The prevalence of seroconversion postvaccination to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is weaker in recipients of a solid-organ transplant compared with healthy individuals. The impact of different immunosuppressive therapies on the immunologic response after SARS-CoV-2 mRNA vaccines in kidney transplant recipients (KTx) is still ill-defined.
We assessed the specific humoral immunity response after SARS-CoV-2 mRNA vaccination in KTx receiving belatacept compared to KTx receiving tacrolimus.
Between February 2011 and April 2021, we included patients who had received consecutive KTx (at least 1-y posttransplantation) from our outpatient clinic and had received 2 doses of SARS-CoV-2 mRNA vaccine (BNT162b2 or Moderna COVID-19). Maintenance immunosuppression was based on either belatacept (every 4 wk) or tacrolimus, in addition to mycophenolic acid. At the time of the first vaccination, patients had a negative SARS-CoV-2 serology. For those receiving belatacept, the SARS-CoV-2 mRNA vaccination was performed at day 21 post–belatacept infusion (to reduce impact on immunogenicity). Blood samples were collected at the time of the first vaccination, 1 mo after the first, the second, and the third vaccination. Humoral immune response was assessed using an enzyme immunoassay against the S1 domain of the SARS-CoV-2 spike protein (Wantai Biological Pharmacy Enterprise Co., Beijing, China).
Fifty-seven patients were recruited: mean age was 62 ± 13 y. Immunosuppression was belatacept for 41 patients (72%) and tacrolimus for 16 patients (28%). Eighteen (31.5%) patients were female. Mean time from kidney transplantation was 122 ± 81 mo in the belatacept group and 174 ± 346 mo in the tacrolimus groups (P = 0.56).
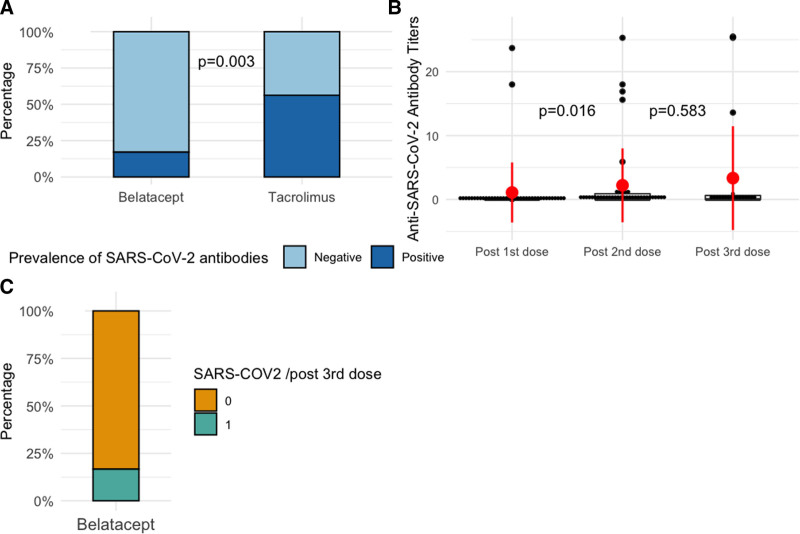
Overall, 21 patients (36%) had a positive immune response post–SARS-CoV-2 vaccination (after the third dose). Of these, after the second dose, 7 (17%) belatacept-treated patients and 9 (56%) tacrolimus-treated patients were tested positive for anti–SARS-CoV-2 antibodies (P = 0.003). After the third vaccination, 20 belatacept patients were assessed: 4 patients were positive (20%). Of these, 1 was positive after the second dose, and 3 were negative. Two positive patients after the second dose of vaccine have lost their immunity after the third dose.
The anti–SARS-CoV-2 antibody titer increased significantly with the number of vaccinations (Figure 1), that is, 1.1 ± 4.7 after the first, 2.2 ± 5.7 after the second, and 3.3 ± 8 after the third SARS-CoV-2 mRNA vaccination.
FIGURE 1.
Response to SARS-CoV-2 vaccination in Belatacept Kidney transplant recipients. A, Comparison of anti–severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) mRNA positive immune responses in kidney transplant recipients at month 3 after vaccination who were either on belatacept or on tacrolimus immunosuppressive regimen. B, Anti–SARS-CoV-2 mRNA antibody titers in kidney transplant recipients who received belatacept. The dot-plot shows all anti–SARS-CoV-2 mRNA titers at months 2 and 3 after vaccination. The red point shows the average; the red bar represents the interquartile. C, anti–SARS-CoV-2 mRNA positive immune responses in belatacept-treated kidney transplant recipients after the third vaccination.
The immune response to SARS-CoV-2 vaccination is lower in KTx recipients compared with healthy populations.1 Herein, we confirm a low immune response (36%) after SARS-CoV-2 vaccination in KTx. In addition, KTx receiving belatacept had a significantly lower antibody response versus tacrolimus.
This trend was also found by Chavarot et al (2021), who reported an antibody positivity of 5.7% in KTx receiving belatacept.2 However, in that study, the SARS-CoV-2 vaccination was given at the same time as belatacept infusion, which possibly minimized vaccine immunogenicity. Ou et al (2021) reported on 609 KTx. Of these, 19 had received belatacept-based immunosuppression, but only 5% of these patients had anti–SARS-CoV-2 antibodies.3 In our study, all patients received a vaccination at 21 d after belatacept infusion. This may explain the improved response to vaccination (ie, 17%) after the second vaccination. The serological Wantai assay is correlated to virus-neutralizing antibodies and is one with the higher sensitivity, which means that our results are not underestimated.4,5
In conclusion, belatacept significantly reduced the immune response to SARS-CoV-2 mRNA vaccination; however, delaying SARS-CoV-2 vaccination until 21 d after a belatacept infusion may improve immunogenicity.
Footnotes
These two authors contributed equally to the paper.
The authors declare no funding or conflicts of interest.
J.N. performed statistical analysis correction and wrote the paper. A.L. and W.B. collected the data and performed the statistical analysis. J.L. performed virologic data collection. D.L. clinical management, patient information, and correction of the manuscript. L.R. had the idea, conception design, and final approval.
REFERENCES
- 1.Bertrand D, Hamzaoui M, Lemée V, et al. Antibody and T cell response to SARS-CoV-2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol. 2021;32:ASN.2021040480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chavarot N, Ouedrani A, Marion O, et al. Poor Anti-SARS-CoV-2 humoral and T-cell responses after 2 injections of mRNA vaccine in kidney transplant recipients treated with belatacept. Transplantation. 2021;105:e94–e95. [DOI] [PubMed] [Google Scholar]
- 3.Ou MT, Boyarsky BJ, Chiang TPY, et al. Immunogenicity and reactogenicity after SARS-CoV-2 mRNA vaccination in kidney transplant recipients taking belatacept. Transplantation. 2021;105:2119–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bal A, Pozzetto B, Trabaud M-A, et al. ; COVID SER Study Group. Evaluation of high-throughput SARS-CoV-2 serological assays in a longitudinal cohort of patients with mild COVID-19: clinical sensitivity, specificity, and association with virus neutralization test. Clin Chem. 2021;67:742–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harritshøj LH, Gybel-Brask M, Afzal S, et al. Comparison of 16 serological SARS-CoV-2 immunoassays in 16 clinical laboratories. J Clin Microbiol. 2021;59:e02596–e02520. [DOI] [PMC free article] [PubMed] [Google Scholar]