Abstract
Objective:
To explore the demographic and clinical variables associated with intraocular pressure lowering after cataract extraction alone (CE) or in combination with iStent placement (CE-IS).
Design:
Retrospective data extraction and survival analysis of consecutive patients identified over a 2-year period.
Subjects:
Patients with mild to moderate glaucoma who underwent CE (48 eyes from 32 patients) or CE-IS (61 eyes from 37 patients) were analyzed.
Methods:
Inability to reduce the number of medications or the IOP by at least 20% compared to baseline on 2 consecutive visits was considered surgical failure. Using Cox proportional hazards models, survival analysis was performed, and demographic and clinical variables were evaluated as risk factors.
Main Outcome Measures:
Time to failure after surgical procedure.
Results:
CE-IS had lower odds of failure than CE (HR=2.01, p=0.047). In Caucasian subjects, CE-IS had greater odds of success as compared to CE alone (HR=2.86, p=0.007). For non-Caucasian subjects there was no difference in the outcomes for the 2 procedures (HR 0.59, p=0.48). In the multivariate analysis, non-Caucasian race (HR=8.75, p=0.0002) and longer axial length (HR=1.61, p=0.03) were associated with greater hazard of failure after CE-IS. In the CE group, greater odds of failure were associated with steeper corneal curvature (HR=1.74, p=0.008), shallower anterior chamber (HR=0.22, p=0.008), and longer axial length (HR=1.58, p=0.01).
Conclusions:
Addition of iStent to CE improved the survival of IOP lowering success in Caucasians but not in non-Caucasians. Associations between IOP lowering after CE and biometric parameters may allow for leveraging these clinical parameters for better case selection for these procedures.
Introduction:
Intraocular pressure (IOP) remains the strongest risk factor for development and progression of glaucomatous visual field loss. The management of glaucoma relies heavily on sustained reduction of IOP.1 Topical ocular hypotensive medications or laser trabeculoplasty are typically considered first-line therapies for IOP lowering. In patients with inadequately controlled IOP, trabeculectomy or ab externo valve placement is considered. However, these procedures are associated with a high incidence of short and long-term complications, including infection, hypotony, scarring, and need for further surgeries.2 Trabecular bypass and scaffolding implants that are typically placed at the time of cataract surgery constitute some of the minimally invasive glaucoma surgeries (MIGS), intended to avoid the complications associated with traditional aqueous shunting procedures. In 2012, the iStent implant (Glaukos Corporation, CA, USA; hereafter referred to as stent) became the first FDA-approved ab interno device to be used for IOP lowering in combination with cataract extraction. Compared to cataract extraction alone, the addition of the stent improved IOP lowering or reduced the number of glaucoma medications in mild to moderate glaucoma patients.3
Since the introduction of the stent, several studies have described its limitations, such as the surgical complexity of implantation, its need to be combined with cataract surgery, and its inability to lower IOP as well as ab externo procedures.3–6 As a result, the lower rate of surgical complications is offset by lower efficacy in IOP lowering with MIGS procedures. To be able to identify the patient characteristics most likely to have an IOP lowering benefit from cataract extraction either with or without MIGS will have significant value in guiding patient selection for these procedures. Even though the risk factors for failure such as race and prior procedures are relatively well known for conventional glaucoma procedures, these have not been well characterized for MIGS procedures. Additionally, ab interno trabecular bypass glaucoma procedures, in theory, should be able to minimize the perils of conjunctival scarring seen in conventional glaucoma surgeries.
This study investigates the success in IOP lowering and medication reduction with cataract extraction with or without stent implantation in mild to moderate glaucoma patients in an academic practice. With an exploratory analysis, clinical covariates were identified that were predictive of IOP lowering success with either of the 2 procedures evaluated.
Methods:
This retrospective cohort study was approved by the University of Nebraska Medical Center Institutional Review Board. Subjects with a diagnosis of ocular hypertension or mild to moderate glaucoma who underwent cataract surgery with stent implantation (CE-IS) or cataract surgery alone (CE) from 2015–2017 at the University of Nebraska Medical Center were included in this study. In patients with surgery performed on both eyes, each eye was included in the study. Specific reasons for exclusion of initially screened cases from analysis are included in the results section.
All available IOP data from up to one year prior to the surgical procedure to the last available follow up visit were extracted from patient records. If more than one IOP was recorded on a given day, the one recorded by the attending or resident physician was included for analysis. All IOPs were recorded using either Goldmann applanation tonometry or pneumatonometry, though the method varied for the same patient from visit to visit. Number of topical IOP lowering medications being used at each of these visits was also recorded. Combination glaucoma medications were recorded as two medications. Oral acetazolamide or methazolamide use was also counted in the study as a single glaucoma medication. Demographic data including age, sex, and race/ethnicity were recorded. Known history of prior laser trabeculoplasty and biometric data including keratometry, anterior chamber depth, and axial length were collected as well. Systemic comorbidities of hypertension, hyperlipidemia, and diabetes were recorded from the patient problem list available in the electronic medical records. Operative and clinic notes were reviewed to confirm the procedure performed (CE alone or CE-IS).
The average of all available IOPs up to one year prior to the surgical procedure was considered the baseline IOP for that individual, and the median number of medications in that period was used as the baseline number of medications. Eyes were evaluated for failure on all visits after the first 30 days post operatively. This was done to allow for resolution of any transient post-operative hyphema and effects of post-operative steroids, as these would be considered the normal post-operative course for these procedures. At each clinic visit after 30 days, IOP change was calculated as percentage change from baseline and medication change was noted as numerical change from baseline number of medications. Surgical failure was defined as inability to lower the number of medications from baseline or reduce the IOP by at least 20% compared to baseline on two consecutive visits. If the failure criteria were met on the last available visit with no subsequent visit for confirmation, the eye was categorized as a surgical failure. When the failure criteria were met on two consecutive visits, time to failure was recorded as that of the first of these visits. Subjects who had less than 30 days of post-operative follow-up (minimum required period to be at risk for failure) were excluded from the analysis.
Primary data analysis was done using survival analysis methods with time to failure being the primary outcome variable. Primary comparator groups were based on the surgical procedure performed, namely CE or CE-IS. Effect of covariates on surgical success was analyzed separately for each group. SAS version 9.4 (Cary, NC) was used for all data analysis. A p value less than 0.05 was considered statistically significant. Descriptive analysis was performed on all variables. Summary measures including mean, standard deviation, and quartiles were obtained for all continuous variables. Frequency distributions were obtained for all categorical variables. In this report, IOP and medication use data over time are presented as spaghetti plots of individual subjects with a mean overlay plot using a fitted smoothed line using a spline routine. Univariate effects of variables on time to failure was studied using Kaplan Meier curves and log rank test and hazard ratios estimated using Cox proportional hazards (PH) model. Quartiles of continuous variables and categories of categorical variables were used for generating the Kaplan Meier curves. Quartile conversion of continuous variables was not required for the Cox PH model. Variables with p value less than 0.05 on univariate analysis and those considered to be clinically relevant confounders on secondary analysis were entered in the multivariate analysis. To account for the effect of inclusion of both eyes of some of the subjects in the analysis, robust sandwich estimate of Lin and Wei was used for the covariance matrix (using COVS(AGGREGATE) option in PHREG procedure in SAS).7
Results:
A total of 64 eyes (39 subjects) undergoing CE-IS were initially identified for inclusion in the study. Three eyes (2 subjects) were excluded because of subjects being lost to follow up (at less than 30 days after surgery). As expected, all CE-IS eyes had preoperative documentation of open angles available in the medical records. In the CE group, 65 eyes (44 subjects) were initially identified for inclusion in the study. Upon a further detailed review of the chart, 17 eyes of 12 subjects were excluded for the reasons as follows. In 3 eyes (3 subjects) stent implantation was attempted after CE but was unsuccessful. In 4 eyes (3 subjects), there was history of past laser iridotomy with persistent angle closure, one due to peripheral anterior synechiae and 3 due to plateau iris configuration. Another 4 eyes (3 subjects) had a history of prior laser iridotomy with gonioscopy not available in the documentation. Of these 3 subjects, one (2 excluded eyes) was also using systemic prednisolone and another (one excluded eye) had proliferative diabetic retinopathy documented in the chart. Two subjects (4 eyes) were excluded as they had newly diagnosed narrow angles with iridotomy deferred due to planned cataract surgery. Two eyes (one subject) with a history of anterior uveitis and peripheral anterior synechiae on gonioscopy were also excluded from analysis. For the analysis presented in this report, 61 eyes from 37 patients were included in the CE-IS group, and 48 eyes from 32 patients were included in the CE group. Among the analyzed subjects, systemic comorbidities data and biometry data was not available for one subject each in CE-IS group. Anterior chamber depth data was not available for 2 additional subjects in the CE group.
For the cases included in analysis, the median available follow up was 298 days (range 31–808 days). A comparison of the baseline demographic and clinical characteristics of the 2 study groups is presented in Table 1. There was no significant difference in age, sex, race or ethnicity, preoperative IOP, or number of medications between the 2 study groups. A higher proportion of CE-IS eyes had a history of prior laser trabeculoplasty.
Table 1.
Baseline demographic and clinical characteristics of the study population
| Cataract extraction with iStent 61 eyes (37 patients) |
Cataract extraction alone 48 eyes (32 patients) |
p valuea | |
|---|---|---|---|
| Age (years) | 71.0 ± 8.1 | 74.2 ± 7.2 | 0.09 |
| Male sex % (n) | 29.7% (11) | 43.8% (14) | 0.32 |
| Caucasian race % (n) | 86.5% (32) | 71.9% (23) | 0.15 |
| Pre-operative IOP (mm Hg) | 17.26 ± 3.1 | 17.64 ± 2.3 | 0.48 |
| Pre-operative number of medications | 1.5 ± 1.1 | 1.2 ± 1.1 | 0.22 |
| Prior laser trabeculoplasty % (n) | 31.1% (19) | 8.3% (4) | 0.004 |
| Diabetes % (n) | 19.4% (7) | 18.8% (6) | 0.99 |
| Hypertension % (n) | 55.5% (20) | 75.0% (24) | 0.13 |
| Hyperlipidemia % (n) | 38.9% (14) | 43.8% (14) | 0.81 |
p values obtained using Student’s t-test for continuous variables and Fisher’s exact test for categorical variables. Data on systemic comorbidities was not available for one subject in the CE-IS group.
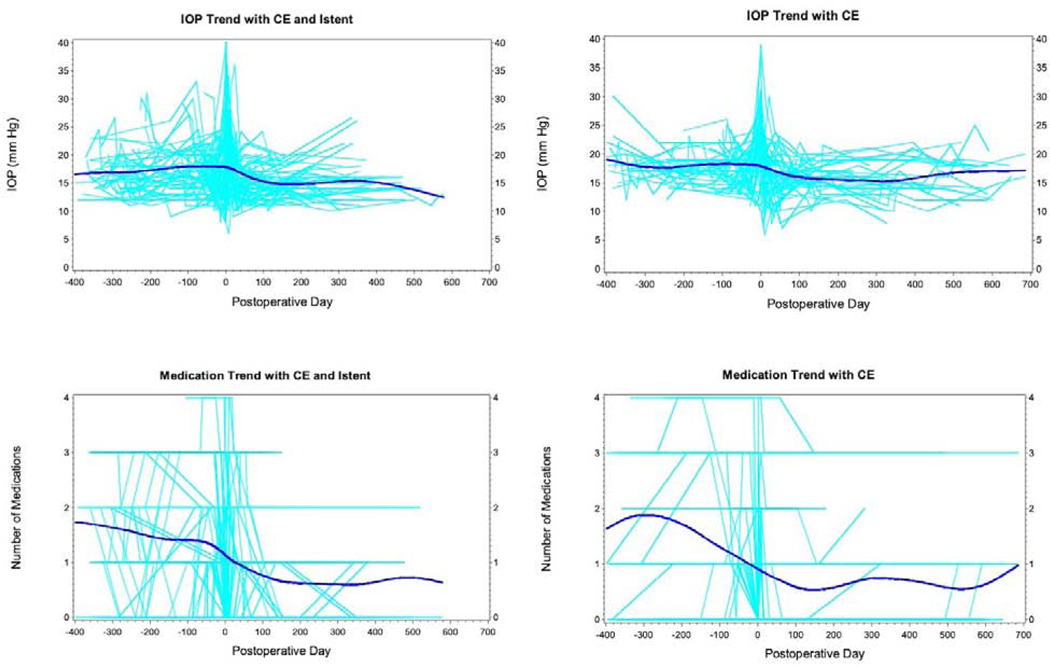
Figure 1 shows the IOP and medication trend over time for all individual subjects and the overall trend for the same in the 2 study groups over time. Overall, there was a 2–3 mmHg reduction in IOP over the first year as compared to the IOP in the year preceding the surgery. There was approximately 1 less medication used in the first year after the procedure in both study groups.
Figure 1.
Trends in intraocular pressure and number of medications over time in the cataract surgery (CE) with iStent and cataract surgery without iStent. The plots show individual cases (light blue) with the trendline (dark blue) for overall trend generated using a smoothed curve with spline routine.
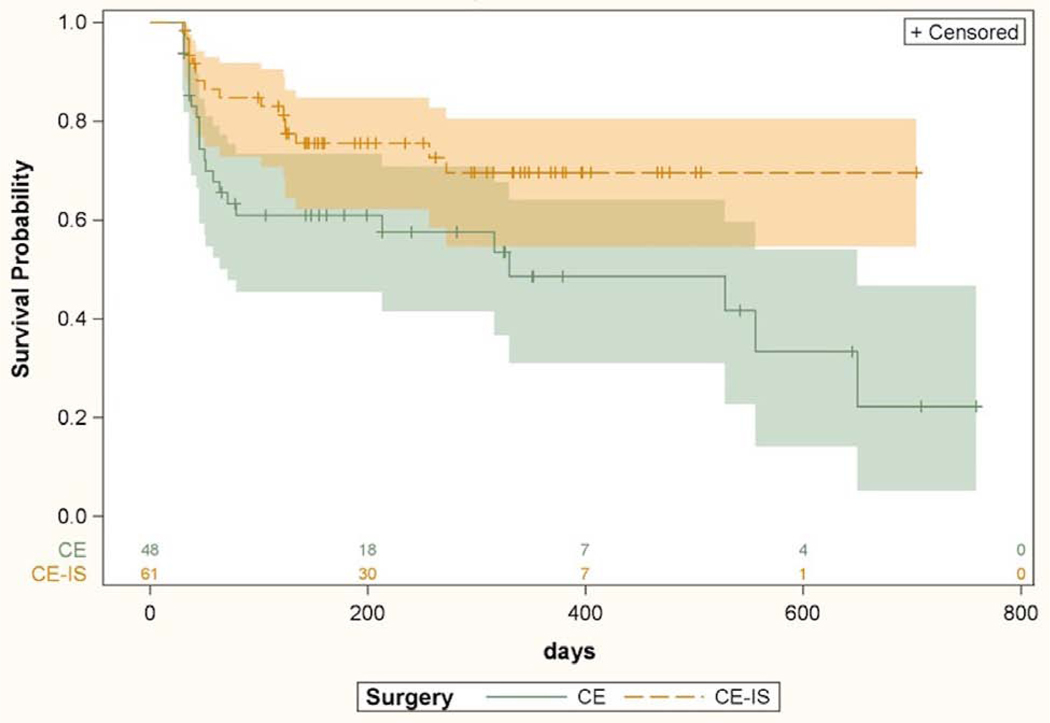
Kaplan Meier survival curves for the CE and CE-IS groups are shown in Figure 2. Patients undergoing CE-IS had lower odds of failure as compared to patients in the CE group (HR 2.01, p=0.047).
Figure 2.
Kaplan-Meier survival curves with 95% confidence interval comparing surgical success among all patients between cataract surgery with iStent and cataract surgery without iStent. Univariate Cox proportional hazard model based hazard ratio was 2.01 (CI 1.01–4.01, p=0.047). CE = cataract extraction; CE-IS = cataract extraction with iStent. Numbers above X axis indicate number of eyes at risk at each time point.
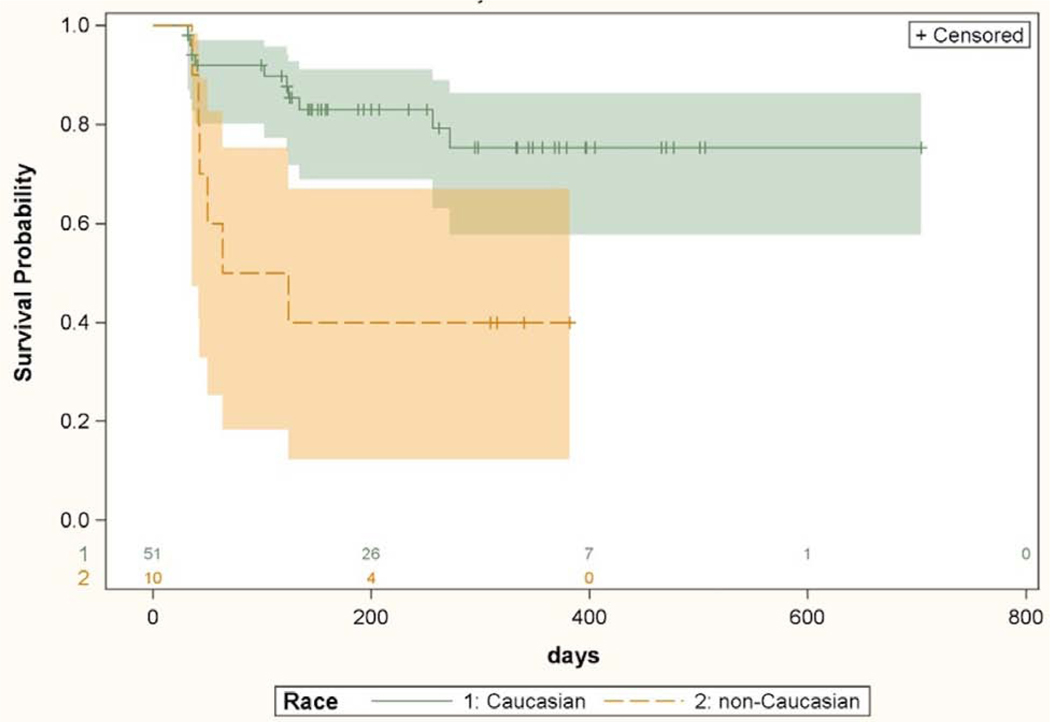
Univariate Cox proportional hazards model analysis of risk factors for failure in the CE-IS group is displayed in Table 2. A greater hazard of failure was associated with non-Caucasian race (HR 3.65, p=0.01) and lower number of pre-operative medications (HR 0.58, p=0.03). A comparison of Kaplan-Meier curves demonstrating the surgical success of CE-IS between Caucasian and non-Caucasian subjects is presented in Figure 3. To evaluate for systematic differences between the Caucasian and non-Caucasian population, baseline demographic and clinical variables were compared between the two groups (Table 3). Caucasians subjects had lower pre-operative IOP (16.9 ± 3.2 vs 19.0 ± 1.7, p=0.009). Nearing significance were older age (71.9 ± 7.4 vs 65.7 ± 11.2 years, p=0.07) and longer axial length (25.0 ± 1.6 vs 23.9 ± 1.4 mm, p=0.07) in the Caucasian subjects. No other variable was different between the 2 groups. Variables entered in the multivariate analysis were those that were statistically significant in the univariate analysis and others that needed to be controlled for because of possible different distributions in the 2 race-based groups. In the multivariate analysis, non-Caucasian race was associated with increased odds of surgical failure compared with Caucasians (HR 8.75, p=0.0002). A greater axial length was also associated with higher risk of surgical failure (HR 1.61, p=0.03) after controlling for other variables in the model. Results of multivariate analysis in the CE-IS group are included in Table 2.
Table 2.
Univariate and multivariate Cox proportional hazards model analysis of risk factors in patients undergoing cataract surgery with iStent
| Variable (reference) | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | Confidence interval | p valuea | Hazard Ratio | Confidence interval | p valuea | |
| Age (1 year) | 0.99 | 0.92–1.07 | 0.88 | 1.04 | 0.98–1.10 | 0.22 |
| Sex (female) | 1.60 | 0.60–4.27 | 0.35 | |||
| Race (Caucasian) | 3.65 | 1.29–10.33 | 0.01 | 8.75 | 2.80–27.31 | 0.0002 |
| Pre-operative IOP (1 mmHg) | 1.09 | 0.95–1.26 | 0.22 | 1.06 | 0.88–1.29 | 0.52 |
| Pre-operative number of medications (1 medication) | 0.58 | 0.36–0.95 | 0.03 | 0.63 | 0.33–1.21 | 0.16 |
| Selective laser trabeculoplasty | 0.40 | 0.13–1.21 | 0.10 | |||
| Corneal curvature (1 diopter) | 0.86 | 0.68–1.09 | 0.22 | |||
| Axial length (1 mm) | 1.29 | 0.93–1.78 | 0.13 | 1.61 | 1.05–2.47 | 0.03 |
| Anterior chamber depth (1 mm) | 1.53 | 0.52–4.53 | 0.45 | |||
| Hypertension | 1.44 | 0.45–4.63 | 0.54 | |||
| Diabetes | 1.77 | 0.60–5.27 | 0.30 | |||
| Hyperlipidemia | 1.37 | 0.48–3.96 | 0.56 | |||
p values obtained using Cox proportional hazards model.
Figure 3.
Kaplan-Meier survival curves with 95% confidence intervals comparing success of cataract surgery with iStent implantation between Caucasians and non-Caucasians. Univariate Cox proportional hazard model based hazard ratio was 3.65 (CI 1.29–10.33, p=0.01). Numbers above X axis indicate number of eyes at risk at each time point.
Table 3.
Comparison of demographic and biometric variables between Caucasian and non-Caucasian subjects in the cataract extraction with iStent group.
| Caucasians 51 eyes (32 subjects) |
Non-Caucasians 10 eyes (5 subjects) |
p valuea | |
|---|---|---|---|
| Age (years) | 71.9 ± 7.4 | 65.7 ± 11.2 | 0.07 |
| Corneal curvature (diopters) | 43.7 ± 1.8 | 43.9 ± 1.5 | 0.72 |
| Axial length (mm) | 25.0 ± 1.6 | 23.9 ± 1.6 | 0.07 |
| Anterior chamber depth (mm) | 3.23 ± 0.4 | 3.11 ± 0.6 | 0.72 |
| Pre-operative number of medications | 1.6 ± 1.2 | 1.2 ± 0.7 | 0.43 |
| Pre-operative IOP (mm Hg) | 16.9 ± 3.2 | 19.0 ± 1.7 | 0.009 |
p values calculated using Mann-Whitney test.
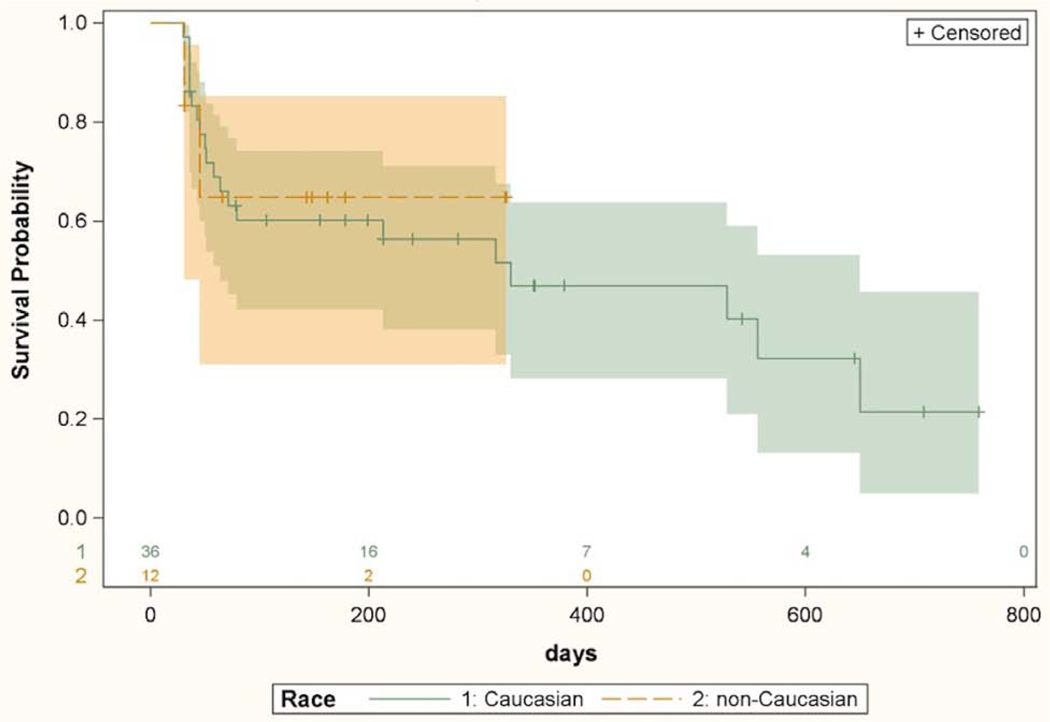
Univariate Cox proportional hazards model analysis of patients in the CE group (Table 4) found a greater hazard of failure associated with lower pre-operative IOP (HR 0.81, p=0.04), steeper corneal curvature (HR 1.42, p=0.03), shallower anterior chamber depth (HR 0.28, p=0.01), and presence of systemic hypertension (p=0.008). No failure events were encountered in the non-hypertensive group. This precluded calculation of a hazard ratio based on application of the proportional hazards model to this variable. Therefore, log rank test was used to determine statistical significance of effect of hypertension on the survival curves, and the variable was not used in the multivariate model. In the multivariate model (Table 4), greater hazard of failure was associated with a longer axial length (HR=1.58, p=0.01), steeper corneal curvature (HR=1.74, p=0.008) and shallower anterior chamber (HR=0.22, p=0.008). There was no difference in survival of surgical success between Caucasian and non-Caucasian subjects in the CE group. A Kaplan-Meier curve demonstrating comparable CE surgical success between Caucasians and non-Caucasians is shown in Figure 4.
Table 4.
Univariate and multivariate Cox proportional hazards model analysis of risk factors for failure in patients undergoing cataract surgery alone
| Variable (reference) | Univariate model | Multivariate model | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | Confidence interval | p value | Hazard Ratio | Confidence interval | p value | |
| Age (1 year) | 0.98 | 0.92–1.04 | 0.48 | |||
| Sex (female) | 1.35 | 0.52–3.47 | 0.54 | |||
| Race (Caucasian) | 0.91 | 0.22–3.67 | 0.89 | 0.50 | 0.13–1.91 | 0.31 |
| Pre-operative IOP (1 mmHg) | 0.81 | 0.65–1.00 | 0.04 | 0.87 | 0.74–1.02 | 0.09 |
| Pre-operative number of medications (1 medication) | 0.55 | 0.24–1.26 | 0.15 | |||
| Selective laser trabeculoplasty | 0.81 | 0.30–2.16 | 0.68 | |||
| Keratometry (1 diopter) | 1.42 | 1.03–1.95 | 0.03 | 1.74 | 1.15–2.62 | 0.008 |
| Axial length (1 mm) | 0.98 | 0.73–1.30 | 0.88 | 1.58 | 1.10–2.29 | 0.01 |
| Anterior chamber depth (1 mm) | 0.28 | 0.11–0.74 | 0.01 | 0.22 | 0.07–0.68 | 0.008 |
| Hypertensiona | 0.008 | |||||
| Diabetes | 0.68 | 0.21–2.20 | 0.52 | |||
| Hyperlipidemia | 1.32 | 0.55–3.15 | 0.53 | |||
p value calculated using log rank test. All other p values obtained using Cox proportional hazards model. See text for rationale.
Figure 4.
Kaplan-Meier survival curves with 95% confidence intervals comparing IOP lowering success of cataract surgery alone between Caucasians and non-Caucasians. Univariate Cox proportional hazard model based hazard ratio was 0.91 (CI 0.22–3.67, p=0.89). Numbers above X axis indicate number of eyes at risk at each time point.
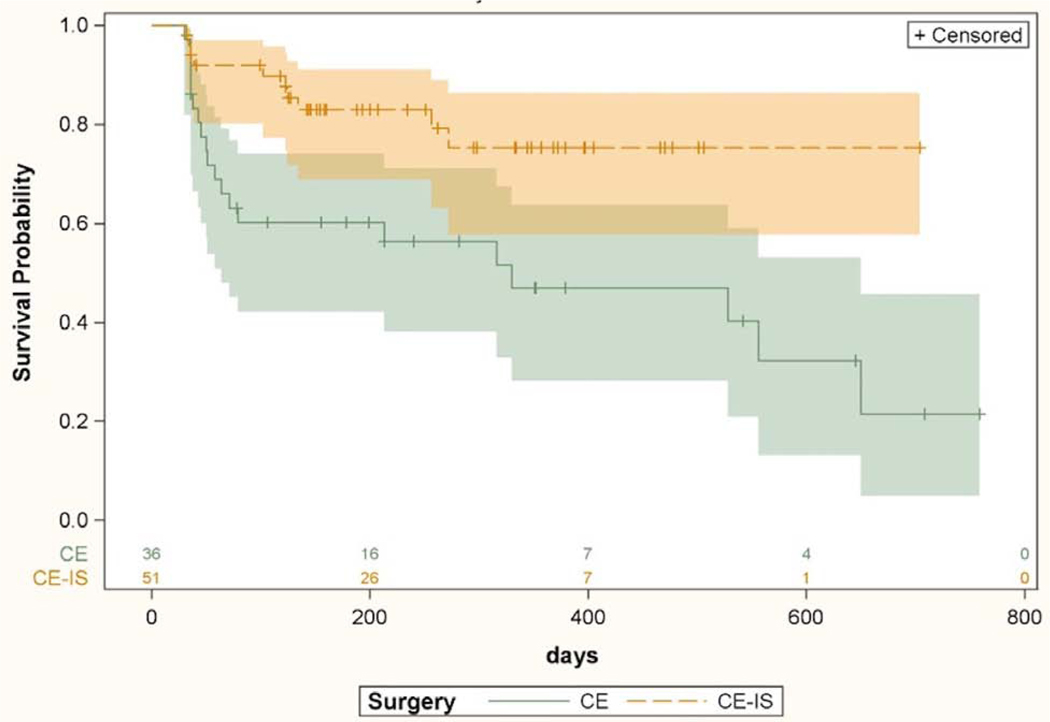
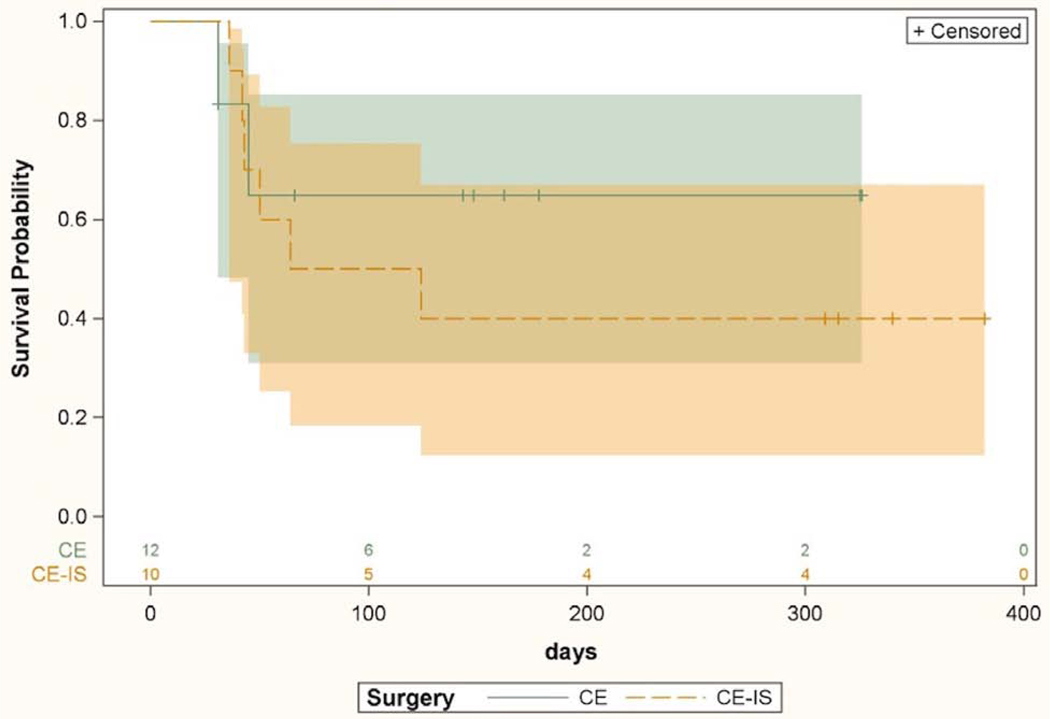
Surgical success for the 2 procedures was compared separately in the 2 race groups. Kaplan-Meier curves comparing surgical success of CE-IS and CE among Caucasians is shown in Figure 5. Patients in the CE-IS group were more likely to maintain surgical success compared to CE alone (HR 2.86, CI 1.33–6.14, p=0.007). Surgical success for the 2 procedures among non-Caucasians is shown in Figure 6. There was no difference between the survival curves for CE-IS and CE in this group (HR 0.59, CI 0.13–2.54, p=0.48).
Figure 5.
Kaplan-Meier survival curves with 95% confidence intervals comparing IOP lowering success of cataract surgery with iStent and cataract surgery without iStent in Caucasian subjects only. Univariate Cox proportional hazard model based hazard ratio was 2.86 (CI 1.33–6.14, p=0.007). CE = cataract extraction; CE-IS = cataract extraction with iStent. Numbers above X axis indicate the number of eyes at risk at each time point.
Figure 6.
Kaplan-Meier survival curves with 95% confidence intervals comparing IOP lowering success of cataract surgery with iStent and cataract surgery without iStent in non-Caucasian subjects only. Univariate Cox proportional hazard model based hazard ratio was 0.59 (CI 0.13–2.54, p=0.48). CE = cataract extraction; CE-IS = cataract extraction with iStent. Numbers above X axis indicate the number of eyes at risk at each time point.
Discussion:
Given our current understanding of pathophysiology of elevated IOP in glaucoma, reducing the resistance in the trabecular outflow pathway should be a robust approach to treatment. However, the real-world outcomes of this approach have not met the high expectations based on the theory behind these procedures. A priori risk stratification of patients undergoing CE or CE-IS can help enhance the benefit to risk ratio and help with better case selection for either procedure. Our retrospective analysis presented here provides preliminary clues as to what demographic and clinical variables may be of value for such models in the future.
In this study, we demonstrated improved IOP lowering success in patients undergoing the combined CE-IS procedure compared to CE alone. However, we found that this advantage of CE-IS over CE was not seen in our small sample of non-Caucasian patients. The non-Caucasian group consisted of mostly Black subjects in both CE-IS and CE patients. The incremental benefit of adding a stent to CE was much more apparent in the Caucasian group. This suggests that the reported IOP lowering outcomes of Schlemm’s canal stenting and trabecular bypass procedures may be significantly affected by the racial composition of the study population, with predominantly Caucasian samples reporting higher success rates as compared to the ones primarily consisting of non-Caucasian subjects. The IOP lowering benefit of CE alone was equivalent in the 2 race-based groups in our study. Even though favorable results for trabecular bypass and non-penetrating glaucoma surgery have been reported in predominantly non-Caucasian populations, to the best of our knowledge, an effect of race on outcomes after MIGS procedures has not been reported in the literature.8,9 In prior reports, patients were either not stratified by race or ethnicity, or all patients were noted to be Caucasian. In a prior report by Seibold et al, Caucasians tended to be more likely to achieve surgical success, however this difference did not reach statistical significance (HR 0.29, CI 0.06–1.29, p=0.10).10 In comparison to that study, our study has a larger sample and longer follow up which may have helped reach statistical significance.
Our method of determining a baseline IOP medications and use is one of several possible methods that can be used for this purpose. Similar to prior large studies on IOP-lowering effects of CE in ocular hypertensive subjects we selected an average of multiple presurgical visits as our baseline.11 An alternate method of setting a baseline IOP can be to use the data from last visit before the procedure for determining the baseline IOP and medications. When the data was analyzed using this alternate method for setting baseline IOP and medications, there was no change in the substantive conclusions of the final multivariate models used for CE-IS or CE data (Supplemental tables 1 and 2). We recognize that 20% IOP lowering may be more difficult to achieve for subjects with lower baseline IOP. This may have been at least partially offset by allowing for medication reduction as a criterion for achieving success. In a previous report on the effect of washout of IOP lowering medications, at a baseline IOP very similar to our cohort, a single medication use was found to have a 23 ± 12% IOP lowering effect.12 Even though we do not have washout IOP available in this study the medicated IOP in this cohort is only slightly lower than those reported in several large randomized MIGS trials.3,13,14
A higher failure rate of conventional filtration procedures in Black patients may be related to a greater number of fibroblasts and macrophages in the conjunctiva of these patients.15 Angle surgical procedures are mechanistically expected to avoid the familiar hazards of the subconjunctival space. The cause of failure of angle surgical procedures is not yet established. Young et al have demonstrated reduced cellularity, fibrotic and basement membrane type deposition in the trabecular meshwork adjacent to prior stent implantation.16 It is possible that post-operative downstream changes in the trabecular outflow may contribute to surgical failure despite successfully eliminating the resistance at the inner wall of Schlemm’s canal, as has been suggested in a prior case report.17 The likely mediators for such a mechanism can be humoral in nature, such as inflammatory markers in the aqueous humor. We are not aware of any known racial differences in the composition of aqueous humor. A prior study evaluating transforming growth factor beta 2 did not find any significant differences in its aqueous concentration between Caucasian and Black subjects.18
Systemic hypertension is more common and more severe in Black patients compared to the general population. Several mediators have been proposed for a greater vulnerability of Black patients to hypertension.19 A greater propensity for vasoreactivity and vascular sclerosis, akin to vulnerability to systemic hypertension in Black patients, can potentially affect the distal outflow pathways. However, contrary to this speculation on a possible mechanism, our study did not find hypertension to be a risk factor for failure. A prior study by Irshad et al found the Schlemm’s canal to be more posteriorly located in eyes of Black patients as compared to Caucasians.20 Other than this report, there are no known race-based differences in the distal outflow pathways. A study comparing aqueous humor dynamics between Black and Caucasian patients with primary open angle glaucoma and ocular hypertension also did not find any significant differences.21 Future research into differences in aqueous humor composition and flow physiology between different race groups may provide greater insights into the causes for failure of trabecular stenting procedures.
In evaluating the effects of systemic comorbidities on surgical success, our study found hypertension to be a risk factor for failure to lower IOP in CE alone, but not for combined CE-IS. Diabetes and hyperlipidemia also were not found to be risk factors for failure. In the hypertensive subset of the study population, the lower hazard of failure with CE-IS was no different from that of the population as a whole (HR 2.35, CI 1.10–5.02, p=0.03). Further research may help determine if the lower odds of IOP lowering with CE in hypertensive subjects is related to some shared pathophysiological effects of hypertension itself or the medications being used to treat it.
Longer axial length was associated with an increased risk of failure after CE and CE-IS. Although all eyes included in this study had open angles on preoperative gonioscopy, shorter eyes may be more likely to have IOP lowering after CE, similar to eyes with narrow angles. Surprisingly, among patients with CE alone, greater corneal curvature and lower anterior chamber depth were associated with increased risk of failure. Empirically, one would expect a better IOP lowering effect of CE in eyes with shallower chambers, as seen in previous studies.22,23 However, the results of the analysis were contrary to this expectation. This suggests that there may be more to IOP lowering with CE than a mere deepening of the anterior chamber. Consistent with this speculation, Coh et al evaluated the effect of preoperative biometric parameters on IOP lowering after CE in glaucomatous and non-glaucomatous eyes.24 At 4 months, they found shallower anterior chambers to be associated with greater IOP lowering only in non-glaucomatous eyes, but not in glaucomatous eyes. Also found was a significant effect of glaucoma diagnosis on the anterior chamber depth-IOP association with a modeled 2.19 mmHg lesser IOP lowering associated with glaucoma diagnosis. Glaucoma patients with certain lens positioning in the eye may have greater IOP lowering benefit from CE. Based on the results of this study’s multivariate analysis, an eye with a combination of shorter axial length, flatter cornea and deeper anterior chamber is expected to have the most IOP lowering benefit from CE.
Our study has several limitations that need to be considered when interpreting the findings. This is a retrospective review of surgical success in a single academic center. However, the severity of pathology and practice patterns at this institution likely reflect those of any academic center in the United States. The sample size is small with a much larger proportion of Caucasian subjects as compared to non-Caucasians, reflective of the population demographics in the service area. To have a large enough sample to make meaningful comparisons, the non-Caucasian group consisted of several racial and ethnic groups. Future prospective studies can address this limitation by targeted enrollment of specific racial and ethnic groups of interest. The current study has all the limitations of a retrospective study with potential for selection bias and cohort effects such as the possibility of CE-IS group having greater pathology than CE cases. To minimize any potential systematic biases, the study had consecutive inclusion of all eligible cases and contemporary controls. There were no exclusions in the CE-IS group for any reason other than loss to follow up. A larger number of exclusions in the CE-IS group was to only include cases that would have been otherwise clinically eligible for inclusion in CE-IS group. The two groups were well matched for most demographic and clinical characteristics with the exception of history of prior SLT, which was as expected, more prevalent in the CE-IS group. This likely reflects a selection bias for cases more in need of IOP lowering for both SLT and stent. However, we did not find any effect of prior SLT on outcomes in either group in this study, which limits the possibility of this being a potential source of bias in our results, though the possibility cannot be absolutely ruled out. It is also important to keep in mind that some of the baseline variables may not have reached statistically significant difference between the 2 groups because of the relatively small sample size of the study. Because of the retrospective design and the absence of any washout prior to surgery, the true IOP lowering effect of either procedure cannot be deduced from these data and could be much different from that reported here. However, outside of the setting of a clinical trial, such data is rarely available to a treating clinician, and treatment decisions and determination of success or failure must be made based on the available clinical data. In that sense, the current study is more reflective of the real-world effectiveness of the procedures evaluated rather than their true efficacy. In addition, because of the retrospective design, we are not able to control for the variability introduced by different methods of tonometry and different time of IOP measurement at different visits. The systemic co-morbidities were extracted from the problem list available in the patient’s electronic medical records. Patient’s general medical care notes were not reviewed, and current treatment was not controlled for. This was outside the primary scope of this preliminary study. Therefore, the possibility of association between outcomes and adequacy of management of systemic comorbidity such as hypertension, diabetes or hyperlipidemia cannot be ruled out. As with any study, the generalizability of the findings of this study is limited to the risk factors evaluated here and the range of variables encountered in this population. Despite these limitations, the current study found clinically and statistically relevant variables associated with IOP lowering success of CE and CE-IS.
The reported findings suggest that there are significant differences in the success of CE-IS but not CE alone based on race. Further research into potential racial differences in wound healing processes at the surgical site or compensatory adjustments in distal outflow pathways may help explain the findings of this study. Certain anatomic configurations of the eye measurable by biometry are associated with success of CE and CE-IS. These variables can be viable candidates for inclusion in possible personalized models for estimating success probability and thereby individualize the surgical recommendations for either procedure. They also can be used to prospectively evaluate and explore other local, humoral or genetic factors to help optimize the IOP lowering outcomes with trabecular bypass procedures.
Supplementary Material
This survival analysis demonstrates that the addition of the iStent to cataract extraction improves the survival of IOP lowering success in Caucasian but not in non-Caucasian patients with open angle glaucoma.
Acknowledgments
Financial support: Supported by NEI K23EY023266
Meeting presentations: Data in this paper were previously presented as poster abstracts at: the Association for Research in Vision and Ophthalmology 2018 Annual Meeting, Honolulu, HI, April 2018; and the American Glaucoma Society 28th Annual Meeting, New York, NY. March 2018.
Footnotes
Conflicts of Interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Boland MV, Ervin A-M, Friedman DS, et al. Comparative Effectiveness of Treatments for Open-Angle Glaucoma: A Systematic Review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;158(4):271. [DOI] [PubMed] [Google Scholar]
- 2.Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC. Postoperative Complications in the Tube Versus Trabeculectomy (TVT) Study During Five Years of Follow-up. Am J Ophthalmol. 2012;153(5):804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samuelson TW, Katz LJ, Wells JM, Duh YJ, Giamporcaro JE. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118(3):459–467. [DOI] [PubMed] [Google Scholar]
- 4.Craven ER, Katz LJ, Wells JM, Giamporcaro JE. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: Two-year follow-up. J Cataract Refract Surg. 2012;38(8):1339–1345. [DOI] [PubMed] [Google Scholar]
- 5.Chen DZ, Sng CCAA. Safety and Efficacy of Microinvasive Glaucoma Surgery. Vol 2017.; 2017:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agrawal P, Bradshaw SE. Systematic Literature Review of Clinical and Economic Outcomes of Micro-Invasive Glaucoma Surgery (MIGS) in Primary Open-Angle Glaucoma. Ophthalmol Ther. 2018;7(1):49–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin DY, Wei LJ. The robust inference for the cox proportional hazards model. J Am Stat Assoc. 1989;84(408):1074–1078. [Google Scholar]
- 8.Gallardo MJ, Supnet RA. Three-year outcomes of combined trabecular micro-bypass and phacoemulsification in a predominantly hispanic population with primary open-angle glaucoma. Clin Ophthalmol. 2019;13:869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grieshaber MC, Pienaar A, Olivier J, Stegmann R. Comparing two tensioning suture sizes for 360° viscocanalostomy (canaloplasty): A randomised controlled trial. Eye. 2010;24(7):1220–1226. [DOI] [PubMed] [Google Scholar]
- 10.Seibold LK, Gamett KM, Kennedy JB, et al. Outcomes after combined phacoemulsification and trabecular microbypass stent implantation in controlled open-angle glaucoma. J Cataract Refract Surg. 2016;42(9):1332–1338. [DOI] [PubMed] [Google Scholar]
- 11.Mansberger SL, Gordon MO, Jampel H, et al. Reduction in intraocular pressure after cataract extraction: the Ocular Hypertension Treatment Study. Ophthalmology. 2012;119(9):1826–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jampel HD, Chon BH, Stamper R, et al. Effectiveness of intraocular pressure-lowering medication determined by washout. JAMA Ophthalmol. 2014;132(4):390–395. [DOI] [PubMed] [Google Scholar]
- 13.Samuelson TW, Chang DF, Marquis R, et al. A Schlemm Canal Microstent for Intraocular Pressure Reduction in Primary Open-Angle Glaucoma and Cataract: The HORIZON Study. Ophthalmology. 2019;126(1):29–37. [DOI] [PubMed] [Google Scholar]
- 14.Johnson T V, Jampel HD. Intraocular Pressure Following Prerandomization Glaucoma Medication Washout in the HORIZON and COMPASS Trials. Am J Ophthalmol. 2020;216:110–120. [DOI] [PubMed] [Google Scholar]
- 15.Broadway D, Grierson I, Hitchings R. Racial differences in the results of glaucoma filtration surgery: Are racial differences in the conjunctival cell profile important? Br J Ophthalmol. 1994;78(6):466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young CEC, Ammar DA, Seibold LK, Pantcheva MB, SooHoo JR, Kahook MY. Histopathologic Examination of Trabecular Meshwork Changes after Trabecular Bypass Stent Implantation. J Glaucoma. 2018;27(7):606–609. [DOI] [PubMed] [Google Scholar]
- 17.Shah M, Campos-Möller X, Werner L, Mamalis N, Ahmed IIK. Midterm failure of combined phacoemulsification with trabecular microbypass stenting: Clinicopathological analysis. J Cataract Refract Surg. 2018;44(5):654–657. [DOI] [PubMed] [Google Scholar]
- 18.Trivedi RH, Nutaitis M, Vroman D, Crosson CE. Influence of Race and Age on Aqueous Humor Levels of Transforming Growth Factor-Beta 2 in Glaucomatous and Nonglaucomatous Eyes. J Ocul Pharmacol Ther. 2011;27(5):477–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nesbitt SD. Hypertension in black patients: Special issues and considerations. Curr Cardiol Rep. 2004;6(6):416–420. [DOI] [PubMed] [Google Scholar]
- 20.Irshad FA, Mayfield MS, Zurakowski D, Ayyala RS. Variation in Schlemm’s Canal Diameter and Location by Ultrasound Biomicroscopy. Ophthalmology. 2010;117(5):916–920. [DOI] [PubMed] [Google Scholar]
- 21.Beltran-Agullo L, Alaghband P, Rashid S, et al. Comparative Human Aqueous Dynamics Study between Black and White Subjects with Glaucoma. Investig Opthalmology Vis Sci. 2011;52(13):9425. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi K, Hayashi H, Nakao F, Hayashi F. Effect of cataract surgery on intraocular pressure control in glaucoma patients. J Cataract Refract Surg. 2001;27(11):1779–1786. [DOI] [PubMed] [Google Scholar]
- 23.Shin HC, Subrayan V, Tajunisah I. Changes in anterior chamber depth and intraocular pressure after phacoemulsification in eyes with occludable angles. J Cataract Refract Surg. 2010;36(8):1289–1295. [DOI] [PubMed] [Google Scholar]
- 24.Coh P, Moghimi S, Chen RI, et al. Lens Position Parameters as Predictors of Intraocular Pressure Reduction After Cataract Surgery in Glaucomatous Versus Nonglaucomatous Eyes. Investig Opthalmology Vis Sci. 2016;57(6):2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.