The most effective approach to fight COVID-19 infection is prevention by targeted vaccines that prime the immune system prior to a first encounter with the virus. The BNT162b2 messenger RNA (mRNA) vaccine encoding the SARS-CoV-2 full length spike generates antibodies that prevent the entry of the virus into cells by blocking either ACE2–RBD binding interactions or spike-mediated membrane fusion [1]. The vaccine proved efficient in a large multinational trial, including 21,720 subjects vaccinated with BNT162b2. The study demonstrated 95% efficacy in preventing COVID-19 disease [2]. As the process of vaccination against COVID-19 is currently spreading all over the world, it is of importance to assess the longevity of the humoral immune response to the vaccination. A high percentage of protective antibody responses was reported 7 days after full vaccination with BNT162b2 [3]. In a very recent study anti-RBD IgG was detected in 100% of fully vaccinated individuals at days 33-55 for both the Moderna and Pfizer cohorts [4]. To better understand the longevity of the humoral response, we conducted a prospective longitudinal study among a cohort of healthy vaccinees up to eight months from the second vaccine dose. The study was approved by Sheba Medical Center Institutional Review Board (approval number Sheba.SMC-8182-21), and all participants signed written informed consent. All participants had no prior infection with SARS-CoV-2, and no evidence of infection during the study. Subjects received two PfizerBNT162b2 vaccine doses, 21 days apart, delivered in the deltoid muscle. SARS-CoV-2 anti-spike IgG levels were measured at least 28 days following the second vaccine dose at two or more time points and spike IgG titer average t1/2 was calculated using linear regression from log2-transformed data for each pair, according to the following equation t1/2=Elapsed time*log2/log[Beginning amount/Ending amount]. Immunoassay for the detection of SARS-CoV-2 IgG antibodies was performed using anti-SARS-CoV-2 QuantiVac ELISA IgG (Euroimmun, Lubeck, Germany) based on the S1 domain of the spike protein. Cut-off for positive IgG level was determined as >35.2 binding antibody units (BAU)/ml. The test has a sensitivity of 93.2% % and a specificity of 100%. Correlation with neutralization tests was reported to be 98.2% (https://www.euroimmun.de/en/).
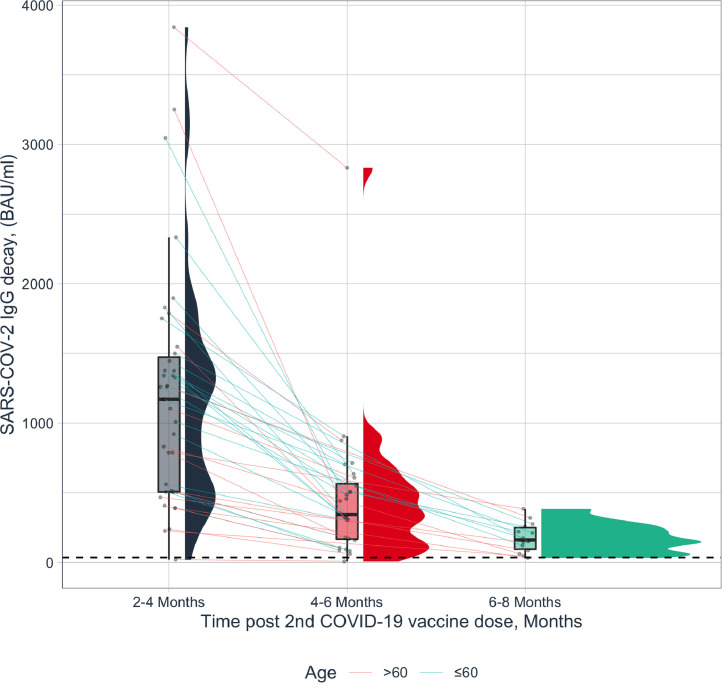
Thirty-nine vaccinated subjects, 9 males, 30 females, mean±SD age 54.3±15.24 years, median 57.8 years, range 25.9-89.0 years, followed for median 4.3 months, range 1.0 - 8.3 months after the second vaccine dose, were included in the study. The time interval between the first and second paired IgG tests was median 83 days, (25 IQR-75 IQR) 64-98 days, range 20 to 142 days. Spike IgG titers (BAU/ml) were heterogeneous among subjects, median 921, (25 IQR-75 IQR) 505–1376, range 19.2-3840.0). The decline from the first to the second time point in spike IgG titers (BAU/ml) was median 617.6, (25 IQR-75 IQR) 300.8-960.0, range 12.8-2764.8). Fig. 1 demonstrates the durability of paired assessments of circulating antibody titers based on data ≥28 days post the second vaccine dose. The antibody responses decay kinetics showed median t1/2 of 45 days, (25 IQR-75 IQR) 38.2-62.3 days. SARS-CoV-2 IgG titers inversely correlated with age (r= -0.323), and SARS-CoV-2 IgG decline corrected for baseline level correlated with age ≥60 years (r= 0.277), while in younger subjects <60 years an inverse correlation was observed (r= -0.502). No significant differences were observed in the decay kinetics in relation with gender. Our findings demonstrate that anti-S1 IgG levels determined across 1 to 8 months after full vaccination, waned with an estimated half-life of 45 days. Age was associated with lower post-vaccination anti-S1 IgG titers and with accelerated IgG decrease over time. Moreover, we calculated that within 225 days (5*t1/2), IgG will decrease below detection level, raising the need for reconsidering the current mRNA vaccine regiments. In comparison, seasonal coronavirus protective immunity in convalescents was reported to be similarly short-lasting up to one year [5]. However, for SARS-CoV and MERS, IgG antibodies remained detectable in 100% and 74.2% of subjects respectively, for 1-3 years post-infection [6]. Our group recently reported that 85% of convalescent COVID-19 subjects had a protective IgG level that prevailed over a period of up to 9 months post-infection, regardless of age or gender [7].
Fig. 1.
Paired circulating antibodies to SARS-CoV-2 over time in healthy vaccinated subjects. Longitudinal spike IgG (n=39, BAU/ml), median t1/2=45 days; 25-75 IQR 38.2-62.3 days. The red line indicates the cutoff. Subjects aged >60 years are depicted in red, subjects aged ≤ 60 years in light blue.
The question of long‐lasting and protective vaccine immunity against SARS‐CoV‐2 is of major importance as the decay suggests the need for an additional booster. A decrease in IgG levels impairs the equilibrium between COVID-19 viral load during exposure and humoral protection. The induction and decay of antigen-specific IgG response to COVID-19 mRNA vaccine are consistent with the known serum T½ of various IgG subclasses immunoglobulin [8]. Among fully vaccinated health care workers, the occurrence of SARS-CoV-2 infection correlated with lower neutralizing antibody titers during the peri-infection period [9]. Older vaccinees, aged ≥ 60 years, demonstrated a more profound decrease in IgG levels over time and are therefore at increased risk for infection with COVID-19 and COVID-19 variants. The Israeli healthcare authorities in accordance recently launched the third vaccination booster in people older than 60 years of age.
CRediT authorship contribution statement
Anat Achiron: Data curation, Funding acquisition, Writing – original draft, Funding acquisition, Writing – review & editing. Mathilda Mandel: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. Sapir Dreyer-Alster: Visualization, Software, Data curation, Writing – review & editing. Gil Harari: Formal analysis, Data curation, Writing – review & editing. Michael Gurevich: Data curation, Supervision, Writing – review & editing.
Declaration of Competing Interest
Anat Achiron, MD, PhD - no conflicts of interest.Mathilda Mandel, MD, MHA - no conflicts of interest. Sapir Dreyer-Alster, BSc - no conflicts of interest Gil Harari, PhD - no conflicts of interest. Michael Gurevich - no conflicts of interest.
Funding/Support
This work was funded by research grants from the Sheba Medical Center (GN. 04027-10/1001493), and the Laura Schwarz-Kipp Research Fund for Autoimmune Diseases (GN. 0601253231), Sackler School of Medicine, Tel-Aviv University, Israel.
References
- 1.Vogel A.B., Kanevsky I., Che Y., et al. BNT162b vaccines are immunogenic and protect non-human primates against SARS-CoV-2. bioRxiv. 2020;12 [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei J., Stoesser N., Matthews P.C., et al. COVID-19 Infection Survey team. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat Microbiol. 2021;21:1–10. doi: 10.1038/s41564-021-00947-3. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bliden K.P., Liu T., Sreedhar D., et al. Evolution of Anti-SARS-CoV-2 IgG Antibody and IgG Avidity Post Pfizer and Moderna mRNA Vaccinations. medRxiv. 2021;06(28) [Google Scholar]
- 5.Edridge A.W.D., Kaczorowska J., Hoste A.C.R., et al. Seasonal coronavirus protective immunity is short-lasting. Nat Med. 2020;26:1691–1693. doi: 10.1038/s41591-020-1083-1. [DOI] [PubMed] [Google Scholar]
- 6.Huang A.T., Garcia-Carreras B., Hitchings M.D.T., et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun. 2020;11:4704. doi: 10.1038/s41467-020-18450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Achiron A., Gurevich M., Falb R., Dreyer-Alster S., Sonis P., Mandel M. SARS-CoV-2 antibody dynamics and B-cell memory response over time in COVID-19 convalescent subjects. Clin Microbiol Infect. 2021;S1198-743X(21) doi: 10.1016/j.cmi.2021.05.008. 00229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mankarious S., Lee M., Fischer S., et al. The half-lives of IgG subclasses and specific antibodies in patients with primary immunodeficiency who are receiving intravenously administered immunoglobulin. J Lab Clin Med. 1988;112:634–640. [PubMed] [Google Scholar]
- 9.Bergwerk M., Gonen T., Lustig Y., et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021 doi: 10.1056/NEJMoa2109072. Jul 28. [DOI] [PMC free article] [PubMed] [Google Scholar]