Supplemental Digital Content is available in the text.
Kidney transplant recipients (KTRs) have an increased risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mortality1 and a markedly decreased immune response after 2 doses of mRNA vaccination.2 The optimal vaccination strategy for KTRs without sufficient immune response after standard vaccination protocols remains unknown. Here, we report combined cellular and humoral response rates after a third dose of the BNT162b2 mRNA vaccine (Tozinameran; Pfizer–BioNTech COVID-19 vaccine).
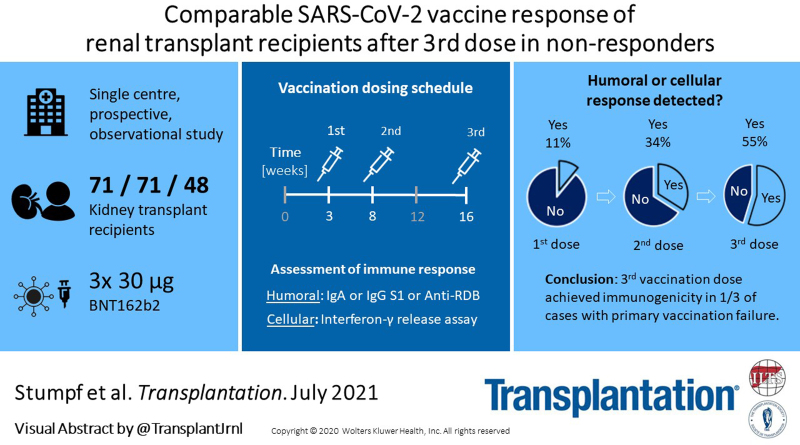
In this single-center study (subanalysis of the DIA-Vacc cohort3), we reported KTRs’ response rates after the first (T1), second (T2), and third (T3) dose of BNT162b2 vaccination. Seventy-one KTRs (mean age ± SD, 57 ± 14.4 y, 63% men) received first and second vaccinations with BNT162b2 at a 3-wk interval. Forty-eight KTRs with insufficient humoral response after 2 vaccinations received the third dose of BNT162b2 vaccine 68 ± 1 d after the second vaccination. Immune responses to the third vaccination were assessed 4 wk after application (T3; Figure 1A). Humoral response was determined using immunoglobulin (Ig) A and IgG antibody ELISAs against spike S1 protein and IgG ELISA against the receptor-binding domain (RBD). To exclude SARS-CoV-2 contact before or after vaccination, IgG antibodies against the nucleocapsid protein subunit were analyzed in parallel.
FIGURE 1.
Scheduling, composition, and immunogenicity. A, Study schedule. T0, T1, T2, and T3 are corresponding time points at wk 0, 3, 8, 12, and 16. B, Immunogenicity after first, second, and third dose of BNT162b2 (T1–3) showing cellular and humoral vaccination response frequencies. C, Immunogenicity 4 wk after the third dose of BNT162b2; humoral response indicating de novo IgA or IgG development against spike S1 at T3. The cellular immune response is a positive IGRA ≥100 mIU/mL. D, Anti-SARS-CoV-2 IgA spike S1 antibody ratio at the different time points T1–3. Threshold to positivity is marked by a dashed gray line. E, Anti-SARS-CoV-2 IgG spike S1 antibody measurements at the different time points T1–3. Threshold to positivity is marked by a dashed gray line. F, IGRA at the different time points T1–3. Threshold to positivity is marked by a dashed gray line. All antibody ELISAs are commercially available (EUROIMMUN Medizinische Labordiagnostika AG, Lübeck, Germany). A positive serologic response was defined by de novo antibody development (seroconversion) at T1–3. Thresholds for positive antibody readings were in the case of IgA anti-spike S1 and IgG anti-NCP ≥1.1[ratio], for IgG anti-RBD (analyzed at T2 and T3 only) ≥35 [% inhibition], and IgG anti-spike S1 ≥35.2 [BAU/mL]. The cellular immune response to vaccination was assessed by SARS-CoV-2-specific IGRA (EUROIMMUN-SARS-CoV-2-IGRA [for research purposes only], positive at ≥100 mIU/mL) at all time points. BAU/mL, binding antibody units per milliliter; BNT162b2 mRNA, Pfizer–BioNTech COVID-19 vaccine (tozinameran); IgA, immunoglobulin A; IgG, immunoglobulin G; IGRA, interferon-γ release assay; NCP, nucleocapsid protein subunit; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; spike S1, spike subunit S1.
Causes of end-stage renal disease were glomerulonephritis in 27%; hypertensive, diabetic, or vascular disease in 18%; cystic kidney disease in 13%; vasculitis in 3%; and unknown cause in ~39% of cases. Immunosuppressive therapy included a calcineurin inhibitor in 87%, mycophenolic acid in 73%, or a mechanistic target of rapamycin inhibitor in 24%, while only 38% of KTRs had glucocorticosteroids as maintenance immunosuppression. The median time after transplantation was 7.5 ± 6 y.
Cumulative humoral response rates in all 71 KTRs were 6% (T1), 32% (T2), and 55% (T3; Figure 1B). Cellular response was 11% at T1 (n = 7/63 patients, no T1 results were reported for 8 patients) and 34% at T2 (n = 23/68 patients, no T2 results were reported for 3 patients; Figure 1B). At T3, cellular response was present in 26% (n = 9/35 patients, no T3 results were reported for 13 patients) and 40% showed a total humoral response (Figure 1C). Among the patients with a humoral response, frequencies of RBD antibodies increased to 94% after the third vaccination (T3; n = 15/16 patients) compared with 56% at T2 (n = 10/18 patients; Figure 1C; additional accurate ELISA/interferon-γ release assay readings; Figure 1D–F).
The humoral response rates after a third mRNA vaccination to SARS-CoV-2 reported here are consistent with a recent publication by Kamar et al.4 We demonstrate that not only the humoral but also the cellular vaccination response rates to a third dose in primary nonresponders are at least comparable with de novo responses after 2 vaccinations. The frequency of neutralizing RBD antibody responses even seemed to be improved. These data do not support the concept of T-cell exhaustion after 2 vaccinations due to high antigen presentation as previously described for viral infections including SARS-CoV-2.4,5
In our view, adapted vaccination protocols with additional vaccinations or higher vaccine doses in KTRs taking immunosuppressants should be encouraged and further investigated.
ACKNOWLEDGMENTs
The authors acknowledge DIA-Vacc Investigators. EUROIMMUN Medizinische Labordiagnostika AG, Lubeck, Germany provided antibody ELISAs and interferon-gamma release assays for this study.
Footnotes
The authors declare no conflicts of interest.
This study was funded by the Else Kröner Fresenius Stiftung, Bad Homburg v. d. H. (grant number Fördervertrag EKFS 2021_EKSE.27).
ClinicalTrials.gov Identifier: NCT04799808.
According to the professional code of conduct for doctors (§15) the clinical trial was submitted to the ethical institutional review boards at Technische Universität Dresden (TU Dresden) responsible for the coordinating investigator (BO-EK-45012021), as well as at the University of Leipzig (046/21-lk) and Saxon Medical Association (Sächsische Landesärztekammer – EK-BR-10/21-1) responsible for further participating trial sites.
J. Stumpf and C.H. contributed to study design, data collection, data interpretation, and drafting of the article. W.T., A.P., R.R., A.S., F.G., F.K., H.K., P.A., J. Sradnick, K.F., and T.T. were involved in data acquisition and collection, study organization, or data interpretation. J. Stumpf, W.T., A.S., and F.G. were involved in statistical analysis or data management of the study. J. Stumpf, A.P., R.R., and C.H. were involved in patient recruitment and data collection. All authors have approved the final version for submission.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Hilbrands LB, Duivenvoorden R, Vart P, et al. ; ERACODA Collaborators. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant. 2020;35:1973–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stumpf J, Siepmann T, Lindner T, et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur. 2021;9:100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamar N, Abravanel F, Marion O, et al. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutiérrez-Bautista JF, Rodriguez-Nicolas A, Rosales-Castillo A, et al. Negative clinical evolution in COVID-19 patients is frequently accompanied with an increased proportion of undifferentiated Th cells and a strong underrepresentation of the Th1 subset. Front Immunol. 2020;11:596553. [DOI] [PMC free article] [PubMed] [Google Scholar]