Abstract
Aims
Insulin sensitivity and insulin secretion can be estimated by multiple indices from fasting blood samples or blood samples obtained during oral glucose tolerance tests. The test-retest reliability of these indices in repeated measurements within the same individuals can strongly vary.
Methods
We analyzed data of persons without diabetes who underwent two repeated OGTTs. For each measurement pair, we calculated multiple commonly used indices for the assessment of insulin secretion and insulin sensitivity. We then evaluated the coefficient of variation (standard deviation/mean) and discriminant ratio for each index.
Results
89 persons underwent two OGTTs with a median interval of 86 days (IQR 64–249). Among indices of insulin sensitivity derived from fasting blood samples, the revised quantitative insulin sensitivity check index had the smallest coefficient of variation (2.8 ± 2.1%) whereas the C-peptide based homeostasis model assessment 2 had the highest discriminant ratio (1.97 (1.65–2.39)). As for insulin sensitivity indices that are based on OGTT, the oral glucose insulin sensitivity index had the smallest coefficient of variation (6.5 ± 5.1%). The highest discriminant ratio was found for the non-esterified fatty acids-based insulin sensitivity index (NEFA-ISI, 2.70 (2.30–3.22)). For the assessment of insulin secretion from fasting variables, the lowest mean coefficient of variation was found for C-peptide based homeostasis model assessment 2 beta with 10.8 ± 8% and the highest discriminant ratio for the C-peptide / Glucose-Ratio (2.18 (1.84–2.63)). Among indices assessing insulin secretion from an OGTT, the lowest coefficient of variation was found for the ratio of the areas under the C-peptide and glucose curves from 0 to 120 minutes with 11.3 ± 9.7%.
Conclusion
The data reveal large differences in the reproducibility and the discrimination capability of different indices that assess insulin sensitivity or insulin secretion. Our findings can aid the selection of an appropriate index in clinical studies.
Introduction
About 422 million people worldwide have diabetes mellitus with numbers expected to rise. Impaired insulin secretion and insulin resistance are the two key abnormalities which underlie the pathogenesis of type 2 diabetes.
The gold standard measurement of insulin secretion and insulin sensitivity are the hyperglycemic clamp and the hyperinsulinemic euglycemic clamp [1, 2], respectively. However, these methods are relatively invasive, labor intensive, as well as time-consuming and therefore not feasible in larger clinical studies. Therefore, different approaches to substitute the clamp technique for the assessment of insulin secretion or insulin sensitivity have been proposed. These methods estimate insulin secretion or insulin sensitivity from variables related to these modalities such as insulin, C-peptide, glucose or non-esterified fatty acid (NEFA) levels. NEFAs are tightly linked to insulin sensitivity due to insulin’s role in the suppression of lipolysis [3–5]. Some of the estimates utilize only measurements from fasting blood samples while others employ variables obtained during standardized metabolic challenges such as the oral glucose tolerance test (OGTT). Based on the type of estimate and underlying blood specimen, the measures can capture different aspects of insulin sensitivity or insulin secretion. The OGTT is widely used in clinical practice and derived measures quantify the efficiency of glucose utilization under standard conditions including both hepatic as well as muscle insulin sensitivity [6, 7]. In contrast, insulin sensitivity indices that are solely based on fasting parameters reflect mostly hepatic insulin sensitivity [8]. Most indices of insulin sensitivity and some for insulin secretion have already been evaluated for correlation with gold standard measurements [9, 10].
Certainly, there is an inherent biological and analytical variability in every measurement [11, 12]. In addition to the desired feature to be measured, glycemic traits are highly influenced by other confounding factors such as quality of sleep, subclinical conditions or inflammation [13, 14]. Glycemic variables obtained during an OGTT are further impacted by the speed of gastric emptying [15], rate of incretin release [16] and potential interactions with the gut microbiome. Therefore, test-retest reliability, i.e. the intra-individual concordance of subsequent measurements, can strongly vary among these indices. Though, there is still lack of scientific data regarding this variability. Our work therefore aims to compare the reproducibility for different frequently reported indices for insulin sensitivity and insulin secretion using highly standardized repeated measurements within the same subjects.
Methods
Subjects
We analyzed data from 120 persons who underwent two or more oral glucose tolerance tests (OGTT) with a time difference of 4 to 363 days. Subjects analyzed in the current study are participants of a number of clinical studies. These include the Tuebingen Family Study (TUEF, n = 15) [17], Tuebinger-Lebensstil-Interventions-Programm (TULIP, n = 4), Prädiabetes Lebensstil Interventions-Studie (PLIS, NCT01947595, n = 2), Deutsche Studie Schwangerschaftsdiabetes (PREG, n = 16, NCT04270578) as well as 14 other projects (NCT02991365 (n = 1), NCT03151590 (n = 7), NCT03231839 [18] (n = 1), NCT 04372849 (n = 2), NCT01635114 [19] (n = 22), NCT03615209 (n = 15), NCT 03590561 (n = 3), and DRKS00012996 (n = 1)). The research took place in Tuebingen, Baden-Wurttemberg, Germany, between August 2002 and March 2019. The first OGTT was performed between 08/2002 and 02/2019, the second between 05/2003 and 03/2019. Written informed consent was obtained from all participants. A vote of approval for all studies has been obtained from the ethics committee of the University Hospital of Tuebingen.
Persons who had diabetes mellitus defined as either an HbA1c ≥6.5%, fasting glucose ≥7mmol/l or a post-challenge glucose ≥11.1mmol/l at 120 minutes after glucose challenge, who underwent a lifestyle modification or who were pregnant were previously excluded. Furthermore, persons who showed a difference of three or more kilograms body weight between the two glucose tolerance tests (n = 31) were excluded. For persons who underwent more than two oral glucose tolerance tests, the two tests with the lowest time interval were analyzed. Data of 89 persons in accordance with 178 OGTTs remained.
Oral glucose tolerance test and laboratory measurements
All participants underwent an OGTT with a standardized 75 g glucose solution (Accu-Check Dextro, Roche) after overnight fasting. Venous plasma and serum samples were obtained before ingestion (at minute 0) and 30, 60, 90 and 120 minutes after the glucose challenge. All blood samples were immediately put on ice and the serum was centrifuged within two hours. Plasma glucose was determined in an ADVIA 1800 autoanalyzer (Siemens Healthcare Diagnostics, Erlangen, Germany). Plasma insulin and C-peptide were measured by an immunoassay with the ADVIA Centaur XP Immunoassay System (Siemens Healthineers, Eschborn, Germany). NEFA concentrations were measured enzymatically (WAKO Chemicals, Neuss, Germany) using the ADVIA 1800 analyzer (Siemens Healthcare Diagnostics, Eschborn, Germany). C-reactive protein levels were measured via immunoturbidimetry with the wide range reagents using the ADVIA 1200 analyzer (Siemens, Eschborn, Germany).
Indices
We used the following common indices for insulin sensitivity and insulin secretion for our study. The indices used are without any claim to completeness.
Calculated indices to measure insulin sensitivity using parameters from fasting state are:
- Quantitative insulin sensitivity check index [20] (QUICKI):
- Revised quantitative insulin sensitivity check index [21] (revised QUICKI):
-
C-peptide based homeostasis model assessment 2 sensitivity [22] (HOMA2-SInsulin):
see HOMA2Calculator [23]
-
C-peptide based homeostasis model assessment 2 sensitivity [22] (HOMA2-SC-Peptide):
see HOMA2Calculator [23]
Calculated indices to measure insulin sensitivity using parameters from oral glucose tolerance tests are:
- Belfiore index [26] (Belfiore):
- Non-esterified fatty acids insulin sensitivity index [27] (NEFA-ISI):
- Gutt insulin sensitivity index [28] (Gutt):
- Matsuda insulin sensitivity index [7] (Matsuda-ISI):
- Stumvoll insulin sensitivity index [29] (Stumvoll-ISI):
- Stumvoll metabolic clearance rate [29] (Stumvoll-MCR):
Calculated indices to measure insulin secretion using parameters from fasting state are:
-
C-peptide based homeostasis model assessment 2 beta [22] (HOMA2-BC-Peptide):
see HOMA2Calculator [23]
-
insulin based homeostasis model assessment 2 beta [22] (HOMA2-BInsulin):
see HOMA2Calculator [23]
- C-Peptide/Glucose-Ratio (CGR):
whereby blood glucose levels are used in mg/dl
Calculated indices to measure insulin secretion using parameters from oral glucose tolerance tests are:
Area under the curve (calculated by using the trapezoidal rule) of C-peptide from 0 to 30 minutes (AUCC-Peptide0-30)
Area under the curve of C-peptide from 0 to 120 minutes (AUCC-Peptide0-120)
Ratio of the area under the curve for insulin from 0 to 30 minutes to the area under the curve for glucose from 0 to 30 minutes (AUCInsulin0-30/AUCGlucose0-30)
Ratio of the area under the curve of insulin from 0 to 120 minutes to the area under the curve of glucose from 0 to 120 minutes (AUCInsulin0-120/AUCGlucose0-120)
Ratio of the area under the curve of C-peptide from 0 to 30 minutes to the area under the curve of glucose from 0 to 30 minutes (AUCC-Peptide0-30/AUCGlucose0-30)
Ratio of the area under the curve of C-peptide from 0 to 120 minutes to the area under the curve of glucose from 0 to 120 minutes (AUCC-Peptide0-120/AUCGlucose0-120)
- Insulinogenic index [30] (IGI):
- Oral disposition index [31] (DI):
It is important to emphasize that indices based on insulin concentration should be differentiated from indices based on C-peptide concentration. Whereas indices based on insulin concentration reflect a combination of insulin secretion and insulin clearance and therefore can be seen as a surrogate index of the “body insulin response” to glucose, indices based on C-peptide reflect insulin secretion only and can therefore be seen as an index of “beta cell function”.
The different indices with the respective necessary measurements are summarized in Table 1.
Table 1. Calculation of the analyzed indices.
| Index | timepoints | variables needed | equation | ||
|---|---|---|---|---|---|
| Insulin secretion | fasting | HOMA2-B C-peptide | 0 | Glucose, C-peptide | https://www.dtu.ox.ac.uk/homacalculator/index.php |
| HOMA2-B Insulin | 0 | Glucose, Insulin | https://www.dtu.ox.ac.uk/homacalculator/index.php | ||
| CGR | 0 | Glucose, C-Peptide |
|
||
| OGTT | AUC C-peptide 0-120/AUC Glucose 0–120 | 0, 120 | Glucose, C-peptide | AUCC-Peptide0-120/AUCGlucose0-120 | |
| AUC C-peptide 0–120 | 0, 120 | C-peptide | AUCC-Peptid0-120 | ||
| AUC C-peptide 0-30/AUC Glucose 0–30 | 0, 30 | Glucose, C-peptide | AUCC-Peptide0-30/AUCGlucose0-30 | ||
| AUC C-peptide 0–30 | 0, 30 | C-peptide | AUCC-Peptide0-30 | ||
| AUC Insulin 0-120/AUC Glucose 0–120 | 0, 30 | Glucose, Insulin | AUCInsulin0-120/AUCGlucose0-120 | ||
| AUC Insulin 0-30/AUC Glucose 0–30 | 0, 30 | Glucose, Insulin | AUCInsulin0-30/AUCGlucose0-30 | ||
| IGI | 0, 30 | Glucose, Insulin |
|
||
| DI | 0, 30 | Glucose, Insulin |
|
||
| Insulin sensitivity | fasting | revised QUICKI | 0 | Glucose, Insulin, NEFA |
|
| QUICKI | 0 | Glucose, Insulin |
|
||
| HOMA2-S C-peptide | 0 | Glucose, C-peptide | https://www.dtu.ox.ac.uk/homacalculator/index.php | ||
| HOMA2-S Insulin | 0 | Glucose, Insulin | https://www.dtu.ox.ac.uk/homacalculator/index.php | ||
| OGTT | OGIS | 0, 90, 120 | Glucose, Insulin, height, body weight, glucose dose [g] |
whereby f is a complex function which can be programmed on a spreadsheet[30] |
whereby f is a complex function which can be programmed on a spreadsheet[30] |
| Belfiore | 0(, 60), 120 | Glucose, Insulin |
|
||
| NEFA-ISI | 0, 60, 120 | Insulin, NEFA, BMI |
|
||
| Gutt | 0, 120 | Glucose, Insulin, body weight | |||
| Matsuda-ISI | 0, 30, 60, 90, 120 | Glucose, Insulin |
|
||
Statistics
To investigate if there is an association between the coefficient of variation of an index and the time-span between measurements within the prespecified one-year time interval, we fitted linear regression models (CoVIndex ~ Δtime (days)) for all investigated indices using a multiple-testing corrected α = 0.0024 threshold (see S1 Table).
We computed the coefficient of variation (standard deviation/ mean) for each measurement pair to determine the reliability of repeated measurements.
The coefficient of variation is a well-established method for assessing the imprecision of a test. To also account for the capability of a given test to discriminate different subjects in relation to the robustness of within subject measurements, we also calculated the discriminant ratio (DR) as proposed Levy et. al [32] as follows:
Where MSB and MSW are the between-subject and within subject mean squares respectively and k is the number of repeated measurements within subject. The discriminant ratio is higher when the differences between subjects are higher and within-subjects are lower. Confidence intervals for discriminant ratios were computed using non-central F distributions and differences between the discriminant ratios in the index groups were tested using Q-statistic, as recommended [32].
All computations were performed with R version 3.6.3 [33].
Literature research
To assess how often different indices are used, we researched the literature via the ISI web of knowledge using the search function title. As key words we used the full title of each original article. The original articles are cited in the upper part (see section Methods-Indices). We looked for the number of citations of the original articles of each investigated index, where possible. All studies found were included. The literature research was conducted on 26th of February 2020. Unfortunately, it was not possible to differentiate various HOMA2 indices in our literature research. Due to difficulties in identifying indices containing areas under curve for C-peptide, insulin and glucose in the literature, it was not possible to assess the citation number for these indices.
Results
We analyzed data of 178 OGTTs from 89 persons (51 females, 38 males). For each of them, data from two OGTTs with a median of 86 days (IQR 64–249) between the two measurements were analyzed. Participants had a median age of 42 years (IQR 29–57) and a median body mass index (BMI) of 27.9 kg/m2 (IQR 23.4–32.4). An overview of the participants`characteristics is given in Table 2.
Table 2. Characteristics of participants.
| characteristic | |
|---|---|
| Total number of participants | 89 |
| Sex [n] | |
| male | 38 |
| female | 51 |
| Age [years] | |
| median (interquartile range) | 42 (29–57) |
| range | 18–72 |
| BMI [kg/m 2 ] | |
| median (interquartile range) | 27.9 (23.4–32.4) |
| range | 17.7–42.0 |
| time difference [days] | |
| median (interquartile range) | 86 (64–249) |
| range | 4–363 |
In Table 3 the mean and the standard deviation of the coefficient of variation as well as the discriminant ratio for each calculated index arranged by insulin secretion or insulin sensitivity as well as by the sampling method (fasting or OGTT) are shown. Moreover, the mean per group is shown here.
Table 3. Coefficient of variation and discriminant ratio index-wise and per group.
| Index | CoV mean1 | CoV sd2 | DR (95% CI)3 | ||
| Insulin secretion | fasting | HOMA2-B C-peptide | 0.108 | 0.080 | 1.69 (1.39–2.07) |
| CGR | 0.134 | 0.101 | 2.18 (1.84–2.63) | ||
| HOMA2-B Insulin | 0.142 | 0.116 | 1.58 (1.28–1.96) | ||
| group4 | 0.128 | 0.099 | 1.82 (1.50–2.22) | ||
| OGTT | AUC C-peptide 0-120/AUC Glucose 0–120 | 0.113 | 0.097 | 2.13 (1.79–2.57) | |
| AUC C-peptide 0–120 | 0.127 | 0.095 | 2.28 (1.93–2.74) | ||
| AUC C-peptide 0-30/AUC Glucose 0–30 | 0.155 | 0.157 | 1.71 (1.41–2.09) | ||
| AUC C-peptide 0–30 | 0.160 | 0.157 | 1.79 (1.48–2.18) | ||
| AUC Insulin 0-120/AUC Glucose 0–120 | 0.161 | 0.112 | 2.52 (2.14–3.01) | ||
| AUC Insulin 0-30/AUC Glucose 0–30 | 0.198 | 0.158 | 1.76 (1.45–2.14) | ||
| IGI | 0.273 | 0.233 | 0.57 (0.23–0.86) | ||
| DI | 0.288 | 0.252 | 0.12 (0–0.53) | ||
| group | 0.184 | 0.158 | 2.03 (1.30–2.02) | ||
| Insulin sensitivity | fasting | revised QUICKI | 0.028 | 0.021 | 1.97 (1.65–2.39) |
| QUICKI | 0.044 | 0.036 | 1.94 (1.62–2.34) | ||
| HOMA2-S C-peptide | 0.149 | 0.117 | 1.97 (1.65–2.39) | ||
| HOMA2-S Insulin | 0.210 | 0.173 | 1.40 (1.12–1.75) | ||
| group | 0.108 | 0.087 | 1.82 (1.51–2.22) | ||
| OGTT | OGIS | 0.065 | 0.051 | 1.99 (1.67–2.40) | |
| Belfiore | 0.112 | 0.096 | 1.52 (1.23–1.87) | ||
| NEFA-ISI | 0.128 | 0.085 | 2.70 (2.30–3.22) | ||
| Gutt | 0.173 | 0.140 | 1.24 (0.97–1.56) | ||
| Matsuda-ISI | 0.188 | 0.150 | 2.16 (1.82–2.59) | ||
| group | 0.133 | 0.104 | 1.92 (1.59–2.33) | ||
1 mean coefficient of variation.
2 standard deviation of the coefficient of variation.
3 discriminant ratio with the corresponding 95% confidence interval.
4 representing the mean values for each group (insulin secretion fasting, insulin secretion OGTT, insulin sensitivity fasting, insulin sensitivity OGTT).
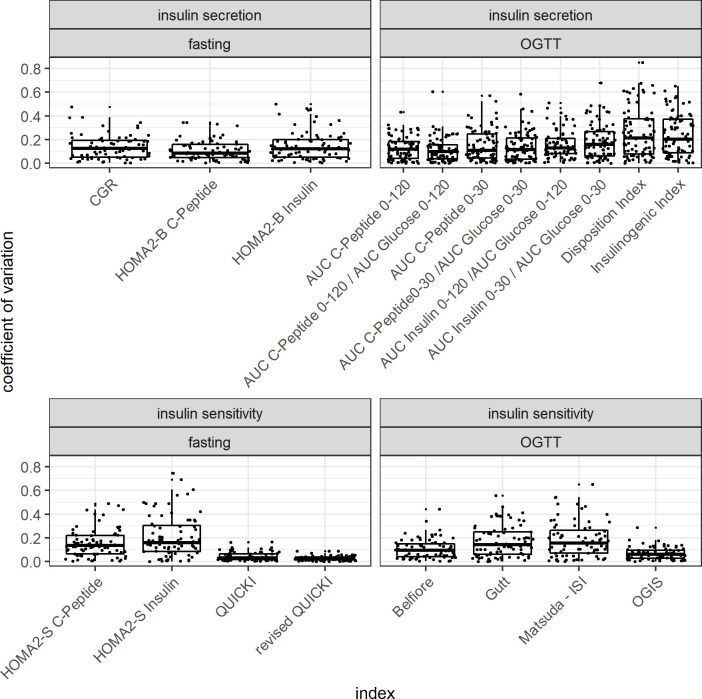
For the indices derived from fasting blood that assess insulin sensitivity, the smallest mean coefficient of variation was found for revised QUICKI at 2.8% (± 2.1%). With 4.4% (± 3.6%) QUICKI also showed a low coefficient of variation while HOMA2-SC-Peptide with 14.9% (± 11.7%) and HOMA2-SInsulin with 21.0% (± 17.3%) demonstrate larger coefficients of variation.
There were no differences among discriminant ratios in the group of fasting-based insulin sensitivity indices (p = 0.07, see Fig 2C).
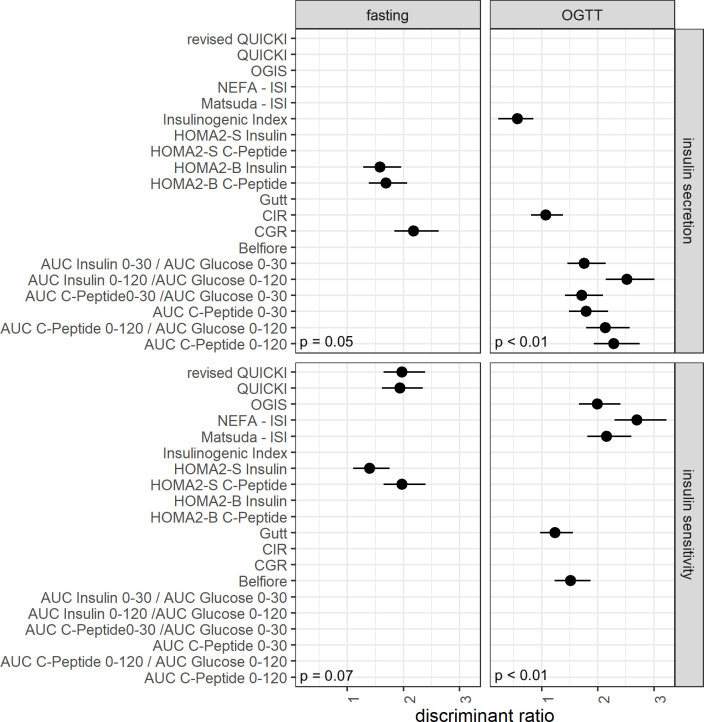
Fig 2. Discriminant ratio and corresponding confidence intervals of the analyzed indices of insulin secretion and insulin sensitivity.
The disposition index had been omitted due to a very low point estimate (0.12) and a failure to compute the confidence interval. The discriminant ratios and their confidence intervals, as well as the p-values of the tests of differences within groups were computed as proposed by Levy at al [32].
For indices that use measurements from oral glucose tolerance tests to measure insulin sensitivity, the smallest coefficient of variation was found for OGIS with 6.5% (± 5.1%) followed by Belfiore with 11.2% (± 9.6%), NEFA-ISI with a mean coefficient of variation of 12.8% (± 8.5%) and Gutt with 17.3% (± 14.0%). With a coefficient of variation of 18.8% (± 15.0%), the Matsuda-ISI had the highest figure in this group.
The highest discriminant ratio was found for NEFA-ISI (2.70 (2.30–3.22) followed by Matsuda-ISI (2.16 (1.82–2.59) and OGIS (1.99 (1.67–2.40)). With 1.52 (1.23–1.87) Belfiore showed a lower discriminant ratio followed by Gutt with 1.24 (0.97–1.56).
For the indices for insulin secretion that use fasting variables, the lowest coefficient of variation was found for HOMA2-BC-Peptide with 10.8% (± 8%) followed by CGR with 13.4 (± 10.1%) and HOMA2-BInsulin with 14.2% (± 11.6%) whereas the highest discriminant ratio was found for CGR with 2.18 (1.84–2.63).
Comparing the indices using OGTT-based variables to assess insulin secretion, the smallest coefficient of variation at 11.3% (± 9.7%) was found for AUCC-Peptide0-120/AUCGlucose0-120. The different coefficients of variation of AUCs are shown in Table 3. The largest coefficient of variation for measuring insulin secretion from OGTT-derived variables was demonstrated for IGI with 27.3% (± 23.3%) and DI with 28.8% (± 25.2%).
We have also performed a sensitivity analysis of the coefficients of variation after excluding female premenopausal participants (see S2 and S3 Tables).
With regard to the discriminant ratio, the highest value was found for AUCInsulin 0-120/AUCGlucose0-120 with 2.52 (2.14–3.01) and the lowest for DI (0.12 (0–0.53). Taking both, the coefficient of variation and the discriminant ratio into account, CGR and HOMA2-BC-Peptide seem to be superior to HOMA2-BInsulin in the group assessing insulin secretion using fasting values. Furthermore, AUCC-Peptide0-120/AUCGlucose0-120, AUCC-Peptide0-120 and AUCInsulin0-120/AUCGlucose0-120 seem to be superior to the other OGTT-based tests assessing insulin secretion.
For indices assessing insulin sensitivity using fasting variables, HOMA2-SInsulin shows the highest coefficient of variation and the lowest discriminant ratio. The best performing index for discrimination between different subjects in relation to its variability is the NEFA-ISI with a low coefficient of variation.
Fig 1 shows a boxplot of the coefficients of variation of the different indices arranged by the fore mentioned order. Fig 2 shows the discriminant ratios and the corresponding confidence interval for each index.
Fig 1. Boxplot showing coefficients of variation of the analyzed indices of insulin secretion and insulin sensitivity.
A groupwise aggregation of different coefficients of variation (showed a mean of 12.8% (± 9.9%) in the insulin secretion from fasting measures group, a mean of 18.4 ± 15.8% in the insulin secretion from OGTT measures group, a mean of 10.8 ± 8.7% in the insulin sensitivity fasting group and a mean of 13.3% (± 10.4%) in the insulin sensitivity OGTT group.
An overview of the mean discriminant ratios for each group shows a mean discriminant ratio of 1.82 (1.50–2.22) for the insulin secretion fasting group and with 2.03 (1.30–2.02) a higher discriminant ratio for the insulin secretion OGTT group. In the insulin sensitivity OGTT group we found a discriminant ratio of 1.92 (1.59–2.33) and a lower discriminant ratio in the insulin sensitivity fasting group with 1.82 (1.51–2.22).
Our literature research yielded inter alia 3415 citations of the original paper of Matsuda, 2452 citations of the original paper of QUICKI, 1198 of the original paper of HOMA2 and 1103 citations of the original paper of DI. The original paper of revised QUICKI was cited 168 times, that of NEFA-ISI 11 times. An overview of the number of citations per year (calculated by the total number of citations divided by the years since first publication) in relation to the coefficients of variation for each group is given in S1 Fig.
Discussion
With a coefficient of variation range between 2.8 to 28.8%, our data show large differences in the reproducibility of different indices measuring insulin sensitivity and insulin secretion. The intra-individual coefficient of variation of indices using fasting variables is smaller than that of indices using variables measured during an OGTT for both insulin sensitivity and insulin secretion (see Table 3). Schousboe et al. already described a larger coefficient of variation in indices measuring insulin sensitivity and secretion than in 2h post-load glucose and in fasting blood glucose [34]. The difference may be due to the greater number of measurements for the OGTT based variables generating more variance and due to variable passage and resorption of the glucose solution after ingestion [34, 35]. Change of insulin sensitivity through the menstrual cycle is controversially discussed as a potential further source of intraindividual variation [36, 37]. To address this, we excluded female premenopausal participants in an additional analysis (see S2 and S3 Tables).
The results were comparable to the whole group.
In addition to coefficient of variation, we also computed the discriminant ratio of all indices. The discriminant ratio as recommended by Levy et. Al [32] is an excellent instrument which not only incorporates the imprecision of a test but quantifies this in relation to the capability to distinguish between different subjects.
Insulin sensitivity
Revised QUICKI [21] and the original QUICKI [20] showed the smallest coefficients of variation among the indices estimating insulin sensitivity from fasting variables as well as compared to all evaluated indices. Of note, revised QUICKI has been shown to have the highest correlation with gold standard measurements in a meta-analysis [9]. In addition to fasting glucose and insulin, revised QUICKI uses the fasting level of NEFA. NEFA are not measured routinely in most metabolic studies and require precise pre-analytics in order to avoid in vitro lipolysis [38].
Generally, insulin concentrations in the bloodstream have a large biological variability due to the hormones short serum half-life, its pulsatile secretion [39], a marked first-pass effect before reaching systemic circulation. In addition to the biological variance, there is a considerable analytical variability of laboratory insulin measurement approaches [10]. Of note, HOMA2-SInsulin [22] had higher coefficients of variation than QUICKI, despite calculating insulin sensitivity from the same variables.
The lowest coefficient of variation to measure insulin sensitivity using dynamic parameters was found for OGIS [24]. OGIS has shown good agreement with the hyperinsulinemic euglycemic clamp and a better correlation in comparison to other indices [24, 40]. Another advantage is that blood sampling is only required at three different time-points during the OGTT (minutes 0, 90 and 120). However, a limitation of OGIS is the complex function, that can, however, be circumvented by web-based calculators and available Excel-sheets [25].
Both the Belfiore [26] and the NEFA-ISI [27] use NEFA levels for the assessment of insulin sensitivity. They exhibit somewhat larger coefficients of variation than OGIS. NEFA-ISI only comprises insulin and NEFA levels at different time-points and it is the only insulin sensitivity index that does not utilize glucose concentrations at all. This makes it more accurate to measure insulin sensitivity in special situations like pregnancy, a state with physiologically lower glucose levels that often hinders comparison of pregnant with non-pregnant women.
The Matsuda index [7] correlates well with insulin sensitivity assessed from euglycemic hyperinsulinemic clamp [41]. It is calculated from fasting glucose and insulin levels and the mean of glucose and insulin during OGTT [7]. Matsuda, computed from 5-point OGTT data, has shown an intraindividual coefficient of variation of 18.8% which is the largest in the group of indices measuring insulin sensitivity with OGTT parameters. The higher number of measured points may explain the higher coefficient of variation as well as the fore mentioned higher variability of insulin and glucose levels.
When comparing the computed coefficients of variations and the discriminant ratios between groups, fasting-based indices showed lower coefficients of variation (10.8%) but also lower discriminant ratios (1.82) than OGTT-based indices (coefficient of variation 13.3% and discriminant ratio 1.92). This might indicate that the indices using fasting values might be more stable during repetitive measurements, but have less power to discriminate metabolic differences between subjects.
Stumvoll-ISI and Stumvoll-MCR incorporate demographic data as age, sex and BMI in addition to glucose and insulin levels obtained during an OGTT. These are reliable indices [29]. Unfortunately, in our group of subjects we found several negative indices in persons with high insulin levels 120 minutes after glucose load so that we excluded these indices from these analyses.
Insulin secretion
The lowest coefficient of variation in the measurement of insulin secretion from fasting parameters was shown for HOMA2-BC-Peptide. This is most likely due to the above mentioned difficulties in insulin measurements. C-peptide and insulin are secreted in equimolar amounts based on their cleavage out of proinsulin. It is a more stable parameter than insulin and has insignificant clearance by the liver and a longer half-life than insulin [42]. Therefore C-peptide levels better approximate pancreatic insulin secretion than insulin levels [43], and so the use of C-peptide to compute HOMA2-B has clear advantages. In clinical practice, it has been recently suggested that a simple ratio of plasma C-peptide over plasma glucose in mg/dl could result in a straight fore ward classification of insulin secretion capacity. Our data show that this ratio has a comparable coefficient of variation to the other indices of this category.
The OGTT had been described as an acceptable compromise to assess ß-cell function [44]. When estimating insulin secretion from dynamic parameters during an oral glucose challenge the lowest coefficient of variation was found for AUCC-Peptide0-120/AUCGlucose0-120 (11.3% ± 9.7%). In comparison, the coefficient of variation of AUCInsulin0-120/AUCGlucose0-120 was larger (16.1% ± 11.2). This is well in line with the noted superiority of C-peptide over direct insulin measurements. In this category of insulin secretion from dynamic parameters during an oral glucose challenge the highest coefficient of variation was found for DI. This index has advantages as it is an early marker of an inadequate compensation of beta cell function [45]. The assumption on which the measurement was established is that all measurements of insulin sensitivity or response show a hyperbolic relationship. But there are discussions if this assumption is sufficient [31]. Besides, the variability of insulin levels may be a source of high variability as well.
As for the group of indices measuring insulin sensitivity, our study shows the same differences when comparing the computed coefficient of variation and the discriminant ratios in the insulin secretion groups. The group based on fasting values shows a smaller coefficient of variation (12.8%) and a smaller discriminant ratio (1.82) than the OGTT group (coefficient of variation 15.8% and discriminant ratio 2.03)
Utzschneider et al. already described a high within-subject variability of various indices and proposed integrated measurements using multiple time points and C-peptide levels to reduce variability [46]. The study population Utzschneider et al. describe consists of overall 37 persons of whom some had normal glucose tolerance, some had impaired glucose tolerance and some had diabetes–with or without medication. Our study conversely just analyzed people without diabetes to ensure a better comparability. Moreover, our study population was larger so that the generalisability should be better. Despite the differences in the sample size and the study population, all in all, our data is in line with the findings of Utzschneider`s study with a high coefficient of variation for insulinogenic index and AUCs with smaller coefficients of variation. One could therefore assume that the variability of the different indices is independent of the glucose levels. This should be investigated by other studies.
Studies comparing the coefficient of variation and the discriminant ratio at the same time are rare. The only study which we are aware of is by Mather et al. [10]. Mather et al. measured the repeatability of different tests of insulin sensitivity. In their investigation logHOMA-IR and QUICKI provided the best results with a low coefficient of variation and a high discriminant ratio.
Similar results were found in our study. QUICKI and also revised QUICKI showed a low coefficient of variation concomitant with a high discriminant ratio. Additionally, we investigated more indices and also the insulin secretion. In the insulin sensitivity group OGIS and NEFA-ISI are two important indices which seem to be superior to the other OGTT-based insulin sensitivity indices.
The discussed indices are commonly used indices. However we did not cover one group of indices in our work, the indices computed by mathematical modeling. For example Mari et al. established models for beta-cell assessment [44, 47]. Most indices based on mathematical modelling require a more frequent glucose sampling, especially a measurement point at 15 minutes.
Use of indices in the literature
QUICKI is used very often, most likely as it only requires one fasting blood sampling. Our literature research showed 2,452 citations, i.e. the second highest number of citations of all used indices. Conversely, the original paper of revised QUICKI was cited just 168 times. Although OGIS has shown good agreement with the hyperinsulinemic euglycemic clamp and we now verified a favorable test-retest coefficient of variation, it is not used applied very frequently. This might be partly due to its complexity, despite the available web-based calculators and Excel-sheets [25]. With 514 citations of the original paper in our literature research it is less cited than the Matsuda index. Matsuda index with 3415 citations is the most cited paper in this category and among all evaluated indices. Of note, this top-cited index shows the highest coefficient of variation.
Limitations
Our study investigated several indices for reliability during repeated measurements. Due to the single-center design, our highly standardized study settings and laboratory measurements, we potentially reduced variance that can originate from methodological and pre-analytic and analytical differences in routine clinical use. Our work only addressed the coefficient of variation and discrimination ratios of the indices and did not compare how these indices relate to gold-standard measurements. Furthermore we did not cover the group of secretion/sensitivity indices computed by mathematical modeling. Most indices based on mathematical modeling require a more frequent glucose sampling. Another limitation may lay in the small number of OGTTs which could restrict the generalisability of our data.
Summary and outlook
Reliable estimation of insulin sensitivity and secretion is crucial when investigating glucose metabolism in clinical research. Furthermore, measurement of these key metabolic features recently gained clinical importance with the introduction of newly identified subphenotypes of prediabetes and diabetes and their potential impact towards more personalized strategies for diabetes therapy. Our current work can aid the selection of the appropriate index in upcoming clinical studies on insulin sensitivity or insulin secretion.
Supporting information
Coefficients of variation of the analyzed indices in relation to the number of citations of the original article per year calculated by the total number of citations divided by the years since first publication. Indices that we could not found or differentiate in our literature research or which are not published yet are presented in red using random values for the y axis. Abbreviations: C = C-peptide; I = Insulin; G = Glucose.
(TIF)
Samples with a time span more than one year between the two OGTTs are already excluded. Linear regression models (CoVIndex ~ Δtime (days)) for all investigated indices using a multiple-testing corrected α = 0.0024 threshold.
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank all research volunteers for their participation. Especially we want to thank Riccardo Bonadonna for his idea of adding the discriminant ratio to our work.
Data Availability
The minimal anonymized data set necessary to replicate our study findings can be found at: https://figshare.com/articles/dataset/dataset_out_xlsx/14654124.
Funding Statement
This study was partly supported by a grant (01GI0925) from the Federal Ministry of Education and Research (BMBF) to the German Center for Diabetes Research (DZD e.V.). We acknowledge support by the Deutsche Forschungsgemeinschaft and the Open Access Publishing Fund of the University of Tübingen. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bergman RN, Finegood DT, Ader M. Assessment of Insulin Sensitivity in Vivo *. Endocr Rev. 1985. Jan;6(1):45–86. doi: 10.1210/edrv-6-1-45 [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol-Endocrinol Metab. 1979. Sep 1;237(3):E214. doi: 10.1152/ajpendo.1979.237.3.E214 [DOI] [PubMed] [Google Scholar]
- 3.Stefan N, Fritsche A, Haring H, Stumvoll M. Effect of Experimental Elevation of Free Fatty Acids on Insulin Secretion and Insulin Sensitivity in Healthy Carriers of the Pro12Ala Polymorphism of the Peroxisome Proliferator-Activated Receptor- 2 Gene. Diabetes. 2001. May 1;50(5):1143–8. doi: 10.2337/diabetes.50.5.1143 [DOI] [PubMed] [Google Scholar]
- 4.Boden G. Obesity, insulin resistance and free fatty acids: Curr Opin Endocrinol Diabetes Obes. 2011. Apr;18(2):139–43. doi: 10.1097/MED.0b013e3283444b09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, et al. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest. 1989. Jul 1;84(1):205–13. doi: 10.1172/JCI114142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA. Muscle and Liver Insulin Resistance Indexes Derived From the Oral Glucose Tolerance Test. Diabetes Care. 2007. Jan 1;30(1):89–94. doi: 10.2337/dc06-1519 [DOI] [PubMed] [Google Scholar]
- 7.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999. Sep 1;22(9):1462–70. doi: 10.2337/diacare.22.9.1462 [DOI] [PubMed] [Google Scholar]
- 8.DeFronzo RA, Ferrannini E, Simonson DC. Fasting hyperglycemia in non-insulin-dependent diabetes mellitus: Contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metabolism. 1989. Apr 1;38(4):387–95. doi: 10.1016/0026-0495(89)90129-7 [DOI] [PubMed] [Google Scholar]
- 9.Otten J, Ahrén B, Olsson T. Surrogate measures of insulin sensitivity vs the hyperinsulinaemic–euglycaemic clamp: a meta-analysis. Diabetologia. 2014. Sep;57(9):1781–8. doi: 10.1007/s00125-014-3285-x [DOI] [PubMed] [Google Scholar]
- 10.Mather KJ, Hunt AE, Steinberg HO, Paradisi G, Hook G, Katz A, et al. Repeatability Characteristics of Simple Indices of Insulin Resistance: Implications for Research Applications. J Clin Endocrinol Metab. 2001. Nov 1;86(11):5457–64. doi: 10.1210/jcem.86.11.7880 [DOI] [PubMed] [Google Scholar]
- 11.Kroll MH. Biological variation of glucose and insulin includes a deterministic chaotic component. Biosystems. 1999. Jun 1;50(3):189–201. doi: 10.1016/s0303-2647(99)00007-6 [DOI] [PubMed] [Google Scholar]
- 12.Perich C, Minchinela J, Ricós C, Fernández-Calle P, Alvarez V, Doménech MV, et al. Biological variation database: structure and criteria used for generation and update. Clin Chem Lab Med. 2015. Feb;53(2):299–305. doi: 10.1515/cclm-2014-0739 [DOI] [PubMed] [Google Scholar]
- 13.Van Cauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev. 1997. Oct;18(5):716–38. doi: 10.1210/edrv.18.5.0317 [DOI] [PubMed] [Google Scholar]
- 14.Van Cauter E, Blackman JD, Roland D, Spire JP, Refetoff S, Polonsky KS. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest. 1991. Sep;88(3):934–42. doi: 10.1172/JCI115396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson DG, Wingate DL, Thomas M, Harrison D. Gastric emptying as a determinant of the oral glucose tolerance test. Gastroenterology. 1982. Jan 1;82(1):51–5. [PubMed] [Google Scholar]
- 16.Dupre J, Ross SA, Watson D, Brown JC. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab. 1973. Nov;37(5):826–8. doi: 10.1210/jcem-37-5-826 [DOI] [PubMed] [Google Scholar]
- 17.Stumvoll M, Tschritter O, Fritsche A, Staiger H, Renn W, Weisser M, et al. Association of the T-G Polymorphism in Adiponectin (Exon 2) With Obesity and Insulin Sensitivity: Interaction With Family History of Type 2 Diabetes. Diabetes. 2002. Jan 1;51(1):37–41. doi: 10.2337/diabetes.51.1.37 [DOI] [PubMed] [Google Scholar]
- 18.Willmann C, Heni M, Linder K, Wagner R, Stefan N, Machann J, et al. Potential effects of reduced red meat compared with increased fiber intake on glucose metabolism and liver fat content: a randomized and controlled dietary intervention study. Am J Clin Nutr. 2019. Feb 1;109(2):288–96. doi: 10.1093/ajcn/nqy307 [DOI] [PubMed] [Google Scholar]
- 19.Kantartzis K, Fritsche L, Bombrich M, Machann J, Schick F, Staiger H, et al. Effects of resveratrol supplementation on liver fat content in overweight and insulin‐resistant subjects: A randomized, double‐blind, placebo‐controlled clinical trial. Diabetes Obes Metab. 2018. Jul;20(7):1793–7. doi: 10.1111/dom.13268 [DOI] [PubMed] [Google Scholar]
- 20.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative Insulin Sensitivity Check Index: A Simple, Accurate Method for Assessing Insulin Sensitivity In Humans. J Clin Endocrinol Metab. 2000. Jul 1;85(7):2402–10. doi: 10.1210/jcem.85.7.6661 [DOI] [PubMed] [Google Scholar]
- 21.Perseghin G, Caumo A, Caloni M, Testolin G, Luzi L. Incorporation of the Fasting Plasma FFA Concentration into QUICKI Improves Its Association with Insulin Sensitivity in Nonobese Individuals. J Clin Endocrinol Metab. 2001. Oct 1;86(10):4776–81. doi: 10.1210/jcem.86.10.7902 [DOI] [PubMed] [Google Scholar]
- 22.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998. Dec;21(12):2191–2. doi: 10.2337/diacare.21.12.2191 [DOI] [PubMed] [Google Scholar]
- 23.HOMA2 Calculator [Internet]. Available from: https://www.dtu.ox.ac.uk/homacalculator/index.php
- 24.Mari A, Pacini G, Murphy E, Ludvik B, Nolan JJ. A Model-Based Method for Assessing Insulin Sensitivity From the Oral Glucose Tolerance Test. Diabetes Care. 2001. Mar 1;24(3):539–48. doi: 10.2337/diacare.24.3.539 [DOI] [PubMed] [Google Scholar]
- 25.OGIS—insulin sensitivity from the oral glucose test [Internet]. Available from: http://webmet.pd.cnr.it/ogis/ogis.php
- 26.Belfiore F, Iannello S, Volpicelli G. Insulin sensitivity indices calculated from basal and OGTT-induced insulin, glucose, and FFA levels. Mol Genet Metab. 1998. Feb;63(2):134–41. doi: 10.1006/mgme.1997.2658 [DOI] [PubMed] [Google Scholar]
- 27.Wagner R, Fritsche L, Heni M, Fehlert E, Stefan N, Staiger H, et al. A novel insulin sensitivity index particularly suitable to measure insulin sensitivity during gestation. Acta Diabetol. 2016. Dec 1;53(6):1037–44. doi: 10.1007/s00592-016-0930-5 [DOI] [PubMed] [Google Scholar]
- 28.Gutt M, Davis CL, Spitzer SB, Llabre MM, Kumar M, Czarnecki EM, et al. Validation of the insulin sensitivity index (ISI(0,120)): comparison with other measures. Diabetes Res Clin Pract. 2000. Mar;47(3):177–84. doi: 10.1016/s0168-8227(99)00116-3 [DOI] [PubMed] [Google Scholar]
- 29.Stumvoll M, Mitrakou A, Pimenta W, Jenssen T, Yki-Järvinen H, Haeften TV, et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care. 2000. Mar 1;23(3):295–301. doi: 10.2337/diacare.23.3.295 [DOI] [PubMed] [Google Scholar]
- 30.Seltzer HS, Allen EW, Herron AL, Brennan MT. Insulin Secretion in Response to Glycemic Stimulus: Relation of Delayed Initial Release to Carbohydrate intolerance in Mild Diabetes Mellitus. J Clin Invest. 1967. Mar 1;46(3):323–35. doi: 10.1172/JCI105534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Utzschneider KM, Prigeon RL, Faulenbach MV, Tong J, Carr DB, Boyko EJ, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care. 2009. Feb;32(2):335–41. doi: 10.2337/dc08-1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy J, Morris R, Hammersley M, Turner R. Discrimination, adjusted correlation, and equivalence of imprecise tests: application to glucose tolerance. Am J Physiol-Endocrinol Metab. 1999. Feb 1;276(2):E365–75. [DOI] [PubMed] [Google Scholar]
- 33.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; [Internet]. Vienna, Austria; 2020. Available from: https://www.R-project.org/. [Google Scholar]
- 34.Schousboe K, Henriksen JE, Kyvik KO, Sørensen TIA, Petersen PH. Reproducibility of S-insulin and B-glucose responses in two identical oral glucose tolerance tests. Scand J Clin Lab Invest. 2002. Jan;62(8):623–30. doi: 10.1080/003655102764654358 [DOI] [PubMed] [Google Scholar]
- 35.Heine RJ, Hanning I, Morgan L, Alberti KG. The oral glucose tolerance test (OGTT): effect of rate of ingestion of carbohydrate and different carbohydrate preparations. Diabetes Care. 1983. Oct;6(5):441–5. doi: 10.2337/diacare.6.5.441 [DOI] [PubMed] [Google Scholar]
- 36.Yeung EH, Zhang C, Mumford SL, Ye A, Trevisan M, Chen L, et al. Longitudinal Study of Insulin Resistance and Sex Hormones over the Menstrual Cycle: The BioCycle Study. J Clin Endocrinol Metab. 2010. Dec;95(12):5435–42. doi: 10.1210/jc.2010-0702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bingley CA, Gitau R, Lovegrove JA. Impact of menstrual cycle phase on insulin sensitivity measures and fasting lipids. Horm Metab Res Horm Stoffwechselforschung Horm Metab. 2008. Dec;40(12):901–6. doi: 10.1055/s-0028-1082081 [DOI] [PubMed] [Google Scholar]
- 38.Zambon A, Hashimoto SI, Brunzell JD. Analysis of techniques to obtain plasma for measurement of levels of free fatty acids. J Lipid Res. 1993. Jan 6;34(6):1021–8. [PubMed] [Google Scholar]
- 39.Matthews DR, Lang DA, Burnett MA, Turner RC. Control of pulsatile insulin secretion in man. Diabetologia. 1983. Apr;24(4):231–7. doi: 10.1007/BF00282705 [DOI] [PubMed] [Google Scholar]
- 40.Mari A, Pacini G, Brazzale AR, Ahrén B. Comparative evaluation of simple insulin sensitivity methods based on the oral glucose tolerance test. Diabetologia. 2005. Apr 1;48(4):748–51. doi: 10.1007/s00125-005-1683-9 [DOI] [PubMed] [Google Scholar]
- 41.Malita FM, Karelis AD, St-Pierre DH, Garrel D, Bastard JP, Tardif A, et al. Surrogate indexes vs. euglycaemic-hyperinsulinemic clamp as an indicator of insulin resistance and cardiovascular risk factors in overweight and obese postmenopausal women. Diabetes Metab. 2006. Jun;32(3):251–5. doi: 10.1016/s1262-3636(07)70276-8 [DOI] [PubMed] [Google Scholar]
- 42.Jones AG, Hattersley AT. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet Med. 2013. Jul;30(7):803–17. doi: 10.1111/dme.12159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tura A, Kautzky-Willer A, Pacini G. Insulinogenic indices from insulin and C-peptide: Comparison of beta-cell function from OGTT and IVGTT. Diabetes Res Clin Pract. 2006. Jun 1;72(3):298–301. doi: 10.1016/j.diabres.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 44.Mari A, Ferrannini E. β-cell function assessment from modelling of oral tests: an effective approach. Diabetes Obes Metab. 2008;10(s4):77–87. [DOI] [PubMed] [Google Scholar]
- 45.Lorenzo C, Wagenknecht LE, Rewers MJ, Karter AJ, Bergman RN, Hanley AJG, et al. Disposition Index, Glucose Effectiveness, and Conversion to Type 2 Diabetes: The Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care. 2010. Sep 1;33(9):2098–103. doi: 10.2337/dc10-0165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Utzschneider KM, Prigeon RL, Tong J, Gerchman F, Carr DB, Zraika S, et al. Within-subject variability of measures of beta cell function derived from a 2 h OGTT: implications for research studies. Diabetologia. 2007. Nov 5;50(12):2516–25. doi: 10.1007/s00125-007-0819-5 [DOI] [PubMed] [Google Scholar]
- 47.Mari A, Tura A, Gastaldelli A, Ferrannini E. Assessing Insulin Secretion by Modeling in Multiple-Meal Tests: Role of Potentiation. Diabetes. 2002. Feb 1;51(suppl 1):S221–6. doi: 10.2337/diabetes.51.2007.s221 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Coefficients of variation of the analyzed indices in relation to the number of citations of the original article per year calculated by the total number of citations divided by the years since first publication. Indices that we could not found or differentiate in our literature research or which are not published yet are presented in red using random values for the y axis. Abbreviations: C = C-peptide; I = Insulin; G = Glucose.
(TIF)
Samples with a time span more than one year between the two OGTTs are already excluded. Linear regression models (CoVIndex ~ Δtime (days)) for all investigated indices using a multiple-testing corrected α = 0.0024 threshold.
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
The minimal anonymized data set necessary to replicate our study findings can be found at: https://figshare.com/articles/dataset/dataset_out_xlsx/14654124.