Abstract
Background
Epidemiological data associate high levels of combustion-derived particulate matter (PM) with deleterious respiratory outcomes, but the mechanism underlying those outcomes remains elusive. It has been acknowledged by the World Health Organization that PM exposure contributes to more than 4.2 million all-cause mortalities worldwide each year. Current literature demonstrates that PM exacerbates respiratory diseases, impairs lung function, results in chronic respiratory illnesses, and is associated with increased mortality. The proposed mechanisms revolve around oxidative stress and inflammation promoting pulmonary physiological remodeling. However, our previous data found that PM is capable of inducing T helper cell 17 (Th17) immune responses via aryl hydrocarbon receptor (Ahr) activation, which was associated with neutrophilic invasion characteristic of steroid insensitive asthma.
Methods
In the present study, we utilized a combination of microarray and single cell RNA sequencing data to analyze the immunological landscape in mouse lungs following acute exposure to combustion derived particulate matter.
Results
We present data that suggest epithelial cells produce specific cytokines in the aryl hydrocarbon receptor (Ahr) pathway that inform dendritic cells to initiate the production of pathogenic T helper (eTh17) cells. Using single-cell RNA sequencing analysis, we observed that upon exposure epithelial cells acquire a transcriptomic profile indicative of increased Il-17 signaling, Ahr activation, Egfr signaling, and T cell receptor and co-stimulatory signaling pathways. Epithelial cells further showed, Ahr activation is brought on by Ahr/ARNT nuclear translocation and activation of tyrosine kinase c-src, Egfr, and subsequently Erk1/2 pathways.
Conclusions
Collectively, our data corroborates that PM initiates an eTh17 specific inflammatory response causing neutrophilic asthma through pathways in epithelial, dendritic, and T cells that promote eTh17 differentiation during initial PM exposure.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-021-01867-w.
Keywords: Combustion derived particulate matter, EPFRs, ScRNA sequencing, Aryl hydrocarbon receptor, Th17
Background
Evidence links exposure to elevated levels of PM with deleterious health effects. Combustion-derived PM is generated by a variety of processes (e.g., burning of diesel/gasoline, stoves, cigarettes, etc.) and has been labeled a group 1 carcinogen by the World Health Organization in order to maintain and protect air quality and human health [21]. Particulate matter is generally categorized into three groups based on the diameter of the particles: course particulate matter, with a mean aerodynamic diameter < 10 µm, fine particulate matter, with an aerodynamic diameter < 2.5 µm; and ultra-fine particulate matter, with an aerodynamic diameter < 0.1 µm. While PM2.5 levels are generally below the national standard of 35 µg/m3 over a 24-h period, as advised by the U.S. Environmental Protection Agency (EPA), in major cities such as Los Angeles and in households, the levels can periodically exceed 150 µg/m3 [24, 61]. Even slight increases in PM2.5 levels show deleterious effects, as a study in 2013 demonstrated a correlation of more than 300,000 patients over nine countries and lung cancer frequency increasing by 36% per 10 µg/m3 increase [47]. It has been shown that organic pollutants chemically bond through transition metals, acting as intermediaries for environmentally persistent free radicals EPFRs [14, 15, 19, 31, 64]. The stabilized free radicals, as well as the transition metals present in particles, increase the production of reactive oxygen species (ROS) through Fenton chemistry, perpetuate the existence and stabilization of free radicals, and induce airway injury and inflammation [4, 22, 32, 34]. Increased ROS results in damage to tissues and disruption of cellular structure, inducing or exacerbating inflammatory responses. Many studies have demonstrated that acute exposure to elevated levels of PM elicits an inflammatory response within the lung and systemically causes oxidative stress. In human studies, it has been demonstrated that exposure to PM elicits increases in Il-6, Gm-csf, Il-1β, C-reactive protein, fibrinogen, and Tnf-α [62], as well as increases in pulmonary neutrophil numbers [45]. The activation of these pro-inflammatory cytokines and neutrophil invasion have been associated with increase in morbidity and mortality rates. Studies have also shown that increases of 10 µg/m3 in major cities corresponded to increases of up to 67% of all-cause mortality rates, as well as increased risk of atherosclerosis, immunological modifications, pulmonary oxidative stress, and a faster progression of chronic obstructive pulmonary disease (COPD) and cardiovascular diseases [40]. In addition, experiments with mouse models have shown PM activates NLRP3 inflammasome [30], promotes lung fibrosis [71], disturbs inflammatory cytokine homeostasis associated with changes in trace metal levels [39], compromises the antioxidant defense response [38], and increases the severity of respiratory infections [28, 49]. Therefore, the mechanism and response to PM is critical knowledge to fully understand PM exposure linked increase of morbidity and mortality.
In the current study, we determined the possible mechanistic pathways responsible for PM induced pathogenic T helper 17 (eTh17) response through epithelial activation of Ahr induced cytokines, dendritic cell cytokine activation of eTh17 specific cytokines, and gamma delta/natural killer T cell production of eTh17 specific cytokines. This is contrary to the activation of regulatory Th17 cells (rTh17) which have been shown to upregulate Ahr and IL-10 consequently allowing for overproduction of pro-inflammatory cytokines and neutrophil recruitment. We present data that suggest epithelial cells produce specific cytokines in the aryl hydrocarbon receptor (Ahr) pathway that inform dendritic cells to initiate the production of eTh17 cells.
Materials and methods
Particulate matter exposure
Both male and female C57BL/6J (Jackson) mice (aged 8–10 weeks) were used for the experiments. All mice were given free access to rodent chow and water and were maintained in a 12-h light-cycle environment. All animal protocols were written according to Policy for the Care and Use of Laboratory Animals and approved by the LSU Institutional Animal Care and Use Committee at Louisiana State University. We used a lab generated PM that contains EPFRs known as MCP230 (PM) with a mean aerodynamic diameter < 0.2 µm created and characterized by Dr. Lomnicki at Louisiana State University, as we have previously published [28]. Particles were suspended at a concentration of 1 mg/ml in sterile saline with 0.02% tween-80 PM particle solution was sonicated for 2 min at 30-s intervals on ice with a probe sonicator set to 50% amplitude. Mice were exposed to 50 µl of particle solution (vehicle) or 50 µl of PM for 4 h via oropharyngeal aspiration (OA), as previously described [27]. 50 µl OA exposure is based on the efficiency of instillation into the lungs and results in an inhalation exposure equivalent of 200 µg/m3 [53].
RT2 PCR analysis
Following exposure, mice were euthanized, and their lungs were subjected to retrograde perfusion with 2 ml Hank’s Balanced Salt solution (HBSS) to remove red blood cells. We followed the manufactures protocol for RNA isolation and purification. We used the RT2 PCR kit for mouse drug metabolism (Catalog No. 330231) to analyze 84 genes related to the metabolism of PM particles in the lungs of (n = 10) mice. Analysis of the data was performed using the RT2 Profiler PCR Data Analysis tools on Qiagen’s website. We used the CT cutoff of 35 and the full panel geometric mean normalization method available in Qiagen’s analysis tool. Significance was calculated based on a Student’s t-test as p-value < 0.05.
Single-cell dissociation of C57BL/6J mice
Following exposure, mice were euthanized, and their lungs were subjected to retrograde perfusion with 2 ml Hank’s Balanced Salt solution (HBSS) to remove red blood cells. The isolated lungs were dissociated using the gentle MACS Dissociator (Miltenyi Biotec) in 2 ml pre-warmed (37 °C) digest buffer (2 mg/ml Type 2 Collagenase, 1 mg/ml ProNase E, 62.5 U/ml DNAse 1, and 5 mM CaCl2 made up in DPBS without added calcium and magnesium) per 100 mg tissue. The lungs were further dissociated for 5 min using a 1000-µl pipette with an additional 1 ml pre-warmed digest buffer added. The lungs were then finely dissociated into a single-cell suspension using a ThermoMixer (Eppendorf) pre-warmed to 37 °C at 1200 RPM for 5 min. To remove clumps, the single cell suspension was passed through a 23-gauge needle and then filtered using 40 µM filter placed on top of a 50 ml conical tube and rinsed with 2 ml of 10% heat inactivated FBS/PBS solution. The resulting cell suspension was again filtered through a 40-µM filter to ensure that no clumping cells remained. Finally, the suspension was centrifuged at 1200G for 5 min, supernatant was removed, and cell pellet was suspended in 10 ml of 1% FBS/PBS solution. Barcoding of single cells was done using the Drop-seq protocol Version 3.1, by Dr. Steven Potter (Cincinnati Children’s Hospital), with a cell suspension of 100 cells/µl as previously described [46].
Bioinformatics
After the droplets were sequenced on Illumina Nextseq500, we utilized the Drop-seq tools-2.3.0 pipeline to tag cell barcodes, tagged molecular barcodes, trimmed a 5ʹ primer sequence, trimmed a 3ʹ polyA sequence, converted the SAM filetype to Fastq, used STAR to align the sequences, sorted STAR alignment in queryname order, merged STAR alignment to M24 (GRCm38.p6) mouse genome, tagged SAM to recover cell/molecular barcodes, added gene/exon and other annotation tags, and conducted barcode repair. For downstream analysis, we removed cells with < 300 detected genes (transcript count > 0) and > 10% of transcript counts mapped to mitochondrial genes, which is indicative of broken cellular membranes, and removed genes with transcripts detected in < 3 cells in Seurat which is standard procedure (version 3.1.2;) [10, 41, 57]. After combining our vehicle and PM samples, this left us with 15,813 genes × 6118 cells and 15,851 genes × 5337 cells, respectively. The data were then processed using the sctransform normalization method with the standard Seurat data integration protocol. Clustering was performed with Seurat’s UMAP using significant principal components (PCs) determined by a JackStraw plot. PCs used to construct the UMAP were p < 0.05 and the elbow plot was used to determine the cutoff; the elbow and jackstraw plot are presented as Additional file 1. Marker genes differentiating each cluster were determined using Seurat’s FindAllMarkers function with the default settings. We used SingleR and the Immgen mouse genome database to classify our cell types by referencing the marker genes, derived from the FindAllMarkers function, with the Immgen database [1]. Differentially expressed genes were identified using the FindMarkers function in Seurat, which applied the Wilcoxon rank-sum test with a false discovery rate (FDR) < 0.05. The differential expression of each cell type was analyzed using R Clusterprofiler for gene ontology analysis, gene set enrichment analysis, and Kyoto Encyclopedia of Genes and Genomes (Kegg) pathway enrichment [70]. Genes in enriched pathways were individually identified to be activation factors or shown to increase signaling within the enriched pathway. The significance reported here was adjusted using Benjamini–Hochberg correction which controls the FDR and thus significance was determined to be FDR < 0.05 in the gene ontology analysis and gene enrichment analysis.
Results
RT2 profiler array of EPFR containing ultrafine particle exposure in vivo
We and others have observed PM affecting changes to epithelial cell integrity resulting in pulmonary neutrophilic infiltration, and metabolic dysregulation [2, 5, 33, 36, 40, 67]. However, the specific mechanisms by which PM causes these deleterious effects have yet to be discovered. To elucidate the effects that exposure of EPFR containing ultrafine particles (PM) has on the transcriptomic profile of mouse lungs, we exposed C57BL/6J adult mice to a laboratory generated particle with properties mimicking combustion derived PM but with defined chemical speciation (PM) and analyzed the lungs by the RT2 Mouse Drug Metabolism profiler PCR array. The PCR array showed 12 genes that were significantly increased following 4-h exposure to PM (Table 1). We saw significant increases in mRNA of Cyp1a1, Cyp4b1, Gpx2, Mt2, and Aldh1a1. Using pathway analysis in the Database for Annotation, Visualization and Integrated Discovery (DAVID) identified tryptophan metabolism, steroid hormone biosynthesis, retinol metabolism, glutathione metabolism, and thyroid hormone synthesis enrichment, suggesting that exposure to PM increases oxidative stress and elicits an antioxidant response.
Table 1.
The RT2 Profiler array shows significant (p < 0.05) gene regulation of Ahr cytokines in PM compared to vehicle exposed (n = 5) mice after 4-h post exposure (n = 5)
| Gene symbol | Name | Log2 fold change | p-value |
|---|---|---|---|
| Aoc1 | Amine oxidase, copper-containing 1 | 1.63 | 0.001 |
| Aldh1a1 | Aldehyde dehydrogenase family 1, subfamily A1 | 1.44 | 0.006 |
| Comt | Catechol-O-methyltransferase | 1.68 | 0.040 |
| Cyp1a1 | Cytochrome P450, family 1, subfamily a, polypeptide 1 | 4.91 | 0.010 |
| Cyp4b1 | Cytochrome P450, family 4, subfamily b, polypeptide 1 | 1.48 | 0.011 |
| Gpx2 | Glutathione peroxidase 2 | 2.72 | 0.001 |
| Gsr | Glutathione reductase | 1.94 | 0.005 |
| Hk2 | Hexokinase 2 | 1.70 | 0.014 |
| Mt2 | Metallothionein 2 | 2.58 | 0.005 |
| Nqo1 | NAD(P)H dehydrogenase, quinone 1 | 1.69 | 0.004 |
| Pon1 | Paraoxonase 1 | 1.43 | 0.043 |
| Pon3 | Paraoxonase 3 | 1.26 | 0.039 |
ScRNA-seq of in vivo exposure to PM pinpoints a distinct gene expression signature
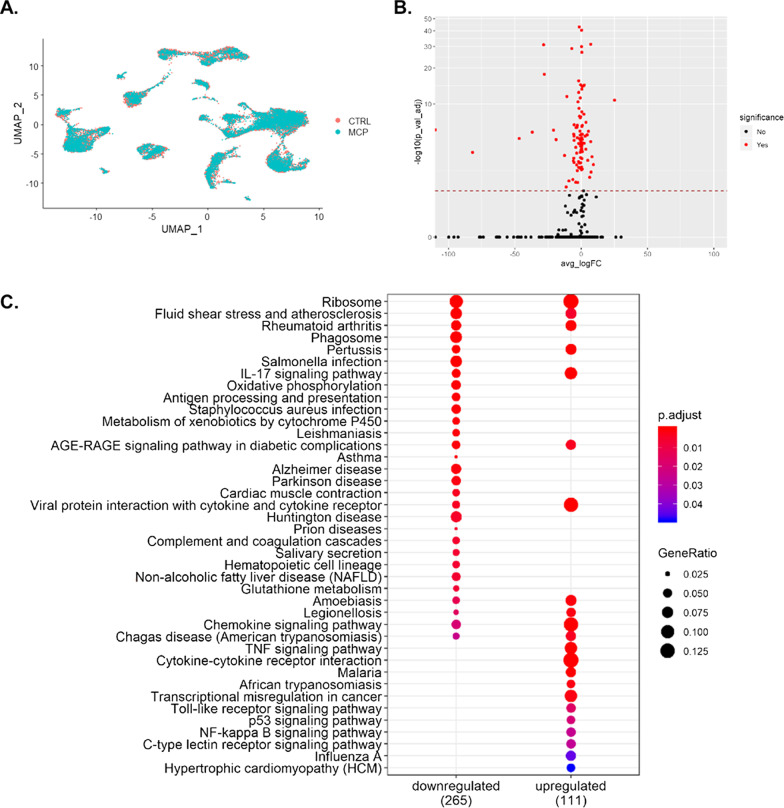
To determine from where the PM-induced expression of Cyp1a1 originated, we performed scRNA-seq using the dropseq protocol established by the McCarrol Lab [41]. This allows us to understand the mRNA changes at a cellular level and explore each cell’s function, as the transcriptional program is a large determinant of the function. Following quality-control and pre-processing steps, selecting cells with at least three transcripts and 200 genes, we retained more than 6000 cells from our vehicle exposed and more than 5000 cells from our PM exposed groups (Fig. 1a). Comparing all RNA in PM-exposed to vehicle-exposed cells, over 18,000 distinct genes were detected overall, with 210 found to be differentially expressed (Fig. 1b). The analysis of the differentially expressed genes (DEGs) in the KEGG database indicated strong correlations of an upregulated IL17 signaling pathway, viral protein interaction with cytokine and cytokine receptors, chemokine signaling, Tnf signaling, toll-like receptor signaling, and P53 signaling pathways (Fig. 1c). Interestingly, this suggests an increase in T-cell response; however, one of the downregulated pathways is antigen processing and presentation. Among the other downregulated pathways are pathways such as salmonella infection, phagosome, oxidative phosphorylation, and Staphylococcus aureus infection.
Fig. 1.
Exposure to PM induces transcriptomic changes across the entire lung at 4-h post exposure. A UMAP plot of all vehicle-exposed cells compared to PM-exposed cells showing a conserved cellular heterogeneity during 4-h timepoint. B Volcano plot showing fold change of 210 differentially expressed genes (DEG) over vehicle-exposed that were FDR < 0.05. C KEGG pathway analysis illustrating an increase in Il17 signaling, Tnf signaling, toll-like receptor signaling, and P 53 signaling
In vivo exposure to PM evokes heterogenous responses from epithelial and dendritic cells
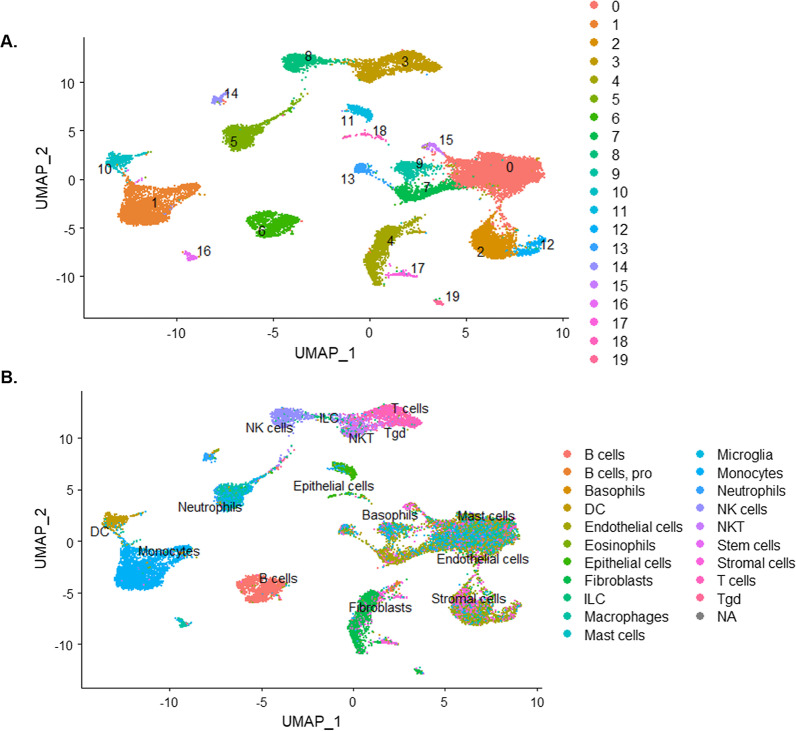
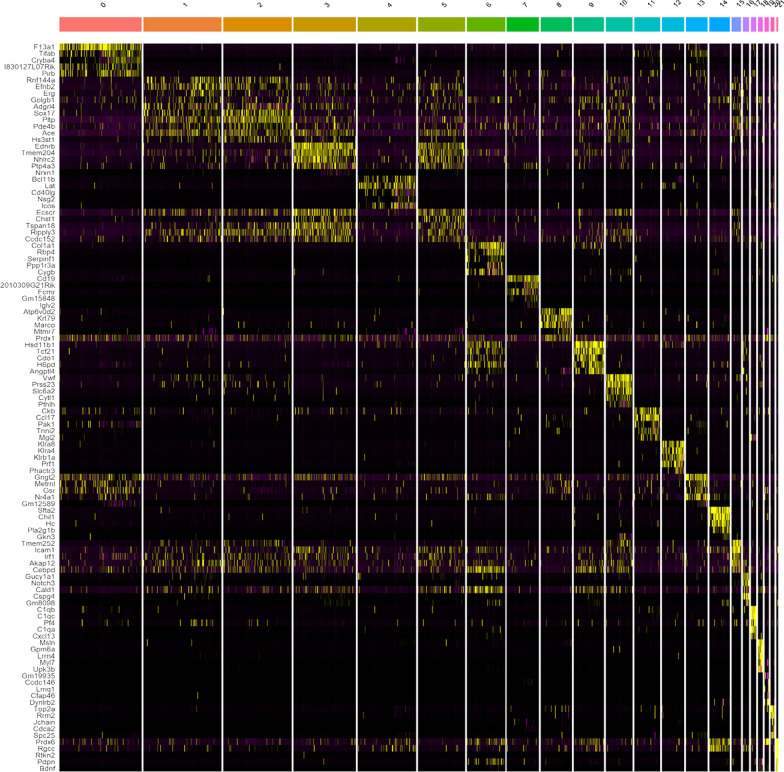
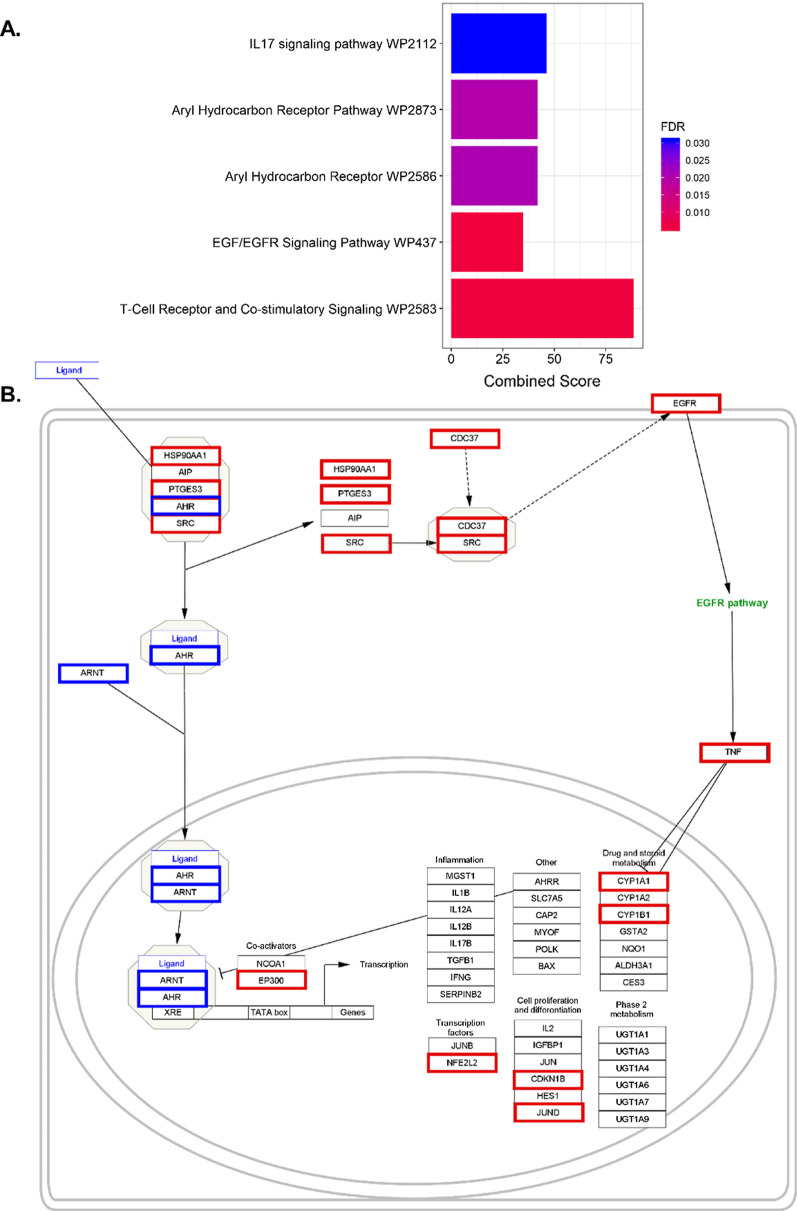
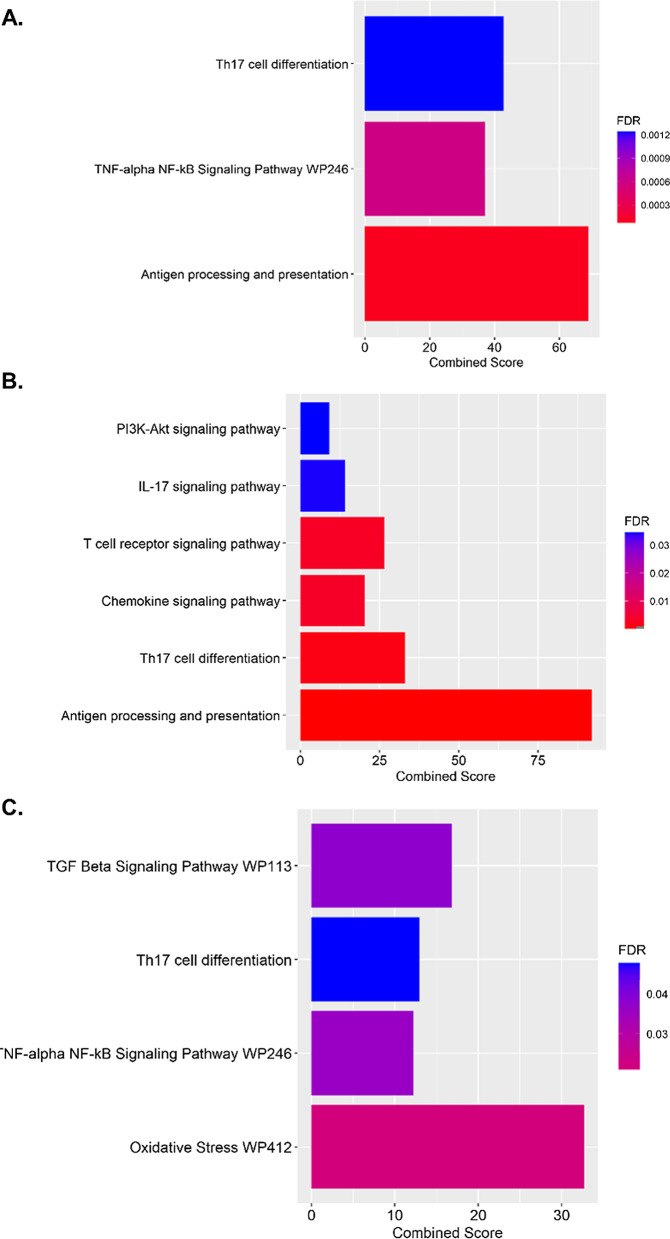
In comparing all mRNA of PM-exposed to vehicle-exposed cells in this study, we see significant changes to the function of epithelial cells and dendritic cell. To explore the underlying mechanisms, we focused on these specific cells. Utilizing Seurat [10, 57] and UMAP, we clustered the single cells into 20 distinct clusters (Fig. 2a). We found the top five differential gene expression profiles for each of the different identity clusters (Fig. 3) and passed those gene expression profiles through the Immgen database using the SingleR [1] to label the cells (Fig. 2b). This allows us to correctly identify the cell clusters in an unsupervised manner, while reducing biases and to evaluate the differential gene expressions across clusters of PM-exposed mice to vehicle-exposed mice. Through the use of Enrichr, we could ascertain that significant biological pathways were affected by short-term exposure to PM. The differential expression profile between vehicle- and PM-exposed epithelial cells was filtered for positively related gene expression and processed in Enrichr. From Enrichr, we saw five pathways directly related to the initiation of eTh17 cells. We saw increases in Il-17 signaling, Ahr activation, Egfr signaling, and T cell receptor and co-stimulatory signaling pathways (Fig. 4a) in our epithelial cluster from Fig. 3. Here, we show that the transcription profile suggests an upregulation in cytokines that are activated via the Ahr pathway specifically in epithelial cells (Fig. 4b).
Fig. 2.
Single-cell RNA sequencing demonstrated 20 distinct cell clusters in PM-exposed mice lungs. A UMAP plot of the 20 distinct cell clusters. B UMAP plot labeled by single-cell passed through SingleR program for cell identification
Fig. 3.
Heatmap of clusters from previous figure that show the top five genes that discriminate each individual cluster
Fig. 4.
Enrichr pathway analysis showing A upregulated pathways in epithelial cells (characterized in Fig. 3) from lungs of mice exposed to PM compared to vehicle exposed. FDR (based on Bonferroni correction) < 0.05. B Ahr pathway that illustrates the upregulated genes in red and downregulated genes in blue showing an upregulation in Egfr and xenobiotic response elements Cyp1a1 and Cyp1b1. Combined score is a combination of the p-value and z-score (calculated by using a modification to Fisher’s exact test) calculated by multiplying the two scores
Ahr activation is known to induce c-src-dependent stimulation of Egfr and its downstream target Erk1/2 [20, 69]. This then leads to the upregulation of similar cytokines to Ahr/Arnt xenobiotic response elements like Cyp1a1, Cyp1b1, and Cox-2 [20, 69].
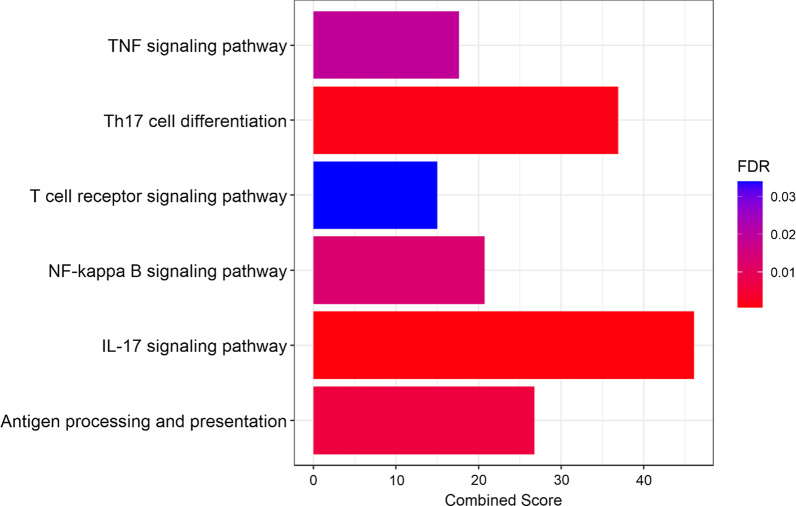
Studies of allergic asthma in mice have demonstrated that Il-17-induced airway neutrophilic asthma was dependent upon Tnf and NF-κB induction [23, 54, 60, 63]. Looking at the dendritic cell cluster from Figs. 2 and 3, we see five pathways that were significantly (FDR < 0.05) upregulated with respect to eTh17 differentiation and subsequent neutrophilic asthma. Through Enrichr and KEGG, we see increases in Th17 cell differentiation, Il-17 signaling, Tnf signaling, NF-κB signaling, and antigen processing and presentation (Fig. 5). This correlates with other published data and demonstrates that the Th17 response is, in part, initiated from cytokine production in dendritic cells. Therefore, this shows a potential mechanism by which dendritic cells promote an Th17 cell response through Il-17, Tnf-α, and NFκB up-regulation, which synergistically activates eTh17 cells.
Fig. 5.
Enrichr pathway analysis showing upregulated eTh17 cell pathways in dendritic cells (characterized in Fig. 3) from lungs of mice exposed to PM compared to vehicle exposed. Significance was tested by Wilcoxon rank sum test with Bonferroni correction FDR < 0.05. Combined score is a combination of the p-value and z-score (calculated by using a modification to Fisher’s exact test) calculated by multiplying the two scores
ScRNA-seq identifies several distinct T cell clusters
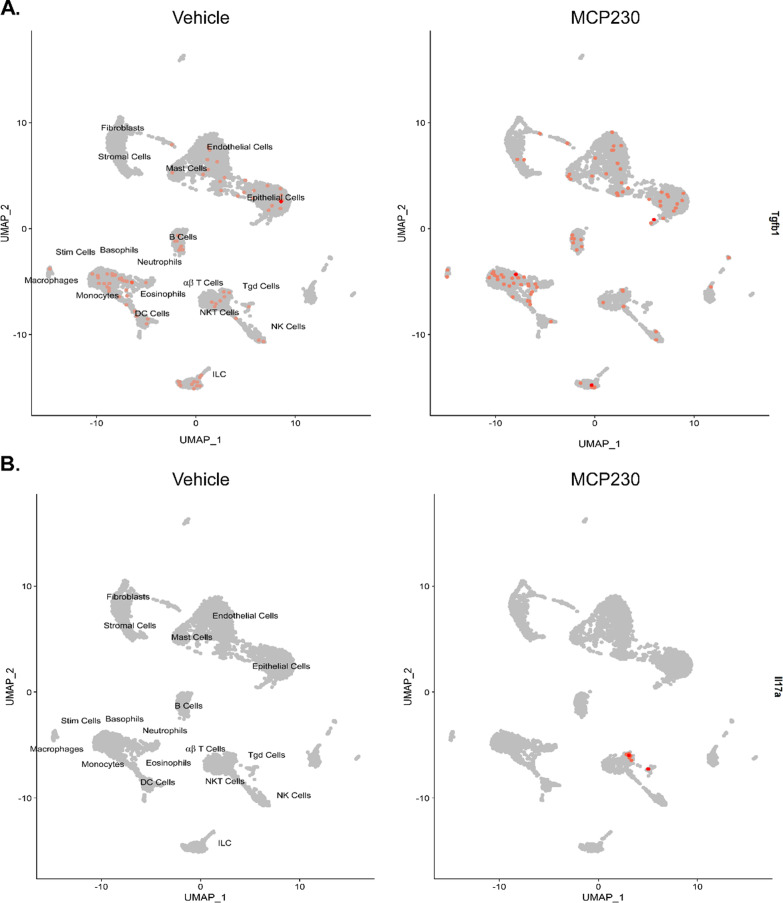
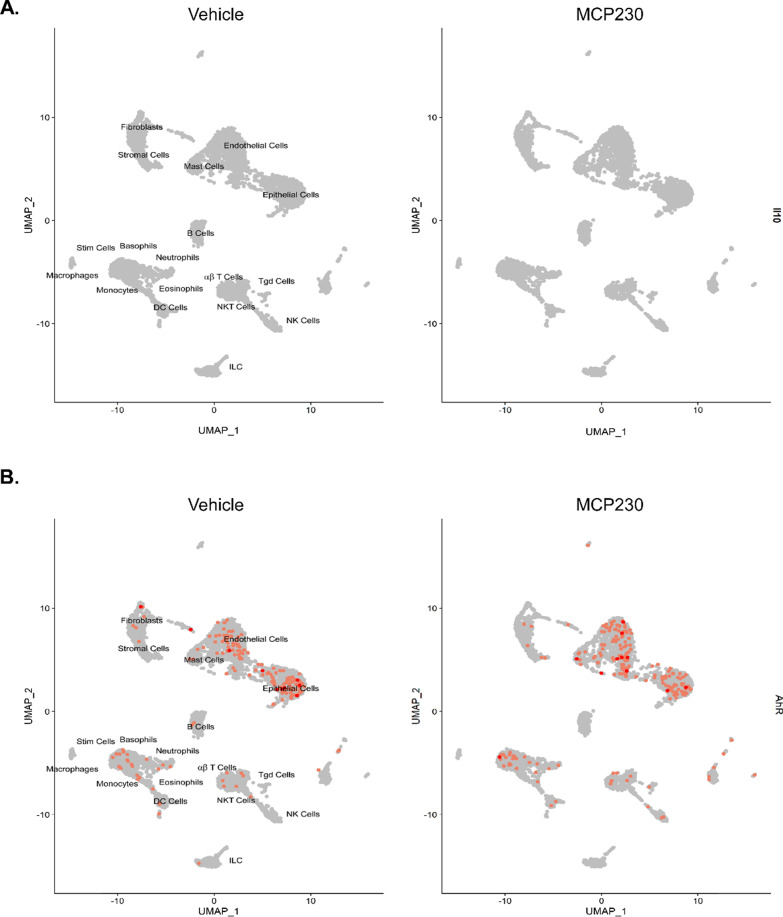
Unsupervised clustering using the UMAP protocol found three distinct T-cell clusters, namely, gamma–delta, NK, and alpha–beta T cells. Using the differential expression profile between vehicle and PM exposure, Enrichr identified pathways specific to Th17 differentiation and pro-inflammatory pathways associated with pulmonary eTh17 responses. In all three T cell populations, we observed an upregulation in Th17 cell differentiation (Fig. 6). Alpha–beta and gamma–delta T cells demonstrated upregulations in the Tnf-alpha NF-κB pathway, which, as previously mentioned, synergistically activates eTh17 cells. In addition, Il-17 signaling, PI3K–Akt signaling, and Tgf-β pathways were upregulated in NK T cells and gamma delta T cells, respectively. As PI3K–Akt and Tgf-β signaling are essential the induction of eTh17 cells, we can see specifically that the alpha–beta cells are already producing Il-17a while epithelial cells, neutrophils, and monocytes produce Tgf-β further demonstrating the induction of eTh17 cells (Fig. 7a, b). Meanwhile, across all cells we see no induction of Il-10 demonstrating a distinct lack of rTh17 cells (Fig. 8a). Furthermore, we can see in our cellular map that Ahr expression shows no significant change with PM exposure further suggesting that the Ahr activation previously seen may not be due to increased Ahr expression (Fig. 8b) but rather Ahr activation through an alternative pathway as we show in Fig. 4.
Fig. 6.
Distinct T-cell populations (characterized in Fig. 3) show dysregulation across immunological pathways essential to eTh17 cell cytokine production. A Upregulated Th17 specific pathways associated with PM exposure in alpha–beta T cells. B Upregulated Th17 specific pathways associated with PM exposure in NK T cells. C Upregulated Th17 specific pathways associated with PM exposure in gamma–delta T cells. Significance was tested by Wilcoxon rank sum test with Bonferroni correction FDR < 0.05. Combined score is a combination of the p-value and z-score (calculated by using a modification to Fisher’s exact test) calculated by multiplying the two scores
Fig. 7.
Gene expression of cytokines that induce eTh17 cells in all cells from Vehicle exposed and PM exposed mice. A Upregulated Transforming growth factor beta (Tgfb1) was associated with PM exposure in epithelial and endothelial cells. B Upregulated Interleukin 17 (IL17a) was associated with PM exposure in alpha–beta T cells. RNA expression levels (colored dots; scale bar on the right) are mapped onto the cells in which they are expressed. Significance was tested by Wilcoxon rank sum test with Bonferroni correction FDR < 0.05
Fig. 8.
Gene expression of IL10 and Ahr in all cells A IL-10 wasn’t expressed in any lung cell demonstrating a lack of rTh17 differentiation. B No significant changes in Ahr expression in PM exposure. Significance was tested by wilcoxon rank sum test with Bonferroni correction FDR < 0.05
Discussion
Several epidemiological studies have demonstrated the deleterious effects of short-term exposure to combustion derived PM. Although the association of PM and respiratory illness is well established, the underlying mechanisms are not fully understood. Some proposed mechanisms by which PM elicits immune responses have been demonstrated, but missing links as to the specific trajectory that PM follows to trigger pulmonary damage remain. For example, it is well known that PM dictates human airway epithelial cells to express inflammatory cytokines through the NF-κB pathway [13, 55, 56, 65]. In addition, PM has been shown to increase oxidative stress through the activation of inflammatory cells along with being able to directly generate ROS from the surface of PM [29, 68]. In this study, we demonstrate the effects that elevated levels of PM exposure has on lung cells during innate immune response and the specific pathways that PM alters during innate exposure.
First, we utilized the mouse drug metabolism profiler of the RT2 PCR Qiagen arrays to show the activation of genes controlled by Ahr in response to short-term exposure of PM, which showed upregulation of tryptophan metabolism, steroid hormone, biosynthesis, retinol metabolism, glutathione metabolism, and thyroid hormone synthesis. This upregulation suggests that exposure to PM elicits an antioxidant response and increases oxidative stress. Since Cyp1a1 was highly upregulated in our RT2 profiler array, we looked at Ahr, the transcription factor that drives Cyp1a1 expression, and found in our single-cell analysis that epithelial cells had multiple genes upregulated in the Ahr wikipathway, namely, Hsp90aa1, Jund, Ptges3, Cdc37, Nfe2l2, Gclc, Cd36, Tnf, and Egfr1. This is interesting, as it shows a potential alternative pathway to Cyp1a1 expression to PM acting as a ligand, as we previously thought [11, 27, 44]. Ahr activation has recently been studied for its role in Th17 activation and consequently in hypercytokinemia, a severe immune reaction in which the body releases too many cytokines, as an early host response to respiratory infections. Understanding this Ahr activation is essential to elucidating the specific downstream effects prompted by PM exposure.
Since eTh17 cell differentiation is dependent upon Tnf, TGF-β, Il-6, and Il-8 induction, we looked at our single cell analysis to trace what cells are involved in the PM induced neutrophilic asthma. In epithelial cells (Fig. 4), we show distinct changes affecting a multiplicity of molecular pathways including Il-17 signaling, Ahr activation, and Egfr signaling pathways. Epithelial cells are the first cells to encounter foreign objects such as PM in the lungs. So, the activation of specific pathways in epithelial cells will inform further responses in the immune system through cytokine initiation. Previous studies have established that PM promotes bacterial invasion of airway epithelial cells by attenuating ROS, destroying tight junctions, and causing epithelial to mesenchymal transitions [12, 50, 59]. In this paper, we present data suggesting that PM induced epithelial transcriptomic changes are responsible for the activation of Ahr through Ahr/Arnt activation/nuclear translocation and an increase in Egfr expression, ultimately resulting in neutrophilic asthma as we have previously shown [27]. Ahr ligand activation leads to the production of xenobiotic response elements such as Cyp1a1, Cyp1a2, and Cyp1b1, however, the ligand activation of Ahr also releases the non-receptor tyrosine kinase c-src in the cytoplasm. C-src kinase translocates to the cell membrane where it activates Egfr as we see in Fig. 4b [9, 16]. Egfr activation further leads to Erk1/2 pathway activation promoting the transcription of cox-2 which has been shown to be essential in eTh17 differentiation [17, 37]. This suggests that there could be new potential therapeutic targets for neutrophilic asthma treatment in the form of blocking Egfr or the activation of c-src.
Following the upregulation of Ahr specific cytokines and Th17 specific cytokines in epithelial cells we see that dendritic cells further produce cytokines involved in Th17 cell differentiation, Il-17 signaling, Tnf signaling, NF-κB signaling, and antigen processing and presentation pathways. Neutrophilic infiltration is dependent on Il-17 signaling through Il-17 induced proinflammatory cytokines and chemokines in lung structural cells promoting neutrophilic infiltration [66]. Both Il-17 and Tnf have been shown to induce Il-6 and Il-8 which are essential to eTh17 cell differentiation. With anti-Tnf therapy in severe asthmatic patients there was a decrease in sputum neutrophil levels, but not pulmonary neutrophilia [3, 7, 25, 51]. Thus, the eTh17-Tnf axis may be involved in the development of neutrophilic asthma.
There were other significant differences in the expression profiles between the three distinct T-cell clusters found in our single-cell RNA sequencing analysis. We found αβ and γδ T cells demonstrate upregulations in the Tnf-α, NF-κB and Th17 differentiation pathways. In addition to Th17 differentiation, we further found upregulation of Il-17 signaling in NKT cells, while seeing upregulation of PI3K–Akt and Tgf-β signaling in γδ T cells. PI3K–Akt can induce IL-6 and IL-8 and likewise Tgf-β have been shown to increase Th17-associated cytokine secretion and T-cell differentiation toward Th17 cells [8]. NKT cells have the ability to produce Il-10, Il-13, Ifn-ɣ, and Tgf-β [48]. Il-10 is vital in the differentiation of rTh17 cells while Il-13, Ifn-ɣ, and Tgf-β are pro-inflammatory cytokines that further stimulates eTh17 cell differentiation. This signifies how early lymphocytes such as NKT, γδ T, and αβ T cells inform the immune response later and can promote neutrophilic asthma [35, 42].
Th17 cells have been known to play both protective and pathogenic roles in various diseases [6, 18, 26, 43, 52, 58]. Therefore, future research would be to focus on the transcriptomic changes at different times throughout exposure to fully map the immune response and cellular composition changes associated with PM exposure.
Finally, it is important to point out that although this data is in line (i.e., Th17 responses following exposure to EPFR containing PM) with our previously published works, there are limitations to this study. First, this is an analysis of complex biological datasets at a static time point in response to an injurious event in vivo. Second, enzymatic digestions used to isolate these cells may modify the transcriptome affecting the resulting data. Third, there are many computational steps involved in analyzing such scRNA data including identification/mapping of corresponding cell populations across data sets, normalization and reduction of dimensionality—each with its own assumptions. Fourth, this work was done on a relatively small number of samples due to cost and only looked at poly A RNA excluding non-coding RNA.
Conclusions
Our study shows the diverse and distinct transcriptome changes of epithelial, dendritic, and T-cells brought on by exposure to elevated levels of PM. Our data unveils potential pathways and gene expressions explaining the phenomena of neutrophilic asthma that we and others have previously observed following PM exposure in mice and humans [35, 42, 60]. While other studies have shown an innate immune response to PM exposure through microarray data, our study is the first to investigate the innate immune response on a cellular level through single-cell RNA sequencing.
Supplementary Information
Additional file 1: Figure S1. Elbow plot and jackstraw plot of principle components to determine the optimal number of PCs to construct the UMAP plot
Acknowledgements
We would also like to thank Dr. Richard Carmouche for helping us with the dropseq protocol and analysis.
Abbreviations
- PM
Particulate matter
- EPFR
Environmentally persistent free radicals
- Ahr
Aryl hydrocarbon receptor
- ScRNA
Single cell RNA sequencing
- Th17
T helper cell subset 17
- eTh17
Pathogenic T helper 17 cells
- rTh17
Regulatory T helper 17 cells
- ROS
Reactive oxygen species
- COPD
Chronic obstructive pulmonary disease
- OA
Oropharyngeal aspiration
- UMAP
Uniform Manifold Approximation and Projection
- NK
Natural killer cells
- NKT
Natural killer T cells
Authors’ contributions
JH wrote and performed the experiments and data analysis for all the data. MG performed experiments and edited the paper. VP also performed the experiments and helped with data analysis. SP performed the dropseq protocol and library preparation as well as guidance for analysis and editing of the paper. JH and SC wrote the paper, provided analysis insight, and designed the project. All authors read and approved the final manuscript.
Funding
This work was supported by a grant awarded to Dr. Stephania Cormier from the National Institutes of Health Grants (R01AI090059 and R01ES015050 and P42ES013648).
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the SRA Accession database: PRJNA666321.
Declarations
Ethics approval and consent to participate
All animal protocols were written according to Policy for the Care and Use of Laboratory Animals and approved by the LSU Institutional Animal Care and Use Committee at Louisiana State University.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aran D, Looney AP, Liu L, Wu E, Fong V, Hsu A, et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat Immunol. 2019;20(2):163–172. doi: 10.1038/s41590-018-0276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson RW, Anderson HR, Sunyer J, Ayres J, Baccini M, Vonk JM, et al. Acute effects of particulate air pollution on respiratory admissions: results from APHEA 2 project. Air Pollution and Health: a European Approach. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1860–1866. doi: 10.1164/ajrccm.164.10.2010138. [DOI] [PubMed] [Google Scholar]
- 3.Babu KS, Davies DE, Holgate ST. Role of tumor necrosis factor alpha in asthma. Immunol Allergy Clin N Am. 2004;24(4):583–597, v–vi. doi: 10.1016/j.iac.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Balakrishna S, Lomnicki S, McAvey KM, Cole RB, Dellinger B, Cormier SA. Environmentally persistent free radicals amplify ultrafine particle mediated cellular oxidative stress and cytotoxicity. Part Fibre Toxicol. 2009;6:11. doi: 10.1186/1743-8977-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behndig AF, Mudway IS, Brown JL, Stenfors N, Helleday R, Duggan ST, et al. Airway antioxidant and inflammatory responses to diesel exhaust exposure in healthy humans. Eur Respir J. 2006;27(2):359–365. doi: 10.1183/09031936.06.00136904. [DOI] [PubMed] [Google Scholar]
- 6.Bermejo-Martin JF, Ortiz de Lejarazu R, Pumarola T, Rello J, Almansa R, RamC-rez P, et al. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit Care (Lond, Engl) 2009;13(6):R201–R201. doi: 10.1186/cc8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry MA, Hargadon B, Shelley M, Parker D, Shaw DE, Green RH, et al. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N Engl J Med. 2006;354(7):697–708. doi: 10.1056/NEJMoa050580. [DOI] [PubMed] [Google Scholar]
- 8.Bikker A, Hack CE, Lafeber FP, van Roon JA. Interleukin-7: a key mediator in T cell-driven autoimmunity, inflammation, and tissue destruction. Curr Pharm Des. 2012;18(16):2347–2356. doi: 10.2174/138161212800165979. [DOI] [PubMed] [Google Scholar]
- 9.Blankenship A, Matsumura F. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-induced activation of a protein tyrosine kinase, pp60src, in Murine hepatic cytosol using a cell-free system. Mol Pharmacol. 1997;52(4):667–675. doi: 10.1124/mol.52.4.667. [DOI] [PubMed] [Google Scholar]
- 10.Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol. 2018;36(5):411–420. doi: 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castaneda AR, Pinkerton KE, Bein KJ, Magana-Mendez A, Yang HT, Ashwood P, Vogel CFA. Ambient particulate matter activates the aryl hydrocarbon receptor in dendritic cells and enhances Th17 polarization. Toxicol Lett. 2018;292:85–96. doi: 10.1016/j.toxlet.2018.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Liu J, Zhou J, Wang J, Chen C, Song Y, Pan J. Urban particulate matter (PM) suppresses airway antibacterial defence. Respir Res. 2018;19(1):5–5. doi: 10.1186/s12931-017-0700-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dagher Z, Garcon G, Billet S, Verdin A, Ledoux F, Courcot D, et al. Role of nuclear factor-kappa B activation in the adverse effects induced by air pollution particulate matter (PM2.5) in human epithelial lung cells (L132) in culture. J Appl Toxicol. 2007;27(3):284–290. doi: 10.1002/jat.1211. [DOI] [PubMed] [Google Scholar]
- 14.Dellinger B, Pryor WA, Cueto R, Squadrito GL, Hegde V, Deutsch WA. Role of free radicals in the toxicity of airborne fine particulate matter. Chem Res Toxicol. 2001;14(10):1371–1377. doi: 10.1021/tx010050x. [DOI] [PubMed] [Google Scholar]
- 15.Diociaiuti M, Balduzzi M, De Berardis B, Cattani G, Stacchini G, Ziemacki G, et al. The two PM2.5 (Fine) and PM2.5–10 (Coarse) fractions: evidence of different biological activity. Environ Res. 2001;86(3):254–262. doi: 10.1006/enrs.2001.4275. [DOI] [PubMed] [Google Scholar]
- 16.Enan E, Matsumura F. Identification of c-Src as the integral component of the cytosolic Ah receptor complex, transducing the signal of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) through the protein phosphorylation pathway. Biochem Pharmacol. 1996;52(10):1599–1612. doi: 10.1016/S0006-2952(96)00566-7. [DOI] [PubMed] [Google Scholar]
- 17.Fritsche E, Schäfer C, Calles C, Bernsmann T, Bernshausen T, Wurm M, et al. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc Natl Acad Sci. 2007;104(21):8851–8856. doi: 10.1073/pnas.0701764104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gagliani N, Amezcua Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW, et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523(7559):221–225. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez-Flecha B. Oxidant mechanisms in response to ambient air particles. Mol Aspects Med. 2004;25(1–2):169–182. doi: 10.1016/j.mam.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 20.Haarmann-Stemmann T, Bothe H, Abel J. Growth factors, cytokines and their receptors as downstream targets of arylhydrocarbon receptor (AhR) signaling pathways. Biochem Pharmacol. 2009;77(4):508–520. doi: 10.1016/j.bcp.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Hamra GB, Guha N, Cohen A, Laden F, Raaschou-Nielsen O, Samet JM, et al. Outdoor particulate matter exposure and lung cancer: a systematic review and meta-analysis. Environ Health Perspect. 2014;122(9):906–911. doi: 10.1289/ehp.1408092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harmon AC, Hebert VY, Cormier SA, Subramanian B, Reed JR, Backes WL, Dugas TR. Particulate matter containing environmentally persistent free radicals induces AhR-dependent cytokine and reactive oxygen species production in human bronchial epithelial cells. PLoS ONE. 2018;13(10):e0205412. doi: 10.1371/journal.pone.0205412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartupee J, Liu C, Novotny M, Li X, Hamilton T. IL-17 enhances chemokine gene expression through mRNA stabilization. J Immunol. 2007;179(6):4135–4141. doi: 10.4049/jimmunol.179.6.4135. [DOI] [PubMed] [Google Scholar]
- 24.Hertz-Picciotto I, Baker RJ, Yap PS, Dostal M, Joad JP, Lipsett M, et al. Early childhood lower respiratory illness and air pollution. Environ Health Perspect. 2007;115(10):1510–1518. doi: 10.1289/ehp.9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howarth PH, Babu KS, Arshad HS, Lau L, Buckley M, McConnell W, et al. Tumour necrosis factor (TNFalpha) as a novel therapeutic target in symptomatic corticosteroid dependent asthma. Thorax. 2005;60(12):1012–1018. doi: 10.1136/thx.2005.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaligama S, Patel VS, Wang P, Sallam A, Harding J, Kelley M, et al. Radical containing combustion derived particulate matter enhance pulmonary Th17 inflammation via the aryl hydrocarbon receptor. Part Fibre Toxicol. 2018;15(1):20. doi: 10.1186/s12989-018-0255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaligama S, Saravia J, You D, Yadav N, Lee GI, Shrestha B, Cormier SA. Regulatory T cells and IL10 suppress pulmonary host defense during early-life exposure to radical containing combustion derived ultrafine particulate matter. Respir Res. 2017;18(1):15. doi: 10.1186/s12931-016-0487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jantzen K, Møller P, Karottki DG, Olsen Y, Bekö G, Clausen G, et al. Exposure to ultrafine particles, intracellular production of reactive oxygen species in leukocytes and altered levels of endothelial progenitor cells. Toxicology. 2016;359–360:11–18. doi: 10.1016/j.tox.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Jia H, Liu Y, Guo D, He W, Zhao L, Xia S. PM2.5-induced pulmonary inflammation via activating of the NLRP3/caspase-1 signaling pathway. Environ Toxicol. 2021;36(3):298–307. doi: 10.1002/tox.23035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jimenez JL, McRae GJ, Nelson DD, Zahniser MS, Kolb CE. Remote sensing of NO and NO2 emissions from heavy-duty diesel trucks using tunable diode lasers. Environ Sci Technol. 2000;34(12):2380–2387. doi: 10.1021/es9911622. [DOI] [Google Scholar]
- 32.Kadiiska MB, Gladen BC, Baird DD, Dikalova AE, Sohal RS, Hatch GE, et al. Biomarkers of oxidative stress study: are plasma antioxidants markers of CCl4 poisoning? Free Radic Biol Med. 2000;28(6):838–845. doi: 10.1016/S0891-5849(00)00198-2. [DOI] [PubMed] [Google Scholar]
- 33.Knol AB, de Hartog JJ, Boogaard H, Slottje P, van der Sluijs JP, Lebret E, et al. Expert elicitation on ultrafine particles: likelihood of health effects and causal pathways. Part Fibre Toxicol. 2009;6:19. doi: 10.1186/1743-8977-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee GI, Saravia J, You D, Shrestha B, Jaligama S, Hebert VY, et al. Exposure to combustion generated environmentally persistent free radicals enhances severity of influenza virus infection. Part Fibre Toxicol. 2014;11:57–57. doi: 10.1186/s12989-014-0057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee K-A, Kang M-H, Lee Y-S, Kim Y-J, Kim D-H, Ko H-J, Kang C-Y. A distinct subset of natural killer T cells produces IL-17, contributing to airway infiltration of neutrophils but not to airway hyperreactivity. Cell Immunol. 2008;251(1):50–55. doi: 10.1016/j.cellimm.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Leikauf GD, Kim S-H, Jang A-S. Mechanisms of ultrafine particle-induced respiratory health effects. Exp Mol Med. 2020;52(3):329–337. doi: 10.1038/s12276-020-0394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H, Bradbury JA, Dackor RT, Edin ML, Graves JP, DeGraff LM, et al. Cyclooxygenase-2 regulates Th17 cell differentiation during allergic lung inflammation. Am J Respir Crit Care Med. 2011;184(1):37–49. doi: 10.1164/rccm.201010-1637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X, Wang J, Fan Y, Xu Y, Xie M, Yuan Y, et al. Particulate matter exposure history affects antioxidant defense response of mouse lung to haze episodes. Environ Sci Technol. 2019;53(16):9789–9799. doi: 10.1021/acs.est.9b01068. [DOI] [PubMed] [Google Scholar]
- 39.Liu X, Wang J, Zhou M, Dai Q, Wang Q, Li H, Qian X. Particulate matter exposure disturbs inflammatory cytokine homeostasis associated with changes in trace metal levels in mouse organs. Sci Total Environ. 2020;727:138377. doi: 10.1016/j.scitotenv.2020.138377. [DOI] [PubMed] [Google Scholar]
- 40.Lu F, Xu D, Cheng Y, Dong S, Guo C, Jiang X, Zheng X. Systematic review and meta-analysis of the adverse health effects of ambient PM2.5 and PM10 pollution in the Chinese population. Environ Res. 2015;136:196–204. doi: 10.1016/j.envres.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 41.Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell. 2015;161(5):1202–1214. doi: 10.1016/j.cell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michel M-L, Keller AC, Paget C, Fujio M, Trottein F, o., Savage, P. B., , et al. Identification of an IL-17–producing NK1.1neg iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204(5):995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nikoopour E, Schwartz JA, Huszarik K, Sandrock C, Krougly O, Lee-Chan E, Singh B. Th17 polarized cells from nonobese diabetic mice following mycobacterial adjuvant immunotherapy delay type 1 diabetes. J Immunol. 2010;184(9):4779–4788. doi: 10.4049/jimmunol.0902822. [DOI] [PubMed] [Google Scholar]
- 44.O’Driscoll CA, Gallo ME, Hoffmann EJ, Fechner JH, Schauer JJ, Bradfield CA, Mezrich JD. Polycyclic aromatic hydrocarbons (PAHs) present in ambient urban dust drive proinflammatory T cell and dendritic cell responses via the aryl hydrocarbon receptor (AHR) in vitro. PLoS ONE. 2018;13(12):e0209690. doi: 10.1371/journal.pone.0209690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pope CA, Bhatnagar A, McCracken James P, Abplanalp W, Conklin Daniel J, O’Toole T. Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ Res. 2016;119(11):1204–1214. doi: 10.1161/CIRCRESAHA.116.309279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Potter AS, Steven Potter S. Dissociation of tissues for single-cell analysis. Methods Mol Biol. 2019;1926:55–62. doi: 10.1007/978-1-4939-9021-4_5. [DOI] [PubMed] [Google Scholar]
- 47.Raaschou-Nielsen O, Andersen ZJ, Beelen R, Samoli E, Stafoggia M, Weinmayr G, et al. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE) Lancet Oncol. 2013;14(9):813–822. doi: 10.1016/S1470-2045(13)70279-1. [DOI] [PubMed] [Google Scholar]
- 48.Rachitskaya AV, Hansen AM, Horai R, Li Z, Villasmil R, Luger D, et al. Cutting edge: NKT cells constitutively express IL-23 receptor and RORγt and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol. 2008;180(8):5167–5171. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raza MW, Essery SD, Weir DM, Ogilvie MM, Elton RA, Blackwell CC. Infection with respiratory syncytial virus and water-soluble components of cigarette smoke alter production of tumour necrosis factor alpha and nitric oxide by human blood monocytes. FEMS Immunol Med Microbiol. 1999;24(4):387–394. doi: 10.1111/j.1574-695X.1999.tb01310.x. [DOI] [PubMed] [Google Scholar]
- 50.Rezaee F, Lerner LB, Ivanov AL, Breysse P, Beck LA, Georas SN. Particulate matter induced airway epithelial barrier dysfunction. J Allergy Clin Immunol. 2010;125(2):AB233. doi: 10.1016/j.jaci.2009.12.909. [DOI] [Google Scholar]
- 51.Rouhani FN, Meitin CA, Kaler M, Miskinis-Hilligoss D, Stylianou M, Levine SJ. Effect of tumor necrosis factor antagonism on allergen-mediated asthmatic airway inflammation. Respir Med. 2005;99(9):1175–1182. doi: 10.1016/j.rmed.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 52.Sandquist I, Kolls J. Update on regulation and effector functions of Th17 cells. F1000Res. 2018;7:205. doi: 10.12688/f1000research.13020.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saravia J, You D, Thevenot P, Lee GI, Shrestha B, Lomnicki S, Cormier SA. Early-life exposure to combustion-derived particulate matter causes pulmonary immunosuppression. Mucosal Immunol. 2014;7(3):694–704. doi: 10.1038/mi.2013.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen F, Ruddy MJ, Plamondon P, Gaffen SL. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-alpha-induced genes in bone cells. J Leukoc Biol. 2005;77(3):388–399. doi: 10.1189/jlb.0904490. [DOI] [PubMed] [Google Scholar]
- 55.Shukla A, Timblin C, BeruBe K, Gordon T, McKinney W, Driscoll K, et al. Inhaled particulate matter causes expression of nuclear factor (NF)-kappaB-related genes and oxidant-dependent NF-kappaB activation in vitro. Am J Respir Cell Mol Biol. 2000;23(2):182–187. doi: 10.1165/ajrcmb.23.2.4035. [DOI] [PubMed] [Google Scholar]
- 56.Silbajoris R, Osornio-Vargas AR, Simmons SO, Reed W, Bromberg PA, Dailey LA, Samet JM. Ambient particulate matter induces interleukin-8 expression through an alternative NF-κB (nuclear factor-kappa B) mechanism in human airway epithelial cells. Environ Health Perspect. 2011;119(10):1379–1383. doi: 10.1289/ehp.1103594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM, et al. Comprehensive integration of single-cell data. Cell. 2019;177(7):1888–1902.e1821. doi: 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thevenot PT, Saravia J, Jin N, Giaimo JD, Chustz RE, Mahne S, et al. Radical-containing ultrafine particulate matter initiates epithelial-to-mesenchymal transitions in airway epithelial cells. Am J Respir Cell Mol Biol. 2013;48(2):188–197. doi: 10.1165/rcmb.2012-0052OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomas PS, Yates DH, Barnes PJ. Tumor necrosis factor-alpha increases airway responsiveness and sputum neutrophilia in normal human subjects. Am J Respir Crit Care Med. 1995;152(1):76–80. doi: 10.1164/ajrccm.152.1.7599866. [DOI] [PubMed] [Google Scholar]
- 61.Today U. The smokestack effect. Toxic air and America's schools. USA Today. 2011.
- 62.Tsai D-H, Amyai N, Marques-Vidal P, Wang J-L, Riediker M, Mooser V, et al. Effects of particulate matter on inflammatory markers in the general adult population. Part Fibre Toxicol. 2012;9(1):24. doi: 10.1186/1743-8977-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van den Berg A, Kuiper M, Snoek M, Timens W, Postma DS, Jansen HM, Lutter R. Interleukin-17 induces hyperresponsive interleukin-8 and interleukin-6 production to tumor necrosis factor-alpha in structural lung cells. Am J Respir Cell Mol Biol. 2005;33(1):97–104. doi: 10.1165/rcmb.2005-0022OC. [DOI] [PubMed] [Google Scholar]
- 64.Vejerano EP, Rao G, Khachatryan L, Cormier SA, Lomnicki S. Environmentally persistent free radicals: insights on a new class of pollutants. Environ Sci Technol. 2018;52(5):2468–2481. doi: 10.1021/acs.est.7b04439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang J, Zhang WJ, Xiong W, Lu WH, Zheng HY, Zhou X, Yuan J. PM2.5 stimulated the release of cytokines from BEAS-2B cells through activation of IKK/NF-kappaB pathway. Hum Exp Toxicol. 2019;38(3):311–320. doi: 10.1177/0960327118802628. [DOI] [PubMed] [Google Scholar]
- 66.Wang YH, Wills-Karp M. The potential role of interleukin-17 in severe asthma. Curr Allergy Asthma Rep. 2011;11(5):388–394. doi: 10.1007/s11882-011-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu W, Jin Y, Carlsten C. Inflammatory health effects of indoor and outdoor particulate matter. J Allergy Clin Immunol. 2018;141(3):833–844. doi: 10.1016/j.jaci.2017.12.981. [DOI] [PubMed] [Google Scholar]
- 68.Xia T, Kovochich M, Nel A. The role of reactive oxygen species and oxidative stress in mediating particulate matter injury. Clin Occup Environ Med. 2006;5(4):817–836. doi: 10.1016/j.coem.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 69.Ye M, Zhang Y, Gao H, Xu Y, Jing P, Wu J, et al. Activation of the aryl hydrocarbon receptor leads to resistance to EGFR TKIs in non-small cell lung cancer by activating src-mediated bypass signaling. Clin Cancer Res. 2018;24(5):1227–1239. doi: 10.1158/1078-0432.Ccr-17-0396. [DOI] [PubMed] [Google Scholar]
- 70.Yu G, Wang L-G, Han Y, He Q-Y. clusterProfiler: an R Package for comparing biological themes among gene clusters. OMICS J Integr Biol. 2012;16(5):284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng R, Tao L, Jian H, Chang Y, Cheng Y, Feng Y, Zhang H. NLRP3 inflammasome activation and lung fibrosis caused by airborne fine particulate matter. Ecotoxicol Environ Saf. 2018;163:612–619. doi: 10.1016/j.ecoenv.2018.07.076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Elbow plot and jackstraw plot of principle components to determine the optimal number of PCs to construct the UMAP plot
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the SRA Accession database: PRJNA666321.