Abstract
Background
Non-alcoholic fatty liver disease (NAFLD) patients are at a substantial risk for developing cardiovascular disease (CVD). High-density lipoprotein (HDL) is well known to have protective effects against the development of atherosclerotic CVD. One of the major antiatherogenic effects of HDL is its anti-oxidative function.
Objectives
This study investigated the association of anti-oxidative capacity of HDL with subclinical atherosclerosis in NAFLD and non-NAFLD subjects.
Methods
A total of 143 subjects including 51 NAFLD and 92 control subjects were included in this case–control study. HDL oxidative index (HOI) was determined spectrophotometrically using a cell-free method in the presence of a fluorescent substrate dichlorofluorescein diacetate (DCFDA). Paraoxonase 1 (PON1) activity, superoxide dismutase (SOD) activity, and malondialdehyde (MDA) plasma levels were assessed in both groups.
Results
The NAFLD patients with impaired HDL anti-oxidative function (HOI ≥ 1) had higher MDA levels, aspartate amino transferase (AST), liver stiffness (LS), and carotid intima-media thickness (cIMT) values compared to the controls. HDL oxidative index (HOI) was positively correlated with MDA levels and cIMT and negatively correlated with SOD activity.
Conclusions
Higher circulating levels of MDA were associated with the impaired anti-oxidative function of HDL in NAFLD. The impaired anti-oxidative capacity of HDL might be related to NAFLD severity and subclinical atherosclerosis in NAFLD patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13098-021-00741-5.
Keywords: HDL oxidative index, Non-alcoholic fatty liver disease, Cardiovascular disease, Carotid intima-media thickness
Introduction
Non-Alcoholic Fatty Liver Disease (NAFLD) is the most leading cause of chronic liver disease which affects an estimated 20–30% of the general population [1]. NAFLD is strongly associated with other metabolic conditions such as obesity, type 2 diabetes mellitus, and cardiovascular diseases [1]. Despite liver-related morbidity and mortality, cardiovascular disease (CVD) is the most important cause of mortality in the NAFLD population [2].
NAFLD patients exhibit atherogenic dyslipidemia with low levels of high-density lipoprotein cholesterol (HDL-C) [3]. Several studies demonstrated an association of higher plasma high-density lipoprotein (HDL) cholesterol levels with a lower risk of atherosclerotic cardiovascular disease (CVD) morbidity and mortality. However, recent studies suggest that HDL particle functionality might be a better predictor of CDV risk than HDL cholesterol mass levels [4]. HDL particle has numerous antiatherogenic functions. One of the important anti-atherogenic properties of HDL is its anti-oxidative function, the ability to suppress LDL oxidation which in turn decreases the atherogenicity of LDL particles [5].
NAFLD presence was shown to be associated with the size and functional heterogeneity of HDL particles [6, 7]. NAFLD patients had an abnormal distribution of HDL subpopulations and the lipid composition of HDL particles [7]. Proteomics analysis showed altered protein composition of HDL particles in the NAFLD patients [8]. HDL size differed by fatty liver index and NAFLD patients had lower levels of larger HDL particles [6]. Association of HDL ApoA1 with liver fat content was only found in the HDL lacked apoC3 [9]. This heterogeneity results in the alteration of different HDL functionalities such as anti-oxidative function. Several studies addressed the anti-oxidative function of HDL in CVD and other disorders with high cardiovascular risk [10–13]. Moreover, previous studies showed impaired HDL cholesterol efflux capacity in NAFLD [14, 15]. However, no study evaluated the anti-oxidative function of HDL in NAFLD patients.
Oxidative stress which occurs because of the imbalance between antioxidants and reactive oxygen species (ROS) is involved in the initiation and promotion of NAFLD [16, 17]. One of the mechanisms which cause HDL dysfunction is a modification with lipid peroxidation products such as malondialdehyde (MDA) [18, 19]. It has been shown that MDA impaired HDL’s athero-protective functions. MDA generates lysine adducts on apoA-1 and blocks the cholesterol efflux function of HDL [20]. Incubation of macrophages with MDA modified HDL also led to an increased ability to generate ROS [21]. The antioxidant enzymes including superoxide dismutase and PON1 diminish ROS and lipid peroxidation. Paraoxonase 1 (PON1) associates with HDL and catalyzes the hydrolysis of lipid peroxides [22].
Increasing data have demonstrated that NAFLD patients have abnormal circulating markers of oxidative stress, such as increased malondialdehyde (MDA), superoxide dismutase activity, and PON1 activity [23, 24]. Having a disturbed anti-oxidative system along with a high risk of CVD in these patients suggests that impaired antioxidant activities of HDL, as an important player in CVD, may be the potential link between these phenomena. In this study, we aimed to investigate differences in HDL antioxidative capacity between NAFLD patients and controls. Moreover, we sought to compare carotid intima-media thickness as well as other relevant biomarkers between NAFLD patients and controls with preserved and reduced HDL antioxidative capacity.
Material and methods
Study participants
In the current study, 92 Controls and 51 NAFLD patients were selected from Golestan Cohort Study [25]. The study was a case–control study and approved by the medical ethics committee of the Semnan University of Medical Sciences (Ethics Code: IR.SEMUMS.REC.1397. 331) and all participants gave written informed consent. All participants were male, aged 50–81 years old. The diagnosis of NAFLD was based on abdominal ultrasonography. Subjects were excluded if they met one of the following criteria: a history of alcohol consumption (> 30 g/d), diabetes, viral hepatitis, autoimmune liver disease, hemochromatosis, Wilson’s disease. None of the patients were taking medication that has been reported to induce hepatitis, statins and antioxidants.
Ultrasonography and elastography
Ultrasound assessment was performed using an Accuvix XQ ultrasound unit (Medison, South Korea) equipped with a 3–7 MHz curved array and a 5–12 MHz linear array transducer for the evaluation of liver, abdominal fat, and carotid arteries as previously described [25]. Visceral Adipose Tissue thickness (VAT), carotid intima-media thickness (cIMT), and liver stiffness (LS) were measured as described before [25].
Anthropometric and laboratory evaluations
Anthropometric parameters were measured under standardized protocols. Fasting blood samples were obtained from the participants following overnight fasting. Serum and plasma were isolated and frozen in aliquots at − 80 °C for further analysis. Biochemical parameters including Fasting blood glucose (FBG), serum total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and levels of alanine amino transferase (ALT), aspartate amino transferase (AST), gamma glutamyl transferase (GGT) were measured by automated enzymatic methods using commercial kits (Pars Azmoon, Iran).
Paraoxonase 1 (PON1) enzymatic activity was measured according to a method described before. Paraoxon (Sigma-Aldrich, Germany) was used as the substrate and PON1 activity was assessed by measuring the rate of substrate hydrolysis to p-nitrophenol. The formation of p-nitrophenol was recorded at 412 nm and activity was expressed as μmol p-nitrophenol/L/plasma/min [26]. Superoxide dismutase (SOD) activity was measured by a SOD activity assay kit (Teb Pazhouhan Razi, Iran) following the manufacturer’s instructions. Plasma MDA levels were determined using an MDA assay kit (Teb Pazhouhan Razi, Iran) following the manufacturer’s instructions.
Measurement of high‐density oxidative index (HOI)
HOI measures the ability of apoB-depleted plasma to inhibit LDL oxidation in the presence of dichlorofluorescein diacetate (DCFDA) (Sigma-Aldrich, Germany). A cell-free assay was performed as previously described with some modifications [5]. Before analysis, apolipoprotein (apo) B depleted plasma was prepared as previously described [5]. LDL was diluted in PBS to a final cholesterol concentration of 100 μg/mL and oxidized by CuSO4 (100 μmol/L) for 6 h at 37 °C. Oxidized LDL with a concentration of 1.4 μg/ml, DCFDA with a concentration of 0.725 μg/ml and apo B-depleted plasma from the participants with PBS to a final volume of 175 μl were added and incubated at 37 °C for 60 min. Fluorescence intensity was measured with an excitation wavelength of 485 nm and emission wavelength of 530 nm using the synergy h1 hybrid multi-mode microplate reader (BioTek, USA). The HDL oxidative index (HOI) was calculated as the ratio of fluorescence intensity in the presence of apo‐B‐depleted plasma samples divided by the fluorescence intensity in the absence of apo‐B‐depleted plasma. An HOI < 1 was considered as anti-oxidative HDL function and an HOI ≥ 1 was considered as pro‐oxidative HDL.
Statistical analysis
The Kolmogorov‐Smirnov test was used to test for the normal distribution of data. Categorical data are presented as percentages, continuous data as the mean ± SD or median (IQR) as appropriate. Comparison between two groups was performed using the independent-sample t-test or Mann‐Whitney U test for continuous data and the chi‐squared test for categorical data. Differences among the four subgroups as classified based on the presence of NAFLD and HOI were determined using ANOVA followed by Tukey’s post hoc tests. Pearson correlation was applied to determine the correlations between HOI and the other parameters in the whole study population. Non-normally distributed data were log-transformed before ANOVA and Pearson correlation tests. Significant differences were defined by P < 0.05 (*) or (#), P < 0.01 (**) or (##), and P < 0.0001 (****) or (####). All of the statistical analyses were performed using the IBM SPSS Statistic 27 and GraphPad prism 9.
Results
Basic clinical and laboratory characteristics of the study groups are presented in Table 1. BMI, waist circumference (WC), waist-to-hip ratio, fasting blood glucose (FBG), TG, visceral fat, systolic blood pressure, diastolic blood pressure, AST, ALT, GGT, and LS were significantly higher in the NAFLD group compared with the control group. NAFLD patients had lower HDL-C than the control subjects. There was not any significant difference in age, total cholesterol (TC), LDL-C, and cIMT between the two groups.
Table 1.
Baseline characteristics comparison between NAFLD patients and control subjects
| Parameter | Control (n = 92) | NAFLD (n = 51) | P value |
|---|---|---|---|
| Age (years) | 59.0 (55.0–66.0) | 56.0 (54.0–61.0) | 0.273 |
| BMI (kg/m2) | 24.8 ± 3.6 | 29.3 ± 3.4 | < 0.001 |
| WC (cm) | 93.2 ± 11.1 | 103.6 ± 9.6 | < 0.001 |
| WHR | 0.97 (0.92–1.00) | 0.98 (0.94–1.03) | 0.002 |
| Weight (kg) | 68.7 (61.0–79.5) | 80.7 (73.5–89.0) | < 0.001 |
| TC (mg/dL) | 205.5 ± 39.4 | 207.3 ± 37.0 | 0.779 |
| LDL-C (mg/dL) | 121.6 ± 33.7 | 124.6 ± 28.7 | 0.594 |
| HDL-C (mg/dL) | 57.4 ± 12.5 | 51.9 ± 11.1 | 0.007 |
| TG (mg/dL) | 112.0 (82.0–158.0) | 148.5 (112.5–186.0) | 0.016 |
| Systolic blood pressure (mmHg) | 131.5 ± 21.3 | 144.9 ± 23.7 | 0.001 |
| Diastolic blood pressure (mmHg) | 79.9 ± 11.0 | 86.8 ± 12.4 | 0.001 |
| FBG (mg/dL) | 92.71 ± 9.00 | 99.57 ± 11.21 | < 0.001 |
| Visceral fat (%) | 46.5 ± 20.7 | 70.2 ± 18.0 | < 0.001 |
| AST (U/L) | 18.5 (15.0–22.0) | 22.0 (19.5–27.0) | < 0.001 |
| ALT (U/L) | 15.5 (11.0–22.0) | 31.5 (23.0–41.0) | < 0.001 |
| GGT (U/L) | 22.37 (17.40–29.10) | 28.65 (23.53–38.50) | < 0.001 |
| LS (kPa) | 3.8 (3.3–4.3) | 5.0 (4.2–6.6) | < 0.001 |
| cIMT (mm) | 0.8 (0.75–0.87) | 0.81 (0.74–0.91) | 0.863 |
| HOI | 0.92 ± 0.19 | 0.95 ± 0.24 | 0.405 |
| HOI ≥ 1 frequency (%) | 33.69 | 41.17 | 0.468 |
| SOD (U/mg protein) | 10.7 ± 1.5 | 8.6 ± 2.4 | < 0.001 |
| MDA (µM) | 33.2 (25.0–42.3) | 34.0 (30.4–47.7) | 0.771 |
| PON1 (U/L) | 24.4 (17.7–31.1) | 42.2 (27.7–52.2) | < 0.001 |
| Hypertension (%) | 27.90 | 47.36 | 0.021 |
Comparisons between groups were performed using Independent Student’s t test or Mann–Whitney U test as appropriate. Continuous data are expressed as mean ± standard deviation (SD) or median and (interquartile range), categorical data as percentages
BMI body mass index; WC waist circumference; WHR waist to hip ratio; TC total cholesterol; LDL-C low density lipoprotein cholesterol; HDL-C high density lipoprotein cholesterol; TG triglycerides; AST aspartate amino transferase; ALT alanine amino transferase; GGT gamma glutamyl transferase; LS liver stiffness; cIMT carotid intima-media thickness; MDA malondialdehyde; PON1 paraoxonase 1; SOD superoxide dismutase; HOI HDL oxidative index
P < 0.05 was considered statistically significant
There was not any significant difference in the mean of HOI between NAFLD and control groups (P = 0.405). We found that 61 (66.30%) controls and 30 (58.82%) patients had a preserved anti-oxidative function with an HOI < 1, and 31 (33.69%) controls and 21 (41.17%) patients presented pro‐oxidative HDL serum measurements with an HOI ≥ 1 (P = 0.468). We stratified the subjects into two groups, HOI < 1 and HOI ≥ 1. The subjects with HOI ≥ 1 had higher levels of MDA (P = 0.042), lower PON1 activity (P = 0.027), lower SOD activity (P = 0.010), and higher cIMT values (P = 0.022) (Additional file 1: Figure S1).
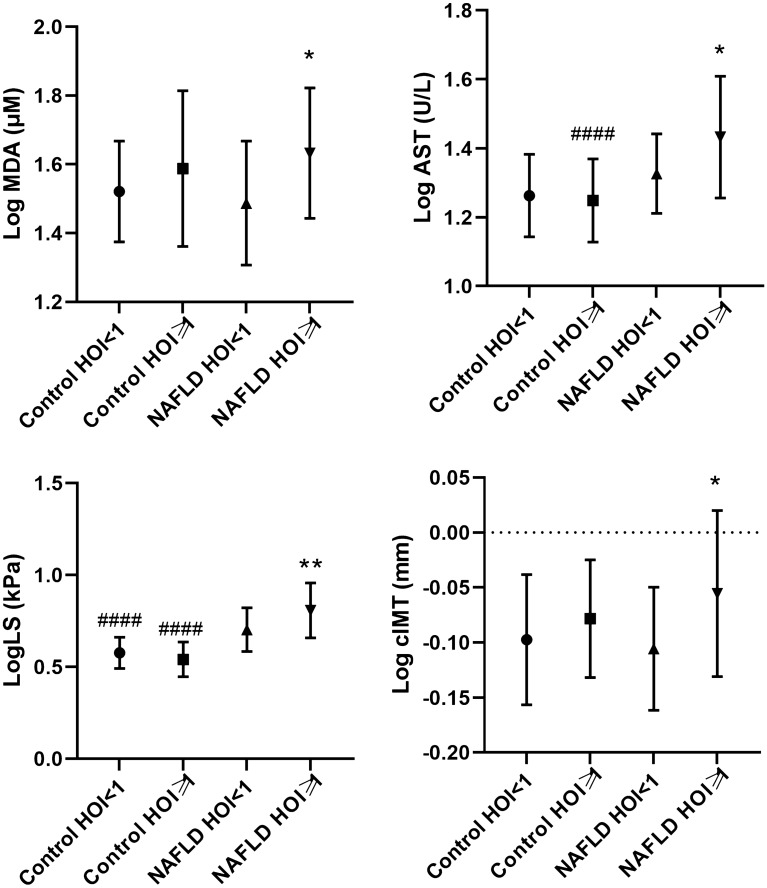
We further stratified the study population into 4 groups according to combined strata of status and HOI above and below 1 and compared MDA, antioxidant enzymes, NAFLD markers, and cIMT (Additional file 1: Table S1). The variables that were significantly different between HOI < 1 and HOI ≥ 1 subgroups of control or NAFLD groups were presented in Fig. 1. Interestingly, NAFLD patients with HOI ≥ 1 had higher MDA (P = 0.043), LS (P = 0.008), AST (P = 0.028) and cIMT (P = 0.030) than the NAFLD patients with HOI < 1.
Fig. 1.
Comparison of AST, LS, cIMT, and MDA in the subjects stratified by a HOI below or above 1 and disease state. ANOVA with Tukey’s post-hoc test was used to compare among four groups. Data were presented as mean ± standard deviation (SD). AST aspartate amino transferase; LS liver stiffness, cIMT carotid intima-media thickness. P < 0.05 was considered statistically significant. *Significant difference between HOI < 1 and HOI ≥ 1. #Significant difference between control and NAFLD groups. Significant differences were defined by P < 0.05 (*) or (#), P < 0.01 (**) or (##), and P < 0.0001 (****) or (####)
Correlation analysis demonstrated that HDL antioxidant capacity (HOI) correlated positively with plasma MDA levels (r = 0.298, P = 0.001) and cIMT (r = 0.197, P = 0.025) in the whole population. There was a significant negative correlation between HOI and the SOD activity (r = − 0.242, P = 0.004) in the whole population (Table 2).
Table 2.
Correlation coefficients between HOI and metabolic and anthropometric parameters
| Parameter | Correlation | P value |
|---|---|---|
| MDA | 0.298 | 0.001 |
| PON1 | − 0.117 | 0.201 |
| SOD | − 0.242 | 0.004 |
| Age | 0.056 | 0.503 |
| cIMT | 0.188 | 0.031 |
| Visceral fat | 0.01 | 0.909 |
| WC | 0.011 | 0.900 |
| BMI | 0.042 | 0.622 |
| WHR | 0.012 | 0.891 |
| AST | 0.059 | 0.488 |
| ALT | 0.067 | 0.432 |
| GGT | 0.075 | 0.374 |
| LS | 0.062 | 0.474 |
| FBG | 0.077 | 0.358 |
| HDL-C | − 0.044 | 0.603 |
| LDL-C | 0.032 | 0.705 |
| TG | − 0.009 | 0.919 |
MDA malondialdehyde; PON1 paraoxonase 1; SOD superoxide dismutase; cIMT carotid intima-media thickness; WC waist circumference; BMI body mass index; WHR waist to hip ratio; AST aspartate amino transferase; ALT alanine amino transferase; GGT gamma glutamyl transferase; LS liver stiffness; HDL-C high density lipoprotein cholesterol; LDL-C low density lipoprotein cholesterol; TG triglycerides
Discussion
Cardiovascular diseases are the main cause of death in NAFLD patients [27]. The impaired anti-oxidative capacity of HDL turned out to be a good predictor of cardiovascular disorders [5]. Our findings showed that impaired anti-oxidative capacity of plasma HDL was associated with higher levels of MDA, hepatic fibrosis markers, and cIMT in NAFLD.
A previous study on the anti-oxidative properties of HDL in patients with coronary syndrome found higher HOI in patients with acute coronary syndrome or stable coronary artery disease compared with controls [11]. Another study reported total HDL antioxidant capacity in systemic lupus erythematosus patients who have an increased risk of cardiovascular diseases were significantly reduced compared to the controls [28]. But there was no significant difference in anti-oxidative properties of HDL in MI patients with or without ST elevation and non-MI participants [10]. We also did not find a significant difference in HOI values between the NAFLD and the control groups. The frequency of HOI ≥ 1 was higher in NAFLD compared to the controls, albeit not significant. Consistently, it has been reported that impaired anti-oxidative capacity of plasma HDL was more frequent in women with polycystic ovary syndrome (PCOS) compared with the control group [13]. These conflicting results might be due to the different study populations and methods used in the studies.
In the current study, we found a higher level of MDA in the NAFLD group compared to the control group. In parallel, previous studies reported increased levels of MDA in the NAFLD patients compared to controls [23, 24, 29]. Additionally, a study showed increased levels of MDA in HDL subfractions isolated from the plasma of acute coronary syndrome patients compared to control subjects and it was along with a pro-inflammatory effect of HDL [30]. Consistently, we found higher levels of plasma MDA in the NAFLD patients with pro-oxidative HDL (HOI ≥ 1) compared to the patients with HOI < 1. Our findings also showed a significant positive correlation between HOI values and MDA levels. Moreover, our results showed higher levels of PON1 paraoxonase activities in the NAFLD patients than in the controls. Conversely, other studies showed lower PON1 serum concentration in the NAFLD group compared to the control group [31, 32]. However, in consistent with our findings, a large population study that used fatty liver index for the diagnosis of NAFLD showed that in men PON-1 activity was significantly higher in the NAFLD population compared to the non-NAFLD population [33]. PON1 has been shown to be critical for the anti-oxidative function of HDL particles. A previous study demonstrated a lower PON-1 activity in patients with an HOI ≥ 1 than the subjects with an HOI < 1 [4]. Although we found lower levels of PON1 paraoxonase activity in all subjects with HO ≥ 1 compared to the subjects with HOI < 1, we didn’t see any significant difference in PON1 between NAFLD patients with HOI ≥ 1 and the patients with HOI < 1. Considering SOD activity, we observed decreased activity of SOD in NAFLD patients. Similarly, other studies reported lower activity of SOD in NAFLD patients [23, 34, 35]. Our findings showed a lower SOD activity in all subjects with HOI ≥ 1 compared to the subjects with HOI < 1 but SOD was not significantly different in the NAFLD patients with HOI ≥ 1 compared to the patients with HOI < 1.
Moreover, we found that NAFLD patients with the impaired antioxidant function of HDL (HOI ≥ 1) had higher AST and LS. Previous studies in NAFLD patients demonstrated the association of circulating oxidative stress biomarkers with disease severity [17]. In the light of the important role of oxidative stress in the progression of NAFLD, one can speculate that NAFLD patients with higher liver enzymes and liver fibrosis have higher levels of pro-oxidative and other detrimental factors which cause HDL dysfunctional. However, owing to the multiple anti-oxidative and anti‐inflammatory properties of HDL, the possibility that an impaired anti-oxidative capacity of HDL may affect disease severity in NAFLD patients cannot be ruled out.
Finally, we observed higher levels of cIMT in the subjects with HDL ≥ 1 compared to the subjects with HOI < 1. We also found a significant positive correlation between HOI and cIMT. However, a previous study on young adults did not find an association between the impaired anti-oxidative capacity of HDL and cIMT [26]. cIMT is considered a surrogate marker of cardiovascular risk [36]. Our findings regarding the association of HOI with cIMT support the notion that impaired anti-oxidative capacity of HDL can be a good predictor of cardiovascular issues. Of note, we found that NAFLD patients with the impaired antioxidant function of HDL (HOI ≥ 1) had higher cIMT. This would suggest that the antioxidant function of HDL might be one of the potential mediators of the relationship between NAFLD and CVD.
There are some limitations to this study. The study was cross-sectional in nature, which does not provide information about possible causal relationships among NAFLD markers, HDL anti-oxidative capacity, and oxidative stress markers. So, further prospective studies are required to specifically address the potential role of HDL anti-oxidative function in the development and progression of NAFLD. We used apolipoprotein B–depleted plasma samples instead of isolated HDL to measure the anti-oxidative capacity of HDL. Polyethylene glycol precipitation is considered as a reproducible and rapid method to extract HDL from plasma samples [5, 11], but there is a possibility that anti-oxidative effects are influenced by other proteins rather than HDL associated proteins. However, a previous study showed comparable HOI values determined with apo B depleted sample and ultracentrifugation isolated HDL.
Conclusions
In summary, our results showed that an impaired anti-oxidative capacity of HDL in NAFLD patients was associated with higher NAFLD markers. This association suggests either this disease state might attenuate the anti-oxidative effect of HDL or HDL anti-oxidative functionality might contribute to NAFLD pathogenesis.
Supplementary Information
Additional file 1: Figure S1. Comparison of cIMT, plasma levels of MDA, PON1 and SOD in the subjects with preserved anti-oxidative HDL capacity (HOI < 1) and impaired anti-oxidative HDL capacity (HOI ≥ 1). The differences between two groups were analyzed by independent Student’s t test and were presented as mean ± SD or median (IQR). MDA malondialdehyde; PON1 paraoxonase 1; SOD superoxide dismutase; cIMT carotid intima-media thickness. Table S1. Comparison of MDA, antioxidant enzymes, NAFLD markers and cIMT in the subjects stratified by a HOI below or above 1 and disease state. ANOVA was used to compare among four groups. Data were presented as mean ± standard deviation (SD). AST aspartate amino transferase; LS liver stiffness; cIMT carotid intima-media thickness; MDA malondialdehyde.
Acknowledgements
The research presented in this article is part of the dissertation of Sara Karami to receive a master’s degree in clinical biochemistry. The authors wish to thank the study participants, doctors, and co-researchers of Digestive Diseases Research Institute of Tehran University of Medical Sciences.
Abbreviations
- NAFLD
Non-alcoholic fatty liver disease
- CVD
Cardiovascular disease
- HDL
High-density lipoprotein
- DCFDA
Dichlorofluorescein diacetate
- PON1
Paraoxonase 1
- HOI
HDL oxidative index
- SOD
Superoxide dismutase (SOD)
- MDA
Malondialdehyde (MDA)
- CIMT
Carotid intima-media thickness
- LDL-C
Low-density lipoprotein cholesterol
- VAT
Visceral adipose tissue thickness
- LS
Liver stiffness
- FBG
Fasting blood glucose
- TC
Total cholesterol
- TG
Triglyceride
- HDL-C
High-density lipoprotein cholesterol
- ALT
Alanine amino transferase
- AST
Aspartate amino transferase
- GGT
Gamma glutamyl transferase
Authors’ contributions
PS designed the study, analyzed the data and wrote the manuscript. AP and HP supervised the study and edited the manuscript. ARR did ultrasonography and fibroscan. SK, FAY, and NS performed the experiments and collected the human subjects. All authors read and approved the final manuscript.
Funding
This work was supported by the Semnan University of Medical Sciences. The funding body played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All participants gave written informed consent. This study was approved by the medical ethics committee of the Semnan University of Medical Sciences (Ethics Code: IR.SEMUMS.REC.1397. 331).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbas Pakdel and Parisa Shabani contributed equally to this work
Contributor Information
Abbas Pakdel, Email: pakdel@semums.ac.ir.
Parisa Shabani, Email: parisa83j@gmail.com.
References
- 1.Vernon G, Baranova A, Younossi Z. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34(3):274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 2.McCullough A, Previs SF, Dasarathy J, Lee K, Osme A, Kim C, et al. HDL flux is higher in patients with nonalcoholic fatty liver disease. Am J Physiol Endocrinol Metab. 2019;317(5):E852–E862. doi: 10.1152/ajpendo.00193.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang K, Shan S, Zheng H, Zhao X, Chen C, Liu C. Non-HDL-cholesterol to HDL-cholesterol ratio is a better predictor of new-onset non-alcoholic fatty liver disease than non-HDL-cholesterol: a cohort study. Lipids Health Dis. 2018;17(1):1–8. doi: 10.1186/s12944-017-0646-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schrutka L, Distelmaier K, Hohensinner P, Sulzgruber P, Lang IM, Maurer G, et al. Impaired high-density lipoprotein anti-oxidative function is associated with outcome in patients with chronic heart failure. J Am Heart Assoc. 2016 doi: 10.1161/JAHA.116.004169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schrutka L, Distelmaier K, Hohensinner P, Sulzgruber P, Lang IM, Maurer G, et al. Impaired high-density lipoprotein anti-oxidative function is associated with outcome in patients with chronic heart failure. J Am Heart Assoc. 2016;5(12):e004169. doi: 10.1161/JAHA.116.004169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amor AJ, Pinyol M, Solà E, Catalan M, Cofán M, Herreras Z, et al. Relationship between noninvasive scores of nonalcoholic fatty liver disease and nuclear magnetic resonance lipoprotein abnormalities: a focus on atherogenic dyslipidemia. J Clin Lipidol. 2017;11(2):551–61.e7. doi: 10.1016/j.jacl.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Orozco Morales JA, Medina Urrutia AX, Torres Tamayo M, Jorge Galarza E, Reyes Barrera J, Díes Suarez P, et al. Effects of fatty liver on the size and composition of high-density lipoprotein cholesterol subpopulations in adolescents with type 2 diabetes mellitus. Pediatr Diabetes. 2020;21(7):1140–1149. doi: 10.1111/pedi.13103. [DOI] [PubMed] [Google Scholar]
- 8.Rao PK, Merath K, Drigalenko E, Jadhav AYL, Komorowski RA, Goldblatt MI, et al. Proteomic characterization of high-density lipoprotein particles in patients with non-alcoholic fatty liver disease. Clin Proteom. 2018;15:10. doi: 10.1186/s12014-018-9186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morze J, Koch M, Aroner SA, Budoff M, McClelland RL, Mukamal KJ, et al. Associations of HDL subspecies defined by ApoC3 with non-alcoholic fatty liver disease: the multi-ethnic study of atherosclerosis. J Clin Med. 2020 doi: 10.3390/jcm9113522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Annema W, Willemsen HM, de Boer JF, Dikkers A, van der Giet M, Nieuwland W, et al. HDL function is impaired in acute myocardial infarction independent of plasma HDL cholesterol levels. J Clin Lipidol. 2016;10(6):1318–1328. doi: 10.1016/j.jacl.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Patel PJ, Khera AV, Jafri K, Wilensky RL, Rader DJ. The anti-oxidative capacity of high-density lipoprotein is reduced in acute coronary syndrome but not in stable coronary artery disease. J Am Coll Cardiol. 2011;58(20):2068–2075. doi: 10.1016/j.jacc.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 12.Morgantini C, Natali A, Boldrini B, Imaizumi S, Navab M, Fogelman AM, et al. Anti-inflammatory and antioxidant properties of HDLs are impaired in type 2 diabetes. Diabetes. 2011;60(10):2617–2623. doi: 10.2337/db11-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Zhang Y, Liu H, Bai H, Wang Y, Jiang C, et al. Antioxidant properties of high-density lipoproteins are impaired in women with polycystic ovary syndrome. Fertil Steril. 2015;103(5):1346–1354. doi: 10.1016/j.fertnstert.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 14.Fadaei R, Poustchi H, Meshkani R, Moradi N, Golmohammadi T, Merat S. Impaired HDL cholesterol efflux capacity in patients with non-alcoholic fatty liver disease is associated with subclinical atherosclerosis. Sci Rep. 2018;8(1):11691. doi: 10.1038/s41598-018-29639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Berg EH, Gruppen EG, Ebtehaj S, Bakker SJL, Tietge UJF, Dullaart RPF. Cholesterol efflux capacity is impaired in subjects with an elevated Fatty Liver Index, a proxy of non-alcoholic fatty liver disease. Atherosclerosis. 2018;277:21–27. doi: 10.1016/j.atherosclerosis.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 16.Wang B, Yang RN, Zhu YR, Xing JC, Lou XW, He YJ, et al. Involvement of xanthine oxidase and paraoxonase 1 in the process of oxidative stress in nonalcoholic fatty liver disease. Mol Med Rep. 2017;15(1):387–395. doi: 10.3892/mmr.2016.6025. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z, Tian R, She Z, Cai J, Li H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radical Biol Med. 2020;152:116–141. doi: 10.1016/j.freeradbiomed.2020.02.025. [DOI] [PubMed] [Google Scholar]
- 18.Ito F, Ito T, Suzuki C, Yahata T, Ikeda K, Hamaoka K. The application of a modified d-ROMs test for measurement of oxidative stress and oxidized high-density lipoprotein. Int J Mol Sci. 2017;18(2):454. doi: 10.3390/ijms18020454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monserrat-Mesquida M, Quetglas-Llabrés M, Abbate M, Montemayor S, Mascaró CM, Casares M, et al. Oxidative stress and pro-inflammatory status in patients with non-alcoholic fatty liver disease. Antioxidants. 2020 doi: 10.3390/antiox9080759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shao B, Pennathur S, Pagani I, Oda MN, Witztum JL, Oram JF, et al. Modifying apolipoprotein A-I by malondialdehyde, but not by an array of other reactive carbonyls, blocks cholesterol efflux by the ABCA1 pathway. J Biol Chem. 2010;285(24):18473–18484. doi: 10.1074/jbc.M110.118182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schill RL, Knaack DA, Powers HR, Chen Y, Yang M, Schill DJ, et al. Modification of HDL by reactive aldehydes alters select cardioprotective functions of HDL in macrophages. FEBS J. 2020;287(4):695–707. doi: 10.1111/febs.15034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sehitogulları A, Aslan M, Sayır F, Kahraman A, Demir H. Serum paraoxonase-1 enzyme activities and oxidative stress levels in patients with esophageal squamous cell carcinoma. Redox Rep. 2014;19(5):199–205. doi: 10.1179/1351000214Y.0000000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arya A, Azarmehr N, Mansourian M, Doustimotlagh AH. Inactivation of the superoxide dismutase by malondialdehyde in the nonalcoholic fatty liver disease: a combined molecular docking approach to clinical studies. Arch Physiol Biochem. 2019 doi: 10.1080/13813455.2019.1659827. [DOI] [PubMed] [Google Scholar]
- 24.Świderska M, Maciejczyk M, Zalewska A, Pogorzelska J, Flisiak R, Chabowski A. Oxidative stress biomarkers in the serum and plasma of patients with non-alcoholic fatty liver disease (NAFLD) Can plasma AGE be a marker of NAFLD? Oxidative stress biomarkers in NAFLD patients. Free Radic Res. 2019;53(8):841–50. doi: 10.1080/10715762.2019.1635691. [DOI] [PubMed] [Google Scholar]
- 25.Merat S, Poustchi H, Hemming K, Jafari E, Radmard AR, Nateghi A, et al. PolyPill for prevention of cardiovascular disease in an urban iranian population with special focus on nonalcoholic steatohepatitis: a pragmatic randomized controlled trial within a cohort (polyiran—liver)—study protocol. Arch Iran Med. 2015;18(8):515–523. [PubMed] [Google Scholar]
- 26.Breton CV, Yin F, Wang X, Avol E, Gilliland FD, Araujo JA. HDL anti-oxidant function associates with LDL level in young adults. Atherosclerosis. 2014;232(1):165–170. doi: 10.1016/j.atherosclerosis.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SB, Park G-M, Lee J-Y, Lee BU, Park JH, Kim BG, et al. Association between non-alcoholic fatty liver disease and subclinical coronary atherosclerosis: an observational cohort study. J Hepatol. 2018;68(5):1018–1024. doi: 10.1016/j.jhep.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Gaál K, Tarr T, Lőrincz H, Borbás V, Seres I, Harangi M, et al. High-density lipopoprotein antioxidant capacity, subpopulation distribution and paraoxonase-1 activity in patients with systemic lupus erythematosus. Lipids Health Dis. 2016;15(1):1–8. doi: 10.1186/s12944-016-0229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito F, Ito T. High-density lipoprotein (Hdl) triglyceride and oxidized HDL: new lipid biomarkers of lipoprotein-related atherosclerotic cardiovascular disease. Antioxidants. 2020;9(5):362. doi: 10.3390/antiox9050362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carnuta MG, Stancu CS, Toma L, Sanda GM, Niculescu LS, Deleanu M, et al. Dysfunctional high-density lipoproteins have distinct composition, diminished anti-inflammatory potential and discriminate acute coronary syndrome from stable coronary artery disease patients. Sci Rep. 2017;7(1):1–13. doi: 10.1038/s41598-017-07821-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milaciu MV, Vesa ȘC, Bocșan IC, Ciumărnean L, Sâmpelean D, Negrean V, et al. Paraoxonase-1 serum concentration and PON1 gene polymorphisms: relationship with non-alcoholic fatty liver disease. J Clin Med. 2019;8(12):2200. doi: 10.3390/jcm8122200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samy W, Hassanian MA. Paraoxonase-1 activity, malondialdehyde and glutathione peroxidase in non-alcoholic fatty liver disease and the effect of atorvastatin. Arab J Gastroenterol. 2011;12(2):80–85. doi: 10.1016/j.ajg.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 33.van den Berg EH, Gruppen EG, James RW, Bakker SJL, Dullaart RPF. Serum paraoxonase 1 activity is paradoxically maintained in nonalcoholic fatty liver disease despite low HDL cholesterol. J Lipid Res. 2019;60(1):168–175. doi: 10.1194/jlr.P088997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koruk M, Taysi S, Savas MC, Yilmaz O, Akcay F, Karakok M. Oxidative stress and enzymatic antioxidant status in patients with nonalcoholic steatohepatitis. Ann Clin Lab Sci. 2004;34(1):57–62. [PubMed] [Google Scholar]
- 35.Videla LA, Rodrigo R, Orellana M, Fernandez V, Tapia G, Quiñones L, et al. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin Sci. 2004;106(3):261–268. doi: 10.1042/CS20030285. [DOI] [PubMed] [Google Scholar]
- 36.Willeit P, Tschiderer L, Allara E, Reuber K, Seekircher L, Gao L, et al. Carotid intima-media thickness progression as surrogate marker for cardiovascular risk: meta-analysis of 119 clinical trials involving 1,00,667 patients. Circulation. 2020;142(7):621–642. doi: 10.1161/CIRCULATIONAHA.120.046361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Comparison of cIMT, plasma levels of MDA, PON1 and SOD in the subjects with preserved anti-oxidative HDL capacity (HOI < 1) and impaired anti-oxidative HDL capacity (HOI ≥ 1). The differences between two groups were analyzed by independent Student’s t test and were presented as mean ± SD or median (IQR). MDA malondialdehyde; PON1 paraoxonase 1; SOD superoxide dismutase; cIMT carotid intima-media thickness. Table S1. Comparison of MDA, antioxidant enzymes, NAFLD markers and cIMT in the subjects stratified by a HOI below or above 1 and disease state. ANOVA was used to compare among four groups. Data were presented as mean ± standard deviation (SD). AST aspartate amino transferase; LS liver stiffness; cIMT carotid intima-media thickness; MDA malondialdehyde.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.