Abstract
Aspirin has been reported for its anti-tumor activity, however, there are few studies on its effects in lung cancer. The present study found that aspirin had a dual role in the proliferation of human lung cancer PC-9 (formerly known as PC-14) and A549 cells, and in human colon cancer HCT116 cells. The cells were treated with 0, 1, 2, 4, 8 and 16 mM aspirin for 24-72 h or 7-12 days and cell proliferation was examined by MTT and colony formation assay. In order to explore the relationship between the proliferation-enhancing effect of low-dose aspirin and mitogen-activated protein kinase (MAPK) signaling activation, PC-9 cells were pretreated with 10 µM PD98059 (a specific inhibitor of ERK), SB203580 (a specific inhibitor of p38) and SP600125 (a specific inhibitor of JNK) for 30 min respectively. Western blot assay was performed to detect the activation of MAPK members in PC-9 cells. Cellular apoptosis was detected using flow cytometer-based Annexin V/propidium iodide dual staining. An assessment of MAPK inhibitors was performed to further validate the role of JNK, p38 and ERK in aspirin-promoted PC-9 cell growth. It was demonstrated that aspirin could promote the growth of human PC-9 lung cancer cells and induced MAPK activation at low concentrations. All the MAPK inhibitors tested (PD98059, SB203580 and SP600125) were able to inhibit the aspirin-induced proliferation of PC-9 cells.
Keywords: aspirin, mitogen-activated protein kinase, ERK, p38, JNK, lung cancer
Introduction
Cancer remains an important global public health problem and lung cancer is a very common cause of malignant tumor development (1). With the gradually deteriorating natural environment due to continuous development and industrialization, the global morbidity and mortality of lung cancer in the past decade has increased, with serious implications for the quality of human life (2). A number of factors are involved in lung cancer progression, such as mutation in tumor suppressor genes, cell proliferation, angiogenesis and chronic inflammation (3). The increased understanding of disease mechanism has led to the development of a number of new antitumor agents and associated therapies, the majority of which have been clinically applied. Aspirin has been a cornerstone in cardiovascular disease prevention since the late 1980s (4) and the antitumor effect of aspirin has received increasing attention. Experimental, epidemiological and clinical data from the last two decades have supported the hypothesis that aspirin possesses anti-cancer properties and its use may also reduce the lifetime probability of developing or dying from a number of cancers (5,6). The mechanism of action of aspirin as an antineoplastic agent is multifold through its anti-platelet effect, inhibition of the enzyme cyclooxygenase (COX), as well as through COX-independent mechanisms and its impact on multiple metabolic signaling pathways are considered critical to its anticancer effects (7-9). Aspirin exerts a proliferation-inhibiting and apoptosis-inducing potential on a number of cancer cell lines, such as hepatocellular carcinoma cells (9). However, it remains to be elucidated whether aspirin has similar inhibitory effect on lung cancer cells.
Mitogen-activated protein kinase (MAPK) signaling pathways are involved in mediation of processes of cell growth, survival and death (10-12). There are three members in the MAPK family; JNK, p38MAPK and ERK. They have diverse cellular functions and are controlled separately by relatively independent upstream signaling molecules (13). These factors are serine/threonine protein kinases that regulate various cellular activities, including proliferation, differentiation, apoptosis, survival, inflammation and innate immunity (14,15). The abnormal activation of MAPK signaling pathways contributes to the pathogenesis of diverse human diseases including cancer (14,16,17). All the MAPK family members can be activated in response to various endogenous and exogenous stresses. Upon stimulation, the phosphorylated/activated ERK can change gene expression to promote cell growth, differentiation or mitosis (18). In some cases, ERK uses JNK as its final mediator to stimulate cell proliferation. p38MAPK appears to play a major role in apoptosis, differentiation, survival, proliferation, development, inflammation and other stress responses (19).
In the current study, it was found that although a high-dose of aspirin showed remarkable tumor suppression in PC-9 lung cancer cells, a relatively low dose of this agent exhibited a marked growth-promoting effect on PC-9 cells. As for the growth-promoting mechanism of low-dose aspirin, it was shown that all the MAPK signaling was involved.
Materials and methods
Materials
Aspirin was freshly dissolved in DMSO (<0.25% final concentration) before assays. DMEM medium and fetal bovine serum (FBS) were obtained from Thermo Fisher Scientific, Inc. Dimethyl sulfoxide (DMSO), MTT and crystal violet were purchased from Sigma-Aldrich (Merck KGaA). The fluorescein isothiocyanate (FITC) Annexin V and propidium iodide (PI) kit for apoptosis detection was obtained from Multisciences (Lianke) Biotech Co., Ltd., anti-ERK (cat. no. WL01864), anti-phosphorylated (p)-ERK (Thr202/Tyr204; cat. no. WLP1512), anti-JNK (cat. no. WL01295), anti-p-JNK (cat. no. WL01813), anti-p38 (cat. no. WL00764), anti-p-p38 (cat. no. WLP1576), anti-cleaved-caspase3 (cat. no. WL02117), anti-Bax (cat. no. WL01637),anti-Bcl-2 antibodies (cat. no. WL01556) and secondary antibodies (cat no. WLA023) were purchased from Wanleibio Co., Ltd. PD98059 (an ERK inhibitor), SP600125 (a JNK inhibitor) and SB203580 (a p38 inhibitor) were purchased from MedChem Express. Penicillin-streptomycin solution, 100X (cat. no. P1400) and anti-GAPDH (cat. no. K106389P) were purchased from Beijing Solarbio Science and Technology Co., Ltd.
Cell culture
Human lung cancer cell lines PC-9 (formerly known as PC-14: Please see the following website for further details https://web.expasy.org/cellosaurus/CVCL_1640) and A549 were obtained from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences. Human colon cancer cell line HCT116 was gifted by Professor Zhang Yu of the Institute of Biological Sciences, Jinzhou Medical University (Jinzhou, China). Cells were maintained in DMEM with 10% FBS, 1% penicillin-streptomycin solution at 37˚C in a humidified atmosphere of 5% CO2. All cells were tested to ensure that they were mycoplasma contamination-free using a PCR-based assay. The cells in single drug groups were treated with 0.25% DMSO (control group) and different concentrations of aspirin (1, 2, 4, 8 and 16 mM). The inhibitor groups were pre-incubated with PD98059 (10 µM), SP600125 (10 µM) or SB203580 (10 µM) respectively and then treated with different concentrations of aspirin.
Colony formation experiments
For this experiment, 600 cells were seeded in six-well plates and treated with various concentrations of aspirin in the presence or absence of MAPK inhibitors for 7 days in single drug groups. For inhibitor groups, cells were pre-treated with a selective MAPK inhibitor (the ERK inhibitor PD98059, the JNK inhibitor SP600125 and the p38 MAPK inhibitor SB203580) for 30 min respectively and then treated with aspirin for 7 days. Control cells were treated with an equal amount of DMSO (0.25% v/v). The culture was terminated when colonies were visible to the naked eye in the culture dish. Then the supernatant was discarded by aspiration and the cells were washed twice with PBS before 4% paraformaldehyde (1 ml) was slowly poured into in each petri dish to fix cells for 20 min at room temperature. The fixative was then discarded and an appropriate amount of crystal violet added (0.1%) for 5-10 min at room temperature before finally being slowly washed away with water. Images were obtained with a camera and ImageJ version 1.8.0.112 (National Institutes of Health) was used to calculate the colony area. Every image includes a full well to ensure the same focal length. The software was used to count the area of blue colony pixels, a common threshold was set. If the gray level was greater than this threshold the area was counted as a colony and if it was less than this value, it was counted as a background.
MTT assay
MTT colorimetric assay was used to detect the effect of aspirin on cell viability. PC-9, A549 and HCT116 cells were seeded in 96-well plates (2,000 cells/well). After adherence, the cells were treated with different concentrations of aspirin (0, 1, 2, 4, 8 and 16 mM) for different time points (0, 24, 48 and 72 h). Subsequently, MTT (5 mg/ml) was added and the plates incubated in an atmosphere of 5% CO2/95% air at 37˚C for 4 h. The supernatant was discarded and 150 µl of DMSO was added and agitated at 37˚C for 10 min until the crystals were sufficiently dissolved. The absorbance values were measured at 570 nm using a microplate reader (Bio-Rad Laboratories, Inc.) at different time points (0, 24, 48 and 72 h).
Western blot analysis
Western blot analyses were performed as previously described (20). The cells were cultured, harvested and lysed on ice for 30 min in an appropriate lysis buffer (120 mM NaCl, 40 mM Tris (pH 8.0) and 0.1% NP-40) and were then centrifuged at 13,000 x g for 15 min at 4˚C. Protein samples were quantified using a BCA kit (Thermo Fisher Scientific, Inc.). Lysates from each sample were mixed with 5X sample buffer (0.375 M Tris-HCl, 5% SDS, 5% β-mercaptoethanol, 50% glycerol and 0.05% bromophenol blue, pH 6.8) and were heated to 95˚C for 5 min. Equal amounts (30 µg) of protein lysate were separated by 12% SDS-PAGE and were transferred onto polyvinylidene fluoride membranes. The membranes were then washed with Tris-buffered saline (10 mM Tris, 150 mM NaCl) containing 0.05% Tween-20 (TBST) and were blocked in TBST containing 5% BSA (Beijing Solarbio Science & Technology Co., Ltd.) for 2 h at room temperature. Next, the membranes were incubated with their respective specific primary antibodies (ERK, p-ERK, JNK, p-JNK, p38, p-p38, Bcl-2, Bax at a dilution of 1:500; GAPDH at a dilution of 1:3,000) overnight at 4˚C. After three washes in TBST, the membranes were incubated with the appropriate secondary antibody conjugated to horseradish peroxidase (cat no. WLA023; 1:3,000) for 1 h at room temperature. Then the membranes were washed again and an enhanced chemiluminescence-based ECL detection kit (Cyanagen Srl) was used. ImageJ version 1.8.0.112 (National Institutes of Health) was used for densitometry. Data of specific protein levels are presented relative to the control. All experiments were repeated at least three times.
Apoptosis assessment
The Annexin V/PI double staining assay recognizes the externalization of phosphatidylserine on the cell membrane, a hallmark of apoptotic cells. In brief, 1x105 cells were seeded on a 24-well plate and treated with aspirin alone or 30 min pre-treatment of an ERK inhibitor PD98059 or JNK inhibitor SP00125 or P38 inhibitor SB203580 for 24 h. Cells were dissociated with trypsin, harvested and washed with PBS. The cells were resuspended in 1X binding buffer (100 µl) and incubated with 1.4 µl of Annexin V-FITC (200 µg/ml) and 5 µl of PI (30 µg/ml) at room temperature for 10 min. The cells stained with Annexin V/PI were analyzed by a flow cytometer (BD Immunocytometry Systems). The amounts of early apoptosis and late apoptosis were determined as the percentage of Annexin V+/PI- or Annexin V+/PI+ cells, respectively. FlowJo version 10.5.3 (FlowJo LLC) was used for analysis.
Cell cycle analysis
For cell cycle analysis, cells were harvested and fixed in 70% (v/v) ethanol and then washed with PBS. All cells were stained with PI solution and the DNA content of each cell was detected by flow cytometry (21). ModFit LT 4.0 (Verity Software House) was used for analysis.
Statistical analysis
All statistical differences were evaluated using one-way analysis of variance followed by Dunnet's test. All experiments were independently performed three times. Data are presented as the mean ± SD and were analyzed using SPSS 21.0 software (IBM Corp.). P<0.05 was considered to indicate a statistically significant difference.
Results
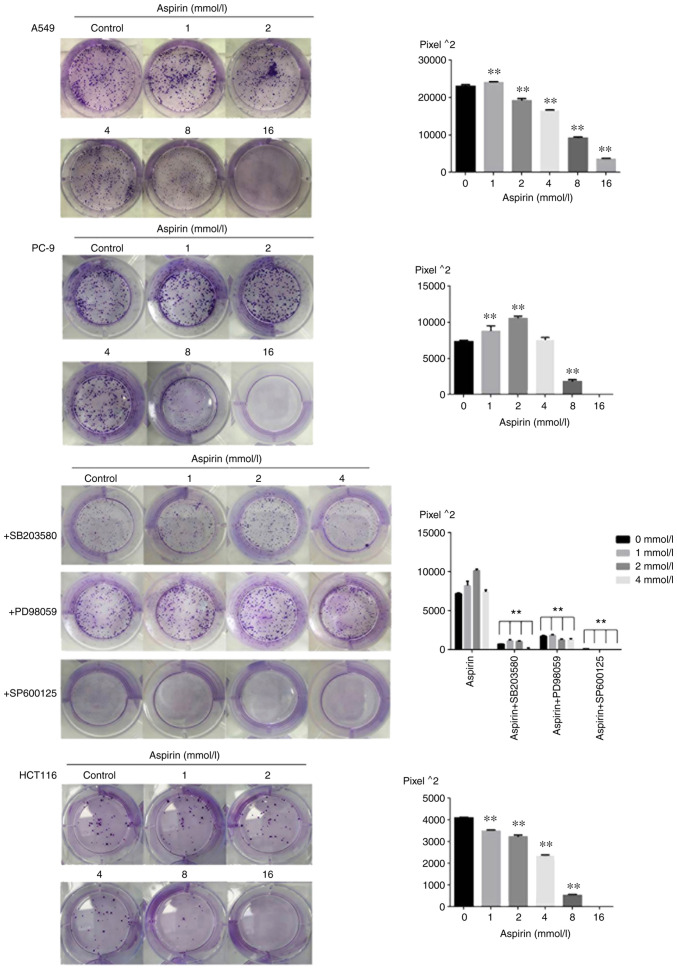
Effects of aspirin on colony formation area of PC-9 cells
The present study examined the effects of aspirin on the proliferation of PC-9, A549 and HCT116 cells by colony formation experiments. Cells were exposed to various concentrations (0, 1, 2, 4, 8 and 16 mM) of aspirin for 7-12 day. Almost no colony formation was observed at the concentration of 16 mM. The results of colony formation are shown as Fig. 1; aspirin significantly promoted the proliferation of PC-9 human lung cancer cells at low concentrations between 1 and 4 mM and significantly inhibited the proliferation at the concentrations of 8 and 16 mM. Aspirin-treated colony formation area at 1, 2 and 4 mM were 1.16-, 1.44- and 1.01-fold relative to the control when 600 cells were seeded per well. Aspirin slightly promoted colony growth of A549 cells at 1 mM concentration and inhibited colony growth of HCT116 cells at various concentrations.
Figure 1.
Colony formation area of PC-9, A549 and HCT116 cells treated with different concentrations of aspirin for 7-12 days (n=3, mean ± standard deviation; **P<0.01 vs. control and Effects of MAPK inhibitors on the colony formation of PC-9 cells upon aspirin stimulation). The size of the colony formation area is indirectly expressed through the number of image pixels.
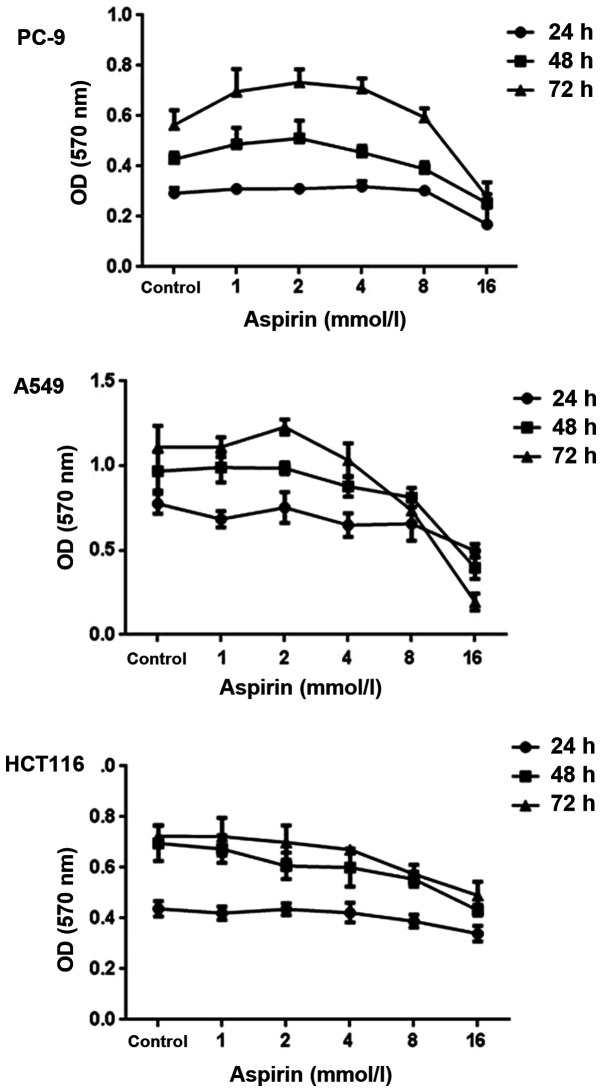
Effects of aspirin on vitality of PC-9 cells
The effects of aspirin on the growth of PC-9 human lung cancer cells were examined by MTT assay. Cells were exposed to various concentrations (0, 1, 2, 4, 8 and 16 mM) of aspirin for 0, 24, 48 and 72 h. As shown in Fig. 2, aspirin-treated cell vitality was significantly increased at the concentrations of 1, 2 and 4 mM and inhibited at 8 and 16 mM. After 48 h of exposure, aspirin induced 13.8% growth promotion at the concentrations of 1 mM, 19.4% at 2 mM and 6.31% at 4 mM, respectively. After 72 h of exposure, aspirin induced 23.4% growth promotion at the concentrations of 1 mM, 29.9% at 2 mM and 25.6% at 4 mM, respectively. It was also observed that 2 mM aspirin has a vitality-promotion at 72 h in A549 cells but no such effect in HCT116 cells.
Figure 2.
Cell viability at different concentrations of aspirin in PC-9, A549 and HCT116 cells at 0, 24, 48 and 72 h was assessed by MTT assay (n=3, mean ± standard deviation).
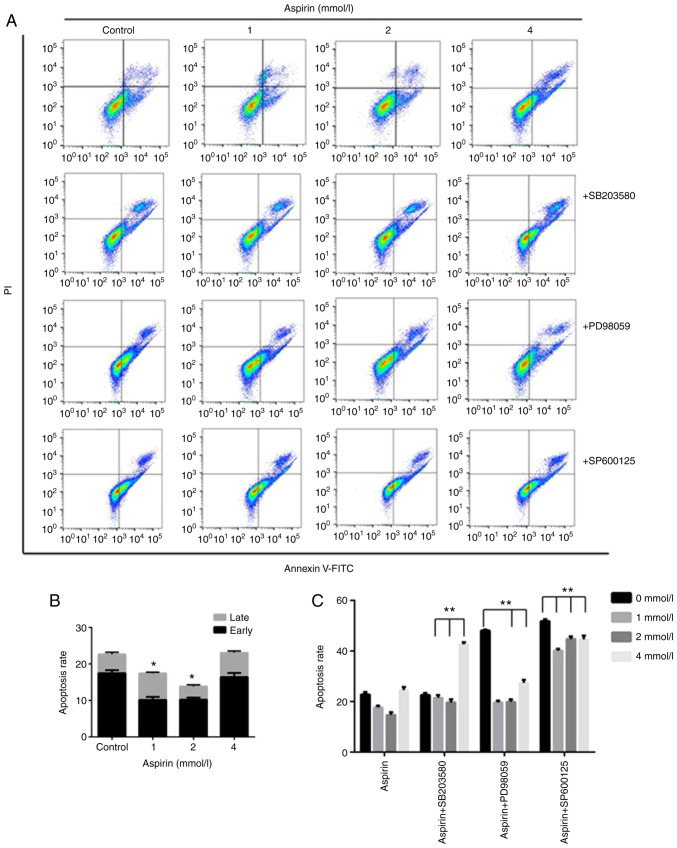
Effects of aspirin on apoptosis in PC-9 cells
To quantify the extent of apoptotic cells, flow cytometry analysis was performed using double staining with Annexin V and PI. The Annexin V-/PI- population was considered to represent unaffected cells, Annexin V+/PI- as early apoptosis, Annexin V+/PI+ as late apoptosis and Annexin V-/PI+ as necrosis. The results showed that low-dose aspirin-treated cells significantly decrease the percentage of apoptotic cells compared with untreated control cells (Fig. 3A and B). In detail, early apoptotic cell populations were decreased 7.3% at the concentration of 1 mM, 6.8% at 2 mM compared with 17.6% of the control. The total apoptotic cell populations were also decreased at 4.99 and 8.25% at 1 and 2 mM, respectively, compared with 22.78% of the control.
Figure 3.
Effect of low-dose aspirin and MAPK inhibitors on the apoptosis of PC-9 cells. (A) Flow cytometric analysis of PC-9 human lung cancer cells incubated with aspirin and MAPK inhibitors for 24 h. The right bottom quadrant represents Annexin V-stained cells (early-phase apoptotic cells). The top right quadrant represents PI and Annexin V-stained cells (late-phase apoptotic cells). (B) Quantitative results of the aspirin group in (A) and in the absence of MAPK inhibitors. (C) Quantitative results of cell apoptosis in (A). (n=3, mean ± standard deviation; *P<0.05 and **P<0.01 vs. control cells).
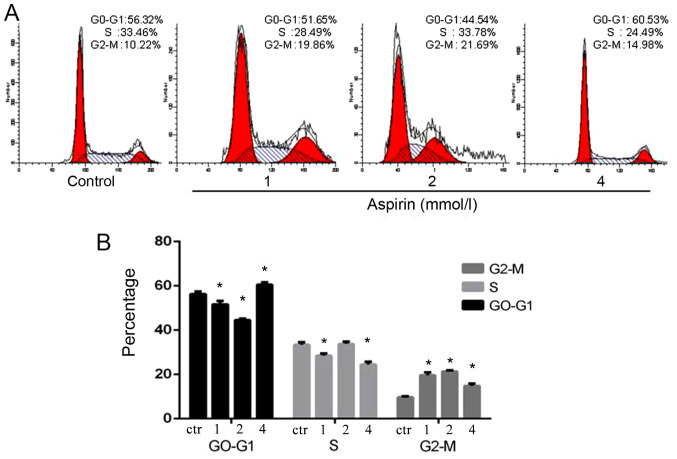
Effects of aspirin on cell cycle phase distribution in PC-9 cells
The fluorescence-activated cell sorting analysis clearly indicated that low-dose aspirin could promote G1/S-phase transition in PC-9 cells. The stimulation of aspirin changed the cell proliferation cycle, which led to more cells in the 4N phase. However, it suggested that low-dose aspirin could facilitate cells into G2-phase of cell cycle (Fig. 4).
Figure 4.
Aspirin accelerates G1/S-phase progression in PC-9 cells. (A) The Fluorescence-activated cell sorting analysis indicated that aspirin could promote G1/S phase transition in PC-9 cells. (B) Quantitative results of (A). *P<0.05 vs. control.
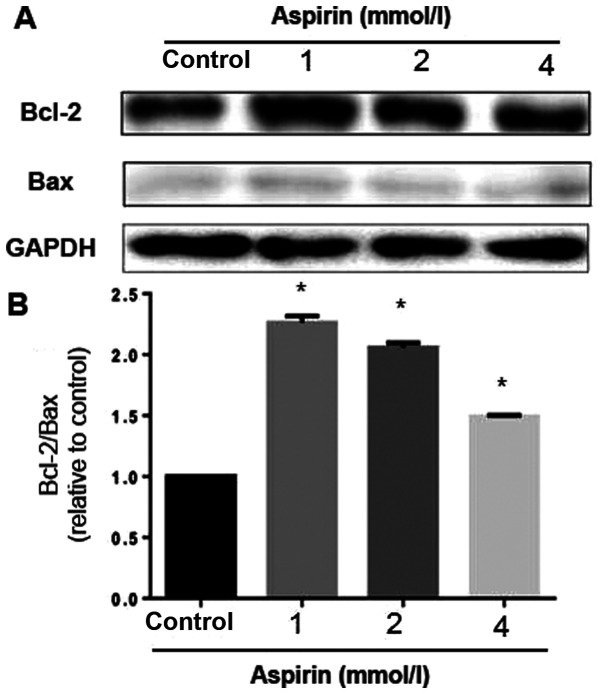
Effects of aspirin on the expression of Bcl-2 family proteins
To study the effects of low-dose aspirin on PC-9 cells, the expression levels of a number of apoptosis regulatory proteins, including Bcl-2 and Bax, were examined. The mitochondrial pathway is an important apoptosis pathway as it regulates the apoptotic cascade via a convergence of signaling at the mitochondria (22). Bcl-2 interacts with the mitochondrial plasma membrane and prevents mitochondrial membrane pores from opening during apoptosis, blocking the signals of apoptotic factors, such as Bax (23). Low-dose aspirin decreased Bax expression but increased the expression of Bcl-2. A densitometric analysis of the bands revealed that low-dose aspirin caused a decreased Bax/Bcl-2 ratio (Fig. 5). These results suggested that aspirin can reduce apoptosis through the regulation of apoptosis-related protein expression in PC-9 cells at low concentration.
Figure 5.
Effects of low-dose aspirin on expression of apoptosis-related proteins in PC-9 human lung cancer cells. (A) Bcl-2 and Bax were detected by western blotting with the corresponding antibodies.(B) Densitometric analysis of protein bands in (A). *P<0.05 vs. control.
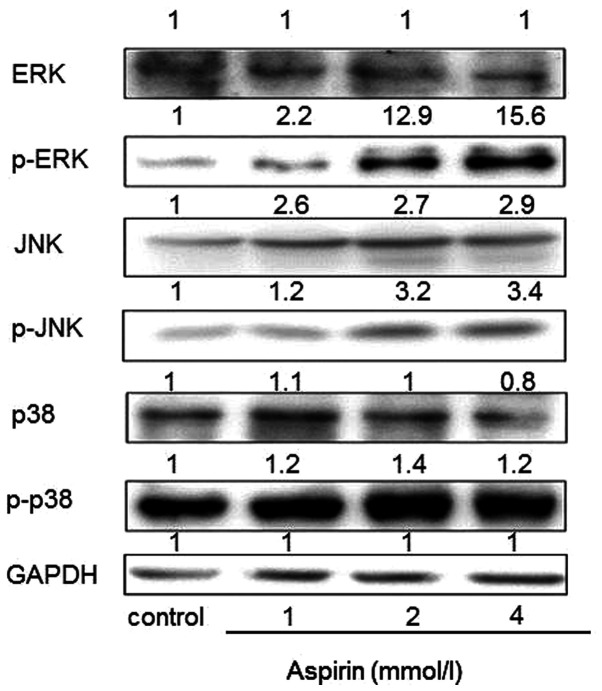
Effects of aspirin on the expression of MAPK in PC-9 cells
MAPKs signaling cascades, including JNK, ERK and p38 kinase, are found in all eukaryotes and have been demonstrated to play a central role in regulating cell proliferation, differentiation and apoptosis (24). To further determine whether MAPKs are involved in the low-dose aspirin-induced PC-9 cells proliferation promotion, the phosphorylation expression levels of MAPKs were examined. As shown in Fig. 6, low-dose aspirin treatment for 24 h significantly increased phosphorylation of JNK, ERK and p38 MAPK. Total protein levels of ERK and p38 remained constant. Notably, total protein levels of JNK were markedly increased.
Figure 6.
Regulation of MAPKs in aspirin-treated PC-9 human lung cancer cells. JNK, ERK and p38 and their phosphorylated expression forms were detected by western blot analysis with corresponding antibodies. MAPKs, mitogen-activated protein kinases; p-, phosphorylated.
Effects of MAPK inhibitor on growth promotion of PC-9 cells induced by low-dose aspirin
In order to investigate the significance of MAPK activation in the low-dose aspirin treatment, PC-9 cells were treated with low-dose aspirin in the presence or absence of SP600125, PD98059 or SB203580. As shown in Fig. 3A and C the treatment of MAPK inhibitor SP600125, PD98059 and SB203580 effectively prevented the aspirin-induced growth promotion. The results of double staining with Annexin V and PI showed that treatment with low-dose aspirin in PC-9 cells in presence of MAPK inhibitor significantly increased the percentage of apoptotic cells compared with that in absence of MAPK inhibitor. The effects of MAPK inhibitors on low-dose aspirin-induced proliferation promotion in PC-9 cells was also examined by the colony formation as shown in Fig. 1. The results showed that the colony formation area of the inhibitor group was smaller than that of the aspirin group.
Discussion
Aspirin can be regarded as one of the most commonly used synthetic drugs in human history. It was originally developed and sold at the end of the 19th century, mainly for the treatment of inflammatory diseases. However, its mechanism of action remains to be determined. Low doses of aspirin are also used for primary and secondary prevention of cardiovascular disease to prevent antithrombotic formation (25). Previous findings have shown that aspirin has a role in reducing cancer risk, but whether it can be used as an anticancer drug for cancer patients was unknown until recently (26). Based on the safety of aspirin for clinical use over the years and its anti-tumor activity (25), it was investigated whether aspirin can also play a role in lung cancer treatment and the effect of aspirin on lung cancer PC-9 cells observed. However, the results of the present study indicated that aspirin stimulated the proliferation of PC-9 cells at low concentrations and significantly inhibited PC-9 cells growth at 8 mM or higher concentrations.
Apoptosis is an important phenomenon due to its maintenance of cellular homeostasis by regulating cell division and cell death. There is increasing evidence that the processes of neoplastic transformation, progression and metastasis involve alterations in the normal apoptotic pathways (27). Furthermore, the majority of chemotherapeutic agents as well as radiation utilize the apoptotic pathway to induce cancer cell death (28). The present study investigated apoptosis by flow cytometry in PC-9 lung cancer cells which were treated with various concentrations of aspirin in the presence or absence of SP600125, PD98059 and SB203580. The results showed that treatment of PC-9 cells with low-dose aspirin significantly decreased the percentage of apoptotic cells compared with untreated control cells and the treatment of MAPK inhibitor (SP600125, PD98059 and SB203580) effectively prevented the aspirin-induced growth promotion. Low-dose aspirin treatment for 24 h significantly increased the phosphorylation of JNK, ERK and p38 MAPK in PC-9 cells. These results indicated that low-dose aspirin-induced proliferation promotion in PC-9 may be involved in the activation of MAPK.
At present, apoptotic signals have two main pathways: the extrinsic or death receptor pathway and the intrinsic or mitochondrial-mediated pathway (29). The mitochondrial-related pathway is regulated by anti-apoptotic (Bcl-2, Bcl-x and Bcl-XL) and pro-apoptotic members (Bax, Bcl-2 homologous antagonist/killer, BH3 interacting-domain death agonist, Bad and Bcl-2-like protein 11) of the Bcl-2 family (30). The anti-apoptotic proteins on the outer membranes of mitochondria maintain the integrity of the mitochondria by inhibiting apoptosis in the presence of various apoptotic stimuli (31). Therefore, the balance between the expression levels of Bax and Bcl-2 is important to cell survival as well as death. Data from the present study showed that aspirin-induced PC-9 growth promotion was associated with the downregulation of Bax protein as well as the upregulation of Bcl-2 expression. Previous findings have also suggested that the Bax/Bcl-2 ratio is a determining factor in regulating the apoptotic process (32). An analysis of the present data indicated that low-dose aspirin may decrease the Bax/Bcl-2 ratio in PC-9 cells, which suggested that low-dose aspirin has anti-apoptotic function in PC-9 cells.
The results of apoptosis were consistent with the results of cell cycle analysis. Low concentrations of aspirin promoted G1/S phase to G2 phase of the cell cycle process and promoted cell proliferation, which was consistent with the MTT and colony experiment. This once again validated the hypothesis of the present study. The comparative results of inhibitor group and the single drug group also showed that MAPK inhibitors can prevent the low-dose aspirin-caused proliferation and promotion of PC-9 cells in the MTT and colony formation experiments.
The present study identified that aspirin promoted the growth of human PC-9 lung cancer cells at low concentrate, particularly <4 mM. Low-dose aspirin treatment for 24 h significantly increased phosphorylation of JNK, ERK and p38 MAPK in PC-9 cells. All of the above results indicated that low-dose aspirin could stimulate the proliferation of lung cancer PC-9 cells through activation of MAPK signaling.
In summary, the present study identified that low-dose aspirin has the potential to promote the proliferation of PC-9 lung cancer cells and that its mechanism was related to MAPK activation. Low-dose aspirin (75-300 mg/day) is used as a basic medication to treat cardiovascular and cerebrovascular disease. However, the results of the present study suggested that the use of low-dose aspirin may pose a risk of cancer progression in lung cancer and a cardiovascular co-morbidity. The conclusions of the present study need further verification.
The results of the present study suggested that the activation of MAPK signaling, at least partially, was responsible for low-dose aspirin-induced proliferation promotion. By contrast, high-dose aspirin attenuated the proliferation of PC-9 cells. These results may suggestion caution should be exercised when employing aspirin in the clinic.
Acknowledgements
The authors thank Ms. Danyang Zhang (The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, China) for experimental guidance and Dr Lei Zhou (The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, China) for language assistance.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HD, YQ and HL conceived and designed the study; YQ performed the experiments and wrote the paper. HD revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Zhang SW, Zheng RS, Yang ZX, Zeng HM, Sun KX, Gu XY, Li H, Chen WQ, He J. Trend analysis on incidence and age at diagnosis for lung cancer in cancer registration areas of China, 2000-2014. Zhonghua Yu Fang Yi Xue Za Zhi. 2018;52:579–585. doi: 10.3760/cma.j.issn.0253-9624.2018.06.005. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics. Adv Exp Med Biol. 2016;893:1–19. doi: 10.1007/978-3-319-24223-1_1. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Guo Z. The role of sulfur in platinum anticancer chemotherapy. Anticancer Agents Med Chem. 2007;7:19–34. doi: 10.2174/187152007779314062. [DOI] [PubMed] [Google Scholar]
- 4.Kim J, Becker RC. Aspirin dosing frequency in the primary and secondary prevention of cardiovascular events. J Thromb Thrombolysis. 2016;41:493–504. doi: 10.1007/s11239-015-1307-2. [DOI] [PubMed] [Google Scholar]
- 5.Okada S, Morimoto T, Ogawa H, Sakuma M, Matsumoto C, Soejima H, Nakayama M, Doi N, Jinnouchi H, Waki M, et al. Effect of aspirin on cancer chemoprevention in Japanese patients with type 2 diabetes: 10-Year observational follow-up of a randomized controlled trial. Diabetes Care. 2018;41:1757–1764. doi: 10.2337/dc18-0368. [DOI] [PubMed] [Google Scholar]
- 6.Hua H, Zhang H, Kong Q, Wang J, Jiang Y. Complex roles of the old drug aspirin in cancer chemoprevention and therapy. Med Res Rev. 2019;39:114–145. doi: 10.1002/med.21514. [DOI] [PubMed] [Google Scholar]
- 7.Di Francesco L, López Contreras LA, Sacco A, Patrignani P. New insights into the mechanism of action of aspirin in the prevention of colorectal neoplasia. Curr Pharm Des. 2015;21:5116–5126. doi: 10.2174/1381612821666150915110706. [DOI] [PubMed] [Google Scholar]
- 8.Usman MW, Luo F, Cheng H, Zhao JJ, Liu P. Chemopreventive effects of aspirin at a glance. Biochim Biophys Acta. 2015;1855:254–263. doi: 10.1016/j.bbcan.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Yang G, Wang Y, Feng J, Liu Y, Wang T, Zhao M, Ye L, Zhang X. Aspirin suppresses the abnormal lipid metabolism in liver cancer cells via disrupting an NFκB-ACSL1 signaling. Biochem Biophys Res Commun. 2017;486:827–832. doi: 10.1016/j.bbrc.2017.03.139. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Wei X, Wu Y, Wang Y, Qiu Y, Shi J, Zhou H, Lu Z, Shao M, Yu L, et al. Giganteaside D induces ROS-mediated apoptosis in human hepatocellular carcinoma cells through the MAPK pathway. Cell Oncol (Dordr) 2016;39:333–342. doi: 10.1007/s13402-016-0273-9. [DOI] [PubMed] [Google Scholar]
- 11.Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 2015;35:600–604. doi: 10.3109/10799893.2015.1030412. [DOI] [PubMed] [Google Scholar]
- 12.Liang Z, Chi YJ, Lin GQ, Luo SH, Jiang QY, Chen YK. MiRNA-26a promotes angiogenesis in a rat model of cerebral infarction via PI3K/AKT and MAPK/ERK pathway. Eur Rev Med Pharmacol Sci. 2018;22:3485–3492. doi: 10.26355/eurrev_201806_15175. [DOI] [PubMed] [Google Scholar]
- 13.Lei YY, Wang WJ, Mei JH, Wang CL. Mitogen-activated protein kinase signal transduction in solid tumors. Asian Pac J Cancer Prev. 2014;15:8539–8548. doi: 10.7314/apjcp.2014.15.20.8539. [DOI] [PubMed] [Google Scholar]
- 14.Kim EK, Choi EJ. Compromised MAPK signaling in human diseases: An update. Arch Toxicol. 2015;89:867–882. doi: 10.1007/s00204-015-1472-2. [DOI] [PubMed] [Google Scholar]
- 15.Geest CR, Coffer PJ. MAPK signaling pathways in the regulation of hematopoiesis. J Leukoc Biol. 2009;86:237–250. doi: 10.1189/jlb.0209097. [DOI] [PubMed] [Google Scholar]
- 16.Liu ZJ, Xiao M, Balint K, Smalley KS, Brafford P, Qiu R, Pinnix CC, Li X, Herlyn M. Notch1 signaling promotes primary melanoma progression by activating mitogen-activated protein kinase/phosphatidylinositol 3-kinase-Akt pathways and up-regulating N-cadherin expression. Cancer Res. 2006;66:4182–4190. doi: 10.1158/0008-5472.CAN-05-3589. [DOI] [PubMed] [Google Scholar]
- 17.Rehman SK, Li SH, Wyszomierski SL, Wang Q, Li P, Sahin O, Xiao Y, Zhang S, Xiong Y, Yang J, et al. 14-3-3ζ orchestrates mammary tumor onset and progression via miR-221-mediated cell proliferation. Cancer Res. 2014;74:363–373. doi: 10.1158/0008-5472.CAN-13-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tibbles LA, Woodgett JR. The stress-activated protein kinase pathways. Cell Mol Life Sci. 1999;55:1230–1254. doi: 10.1007/s000180050369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- 20.Ryu MJ, Chung HS. [10]-Gingerol induces mitochondrial apoptosis through activation of MAPK pathway in HCT116 human colon cancer cells. In Vitro Cell Dev Biol Anim. 2015;51:92–101. doi: 10.1007/s11626-014-9806-6. [DOI] [PubMed] [Google Scholar]
- 21.Wu T, Ren MX, Chen GP, Jin ZM, Wang G. Rrp15 affects cell cycle, proliferation, and apoptosis in NIH3T3 cells. FEBS Open Bio. 2016;6:1085–1092. doi: 10.1002/2211-5463.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Estaquier J, Vallette F, Vayssiere JL, Mignotte B. The mitochondrial pathways of apoptosis. Adv Exp Med Biol. 2012;942:157–183. doi: 10.1007/978-94-007-2869-1_7. [DOI] [PubMed] [Google Scholar]
- 23.Ryu MJ, Kim AD, Kang KA, Chung HS, Kim HS, Suh IS, Chang WY, Hyun JW. The green algae Ulva fasciata Delile extract induces apoptotic cell death in human colon cancer cells. In Vitro Cell Dev Biol Anim. 2013;49:74–81. doi: 10.1007/s11626-012-9547-3. [DOI] [PubMed] [Google Scholar]
- 24.Stadheim TA, Kucera GL. c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for mitoxantrone- and anisomycin-induced apoptosis in HL-60 cells. Leuk Res. 2002;26:55–65. doi: 10.1016/s0145-2126(01)00099-6. [DOI] [PubMed] [Google Scholar]
- 25.Sarbacker GB, Lusk KA, Flieller LA, Van Liew JR. Aspirin use for the primary prevention of cardiovascular disease in the elderly. Consult Pharm. 2016;31:24–32. doi: 10.4140/TCP.n.2016.24. [DOI] [PubMed] [Google Scholar]
- 26.Pasche B, Wang M, Pennison M, Jimenez H. Prevention and treatment of cancer with aspirin: Where do we stand? Semin Oncol. 2014;41:397–401. doi: 10.1053/j.seminoncol.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldar S, Khaniani MS, Derakhshan SM, Baradaran B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac J Cancer Prev. 2015;16:2129–2144. doi: 10.7314/apjcp.2015.16.6.2129. [DOI] [PubMed] [Google Scholar]
- 28.Suvarna V, Singh V, Murahari M. Current overview on the clinical update of Bcl-2 anti-apoptotic inhibitors for cancer therapy. Eur J Pharmacol. 2019;862(172655) doi: 10.1016/j.ejphar.2019.172655. [DOI] [PubMed] [Google Scholar]
- 29.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- 30.Peña-Blanco A, García-Sáez AJ. Bax, Bak and beyond - mitochondrial performance in apoptosis. FEBS J. 2018;285:416–431. doi: 10.1111/febs.14186. [DOI] [PubMed] [Google Scholar]
- 31.Nagappan A, Park KI, Park HS, Kim JA, Hong GE, Kang SR, Lee DH, Kim EH, Lee WS, Won CK, et al. Vitamin C induces apoptosis in AGS cells by down-regulation of 14-3-3σ via a mitochondrial dependent pathway. Food Chem. 2012;135:1920–1928. doi: 10.1016/j.foodchem.2012.06.050. [DOI] [PubMed] [Google Scholar]
- 32.Leung HW, Yang WH, Lai MY, Lin CJ, Lee HZ. Inhibition of 12-lipoxygenase during baicalein-induced human lung nonsmall carcinoma H460 cell apoptosis. Food Chem Toxicol. 2007;45:403–411. doi: 10.1016/j.fct.2006.08.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.