Abstract
Introduction and hypothesis
Post-operative urinary retention is a common problem affecting close to half of all women undergoing pelvic reconstructive surgery. This was an exploratory analysis that was aimed at identifying factors associated with an inability to learn clean intermittent self-catheterization (CISC) after a failed post-operative retrograde voiding trial (RGVT).
Methods
We performed a retrospective case–control study of women who underwent pelvic organ prolapse or urinary incontinence surgery within a single division from 2016 to 2018. We compared women who could learn CISC with those unable to learn and discharged home with an indwelling catheter (IC). Analyses were carried out using Fisher’s exact test, the Mann–Whitney U test, the Chi-squared test, and the t test with logistic regression.
Results
Of the 202 women who failed their RGVT, 134 (66.3%) were able to learn CISC and 68 (33.7%) were not. Older age, urinary incontinence, diabetes and colpectomy/colpocleisis were associated with an inability to learn CISC (p < 0.05). Women with an IC were more likely to have an office visit related to catheter care (65.7% vs 5.2%, p < 0.001). A UTI within 30 days of surgery was more common with CISC (16.4% vs 6.0%, p = 0.037). In a multivariate logistic regression model, each increasing year of age was associated with a 1.036-fold decrease in the ability to learn CISC (aOR 1.036, 95% CI 1.002–1.071; p = 0.04).
Conclusions
Increasing age was the only variable identified on multivariate logistic regression as a risk factor for failure to learn CISC. Further studies are needed to identify barriers to learning post-operative self-catheterization.
Keywords: Pelvic organ prolapse, Post-operative urinary retention, Self-catheterization
Introduction
Post-operative urinary retention is a common problem affecting up to 40% of women who undergo reconstructive pelvic surgery [1, 2]. Common clinical estimates based on retrospective studies have reported rates of post-operative urinary retention for prolapse surgery to be between 27 and 32% [3-5]. Post-operative urinary retention is diagnosed by an assessment of the post-void residual volume. This can be done using a retrograde or spontaneous bladder fill or with a suprapubic catheter that has been clamped prior to voiding [1, 2]. Once identified, post-operative urinary retention should be treated to avoid bladder overdistention, urinary tract infection, and detrusor dysfunction [1, 6].
Studies evaluating optimal treatment of post-operative retention favor clean intermittent catheterization (CISC) over indwelling catheter (IC) placement, as CISC has been found to be associated with a decreased risk of bacteriuria and a faster return to normal voiding [7]. There has been a movement toward same-day discharge after urogynecologic procedures with demonstrated safety among both minimally invasive sacrocolpopexy and vaginal surgeries [8, 9]. For patients undergoing same-day discharge, optimal treatment requires a patient to have the motor, visual, and mental capacity to learn CISC. Although small studies have demonstrated the feasibility of post-operative CISC teaching, studies examining factors influencing patient ability to learn CISC are limited and primarily draw from the spinal cord injury literature [10-12]. Specific factors influencing patient ability to learn CISC in a urogynecologic surgical population remain unknown.
This was a retrospective, exploratory analysis that sought to identify factors associated with an inability to learn CISC after failed post-operative retrograde voiding trial (RGVT) in patients undergoing same-day discharge pelvic reconstructive surgery. Our long-term goal is to use this information to determine which patients may benefit from pre-operative CISC teaching and to optimize post-operative urinary retention management.
Materials and methods
We performed a retrospective case–control study of women who underwent pelvic organ prolapse or urinary incontinence surgery within a single division between January 2016 and January 2018. Approval by the University of Pittsburgh Institutional Review Board was obtained prior to case identification. Surgeries were performed at one of three hospitals within the University of Pittsburgh Medical Center and by board-certified Female Pelvic Medicine and Reconstructive Surgery (FPMRS) surgeons in the Division of Urogynecology. We included all women who had same-day discharge and failed their post-operative RGVT. Subjects were excluded if a prolonged IC was planned or if the patient was admitted overnight. At our institution, all patients recover in the post-anesthesia care unit immediately after surgery where they spend on average 2–5 h prior to discharge. An RGVT is performed during this time by instilling 300 ml saline into the bladder and then immediately asking the patient to void. They pass the test if they can void 200 ml or at least two thirds of the volume instilled into the bladder. Our division practice is for women who failed their RGVT to be discharged home with prophylactic nitrofurantoin to be taken daily for the duration of their self-catheterization or IC use. A prospective, randomized controlled trial (RCT) looking at prophylactic nitrofurantoin versus placebo in a 1:1 ratio following a failed RGVT was occurring at our institution during the study period. However, because of this study, some women in our cohort may have received placebo rather than antibiotic prophylaxis following their surgery.
Cases included women unable to learn CISC who were discharged home with an IC. Controls were women discharged having successfully learned to perform CISC. CISC teaching was done in the post-operative anesthesia area by a registered nurse. Patients were considered unable to learn CISC if they were unable to perform CISC on multiple attempts. All women were contacted on post-operative day 1 by our nursing staff to assess pain, mobility, and voiding. Women were given multiple catheters with instructions for single use; however, it was not assessed in the post-operative period whether they used each catheter only once or multiple times. For CISC patients, staff would call daily to learn how often patients were performing CISC and their post-void residuals. Women would be instructed to discontinue CISC when post-void residuals were < 100 ml on two consecutive occasions. Women with an IC would undergo a repeat RGVT in the office or at home by a home health care nurse between post-operative days 3 and 7 with catheter removal if appropriate. The timing of the repeat RGVT and the decision of an office visit or home health care nurse were often determined by patient preference.
Electronic health records were reviewed by physician investigators and data extracted regarding demographics, medical and surgical history, pre-operative examinations, procedural data (i.e., surgery performed, hospital, surgeon, type of anesthesia, case order, and intra-operative complications), and post-operative information such as RGVT results, days of catheterization, related office visits, or post-operative urinary tract infections (UTIs). In 2017, our institution began offering an additional pre-operative visit that addressed procedure-related risks and benefits, surgical expectations, post-operative management, and the possibility of urinary retention for women undergoing major pelvic organ prolapse surgery. It was noted whether the patients in our cohort attended an additional pre-operative counseling visit prior to their surgery. A patient was considered to have a post-operative UTI if they had symptoms plus treatment with an antibiotic or symptoms plus a positive urine culture and treatment with an antibiotic within 30 days of surgery. The decision to include patients treated empirically based on symptoms is consistent with previous literature that looked at post-operative UTIs [13, 14]. All other post-operative metrics such as readmission or emergency department visits were included if within 30 days of surgery. Duration of catheter use was determined by a review of daily nursing phone calls that documented whether the patient had an IC or was performing CISC.
We used a convenience sample of all patients who failed their post-operative RGVT within a 2-year time frame. Statistical analysis was performed using SPSS version 22.0 (IBM Corporation, Armonk, NY, USA). Continuous variables were analyzed using t test or Mann–Whitney U test where appropriate. Categorical variables were analyzed using Chi-square or Fisher’s exact tests. Bivariate analyses were carried out to determine relationships between variables and the inability to learn CISC, and multivariate logistic regression was performed to control for potential confounders.
Results
There were 1011 urogynecology cases between January 2016 and December 2017 where a post-operative RGVT was performed (Fig. 1). Of these cases, 232 were admitted and 28 had a planned prolonged IC. There were 202 out of 751 (26.9%) eligible patients who failed their RGVT and were discharged home with either CISC or IC. Of these 202 women, two thirds were able to learn CISC (134, 66.3%) and one third were discharged home with an IC (68, 33.7%). In our sample, mean age was 64.6 years (± 12.4), 95.5% were Caucasian, 69.2% were never smokers, and 42.8% had a prior hysterectomy. Of those with prolapse, 58.4% were stage III and 11.7% were stage IV. Table 1 contains demographic information by group. The percentage of women who attended a pre-operative counseling appointment was similar between groups (p = 0.54). Overall, 1.5% of the IC group and 2.2% of the CISC group had been taught CISC prior to surgery (p = 0.72) and 0% of the IC group and 4.5% of the CISC group had performed CISC previously (p = 0.08).
Fig. 1.
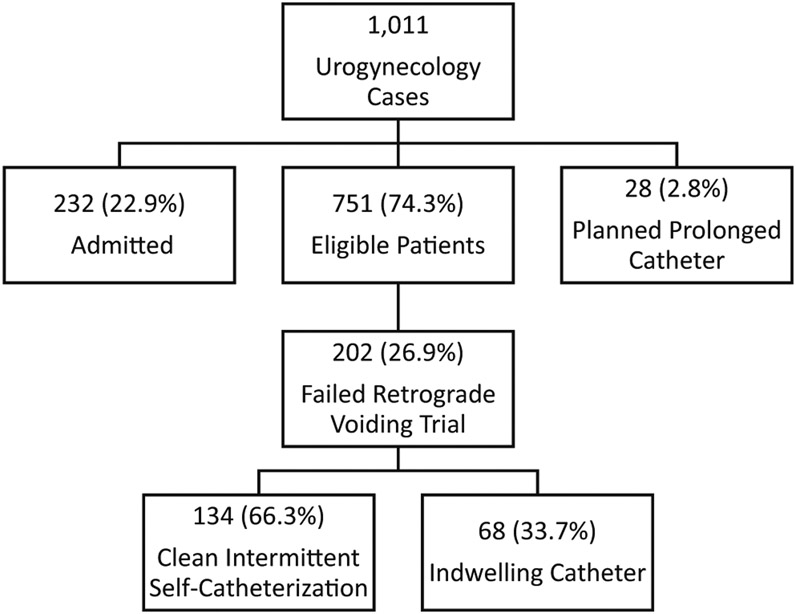
Patient flow diagram
Table 1.
Demographics and clinical characteristics
| Indwelling catheter (N = 68) | CISC (N = 134) | p | |
|---|---|---|---|
| Age (years), mean ± SD | 69.5 ± 12.3 | 62.1 ± 11.8 | <0.0001 |
| Age group, n (%) | 0.0002 | ||
| <50 years | 6 (9.0) | 30 (22.4) | |
| 50–65 years | 12 (17.9) | 47 (35.1) | |
| >65 years | 49 (73.1) | 57 (42.5) | |
| BMI, mean ± SD | 27.0 ± 0.6 | 27.0 ± 0.4 | 0.97 |
| White ethnicity, n (%) | 62 (92.5) | 130 (97.0) | 0.14 |
| Current smoker, n (%) | 5 (7.5) | 5 (3.7) | 0.48 |
| Gravity, median (range) | 3 (2–4) | 3 (2–4) | 0.77 |
| Parity, median (range) | 3 (2–3) | 2 (2–3) | 0.24 |
| Diabetes, n (%) | 13 (19.4) | 11 (8.2) | 0.021 |
| Anxiety, n (%) | 10 (14.9) | 13 (9.7) | 0.27 |
| Arthritis, n (%) | 22 (32.8) | 32 (23.9) | 0.18 |
| Neurologic condition, n (%)a | 6 (9.0) | 10 (7.5) | 0.71 |
| History of incontinence, n (%) | 34 (50.7) | 90 (67.2) | 0.024 |
| Prior hysterectomy, n (%) | 31 (46.3) | 55 (41.0) | 0.48 |
| Prior prolapse surgery, n (%) | 13 (19.4) | 26 (19.4) | 1.00 |
| Prior incontinence surgery, n (%) | 4 (6.0) | 19 (14.2) | 0.10 |
| Pre-operative anticholinergic use, n (%) | 7 (10.5) | 16 (11.9) | 0.75 |
| Pre-operative POP-Q stage, n (%) | 0.26 | ||
| II | 13/59 (22.0) | 26/95 (27.4) | |
| III | 39/59 (66.1) | 51/95 (56.7) | |
| IV | 7/59 (11.9) | 11/95 (11.6) | |
| Pre-operative counseling appointment, n (%) | 27 (40.3) | 48 (35.8) | 0.54 |
| Pre-operative PVR (mL), mean ± SD | 91.7 ± 113.6 | 89.9 ± 97.2 | 0.90 |
| Pre-operative CISC teaching, n (%) | 1 (1.5) | 3 (2.2) | 0.72 |
| Ever performed CISC, n (%) | 0 (0.0) | 6 (4.5) | 0.18 |
BMI body mass index, CISC clean intermittent self-catheterization, PVR post-void residual
Neurologic conditions include Parkinson’s disease, multiple sclerosis, history of stroke
Increasing age was associated with the inability to learn CISC (p < 0.001, Table 1). When broken down into age categories, this difference was most pronounced for women over age 65 with 73.1% of women unable to learn CISC being over age 65 compared with 42.5% of the CISC group being over age 65 (p = 0.0002). On bivariate analysis, women with urinary incontinence or a past medical history of diabetes were less likely to learn CISC (p < 0.05). A past medical history of arthritis, neurologic disorder or anxiety was not different between groups. The percentage of women who underwent hysterectomy or a major prolapse procedure was not significantly different between groups. Women who underwent a colpectomy and colpocleisis were more likely to be discharged home with an IC (p < 0.001). Conversely, women who underwent incontinence surgery were less likely to be discharged home with an IC (p = 0.03). Women who underwent oophorectomy were also less likely to learn CISC (p = 0.035), but this trend did not persist for salpingectomy alone. There were differences in the mean age of our subpopulations. Specifically, patients who underwent oophorectomy were older than those who did not have an oophorectomy (oophorectomy mean age 68.6 ± 9.5 years vs nonoophorectomy mean age 63.7 ± 12.8 years). Similarly, colpectomy and colpocleisis patients were on average older (colpocleisis mean age 78.7 ± 6.1 years vs no colpocleisis mean age 61.2 ± 11.4 years) whereas those undergoing an incontinence procedure were younger (incontinence procedure age 59.2 ± 13.2 years vs no incontinence procedure age 67.7 ± 10.7 years). There were no significant differences in estimated blood loss and case length between the two groups, even when controlling for hysterectomy and prolapse surgery. Additionally, type of anesthesia and post-operative morphine equivalent units were similar between groups (all p > 0.05).
Women had slightly longer catheter use when discharged home with an IC when compared with CISC (p = 0.001, Table 2). Women with an IC were significantly more likely to require an office visit for catheter management than those who learned CISC (p < 0.001). The majority of post-operative catheter visits were for a repeat RGVT in the IC group and for difficulty performing CISC in the CISC group. Those discharged using CISC were more likely to be treated for a UTI within 30 days of surgery than those in the IC group (16.4% CISC vs 6.0% IC, p = 0.045). All 4 UTIs in the IC group had a positive urine culture. Of those in the CISC group who were treated for UTI, 9 were treated empirically based on symptoms (with 4 ultimately having a negative urine culture result) and 13 had symptoms with a positive urine culture. The difference in UTI between groups was not significant when those treated empirically prior to a negative urine culture result were excluded (p = 0.11, Table 2). There was no difference in intra-operative or post-operative complications, re-admissions or emergency department visits within 30 days (allp > 0.05).
Table 2.
Intra-operative and post-operative characteristics
| Indwelling catheter (n = 68) | CISC (n = 134) | p | |
|---|---|---|---|
| Hysterectomy, n (%) | 0.84 | ||
| No | 42 (62.7) | 85 (63.4) | |
| Vaginal | 13 (19.4) | 22 (16.4) | |
| Minimally invasive | 12 (17.9) | 27 (20.2) | |
| Major prolapse surgery, n (%) | |||
| Minimally invasive sacrocolpopexy | 12 (17.9) | 23 (17.2) | 0.90 |
| Native tissue repair | 19 (28.4) | 34 (25.3) | 0.65 |
| Colpectomy/colpocleisis | 21 (31.3) | 14 (10.4) | <0.001 |
| Incontinence surgery, n (%) | 17 (25.4) | 55 (41.0) | 0.03 |
| General procedure, n (%) | |||
| Stents | 17 (25.4) | 43 (32.1) | 0.33 |
| Oophorectomy | 17 (25.4) | 18 (13.4) | 0.035 |
| Salpingectomy | 24 (35.8) | 43 (32.1) | 0.60 |
| LOA | 4 (6.0) | 9 (6.7) | 1.00 |
| Mesh removal | 4 (6.0) | 5 (3.7) | 0.48 |
| Estimated blood loss (mL), mean ± SD | 67.5 ± 61.3 | 61.1 ± 65.2 | 0.22 |
| Case length (h), mean ± SD | 2.0 ± 0.9 | 1.8 ± 1.1 | 0.30 |
| Case ended after noon, n (%) | 36 (53.7) | 66 (49.3) | 0.55 |
| ERAS, n (%) | 33 (49.3) | 53 (39.6) | 0.19 |
| Pre-operative scopolamine patch, n (%) | 6 (9.0) | 24 (17.9) | 0.09 |
| Anesthesia, n (%) | 0.052 | ||
| GETA | 54 (80.6) | 105 (78.4) | |
| Laryngeal | 1 (1.5) | 1 (0.8) | |
| Spinal | 4 (6.0) | 1 (0.8) | |
| Sedation | 8 (11.9) | 27 (20.2) | |
| Local anesthetic | 55 (82.1) | 105 (78.4) | 0.54 |
| Intraoperative complication | 1 (1.5) | 1 (0.7) | 0.62 |
| PACU narcotic use (morphine EqU), mean ± SD | 6.7 ± 9.6 | 7.6 ± 11.6 | 0.49 |
| Days CISC or Foley, mean ± SD | 5.4 ± 2.9 | 4.8 ± 4.9 | 0.0014 |
| Post-operative office visit related to CISC or catheter care, n (%) | 44 (65.7) | 7 (5.2) | <0.001 |
| UTI within 30 days, n (%) | 4 (6.0) | 18 (13.4) | 0.11 |
| ED visit within 30 days, n (%) | 6 (9.0) | 9 (6.7) | 0.57 |
| Post-operative complication, n (%) | 6 (9.0) | 9 (6.7) | 0.57 |
| Re-admission, n (%) | 3 (4.5) | 5 (3.7) | 0.80 |
Native tissue repair includes uterosacral ligament suspension and sacrospinous ligament fixation
Incontinence surgery included midurethral slings, periurethral bulking, and Burch procedure
LOA lysis of adhesions, ERAS extended recovery after surgery, GETA general endotracheal anesthesia, PACU post-anesthesia care unit, UTI urinary tract infection, ED emergency department
We included the following variables in our multivariate regression model: age, race, gravidity, parity, diabetes history, colpectomy and colpocleisis, oophorectomy, incontinence procedure, extended recovery after surgery (ERAS) protocol, total operative time, and type of anesthesia. Backward selection was used to yield our final model, which included age, performance of an incontinence procedure, ERAS protocol, and total operative time. After controlling for the above variables, only age was significant, with each increasing year of age being associated with a 1.036-fold increase in the inability to learn CISC (aOR 1.036, 95% CI 1.002–1.071, p = 0.04). Although significant in the bivariate analysis, incontinence status, diabetes, and colpectomy and colpocleisis performed were no longer significant in the final model.
Discussion
Our cohort of same-day discharge urogynecology patients had a post-operative urinary retention rate of 27%, which is consistent with published literature [3-5]. Older age, urinary incontinence, past medical history of diabetes, and undergoing a colpectomy and colpocleisis were all associated with the inability to learn CISC among women with post-operative urinary retention following pelvic reconstructive surgery. However, only age was significant in the final logistic regression model, with an increasing age corresponding to an inability to learn CISC.
Overall, one third of our population was unable to learn CISC and discharged home with an IC. Some of our hypothesized barriers, such as procedure end time, baseline anticholinergic use, and post-operative narcotic use did not show any difference in ability to learn CISC. Oophorectomy and colpectomy/colpocleisis were associated with failure to learn CISC, which is likely due to older age in their populations as this was not significant in the final regression model. Age alone likely does not account for the number of patients unable to learn CISC and we suspect that other barriers exist to explain this difference.
Women in our study had a shorter duration of catheterization when performing CISC than when using an IC, with a difference of 0.6 days (p = 0.001); however, it is unlikely that this is clinically significant. Duration of CISC is based on a daily assessment of post-void residual volumes that serves as a proxy for bladder function, but duration of IC is determined by repeat RGVT, which is dependent on the patient’s schedule and ability to present for the repeat test. This makes the duration of IC somewhat arbitrary in our population. Patients with an IC had a significantly higher rate of post-operative visits related to catheter care, as might be expected (IC 65.7% vs CISC 5.2%, p < 0.001). This difference in office follow-up has consequences for both patient burden and costs. Further analysis that compares the costs of both methods while considering office visits, numbers of catheters used, and cost of UTI treatment is warranted.
When excluding negative urine culture results, women in our study had a higher rate of UTI in the CISC group than in the IC group, although this did not reach statistical significance (13.4% vs 6.0%, p = 0.11). This contrasts with previous studies. A RCT looked at rates of bacteriuria and UTI following vaginal prolapse surgery and found both a higher rate of bacteriuria (38% vs 14%, p = 0.02) and a higher rate of UTI in the IC group (33% vs 12%, p = 0.03) [7]. The patients in the RCT differed from our population in that they were all inpatients following surgery and catheterized by trained nursing staff no less than every 6 h [7]. Despite teaching by nursing staff with the assistance of home health care we cannot ensure a sterile technique with CISC. Additionally, we do not know whether patients in this study used their catheter once or multiple times to reduce supply costs. However, a recent Cochrane review of IC for long-term retention determined that there was insufficient evidence to determine whether the incidence of UTI is affected by multi-use catheters or an aseptic technique [15]. Further pragmatic studies looking at home CISC in this patient population are warranted.
Our overall UTI rate in the IC group was lower than in previously published literature [7, 16]. This may be partially explained by the retrospective nature of this study, which may have missed women who presented to an outside facility for diagnosis and treatment. However, it is also possible that the rate of UTI was overestimated in the CISC group, making it difficult to draw definitive conclusions. The act of performing CISC may cause urethral irritation, which could have prompted some patients to seek treatment for presumed infection, thus giving a falsely elevated rate of UTI in this group. Our UTI diagnosis was made from a positive culture or symptoms followed by antibiotic treatment, which may overestimate the true prevalence; however, this is a commonly used definition of post-operative UTI in urogynecologic literature and reflects real-life clinical management, where some patients are treated empirically prior to culture results [13, 14].
Most of our patients were discharged with prophylactic nitrofurantoin to be taken daily until they were no longer performing CISC or using an IC. Dieter et al. showed in their RCT of nitrofurantoin versus placebo that there was no difference in post-operative UTI rates following prolapse surgery that required post-operative catheterization [13]. A recent multi-center RCT looking at prophylactic nitrofurantoin versus placebo among women who underwent pelvic reconstructive surgery and failed their RGVT was occurring at our institution during our study period. Some of the patients in our cohort participated in this study, but we do not have access to information about which treatment arm they received. Ultimately, the results of this trial showed that the frequency of UTI was 17% overall and did not differ by treatment group or when method of catheterization was assessed [17]. Owing to the randomization of study participants, some women in our cohort would have received placebo rather than nitrofurantoin upon discharge. The remainder of patients in this study would have received nitrofurantoin, as was division practice at that time. The similar rates of UTIs in previous studies looking at antibiotic prophylaxis suggests that the small number of women who received placebo may not have made a difference to UTI outcomes [13, 17]. However, the inability to confirm nitrofurantoin versus placebo for a small portion of our cohort is a limitation related to a concurrent study at our institution during this time.
More than one third of our sample underwent a pre-operative counseling visit prior to their surgery, but this did not appear to have an impact on whether the patient could learn CISC. Routine pre-operative counseling visits for major pelvic organ prolapse procedures were instituted by our division in 2017 to better prepare patients for their surgical experience. An important discussion during these visits concerns the possibility of voiding dysfunction and catheter use. This suggests that an attempt at increased pre-operative preparedness might not have improved a patient’s ability to learn CISC in our population. Post-operative retention and catheter care have previously been linked to patient satisfaction in a urogynecology population [18]. Brubaker et al. have previously reported that although most women felt prepared for pelvic organ prolapse surgery, 27% did not feel prepared to cope with a catheter at home [19]. Moreover, despite the need for a catheter being a known event following pelvic organ prolapse and incontinence surgery, 6% of their population considered it a surgical complication [19]. Because of the retrospective design of this study, we cannot ascertain whether the pre-operative counseling visit had a positive impact on patients’ ability to cope with an IC or self-catheterization and is a possible avenue for future research.
Strengths of this study include the relatively large sample size and a heterogeneous population in terms of urogynecologic procedures. All cases were performed by one of eight board-certified FPMRS surgeons. Previous literature surrounding post-operative urinary retention in urogynecologic procedures has focused on risk factors for failed RGVT [3, 20]. Our study uniquely looks at risk factors in learning CISC after a failed RGVT.
Limitations of this study include the retrospective design, which restricts our analysis to information available in the medical record only. Given this limitation, we were unable to assess for patient preferences, past experiences (either personal, a friend or family member) with catheterization or overall discomfort with one method over the other. Any of these factors could have influenced a woman’s ability or willingness to learn CISC in the post-operative period. We also did not have access to the experience of the post-operative nurse who was performing the CISC teaching or the amount of time he or she spent with the patient. Our outcomes were measured at 30 days, which is within the published range for post-operative UTIs and complications, but may have missed some patients who had a UTI or complication between 30 days and 6 weeks post-operatively [13, 15].
In conclusion, increasing age was the only variable identified on multivariate logistic regression as a risk factor for failure to learn CISC for post-operative urinary retention following pelvic reconstructive surgery. As one-third of our sample was unable to learn CISC, barriers other than age alone likely exist. In our population, women who performed CISC had a higher rate of UTI. Further studies are needed to both identify additional barriers to CISC and study this catheterization practice in an outpatient surgical setting.
Acknowledgements
Our research was supported by the National Institutes of Health through Grant Number UL1TR001857.
Footnotes
Conflicts of interest The authors report that they have no conflicts of interest.
Our research was presented as a poster abstract at the American Urogynecologic Society Meeting; Chicago IL, USA; 9 October to 13 October 2018
References
- 1.Geller EJ. Prevention and management of postoperative urinary retention after urogynecologic surgery. Int J Women’s Health. 2014;6:829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walters MD, Karram MM, editors. Urogynecology and reconstructive pelvic surgery. 4th ed. Philadelphia: Elsevier; 2015. [Google Scholar]
- 3.Hakvoort RA, Dijkgraaf MG, Burger MP, Emanuel MH, Roovers JP. Predicting short-term urinary retention after vaginal prolapse surgery. Neurourol Urodyn. 2009;28(3):225–8. [DOI] [PubMed] [Google Scholar]
- 4.Turner LC, Kantartzis K, Shepherd JP. Predictors of postoperative acute urinary retention in women undergoing minimally invasive sacral colpopexy. Female Pelvic Med Reconstr Surg. 2015;21(1): 39–42. [DOI] [PubMed] [Google Scholar]
- 5.Book NM, Novi B, Novi JM, Pulvino JQ. Postoperative voiding dysfunction following posterior colporrhaphy. Female Pelvic Med Reconstr Surg. 2012;18(1):32–4. [DOI] [PubMed] [Google Scholar]
- 6.Madersbacher H, Cardozo L, Chapple C, et al. What are the causes and consequences of bladder overdistension? ICI-RS 2011. Neurourol Urodyn. 2012;31(3):317–21. [DOI] [PubMed] [Google Scholar]
- 7.Hakvoort RA, Thijs SD, Bouwmeester FW, et al. Comparing clean intermittent catheterisation and transurethral indwelling catheterisation for incomplete voiding after vaginal prolapse surgery: a multicentre randomised trial. BJOG. 2011;118(9):1055–60. [DOI] [PubMed] [Google Scholar]
- 8.Kisby CK, Polin MR, Visco AG, Siddiqui NY. Same-day discharge after robotic-assisted sacrocolpopexy. Female Pelvic Med Reconstr Surg. 2018; DOI: 10.1097/SPV.0000000000000573. [DOI] [PubMed] [Google Scholar]
- 9.Carter-Brooks CM, Du AL, Ruppert KM, Romanova AL, Zyczynski HM. Implementation of a urogynecology-specific enhanced recovery after surgery (ERAS) pathway. Am J Obstet Gynecol. 2018;219(5):495.e1–495.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bickhaus JA, Drobnis EZ, Critchlow WA, Occhino JA, Foster RT Sr. The feasibility of clean intermittent self-catheterization teaching in an outpatient setting. Female Pelvic Med Reconstr Surg. 2015;21(4):220–4. [DOI] [PubMed] [Google Scholar]
- 11.Webb RJ, Lawson AL, Neal DE. Clean intermittent selfcatheterisation in 172 adults. Br J Urol. 1990;65(1):20–3. [DOI] [PubMed] [Google Scholar]
- 12.Cobussen-Boekhorst H, Beekman J, van Wijlick E, Schaafstra J, van Kuppevelt D, Heesakkers J. Which factors make clean intermittent (self) catheterisation successful? J Clin Nurs. 2016;25(9-10):1308–18. [DOI] [PubMed] [Google Scholar]
- 13.Dieter AA, Amundsen CL, Edenfield AL, et al. Oral antibiotics to prevent postoperative urinary tract infection: a randomized controlled trial. Obstet Gynecol. 2014;123(1):96–103. [DOI] [PubMed] [Google Scholar]
- 14.Nygaard I, Brubaker L, Chai TC, et al. Risk factors for urinary tract infection following incontinence surgery. Int Urogynecol J. 2011;22(10):1255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prieto J, Murphy CL, Moore KN, Fader M. Intermittent catheterisation for long-term bladder management. Cochrane Database Syst Rev. 2014;8:CD006008. [DOI] [PubMed] [Google Scholar]
- 16.Sutkin G, Alperin M, Meyn L, Wiesenfeld HC, Ellison R, Zyczynski HM. Symptomatic urinary tract infections after surgery for prolapse and/or incontinence. Int Urogynecol J. 2010;21(8): 955–61. [DOI] [PubMed] [Google Scholar]
- 17.Lavelle ES, et al. Nitrofurantoin prophylaxis in women undergoing catheterization for acute postoperative urinary retention after pelvic reconstructive surgery: a randomized, double-blind, placebo-controlled trial. American Urogynecologic Society Pelvic Floor Disorders Week. Chicago; October 9–13, 2018. [Google Scholar]
- 18.Elkadry EA, Kenton KS, FitzGerald MP, Shott S, Brubaker L. Patient-selected goals: a new perspective on surgical outcome. Am J Obstet Gynecol. 2003;189(6):1551–7, discussion 1557-8. [DOI] [PubMed] [Google Scholar]
- 19.Brubaker L, Litman HJ, Rickey L, et al. Surgical preparation: are patients "ready" for stress urinary incontinence surgery? Int Urogynecol J. 2014;25(1):41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chong C, Kim HS, Suh DH, Jee BC. Risk factors for urinary retention after vaginal hysterectomy for pelvic organ prolapse. Obstet Gynecol Sci. 2016;59(2):137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]


