Abstract
Shigellosis in men who have sex with men (MSM) is caused by multidrug resistant Shigellae, exhibiting resistance to antimicrobials including azithromycin, ciprofloxacin and more recently the third-generation cephalosporins. We sequenced four bla CTX-M-27-positive MSM Shigella isolates (2018–20) using Oxford Nanopore Technologies; three S. sonnei (identified as two MSM clade 2, one MSM clade 5) and one S. flexneri 3a, to explore AMR context. All S. sonnei isolates harboured Tn7/Int2 chromosomal integrons, whereas S. flexneri 3a contained the Shigella Resistance Locus. All strains harboured IncFII pKSR100-like plasmids (67-83kbp); where present bla CTX-M-27 was located on these plasmids flanked by IS26 and IS903B, however bla CTX-M-27 was lost in S. flexneri 3a during storage between Illumina and Nanopore sequencing. IncFII AMR regions were mosaic and likely reorganised by IS26; three of the four plasmids contained azithromycin-resistance genes erm(B) and mph(A) and one harboured the pKSR100 integron. Additionally, all S. sonnei isolates possessed a large IncB/O/K/Z plasmid, two of which carried aph(3’)-Ib/aph(6)-Id/sul2 and tet(A). Monitoring the transmission of mobile genetic elements with co-located AMR determinants is necessary to inform empirical treatment guidance and clinical management of MSM-associated shigellosis.
Keywords: antimicrobial resistance, CTX-M, ESBL, MSM, public health, Shigella
Data Summary
Whole genomes sequencing projects for strains 598 080 and 607 387 are deposited in GenBank under the accession numbers JAENSM000000000 and JAEMEC000000000 respectively. For strain 888 048, the chromosomal sequence can be retrieved with the accession CP066809 and its plasmids under accessions MW396860-3. For strain 893 916, the chromosomal sequence is deposited under accession CP066810 and plasmid sequences under accessions MW396858-9 and MW396864. Further information can be found in Table S4 (available in the online version of this article).
Impact Statement.
Outbreaks of Shigella , a gastrointestinal pathogen, have been known to occur in men who have sex with men (MSM) globally since the 1970s. Increasing prevalence of multidrug resistance in Shigellae is concerning due to the challenge this poses to current antimicrobial treatment options. This includes the emergence of extended-spectrum beta-lactamase (ESBL)-producing strains which are resistant to ceftriaxone due to plasmid-mediated CTX-M determinants. As few CTX-M plasmids have been described, we apply a combination of short- and long-read sequencing with Illumina and MinION technologies to assemble complete plasmids in four Shigella isolates associated with MSM transmission in the United Kingdom to investigate the location of antimicrobial resistance (AMR) genes. We find bla CTX-M-27 is inserted into plasmids with high identity to known plasmids which have previously driven shigellosis epidemics worldwide and typically carry azithromycin resistance elements. We compare isolate differences in AMR gene context, the impact of insertion sequences on their plasmids and discuss how resistance profiles relate to MSM clade. Our findings highlight the necessity of increased public health monitoring of AMR in Shigella at different hierarchies; the whole genome, mobile genetic element and single gene level to understand resistance dissemination and inform effective treatment options.
Introduction
Shigella is a Gram-negative genus comprising four species; Shigella dysenteriae , Shigella boydii , Shigella sonnei and Shigella flexneri, all etiological agents of shigellosis [1]. Shigella spp. are highly contagious [2] and primarily transmitted via the faecal-oral route through direct person-person contact or contaminated food or water [3]. Presence of a large virulence plasmid (pINV) encoding a type III secretion system supports Shigella invasion of colonic epithelial cells, generation of localised inflammation and necrosis, which combined with expression of genes on chromosomal pathogenicity islands (PAIs) generates characteristic symptoms of abdominal cramps and mucoid diarrhoea [4, 5]. Shigellosis accounts for a significant proportion of the global diarrhoea burden; despite mortality decreases since the 1990s, ~125 m estimated cases occur annually, mainly in children <5 years in Asia and Africa [6, 7].
Historically, Shigella infections in higher-income countries such as the United Kingdom (UK) were mainly associated with travellers returning from endemic areas [8]. However, since 2009 the UK has observed sustained epidemics of domestically-acquired multidrug resistant (MDR) Shigella serotypes S. flexneri 3a and 2a and S. sonnei, attributed to transmission in men who have sex with men (MSM) [9]. Ciprofloxacin, azithromycin, and ceftriaxone are now first and second-line treatment options [10] due to chromosomal acquisition of antimicrobial resistance genes (ARGs) relevant to earlier drugs. This includes the class II integron Tn7/Int2 (trimethoprim and aminoglycosides), class I Shigella Resistance Locus (SRL) (chloramphenicol, ampicillin and tetracycline) and additional sulphonamide resistance via the small spA plasmid [11–13]. However, ciprofloxacin use is restricted by step-wise chromosomal mutations in the quinolone resistance determining regions (QRDR) gyrA and parC [14] and intercontinental dissemination of azithromycin ARGs on a single large IncF plasmid, pKSR100, has directly enhanced previous MSM Shigella outbreaks [15].
Extended spectrum β-lactamase (ESBL) genes have implications for failure of ceftriaxone, one of the few remaining options, and were relatively rare in domestically-acquired Shigella in the UK [8]. However, in 2015 Public Health England (PHE) described an ESBL-producing S. sonnei MSM cluster [16] conferred by a pKSR100-like plasmid (p183660), which had acquired bla CTX-M-27. This element recently drove a prolonged S. sonnei outbreak among MSM in Victoria, Australia [17] but few CTX-M Shigella plasmids have been characterised [18]; seemingly only one other has been described in detail, in a Swiss S. sonnei isolate [19].
Assembly of these plasmids is important for AMR surveillance but is undermined by the failure of short-read sequencing to span repetitive mobile genetic element (MGE) regions, preventing ARG location determination [20]. Advent of long-read sequencing such as the Oxford Nanopore Technologies (Nanopore) platform, in combination with Illumina-mediated polishing, overcomes this to provide accurate genomes with complete plasmids [21, 22].
In this study we report comparative genomics of four MSM-associated Shigella strains (three S. sonnei, one S. flexneri 3a) in England initially characterised as bla CTX-M-27-positive. Availability of complete ESBL-producing Shigella genomes helps determine structural MGE arrangement, genomic ARG context and identify insertion sequences (IS) involved in their reorganisation. Together this helps elucidate how MGE evolution is tied to pathogenic persistence, emphasising the importance of responsible antimicrobial stewardship.
Experimental procedures
Short-read sequencing (Illumina HiSeq 2500) and data processing
Four strains of Shigella spp. ( Shigella sonnei , n=3, Shigella flexneri 3a, n=1) from MSM were selected as representatives of clusters of all Shigella species in the PHE database with the bla CTX-M-27 determinant and characterised in this study (Table 1). If the isolate formed part of a cluster, the earliest isolate was selected with respect to faecal sample collection date (or date of isolation if sample data were unavailable). For Illumina sequencing, Genomic DNA was extracted from cultures using the QIAsymphony system (Qiagen). The sequencing library was prepared using the Nextera XP kit (Illumina) for sequencing on the HiSeq 2500 instrument (Illumina), run with the fast protocol. FASTQ reads were processed using Trimmomatic v0.27 [23] to remove bases with a PHRED score of <30 from the leading and trailing ends, with reads <50 bp after quality trimming discarded.
Table 1.
Epidemiological information related to the four Shigella isolates associated with men who have sex with men (MSM) that were whole genome sequenced with Nanopore and Illumina technologies in this study. S. sonnei MSM clade is reported according to [8] and S. sonnei lineage is included according to [47]
|
Isolate ID |
598 080 |
607 387 |
888 048 |
893 916 |
|---|---|---|---|---|
|
Species |
S. flexneri 3a |
|||
|
Region |
London |
London |
London |
London |
|
Sex |
M |
M |
M |
M |
|
Age |
22 |
50 |
50 |
36 |
|
Receipt date (M/Y) |
08/18 |
09/18 |
02/20 |
02/20 |
|
S. sonnei MSM clade |
2 |
2 |
– |
5 |
|
S. sonnei lineage |
3.7.29.1.4.1 (Global III VN2.KH1.Aus) |
3.7.29.1.4.1 (Global III VN2.KH1.Aus) |
– |
3.6.1.1.2 (CipR.MSM5) |
Whole genome long-read sequencing (Nanopore) and data processing
Samples were subsequently sequenced using Oxford Nanopore technologies. Genomic DNA was extracted and purified using the Fire Monkey DNA extraction kit (Revolugen) with subtle modifications to manufacturer’s instructions including removal of vortexing steps. Genomic DNA for each extract was quantified using a Qubit and the HS (high sensitivity) dsDNA assay kit (Thermofisher Scientific), following manufacturer’s instructions. Library preparation was performed using the Rapid barcoding kit SQK-RBK004 (Oxford Nanopore Technologies). The prepared libraries were loaded onto a FLO-MIN106 R9.4.1 flow cell (Oxford Nanopore Technologies) and sequenced using the MinION (Oxford Nanopore Technologies) for 36 h and further processed as follows (Fig. S1). Data produced in a raw FAST5 format was basecalled and de-multiplexed with Guppy v3.2.10 using the FAST protocol (Oxford Nanopore Technologies) into FASTQ format and de-multiplexed into each samples’ respective barcode.
Read metrics prior to and following filtering were determined with NanoPlot v1.8.1 [24]. Adapter sequences were trimmed from raw Nanopore reads and chimeric reads discarded to prevent cross-barcode contamination using Porechop v0.2.4 (https://github.com/rrwick/Porechop). Read filtering was conducted with Filtlong v0.2.0 (https://github.com/rrwick/Filtlong) to yield 30× theoretical coverage of the Shigella genome (~4.7 Mb) with the longest reads using the following parameters; min_length=1000, keep_percent=90, length_weight=10, target_bases=141 Mb.
De novo assembly, polishing and reorientation
All genomes were de novo assembled from filtered ONT FASTQ files and two assemblers were compared for each strain. First, long-read assembly was performed with Flye v2.7.1 [25] with an input genome size of 4.7 Mb and use of the --plasmids parameter to rescue any small unassembled replicons. A hybrid assembly combining paired-end Illumina and Nanopore reads was also generated with Unicycler v0.4.8 [26]. The resulting assemblies were compared for contiguity (total contig number and contig N50) and plasmid recovery and the Flye-generated assembly was utilised for further investigation in all cases (Table S2). Assemblies were then polished to reduce complex break, insertion and base-level substitution errors; Illumina FASTQ reads were mapped to the draft assembly with the Burrows-Wheeler Aligner (BWA) v0.7.17 MEM algorithm [27] and Samtools v1.7 [28] with the -F 256 parameter to ignore non-primary aligning reads. This was achieved with two rounds of the Pilon v1.23 –fix all function [29] with the following parameters; minimum depth 0.05, minimum quality 30 and minimum mapping quality 30. This was followed by two rounds of Racon v1.4.13 [30] with a match score of 8, average base quality threshold for windows of 30, mismatch score of −6 and gap penalty of −8. The final assembly quality was determined by QUAST v5.0.2 [31]. Where possible, contigs were reoriented on dnaA or repA using the Circlator v1.5.5 fixstart function [32]. As one assembly (607387) was poorly resolved, contigs were re-ordered according to the high quality S. sonnei 53G reference (GenBank NC_016822.1) using Mauve v2.4.0 [33].
Annotation, MLST and plasmid comparison
FASTA files were annotated with Prokka v1.14.6 [34] using a protein reference guide of pKSR100 (Accession LN624486) proteins and those identified at high confidence by ResFinder; in cases of ambiguity proteins were checked manually with the non-redundant blastp database. Then mlplasmids v1.1.0 [35] and blastn were used to predict chromosomal or plasmid contig origin. Multi-Locus Sequence Typing (MLST) was performed using the Centre for Genomic Epidemiology (CGE) database E. coli scheme #1 based on allelic profiles of seven housekeeping genes: adk, fumC, gyrB, icd, mdh, purA and recA [36]. IncFII plasmids were compared using blastn v2.10.1 with default parameters for percent identity and query cover to pKSR100 (GenBank LN624486.1), a conjugative plasmid from an S. flexneri strain SF795513 associated with shigellosis in MSM. As isolates were known to harbour bla CTX-M-27 they were also compared to p183660, found previously in S. sonnei , with high identity to pKSR100 and harbouring bla CTX-M-27 (GenBank KX008967.1) [16]. Other plasmids were compared to known plasmids using the non-redundant blastn database. ISsaga [37] was used to annotate IS in pKSR100-like plasmids and to detect overall numbers in genomes.
Antimicrobial resistance and virulence gene identification
The CGE PlasmidFinder-2.0 and pMLST-2.0 Enterobacteriaceae plasmid replicon database [38] was used to detect and type plasmids with default parameters (>95 % nucleotide identity threshold and >60 % query coverage). Acquired ARGs in Shigellae samples were detected in silico from assembled sequences using the ResFinder-3.2 [39] database including known E. coli chromosomal mutations, both with default 90 % identity and 80 % minimum length thresholds but all genes found and reported exhibited >98 % identity and >99 % coverage. The Comprehensive Antimicrobial Resistance Database (CARD) Resistance Gene Identifier tool [40] was also used to identify ARGs and mutations with default parameters (Perfect and Strict hits), as ResFinder does not generally include chromosome-specific genes. To investigate discrepancies in ARG identification, Illumina and Nanopore reads were mapped to bla CTX-M-27 from the CARD database using BWA-MEM v0.7.17 and minimap2 v2.17 [41] respectively with Samtools v1.7 where BAM files were visualised using Tablet v1.19.09.03 [42]. The ISEScan tool v.1.7.2.1 [43] was used to identify terminal inverted repeats associated with IS. Virulence factors were detected within assemblies using VirulenceFinder (https://cge.cbs.dtu.dk/services/VirulenceFinder) with default parameters.
Maximum-likelihood phylogeny
To provide context for the four Shigella isolates, maximum-likelihood phylogenies were created for the S. flexneri 3a isolate and 49 other S. flexneri genomes (50 total) (Table S3), and the S. sonnei isolates with 198 other S. sonnei strains (201 total) from PHE’s Shigella genome collection isolated in England. Representative strains from relevant unique 250 single-linkage hierarchical clusters were randomly selected from different time frames and SnapperDB v0.2.6 (get_the_snsps) [44] was used to generate core variant multiple sequence alignments of 4415 bp and 11 096 bp for S. sonnei and S. flexneri respectively. SnapperDB stores reference-based single nucleotide polymorphism (SNP) variant calls relative to the reference genome, with each isolate given a SNP Address based on single-linkage hierarchical clustering which is a proxy for the genetic distances of isolates in the population. In detail, Illumina reads were aligned to a reference genome, consisting of the S. sonnei strain Ss46 (GenBank accession number NC_007384.1) and S. flexneri 2a strain 2457T (GenBank accession number AE014073.1) using BWA-MEM v0.7.12 as previously described [45]. Sequence alignment maps were sorted and indexed to produce a binary alignment map (BAM) file using Samtools v1.0.18. SNPs were identified using GATK v2.6.5 and only high-quality SNP (mapping quality [MQ] >30, minimum depth >10, variant ratio >0.9) positions were extracted. These polymorphic position alignments were used to infer maximum-likelihood phylogenetic trees using IQ-Tree v2.0.6 [46] with 1000 ultrafast bootstrap approximations. The general time reversible model plus ascertainment bias correction was used to correct branch length overestimation due to absence of constant sites.
Lineage typing
Discriminatory, lineage specific SNPs were defined based on the phylogenetic lineage assignment in Baker et al. [8] and extracted directly from SnapperDB v0.2.5. To ensure greater utility to public health researchers, lineage typing of S. sonnei isolates (including publicly available AUSMDU00008333 (GenBank accession ERA1715196) and 183 660 (GenBank accession SRX1766927)) was also performed according to a newly described standard scheme [47] with Mykrobe v0.9.0 (predict) software [48] using the --ont parameter with trimmed FASTQ reads as input.
Metadata and ARG presence for isolates used for phylogenetic context
Not all isolates included for context were MSM-linked, however shigellosis cases that were (i) male, (ii) adults and (iii) had not reported recent foreign travel were inferred to be likely associated with sexual transmission among MSM. Presence of genes conferring resistance to azithromycin (erm(B), mph(A)) and mutations in the quinolone resistance-determining regions (QRDR) of gyrA and parC for all isolates included in the two phylogenetic trees were extracted from a previously published study [45]. This excludes S. sonnei isolate 893 916 where ResFinder results were used to determine presence of these genes and mutations. Detection of bla CTX-M-27 within all genomes was determined using a mapping-based approach known as GeneFinder v2.2 (https://github.com/phe-bioinformatics/gene_finder) with default parameters. GeneFinder utilises Bowtie2 v2.1 [49] and Samtools v1.0.18 [28] to align Illumina query sample reads to a reference.1.1.
Visualisation tools
Graphical comparison of chromosomal islands was performed using EasyFig v2.2 [50] and SRL genomic features of S. flexneri 3a were visualised with GView 1.7 [51]. blast Ring Image Generator (BRIG) [52] with blast v2.10.1 was utilised to visualise similarity of plasmids to pKSR100 and p183660, the latter of which was used as a reference. BRIG was also used to compare IncB/O/K/Z plasmids to a plasmid identified with high similarity by blastn, pAUSMDU00008333_3 (GenBank LR213460.1); isolate 607 387 was used as a reference due to it being the largest plasmid and virulence plasmids (pINV) were compared to one another other. The R package ggtree [53] was used to midpoint root maximum-likelihood trees, annotate tip nodes and visualise trees with isolate metadata and resistance gene presence/absence as an adjacent heatmap.
Results
Genomic features and assemblies
Four MSM-associated Shigella isolates (three S. sonnei; two 2018, one 2020 and one S. flexneri 3a, 2020) were selected for long-read sequencing due to bla CTX-M-27 presence in initial Illumina reads (Table 1). In all cases Flye outperformed Unicycler in creating more contiguous assemblies (Tables 2 and S2); detailed contig-level information for each isolate is available in Table S4.
Table 2.
Assembly statistics, genomic features and predicted number of insertion sequence (IS) elements of the three S. sonnei isolates and one S. flexneri 3a isolate. Genomes were constructed with Flye, where Illumina short reads were utilised to polish a draft long-read assembly to yield a more complete and accurate final assembly
|
Isolate ID |
598 080 |
607 387 |
888 048 |
893 916 |
|---|---|---|---|---|
|
Species |
S. flexneri 3a |
|||
|
MLST |
152 |
152 |
245 |
152 |
|
Total contigs |
6 (2 Chromosomal contigs, 3 plasmids, 1 likely plasmid contig) |
37 (24 likely Chromosomal contigs, 4 plasmids, 9 likely plasmid contigs) |
5 (1 Chromosomal contig, 2 plasmids, 2 likely plasmid contigs) |
4 (1 Chromosomal contig, 3 plasmids) |
|
Total size (bp) |
5 282 682 |
5 315865 |
4 839 032 |
5 196 904 |
|
Largest contig (bp) |
4 879 016 |
2 500 534 |
4 519 004 |
4 813 904 |
|
N50 |
4 879 016 |
897 518 |
4 519 004 |
4 813 904 |
|
L50 |
1 |
2 |
1 |
1 |
|
GC content (%) |
50.86 |
50.82 |
50.68 |
50.84 |
|
Total CDS |
5533 |
5554 |
5174 |
5462 |
|
rRNA |
22 |
22 |
23 |
22 |
|
tRNA |
102 |
101 |
98 |
96 |
|
tmRNA |
1 |
1 |
1 |
1 |
|
Acquired resistance genes (ResFinder) |
11 |
9 |
7 |
11* |
|
Resistance genes (CARD)† |
59 |
58 |
47 |
60 |
|
Virulence factors (VFDB) |
14 |
13 |
12 |
13 |
|
Predicted IS number |
577 |
520 |
504 |
588 |
|
Estimated different IS |
45–48 |
38–43 |
43–47 |
43–44 |
|
SRR number (Illumina) |
SRR7842065 |
SRR7892120 |
SRR11096691 |
SRR11206407 |
|
SNP address |
1.3.197.460. 1360.1582. 2770 |
1.3.197.460. 1360.1582. 2813 |
35.43.43. 43.1189. 2224.2978 |
1.1.1.1.377. 394.3945 |
*Two mutations in gyrA are counted as two genes.
†Perfect and Strict hits only, total includes those also found by ResFinder.
Virulence determinants and known virulence plasmid, pINV
Detection of virulence factors revealed small differences between strains and no genes encoding Shigatoxin (Table 3). All isolates harboured the large ~220 kbp virulence plasmid pINV (Fig. S5); pivotal for Shigella pathogenic evolution through acquisition of genes such as the conserved 30kbp mxi-spa locus encoding a type III secretion system, facilitating host invasion and spread [54]. pINV carried ipaD, capU and virF, with the S. flexneri isolate containing an additional sepA gene, thought to intensify intestinal fluid accumulation [55]. S. sonnei isolates displayed similar virulence profiles to S. flexneri but lacked sat, instead possessing lpfA, sigA and senB, hallmarks of S. sonnei virulence [56].
Table 3.
Virulence profiles of four Shigella isolates sequenced in this study, as determined by VirulenceFinder from whole genome assemblies. x indicates virulence factor present, – indicates virulence factor absent
|
Virulence factor |
598 080 (S. sonnei) |
607 387 (S. sonnei) |
888 048 ( S. flexneri 3a) |
893 916 ( S. sonnei ) |
|---|---|---|---|---|
|
Invasion protein (ipaD) |
x |
x |
x |
x |
|
Hexosyltransferase homlog (capU) |
x |
x |
x |
x |
|
VirF transcriptional activator (virF) |
x |
x |
x |
x |
|
Shigella extracellular protein A (sepA) |
– |
– |
x |
– |
|
Glutamate decarboxylase (gad) |
x |
x |
x |
x |
|
Invasion plasmid antigen (ipaH) |
x |
x |
x |
x |
|
Aerobactin synthetase (iucC) |
x |
x |
x |
x |
|
Ferric aerobactin receptor (iutA) |
x |
x |
x |
x |
|
Secreted autotransporter toxin (sat) |
– |
– |
x |
– |
|
Iron transport protein (sitA) |
x |
x |
x |
x |
|
Tellurium ion resistance protein (terC) |
x |
x |
x |
x |
|
Outer membrane complement resistance protein (traT) |
x |
x |
x |
x |
|
Long polar fimbriae (lpfA) |
x |
x |
– |
x |
|
Shigella IgA-like protease homolog (sigA) |
x |
x |
– |
x |
|
Enterotoxin ShET-2 (senB) |
x |
x |
– |
x |
|
Endonuclease colicin E2 (celb) |
x |
x |
– |
– |
Shared chromosomal AMR determinants among Shigella species
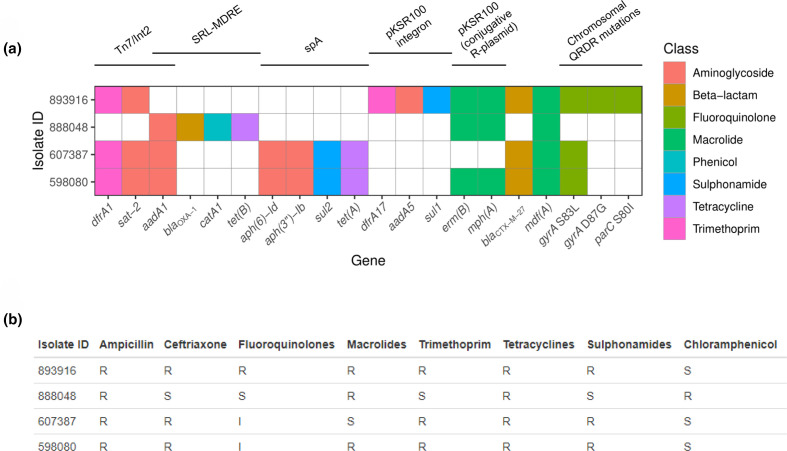
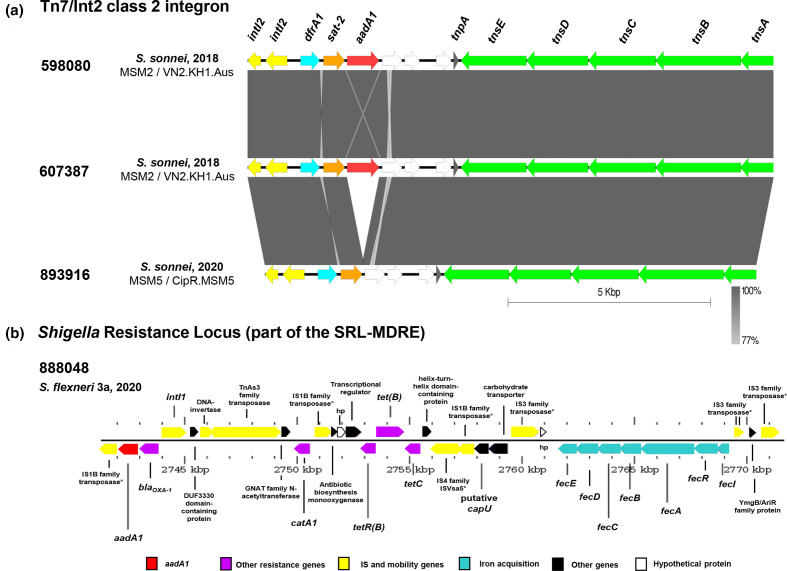
All isolates were genotypically and phenotypically MDR (resistant to ≥3 antimicrobial classes) and harboured known Shigella MGEs (Fig. 1) according to [8] (Table S5). Sequence analysis showed the presence of chromosomal class 2 IntI2/Tn7 integrons in all S. sonnei isolates. For 598 080 and 607 387 this comprised the canonical ARG cassette organisation dfrA1-sat2-aadA1 (Fig. 2a), conferring trimethoprim, streptothricin and streptomycin resistance respectively, however 893 916 lacked aadA1. This was unexpected as IntI2 is unable to alter the array due to an internal stop codon, usually leading to constant arrangements. All S. sonnei isolates harboured a 3′ tns transposition gene segment (tnsA-E) inserted adjacent to glutamine-fructose-6-phosphate aminotransferase, glmS, suggesting a single acquisition event. No such cassette was present in the S. flexneri isolate, which instead harboured aadA1 on a class one integron, the SRL (Fig. 2b) alongside bla OXA-1 , catA1 and tet(B) genes. These confer resistance to streptomycin, β-lactams, chloramphenicol and tetracyclines alongside a ferric uptake transport system (fecI-R with downstream structural fecABCDE genes), all integrated into tRNA-Ser.
Fig. 1.
(a) Binary heatmap of antimicrobial resistance genes (ARGs) and chromosomal mutations present in four Shigella strains identified by ResFinder-3.2; S. sonnei (598 080, 607 387 and 893 916) and S. flexneri 3a (888048). Where a gene is present (at a threshold of >98 % identity and >99 % query coverage), the tile is coloured by the antimicrobial class it confers resistance to. The gene sat-2 is not included in the ResFinder database but is included in this figure and chromosomal mutations in quinolone resistance determining region (QRDR) genes gyrA and parC are shown on the right. Known mobile genetic elements in Shigella are denoted above, as previously defined by Baker et al. [8]. (b) Phenotypic resistance profiles of the four isolates to antimicrobial classes. R=Resistant, S=Susceptible, I=Intermediate.
Fig. 2.
Chromosomal resistance islands identified in four MSM-associated Shigella isolates through sequencing with Oxford Nanopore Technologies. (a) blast comparisons of Tn7/Int2 integron antibiotic resistance gene cassettes found in all strains of S. sonnei . The three have similar arrangements, with 893 916 lacking the 3′ aadA1 aminoglycoside resistance gene. IntI2, an integrase, is shown in yellow and is non-functional. Genes dfrA1: dihydrofolate reductase (blue); sat-2: streptothricin acetyltransferase (orange); aadA1; aminoglycoside adenyltransferase (red); encoding resistance to trimethoprim, streptothricin and streptomycin respectively. Hypothetical proteins are shown in white and Tn7 transposition genes shown in green. The direction of the arrow shows the direction of gene coding and grey gradient legend shows blast identity between sequences. Figure produced with Easyfig v2.2.5 [50]. (b) Genomic organisation of the chromosomal Shigella Resistance Locus, part of the Multiple Drug Resistance Element (SRL-MDRE) present only in the S. flexneri 3a strain 888 048. This harbours aadA1, blaOXA-1, catA1 and tet genes encoding resistance to aminoglycosides, ampicillin, chloramphenicol and tetracycline respectively. aadA1 is shown in red due to commonality to Tn7/Int2, other resistance genes are shown in purple. The iron acquisition system is shown in turquoise, mobility related genes in yellow, other genes in black and hypothetical proteins in white. Figure produced with Gview [51].
Ciprofloxacin resistance is conferred by chromosomal Quinolone Resistance Determining Region (QRDR) mutations [8]. The two 2018 S. sonnei strains harboured single gyrA mutations (S83L) conferring reduced susceptibility to fluoroquinolones and are concerning as this can act as a prerequisite for successive stepwise mutations [57], implying possible evolution into fully resistant clones. Contrastingly, the 2020 S. sonnei strain had triple mutations (gyrA S83L; D87G, parC S80I) conferring high-level resistance. No QRDR mutations were found in S. flexneri 3a and, overall, no plasmid-encoded quinolone ARGs were detected.
All isolates harboured chromosomal class C β-lactamases ampC (between frdD and blc genes) and ampH (between sbmA and iprA) (Fig. 1b) promoting cephalosporin resistance [58]. These results are all concordant with phenotypic data, excluding the finding that 893 916 was phenotypically resistant to tetracyclines, despite no tet genes being found by ResFinder or CARD (Figs 1b and S2).
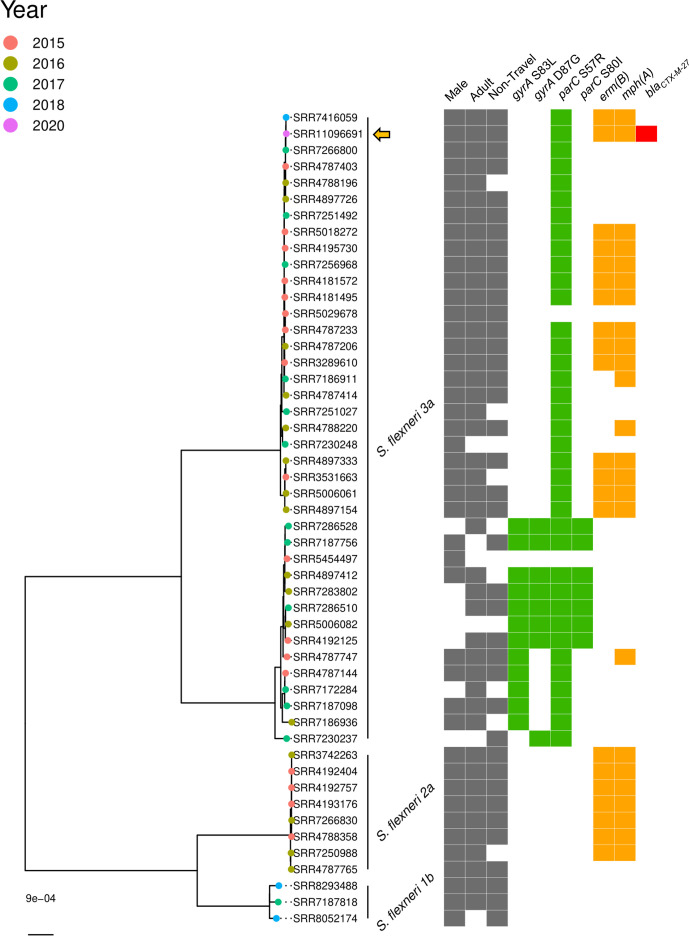
Phylogenetic analysis and clustering within MSM clades
To determine evolutionary relationships of strains within Shigella , we undertook separate analyses of 50 S . flexneri isolates and 201 S . sonnei strains based on SNP differences. The S. flexneri 3a isolate clustered within an S. flexneri 3a clade with high genetic homogeneity that emerged in 2015 and persists in the MSM population to date (Fig. 3), suggesting a sustained outbreak with possibly one transmission chain. Previous phylodynamic analysis has shown decreases in S. flexneri 3a cases and effective population size over time in English MSM, reflected in decreased sample diversity [45] as displayed here. Isolate 888 048 was the only S. flexneri isolate with presence of bla CTX-M-27, within a clade where almost all isolates harbour single parC mutation S57R and the majority contain both erm(B) and mph(A), though some isolates exhibit neither macrolide resistance determinant or mph(A) alone.
Fig. 3.
Maximum-likelihood phylogeny of S. flexneri 3a isolate 888 048 (SRR11096691, indicated by a filled orange arrow) sequenced with Nanopore technologies in this study, compared to other S. flexneri isolates in Public Health England’s collections. To place the isolate in context, 49 other S. flexneri isolates that are representatives from relevant SNP (single nucleotide polymorphism) clusters were included in the comparison. MSM clades associated with S. flexneri 1b, S. flexneri 2a and S. flexneri 3a are labelled. Isolates are labelled by SRR number and tip nodes are coloured by year of receipt. Adjacent grey blocks in each of the first three columns indicate the sample was isolated from a male, an adult, and that the illness was non-travel related (left-right). Grey blocks in these three lanes for an isolate implies the infection may be associated with MSM transmission. Presence of antimicrobial resistance genes concerning fluoroquinolones (mutations in gyrA and parC, green) and macrolides (erm(B) and mph(A), orange) are taken from Bardsley et al. [45] and presence of blaCTX-M-27 was determined using GeneFinder. Gene presence is indicated by a coloured tile in the relevant column. ML trees are midpoint rooted and scale bar indicates SNPs.
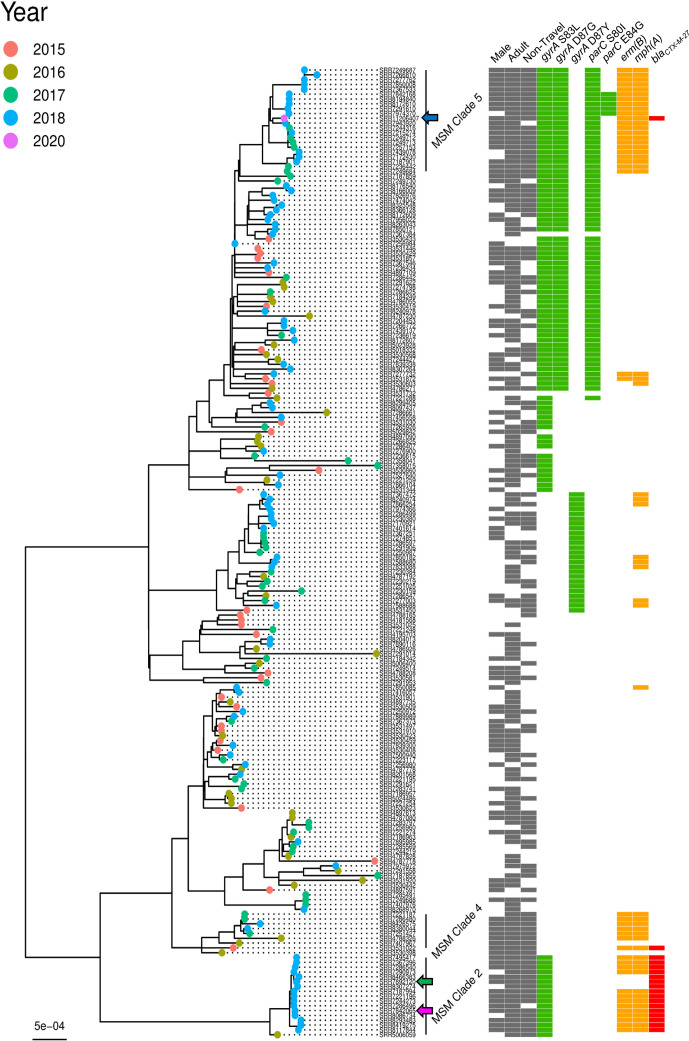
S. sonnei is divided into four lineages, one of which (lineage III) disseminated globally after MDR acquisition [11], and comprises five MSM-specific clades [8]. The two 2018 S. sonnei isolates (green and pink triangles) clustered within MSM clade 2 alongside mostly 2018 isolates (Fig. 4). This clade is known to have acquired pKSR100 (with erm(B) and mph(A)) on multiple occasions, have single QRDR mutations and links to Australian MSM but has a declining effective population with stable case rates [45]. Lineage typing according to a new scheme denotes 598 080 and 607 387 to be part of the VN2.KH1.Aus lineage (genotype 3.7.29.1.4.1); Australian isolates that emerged from the Khanh Hoa region of Vietnam [47].
Fig. 4.
Maximum-likelihood phylogeny of three S. sonnei isolates known to be associated with men who have sex with men that were sequenced using Nanopore technologies in this study, compared to other isolates in Public Health England’s collections. To place the isolates in context, 198 other S. flexneri isolates that are representatives from relevant SNP (single nucleotide polymorphism) clusters were included in the comparison. S. sonnei isolates investigated in detail in this study are as follows; 598 080 (SRR7842065, pink filled arrow), 607 387 (SRR7892120, green filled arrow), 893 916 (SRR11206407, blue filled arrow). Isolates are labelled by SRR number and tip nodes are coloured by year of receipt. S. sonnei MSM clades are labelled as per those described in Baker et al. [8]. Adjacent grey blocks in each of the first three columns (left-right) indicate the sample was isolated from a male, an adult, and that the illness was non-travel related (left-right). Grey blocks in these three lanes for an isolate implies the infection may be associated with MSM transmission. Presence of antimicrobial resistance genes concerning fluoroquinolones (mutations in gyrA and parC, green) and macrolides (erm(B) and mph(A), orange) are taken from Bardsley et al. [45] and presence of blaCTX-M-27 was determined using GeneFinder. Gene presence is indicated by a coloured tile in the relevant column. ML trees are midpoint rooted and scale bar indicates SNPs.
Contrastingly, the 2020 isolate clustered within MSM clade 5 isolates collected from 2017 to 2020. Members are known to harbour pKSR100 and concerning triple QRDR mutations (some exhibited an additional parC E84G mutation) and have an increasing effective population size since 2017 [45]. When utilising the standard typing scheme, 893 916 was part of the CipR.MSM5 sublineage (genotype 3.6.1.1.2), which emerged from South Asia in the early 2000s, is linked to MSM communities and is responsible for the majority of fluoroquinolone resistant S. sonnei infections in Australia, England and the USA [47].
We found bla CTX-M-27 is not restricted to one S. sonnei MSM clade and may have been acquired on multiple occasions; 893 916 is the only MSM clade 5 isolate with this element whereas it is present in all MSM clade 2 and only one MSM clade 4 isolate (Fig. 4). bla CTX-M-27 is only found in isolates with both erm(B) and mph(A) (likely co-located on the same plasmid) or neither determinant. Clustering of non-MSM cases within MSM clades could indicate community acquisition from MSM (e.g. shared households), or onward transmission to MSM from travel-related cases.
S. sonnei harbour AMR determinants on IncB/O/K/Z plasmids
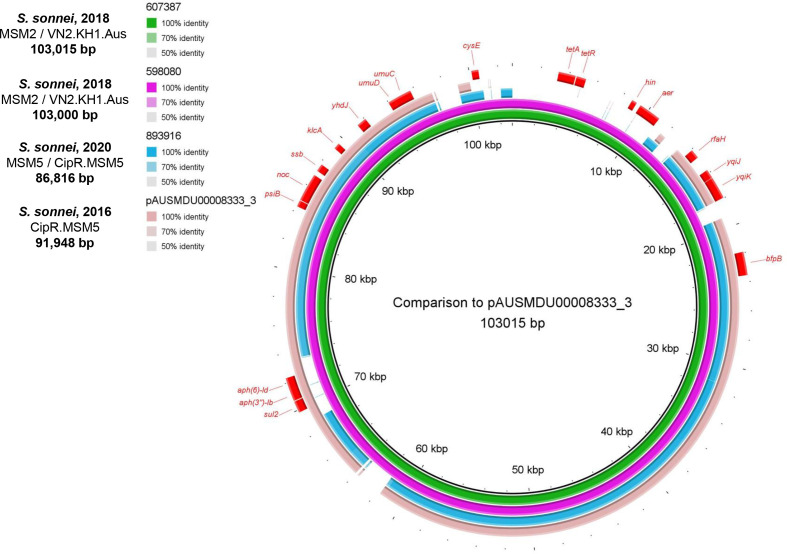
PlasmidFinder revealed a large IncB/O/K/Z plasmid (86-103 kbp) in all S. sonnei isolates with high nucleotide similarity (96.3–99.5% identity/79-91 % coverage) to a plasmid circulating in Australian MSM from a 2 year population-level study (2016–18), AUSMDU00008333 plasmid 3 [59] (Figs 5 and S4). Compared to this 2016 reference, 598 080 and 607 307 share an AMR region harbouring resistance to aminoglycosides (aph(6)-Id and aph(3′)-Ib) and sulphonamides (sul2), however the two 2018 S . sonnei isolates had acquired additional tetracycline tetR/tet(A) genes. Notably, the Australian MSM plasmid harboured bla TEM-1c, but this element was not present in this study and the plasmid within 893 916 did not carry AMR determinants, suggesting other genes present on this plasmid are sufficient to outweigh any reproductive fitness cost.
Fig. 5.
blast comparisons of IncB/O/K/Z plasmids for three MSM S. sonnei isolates in England, obtained with Nanopore sequencing. These are compared to a plasmid known to be associated with MSM in Australia, pAUSMDU00008333 plasmid 3 (LR213460.1). Three S. sonnei plasmids are from this study (isolates 598080; pink ring, 607387; green ring and 893916; blue ring) and the outer peach coloured ring is pAUSMDU00008333 plasmid 3. Genes present are shown in red on the outer ring (excluding hypothetical proteins). aph(3′)-Ib (strA): Aminoglycoside 3′-phosphotransferase; aph(6)-Id (strB): Aminoglycoside O-phosphotransferase; sul2: Dihydropteroate synthase; tet(A): Tetraycline efflux protein; psiB: Plasmid SOS inhibition protein B; noc: Nucleoid occlusion protein; ssb: Single-stranded DNA-binding protein; Anti-Restriction protein; yhdJ: DNA adenine methyltransferase; umuD and umuC - UV mutagenesis and repair proteins; cysE: Serine acetyltransferase; hin: DNA-invertase; aer: Aerotaxis receptor; rfaH: Transcription antitermination protein; yqiJ and yqiK: Inner membrane proteins; bfpB: Outer membrane lipoprotein. Figure produced with blast Ring Image Generator (BRIG) [52] and gradient of blast identity is shown in the legend.
Analysis of IncFII plasmids and genomic context of the bla CTX-M-27 gene
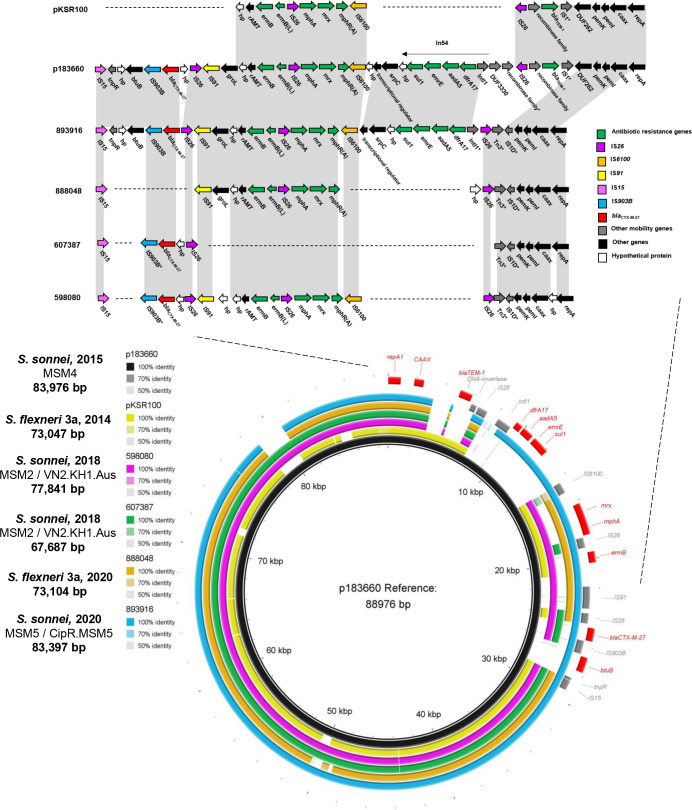
pMLST revealed all four strains harboured a large IncFII ([F2:A-:B-]) plasmid (67-83kbp). They demonstrated high similarity to known plasmid pKSR100 (98–99.5% identity/80-90 % cover), which has facilitated azithromycin resistance dissemination between Shigella MSM lineages [15]. Since isolates were known to harbour bla CTX-M-27 they were compared to p183660, a pKSR100-like MSM plasmid which has acquired this element [16], revealing 99.7–99.9% identity/97-98 % cover. These were also compared to each other to understand the organisation and loss of respective ARGs (Fig. 4).
All IncFII plasmids contained a conserved ~35 kbp conjugation-associated tra region allowing broader host dissemination, pemKI toxin-antitoxin system and maintenance genes. Interestingly, ARGs were located on mosaic regions displaying unique structures for each plasmid (Fig. 6). No single ARG was found ubiquitously and no strains contained non-ESBL β-lactamase gene bla TEM-1, present in both references pKSR100 and p183660. Three-quarters of isolates harboured erm(B) and mph(A); the two 2018 S . sonnei isolates (598 080 and 607 387) had similar resistance profiles overall, differing only in loss of both genes by 607 387.
Fig. 6.
blast comparisons of IncFII pKSR100-like plasmids in four Shigella isolates from men who have sex with men (MSM) in England obtained with Nanopore sequencing. Three plasmids are from S. sonnei (isolates 598080: pink ring; 607387: green ring and 893916: blue ring) and one from S. flexneri 3a (888048: orange ring). The second inner ring (yellow) is pKSR100, a known MSM-related plasmid from an S. flexneri 3a infection and the innermost (black) ring is p183660, obtained from a man in England infected with S. sonnei . This has high identity to pKSR100 but had acquired the pKSR100 integron [dfrA17, aadA5, emrE, sul1] and a novel mobile element harbouring bla CTX-M-27. Upper: DUF- denotes domain-containing proteins. * Denotes partial features. Features are not drawn to scale. Lower: Sequences associated with mobility in this region are coloured in grey on an outer ring and antimicrobial resistance and other genes are shown on the outer ring in red. repA1: IncFII repA protein; CAAX: CPBP family intramembrane metalloprotease; blaTEM-1 : β-lactamase; intI1: Class one integrase; dfrA17: Type I dihydrofolate reductase; aadA5: Streptomycin adenyltransferase; emrE: Multidrug transporter; sul1: Dihydropteroate synthase; mrx: Multidrug efflux pump; mph(A): Aminoglycoside phosphotransferase; erm(B): Dimethyladenosine transferase; blaCTX-M-27 : ESBL class A β-lactamase, CTX-M-27 type; btuB: Vitamin B12 transporter. Figure produced with blast Ring Image Generator (BRIG) [52].
Where present, erm(B) was adjacent to its leading peptide (erm(B)(L)) and mph(A) to its regulatory genes, associated with IS26 as part of an IS26-mph(A)-mrx-mphR(A)-IS6100 unit, though IS6100 was not present in S. flexneri . Downstream of erm(B), these genes were always associated with a putative rRNA adenine methyltransferase, a hypothetical protein, a 60 kDa chaperonin (groL) and IS91 (Fig. 6).
The bla CTX-M-27 gene (876 bp) was found on all S. sonnei plasmids but despite initial Illumina-based bla CTX-M-27 detection in S. flexneri 3a (888048), it was not observed in the assembly (Fig. 1). Mapping of trimmed Nanopore reads to a bla CTX-M-27 reference (GenBank AAO61597.1) showed it was not present in these reads (Fig. S3). As this assembly harboured a similar IncFII plasmid with mph(A) and erm(B), this suggests bla CTX-M-27 loss during storage or subculture in the 2 month duration between Illumina and Nanopore sequencing (Table S1), rather than whole plasmid loss or assembly errors (though it is possible bla CTX-M-27 was present on another plasmid that was lost during culture or through mis-assembly). Where present, bla CTX-M-27 was flanked upstream by IS26 and downstream by IS903B. Isolate 893 916 was the only one to harbour the previously described pKSR100 integron present in p183660 with sulphonamide, trimethoprim and aminoglycoside resistance genes [sul1/dfrA17/aadA5] alongside emrE (qacEdelta1), quaternary ammonium compound-resistance protein. This region included heavy metal resistance protein (srpC) and vitamin B12 transporter (btuB) following bla CTX-M-27. As IS91 is found between the pKSR100 integron and mph(A)-erm(B) unit in 893 916 but is lost in 888 048 which lacks this integron while harbouring mph(A)-erm(B), despite being present in 598 080 with the same context, it is not clear which region IS91 is associated with (Fig. 6).
Reorganisation of IncFII plasmids is likely driven by IS26
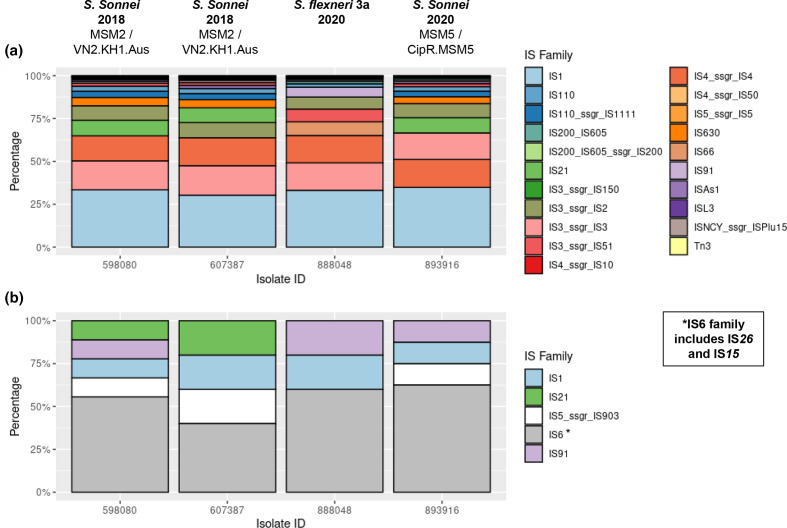
IS elements drive genomic evolution and typically comprise a transposase, catalysing enzymatic mobility, with flanking short terminal inverted repeats (TIRs) (transposase recognition sites) [60]. ISsaga revealed 577, 520 and 588 total predicted IS in S. sonnei genomes (598080, 607387 and 893 916 respectively) and 504 in S. flexneri 3a (Table 2); lower copy numbers in 607 387 are likely due to breaks between contigs. The most frequently identified IS family was IS1, accounting for 28.4, 26.3 and 30.8% in S. sonnei and 28.9% in S. flexneri . Chromosomal IS family proportions are similar between strains (Fig. 7a) but only S. flexneri harbours IS66 and remarkably, no copies of the IS6 family are present chromosomally in any isolates, yet dominate on pKSR100-like plasmids; mainly IS26 (Fig. 7b).
Fig. 7.
The predicted distribution of each IS family identified by ISSaga for each of the four Shigella isolates; S. sonnei (598 080, 607 387 and 893 916) and S. flexneri 3a (888048), by total copy number of each family. S. sonnei lineage is shown below isolation year where applicable. (Note: in some cases there are >1 different IS per family). (a) Distribution of each family within contigs identified as chromosomal sequences for each isolate. (b) Distribution of each IS family within the IncFII pKSR100-like plasmid present within each isolate.
IS26 and IS15 were always flanked by known 14 bp TIR (5′-GGCACTGTTGCAAA-3′) [61], suggesting possible recent acquisition without amelioration. When IS26 integrates, in some circumstances this results in 8 bp target sequence duplication (TSD) and flanking TIRs. No TSDs were manually identified so further study is needed to understand translocation mechanisms and acquisition dynamics. IS concentration on these plasmids and flanking of lost regions by IS suggests possible IS-mediated deletion and microevolution driven by IS insertion into the plasmid backbone.
Discussion
Overall our data show long-read sequencing supports elucidation of genomic AMR context among MSM-associated Shigella strains. This includes ESBL (bla CTX-M-27), borne on a mosaic ARG region within IncFII plasmids which are likely to have been reorganised by IS26. This, together with their phylogenetic position within MSM clades, can aid understanding of how AMR is tied to persistence in the population.
Combinations of mutations and presence of ARGs within isolates in this study promote resistance to all current first-line antimicrobials (macrolides, fluoroquinolones and third-generation cephalosporins) [62]. S. sonnei isolates harboured Tn7/Int2; this facilitated worldwide dissemination of global lineage III by allowing adaptation to multiple antibiotics in the environment [11]. Only the S. flexneri isolate harboured the SRL-MDRE with bla OXA-1 conferring ampicillin resistance, congruent with an Iranian study which suggested S. flexneri bla OXA-1 host preference [63]. The S. flexneri isolate harboured secretion autotransporter toxin sat and sepA which is associated with intense abdominal pain [64]. However, S. sonnei isolates exhibited higher numbers of virulence genes, including senB and sigA associated with bloody diarrhoea and fever respectively [65]. Presence of isolates with high levels of both AMR and virulence factors could be a consequence of clinically severe cases requiring antibiotics and exerting selective pressure and are a public health concern due to the challenge this poses to outbreak management.
Reduced susceptibility to azithromycin (RSA) in Shigella was not present in England in 2002 [66], but has arisen possibly due to pressure from treatment of other STIs in MSM [67] and triple QRDR mutations promoted emergence of fluoroquinolone-resistant S. sonnei in South Asia which later transmitted internationally [14]. ESBL prevalence is increasing, especially in Asia, North America and Europe [68]; WGS of 335 S . sonnei isolates associated with domestic acquisition in England and Wales (2015–16) denotes CTX-M genes in 12% of isolates and bla CTX-M-27 in 2.7% of those, though CTX-M-15 was most common [69]. Use of ceftriaxone to treat shigellosis should be carefully considered, especially in areas with RSA, due to possible co-selection of ESBL with macrolide resistance genes on the same plasmid [15, 59]. There are risks that ESBL-producing strains may spread among HIV-positive MSM with increased biologically susceptibility; use of networking applications is a transmission risk factor [70] so could be utilised for awareness campaigns and contact tracing, as shown effectively during a Berlin MSM hepatitis A outbreak [71].
In this study, aph(6)-Id/aph(3)-Ib/sul2/tet(A) carriage was on a large (~103 kbp) IncB/O/K/Z plasmid with Australian links. bla CTX-M-27-positive S. sonnei has recently driven a prolonged MSM outbreak in Australia [17], with one successful sublineage harbouring an AMR profile consistent with one isolate in this study, 598 080 (mphA, ermB, dfrA1 and sul2 genes with gyrA S83L). Typing of S. sonnei isolates 598 080 and 607 387 determined them as VN2.KH1.Aus; Australian isolates emerging from Kahnh Hoa Subclone 1 [47]. Though not designated as MSM-linked by this scheme, this could represent travel-associated introduction into England and subsequent MSM transmission.
We found bla CTX-M-27 was present on IncFII plasmids with high identity to a known plasmid linked to an English MDR S. sonnei cluster, p183660 (which harbours bla CTX-M-27 and has high identity to pKSR100) [15]. We observed bla CTX-M-27 displace bla TEM-1, and bla CTX-M-27 loss between sequencing rounds was observed in S. flexneri 3a. This gene possibly has lower stability in this species; none of the 49 other S. flexneri isolates harboured bla CTX-M-27 and globally bla CTX-M-27 seems relatively rare in S. flexneri [72]. Alternatively, as this isolate also harboured chromosomal β-lactamases ampC and bla OXA-1, IS activity may have led to rapid plasmid bla CTX-M-27 loss in the absence of antimicrobial pressure. It is interesting that isolate 607 387 lost both mphA and ermB (two macrolide resistance mechanisms; phosphotransferase-mediated modification and methylase ribosome target-site modification) together with two MSM clade 2 members, whereas many S. sonnei isolates in non-MSM clades harboured only mph(A) (no isolate contained erm(B) alone).
Insertion sequences are challenging to identify with short-read data but facilitate ongoing species diversification in Shigella through gene interruption, deletion and genome reorganisation with convergence on streamlined genomes [73]. Hawkey et al. showed expansion of IS1, IS2, IS4, IS600 and IS911 in Shigella genomes; similarly, we showed high proportions of IS1, IS2 and IS4 with differing S. sonnei and S. flexneri IS profiles. IS activity causes potentially deleterious outcomes (e.g. ARG loss) but allows flexibility to create diverse structures; some of which are likely to be selected for [74]. It has been suggested if insertion of IS26 causes no deleterious effects it acts as a ‘founder element’ where further IS26 insertion occurs preferentially next to another [75], enabling rapid novel ARG acquisition. Concordantly, here IS26 preferentially inserted into IncFII plasmids and IS15 was present close to bla CTX-M-27, corresponding to one IS26 copy inserted into another. Similar to our genetic platform, bla CTX-M-27 was flanked by IS26 and IS903B in E. coli ST38 IncF plasmids [76], suggesting a possible CTX-M reservoir. Currently annotated bla CTX-M-27-carrying Shigella plasmids are limited to S. sonnei p183660 (GenBank KX008967.1) and Swiss pEC732_2 (GenBank CP015140.1). Within the latter a contrasting IS26-IS903BΔ-bla CTX-M-27-ISEcp1Δ-IS26 unit was identified [19], though it is possible IS26 was inserted into ISEcp1 (promoting high-level expression) in our study but not identified.
Although the mechanistic basis of bla CTX-M-27 and mph(A)-erm(B) loss is speculative, this could reflect intramolecular IS26 transposition in cis, resulting in deletion of DNA between the IS and target site, leaving one IS copy [77]. IS26 is known to generate 8 bp target site duplications during cointegrate formation [77]; investigating these within additional ESBL plasmids over time would provide insights regarding temporal and spatial dynamics of AMR acquisition and loss.
This study is limited in low isolate numbers from a single region without patient exposure data or investigation of other CTX-M genes in additional isolates in PHE's collection; we cannot extrapolate ARG patterns and their position within MGEs in MSM-related strains over time or characterise risk factors. In S. sonnei there is generally high concordance between WGS ARG presence and phenotype when 100% read coverage and >90 % nucleotide identity thresholds are used [69], though phenotypic data did not correlate with genotype for tetracycline resistance in 893 916 and the underlying mechanism was not investigated. If long-read sequencing becomes viable on a routine scale, this would significantly aid plasmid monitoring to predict AMR dissemination, especially as bla CTX-M-27 seems to have been acquired on multiple occasions.
Conclusions
Shigella spp. are concerning due to their extraordinary ability to acquire and disseminate AMR. As ESBL genes can be propagated by movement on small mobile genetic elements, dissemination of ‘epidemic’ plasmids and via clonal spread, WGS surveillance of all three avenues with international data sharing is necessary to inform coordinated and responsible antimicrobial strategies.
Supplementary Data
Funding information
D.R.G., C.J. and T.J.D., are affiliated to the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Gastrointestinal Infections at University of Liverpool in partnership with Public Health England (PHE), in collaboration with University of Warwick, and are based at PHE. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, the Department of Health and Social Care or Public Health England.
Acknowledgements
Special thanks to Matt Bird for his ongoing encouragement.
Author contributions
Conceptualisation and methodology: L.A.C., D.R.G., C.J., T.J.D., Supervision: L.A.C. Investigation: R.K.L., D.R.G., Visualisation: R.K.L., Writing – original draft: R.K.L., Writing – review and editing: R.K.L., L.A.C., C.J., D.R.G.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: AMR, antimicrobial resistance; ARG, antimicrobial resistance gene; BAM, binary alignment map; BRIG, BLAST Ring Image Generator; CARD, Comprehensive Antimicrobial Resistance Database; ESBL, extended spectrum beta-lactamase; IS, insertion sequence; MDR, multidrug resistant; MDRE, Multiple Drug Resistance Element; MGE, mobile genetic element; MLST, Multi-Locus Sequence Typing; MSM, men who have sex with men; ONT, Oxford Nanopore Technologies; PAI, pathogenicity island; PHE, Public Health England; QRDR, quinolone resistance determining region; SNP, single nucleotide polymorphism; SRL, Shigella Resistance Locus; TIR, terminal inverted repeat; TSD, target sequence duplication.
All supporting data, code and protocols have been provided within the article or through supplementary data files. Five supplementary figures and six supplementary tables are available with the online version of this article.
References
- 1.Chiou CS, Izumiya H, Kawamura M, Liao YS, Su YS, et al. The worldwide spread of ciprofloxacin-resistant Shigella sonnei among HIV-infected men who have sex with men, Taiwan. Clin Microbiol Infect. 2016;22:383. doi: 10.1016/j.cmi.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 2.DuPont HL, Levine MM, Hornick RB, Formal SB. Inoculum size in shigellosis and implications for expected mode of transmission. J Infect Dis. 1989;159:1126–1128. doi: 10.1093/infdis/159.6.1126. [DOI] [PubMed] [Google Scholar]
- 3.Murray K, Reddy V, Kornblum JS, Waechter H, Chicaiza LF, et al. Increasing antibiotic resistance in Shigella spp. From infected New York city residents, New York, USA. Emerg Infect Dis. 2017;23:332–335. doi: 10.3201/eid2302.161203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schroeder GN, Hilbi H. Molecular pathogenesis of shigella spp.: Controlling host cell signaling, invasion, and death by type iii secretion. Clin Microbiol Rev. 2008;21:134–156. doi: 10.1128/CMR.00032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang F, Yang J, Zhang X, Chen L, Jiang Y, et al. Genome dynamics and diversity of Shigella species, the etiologic agents of bacillary dysentery. Nucleic Acids Res. 2005;33:6445–6458. doi: 10.1093/nar/gki954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bardhan P, Faruque ASG, Naheed A, Sack DA. Decrease in shigellosis-related deaths without shigella spp.- specific interventions, Asia. Emerg Infect Dis. 2010;16:1718–1723. doi: 10.3201/eid1611.090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. The Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 8.Baker KS, Dallman TJ, Field N, Childs T, Mitchell H, et al. Genomic epidemiology of Shigella in the United Kingdom shows transmission of pathogen sublineages and determinants of antimicrobial resistance. Scientific Reports. 2018;8 doi: 10.1038/s41598-018-25764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simms I, Field N, Jenkins C, Childs T, Gilbart VL, et al. Intensified shigellosis epidemic associated with sexual transmission in men who have sex with men - Shigella fexneri and s. Sonnei in England, 2004 to end of February 2015. Eurosurveillance. 2015;20:1–5. doi: 10.2807/1560-7917.es2015.20.15.21097. [DOI] [PubMed] [Google Scholar]
- 10.Williams PCM, Berkley JA. Guidelines for the treatment of dysentery (shigellosis): a systematic review of the evidence. Paediatrics and International Child Health. 2018;38 doi: 10.1080/20469047.2017.1409454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holt KE, Baker S, Weill FX, Holmes EC, Kitchen A, et al. Shigella sonnei genome sequencing and phylogenetic analysis indicate recent global dissemination from Europe. Nat Genet. 2012;44:1056–1059. doi: 10.1038/ng.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajakumar K, Bulach D, Davies J, Ambrose L, Sasakawa C, et al. Identification of a chromosomal Shigella flexneri multi-antibiotic resistance locus which shares sequence and organizational similarity with the resistance region of the plasmid NR1. Plasmid. 1997;37:159–168. doi: 10.1006/plas.1997.1280. [DOI] [PubMed] [Google Scholar]
- 13.Ye C, Lan R, Xia S, Zhang J, Sun Q, et al. Emergence of a new multidrug-resistant serotype X variant in an epidemic clone of Shigella flexneri . J Clin Microbiol. 2010;48:419–426. doi: 10.1128/JCM.00614-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung The H, Boinett C, Pham Thanh D, Jenkins C, Weill F-X, et al. Dissecting the molecular evolution of fluoroquinolone-resistant Shigella sonnei . Nat Commun. 2019;10:4828. doi: 10.1038/s41467-019-12823-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker KS, Dallman TJ, Field N, Childs T, Mitchell H, et al. Horizontal antimicrobial resistance transfer drives epidemics of multiple Shigella species. Nature Communications. 2018;9:1–10. doi: 10.1038/s41467-018-03949-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mook P, McCormick J, Bains M, Cowley LA, Chattaway MA, et al. ESBL-Producing and macrolide-resistant Shigella sonnei infections among men who have sex with men, England, 2015. Emerg Infect Dis. 2016;22:1948–1952. doi: 10.3201/eid2211.160653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ingle DJ, Andersson P, Valcanis M, Barnden J, Gonçalves da Silva A, et al. Prolonged outbreak of multidrug-resistant Shigella sonnei harbouring bla CTX-M-27 in Victoria, Australia. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.01518-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muthuirulandi Sethuvel DP, Veeraraghavan B, Vasudevan K, Devanga Ragupathi NK, Murugan D, et al. Complete genome analysis of clinical Shigella strains reveals plasmid pSS1653 with resistance determinants: A triumph of hybrid approach. Gut Pathog. 2019;11:55. doi: 10.1186/s13099-019-0334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campos-Madueno EI, Bernasconi OJ, Moser AI, Keller PM, Luzzaro F, et al. Rapid Increase of CTX-M-Producing Shigella sonnei Isolates in Switzerland: spread of common plasmids and International Clones. Antimicrobial Agents and Chemotherapy. 2020:01057–20. doi: 10.1128/AAC.01057-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arredondo-Alonso S, Willems RJ, van Schaik W, Schürch AC. On the (im)possibility of reconstructing plasmids from whole-genome short-read sequencing data. Microbial Genomics. 2017;3:10. doi: 10.1099/mgen.0.000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.George S, Pankhurst L, Hubbard A, Votintseva A, Stoesser N, et al. Resolving plasmid structures in enterobacteriaceae using the Minion nanopore sequencer: Assessment of Minion and Minion/illumina hybrid data assembly approaches. Microb Genom. 2017;3:e000118. doi: 10.1099/mgen.0.000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sedlazeck FJ, Lee H, Darby CA, Schatz MC. Piercing the dark matter: Bioinformatics of long-range sequencing and mapping. Nat Rev Genet. 2018;19:329–346. doi: 10.1038/s41576-018-0003-4. [DOI] [PubMed] [Google Scholar]
- 23.Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Coster W, D’Hert S, Schultz DT, Cruts M, Van Broeckhoven C. NanoPack: visualizing and processing long-read sequencing data. Bioinformatics. 2018;34:2666–2669. doi: 10.1093/bioinformatics/bty149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolmogorov M, Yuan J, Lin Y, Pevzner PA. Assembly of long, error-prone reads using repeat graphs. Nat Biotechnol. 2019;37:540–546. doi: 10.1038/s41587-019-0072-8. [DOI] [PubMed] [Google Scholar]
- 26.Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol. 2017;13:e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Durbin R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, et al. Pilon: An Integrated Tool for Comprehensive Microbial Variant Detection and Genome Assembly Improvement. PLOS ONE. 2014;9:e112963. doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaser R, Sović I, Nagarajan N, Šikić M. Fast and accurate de novo genome assembly from long uncorrected reads. Genome Res. 2017;27:737–746. doi: 10.1101/gr.214270.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gurevich A, Saveliev V, Vyahhi N, Tesler G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics. 2013;29:1072–1075. doi: 10.1093/bioinformatics/btt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunt M, Silva ND, Otto TD, Parkhill J, Keane JA, et al. Circlator: Automated circularization of genome assemblies using long sequencing reads. Genome Biol. 2015;16:294. doi: 10.1186/s13059-015-0849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Darling AE, Mau B, Perna NT. Progressivemauve: Multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE. 2010;5:e11147. doi: 10.1371/journal.pone.0011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 35.Arredondo-Alonso S, Rogers MRC, Braat JC, Verschuuren TD, Top J, et al. mlplasmids: a user-friendly tool to predict plasmid- and chromosome-derived sequences for single species. Microb Genom. 2018;4:e000224. doi: 10.1099/mgen.0.000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larsen M, Cosentino S, Rasmussen S, Friis C, Hasman H, et al. Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol. 2012;50:1355–1361. doi: 10.1128/JCM.06094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varani AM, Siguier P, Gourbeyre E, Charneau V, Chandler M. ISsaga is an ensemble of web-based methods for high throughput identification and semi-automatic annotation of insertion sequences in prokaryotic genomes. Genome Biol. 2011;12:R30. doi: 10.1186/gb-2011-12-3-r30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carattoli A, Hasman H. Methods in Molecular Biology. Humana Press Inc; 2020. PlasmidFinder and In silico pMLST: Identification and typing of plasmid replicons in whole-genome sequencing (WGS) pp. 285–294. [DOI] [PubMed] [Google Scholar]
- 39.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother. 2012;67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alcock BP, Raphenya AR, Lau TTY, Tsang KK, Bouchard M, et al. CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Research. 2019;48:D517–D525. doi: 10.1093/nar/gkz935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics. 2018;34:3094–3100. doi: 10.1093/bioinformatics/bty191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milne I, Bayer M, Cardle L, Shaw P, Stephen G, et al. Tablet-next generation sequence assembly visualization. Bioinformatics. 2009;26:401–402. doi: 10.1093/bioinformatics/btp666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie Z, Tang H. ISEScan: Automated Identification of Insertion Sequence Elements in Prokaryotic Genomes. Bioinformatics (Oxford, England: 2017. pp. 3340–3347. [DOI] [PubMed] [Google Scholar]
- 44.Dallman T, Ashton P, Schafer U, Jironkin A, Painset A, et al. SnapperDB: a database solution for routine sequencing analysis of bacterial isolates. Bioinformatics. 2018;34:3028–3029. doi: 10.1093/bioinformatics/bty212. [DOI] [PubMed] [Google Scholar]
- 45.Bardsley M, Jenkins C, Mitchell HD, Mikhail AFW, Baker KS, et al. Persistent transmission of shigellosis in England is associated with a recently emerged multidrug-resistant strain of Shigella Sonnei . J Clin Microbiol. 2020;58:3. doi: 10.1128/JCM.01692-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, et al. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol Biol Evol. 2020;37:1530–1534. doi: 10.1093/molbev/msaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hawkey J, Paranagama K, Baker KS, Bengtsson RJ, Weill FX, et al. Global population structure and genotyping framework for genomic surveillance of the major dysentery pathogen, Shigella sonnei . Nature Communications. 2021;12:1–12. doi: 10.1038/s41467-021-22700-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hunt M, Bradley P, Lapierre SG, Heys S, Thomsit M, et al. Antibiotic resistance prediction for Mycobacterium tuberculosis from genome sequence data with Mykrobe. Wellcome Open Res. 2019;4:191. doi: 10.12688/wellcomeopenres.15603.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sullivan MJ, Petty NK, Beatson SA. Easyfig: A genome comparison visualizer. Bioinformatics. 2011;27:1009–1010. doi: 10.1093/bioinformatics/btr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petkau A, Stuart-Edwards M, Stothard P, van Domselaar G. Interactive microbial genome visualization with GView. Bioinformatics. 2010;26:3125–3126. doi: 10.1093/bioinformatics/btq588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alikhan NF, Petty NK, Ben Zakour NL, Beatson SA. blast Ring Image Generator (BRIG): Simple prokaryote genome comparisons. BMC Genomics. 2011;12:402. doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu G. Using ggtree to Visualize Data on Tree-Like Structures. Current Protocols in Bioinformatics. 2020 doi: 10.1002/cpbi.96. [DOI] [PubMed] [Google Scholar]
- 54.Buchrieser C, Glaser P, Rusniok C, Nedjari H, D’Hauteville H, et al. The virulence plasmid pWR100 and the repertoire of proteins secreted by the type III secretion apparatus of Shigella flexneri . Mol Microbiol. 2000;38:760–771. doi: 10.1046/j.1365-2958.2000.02179.x. [DOI] [PubMed] [Google Scholar]
- 55.Faherty C, Harper JM, Shea-Donohue T, Barry EM, Kaper JB, et al. Chromosomal and plasmid-encoded factors of Shigella flexneri induce secretogenic activity ex vivo . PLoS ONE. 2012;7:49980. doi: 10.1371/journal.pone.0049980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mattock E, Blocker AJ. How do the virulence factors of shigella work together to cause disease. Front Cell Infect Microbiol. 2017;7:64. doi: 10.3389/fcimb.2017.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bagel S, Hüllen V, Wiedemann B, Heisig P. Impact of gyrA and parC mutations on quinolone resistance, doubling time, and supercoiling degree of Escherichia coli . Antimicrob Agents Chemother. 1999;43:868–875. doi: 10.1128/AAC.43.4.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ranjbar R, Farahani A. Shigella: Antibiotic-resistance mechanisms and new horizons for treatment. Infect Drug Resist. 2019;12:3137–3167. doi: 10.2147/IDR.S219755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ingle DJ, Easton M, Valcanis M, Seemann T, Kwong JC, et al. Co-circulation of multidrug-resistant Shigella among men who have sex with men in Australia. Clin Infect Dis. 2019;69:1535–1544. doi: 10.1093/cid/ciz005. [DOI] [PubMed] [Google Scholar]
- 60.Yousfi K, Gaudreau C, Pilon PA, Lefebvre B, Walker M, et al. Genetic mechanisms behind the spread of reduced susceptibility to azithromycin in Shigella strains isolated from men who have sex with men in Québec, Canada. Antimicrob Agents Chemother. 2019;63 doi: 10.1128/AAC.01679-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trieu-Cuot P, Labigne-Roussel A, Courvalin P. An IS15 insertion generates an eight-base-pair duplication of the target DNA. Gene. 1983;24:125–129. doi: 10.1016/0378-1119(83)90137-3. [DOI] [PubMed] [Google Scholar]
- 62.Klontz KC, Singh N. Treatment of drug-resistant Shigella infections. Expert Rev Anti Infect Ther. 2015;13:69–80. doi: 10.1586/14787210.2015.983902. [DOI] [PubMed] [Google Scholar]
- 63.Zamanlou S, Ahangarzadeh Rezaee M, Aghazadeh M, Ghotaslou R, Babaie F, et al. Characterization of integrons, extended-spectrum β-lactamases, AmpC cephalosporinase, quinolone resistance, and molecular typing of Shigella spp. Infect Dis (Lond) 2018;50:616–624. doi: 10.1080/23744235.2018.1455222. [DOI] [PubMed] [Google Scholar]
- 64.Medeiros PHQS, Lima AÂM, Guedes MM, Havt A, Bona MD, et al. Molecular characterization of virulence and antimicrobial resistance profile of Shigella species isolated from children with moderate to severe diarrhea in northeastern Brazil. Diagn Microbiol Infect Dis. 2018;90:198–205. doi: 10.1016/j.diagmicrobio.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 65.Sethuvel DPM, Anandan S, Michael JS, Murugan D, Neeravi A, et al. Virulence gene profiles of Shigella species isolated from stool specimens in India: its association with clinical manifestation and antimicrobial resistance. Pathog Glob Health. 2019;113:173–179. doi: 10.1080/20477724.2019.1632062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheasty T, Day M, Threlfall EJ. Increasing incidence of resistance to nalidixic acid in shigellas from humans in England and Wales: Implications for therapy. Clin Microbiol Infect. 2004;10:1033–1035. doi: 10.1111/j.1469-0691.2004.00982.x. [DOI] [PubMed] [Google Scholar]
- 67.The HC, Thanh DP, Holt KE, Thomson NR, Baker S. The genomic signatures of Shigella evolution, adaptation and geographical spread. Nat Rev Microbiol. 2016;14:235–250. doi: 10.1038/nrmicro.2016.10. [DOI] [PubMed] [Google Scholar]
- 68.Bevan E, Jones A, Hawkey P. Global epidemiology of CTX-M β-lactamases: temporal and geographical shifts in genotype. J Antimicrob Chemother. 2017;72:2145–2155. doi: 10.1093/jac/dkx146. [DOI] [PubMed] [Google Scholar]
- 69.Sadouki Z, Day MR, Doumith M, Chattaway MA, Dallman TJ, et al. Comparison of phenotypic and WGS-derived antimicrobial resistance profiles of Shigella sonnei isolated from cases of diarrhoeal disease in England and Wales, 2015. J Antimicrob Chemother. 2017;72:2496–2502. doi: 10.1093/jac/dkx170. [DOI] [PubMed] [Google Scholar]
- 70.Gilbart VL, Simms I, Jenkins C, Furegato M, Gobin M, et al. Sex, drugs and smart phone applications: Findings from semistructured interviews with men who have sex with men diagnosed with Shigella flexneri 3a in England and Wales. Sex Transm Infect. 2015;91:598–602. doi: 10.1136/sextrans-2015-052014. [DOI] [PubMed] [Google Scholar]
- 71.Ruscher C, Werber D, Thoulass J, Zimmermann R, Eckardt M, et al. Dating apps and websites as tools to reach anonymous sexual contacts during an outbreak of hepatitis A among men who have sex with men, Berlin, 2017. Eurosurveillance. 2019;24:1800460. doi: 10.2807/1560-7917.ES.2019.24.21.1800460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu G, Qian H, Tang B, Chen Y, Kang H, et al. Prevalence and characterisation of third-generation cephalosporin-resistant Shigella flexneri isolates from Jiangsu Province, China, 2013–2015. J Glob Antimicrob Resist. 2018;15:283–287. doi: 10.1016/j.jgar.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 73.Hawkey J, Monk JM, Billman-Jacobe H, Palsson B, Holt KE. Impact of insertion sequences on convergent evolution of Shigella species. PLoS Genet. 2020;16:e1008931. doi: 10.1371/journal.pgen.1008931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Partridge SR. Analysis of antibiotic resistance regions in Gram-negative bacteria. FEMS Microbiol Rev. 2011;35:820–855. doi: 10.1111/j.1574-6976.2011.00277.x. [DOI] [PubMed] [Google Scholar]
- 75.Harmer CJ, Moran RA, Hall RM. Movement of IS26-Associated antibiotic resistance genes occurs via a translocatable unit that includes a single IS26 and preferentially inserts adjacent to another IS26. mBio. 2014;5:e01801–14. doi: 10.1128/mBio.01801-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mostafa HH, Cameron A, Taffner SM, Wang J, Malek A, et al. Genomic surveillance of ceftriaxone-resistant Escherichia coli in Western New York suggests the extended-spectrum β-lactamase blaCTX-M-27 is emerging on distinct plasmids in ST38. Front Microbiol. 2020;11:1747. doi: 10.3389/fmicb.2020.01747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.He S, Hickman AB, Varani AM, Siguier P, Chandler M, et al. Insertion sequence IS26 reorganizes plasmids in clinically isolated multidrug-resistant bacteria by replicative transposition. mBio. 2015;6 doi: 10.1128/mBio.00762-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.