Abstract
Adapters are typically viewed as molecules coordinating the recruitment of positive effectors of cell signaling. Herein, we report the identification of Dok-3, a novel adapter molecule belonging to the Dok family. Our studies show that Dok-3 is highly expressed in several hemopoietic cell types, including B cells and macrophages. It undergoes rapid tyrosine phosphorylation in response to immunoreceptor-mediated cellular activation, seemingly as a result of the action of Src family kinases. This phosphorylation induces the binding of Dok-3 to at least two inhibitory molecules, the 5′ inositol phosphatase SHIP and the protein tyrosine kinase Csk. We also demonstrate that augmented expression of wild-type Dok-3 in a B-cell line results in an inhibition of immunoreceptor-mediated nuclear factor of activated T-cells (NFAT) activation and cytokine release, while introduction of a Dok-3 mutant with impaired ability to associate with SHIP and Csk enhances B-cell responsiveness. Taken together, these results indicate that Dok-3 is an adapter involved in the recruitment of inhibitory molecules and that it may play a significant role in the negative regulation of immunoreceptor signaling in hemopoietic cells such as B cells and macrophages.
Immunoreceptors such as the T-cell receptor (TCR) for antigen, the B-cell receptor (BCR) for antigen, and a variety of receptors for the Fc portion of immunoglobulins (Ig), play central roles in antigen-specific and natural immunity (6, 20, 34, 54, 59, 66). Typically, these receptors contain several chains, including ligand-binding subunits and subunits involved in signal transduction. Accumulating data show that immunoreceptors mediate their biological effects via the induction of intracellular protein tyrosine phosphorylation. While they lack intrinsic protein tyrosine kinase (PTK) activity, they possess within their cytoplasmic domain a motif termed the immunoreceptor tyrosine-based activation motif (ITAM), which has the ability to recruit and activate cytoplasmic PTKs.
Two classes of cytoplasmic PTKs have been implicated in immunoreceptor-mediated signal transduction: the Src and Syk/Zap-70 families (11, 15, 16, 59, 63). Genetic and biochemical studies have shown that Src-related enzymes initiate immunoreceptor signaling through their capacity to phosphorylate two conserved tyrosines in the ITAMs. This phosphorylation permits the binding and activation of Syk/Zap-70-related PTKs, which amplify the immunoreceptor-induced signal. Together, Src and Syk/Zap family kinases activate downstream effectors, including phospholipase C (PLC)-γ, the guanine nucleotide exchange factor Vav, phosphatidylinositol (PI) 3′ kinase, and Ras. These targets lead to reorganization of the cytoskeleton, transcriptional activation, and, ultimately, induction of immune functions.
Intracellular signals delivered by PTKs such as Src and Syk/Zap-70 family kinases are coordinated by a class of molecules termed “adapters” or “linkers” (49, 51, 56). Even though these polypeptides lack intrinsic catalytic activity, they possess motifs and domains capable of mediating protein-protein and, in some cases, protein-lipid interactions. As a result, adapters allow the immunoreceptors and their PTKs to come into close proximity with their targets. Several adapters have been found to play pivotal roles during immune-cell activation. For example, SLP-76 and LAT, two adapter molecules expressed in T-cells, are required for proper tyrosine phosphorylation and activation of PLC-γ, intracellular calcium fluxes, and Ras stimulation during T-cell activation (68, 70). In an analogous manner, the B-cell-specific adapter Blnk is required for tyrosine phosphorylation of PLC-γ and activation of Jun N-terminal kinase (JNK) in activated B lymphocytes (28, 35).
Evidence is growing that highly regulated intracellular mechanisms are involved in restricting the duration and/or intensity of immunoreceptor signaling (10, 29, 52, 58, 61, 62). These negative regulators include several protein tyrosine phosphatases (PTPs) like SHP-1, SHP-2, PEP, CD45 and HePTP, the Src homology 2 domain-containing inositol 5′-phosphatase (SHIP), and the protein tyrosine kinase Csk. While the processes orchestrating the involvement of these inhibitors during cellular activation are not fully understood, recent findings have shown that SHP-1, SHP-2, and SHIP are recruited by inhibitory receptors such as killer inhibitory receptors (KIRs) and FcγRIIB, which contain immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in their cytoplasmic domains (5, 19, 20, 61, 62). However, by analogy to positive signaling, it is plausible that intracellular adapter molecules also play an important role in coordinating inhibitory signals. Unfortunately, little is known about these “inhibitory” adapters.
In this paper, we report the cloning and characterization of a novel adapter molecule which we termed Dok-3. Our data show that Dok-3 rapidly becomes tyrosine phosphorylated in response to immunoreceptor stimulation and that, as a consequence, it recruits at least two inhibitory molecules: the inositol phosphatase SHIP and the protein tyrosine kinase Csk. These interactions seem to constitute an inhibitory signal aimed at restricting the intensity of cellular activation.
MATERIALS AND METHODS
cDNA cloning and DNA constructs.
A partial mouse dok-3 cDNA was cloned during a yeast two-hybrid screen using Csk as bait in the presence of the Src kinase (unpublished data). This cDNA encoded the carboxy-terminal domain of Dok-3 (amino acids 267 to 444). Full-length cDNAs were subsequently obtained through a combination of screening of a mouse fetal thymus cDNA library (provided by Louis Matis, Alexion Pharmaceuticals, New Haven, Conn.) and rapid amplification of 5′ cDNA ends (5′ RACE) (utilizing RNA from the BAL17 B-cell line as the template). Both strands of representative cDNAs were sequenced, using the dideoxynucleotide chain termination method (data not shown; GenBank accession number AF23758). A dok-3 cDNA encoding a mutant in which all four tyrosines in the carboxy-terminal domain were replaced by phenylalanines (Dok-3 4F) was produced by PCR. A myc-tagged version of the dok-3 cDNA was also created by PCR. All cDNA variants were verified by sequencing, to ensure that no unwanted mutation had been introduced in the process of their generation (data not shown). The mouse dok cDNA was obtained from Yuji Yamanashi and David Baltimore (Massachusetts Institute of Technology, Cambridge, Mass.) (69), whereas the mouse dok-2 cDNA will be reported elsewhere (our unpublished results).
cDNAs constructs encoding Lck, FynT, Lyn, Syk, Zap-70, and Csk, as well as a variety of Csk mutants, were reported elsewhere (7, 13, 17). The bcr-abl cDNA was obtained from Pierre Laneuville (Royal Victoria Hospital, Montreal, Quebec, Canada). For transient expression in Cos-1 cells, myc-tagged dok-3 cDNAs were cloned in the vector pXM139, which contains the simian virus 40 (SV40) origin of replication and an adenovirus major late promoter. For stable expression in A20 cells, dok-3 cDNAs were inserted in the mammalian expression vector pNT-Neo, which possesses an SRα-based promoter and the neo gene. For transient expression in A20 cells, dok-3 cDNAs (without the myc tag) were cloned in pXM139. pNFAT-luciferase was kindly provided by G. Crabtree (Stanford University, Palo Alto, Calif.).
RNase protection assays.
RNase protection assays were performed using total cellular RNA (25 μg) from various mouse tissues and cell lines, according to a previously published protocol (14). The riboprobes used in these assays corresponded to nucleotides 1847 to 2162 of dok-3, nucleotides 460 to 795 of dok (69), and nucleotides 740 to 1061 of dok-2 (36). For size markers, MspI-digested fragments of pBR322 were end labeled with Klenow DNA polymerase (Life Technologies, Gaithersburg, Md.) and [α-32P]dCTP (New England Nuclear, Boston, Mass.). The integrity of the cellular RNAs used in the assays was verified by electrophoresis of aliquots in agarose-formaldehyde gels and staining with ethidium bromide (data not shown).
Tissues, cells, and transfections.
Mouse tissues were obtained from either C57BL/6 or BALB/c mice. The panel of cell lines used for the RNase protection assays has been described elsewhere (12). A20 is a mouse B-cell lymphoma line that expresses surface IgG (41). It was propagated in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), penicillin, streptomycin, and β-mercaptoethanol. Stable derivatives expressing the neomycin phosphotransferase alone or in combination with wild-type Dok-3 or Dok-3 4F were generated by electroporation (260 V; 960 μF) and selection in medium containing G418 (0.5 mg/ml). Monoclonal cell lines were produced by limiting dilution. J774A is a mouse macrophage-derived cell line. It bears high-affinity receptors for the Fc portion for IgG (FcγRI) and was grown in high-glucose Dulbecco minimal essential medium (MEM) containing 10% FBS and antibiotics. Cos-1 cells were propagated in α-MEM supplemented with 10% FBS and antibiotics. They were transiently transfected by the DEAE-dextran method, as detailed elsewhere (27).
Antibodies.
Polyclonal antibodies against Dok-3 were produced by immunizing rabbits with a bacterial fusion protein (TrpE) encompassing amino acids 363 to 444 of Dok-3. These antibodies efficiently recognized Dok-3 in immunoprecipitations and immunoblots. Importantly, they did not cross-react with Dok and Dok-2 (data not shown). Affinity purification was performed by passing the crude serum over a column containing the immunogen immobilized on Affigel (Bio-Rad Laboratories, Hercules, Calif.). Anti-Dok antibodies were also produced in rabbits, using a TrpE fusion protein bearing residues 374 to 482 of Dok. The two antisera directed against SHIP were generated in a similar manner. The first one was directed against the SH2 domain of SHIP, while the second one recognized amino acids 1125 to 1190. Polyclonal antibodies directed against Shc, Csk, Fyn, Lck, and phosphotyrosine have been characterized previously (1, 18, 23, 24). A mouse monoclonal antibody (MAb) reacting with the Myc-derived epitope (MAb 9E10) has also been described previously (18). The anti-phosphotyrosine mouse MAb 4G10 was purchased from Upstate Biotechnology (Lake Placid, N.Y.), while the anti-Ras-GAP mouse MAb B4F8 was obtained from Santa Cruz Biotechnology (Santa Cruz, Calif.).
Cell stimulation.
A20 B cells (2 × 107 cells/ml) were stimulated for the indicated periods of time at 37°C with either F(ab′)2 fragment (20 μg/ml; Jackson Immunoresearch Laboratories, West Grove, Pa.) or intact (30 μg/ml; ICN Biomedicals, Aurora, Ohio); sheep anti-mouse (SAM) IgG. J774A macrophages (1.5 × 107 cells/ml) were activated by stimulation for 2 min at 37°C with mouse IgG2a (MAb 7G7; 15 μg/ml) followed by F(ab′)2 fragments of SAM IgG (45 μg/ml). After stimulation, A20 and J774A cells were lysed in TNE buffer (1× TNE is 50 mM Tris [pH 8.0], 1% Nonidet P-40, and 2 mM EDTA) supplemented with protease and phosphatase inhibitors, as detailed in an earlier report (18). Lysates were then processed for immunoprecipitations, in vitro binding assays, or immunoblots.
Immunoprecipitations and immunoblots.
Precleared postnuclear lysates from A20, J774A, or transfected Cos-1 cells were immunoprecipitated for 1.5 h with the antibodies specified in the text. Immune complexes were collected with protein A-Sepharose (Amersham Pharmacia Biotech, Baie d'Urfe, Quebec, Canada) precoupled, if necessary, to rabbit anti-mouse (RAM) IgG (Jackson Immunoresearch Laboratories). After three washes, proteins were eluted in sodium dodecyl sulfate (SDS)-containing sample buffer, boiled, and resolved by SDS-polyacrylamide gel electrophoresis. Immunoblotting was performed according to a previously described protocol (64). Unless specified, immunoreactive products were detected using 125I-labeled protein A (Amersham Pharmacia Biotech) or 125I-labeled goat anti-mouse IgG (ICN Biomedicals). In some cases, protein A-horseradish peroxidase and enhanced chemiluminescence reagents (Amersham Pharmacia Biotech) were utilized.
In vitro binding assays.
Glutathione S-transferase (GST) fusion proteins encompassing the phosphotyrosine-binding (PTB) domain of Dok-3 (amino acids 104 to 251) or Shc (amino acids 40 to 209) were generated by cloning the necessary PCR-amplified DNA fragments in the vector pGEX-2T (Amersham Pharmacia Biotech). The integrity of the PCR-amplified segments was verified by sequencing (data not shown). The construct for production of GST-Csk SH2 domains has been described previously (17), whereas that allowing the synthesis of GST-SHIP SH2 domains was obtained from Gerald Krystal (Terry Fox Laboratory, Vancouver, British Columbia, Canada). Induction of GST fusion proteins and purification on agarose-glutathione beads (Sigma-Aldrich Canada, Oakville, Ontario, Canada) were done according to a previously described protocol (50). Binding assays were also performed as outlined elsewhere (50), using lysates from either A20 B cells (4 × 107 cells) or transfected Cos-1 cells (250 μg). After extensive washing, bound proteins were detected by immunoblotting with the indicated antibodies.
IL-2 production.
Pools of at least three independent A20 B-cell transfectants (105 cells in 200 μl) were stimulated for 24 h at 37°C in 96-well plates, in the presence of various concentrations of F(ab′)2 fragments of RAM IgG. As a control, cells were stimulated with the indicated concentrations of phorbol myristate acetate (PMA) and ionomycin. After this period, supernatants were harvested, frozen at −70°C to destroy carryover cells, and assayed for interleukin 2 (IL-2) content using the IL-2-dependent cell line HT-2 (23). All assays were done in triplicate and repeated at least three times. Representative results are shown.
Luciferase assays.
A20 cells (10 × 106) were transfected by electroporation (300 V; 975 μF) with 20 μg of pNFAT-luciferase and 5 μg of pXM139, pXM139-dok-3 wt, or pXM139-dok-3 4F DNA. After 40 h, cells (2 × 106 viable cells) were stimulated for 6 h with 10 μg of F(ab′)2 fragments of SAM IgG/ml. As a control, cells were activated by the combination of PMA (100 ng/ml) and ionomycin (1 μM). Cells were then lysed and assayed for luciferase activity using the luciferase reporter assay system (Promega) and a luminometer (EG&G Berthold). Results are presented as percentages of luciferase activity induced by PMA plus ionomycin. Equivalent numbers of viable cells were also lysed in parallel with boiling sample buffer, for immunoblotting of total cell lysates with anti-Dok-3 antisera.
RESULTS
Dok-3, a novel member of the Dok family of adapter molecules.
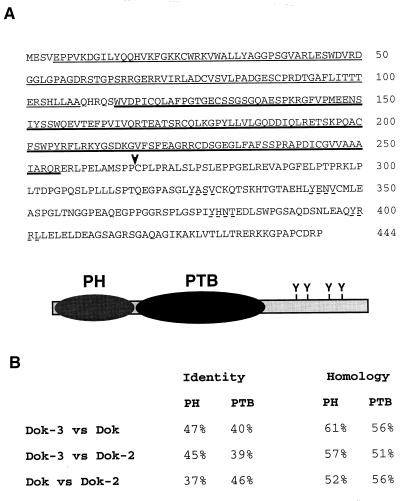
In an attempt to uncover new molecules binding to the SH2 domain of Csk, as described in Materials and Methods, we identified a cDNA corresponding to a previously undescribed molecule. After full-length cDNAs were obtained, database searches demonstrated that this polypeptide (Fig. 1A) was most closely related to the adapters Dok (8, 69) and Dok-2/FRIP/Dok-R (referred to below as Dok-2) (25, 36, 44). Sequence analyses predicted that it contained an amino-terminal pleckstrin homology (PH) domain, a central PTB domain, and a carboxy-terminal region of ∼190 amino acids. Whereas the PH and PTB domains of the novel polypeptide shared extensive homology with those of Dok and Dok-2 (Fig. 1B), there was little or no sequence conservation in the carboxy-terminal domain. In particular, the repeated YXXP motif (where X represents any amino acid) found in Dok and Dok-2 was absent in the new molecule. This motif mediates the binding of Dok and Dok-2 to the SH2 domains of Ras–GTPase-activating protein (GAP) and Nck (40, 45). Nevertheless, the extent of the homology between the PH and PTB domains of the new polypeptide and those of Dok and Dok-2 was typical of proteins belonging to the same family. On this basis, the novel molecule was named Dok-3.
FIG. 1.
Predicted sequence and structure of Dok-3. (A) Amino acid sequence of Dok-3. The predicted sequence of the mouse Dok-3 protein is shown. The putative PH domain (residues 5 to 108) is marked by thin underlining, whereas the presumed PTB domain (amino acids 114 to 255) is indicated by heavy underlining. The four potential tyrosine phosphorylation sites in the carboxy-terminal domain are indicated by discontinuous underlining. An arrowhead indicates the amino-terminal boundary of the fragment identified in the yeast two-hybrid screen. A schematic representation of Dok-3 is shown below the sequence. (B) Comparison of Dok-3 with Dok and Dok-2. The extents of sequence identity and homology between the PH and PTB domains of Dok-3, Dok, and Dok-2 are presented (determined with the BLAST program/BLOSUM 62 matrix).
dok-3 is highly expressed in B cells and macrophages.
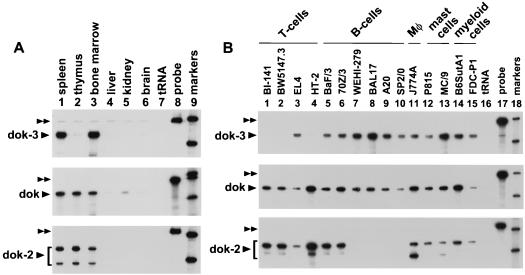
To establish the pattern of expression of dok-3, RNase protection assays were conducted (Fig. 2). Among mouse tissues (Fig. 2A), dok-3 (top panel) was abundantly expressed in the spleen (lane 1) and bone marrow (lane 3) but not in the thymus (lane 2). Little or no dok-3 was found in nonlymphoid organs (lanes 4 to 6). In contrast, dok (middle panel) and dok-2 (bottom panel) were equally present in the spleen (lane 1), thymus (lane 2), and bone marrow (lane 3). In another experiment with RNA samples from various hematopoietic cell lines (Fig. 2B), we found that dok-3 (top panel) was expressed in all B-cell (lanes 5 to 10), macrophage (lane 11), mast cell (lanes 12 and 13), and myeloid cell (lanes 14 and 15) lines tested. However, it was absent from most T-cell lines examined (lanes 1 to 4), with the exception of EL-4 (lane 3). In comparison, dok (middle panel) was observed in all hematopoietic cell types (lanes 1 to 15), whereas dok-2 was expressed broadly in T cells (lanes 1 to 4), macrophages (lane 11), mast cells (lanes 12 and 13), and myeloid cells (lanes 14 and 15), but not in B cells (lanes 5 to 10). The relative absence of dok-2 in B cells was also noted earlier by others (44). In light of our results, we concluded that dok-3 was highly expressed in most hematopoietic cells, including B cells and macrophages. Expression was much less uniform in T cells.
FIG. 2.
Expression pattern of dok-3 in mouse tissues and cell lines. The abundance of dok-3 RNA in various mouse tissues (A) and cell lines (B) was ascertained by RNase protection assay. For comparison, the distribution of dok and dok-2 was examined in parallel. The positions of the undigested riboprobes are indicated by double arrowheads, whereas those of the protected fragments are shown by single arrowheads. The basis for the existence of two protected fragments of different weights in some of the dok-2 samples is not known. For all panels, only the 404 and 309 nucleotide size markers are shown. (A) Exposure times: top panel, 6 h; middle panel, 20 h; bottom panel, 6 h. (B) Exposure times: top panel, 20 h; middle panel, 20 h; bottom panel, 24 h.
Dok-3 undergoes tyrosine phosphorylation in response to immunoreceptor stimulation.
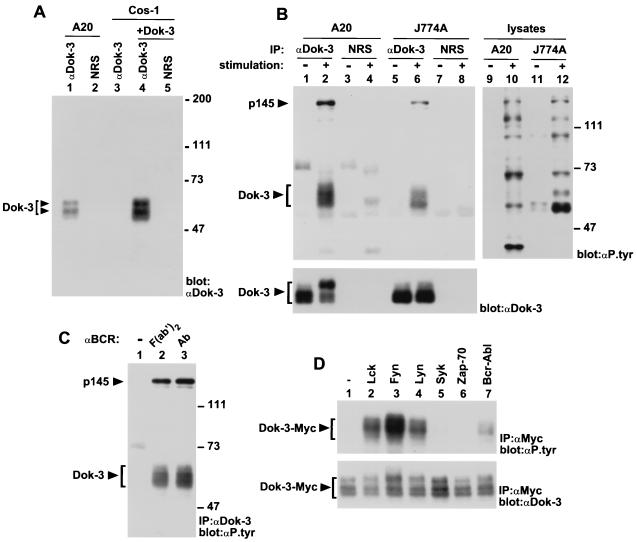
To begin to understand the function of Dok-3, a polyclonal anti-Dok-3 serum was generated in rabbits, using a bacterial fusion protein encompassing amino acids 363 to 444 of Dok-3 as an immunogen. For identification of the Dok-3 protein, the A20 B-cell line was lysed in non-ionic detergent-containing buffer, and postnuclear lysates were immunoprecipitated with either anti-Dok-3 antibodies or normal rabbit serum (Fig. 3A, lanes 1 and 2). After extensive washes, the presence of Dok-3 in these immunoprecipitates was revealed by immunoblotting with anti-Dok-3 antibodies. This analysis showed that, in A20 B cells, the anti-Dok-3 serum (lane 1) precipitated two major immunoreactive species of ∼58 and 62 kDa. These polypeptides were absent in immunoprecipitates obtained with normal rabbit serum (lane 2). To ensure that these products represented the bona fide Dok-3 protein, Cos-1 cells were transfected with a full-length mouse dok-3 cDNA, and the Dok-3 molecules accumulating in these cells were recovered by immunoprecipitation. Dok-3 polypeptides produced in Cos-1 cells (lane 4) exhibited the same electrophoretic mobility as those present in A20 cells (lane 1). Besides confirming the identity of Dok-3, these findings implied that the variations in the apparent molecular mass of Dok-3 in A20 cells were not caused by alternative splicing of the dok-3 gene, but rather by alternative translation initiation or posttranslational modification of the Dok-3 protein.
FIG. 3.
Impact of immunoreceptor stimulation on Dok-3 tyrosine phosphorylation. (A) Identification of the Dok-3 protein. The presence of Dok-3 in A20 B-cells and transfected Cos-1 cells was detected by anti-Dok-3 immunoblotting of the indicated immunoprecipitates. The positions of the two forms of Dok-3 are indicated on the left, while those of prestained molecular mass markers are shown on the right. Exposure: 3 h. NRS, normal rabbit serum. (B) Effect of cellular activation on Dok-3 tyrosine phosphorylation. A20 B cells (2 × 107 cells) were activated by incubation for 2.5 min with F(ab′)2 fragments of SAM IgG, while J774A macrophages (1.5 × 107 cells) were activated by stimulation for 2 min with mouse IgG2a (MAb 7G7) and F(ab′)2 fragments of SAM IgG. The positions of Dok-3 and p145 are indicated on the left; those of prestained molecular weight markers are shown on the right. Exposures: top panel, 24 h (lanes 1 to 8) and 48 h (lanes 9 to 12); bottom panel, 14 h. IP, immunoprecipitation. (C) Effect of FcγRIIB coaggregation on BCR-induced tyrosine phosphorylation of Dok-3. A20 cells were treated as for panel B, except that either F(ab′)2 fragments of SAM IgG (lane 2) or intact SAM IgG (Ab; lane 3) was used for stimulation. The locations of Dok-3 and p145 are shown on the left, whereas those of prestained molecular mass markers are indicated on the right. Exposure: 22 h. (D) Differential tyrosine phosphorylation of Dok-3 by various protein tyrosine kinases. Cos-1 cells were transiently transfected with the indicated cDNAs in the presence of a Myc-tagged version of Dok-3. Adequate expression of the PTKs was documented by parallel immunoblotting of total cell lysates with antibodies against these various molecules (data not shown). The migration of Dok-3-Myc is shown on the left. Exposures: top panel, 4 h; bottom panel, 16 h.
In light of the expression pattern and adapter-like features of Dok-3, we wanted to examine whether this protein might be involved in immunoreceptor signaling (Fig. 3B). To assess this possibility, IgG+ A20 B cells were activated via the BCR with F(ab′)2 fragments of SAM IgG. Additionally, J774A macrophages were triggered through the high-affinity Fc receptor for IgG (FcγRI) using mouse IgG2a followed by F(ab′)2 fragments of anti-mouse IgG. After stimulation, cells were lysed and Dok-3 polypeptides were immunoprecipitated with anti-Dok-3 antibodies. Their phosphotyrosine content was then determined by immunoblotting with anti-phosphotyrosine antibodies (Fig. 3B, top panel). This assay demonstrated that Dok-3 underwent rapid tyrosine phosphorylation (within 2 min) in response to engagement of either BCR (lane 2) or FcγRI (lane 6). In addition, we could observe that immunoreceptor stimulation provoked the appearance of a ∼145-kDa phosphotyrosine-containing polypeptide in Dok-3 immunoprecipitates (lanes 2 and 6) but not in immunoprecipitates obtained with normal rabbit serum (lanes 4 and 8). The nature of this molecule will be addressed below. Lastly, it was evident that activation of A20 B cells, but not of J774A cells, caused a striking retardation in the electrophoretic mobility of Dok-3 (bottom panel; compare lanes 1 and 2). Since this modification persisted at later time points after the disappearance of Dok-3 tyrosine phosphorylation (data not shown), it may be caused by a supplementary alteration of Dok-3 such as serine or threonine phosphorylation.
Previous studies have shown that some of the tyrosine phosphorylation events in activated B cells are augmented upon coaggregation of the BCR with the inhibitory receptor FcγRIIB (9, 41, 65). To assess whether FcγRIIB engagement modulated the extent of Dok-3 tyrosine phosphorylation, A20 cells were stimulated either with F(ab′)2 fragments of SAM IgG or with intact SAM IgG (Fig. 3C). This analysis demonstrated that no marked difference in the extent of tyrosine phosphorylation of Dok-3 existed between the two stimuli (compare lanes 2 and 3).
Two classes of cytoplasmic PTKs, the Src and Syk/Zap-70 families, have been implicated in immunoreceptor-mediated protein tyrosine phosphorylation (11, 15, 59, 63). To discriminate which one(s) may be responsible for Dok-3 tyrosine phosphorylation during B-cell and macrophage activation, the ability of PTKs to provoke Dok-3 tyrosine phosphorylation was examined in a heterologous system (Fig. 3D). A Myc-tagged version of Dok-3 (Dok-3-Myc) was transiently expressed in Cos-1 cells in the presence of various PTKs, and the tyrosine phosphorylation of Dok-3 was monitored by anti-phosphotyrosine immunoblotting of anti-Myc immunoprecipitates (top panel). While this approach has inherent limitations, it can provide useful clues regarding the identity of the PTKs responsible for substrate phosphorylation in more physiological conditions. We found that the Src-related enzymes Lck (lane 2), FynT (lane 3), and Lyn (lane 4) provoked easily detectable tyrosine phosphorylation of Dok-3 in this system. In comparison, Syk (lane 5) and Zap-70 (lane 6) had no effect. Tyrosine phosphorylation of Dok-3 could also be induced by Bcr-Abl (lane 7), an activated version of the Abl protein tyrosine kinase involved in human leukemias. Bcr-Abl has been shown previously to induce tyrosine phosphorylation of Dok and Dok-2 (8, 25, 30, 67, 69). In conclusion, the results shown in Fig. 3 indicated that Dok-3 underwent tyrosine phosphorylation in response to immunoreceptor stimulation. Moreover, they indicated that, at least in a heterologous system, this phosphorylation could be caused by Src family kinases.
Immunoreceptor stimulation induces the association of Dok-3 with the inhibitory molecules SHIP and Csk, but not with Ras-GAP.
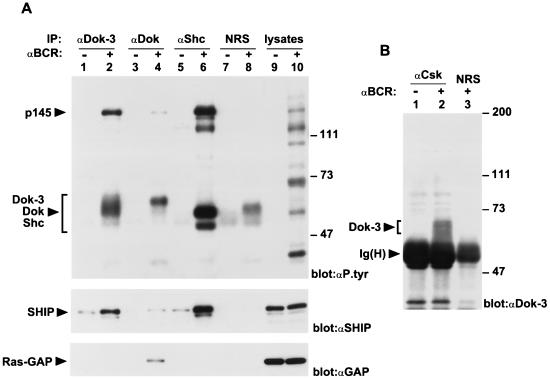
To help establish the possible role of Dok-3 in immunoreceptor signaling, we sought to identify the molecules with which it interacted. As shown above, BCR stimulation induced the association of Dok-3 with a tyrosine-phosphorylated molecule of ∼145 kDa. Considering this finding, we wanted to determine whether the 145-kDa polypeptide was SHIP, an SH2 domain-containing 5′ inositol phosphatase known to undergo tyrosine phosphorylation in response to B-cell activation (9, 22, 37). To test this possibility, anti-Dok-3 immunoprecipitates from resting and activated A20 cells were immunoblotted with anti-SHIP antibodies (Fig. 4A, middle panel). This study indicated that SHIP became associated with Dok-3 in BCR-stimulated A20 B cells (lane 2). A much smaller extent of association existed prior to BCR stimulation (lane 1). Interestingly, we also found that SHIP underwent association with the related adapter Dok in response to B-cell activation (lane 4), albeit in smaller amounts. In agreement with earlier reports (9, 53), SHIP was also complexed to the adapter Shc (lane 6).
FIG. 4.
Interaction of Dok-3 with inhibitory molecules in activated A20 B cells. (A) Association of Dok-3 with SHIP, but not Ras-GAP. A20 B cells were stimulated as described in the legend to Fig. 3B. The ability of Dok-3 to associate with SHIP and Ras-GAP was compared with those of Dok and Shc. The migrations of Dok-3, Dok, Shc, SHIP, and Ras-GAP are indicated on the left; the positions of prestained molecular weight markers are shown on the right. Exposure: 36 h. IP, immunoprecipitation. (B) Association of Dok-3 with Csk. Cells were stimulated as for Fig. 3B, except that lysates from 5 × 107 cells were used for immunoprecipitation. The positions of Dok-3 and the heavy chain of IgG [Ig(H)] are shown on the left, while those of prestained molecular mass markers are indicated on the right. The presence of Dok-3 was revealed by enhanced chemiluminescence. Exposure: 7 s. NRS, normal rabbit serum.
Next, we wished to assess whether Dok-3 was associated with Ras-GAP, a negative regulator of Ras reported to bind to Dok and Dok-2 (25, 44, 67, 69). To this end, the immunoblot membrane was stripped and probed with antibodies directed against Ras-GAP (Fig. 4A, bottom panel). This analysis indicated that, unlike Dok (lane 4), the Dok-3 protein (lane 2) did not associate with Ras-GAP in activated A20 cells. Likewise, Ras-GAP did not form a complex with Shc (lane 6). The inability of Dok-3 to bind Ras-GAP was also demonstrated in transiently transfected Cos-1 cells (data not shown).
As indicated above, we first identified Dok-3 through its capacity to interact with Csk in the yeast two-hybrid system (see Materials and Methods) (unpublished data). This association involved the carboxy-terminal region of Dok-3 (Fig. 1A) and required the coexpression of a Src-related enzyme in the yeast, suggesting that it was mediated by Dok-3 tyrosine phosphorylation. Based on these findings, the ability of Dok-3 to associate with Csk in A20 cells was examined (Fig. 4B). Cells were activated with F(ab′)2 fragments of IgG as detailed above, and the association between Dok-3 and Csk was ascertained by anti-Dok-3 immunoblotting of anti-Csk immunoprecipitates. We found that detectable amounts of Dok-3 could be immunoprecipitated with Csk in activated A20 cells (lane 2) but not in unstimulated cells (lane 1). No Dok-3 was found in immunoprecipitates obtained with normal rabbit serum (lane 3). Taken together, the results of these studies showed that Dok-3 became associated with SHIP and Csk, but not with Ras-GAP, during B-cell activation.
Mechanisms of interaction of Dok-3 with SHIP and Csk.
We wanted to elucidate the mechanism of the association between Dok-3 and SHIP (Fig. 5A and B). Sequence analysis of SHIP has revealed that it possesses an amino-terminal SH2 region, a central lipid phosphatase domain, and a long carboxy-terminal region bearing several sites of tyrosine phosphorylation (22, 37). Because the Dok-3–SHIP interaction was induced by BCR stimulation, it appeared likely that tyrosine phosphorylation of Dok-3 and/or SHIP was the trigger for this association. Possibly, one or more sites of tyrosine phosphorylation on Dok-3 contacted the SH2 domain of SHIP. Alternatively, the PTB domain of Dok-3 could associate with tyrosine-phosphorylated residues on SHIP. Finally, both mechanisms could be involved in the association, as previously documented for the Shc-SHIP interaction (53).
FIG. 5.
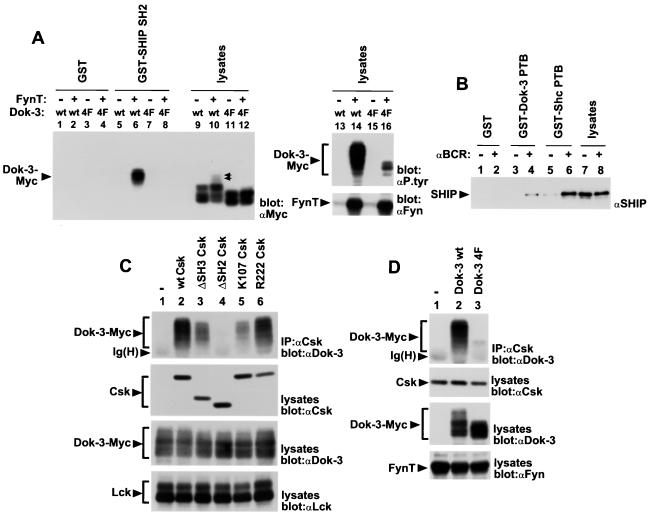
Structural basis for Dok-3–SHIP and Dok-3–Csk interactions. (A) Binding of the SH2 domain of SHIP to Dok-3. The capacity of various fusion proteins to interact in vitro with Myc-tagged variants of Dok-3 from transiently transfected Cos-1 cells was determined. It is noticeable that the electrophoretic mobility of Dok-3 4F (lanes 11 and 12) was faster than that of the wild-type protein (lanes 9 and 10), even in the absence of measurable tyrosine phosphorylation. The exact basis for this difference is not known. Furthermore, the wild-type Dok-3 found to associate with GST-SHIP SH2 comigrated with species of Dok-3 having further retardation in their electrophoretic mobility (indicated by a double arrowhead). The positions of Dok-3-Myc and FynT are indicated on the left. Exposure: 16 h. (B) Interaction of the PTB domain of Dok-3 with SHIP. The ability of various fusion proteins to associate with SHIP from lysates of A20 B cells was examined. The migration of SHIP is shown on the left. Exposure: 36 h. (C) Importance of various domains of Csk for association with Dok-3. The ability of various Csk mutants to bind a Myc-tagged version of Dok-3 was determined by transient transfection in Cos-1 cells. To avoid any interference from Csk, a constitutively activated version of Lck (tyrosine 505-to-phenylalanine 505 Lck) was used to provoke Dok-3 tyrosine phosphorylation. The positions of Dok-3-Myc, Csk, Lck, and the heavy chain of IgG [Ig(H)] are indicated on the left. Exposures (from top to bottom): first panel, 12 h; second panel, 5 h; third panel, 4 h; fourth panel, 12 h. (D) Importance of carboxy-terminal tyrosines of Dok-3 for the interaction with Csk. The capacity of Myc-tagged variants of Dok-3 to associate with Csk was ascertained in transiently transfected Cos-1 cells. To avoid interfering with the activity of FynT, a kinase-inactive version of Csk (lysine 222-to-arginine 222 Csk) was used in this assay. The migrations of Dok-3-Myc, Csk, FynT, and the heavy chain of IgG [Ig(H)] are indicated on the left. Exposures: top three panels, 2.5 h; bottom panel, 16 h.
To address the first possibility, the capacity of the SH2 domain of SHIP to associate with Dok-3 was examined in an in vitro binding assay (Fig. 5A). Myc-tagged versions of either wild-type Dok-3 (Dok-3 wt) or a Dok-3 mutant in which all four tyrosines in the carboxy-terminal region were replaced by phenylalanines (Dok-3 4F) were expressed in Cos-1 cells in the presence or absence of the Src-related enzyme FynT. Cell lysates were then incubated with immobilized GST fusion proteins encompassing the SH2 region of SHIP (GST-SHIP SH2), and bound Dok-3 molecules were detected by immunoblotting with anti-Myc MAb 9E10 (Fig. 5A, lanes 1 to 8). This experiment revealed that the SH2 domain of SHIP associated with wild-type Dok-3 (lane 6), but not with Dok-3 4F (lane 8), in the presence of FynT. No interaction occurred in cells lacking FynT (lanes 5 and 7), or when GST alone was used (lanes 1 to 4). A parallel anti-phosphotyrosine immunoblot of total-cell lysates (lanes 13 to 16, top panel) demonstrated that, in the presence of FynT, the phosphotyrosine content of Dok-3 4F (lane 16) was much lower than that of Dok-3 wt (lane 14). This observation was consistent with the idea that the major site(s) of tyrosine phosphorylation of Dok-3 in vivo was positioned in its carboxy-terminal domain. Obviously, though, phosphopeptide analysis will be required in order to identify these sites more formally.
The ability of the PTB region of Dok-3 to bind SHIP was also investigated (Fig. 5B). For this purpose, an immobilized fusion protein containing the PTB domain of Dok-3 (GST–Dok-3 PTB) was incubated with lysates from either resting or activated A20 B cells, and the associated proteins were probed by immunoblotting with anti-SHIP antibodies. This analysis indicated that the PTB domain of Dok-3 could interact with SHIP from lysates of activated B cells (lane 4), but not unstimulated cells (lane 3). Similar results were obtained with the Shc PTB region (lanes 5 and 6), although, in this case, some association with SHIP existed in the absence of BCR stimulation (lane 5). Combined with the findings of Fig. 5A, these results indicated that Dok-3 had the capacity to bind SHIP through at least two distinct mechanisms. While we attempted to substantiate this concept further by transient expression of Dok-3 and SHIP in Cos-1 cells, we were unfortunately not able to reconstitute SHIP tyrosine phosphorylation in this system (data not shown). The reasons for this limitation are currently being examined.
Next, the structural basis for the interaction between Dok-3 and Csk was evaluated (Fig. 5C and D). Csk is a cytosolic protein tyrosine kinase that contains, from the amino to the carboxy terminus, an SH3 domain, an SH2 region, and a catalytic domain (42). To define which domain(s) was involved in contacting Dok-3, various Csk mutants were coexpressed with Dok-3 in Cos-1 cells, together with a Src family kinase (Lck) to allow Dok-3 tyrosine phosphorylation. The capacity of Csk to bind Dok-3 was monitored by anti-Dok-3 immunoblotting of Csk immunoprecipitates (Fig. 5C, top panel). Under these conditions, wild-type Csk (lane 2) and kinase-inactive Csk (lane 6) efficiently associated with Dok-3. In contrast, a mutant bearing a complete deletion of the SH2 domain (ΔSH2 Csk; lane 4) was unable to interact with Dok-3. A Csk variant carrying a point mutation of a critical arginine in the SH2 domain (arginine 107 to lysine 107 [K107] Csk; lane 5) also showed markedly reduced interaction with Dok-3. However, residual binding could be observed, in keeping with our earlier observation that this point mutation did not fully abolish the ability of the Csk SH2 region to interact with phosphotyrosine-containing proteins in vitro (17). It is interesting that a Csk mutant lacking the SH3 region (ΔSH3 Csk; lane 3) also exhibited partially diminished binding to Dok-3. Whereas this finding did indicate that the Csk SH3 domain was not absolutely essential for the interaction, it suggested that the SH3 region may have a modulatory role. In support of this idea, we and others have reported that the SH3 domain of Csk enhanced the ability of its SH2 region to bind a subset of tyrosine-phosphorylated proteins in vitro (17, 57).
In order to identify the requirements in Dok-3 for its association with Csk, Csk was introduced in Cos-1 cells with either wild-type Dok-3 or Dok-3 4F, in addition to the Src-related kinase FynT (Fig. 5D). The ability of the two molecules to form a complex was subsequently tested as detailed for Fig. 5C. The results of this experiment showed that, in comparison to wild-type Dok-3 (top panel, lane 2), the Dok-3 4F mutant (lane 3) had a dramatically reduced potential to associate with Csk. Together with the results of Fig. 5C, these data suggested that the Dok-3–Csk association was mediated by one or more tyrosine-phosphorylated residues in the carboxy-terminal domain of Dok-3 and by the SH2 domain of Csk.
Overexpression of Dok-3 inhibits immunoreceptor signaling.
The aptitude of Dok-3 to recruit two inhibitory molecules (SHIP and Csk) in response to immunoreceptor stimulation raised the possibility that it may have an inhibitory role in this process. To test this idea, the impact of enforced expression of Dok-3 on BCR-mediated signal transduction was assessed (Fig. 6 to 8). First, A20 B cells were stably transfected by electroporation with cDNA constructs encoding either wild-type Dok-3 or Dok-3 4F. Monoclonal cell lines overexpressing Dok-3 were selected by growth in medium containing G418 and were identified by immunoblotting of total cell lysates with anti-Dok-3 antibodies (data not shown). Derivatives expressing neomycin phosphotransferase alone (Neo) were also used, as controls. All cell lines chosen for subsequent experiments expressed unaltered levels of BCR, FcγRIIB, CD45, and CD40 (data not shown).
FIG. 6.
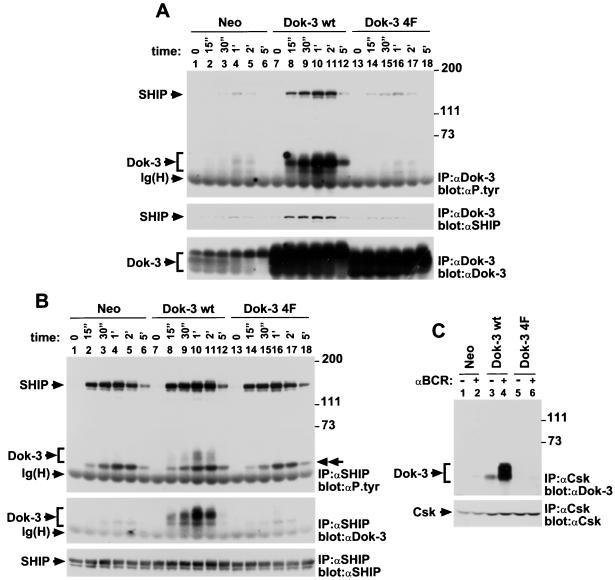
Biochemical consequences of Dok-3 overexpression in A20 B cells. (A) Effect of Dok-3 overexpression on recruitment of SHIP. A20 transfectants (1.5 × 107 cells) were stimulated for the indicated periods of time with F(ab′)2 fragments of SAM IgG. The migrations of SHIP, Dok-3, and the heavy chain of IgG [Ig(H)] are shown on the left, whereas the positions of prestained molecular mass markers are indicated on the right. Exposures: top panel, 16 h; middle panel, 2 h; bottom panel, 7 h. (B) Impact of Dok-3 overexpression on overall SHIP tyrosine phosphorylation. Cells were activated as described for panel A. The migration of the ∼52-kDa SHIP-associated protein likely to represent Shc is indicated by a double arrowhead on the right. The positions of SHIP, Dok-3, and the heavy chain of IgG [Ig(H)] are shown on the left, whereas those of prestained molecular mass markers are indicated on the right. Exposures: top and middle panels, 16 h; bottom panel, 2 h. (C) Effect of Dok-3 overexpression on Csk recruitment. Derivatives of A20 (5 × 107 cells) were activated for 2 min with F(ab′)2 fragments of SAM IgG. The migrations of Dok-3 and Csk are shown on the left; positions of prestained molecular mass markers are indicated on the right. The presence of Dok-3 was detected by enhanced chemiluminescence. Exposures: top panel, 1.5 min; bottom panel, 3 h.
FIG. 8.
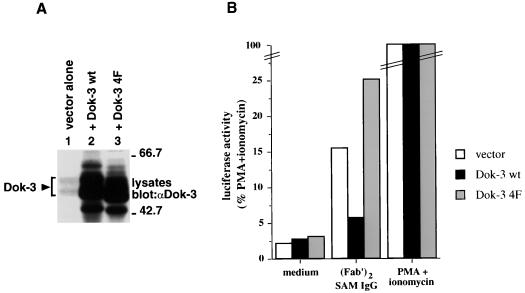
Transient transfection assays. A20 cells were transiently transfected with the indicated cDNAs (5 μg), in the presence of pNFAT-luciferase (20 μg). After 40 h, they were stimulated for 6 h with F(ab′)2 fragments of SAM IgG or the combination of PMA plus ionomycin. (A) Expression levels of Dok-3. Transfected cells were lysed in boiling sample buffer, and the abundance of Dok-3 was measured by anti-Dok-3 immunoblotting of total cell lysates. The migration of Dok-3 is indicated on the left, whereas positions of prestained molecular mass markers are shown on the right. Exposure: 3 h. (B) Luciferase assays. Luciferase activity was measured as described in Materials and Methods. Values are expressed as percentages of maximal stimulation (PMA plus ionomycin). These results are representative of three independent experiments (data not shown).
The influence of Dok-3 overexpression on SHIP recruitment was first evaluated (Fig. 6A). Representative clones expressing Dok-3 levels (bottom panel) that were ∼10 times higher than those in Neo cells were stimulated for the indicated periods of time with F(ab′)2 fragments of SAM IgG. After cell lysis, Dok-3 was recovered by immunoprecipitation with anti-Dok-3 antibodies and probed by immunoblotting with either anti-phosphotyrosine (top panel), anti-SHIP (middle panel), or anti-Dok-3 (bottom panel) antibodies. We found that cells overexpressing wild-type Dok-3 (top panel, lanes 7 to 12) exhibited a marked increase in BCR-induced tyrosine phosphorylation of Dok-3 and Dok-3-bound SHIP, compared to cells expressing the neomycin resistance marker alone (lanes 1 to 6). In contrast, cells containing Dok-3 4F (lanes 13 to 18) displayed no augmentation of Dok-3 tyrosine phosphorylation, even though they expressed comparably elevated amounts of Dok-3 protein (bottom panel; compare lanes 13 to 18 with lanes 7 to 12). They also exhibited a much lower increase in tyrosine phosphorylation of Dok-3-associated SHIP (2-fold), in comparison to transfectants overexpressing wild-type Dok-3 (7-fold). The anti-SHIP immunoblot (middle panel) confirmed that expression of Dok-3 wt (lanes 7 to 12) caused an increase in the quantity of SHIP recruited by Dok-3. Little or no increment was observed in cells containing Dok-3 4F (lanes 13 to 18).
Given these results, we wanted to ascertain whether Dok-3 overexpression influenced the overall tyrosine phosphorylation of SHIP in the cell (Fig. 6B). A20 cells were stimulated as outlined for Fig. 6A, and lysates were immunoprecipitated with anti-SHIP antibodies. A subsequent anti-phosphotyrosine immunoblot (top panel) revealed that the increased Dok-3 levels had only a small (∼1.5-fold), albeit reproducible, effect on total SHIP tyrosine phosphorylation. Moreover, it did not affect the ability of SHIP to associate with a ∼52-kDa tyrosine-phosphorylated polypeptide likely to represent Shc (9). As expected, reprobing of the immunoblot membrane with anti-Dok-3 antibodies (middle panel) indicated that SHIP immunoprecipitates contained increased quantities of Dok-3 in cells expressing wild-type Dok-3 (lanes 7 to 12), but not Dok-3 4F (lanes 13 to 18).
The effect of Dok-3 overexpression on the recruitment of Csk was also examined (Fig. 6C). Cells were stimulated for 2 min with SAM F(ab′)2 fragments, and Csk was recovered from cell lysates by immunoprecipitation. Its interaction with Dok-3 was monitored by anti-Dok-3 immunoblotting (top panel). This experiment demonstrated that enhanced expression of wild-type Dok-3 caused a marked increase in the extent of association between Dok-3 and Csk in activated A20 cells (compare lane 4 with lane 2). In contrast, introduction of Dok-3 4F (lane 6) had no appreciable effect.
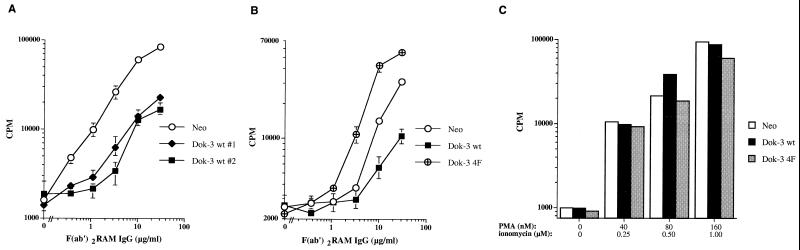
Previous studies have shown that A20 cells secrete significant quantities of IL-2 in response to BCR stimulation (41). Hence, to address the functional impact of Dok-3 on B-cell activation, the influence of its overexpression on IL-2 secretion was characterized (Fig. 7). Pools of A20 derivatives expressing the neomycin resistance marker alone (Neo), wild-type Dok-3, or Dok-3 4F were stimulated with increasing concentrations of F(ab′)2 fragments of RAM IgG. After 24 h, supernatants were recovered and assayed for IL-2 production using a bioassay. This study revealed that A20 cells overexpressing wild-type Dok-3 (Fig. 7A) exhibited a clear reduction in BCR-triggered cytokine production. This effect was observed in the two pools of Dok-3-overexpressing cells tested, and at all concentrations of anti-BCR antibodies. In contrast to cells overexpressing wild-type Dok-3, however, derivatives containing Dok-3 4F demonstrated augmented BCR-induced IL-2 secretion (Fig. 7B). Similar results were obtained when individual clones, and not pools of clones, were tested in these assays (data not shown). To ensure that these effects were not due to clonal variation, the impact of stimulation of these transfectants with PMA plus ionomycin, two pharmacological agents bypassing the proximal stages of BCR signaling, was ascertained (Fig. 7C). This experiment showed that the pools of transfectants expressing wild-type Dok-3 or Dok-3 4F responded to PMA and ionomycin in a manner comparable to the Neo cells, at all concentrations tested.
FIG. 7.
Functional consequences of stable Dok-3 overexpression in A20 B cells. (A and B) Pools of at least three independent stable transfectants of each group were activated for 24 h with the indicated concentrations of F(ab′)2 fragments of RAM IgG. IL-2 release was measured in a standard bioassay, using the HT-2 indicator cell line. Standard deviations are indicated by error bars. (C) Cells were stimulated for 24 h with the indicated concentrations of PMA plus ionomycin. IL-2 release was subsequently measured as described in the legend for panels A and B.
Lastly, the impact of Dok-3 on BCR signaling was examined in transient transfection assays (Fig. 8). A20 cells were transfected by electroporation with pXM139 alone or pXM139 variants bearing the dok-3 cDNAs, in combination with the plasmid pNFAT-luciferase, which contains three copies of an NFAT-binding site linked to a luciferase reporter. After 40 h, cells were stimulated for 6 h with F(ab′)2 fragments of SAM IgG and assayed for luciferase activity, as detailed in Materials and Methods (Fig. 8B). This analysis revealed that transient overexpression of wild-type Dok-3 inhibited (∼2.5- to 3-fold) BCR-induced NFAT activation in A20 cells. By comparison, introduction of Dok-3 4F caused an increase (∼1.5- to 2-fold) in NFAT activity. Importantly, both wild-type Dok-3 and Dok-3 4F were expressed in comparable quantities in these transfectants (Fig. 8A; compare lanes 2 and 3). In addition to confirming the results obtained with the stable transfections, these data indicated that Dok-3 inhibited BCR signaling by acting at a level proximal to activation of the transcription factor NFAT.
DISCUSSION
In this paper, we report the identification of a novel adapter molecule belonging to the Dok family, which we termed Dok-3. RNase protection assays showed that dok-3 is widely expressed in hematopoietic cells, including B cells, macrophages, and myeloid cells, but not in T cells. The expression pattern of dok-3 is clearly different from those of dok and dok-2. While the two previously known dok-related molecules also abound in cells of hematopoietic lineages, it is noteworthy that, unlike dok-3, both dok and dok-2 accumulate widely in T cells. Moreover, in contrast to dok-3, dok-2 is not contained in B cells (this report) (44). Given these differences, it appears likely that the three Dok-related proteins serve distinct purposes in hematopoietic cells.
In order to address the role of Dok-3 in hematopoietic cells, its regulation during immunoreceptor-mediated signal transduction was examined. These studies showed that engagement of BCR on B cells or FcγRI on macrophages provoked a rapid increase in Dok-3 tyrosine phosphorylation. Through site-directed mutagenesis, indirect evidence was adduced that this phosphorylation principally occurred at one or more of four tyrosines in the carboxy-terminal region of Dok-3, i.e., tyrosines 325 (YASV), 343 (YENV), 378 (YHNT), and 399 (YRRL). Interestingly, none of these tyrosines is contained within a YXXP motif, which was found by others to mediate the binding of Dok and Dok-2 to the SH2 domain of Ras-GAP (40, 45). In agreement with this finding, we were not able to observe any interaction between Dok-3 and Ras-GAP in either activated B cells (this report) or transiently transfected Cos-1 cells (data not shown).
Instead, we found that immunoreceptor stimulation on B cells and macrophages triggered the association of Dok-3 with a 145-kDa tyrosine-phosphorylated protein. Subsequent studies revealed that this polypeptide was SHIP, a 5′ inositol phosphatase implicated in the negative regulation of immunoreceptor signaling (9, 31, 33, 38, 46, 47). Using in vitro binding analyses, it was shown that this interaction could occur through two distinct mechanisms, involving putative sites of tyrosine phosphorylation on Dok-3 and the SHIP SH2 domain on the one hand, and the PTB domain of Dok-3 and tyrosine-phosphorylated residues on SHIP on the other hand. A similar “bidentate” mode of association was proposed to link the adapter Shc to SHIP (53). However, it should be pointed out that mutation of the four carboxy-terminal tyrosines of Dok-3 essentially abolished the ability to bind SHIP in activated A20 B cells. Therefore, it is clear that the PTB region of Dok-3 was insufficient to allow a stable association between the two molecules and that tyrosine phosphorylation of Dok-3 was probably necessary to induce this interaction.
Immunoreceptor stimulation also induced the association of Dok-3 with Csk, a PTK involved in the negative regulation of Src family kinases (15). Structure-function analyses performed in Cos-1 cells indicated that this interaction was mediated by the carboxy-terminal tyrosines of Dok-3 and by the SH2 domain of Csk. This idea was further supported by the finding that overexpression of wild-type Dok-3, but not Dok-3 4F, increased the extent of association of Csk with Dok-3 in BCR-stimulated A20 cells. We wish to mention, though, that larger quantities of cellular protein were needed for immunoprecipitation, and enhanced chemiluminescence had to be used, in order to reveal the interaction between Dok-3 and Csk. Whereas the exact significance of this observation is unclear, it may be argued that tyrosine-phosphorylated Dok-3 had a greater affinity for SHIP than for Csk, perhaps as a result of the ability of Dok-3 and SHIP to associate through two distinct interactions.
Considering the capacity of Dok-3 to recruit at least two inhibitory molecules (SHIP and Csk) in response to immunoreceptor stimulation, its functional impact on cellular activation was ascertained. Transfection experiments in A20 B cells demonstrated that increased expression of wild-type Dok-3 provoked inhibition of BCR-mediated NFAT activation and IL-2 secretion. In contrast, expression of comparable amounts of Dok-3 4F caused an enhancement of antigen receptor-induced NFAT stimulation and cytokine release. Since Dok-3 4F was defective in the ability to bind SHIP and Csk, it is likely that this impact was due to interference with the function of endogenous Dok-3 molecules (a “dominant-negative” effect). Thus, in combination, these studies supported the idea that Dok-3 may be a negative regulator of BCR signaling and that this function may relate to its ability to associate with SHIP and Csk.
It should be pointed that Dok-3 overexpression in A20 B cells did not cause a global reduction of BCR-induced protein tyrosine phosphorylation (data not shown). Furthermore, it had no appreciable impact on BCR-induced calcium fluxes, mitogen-activated protein kinase activation, or Akt activation (our unpublished results). Obviously, these observations raised the possibility that Dok-3 mediated its inhibitory impact by a mechanism distinct from recruitment of SHIP and Csk. It is conceivable that other types of negative regulators were recruited by tyrosine-phosphorylated Dok-3. At this time, however, we have been unable to obtain any evidence to support this possibility. Alternatively, it is plausible that Dok-3–SHIP and Dok-3–Csk acted on limited pools of potential intracellular targets in activated cells, presumably those that colocalized with Dok-3. While we favor the latter possibility, the currently available technologies unfortunately limit further assessment of this idea.
Recently, there has been significant interest in understanding the mechanisms involved in the negative regulation of hematopoietic-cell activation. In particular, the importance of SHIP in this process has been the object of intense investigation. This lipid phosphatase has been shown to be a potent inhibitor of cellular activation, through its capacity to reduce the levels of phosphatidylinositol 3,4,5-triphosphate and to prevent the activation of PH domain-containing kinases such as Btk and Akt (2, 4, 39). Most notably, SHIP has been demonstrated to be largely responsible for the inhibitory impact of FcγRIIB in B cells (21, 46–48). Nevertheless, it is also clear that SHIP has an inhibitory role in hematopoietic cells independently from its interaction with FcγRIIB. This idea is especially supported by the observation that SHIP-deficient B cells exhibited enhanced BCR-induced responses even in the absence of FcγRIIB expression (31, 46). While the precise mechanism of regulation of SHIP in this context was not determined, our data suggested that molecules such as Dok-3, Dok, and perhaps Shc may carry out this function.
Based on the currently available data, the following model may be proposed. Following immunoreceptor stimulation, the Src and Syk/Zap-70 families of PTKs induce a protein tyrosine phosphorylation signal that ultimately leads to cellular activation. In response to this signal, Dok-3 is translocated near Src-related kinases, perhaps through an interaction between its PH domain and plasma membrane-associated inositol phospholipids (55). Consequently, Src family kinases induce tyrosine phosphorylation of Dok-3 within its carboxy-terminal domain, which allows for the recruitment of SHIP and Csk via their SH2 domains. The association between Dok-3 and SHIP may be further secured by an interaction involving the PTB domain of Dok-3 and tyrosine-phosphorylated residues on SHIP. Following their recruitment, SHIP and Csk inhibit positive effectors of cell signaling, thereby restricting the duration and/or intensity of cellular activation.
The Dok family was first uncovered as a consequence of the ability of its members to be phosphorylated by several mitogenic and oncogenic PTKs (26). On this basis, it was proposed that Dok-related molecules may be positive effectors of PTK signaling. However, it is striking that these polypeptides have a propensity to associate with inhibitory molecules. We found that Dok-3 associates with SHIP and Csk. Furthermore, others have reported that Dok binds to Ras-GAP and Csk, while Dok-2 interacts with Ras-GAP (8, 26, 43, 69). Our data show that Dok and Dok-2 can also form complexes with SHIP (this report and our unpublished results). Consequently, Dok-related adapters may actually be involved in inhibitory signaling. Our observation that Dok-3 is a negative regulator of B-cell activation is in agreement with this concept. Similarly, others have reported that Dok-2 could inhibit cytokine receptor signaling in T cells and myeloid cells (44). This notion does not exclude the possibility that Dok-related polypeptides can have a positive regulatory role under certain conditions, especially since Dok family members can associate with adapters involved in positive signaling, such as Nck and CrkL (3, 32, 40, 45, 60). In support of this idea, it was found that Dok overexpression in Chinese hamster ovary cells enhanced cellular migration in response to insulin (45). Clearly, more studies will be needed to understand fully the functions of this unique class of adapter molecules.
In summary, we have identified a novel member of the Dok family, which we have named Dok-3. Dok-3 is highly expressed in several hematopoietic cell types including B cells and macrophages. Our studies showed that Dok-3 rapidly becomes tyrosine phosphorylated and associates with the inhibitory molecules SHIP and Csk following immunoreceptor stimulation. Transfection experiments in a B-cell line also indicated that Dok-3 can inhibit immunoreceptor signaling and that this function correlates with its capacity to recruit SHIP and Csk. Together, these findings suggest that Dok-3 plays a significant role in the negative regulation of immunoreceptor signaling in several hematopoietic cell types. Moreover, they provide evidence that Dok-related molecules act as “inhibitory” adapters during cell signaling through their capacity to orchestrate the recruitment of effectors like SHIP, Csk, and/or Ras-GAP.
ACKNOWLEDGMENTS
We thank Gerry Krystal, Yuji Yamanashi, David Baltimore, Pierre Laneuville, Gerry Crabtree, and Lou Matis for their gifts of reagents. We also acknowledge A. Chan for advice regarding the transient transfection assays.
This work was funded by grants from the Medical Research Council of Canada and the National Cancer Institute of Canada to A.V. S. Lemay was supported by a Fellowship from the Kidney Foundation of Canada and by a Joseph Kaufmann Fellowship from the Faculty of Medicine, McGill University, while S. Latour was awarded a Fellowship from the Medical Research Council of Canada. A.V. is a Senior Scientist of the Medical Research Council of Canada.
S. Lemay and D. Davidson contributed equally to this work.
REFERENCES
- 1.Abraham N, Miceli M C, Parnes J R, Veillette A. Enhancement of T-cell responsiveness by the lymphocyte-specific tyrosine protein kinase p56lck. Nature. 1991;350:62–66. doi: 10.1038/350062a0. [DOI] [PubMed] [Google Scholar]
- 2.Aman M J, Lamkin T D, Okada H, Kurosaki T, Ravichandran K S. The inositol phosphatase SHIP inhibits Akt/PKB activation in B cells. J Biol Chem. 1998;273:33922–33928. doi: 10.1074/jbc.273.51.33922. [DOI] [PubMed] [Google Scholar]
- 3.Bhat A, Johnson K J, Oda T, Corbin A S, Druker B J. Interactions of p62(dok) with p210(bcr-abl) and Bcr-Abl-associated proteins. J Biol Chem. 1998;273:32360–32368. doi: 10.1074/jbc.273.48.32360. [DOI] [PubMed] [Google Scholar]
- 4.Bolland S, Pearse R N, Kurosaki T, Ravetch J V. SHIP modulates immune receptor responses by regulating membrane association of Btk. Immunity. 1998;8:509–516. doi: 10.1016/s1074-7613(00)80555-5. [DOI] [PubMed] [Google Scholar]
- 5.Bolland S, Ravetch J V. Inhibitory pathways triggered by ITIM-containing receptors. Adv Immunol. 1999;72:149–177. doi: 10.1016/s0065-2776(08)60019-x. [DOI] [PubMed] [Google Scholar]
- 6.Cambier J C. Antigen and Fc receptor signaling. The awesome power of the immunoreceptor tyrosine-based activation motif (ITAM) J Immunol. 1995;155:3281–3285. [PubMed] [Google Scholar]
- 7.Cao M Y, Huber M, Beauchemin N, Famiglietti J, Albelda S M, Veillette A. Regulation of mouse PECAM-1 tyrosine phosphorylation by the Src and Csk families of protein-tyrosine kinases. J Biol Chem. 1998;273:15765–15772. doi: 10.1074/jbc.273.25.15765. [DOI] [PubMed] [Google Scholar]
- 8.Carpino N, Wisniewski D, Strife A, Marshak D, Kobayashi R, Stillman B, Clarkson B. p62(dok): a constitutively tyrosine-phosphorylated, GAP-associated protein in chronic myelogenous leukemia progenitor cells. Cell. 1997;88:197–204. doi: 10.1016/s0092-8674(00)81840-1. [DOI] [PubMed] [Google Scholar]
- 9.Chacko G W, Tridandapani S, Damen J E, Liu L, Krystal G, Coggeshall K M. Negative signaling in B lymphocytes induces tyrosine phosphorylation of the 145-kDa inositol polyphosphate 5-phosphatase, SHIP. J Immunol. 1996;157:2234–2238. [PubMed] [Google Scholar]
- 10.Chan A C, Desai D M, Weiss A. The role of protein tyrosine kinases and protein tyrosine phosphatases in T cell antigen receptor signal transduction. Annu Rev Immunol. 1994;12:555–592. doi: 10.1146/annurev.iy.12.040194.003011. [DOI] [PubMed] [Google Scholar]
- 11.Chan A C, Shaw A S. Regulation of antigen receptor signal transduction by protein tyrosine kinases. Curr Opin Immunol. 1996;8:394–401. doi: 10.1016/s0952-7915(96)80130-0. [DOI] [PubMed] [Google Scholar]
- 12.Chow L M, Davidson D, Fournel M, Gosselin P, Lemieux S, Lyu M S, Kozak C A, Matis L A, Veillette A. Two distinct protein isoforms are encoded by ntk, a csk-related tyrosine protein kinase gene. Oncogene. 1994;9:3437–3448. [PubMed] [Google Scholar]
- 13.Chow L M, Fournel M, Davidson D, Veillette A. Negative regulation of T-cell receptor signalling by tyrosine protein kinase p50csk. Nature. 1993;365:156–160. doi: 10.1038/365156a0. [DOI] [PubMed] [Google Scholar]
- 14.Chow L M, Ratcliffe M J, Veillette A. tkl is the avian homolog of the mammalian lck tyrosine protein kinase gene. Mol Cell Biol. 1992;12:1226–1233. doi: 10.1128/mcb.12.3.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chow L M, Veillette A. The Src and Csk families of tyrosine protein kinases in hemopoietic cells. Semin Immunol. 1995;7:207–226. doi: 10.1006/smim.1995.0026. [DOI] [PubMed] [Google Scholar]
- 16.Chu D H, Morita C T, Weiss A. The Syk family of protein tyrosine kinases in T-cell activation and development. Immunol Rev. 1998;165:167–180. doi: 10.1111/j.1600-065x.1998.tb01238.x. [DOI] [PubMed] [Google Scholar]
- 17.Cloutier J F, Chow L M, Veillette A. Requirement of the SH3 and SH2 domains for the inhibitory function of tyrosine protein kinase p50csk in T lymphocytes. Mol Cell Biol. 1995;15:5937–5944. doi: 10.1128/mcb.15.11.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cloutier J F, Veillette A. Association of inhibitory tyrosine protein kinase p50csk with protein tyrosine phosphatase PEP in T cells and other hemopoietic cells. EMBO J. 1996;15:4909–4918. [PMC free article] [PubMed] [Google Scholar]
- 19.Daeron M. Building up the family of ITIM-bearing negative coreceptors. Immunol Lett. 1996;54:73–76. doi: 10.1016/s0165-2478(96)02652-1. [DOI] [PubMed] [Google Scholar]
- 20.Daeron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 21.D'Ambrosio D, Fong D C, Cambier J C. The SHIP phosphatase becomes associated with Fc gammaRIIB1 and is tyrosine phosphorylated during ‘negative’ signaling. Immunol Lett. 1996;54:77–82. doi: 10.1016/s0165-2478(96)02653-3. [DOI] [PubMed] [Google Scholar]
- 22.Damen J E, Liu L, Rosten P, Humphries R K, Jefferson A B, Majerus P W, Krystal G. The 145-kDa protein induced to associate with Shc by multiple cytokines is an inositol tetraphosphate and phosphatidylinositol 3,4,5-triphosphate 5-phosphatase. Proc Natl Acad Sci USA. 1996;93:1689–1693. doi: 10.1073/pnas.93.4.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davidson D, Chow L M, Fournel M, Veillette A. Differential regulation of T cell antigen responsiveness by isoforms of the src-related tyrosine protein kinase p59fyn. J Exp Med. 1992;175:1483–1492. doi: 10.1084/jem.175.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davidson D, Cloutier J F, Gregorieff A, Veillette A. Inhibitory tyrosine protein kinase p50csk is associated with protein-tyrosine phosphatase PTP-PEST in hemopoietic and non-hemopoietic cells. J Biol Chem. 1997;272:23455–23462. doi: 10.1074/jbc.272.37.23455. [DOI] [PubMed] [Google Scholar]
- 25.Di Cristofano A, Carpino N, Dunant N, Friedland G, Kobayashi R, Strife A, Wisniewski D, Clarkson B, Pandolfi P P, Resh M D. Molecular cloning and characterization of p56dok-2 defines a new family of RasGAP-binding proteins. J Biol Chem. 1998;273:4827–4830. doi: 10.1074/jbc.273.9.4827. [DOI] [PubMed] [Google Scholar]
- 26.Ellis C, Moran M, McCormick F, Pawson T. Phosphorylation of GAP and GAP-associated proteins by transforming and mitogenic tyrosine kinases. Nature. 1990;343:377–381. doi: 10.1038/343377a0. [DOI] [PubMed] [Google Scholar]
- 27.Fournel M, Davidson D, Weil R, Veillette A. Association of tyrosine protein kinase Zap-70 with the protooncogene product p120c-cbl in T lymphocytes. J Exp Med. 1996;183:301–306. doi: 10.1084/jem.183.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu C, Turck C W, Kurosaki T, Chan A C. BLNK: a central linker protein in B cell activation. Immunity. 1998;9:93–103. doi: 10.1016/s1074-7613(00)80591-9. [DOI] [PubMed] [Google Scholar]
- 29.Gergely J, Pecht I, Sarmay G. Immunoreceptor tyrosine-based inhibition motif-bearing receptors regulate the immunoreceptor tyrosine-based activation motif-induced activation of immune competent cells. Immunol Lett. 1999;68:3–15. doi: 10.1016/s0165-2478(99)00024-3. [DOI] [PubMed] [Google Scholar]
- 30.Gotoh A, Miyazawa K, Ohyashiki K, Toyama K. Potential molecules implicated in downstream signaling pathways of p185BCR-ABL in Ph+ ALL involve GTPase-activating protein, phospholipase C-gamma 1, and phosphatidylinositol 3′-kinase. Leukemia. 1994;8:115–120. [PubMed] [Google Scholar]
- 31.Hashimoto A, Hirose K, Okada H, Kurosaki T, Iino M. Inhibitory modulation of B cell receptor-mediated Ca2+ mobilization by Src homology 2 domain-containing inositol 5′-phosphatase (SHIP) J Biol Chem. 1999;274:11203–11208. doi: 10.1074/jbc.274.16.11203. [DOI] [PubMed] [Google Scholar]
- 32.Holland S J, Gale N W, Gish G D, Roth R A, Songyang Z, Cantley L C, Henkemeyer M, Yancopoulos G D, Pawson T. Juxtamembrane tyrosine residues couple the Eph family receptor EphB2/Nuk to specific SH2 domain proteins in neuronal cells. EMBO J. 1997;16:3877–3888. doi: 10.1093/emboj/16.13.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huber M, Helgason C D, Damen J E, Liu L, Humphries R K, Krystal G. The Src homology 2-containing inositol phosphatase (SHIP) is the gatekeeper of mast cell degranulation. Proc Natl Acad Sci USA. 1998;95:11330–11335. doi: 10.1073/pnas.95.19.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isakov N. ITAMs: immunoregulatory scaffolds that link immunoreceptors to their intracellular signaling pathways. Receptors Channels. 1998;5:243–253. [PubMed] [Google Scholar]
- 35.Ishiai M, Kurosaki M, Pappu R, Okawa K, Ronko I, Fu C, Shibata M, Iwamatsu A, Chan A C, Kurosaki T. BLNK required for coupling Syk to PLC gamma 2 and Rac1-JNK in B cells. Immunity. 1999;10:117–125. doi: 10.1016/s1074-7613(00)80012-6. [DOI] [PubMed] [Google Scholar]
- 36.Jones N, Dumont D J. The Tek/Tie2 receptor signals through a novel Dok-related docking protein, Dok-R. Oncogene. 1998;17:1097–1108. doi: 10.1038/sj.onc.1202115. [DOI] [PubMed] [Google Scholar]
- 37.Lioubin M N, Algate P A, Tsai S, Carlberg K, Aebersold A, Rohrschneider L R. p150Ship, a signal transduction molecule with inositol polyphosphate-5-phosphatase activity. Genes Dev. 1996;10:1084–1095. doi: 10.1101/gad.10.9.1084. [DOI] [PubMed] [Google Scholar]
- 38.Liu Q, Oliveira-Dos-Santos A J, Mariathasan S, Bouchard D, Jones J, Sarao R, Kozieradzki I, Ohashi P S, Penninger J M, Dumont D J. The inositol polyphosphate 5-phosphatase SHIP is a crucial negative regulator of B cell antigen receptor signaling. J Exp Med. 1998;188:1333–1342. doi: 10.1084/jem.188.7.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Q, Sasaki T, Kozieradzki I, Wakeham A, Itie A, Dumont D J, Penninger J M. SHIP is a negative regulator of growth factor receptor-mediated PKB/Akt activation and myeloid cell survival. Genes Dev. 1999;13:786–791. doi: 10.1101/gad.13.7.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lock P, Casagranda F, Dunn A R. Independent SH2-binding sites mediate interaction of Dok-related protein with RasGTPase-activating protein and Nck. J Biol Chem. 1999;274:22775–22784. doi: 10.1074/jbc.274.32.22775. [DOI] [PubMed] [Google Scholar]
- 41.Muta T, Kurosaki T, Misulovin Z, Sanchez M, Nussenzweig M C, Ravetch J V. A 13-amino-acid motif in the cytoplasmic domain of Fc gamma RIIB modulates B-cell receptor signalling. Nature. 1994;369:340. doi: 10.1038/369340a0. [DOI] [PubMed] [Google Scholar]
- 42.Nada S, Okada M, MacAuley A, Cooper J A, Nakagawa H. Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature. 1991;351:69–72. doi: 10.1038/351069a0. [DOI] [PubMed] [Google Scholar]
- 43.Neet K, Hunter T. The nonreceptor protein-tyrosine kinase CSK complexes directly with the GTPase-activating protein-associated p62 protein in cells expressing v-Src or activated c-Src. Mol Cell Biol. 1995;15:4908–4920. doi: 10.1128/mcb.15.9.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nelms K, Snow A L, Hu-Li J, Paul W E. FRIP, a hematopoietic cell-specific rasGAP-interacting protein phosphorylated in response to cytokine stimulation. Immunity. 1998;9:13–24. doi: 10.1016/s1074-7613(00)80584-1. [DOI] [PubMed] [Google Scholar]
- 45.Noguchi T, Matozaki T, Inagaki K, Tsuda M, Fukunaga K, Kitamura Y, Kitamura T, Shii K, Yamanashi Y, Kasuga M. Tyrosine phosphorylation of p62(Dok) induced by cell adhesion and insulin: possible role in cell migration. EMBO J. 1999;18:1748–1760. doi: 10.1093/emboj/18.7.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okada H, Bolland S, Hashimoto A, Kurosaki M, Kabuyama Y, Iino M, Ravetch J V, Kurosaki T. Role of the inositol phosphatase SHIP in B cell receptor-induced Ca2+ oscillatory response. J Immunol. 1998;161:5129–5132. [PubMed] [Google Scholar]
- 47.Ono M, Bolland S, Tempst P, Ravetch J V. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor Fc(gamma)RIIB. Nature. 1996;383:263–266. doi: 10.1038/383263a0. [DOI] [PubMed] [Google Scholar]
- 48.Ono M, Okada H, Bolland S, Yanagi S, Kurosaki T, Ravetch J V. Deletion of SHIP or SHP-1 reveals two distinct pathways for inhibitory signaling. Cell. 1997;90:293–301. doi: 10.1016/s0092-8674(00)80337-2. [DOI] [PubMed] [Google Scholar]
- 49.Pawson T, Scott J D. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- 50.Peri K G, Gervais F G, Weil R, Davidson D, Gish G D, Veillette A. Interactions of the SH2 domain of lymphocyte-specific tyrosine protein kinase p56lck with phosphotyrosine-containing proteins. Oncogene. 1993;8:2765–2772. [PubMed] [Google Scholar]
- 51.Peterson E J, Clements J L, Fang N, Koretzky G A. Adaptor proteins in lymphocyte antigen-receptor signaling. Curr Opin Immunol. 1998;10:337–344. doi: 10.1016/s0952-7915(98)80173-8. [DOI] [PubMed] [Google Scholar]
- 52.Plas D R, Thomas M L. Negative regulation of antigen receptor signaling in lymphocytes. J Mol Med. 1998;76:589–595. doi: 10.1007/s001090050254. [DOI] [PubMed] [Google Scholar]
- 53.Pradhan M, Coggeshall K M. Activation-induced bi-dentate interaction of SHIP and Shc in B lymphocytes. J Cell Biochem. 1997;67:32–42. doi: 10.1002/(sici)1097-4644(19971001)67:1<32::aid-jcb4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 54.Ravetch J V. Fc receptors. Curr Opin Immunol. 1997;9:121–125. doi: 10.1016/s0952-7915(97)80168-9. [DOI] [PubMed] [Google Scholar]
- 55.Rebecchi M J, Scarlata S. Pleckstrin homology domains: a common fold with diverse functions. Annu Rev Biophys Biomol Struct. 1998;27:503–528. doi: 10.1146/annurev.biophys.27.1.503. [DOI] [PubMed] [Google Scholar]
- 56.Rudd C E. Adaptors and molecular scaffolds in immune cell signaling. Cell. 1999;96:5–8. doi: 10.1016/s0092-8674(00)80953-8. [DOI] [PubMed] [Google Scholar]
- 57.Sabe H, Hata A, Okada M, Nakagawa H, Hanafusa H. Analysis of the binding of the Src homology 2 domain of Csk to tyrosine-phosphorylated proteins in the suppression and mitotic activation of c-Src. Proc Natl Acad Sci USA. 1994;91:3984–3988. doi: 10.1073/pnas.91.9.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saito T. Negative regulation of T cell activation. Curr Opin Immunol. 1998;10:313–321. doi: 10.1016/s0952-7915(98)80170-2. [DOI] [PubMed] [Google Scholar]
- 59.Tamir I, Cambier J C. Antigen receptor signaling: integration of protein tyrosine kinase functions. Oncogene. 1998;17:1353–1364. doi: 10.1038/sj.onc.1202187. [DOI] [PubMed] [Google Scholar]
- 60.Tang J, Feng G S, Li W. Induced direct binding of the adapter protein Nck to the GTPase-activating protein-associated protein p62 by epidermal growth factor. Oncogene. 1997;15:1823–1832. doi: 10.1038/sj.onc.1201351. [DOI] [PubMed] [Google Scholar]
- 61.Thomas M L. Of ITAMs and ITIMs: turning on and off the B cell antigen receptor. J Exp Med. 1995;181:1953–1956. doi: 10.1084/jem.181.6.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Unkeless J C, Jin J. Inhibitory receptors, ITIM sequences and phosphatases. Curr Opin Immunol. 1997;9:338–343. doi: 10.1016/s0952-7915(97)80079-9. [DOI] [PubMed] [Google Scholar]
- 63.van Oers N S, Weiss A. The Syk/ZAP-70 protein tyrosine kinase connection to antigen receptor signalling processes. Semin Immunol. 1995;7:227–236. doi: 10.1006/smim.1995.0027. [DOI] [PubMed] [Google Scholar]
- 64.Veillette A, Bookman M A, Horak E M, Bolen J B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988;55:301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- 65.Vuica M, Desiderio S, Schneck J P. Differential effects of B cell receptor and B cell receptor-FcgammaRIIB1 engagement on docking of Csk to GTPase-activating protein (GAP)-associated p62. J Exp Med. 1997;186:259–267. doi: 10.1084/jem.186.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weiss A, Littman D R. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 67.Wisniewski D, Strife A, Wojciechowicz D, Lambek C, Clarkson B. A 62-kilodalton tyrosine phosphoprotein constitutively present in primary chronic phase chronic myelogenous leukemia enriched lineage negative blast populations. Leukemia. 1994;8:688–693. [PubMed] [Google Scholar]
- 68.Yablonski D, Kuhne M R, Kadlecek T, Weiss A. Uncoupling of nonreceptor tyrosine kinases from PLC-gamma1 in an SLP-76-deficient T cell. Science. 1998;281:413–416. doi: 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- 69.Yamanashi Y, Baltimore D. Identification of the Abl- and rasGAP-associated 62-kDa protein as a docking protein, Dok. Cell. 1997;88:205–211. doi: 10.1016/s0092-8674(00)81841-3. [DOI] [PubMed] [Google Scholar]
- 70.Zhang W, Irvin B J, Trible R P, Abraham R T, Samelson L E. Functional analysis of LAT in TCR-mediated signaling pathways using a LAT-deficient Jurkat cell line. Int Immunol. 1999;11:943–950. doi: 10.1093/intimm/11.6.943. [DOI] [PubMed] [Google Scholar]