Abstract
Background and Objectives
Atrial fibrillation (AF) has been associated with cognitive decline and dementia. However, the mechanisms behind these associations are not clear. Examination of cerebrovascular pathology on MRI may shed light on how AF affects the brain. This study aimed to determine whether AF is associated with a broad range of cerebrovascular diseases beyond the well-known association with symptomatic stroke, including silent infarcts and markers of small vessel disease, i.e., cerebral microbleeds (CMBs), white matter hyperintensities (WMHs), and lacunes, in a population-based sample of 70-year-olds.
Methods
Data were obtained from the Gothenburg H70 Birth Cohort Studies, in which individuals are invited based on birthdate. This study has a cross-sectional design and includes individuals born in 1944 who underwent structural brain MRI in 2014 to 2017. AF diagnoses were based on self-report, ECG, and register data. Symptomatic stroke was based on self-report, proxy interviews, and register data. Brain infarcts and CMBs were assessed by a radiologist. WMH volumes were measured on fluid-attenuated inversion recovery images with the Lesion Segmentation Tool. Multivariable logistic regression was used to study the association between AF and infarcts/CMBs, and multivariable linear regression was used to study the association between AF and WMHs.
Results
A total of 776 individuals were included, and 65 (8.4%) had AF. AF was associated with symptomatic stroke (odds ratio [OR] 4.5, 95% confidence interval [CI] 2.1–9.5) and MRI findings of large infarcts (OR 5.0, 95% CI 1.5–15.9), lacunes (OR 2.7, 95% CI 1.2–5.6), and silent brain infarcts (OR 3.5; 95% CI 1.6–7.4). Among those with symptomatic stroke, individuals with AF had larger WMH volumes (0.0137 mL/total intracranial volume [TIV], 95% CI 0.0074–0.0252) compared to those without AF (0.0043 mL/TIV, 95% CI 0.0029–0.0064). There was no association between AF and WMH volumes among those without symptomatic stroke. In addition, AF was associated to CMBs in the frontal lobe.
Discussion
AF was associated with a broad range of cerebrovascular pathologies. Further research is needed to establish whether cerebrovascular MRI markers can be added to current treatment guidelines to further personalize anticoagulant treatment in patients with AF and to further characterize the pathogenetic processes underlying the associations between AF and cerebrovascular diseases, as well as dementia.
Atrial fibrillation (AF) is the most common clinically relevant cardiac arrhythmia, affecting 1% to 4% of the adult population and >13% of persons ≥80 years of age.1 AF has been associated with stroke,2 dementia,3 and mortality, but the mechanisms behind these associations, in the absence of cardiac embolism, are not clear. Suggested mechanisms include systemic inflammation and brain hypoperfusion due to reduced cardiac output.4 In addition, AF shares several risk factors with both stroke and dementia.5
Cerebral small vessel disease (cSVD) affects small arteries, arterioles, capillaries, and small veins6and has been related to cognitive decline, vascular dementia, and stroke.6,7 Different pathologic processes may lead to cSVD such as arteriosclerosis, cerebral amyloid angiopathy (CAA), venous collagenosis, and inflammation.6 Markers of cSVD on brain MRI includes white matter hyperintensities (WMHs), lacunes of presumed vascular origin, and cerebral microbleeds (CMBs).7 The most established risk factors for WMHs are hypertension and advanced age,8 but other cardiovascular risk factors may also play a role.9 Lacunes are highly correlated with WMHs6 and are associated with advanced age and vascular risk factors such as hypertension, smoking, and diabetes.10 For CMBs, different topography has been linked to different etiologies; lobar CMBs have been associated with CAA, while CMBs in the infratentorial and deep brain regions have been associated with atherosclerosis and hypertension.11 Because AF is associated with both symptomatic stroke and silent infarcts (i.e., infarcts on brain imaging without clinical symptoms), it is important to also consider the influence of stroke and silent infarcts when studying the effects of AF on the brain.
This study aimed to determine whether AF is associated with a broad range of cerebrovascular diseases, beyond the well-known association with symptomatic stroke, including silent infarcts and markers of small vessel disease, i.e., CMBs, WMHs, and lacunes, in a population-based sample of 70-year-olds. In addition, we aimed to determine whether the association with WMHs was affected by the presence of stroke and anticoagulant treatment and age at AF onset.
Methods
Participants
Data were obtained from the population-based Gothenburg H70 Birth Cohort Studies (the H70 studies).12 The present study has a cross-sectional design, and in total, 1,667 men and women living in Gothenburg, Sweden, born in 1944 on birthdates ending with 0, 2, 5, and 8 were invited to participate. A total of 1,203 individuals (559 men, 644 women; response rate 72%) were examined in 2014 to 2016,12 and 791 individuals (414 women, 377 men) underwent structural brain MRI in 2014 to 2016. After the exclusion of individuals with multiple sclerosis, normal-pressure hydrocephalus, Parkinson disease, and valvular diseases (mitral valve disease or artificial heart valves), a total of 776 individuals remained for analyses.
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the Regional Ethical Review Board in Gothenburg. Informed consent was obtained from all participants or their relatives if the participant was unable to provide informed consent.
General Examinations
Examinations were conducted by research nurses or medical doctors. Participants were asked about present and past diseases and medications. The interviews also included questions about sudden onset of focal neurologic symptoms, duration of symptoms, and admission to hospital due to stroke. Physical examinations included anthropometry, blood pressure, blood sampling, and ECG. The ECG was coded according to the Minnesota Code (MC), and blood pressure was measured in the right arm after 5 minutes of rest in the seated position with a manual sphygmomanometer. DNA was extracted from blood samples according to standard procedures.12
Neuropsychiatric examinations were performed by psychiatric research nurses or medical doctors and included questions about psychiatric disorders and symptoms and assessments of signs of dementia, including cognitive tests. Proxy interviews were performed by a psychologist or research nurse and included questions about history of stroke symptoms and signs and symptoms of dementia.
Additional data were obtained from the National Patient Register (NPR), containing hospital discharge diagnoses and specialized outpatient care coded according to ICD10-SE.
Brain Imaging
All participants were scanned on a 3.0T Philips Achieva system (Philips Medical Systems, Best, the Netherland) using a protocol including T1, T2, fluid-attenuated inversion recovery, T2*, and diffusion-weighted imaging.12 Large infarcts (>15 mm), lacunes (3–15 mm), intracerebral hemorrhages, and CMBs were assessed by an experienced radiologist (S.Shams)13 blinded to clinical data. Lacunes and CMBs were defined according to the Standards for Reporting Vascular Changes on Neuroimaging.7 CMBs were recorded by location, that is, lobar (temporal, parietal, frontal, occipital) or deep/infratentorial. The fluid-attenuated inversion recovery sequence, which was used in WMH measurements, was acquired with the following parameters: 2.0-mm isotropic resolution sagittal slices, echo time 280 milliseconds, repetition time 4,800 milliseconds, field of view 250 × 250 mm2, flip angle 90°, and 140 slices.
WMH volumes were automatically segmented with the Lesion Segmentation Tool 2.0.15 using a lesion prediction algorithm and running under the statistical parametric mapping software.14,15 A quality control of the lesion segmentation output was applied, and those with invalid segmentation (n = 13) were excluded, leaving 763 individuals for WMH volume analyses. Data were managed and processed through TheHiveDB system.16
Definition of AF and Stroke
The diagnosis of AF was based on self-report, ECG (MC 8-3), and the NPR (ICD10-SE: I48) and included all individuals with a history of AF. Age at AF onset was dichotomized to <65 vs ≥65 years of age and was based on the earliest reported date of AF from self-report, ECG, or the NPR. The diagnosis of symptomatic stroke was based on self- or proxy-reported history of stroke symptoms and the NPR (ICD10-SE I60-I61, I62.9, I63.0–I63.5, I63.8, I63.9, I64, I69.0–I69.1, I69.3–I69.4), as described previously.17 The criteria for stroke according to self- and proxy reports required a clear history of acute focal neurologic symptoms (including aphasia) lasting for >24 hours.
Large infarcts and lacunes on MRI could be either symptomatic or silent. Silent brain infarcts were defined as large infarcts or lacunes on MRI in an individual without history of symptomatic stroke.
Definition of Covariates
Education level was defined as mandatory (corresponding to 7–8 years) or less vs more than mandatory. Heart diseases included heart failure and myocardial infarction. Heart failure was defined through self-report or the NPR (ICD10-SE I11.0, I13.0, I13.2, I50). Myocardial infarction was defined through self-report, the NPR (ICD10-SE I21–I23, I24.1, I25.2, I25.6), or major or moderate Q waves on ECG (MC 1.1–1.2, excluding 1.2.6 and 1.2.8). Hypertension was defined as present medical treatment for hypertension, systolic blood pressure ≥150 mm Hg, or diastolic blood pressure ≥90 mm Hg as suggested by the Eight Joint National Committee in 2014.18 Diabetes mellitus was defined as present treatment with insulin or antidiabetic drug, fasting glucose ≥7.0 mmol/L, or nonfasting glucose ≥11.1 mmol/L. Hypercholesterolemia was defined as present treatment with lipid-lowering medication, total cholesterol >6.2 mmol/L, low-density lipoprotein cholesterol ≥4.1 mmol/L, triglycerides >5.6 mmol/L, or high-density lipoprotein cholesterol <1.0 mmol/L in men or <1.3 mmol/L in women.19 Smoking was based on self-report and defined as never smoker, past smoker, or current smoker. Body mass index (BMI) was defined as weight in kilograms divided by the square of height in meters. Alcohol risk consumption was defined according to the National Institute on Alcohol Abuse and Alcoholismguidelines20 as >98 g alcohol/wk based on self-reported consumption during the last month.
Definition of Diagnoses Used for Exclusion
Dementia was diagnosed according to the DSM-III-R using information from psychiatric examinations and proxy reports, as described previously.21 Diagnoses of multiple sclerosis, normal-pressure hydrocephalus, and Parkinson disease were based on self-report and the NPR (ICD10-SE G20, G35, and G91). Valvular diseases (mitral valve disease or artificial heart valves) were obtained from the NPR (ICD10-SE I34, Z952-954, I05, I08, Q23). TIA was based on self- or proxy-reported history of stroke symptoms lasting <24 hours and the NPR (ICD10-SE G45.0–G45.3, G45.8–G45.9).
Statistical Analyses
Independent-sample t test was used for continuous variables, and Fisher exact test and Pearson χ2 test were used for categorical variables when comparing participant characteristics by brain MRI and AF status.
Binary logistic regression was used to study the association between AF and stroke (i.e., symptomatic strokes, large infarcts, lacunes, and silent brain infarcts). To study whether the relation between AF and stroke was affected by sex, interactions for AF and sex were performed. Stratified analyses based on sex were performed regardless of the significance level of the interaction term. Binary logistic regression was used to study the association between AF and CMBs, by location and run separately for men but not for women because only 1 woman with AF had CMBs.
Linear regression was used to study the association between AF and WMH volumes. WMH volumes were adjusted for total intracranial volume (TIV) by dividing WMH volume by TIV. A logarithmic transformation was applied to the adjusted WMH volumes due to nonnormal distribution of the residuals. To study whether the relation between AF and WMHs was affected by sex or stroke status, interactions for AF and sex and for AF and stroke were performed. Stratified analyses based on sex were performed regardless of the significance level of the interaction term. In addition, the sample was stratified into 4 groups based on AF and stroke status; (1) no AF and no stroke, (2) AF and no stroke, (3) no AF and stroke, and (4) AF and stroke. These analyses were corrected for multiple comparison with Bonferroni correction.
Analyses including stroke/infarcts and WMHs were repeated after the exclusion of individuals with dementia. For analyses including silent brain infarcts, individuals with a history of symptomatic stroke (n = 44) were excluded. The analyses including silent brain infarcts were repeated after the additional exclusion of individuals with TIA. Sex, education, heart disease, hypertension, diabetes, hypercholesterolemia, BMI, smoking, alcohol risk consumption, and the APOE ε4 allele were considered possible covariates. In analyses including WMHs and CMBs, stroke and infarcts were also considered possible covariates. Purposeful variable selection was applied in all adjusted models22; that is, all potential covariates were included in univariable models and thereafter in the multivariable models if the values of p were <0.2. In multivariable analyses, covariates with the largest p values were removed one by one until all remaining covariates had p < 0.2. The estimate of the predictors was controlled after each removal, and the covariate was reentered if the estimate changed >15%. Individuals with missing data were omitted from the analyses.
Data Availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
Results
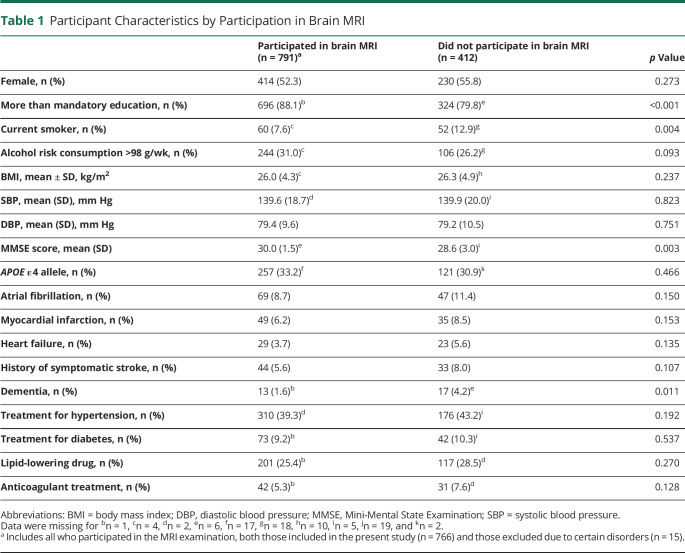
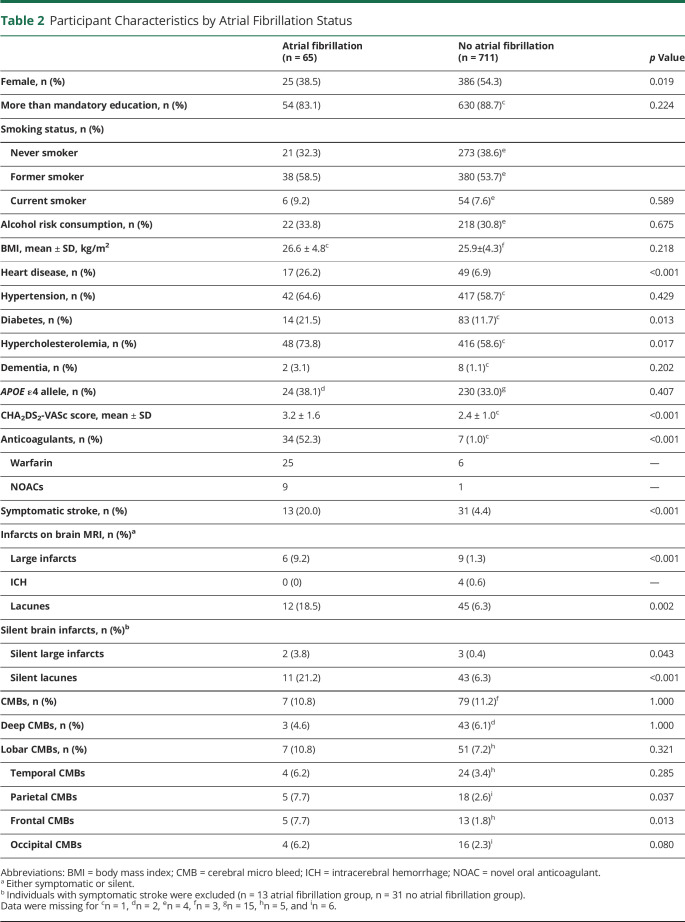
Participants in brain MRI (n = 791) more often had more than mandatory education (88% vs 80%), less often had dementia (1.6% vs 4.2%), and less often were current smokers (8% vs 13%) compared to nonparticipants (n = 412) (Table 1). Among participants in the present study (n = 776 after exclusion of individuals with multiple sclerosis, normal-pressure hydrocephalus, Parkinson disease, and valvular diseases), individuals with AF (n = 65) were more often men and more often had heart disease, diabetes, hypercholesterolemia, higher CHA2DS2-VASc score, and anticoagulant treatment than those without AF (n = 711) (Table 2).
Table 1.
Participant Characteristics by Participation in Brain MRI
Table 2.
Participant Characteristics by Atrial Fibrillation Status
AF and Stroke
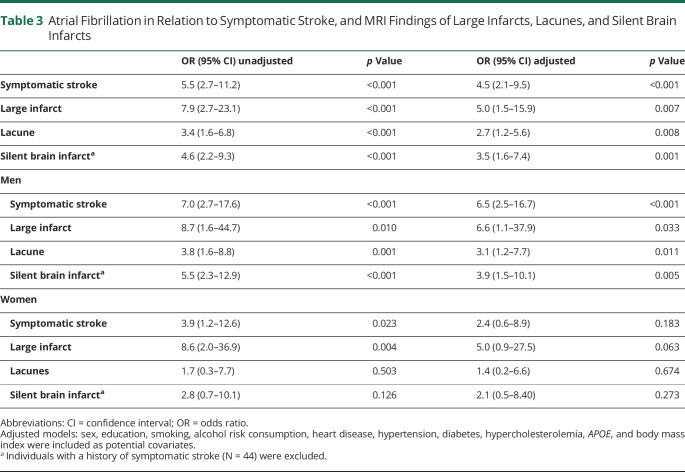
AF was associated with a history of symptomatic stroke (odds ratio [OR] 4.5, 95% confidence interval [CI] 2.1–9.5), large infarcts (OR 5.0, 95% CI 1.5–5.9), lacunes (OR 2.7, 95% CI 1.2–5.6), and silent brain infarcts (OR 3.5, 95% CI 1.6–7.4) (Table 3). Excluding individuals with dementia (and TIA for the analysis of silent brain infarcts) did not affect the results. No interactions were found between AF and sex in relation to symptomatic stroke (p = 0.399 in the adjusted model), large infarcts (p = 0.932), lacunes (p = 0.313), or silent brain infarcts (p = 0.410). However, after exclusion of individuals with dementia, the interaction for AF and sex in relation to silent brain infarcts had a value of p = 0.121. In stratified analyses by sex, AF was associated with symptomatic stroke, large infarcts, lacunes, and silent brain infarcts in men. In women, there were no significant associations between AF and symptomatic stroke, lacunes, and silent brain infarcts, but there was a trend toward an association between AF and large infarcts.
Table 3.
Atrial Fibrillation in Relation to Symptomatic Stroke, and MRI Findings of Large Infarcts, Lacunes, and Silent Brain Infarcts
AF and WMH Volumes
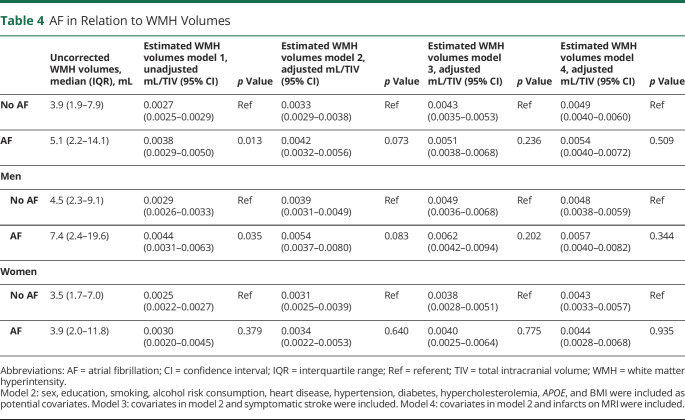
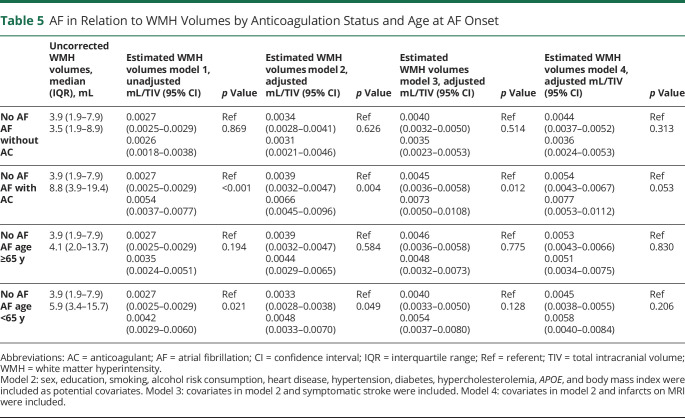
Individuals with AF had larger WMH volumes than those without after adjustment for TIV. However, the association was weakened after additional adjustment for cardiovascular risk factors and prevalent heart diseases. After additional adjustment for symptomatic stroke or MRI findings of infarcts, the association disappeared (Table 4). No interaction was found for AF and sex in relation to WMH volumes (p = 0.654 in the adjusted model including symptomatic stroke). An interaction was found for AF and symptomatic stroke in relation to WMH volumes (p = 0.003 in the adjusted model) but not for AF and lacunes (p = 0.199) or AF and silent brain infarcts (p = 0.912). Individuals with AF and present anticoagulant treatment had larger WMH volumes than individuals without AF after adjustment for cardiovascular risk factors, prevalent heart diseases, and symptomatic stroke. Individuals with AF but without anticoagulation did not have larger WMH volumes compared to individuals without AF (Table 5). Among those with AF and anticoagulant treatment, 85% (n = 29 of 34) had a CHA2DS2-VASc score >1 (for men) or >2 (for women). Among those with AF but without anticoagulant treatment, 73% (n = 22 of 30) had a CHA2DS2-VASc >1 (for men) or >2 (for women). Age at AF onset did not influence WMH volumes after adjustments (Table 5). Excluding individuals with dementia did not affect the results.
Table 4.
AF in Relation to WMH Volumes
Table 5.
AF in Relation to WMH Volumes by Anticoagulation Status and Age at AF Onset
AF, History of Symptomatic Stroke, and WMH Volumes
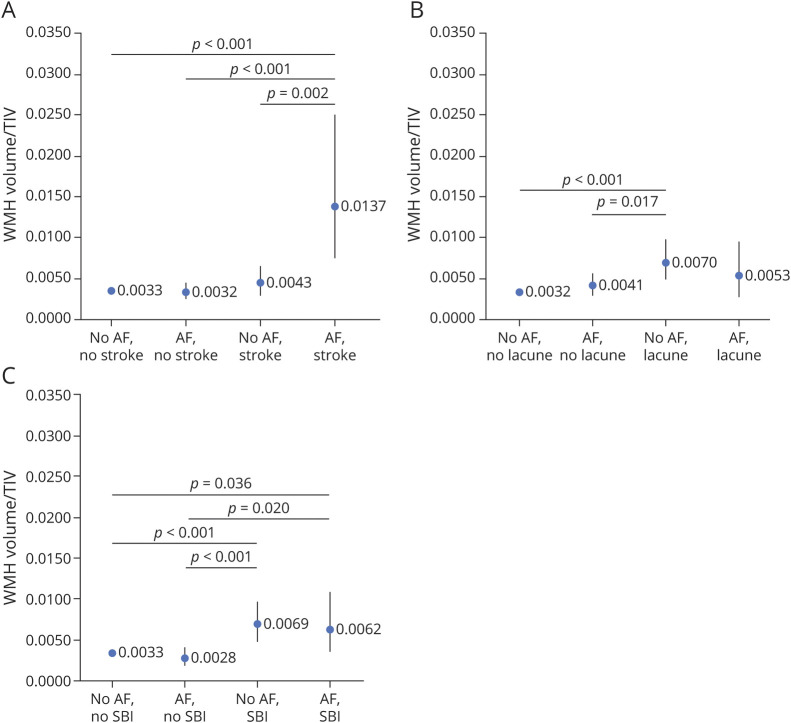
Figure, A shows the estimated WMH volumes divided by TIV in 4 groups: (1) no AF and no symptomatic stroke, (2) AF and no symptomatic stroke, (3) no AF and symptomatic stroke, and (4) AF and symptomatic stroke. Group 4 (AF and symptomatic stroke) had larger WMH volumes compared to all other groups. There were no other differences between the groups. Excluding individuals with dementia did not affect the results.
Figure. Estimated WMH Volume as a Fraction of TIV By AF and Stroke Status.
Estimated white matter hyperintensity (WMH) volume as a fraction of total intracranial volume (TIV) divided into 4 groups by (A) atrial fibrillation (AF) and history of symptomatic stroke status, (B) AF and silent brain infarct (SBI) status, and (C) AF and lacune status. Sex, education, smoking, alcohol risk consumption, heart disease, hypertension, diabetes, hypercholesterolemia, APOE, and body mass index were included as potential covariates. Values of p <0.05 are shown; p < 0.008 is considered statistically significant after Bonferroni correction.
AF, Lacunes, and WMH Volumes
Figure, B shows the estimated WMH volumes in 4 groups: (1) no AF and no lacune, (2) AF and no lacune, (3) no AF and lacune, and (4) AF and lacune. Group 3 (no AF and lacune) had larger WMH volume compared to group 1 (no AF and no lacune). Group 4 did not differ statistically from the other groups. Excluding individuals with dementia did not affect the results.
AF, Silent Brain Infarcts, and WMH Volumes
Figure, C shows the estimated WMH volumes in 4 groups: (1) no AF and no silent brain infarct, (2) AF and no silent brain infarct, (3) no AF and silent brain infarct, and (4) AF and silent brain infarct. Group 3 (no AF and silent brain infarct) had larger WMH volume compared to both groups 1 (no AF and no silent brain infarct) and 2 (AF and no silent brain infarct). Group 4 (AF and silent brain infarct) did not differ statistically from the other groups, but the estimated WMH volume was similar to that of group 3. There were no other differences between the groups. Excluding individuals with dementia or TIA did not affect the results. There were too few individuals with large infarcts on brain MRI for subgroup analyses.
AF and Cerebral Microbleeds
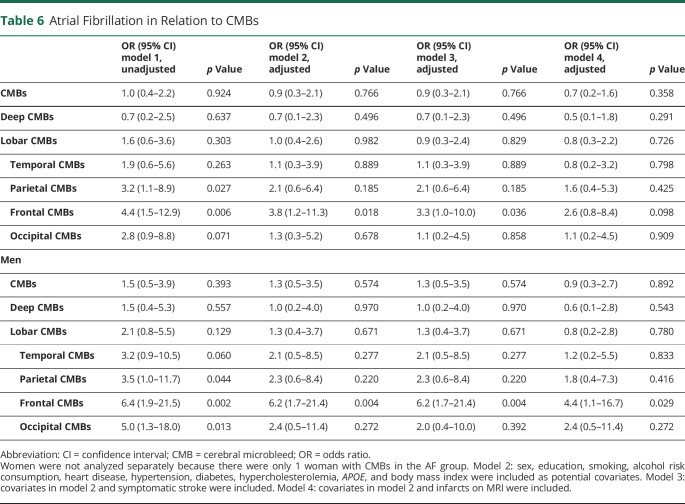
AF was not associated with the presence of CMBs, lobar CMBs, or deep/infratentorial CMBs (Table 6). However, after the CMBs were divided by lobe, AF was associated with CMBs in the parietal, frontal, and occipital lobes in the unadjusted models. In the adjusted models, including symptomatic stroke, AF was associated with CMBs only in the frontal lobe (OR 3.3, 95% CI 1.0–10.0). After adjustment for infarcts on MRI, the association became weaker (OR 2.6, 95% CI 0.8–8.4). Among men, AF was associated with CMBs in the frontal lobe in the adjusted model including symptomatic stroke (OR 6.2, 95% CI 1.7–21.4) and infarcts on MRI (OR 4.4, 95% CI 1.1–16.7). Because only 1 woman with AF had CMBs, we did not analyze the association between AF and CMBs among women.
Table 6.
Atrial Fibrillation in Relation to CMBs
Discussion
We found that AF was associated with history of symptomatic stroke, large infarcts, lacunes, and silent brain infarcts. AF was associated with larger WMH volumes in individuals with symptomatic stroke but not in those without. However, despite the fact that silent brain infarcts and lacunes were associated with larger WMH volumes, AF did not affect these relations. In addition, individuals with AF and anticoagulant treatment had larger WMH volumes than individuals without AF, while individuals with AF but without anticoagulant treatment did not have larger WMH volumes than those without AF. AF was not associated with lobar or deep/infratentorial CMBs except in the frontal lobe, where microbleeds were more common in individuals with AF.
We found that AF was related to both history of symptomatic stroke and large infarcts on brain MRI after adjusting for demographic factors, cardiovascular risk factors, and comorbid conditions. No interactions were found for AF and sex in relation to symptomatic stroke or large infarcts on MRI. Traditionally, the dysrhythmia in AF has been regarded as the major cause of embolic stroke, with an almost 5-fold increased risk of overall stroke.2 In contrast, AF did not increase overall stroke risk in individuals without cardiovascular comorbid conditions in a recent large population-based study.23 Instead, cardiovascular comorbid conditions increased risk of stroke independently of AF.23 It has been suggested that aging and vascular risk factors cause abnormal atrial substrate that may result in both AF and stroke. Once AF develops, it might further increase stroke risk.24 Whether AF on its own has a causal relationship with stroke is still being discussed.23-25 In addition, AF is often asymptomatic and therefore not diagnosed until complications, for example, stroke and heart failure,26 occur, which may overestimate the association between AF and stroke.
After excluding individuals with history of symptomatic stroke, we found that AF was related to a >3-fold increase in the odds of silent brain infarcts after adjustment for several confounding variables. This is in line with a meta-analysis from 2014 showing that AF was related to a >2-fold increase in the odds of silent brain infarcts.27 Because most silent brain infarcts are lacunes,28 it was not surprising that we also found an association between AF and lacunes on brain MRI. The most established risk factors for silent brain infarcts are hypertension and advanced age, but silent brain infarcts have also been associated with different cardiac diseases (e.g., AF, cardiomyopathy, and patent foramen ovale) and procedures such as coronary artery bypass graft surgery, indicating that cardiac factors may play a role.28 No interactions were found for AF and sex in relation to lacunes or silent brain infarcts. However, after stratification for sex, significant associations between AF and lacunes and silent brain infarcts were observed only in men. We have previously reported that the association between AF and dementia in a sample without symptomatic stroke was seen only in men in both the unadjusted and adjusted models.3 In contrast, female sex is a well-recognized risk factor for stroke in patients with AF and is included as such in guidelines of stroke risk (i.e., CHA2DS2-VASc score). Because we did not find an interaction for AF and sex in relation to stroke, we cannot make any conclusions regarding sex differences in the relations.
Our finding that the association between AF and WMHs disappeared after taking stroke and cardiovascular risk factors into account is in line with results from the Mayo Clinic Study of Aging29 and the Atherosclerosis Risk in Communities Neurocognitive Study.30,31 In addition, our finding that AF was not associated with WMHs in individuals without a history of stroke is in line with the Framingham offspring study32 and the German AF Competence NETwork Study.33 However, in our study, individuals with both AF and anticoagulant treatment had larger WMH volumes than individuals without AF after adjustments, including cardiovascular risk factors, heart disease, and symptomatic stroke, while individuals with AF but no anticoagulant treatment did not have larger WMH volumes than individuals without AF. However, this could be due to confounding by indication because current treatment guidelines for AF (CHA2DS2-VASc score) advocate anticoagulant treatment for individuals with more comorbid conditions such as congestive heart failure, hypertension, diabetes, stroke, and vascular diseases. Therefore, even if multiple adjustments were performed, including risk factors in the CHA2DS2-VASc score, individuals with both AF and anticoagulant treatment might have more severe cardiovascular disorders and more comorbid conditions than those with AF alone. We cannot, however, exclude the possibility that anticoagulant treatment may lead to more WMHs due to, for example, minor subclinical bleedings. We take note of a recent study reporting that in individuals with intracerebral hemorrhage, those taking anticoagulants did not have higher WMH burden than those not taking anticoagulants after adjustments for potential confounders.34 It is worth noticing that there were few individuals on novel oral anticoagulant compared to warfarin in individuals with AF in our study.
We found an interaction between AF and symptomatic stroke in relation to WMH volumes. Thus, AF was related to larger WMH volumes in persons with a history of symptomatic stroke but not in those without. In contrast to our results, studies on patients with acute ischemic stroke have not found a higher WMH burden in individuals with AF compared to those without AF.35-38 This discrepancy suggests that AF in itself does not lead to WMHs but accentuates the development of WMHs initiated by the stroke. One explanation might be that a brain affected by stroke is more vulnerable to, for example, hypoperfusion due to AF. AF did not affect the associations between lacunes or silent brain infarcts and WMH volumes. One explanation may be that silent brain infarcts and lacunes often affect small brain areas, thus making the brain less vulnerable to hypoperfusion due to AF. However, due to the small number of individuals with both AF and stroke, our results should be interpreted cautiously.
In comparisons of WMH volumes using automated imaging processing tools (i.e., the Lesion Segmentation Tool) with the Fazekas scale, a cutoff of 0.00496 mL WMH/TIV has been proposed to classify low and high Fazekas WMH burden, indicating a clinically relevant burden.14 In our study, only those with both AF and a history of symptomatic stroke had estimated WMH volumes above this cutoff, while the other groups had estimates around or below this value. Because comorbid conditions may exacerbate clinical outcomes, it is useful to study risk factors and diseases in combinations. For example, it has been suggested that aging and hypertension exacerbate WMH burden and inhibit WMH repair in patients with stroke and that this needs to be accounted for in clinical trials to improve stroke outcomes.39 This study showed that AF also is a risk marker of WMHs in individuals with a history of symptomatic stroke. Because WMHs are associated with unfavorable outcomes such as dementia, depression, and stroke,40 individuals with both AF and a history of symptomatic stroke might be particularly vulnerable to unfavorable outcomes.
We did not find any association between AF and lobar or deep/infratentorial microbleeds in multivariable analyses except in the frontal lobe, where microbleeds were more common in individuals with AF than in those without. In contrast, a case-control study from Japan41 found a higher prevalence of CMBs in patients with AF compared to controls without AF. However, cases differed from controls regarding sex, hypertension, diabetes, and medications. Thus, factors other than AF might have contributed to the higher CMB prevalence. CMB topography has been linked to different pathologies; lobar CMBs are associated with CAA, while deep and infratentorial CMBs are associated with atherosclerosis and hypertension.11 In addition, it has been shown that individuals with Alzheimer dementia have a predominant lobar topography.13 Our finding of an association between AF and CMBs in the frontal lobe could therefore be due to CAA. However, it has been suggested that CAA-related CMBs have a posterior predominance.11,42 The findings that frontal CMBs were more common in individuals with AF and that we did not find an association between AF and other lobar or deep/infratentorial CMBs need to be interpreted cautiously because there were few cases with both AF and CMBs.
Our study has several strengths such as the large population-based sample with MRI, comprehensive examinations performed by health professionals, and the use of multiple sources, including self- and proxy reports, register, physical examinations, and biomarkers. We have previously validated self-reported AF diagnoses in the H70 studies against register data and found a substantial agreement between the 2 sources (κ = 0.61).43 However, some limitations should also be recognized. First, this study has a cross-sectional design, which does not allow us to assess causal relationships. Second, AF may remain undiagnosed in asymptomatic individuals. Our examinations included ECGs to detect silent AF; however, for example, 24-hour Holter monitoring can increase AF detection.44 Third, sample size limits the possibility of doing all warranted subgroup analyses. Fourth, we were not able to examine regional patterns of WMHs. Other studies have reported associations between AF and WMHs in the deep and subcortical white matter45 or periventricular white matter46 only. Fifth, our study only includes 70-year-olds, limiting generalizability to other age groups.
We found that AF was associated with a history of symptomatic stroke and several brain pathologic markers such as large infarcts, lacunes, and silent brain infarcts. AF was associated with larger WMH volumes in individuals with symptomatic stroke but not in those without. In addition, AF was not associated with lobar or deep/infratentorial CMBs except in the frontal lobe, where microbleeds were more common in individuals with AF.
Further research is needed to establish whether cerebrovascular MRI markers can be used as a complement to current treatment guidelines to further personalize anticoagulant treatment in patients with AF and to further characterize the pathogenetic processes underlying the associations between AF and cerebrovascular diseases, as well as dementia.
Glossary
- AF
atrial fibrillation
- BMI
body mass index
- CAA
cerebral amyloid angiopathy
- CI
confidence interval
- CMB
cerebral microbleed
- cSVD
cerebral small vessel disease
- DSM-III-R
Diagnostic and Statistical Manual of Mental Disorders, 3rd edition, revised
- ICD
International Classification of Diseases
- MC
Minnesota Code
- NPR
National Patient Register
- OR
odds ratio
- TIV
total intracranial volume
- WMH
white matter hyperintensity
Appendix. Authors
Footnotes
CME Course: NPub.org/cmelist
Study Funding
The study was financed by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (ALF-716681, ALFGBG-637271, ALFGBG-81392, ALFGBG-771071), the Swedish Research Council (2012-5041, 2015-02830, 2013-8717, 2017-00639, 2018-02201, 2019-01096, 2019-02075), Swedish Research Council for Health, Working Life and Welfare (2013-1202, 2018-00471, AGECAP 2013-2300, 2013-2496), Konung Gustaf V:s och Drottning Victorias Frimurarestiftelse, Hjärnfonden (FO2014-0207, FO2016-0214, FO2018-0214, FO2019-0163, FO2020-0235), Alzheimerfonden (AF-554461, AF-647651, AF-743701, AF-844671, AF-930868), Eivind och Elsa K:son Sylvans stiftelse, Stiftelsen Hjalmar Svenssons forskningsfond, Academy Homecoming Fellowship (V2012/294), the Swedish Alzheimer Foundation, the Swedish Brain Foundation, the Strategic Research Area Neuroscience, Center for Medical Innovation, and Gamla Tjänarinnor.
Disclosure
L. Rydén, S. Sacuiu, H. Wetterberg, J. Najar, X. Guo, S. Kern, A. Zettergren, S. Shams, J.B. Pereira, L.-O. Wahlund, and E. Westman declare no disclosures relevant to the manuscript. I. Skoog has been a speaker for Takeda. Go to Neurology.org/N for full disclosures.
References
- 1.Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nat Rev Cardiol. 2014;11(11):639-654. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke. 1991;22(8):983-988. [DOI] [PubMed] [Google Scholar]
- 3.Rydén L, Zettergren A, Seidu NM, et al. Atrial fibrillation increases the risk of dementia amongst older adults even in the absence of stroke. J Intern Med. 2019;286(1):101-110. [DOI] [PubMed] [Google Scholar]
- 4.Dietzel J, Haeusler KG, Endres M. Does atrial fibrillation cause cognitive decline and dementia? Europace. 2018;20(3):408-419. [DOI] [PubMed] [Google Scholar]
- 5.Manolis TA, Manolis AA, Apostolopoulos EJ, Melita H, Manolis AS. Atrial fibrillation and cognitive impairment: an associated burden or burden by association? Angiology. 2020;71(6):498-519. [DOI] [PubMed] [Google Scholar]
- 6.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9(7):689-701. [DOI] [PubMed] [Google Scholar]
- 7.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin J, Wang D, Lan L, Fan Y. Multiple factors involved in the pathogenesis of white matter lesions. Biomed Res Int. 2017;2017:9372050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moroni F, Ammirati E, Rocca MA, Filippi M, Magnoni M, Camici PG. Cardiovascular disease and brain health: focus on white matter hyperintensities. Int J Cardiol Heart Vasc. 2018;19:63-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regenhardt RW, Das AS, Ohtomo R, Lo EH, Ayata C, Gurol ME. Pathophysiology of lacunar stroke: history's mysteries and modern interpretations. J Stroke Cerebrovasc Dis. 2019;28(8):2079-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yates PA, Villemagne VL, Ellis KA, Desmond PM, Masters CL, Rowe CC. Cerebral microbleeds: a review of clinical, genetic, and neuroimaging associations. Front Neurol. 2014;4:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rydberg Sterner T, Ahlner F, Blennow K, et al. The Gothenburg H70 Birth cohort study 2014-16: design, methods and study population. Eur J Epidemiol. 2019;34(2):191-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shams S, Martola J, Granberg T, et al. Cerebral microbleeds: different prevalence, topography, and risk factors depending on dementia diagnosis: the Karolinska Imaging Dementia Study. AJNR Am J Neuroradiol. 2015;36(4):661-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cedres N, Ferreira D, Machado A, et al. Predicting Fazekas scores from automatic segmentations of white matter signal abnormalities. Aging. 2020;12(1):894-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.UCL. Statistical Parametric Mapping. Accessed January 8, 2021. fil.ion.ucl.ac.uk/spm/. [Google Scholar]
- 16.Muehlboeck JS, Westman E, Simmons A. TheHiveDB image data management and analysis framework. Front Neuroinform. 2014;7:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo X, Östling S, Kern S, Johansson L, Skoog I. Increased risk for dementia both before and after stroke: a population-based study in women followed over 44 years. Alzheimers Dement. 2018;14(10):1253-1260. [DOI] [PubMed] [Google Scholar]
- 18.James PA, Oparil S, Carter BL, et al. Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520. [DOI] [PubMed] [Google Scholar]
- 19.Mayo Clinic. High cholesterol. Accessed April 21, 2021. Available at: mayoclinic.org/diseases-conditions/high-blood-cholesterol/diagnosis-treatment/drc-20350806. [Google Scholar]
- 20.US Department of Health And Human Services. National Institute on Alcohol Abuse and Alcoholism helping patients who drink too much, a clinician’s guide. Accessed December 20, 2020. Available at: pubs.niaaa.nih.gov/publications/practitioner/cliniciansguide2005/guide.pdf. [Google Scholar]
- 21.Skoog I, Nilsson L, Palmertz B, Andreasson LA, Svanborg A. A population-based study of dementia in 85-year-olds. N Engl J Med. 1993;328(3):153-158. [DOI] [PubMed] [Google Scholar]
- 22.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singleton MJ, Imtiaz-Ahmad M, Kamel H, et al. Association of atrial fibrillation without cardiovascular comorbidities and stroke risk: from the REGARDS study. J Am Heart Assoc. 2020;9(12):e016380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamel H, Okin PM, Elkind MS, Iadecola C. Atrial fibrillation and mechanisms of stroke: time for a new model. Stroke. 2016;47(3):895-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malik V, Ganesan AN, Selvanayagam JB, Chew DP, McGavigan AD. Is atrial fibrillation a stroke risk factor or risk marker? An appraisal using the Bradford Hill framework for causality. Heart Lung Circ. 2020;29(1):86-93. [DOI] [PubMed] [Google Scholar]
- 26.Samol A, Masin M, Gellner R, et al. Prevalence of unknown atrial fibrillation in patients with risk factors. Europace. 2013;15(5):657-662. [DOI] [PubMed] [Google Scholar]
- 27.Kalantarian S, Ay H, Gollub RL, et al. Association between atrial fibrillation and silent cerebral infarctions: a systematic review and meta-analysis. Ann Intern Med. 2014;161(9):650-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bang OY. Silent brain infarction: a quiet predictor of future stroke. Precision Future Med. 2018;2(4):167-174. [Google Scholar]
- 29.Graff-Radford J, Madhavan M, Vemuri P, et al. Atrial fibrillation, cognitive impairment, and neuroimaging. Alzheimers Dement. 2016;12(4):391-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berman JP, Norby FL, Mosley T, et al. Atrial fibrillation and brain magnetic resonance imaging abnormalities. Stroke. 2019;50(4):783-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moazzami K, Shao IY, Chen LY, et al. Atrial fibrillation, brain volumes, and subclinical cerebrovascular disease (from the Atherosclerosis Risk in Communities Neurocognitive Study [ARIC-NCS]). Am J Cardiol. 2020;125(2):222-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piers RJ, Nishtala A, Preis SR, et al. Association between atrial fibrillation and volumetric magnetic resonance imaging brain measures: Framingham offspring study. Heart Rhythm. 2016;13(10):2020-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knecht S, Oelschläger C, Duning T, et al. Atrial fibrillation in stroke-free patients is associated with memory impairment and hippocampal atrophy. Eur Heart J. 2008;29(17):2125-2132. [DOI] [PubMed] [Google Scholar]
- 34.Seiffge DJ, Wilson D, Ambler G, et al. Small vessel disease burden and intracerebral haemorrhage in patients taking oral anticoagulants. J Neurol Neurosurg Psychiatry. 2021;92(8):805-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cloonan L, Fitzpatrick KM, Kanakis AS, Furie KL, Rosand J, Rost NS. Metabolic determinants of white matter hyperintensity burden in patients with ischemic stroke. Atherosclerosis. 2015;240(1):149-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L, Simoni M, Küker W, et al. Population-based case-control study of white matter changes on brain imaging in transient ischemic attack and ischemic stroke. Stroke. 2013;44(11):3063-3070. [DOI] [PubMed] [Google Scholar]
- 37.Rost NS, Rahman R, Sonni S, et al. Determinants of white matter hyperintensity volume in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. 2010;19(3):230-235. [DOI] [PubMed] [Google Scholar]
- 38.Ryu WS, Woo SH, Schellingerhout D, et al. Grading and interpretation of white matter hyperintensities using statistical maps. Stroke. 2014;45(12):3567-3575. [DOI] [PubMed] [Google Scholar]
- 39.Xu M, Wang MM, Gao Y, Keep RF, Shi Y. The effect of age-related risk factors and comorbidities on white matter injury and repair after ischemic stroke. Neurobiol Dis. 2019;126:13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rensma SP, van Sloten TT, Launer LJ, Stehouwer CDA. Cerebral small vessel disease and risk of incident stroke, dementia and depression, and all-cause mortality: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;90:164-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saito T, Kawamura Y, Tanabe Y, et al. Cerebral microbleeds and asymptomatic cerebral infarctions in patients with atrial fibrillation. J Stroke Cerebrovasc Dis. 2014;23(6):1616-1622. [DOI] [PubMed] [Google Scholar]
- 42.Rosand J, Muzikansky A, Kumar A, et al. Spatial clustering of hemorrhages in probable cerebral amyloid angiopathy. Ann Neurol. 2005;58(3):459-462. [DOI] [PubMed] [Google Scholar]
- 43.Rydén L, Sigström R, Nilsson J, et al. Agreement between self-reports, proxy-reports and the National Patient Register regarding diagnoses of cardiovascular disorders and diabetes mellitus in a population-based sample of 80-year-olds. Age Ageing. 2019;48(4):513-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramkumar S, Nerlekar N, D'Souza D, Pol DJ, Kalman JM, Marwick TH. Atrial fibrillation detection using single lead portable electrocardiographic monitoring: a systematic review and meta-analysis. BMJ Open. 2018;8(9):e024178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kobayashi A, Iguchi M, Shimizu S, Uchiyama S. Silent cerebral infarcts and cerebral white matter lesions in patients with nonvalvular atrial fibrillation. J Stroke Cerebrovasc Dis. 2012;21(4):310-317. [DOI] [PubMed] [Google Scholar]
- 46.de Leeuw FE, de Groot JC, Oudkerk M, et al. Atrial fibrillation and the risk of cerebral white matter lesions. Neurology. 2000;54(9):1795-1800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.