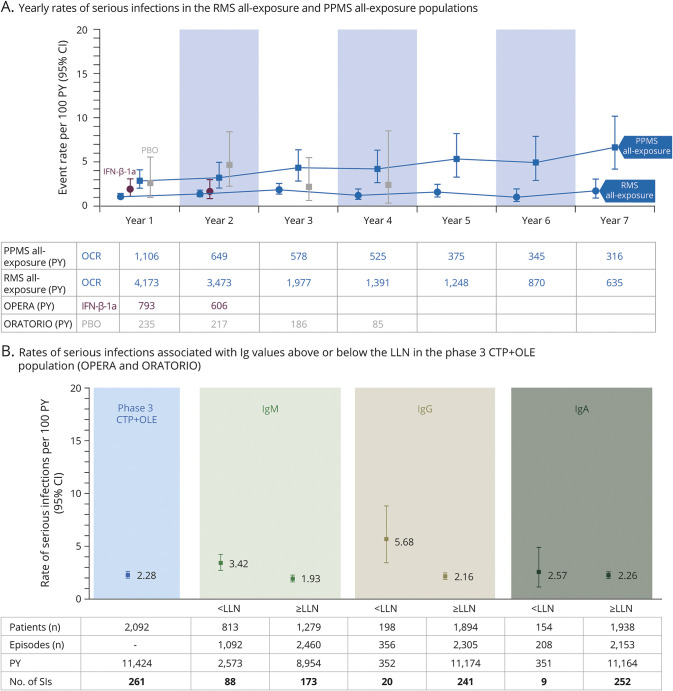
Figure 3. Rate of Serious Infections Over 7 years and Association With Immunoglobulin Levels.
Data cutoff: January 2020. (A) Yearly rates of serious infections (SIs) in patients with relapsing multiple sclerosis (RMS) or primary progressive multiple sclerosis (PPMS) treated with ocrelizumab (OCR) for a period of up to 7 years, during the controlled treatment period (CTP) and associated open-label extension (OLE) periods of the phase 2 and phase 3 studies, including patients originally randomized to comparator (interferon [IFN]-β-1a or placebo [PBO]) who switched to open-label OCR treatment, plus VELOCE, CHORDS, CASTING, OBOE, ENSEMBLE, CONSONANCE (PPMS all-exposure only), and LIBERTO (n = 5,680; 18,218 patient-years [PY]). Yearly rates of SIs of patients who received PBO (gray) or IFN-β-1a (purple) during the CTPs are displayed. Due to the event-driven design of ORATORIO, some patients remained on PBO for up to 4 years. (B) Rates of SIs associated with immunoglobulin G (IgG), immunoglobulin M (IgM), and immunoglobulin A (IgA) values above or below the respective lower limit of normal (LLN) in patients who received any dose of OCR during the CTP and associated OLE periods of the phase 3 trials (OPERA 1, OPERA 2, and ORATORIO). CI = confidence interval.