Abstract
Expression of oncogenic Ras in thyroid cells results in loss of expression of several thyroid-specific genes and inactivation of TTF-1, a homeodomain-containing transcription factor required for normal development of the thyroid gland. In an effort to understand how signal transduction pathways downstream of Ras may be involved in suppression of the differentiated phenotype, we have tested mutants of the Ras effector region for their ability to affect TTF-1 transcriptional activity in a transient-transfection assay. We find that V12S35 Ras, a mutant known to interact specifically with Raf but not with RalGDS or phosphatidylinositol 3-kinase (PI3 kinase) inhibits TTF-1 activity. Expression of an activated form of Raf (Raf-BXB) also inhibits TTF-1 function to a similar extent, while the MEK inhibitors U0126 and PD98059 partially relieve Ras-mediated inactivation of TTF-1, suggesting that the extracellular signal-regulated kinase (ERK) pathway is involved in this process. Indeed, ERK directly phosphorylates TTF-1 at three serine residues, and concomitant mutation of these serines to alanines completely abolishes ERK-mediated phosphorylation both in vitro and in vivo. Since activation of the Raf/MEK/ERK pathway accounts for only part of the activity elicited by oncogenic Ras on TTF-1, other downstream pathways are likely to be involved in this process. We find that activation of PI3 kinase, Rho, Rac, and RalGDS has no effect on TTF-1 transcriptional activity. However, a poorly characterized Ras mutant, V12N38 Ras, can partially repress TTF-1 transcriptional activity through an ERK-independent pathway. Importantly, concomitant expression of constitutive activated Raf and V12N38 Ras results in almost complete loss of TTF-1 activity. Our data indicate that the Raf/MEK/ERK cascade may act in concert with an as-yet-uncharacterized signaling pathway activated by V12N38 Ras to repress TTF-1 function and ultimately to inhibit thyroid cell differentiation.
Ras proteins act as molecular switches that cycle between active GTP-bound and inactive GDP-bound forms and function as essential components of signal transduction pathways that regulate cell growth, morphology, and differentiation (6, 14, 25, 36). Activated ras genes have been implicated in many types of human cancer (4). In several cultured cell lines, expression of constitutively active forms of Ras results in growth factor-independent proliferation, morphological transformation, anchorage-independent growth, and loss of the differentiated phenotype.
Several proteins have been identified as potential effectors of Ras signaling, of which Raf, phosphatidylinositol 3-kinase (PI3K), and guanine nucleotide exchange factors for the Ral family of GTPases are the best characterized (6, 25). The Raf family of serine/threonine kinases (Raf1, Raf-A, and Raf-B) is the best characterized among the Ras effectors (43, 62). Ras interaction with Raf leads to the activation of MEKs (mitogen-activated protein [MAP] kinase kinases or extracellular signal-regulated kinase [ERK] kinases), which, in turn, phosphorylate ERKs, resulting in phosphorylation of cytoplasmic and nuclear targets, among which are several transcription factors (12, 20, 23, 37, 59). Thus, ERKs function as a transition point between signaling proteins and transcriptional regulators, resulting in changes in gene expression. Interestingly, ERKs are likely to phosphorylate different targets depending on the cell type.
Ras can trigger multiple signaling pathways in addition to the Raf/MEK/ERK cascade, which often lead to activation of other MAP kinase family members. It has become progressively clear that integration of multiple functions is required to mimic Ras effects and ultimately lead to full transformation (28, 57).
PI3K is a direct effector of Ras, since its catalytic subunit p110 is recruited by Ras in a GTP-dependent manner (47, 49). This kinase has been implicated in activation of the small GTPase Rac, thus linking Ras with the Rac pathway (50). Besides being an important regulator of the actin cytoskeleton, Rac functions also as an activator of a member of the MAP kinase family, JNK (c-Jun N-terminal-activated kinase; also called stress-activated protein kinase), resulting in induction of gene expression (11, 40).
Another family of putative Ras effectors, RalGDS, and two related proteins (Rfl and Rgl) function as guanine nucleotide exchange factors for the Ras-related GTPases RalA and RalB (22, 30, 55, 61). While the Ral downstream pathway has not been elucidated yet, it is believed that activation of RalGDS may play a role in transformation, since constitutive active forms of RalGDS cooperate with Raf to induce cell transformation (29, 55, 58). Furthermore, Ral dominant-negative mutants inhibit Ras-induced proliferation and cell transformation (39, 55).
Many mutations of the Ras effector region (residues 32 to 40) are known to inhibit the biological function of Ras, blocking its interaction with target proteins. Some single point mutations in this region lead to partial-loss-of-function mutants in which interaction with some effectors, but not with others, is lost. The involvement of multiple effectors in Ras-mediated transformation was demonstrated by using these mutants (26, 50, 57). Three complementation groups have been described, each binding specifically to one effector but not to the others. V12S35 binds to Raf and not to RalGDS or PI3K, whereas Ras V12C40 binds only to PI3K, and V12G37 binds only to RalGDS. Thus, the Ras effector mutants, in combination with constitutively active or dominant negative mutations of downstream molecules, are invaluable tools for determining the specific contribution of each pathway in transformation. Activation of at least two of these functions is thought to be required for neoplastic transformation to occur.
The transcription factor TTF-1 (also named Nkx-2.1 and T/EBP) belongs to the Nkx-2 class of homeodomain-containing proteins that function as regulators of regional specification, cell fate determination, and organ morphogenesis during embryonic development (9, 21). Its expression has been detected in the thyroid follicular cells, in restricted areas of the developing brain, and in the lung bronchial epithelium (35). TTF-1-null mice die at birth, lack the lung parenchyma and the thyroid and pituitary glands, and display severe defects in the ventral area of the forebrain (31). Besides its crucial role in development, TTF-1 is thought to regulate tissue-specific transcription in the adult thyroid gland and lung. The expression of thyroid-specific genes, such as those for thyroglobulin, thyroperoxidase (TPO), thyroid-stimulating hormone receptor, and Na-iodide symporter (NIS), is positively controlled by TTF-1 (8, 9, 15, 17).
Several reports on the high frequency of Ras mutations in both benign and malignant follicular neoplasia suggest that Ras activation is an early event in thyroid tumorigenesis (16, 27, 54). Previous studies have shown that oncogenic Ras causes potent tumorigenic transformation of thyroid follicular cells, resulting in growth factor-independent proliferation, morphological transformation, anchorage-independent growth, and tumor formation when cells are injected into nude mice (10, 18, 19). Interestingly, in Ras-transformed cells, thyroid-specific gene expression is suppressed (10, 18, 19, 33). Loss of the differentiated phenotype is a common feature of malignant transformation. While Ras downstream pathways leading to cell proliferation and morphological transformation have been extensively studied, very little is known about the signaling pathways involved in Ras-mediated suppression of differentiation-specific gene expression. TTF-1 transcriptional activity is suppressed by H-Ras with a mechanism that has not been well understood, since its expression and its binding to DNA are not significantly altered (18). Overexpression of TTF-1 in Ras-transformed thyroid cells is not sufficient to override the inhibitory effect.
In this study we have used Ras effector mutants, in combination with constitutively active signaling molecules, as tools to investigate the signal transduction pathways involved in inactivation of the transcription factor TTF-1. We find that activation of the Raf/MEK/ERK cascade inhibits TTF-1 transcriptional activity. Inhibition may occur through direct TTF-1 phosphorylation by ERK, since TTF-1 is an ERK substrate in vitro and in vivo. Activation of other well-characterized signaling molecules, such as PI3K, RalGDS, Rac, and Rho, has no effect on TTF-1 function. Interestingly, the V12N38 Ras mutant, which is unable to induce ERK activation, acts in concert with Raf to repress TTF-1 transcriptional activity.
MATERIALS AND METHODS
Cell culture and transfection assay.
Rat thyroid follicular FRTL-5 cells were maintained in Coon's modified Ham's F-12 medium (Sigma) supplemented with 5% calf serum (Gibco-BRL) and six growth factors, including thyroid stimulating hormone (1 mU/ml) and insulin (10 μg/ml), as previously described (2, 63). Human embryonic kidney 293 cells were grown in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% calf serum.
Transient transfections in FRTL-5 cells were carried out by the calcium phosphate-DNA precipitation method as previously described (42). Briefly, FRTL-5 cells were plated at a density of 5 × 105 per 60-mm dish, and 48 h later, C5E1b-CAT, TPO-Luc, or NIS-Luc (2.5 μg) or serum-responsive element (SRE)-Luc (6 μg) reporter plasmids were transfected with the different expression vectors as indicated in the figure legends. After 72 h, cell extracts were lysed in lysis buffer (10 mM HEPES [pH 7.9], 400 mM NaCl, 0.1 mM EGTA, 0.5 mM dithiothreitol, 5% glycerol, and 0.5 mM phenylmethylsulfonyl fluoride (PMSF). Luciferase and chloramphenicol acetyltransferase (CAT) activities were measured as described before (5, 42). Briefly, CAT activity was measured by incubation with 5 mM chloramphenicol and 0.1 μCi of [3H]acetyl-coenzyme A (1.4 Ci/mmol; 50 μCi/ml). Reactions were performed in the presence of water-insoluble scintillation fluid (Econofluor-2; Packard Bioscience) at 37°C and counted after 5 h. Luciferase activity was measured in the presence of 0.2 mM d-luciferin (Sigma) in a Lumat LB 9501 luminometer (Berthold). Treatment with kinase inhibitors was performed 24 h after transfection and repeated after 24 h. Drugs were obtained from Calbiochem (staurosporine, bisindolylmaleimide, chelerythrine, KT5720, olomoucine, KN93, rapamycin, tyrphostin, SB203580, and LY294002), Sigma (wortmannin), Promega (U0126), and New England Biolabs (PD098059).
293 cells were transiently transfected with the Lipofectamine reagent (Gibco-BRL) following the manufacturer's instructions. Cells were collected 48 h after transfection unless otherwise indicated in the figure legends.
Expression constructs.
TPO-Luc, a luciferase reporter gene controlled by a 400-bp TPO promoter, has been described previously (17). NIS-Luc is a pGL3 vector containing the luciferase reporter gene under the control of the NIS promoter (−2711 to −1 from the ATG) (44). C5-CAT is an artificial reporter construct in which the cat gene is under the control of an artificial promoter containing five binding sites for the transcription factor TTF-1 (42). The pSRE-Luc reporter construct, containing the SRE, was purchased from Stratagene. RcCMV-TTF-1, ΔS80, ΔS61, ΔS1-24, and ΔS64, encoding wild-type TTF-1, and TTF-1 recombinants containing serine-to-alanine mutations have been described previously (63). A PCR-based method using the QuikChange site-directed mutagenesis kit (Stratagene) was performed to generate serine-to-alanine substitutions in positions 4, 18, 328, and 337 of the wild-type TTF-1 protein. The triple mutant S18ΔS64 was obtained by restriction digestion of RcCMV TTF-1 with BstXI and SmaI. The small insert containing the ΔS64 carboxy terminus was then ligated to the vector containing the S18A amino-terminal region of TTF-1.
The pcDNA3 V12 Ras, V12S35, V12C40, and V12G37 constructs were kindly provided by Julian Downward (50). Plasmids containing V14 Rho and V12 Rac were obtained from Alan Hall (48). An expression vector containing an internal deletion in the Raf cDNA (from amino acids 26 to 302) was provided by John Kyriakis (Raf-BXB). All these cDNAs were cloned blunt in the BamHI site in the pCMV vector to ensure equal expression levels (3). The Ras mutants N26G, Y32V, E37N, and D38N were obtained with the QuikChange site-directed mutagenesis kit (Stratagene) using pCMV-V12 Ras as a template.
Immunoblotting.
Whole-cell lysates of transiently transfected 293 cells were prepared in sample buffer and normalized for equal protein concentration by the Bradford assay (Bio-Rad). A 30-μg amount of protein samples was resolved either by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (SDS–10% PAGE) or on a precast SDS-PAGE gel containing 4 to 15% polyacrylamide (Bio-Rad) and transferred on a polyvinylidene difluoride (PVDF) membrane (Millipore). Nonspecific binding sites were blocked by incubation with 5% nonfat dry milk in phosphate-buffered saline–0.2% Tween 20. Rabbit polyclonal antibodies against TTF-1 were previously obtained in our laboratory and used at approximately 1 μg/ml (18). Anti-H-Ras monoclonal antibodies (F-235) were purchased from Santa Cruz Biotechnology. Anti-phospho-ERK antibodies, recognizing specifically phosphorylated ERKs, were purchased from Santa Cruz Biotechnology. Immune complexes were detected by enhanced chemiluminescence as instructed by the manufacturer (Amersham International).
Protein kinase assay.
For the myelin basic protein (MBP) kinase assay, 293 cells were incubated after transfection for 24 h in DMEM–10% calf serum and then switched to DMEM containing 0.5% calf serum for the last 18 h. Cells were then lysed in ERK lysis buffer (10 mM Tris [pH 7.4], 150 mM NaCl, 1% Triton, 1 mM EDTA, 10% glycerol, 0.5 mM PMSF, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml, 1 mM sodium vanadate, 50 mM NaF, 25 mM β-glycerophosphate). Protein concentration was determined as described above, and 200 μg of total cell extracts was immunoprecipitated with monoclonal 12CA5 antibodies (3.5 μg/200 μg; Boehringer Mannheim) recognizing hemagglutinin (HA)-tagged ERK2. After 2 h, immunocomplexes were recovered on protein A-Sepharose beads (Pharmacia) and washed three times in lysis buffer and twice in kinase buffer (50 mM HEPES-Na, [pH 7.4], 10 mM MgCl2, 5 mM MnCl2). The immunocomplexes were incubated for 20 min at 30°C in kinase buffer containing 7 mM dithiothreitol, 40 μM ATP, and 0.2 μl of [γ-32P]ATP (3,000 Ci/mmol) in the presence of 10 μg of MBP. Samples were subjected to SDS–15% PAGE and autoradiography.
To test for TTF-1 phosphorylation in vitro, 293 cells were transfected with the various RcCMV–TTF-1 mutants, incubated for 24 h in DMEM–10% calf serum, and then switched to DMEM containing 0.5% calf serum for the last 18 h. Cells were then lysed in radioimmunoprecipitation (RIPA) buffer (10 mM Tris [pH 7.4], 150 mM NaCl, 1% NP-40, 0.1% SDS, 0.5% deoxycholate, 0.5 mM PMSF, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml, 1 mM sodium anadate, 50 mM NaF, 25 mM β-glycerophosphate), and immunoprecipitations were carried out with anti-TTF-1 polyclonal antibodies (3.5 μg/200 μg of cell extracts). Immunocomplexes were recovered with protein A-Sepharose, washed three times with RIPA buffer and twice with kinase buffer, and subsequently incubated with mouse recombinant ERK2 (50 U; New England Biolabs) for 20 min at 30°C in the presence of [γ-32P]ATP, as described above. The reaction was stopped by addition of sample buffer supplemented with 5% β-mercaptoethanol. TTF-1 was resolved by SDS–10% PAGE. The gel was fixed and stained with Coomassie blue and subjected to autoradiography.
In vivo labeling experiment.
293 cells were transfected with RcCMV–TTF-1 constructs in the presence or absence of CMV-V12 Ras or CMV-Raf-BXB. The CMV-βGAL plasmid was used as a control. After 24 h, cells were starved for 18 h in 0.2% calf serum unless otherwise indicated in the figure legends. Cells were treated for 4 h with U0126 (50 μM) where indicated and incubated for 30 min in DMEM lacking phosphate and serum (Gibco-BRL). 32Pi (200 μCi/700 μl) was added to the cells for either 45 min or 2 or 4 h, depending on the experiment. When indicated, cells were treated with phorbol 12-myristate 13-acetate (PMA) (100 nM; Sigma). Cell lysates were obtained in RIPA buffer, and TTF-1 was immunoprecipitated with anti-TTF-1 polyclonal antibodies as described above. Immunocomplexes were recovered with protein A-Sepharose beads and washed in RIPA buffer five times. Samples were run on SDS–10% PAGE and either stained with Coomassie blue and subjected to autoradiography or blotted on a PVDF membrane. After transfer, the membrane was air-dried and subjected to autoradiography with BioMax MS films (Kodak). The membrane was subsequently wet in methanol, and immunoblotting was performed as described above.
RESULTS
Transient expression of V12 Ras represses the activity of thyroid-specific promoters.
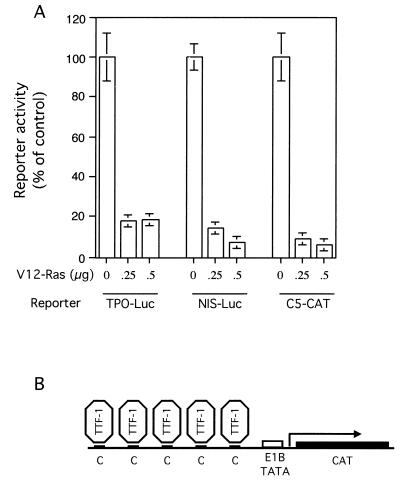
Previous studies on Ras-mediated transformation and loss of differentiation in thyroid cells have been performed with long-term cultures of stably transfected or virally infected cell lines. In the present report, we initially set up a transient-transfection assay to test Ras activity on thyroid-specific transcription under acute conditions. The rat thyroid cell line FRTL-5 was transiently transfected with thyroid-specific promoters driving the expression of the luciferase (Luc) or CAT reporter gene and with a plasmid expressing an oncogenic form of H-Ras (V12 Ras). At 72 h after transfection, the cells were lysed, and the luciferase or CAT activity was measured. As shown in Fig. 1A, expression of V12 Ras resulted in 80 to 90% inhibition of the TPO and NIS promoters. Both TPO and NIS promoters present binding sites for the homeodomain-containing protein TTF-1 but are also regulated by other transcription factors (17, 42, 44). To examine the molecular mechanisms underlying Ras-mediated inhibition of TTF-1 transcriptional activity, a C5-CAT reporter gene was used, containing an artificial promoter carrying five binding sites for TTF-1 placed in tandem upstream of the E1B TATA box (Fig. 1B). This promoter displays strong activity in FRTL-5 cells, and it is activated specifically by TTF-1 in nonthyroid cell lines (42). In transient transfections, the transcription of C5-CAT was almost completely suppressed by the expression of V12 Ras (Fig. 1A). Importantly, this effect was not due to a general inhibition of transcription in FRTL-5 cells, since V12 Ras had no negative effects on several control reporter genes (data not shown). Thus, inhibition of TTF-1 transcriptional activity mediated by activated Ras can be efficiently measured in the transient-transfection assay.
FIG. 1.
Transient expression of V12 Ras inhibits the activity of thyroid-specific promoters. (A) FRTL-5 cells were transiently transfected with 2.5 μg of TPO-Luc, NIS-Luc, or C5-CAT and pCMV encoding V12 Ras at various amounts (0, 0.25, and 0.5 μg). The amount of total DNA was held constant by including the pCMV-βGAL expression vector. After 72 h, cell extracts were prepared, normalized for protein content, and assayed for luciferase (Luc) or CAT activity as described in Materials and Methods. Reporter activity was expressed as a percentage of reporter activity in the absence of V12 Ras. Error bars indicate the standard errors of the means of four independent experiments. (B) Structure of the C5-CAT reporter gene. The artificial promoter, driving the CAT gene, was constructed by inserting five TTF-1 binding sites in tandem in front of the E1B TATA minimal region.
Constitutive activation of the Raf/MEK/ERK cascade results in inhibition of TTF-1 transcriptional activity.
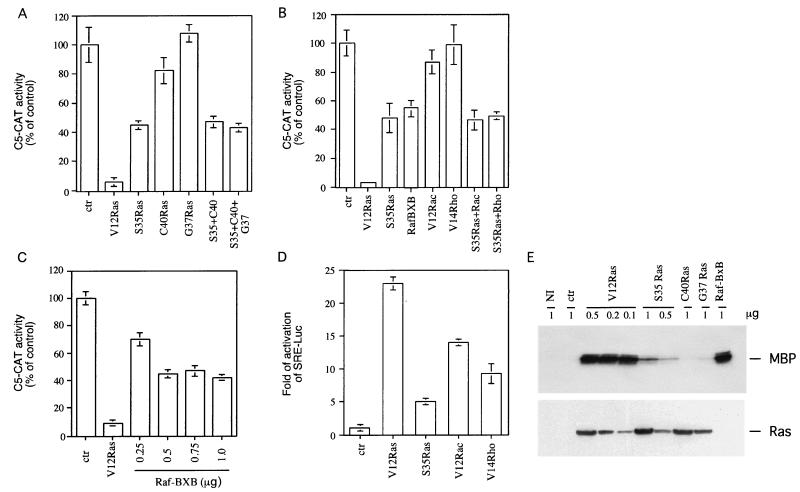
The ras oncogene is responsible for the activation of several downstream effectors, of which Raf, PI3K, and RalGDS are the best characterized. Using V12 Ras mutants activating specifically only one of these effectors, we have explored their involvement in the suppression of TTF-1 transcriptional activity. As shown in Fig. 2A, cells cotransfected with C5-CAT and V12G37 Ras, an effector domain mutant that interacts with RalGDS but not Raf or PI3K, exhibited no reduction in TTF-1 activity. V12C40 Ras, which activates specifically PI3K and Rac, could only weakly inhibit TTF-1 activity (20 to 25% inhibition). In contrast, specific activation of Raf induced by V12S35 Ras significantly altered TTF-1 activity (50 to 55% inhibition), although it could not fully reproduce V12 Ras inhibition. Interestingly, concomitant expression of V12S35 and V12C40 or of the three mutants resulted in the same effect obtained with the V12S35 mutant alone (Fig. 2A).
FIG. 2.
V12S35 Ras and Raf-BXB inhibit TTF-1 transcriptional activity. (A) FRTL-5 cells were transiently transfected with 2.5 μg of C5-CAT and with pCMV encoding V12 Ras (0.2 μg) or the various Ras effector mutants (1 μg) alone or in combination. The amount of Ras-expressing vectors was selected for equal protein expression in 293 cells (see panel E). Preliminary experiments were performed to ensure that maximal activity on the C5-CAT reporter gene was seen for each mutant. (B) FRTL-5 cells were transiently transfected with 2.5 μg of C5-CAT, CMV-βGAL as the control (ctr) (1 μg), V12 Ras (0.2 μg), V12S35 Ras (1 μg), or constitutively active forms of Raf (Raf-BXB; 1 μg), Rac (V12 Rac; 1 μg), and Rho (V14 Rho; 1 μg), either alone or in combination. (C) FRTL-5 cells were transiently transfected with 2.5 μg of C5-CAT, CMV-βGAL as the control (ctr), V12 Ras (0.2 μg), or various concentrations of V12S35 Ras (0.25, 0.5, 0.75, and 1 μg). The amount of total DNA was held constant by including pCMV-βGAL expression vector. These experiments were performed as described in the legend to Fig. 1. For panels A, B, and C, reporter activities were expressed as a percentage of the untreated controls, and error bars indicate the standard errors of the means of four independent experiments. (D) FRTL-5 cells were transiently transfected with 6 μg of SRE-Luc, CMV-βGAL (control [ctr]), and pCMV encoding V12 Ras (0.2 μg), V12S35 Ras (1 μg), V12 Rac (1 μg), and V14 Rho (1 μg). The reporter activity was expressed as fold activation over the untreated control (SRE-Luc transfected with CMV-βGAL). Error bars indicate the standard errors of the means of four independent experiments. (E) 293 cells were transiently transfected with pcDNA3-ERK2 (2 μg) in the presence of various amounts of pCMV vector encoding V12 Ras (0.1, 0.2, or 0.5 μg), V12S35 Ras (0.5 and 1 μg), V12C40 (1 μg), V12G37 (1 μg), Raf-BXB (1 μg), or βGAL as a control (ctr). After 48 h, cell extracts were prepared, and HA-ERK2 was immunoprecipitated with anti-HA epitope-specific antibodies, as described in Materials and Methods. The nonimmune (NI) sample was immunoprecipitated with unrelated control antibodies. Immunoprecipitated ERK2 was then subjected to an in vitro kinase assay with MBP as the substrate. Half of the samples were then run on SDS–4 to 15% PAGE and subjected to autoradiography (upper panel). The lower panel represents an immunoblot with the other half of the samples probed with anti-HA Ras antibody.
These data suggest that the Raf pathway mediates, at least in part, the Ras effects on TTF-1-dependent transcription. However, V12S35 Ras could not fully reproduce the extent of inhibition obtained with V12 Ras. It has been reported that V12S35 Ras is less potent than V12 Ras at inducing Raf activation (50). Upon transient transfection in 293 cells, the ability of the Ras mutants to activate the ERK pathway was tested in an in vitro kinase assay using MBP as the substrate. As shown in Fig. 2E, transient expression of V12S35 Ras only weakly activated ERK2 compared with V12 Ras, even when expressed at higher concentrations. As expected, V12C40 Ras and V12G37 Ras could not activate ERK to any significant extent. It is conceivable that V12S35 Ras is unable to fully repress TTF-1 activity due to its lower ERK activation. To test this hypothesis, we used a constitutive form of Raf (Raf-BXB), capable of strong ERK activation, similar to that obtained with V12 Ras (Fig. 2E). Raf-BXB inhibited C5-CAT transcription to the same extent as V12S35 Ras (Fig. 2B). A dose-response curve revealed that maximum inhibition could be achieved at relatively low levels of Raf-BXB (0.5 μg), and higher DNA concentrations did not result in further inhibition (Fig. 2C). These data suggest that inhibition of TTF-1 activity by the Raf/MAP kinase pathway reaches a maximum at a relatively low level of MAP kinase activation and that full V12 Ras suppression of TTF-1 activity requires the recruitment of additional signaling pathways.
Among the Ras downstream signaling molecules, the small GTPases Rac and Rho have been shown to cooperate with V12S35 Ras to induce transformation in fibroblasts (29, 45, 46, 50). In thyroid cells, stable expression of a constitutive active form of Rac (V12 Rac) results in induction of JNK activity without affecting thyroid cell differentiation (10). To test whether these molecules would alter TTF-1 transcriptional activity, V12 Rac or constitutively active Rho (V14 Rho) was tested alone and in combination with V12S35 Ras. As shown in Fig. 2B, expression of V12 Rac or V14 Rho had no significant effect on TTF-1 activity and could not cooperate with the V12S35 Ras mutant to further inhibit TTF-1. As control, V12 Rac and V14 Rho expression vectors were cotransfected with an SRE reporter to test whether Rac and Rho downstream pathways were indeed activated in thyroid cells under these conditions. The induction of the SRE reporter elicited by activated Rac and Rho was comparable to that in other cell types and to that elicited by V12 Ras (Fig. 2D).
Taken together, these data indicate that Raf activation plays a significant role in suppression of TTF-1 transcriptional activity, while activation of other well-characterized signaling molecules downstream of Ras is not involved in this process.
Staurosporine completely restores C5 promoter activity in the presence of V12 Ras.
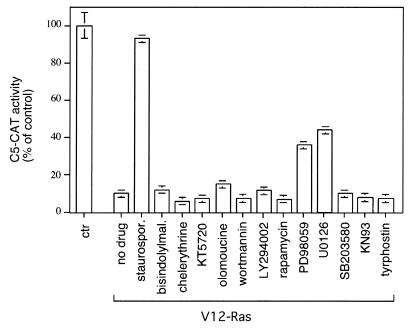
Oncogenic Ras induces the activation of several downstream kinases. To determine the contribution of some known kinases to TTF-1 repression, C5-CAT activity was measured in the presence and absence of Ras upon treatment with an array of potent kinase inhibitors. As shown in Fig. 3, staurosporine, a nonselective kinase inhibitor, could completely suppress the Ras effect on C5-CAT, restoring full promoter activity. In contrast, PI3K inhibitors such as wortmannin and LY294002 had no effect on the C5-CAT reporter gene in the presence or absence of Ras, suggesting that PI3K is not involved in Ras-mediated inhibition of TTF-1. Similarly, the S6 kinase and p38 MAP kinase inhibitors could not prevent Ras repression of TTF-1 activity. Ras inhibitory function was also unaffected by saturating doses of selective inhibitors of protein kinase C, protein kinase A, cyclin-dependent kinase, and tyrosine kinases (Fig. 3). Interestingly, the MEK-specific inhibitors PD98059 and U0126 partially restored C5-CAT activity in the presence of Ras, in agreement with an involvement of ERK activation in this process (Fig. 3).
FIG. 3.
V12 Ras represses TTF-1 transcriptional activity with a kinase-dependent mechanism. FRTL-5 cells were transiently transfected with C5-CAT (2.5 μg) and with pCMV-V12 Ras (0.2 μg) or pCMV-βGAL as a control (ctr). After 24 h, cells were treated with a broad-spectrum kinase inhibitor (staurosporine, 100 nM), protein kinase C inhibitors (bisindolylmaleimide [500 nM] and chelerythrine [10 μM]), a protein kinase A inhibitor (KT5720, 2 μM), a cyclin-dependent kinase inhibitor (olomoucine, 200 μM), PI3K inhibitors (wortmannin [200 nM] and LY294002 [50 μM]), an S60 kinase inhibitor (rapamycin, 50 nM), MEK inhibitors (PD098059 [75 μM] and U0126 [50 μM]), a p38 MAP kinase inhibitor (SB203580, 10 μM), a calmodulin-dependent kinase inhibitor (KN93, 5 μM), or a tyrosine kinase inhibitor (tyrphostin, 50 μM). Treatment was repeated after 24 h. Cells were lysed after 72 h, and CAT activity was measured as described in the legend to Fig. 1. The amount of each drug was tested in preliminary experiments, and the maximum concentration that had minimal effects on cell viability was used. C5-CAT activity was calculated for each point by assuming that the activity of the drug-treated control in the absence of V12 Ras was 100%. Error bars indicate the standard errors of the means of four independent experiments.
Thus, the mechanisms involved in Ras suppression of TTF-1 transcriptional activity require activation of the Raf/MEK/ERK pathway, while other well-characterized kinases are unlikely to be involved in this process. Activation of as-yet-unidentified kinases is required for full inhibition of TTF-1 activity, as suggested by the results of treatment with staurosporine.
Phosphorylation of TTF-1 by ERK in vitro and its phosphorylation in vivo.
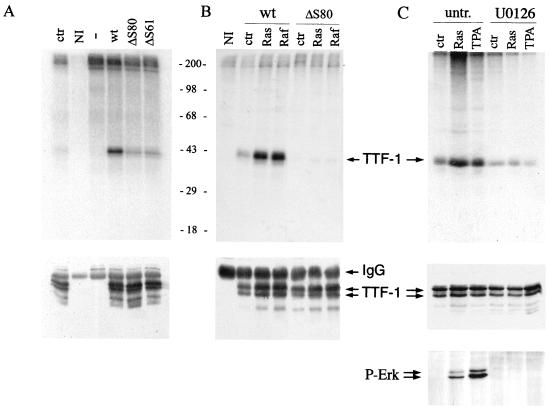
Phosphorylation of transcription factors by MAP kinases is a key event in regulation of many cellular responses. Since TTF-1 contains several minimal consensus sequences (S/TP) for ERK phosphorylation (53, 63), we investigated the possibility that TTF-1 may be a direct substrate. 293 cells were transiently transfected with plasmids expressing various TTF-1 mutants. After 48 h, TTF-1 was isolated by immunoprecipitation, and the immunocomplex was incubated in the presence and absence of bacterially produced activated ERK2 and [γ-32P]ATP. As shown in Fig. 4A (upper panel), wild-type TTF-1 was readily phosphorylated by ERK2, while no phosphorylation could be detected in the absence of purified ERK (control). Similar results were obtained when 293 cells were transfected with HA-ERK2 and with an expression vector for Raf-BXB, followed by ERK-2 immunoprecipitation and an in vitro kinase assay with TTF-1 as a substrate (data not shown).
FIG. 4.
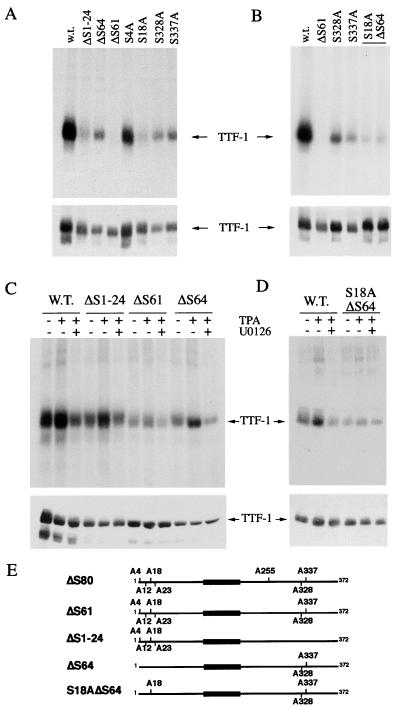
TTF-1 is a direct substrate of ERK2. (A) In vitro kinase assay. 293 cells were transiently transfected with RcCMV encoding wild-type TTF-1 (wt), a mutant carrying seven serine-to-alanine substitutions (ΔS80), a mutant carrying six serine-to-alanine substitutions (ΔS61), or βGAL as a negative control (—). After 48 h, cell lysates were prepared and TTF-1 was immunoprecipitated with anti-TTF-1 polyclonal antibodies or with control antibodies (NI). Immunoprecipitated TTF-1 was then subjected to an in vitro kinase assay by addition of bacterially produced activated ERK2, as described in Materials and Methods. To ensure that the kinase activity seen on TTF-1 was due to ERK and not to an immunoprecipitated kinase, a sample was incubated under the same conditions in the absence of ERK2 as a control (ctr). Samples were then run on SDS–10% PAGE and subjected to autoradiography. The migration of molecular mass standards is indicated (in kilodaltons). Samples were also run on SDS–4 to 15% PAGE gradient gels and subjected to Western blotting with anti-TTF-1 antibodies (lower panel). (B) In vivo labeling experiment. 293 cells were transfected with RcCMV encoding wild-type (wt) or mutant (ΔS80) TTF-1, pCMV encoding V12 Ras (Ras), Raf-BXB (Raf), or βGAL as a control (ctr). After 24 h, cells were metabolically labeled with 32Pi for 4 h and then lysed, as described in Materials and Methods. Cell lysates were subjected to immunoprecipitation with antibodies to TTF-1, run on SDS–10% PAGE, and subjected to autoradiography (upper panel). The migration of molecular mass standards is indicated (in kilodaltons). Samples were run on an SDS–4 to 15% PAGE gradient gel and subjected to immunoblotting with anti-TTF-1 antibodies (lower panel). (C) In vivo labeling experiment. 293 cells were transfected with RcCMV encoding wild-type TTF-1 in the presence or absence of V12 Ras (Ras) or with βGAL as a control (ctr). After 24 h, cells were incubated with DMEM–0.2% calf serum for 18 h and then left untreated (untr.) or treated with U0126 (50 μM) for 4 h. Subsequently, cells were incubated for 45 min with 32Pi in the presence or absence of the phorbol ester PMA (100 nM). Cell lysates were subjected to immunoprecipitation with antibodies to TTF-1, run on SDS–10% PAGE, and subjected to autoradiography (upper panel). As a control, total cell extracts were run on an SDS–4 to 15% PAGE gradient gel and subjected to Western blotting with anti-TTF-1 antibodies (middle panel). In the lower panel, immunoblotting was performed with anti-phospho-specific-ERK antibodies, showing that the expression of activated Ras and PMA treatment induced ERK phosphorylation to a similar extent. Treatment with U0126 completely suppressed ERK phosphorylation. Arrows indicate TTF-1 (∼42 kDa) and the immunoglobulin G heavy chains (IgG). The TTF-1 double pattern in the immunoblot is not due to phosphorylation of the sites taken into consideration in this study, since it is present in the wild type as well as in the ΔS80 mutant. However, TTF-1 phosphorylation occurs predominantly in the upper band, as shown by autoradiography and Western blotting of the same filter.
Previous studies have shown that seven serine residues are the main sites of phosphorylation within the TTF-1 protein (63), as mutation of these serine residues to alanine (ΔS80 mutant [Fig. 5E]) results in suppression of TTF-1 phosphorylation. Indeed, the ΔS80 mutant was almost completely unphosphorylated in the presence of active ERK, suggesting that most of the phosphorylation occurred in those sites (Fig. 4A, upper panel). Similarly, the mutant ΔS61, which is mutated in six of the seven serine residues (Fig. 5E), displayed barely detectable ERK phosphorylation (Fig. 4A, upper panel). Western blotting analysis of the immunocomplexes revealed that equal amounts of TTF-1 protein were assayed in this experiment (Fig. 4A, lower panel).
FIG. 5.
ERK-mediated phosphorylation of the TTF-1 protein occurs at serine residues 18, 328, and 337. (A and B) In vitro kinase assays. 293 cells were transiently transfected with RcCMV encoding wild-type TTF-1 (wt) and various mutants containing serine-to-alanine substitutions (see panel E for details on the specific mutants). After 48 h, cell lysates were prepared and TTF-1 was immunoprecipitated with anti-TTF-1 polyclonal antibodies. Immunoprecipitated TTF-1 was then subjected to an in vitro kinase assay using bacterially produced ERK2 as the active kinase (see Materials and Methods). Samples were then run on a long SDS–10% PAGE gel and then blotted on a PVDF membrane as described in Materials and Methods. After transfer, the membrane was subjected to autoradiography and then used to perform immunoblotting with anti-TTF-1 antibodies (lower panel). (C and D) In vivo labeling experiments. 293 cells were transfected with RcCMV encoding wild-type (wt) or mutant TTF-1 as indicated. After 24 h, cells were incubated with DMEM supplemented with 0.2% calf serum for 18 h and treated (+) with U0126 (50 μM) for 4 h or left untreated (−). Subsequently, cells were incubated for 45 min with 32Pi in the presence (+) or in the absence (−) of the phorbol ester PMA (100 nM) and then lysed as described in Materials and Methods. TTF-1 was immunoprecipitated with anti-TTF-1 polyclonal antibodies. Samples were then run on a long SDS–10% PAGE gel and blotted on a PVDF membrane as described in Materials and Methods. After transfer, the membrane was subjected to autoradiography and then used to perform immunoblotting with anti-TTF-1 antibodies (lower panel). (E) Schematic diagram of the TTF-1 serine-to-alanine mutants. The ΔS80 mutant encodes a full-length TTF-1 protein containing Ser-to-Ala substitutions at residues 4, 12, 18, 23, 255, 328, and 337. The ΔS61 mutant encodes a full-length TTF-1 protein containing Ser-to-Ala substitutions at residues 4, 12, 18, 23, 328, and 337. The ΔS1-24 mutant encodes a full-length TTF-1 protein containing Ser-to-Ala substitutions only at the N terminus at residues 4, 12, 18, and 23. The ΔS64 mutant encodes a full-length TTF-1 protein containing only the two carboxy-terminal Ser-to-Ala substitutions at residues 328 and 337. Finally, the S18AΔS64 mutant encodes a full-length TTF-1 protein containing Ser-to-Ala substitutions at residues 18, 328, and 337. Single-amino-acid substitutions such as S4A (Ser-to-Ala substitution at residue 4), S18A, S328A, and S337A were also tested (panels A and B).
To assess whether TTF-1 phosphorylation could be specifically induced by activation of the Raf/MEK/ERK pathway in vivo, 293 cells were transiently transfected with expression plasmids carrying wild-type TTF-1 or the ΔS80 TTF-1 mutant, alone and in the presence of expression vectors for V12 Ras and Raf-BXB. After 24 h, cells were metabolically labeled with 32Pi for 4 h and then lysed. TTF-1 was immunoprecipitated, and samples were resolved by SDS-PAGE and then subjected to autoradiography. Importantly, phosphorylation of wild-type TTF-1 was strongly induced by V12 Ras and Raf-BXB in vivo, while no detectable phosphorylation could be seen with the ΔS80 mutant (Fig. 4B, upper panel). Western blotting analysis of the immunocomplexes revealed that TTF-1 expression was slightly induced by the presence of V12 Ras and Raf-BXB (Fig. 4B, lower panel), even though this effect could not account for the strong induction of TTF-1 phosphorylation in these samples.
We then asked whether TTF-1 could be phosphorylated in vivo by exogenous stimuli known to activate the ERK pathway, such as the phorbol ester PMA. PMA treatment for 45 min activated the ERK pathway at least as strongly as the exogenous expression of V12 Ras, as judged from Western blotting analysis with anti-phospho-ERK antibodies (Fig. 4C, lower panel). Accordingly, TTF-1 phosphorylation was induced in the presence of PMA to a similar extent as in V12 Ras-expressing cells (Fig. 4C, upper panel). Furthermore, TTF-1 phosphorylation induced by V12 Ras and PMA was completely suppressed in the presence of the MEK-specific inhibitor U0126, demonstrating that their ability to induce TTF-1 phosphorylation was dependent on ERK activation. Immunoblotting analysis of total cell extracts revealed that equal amounts of TTF-1 protein were assayed in this experiment (Fig. 4C, middle panel).
To determine which serine residues of the six mutated in ΔS61TTF-1 are phosphorylated by ERK, we took advantage of the previously described mutants ΔS1-24 and ΔS64. As shown in Fig. 5E, ΔS1-24 carries four serine-to-alanine substitutions at the N terminus, while ΔS64 carries two serine-to-alanine conversions at the C terminus. In an in vitro kinase assay with recombinant ERK as the active kinase, both the ΔS1-24 and S64 mutants showed significant impairment in the ability to function as an ERK substrate, although to different extents (Fig. 5A), suggesting that the N-terminal and the C-terminal sites are both important for ERK phosphorylation. To determine how each single serine contributes to ERK phosphorylation, we generated single point mutations of serine residues 4, 18, 328, and 337, which are ERK phosphorylation sites (SP consensus). Whereas the S4A mutant was phosphorylated to a similar extent as wild-type TTF-1, mutant S18A almost completely lost the ability to be phosphorylated by recombinant ERK. Mutants S328A and S337A were also phosphorylated at significantly lower levels, indicating that S18, S328, and S337 are likely to be ERK targets (Fig. 5A). Indeed, a TTF-1 protein in which all three of the putative ERK sites were converted to alanines (S18AΔS64) was no longer detectably phosphorylated by ERK (Fig. 5B). The relative amount of TTF-1 protein in the immunoprecipitates was monitored by immunoblotting (Fig. 5A and B, lower panels).
To determine whether ERK phosphorylates TTF-1 at S18, S328, and S337 within cells, we tested the mutants described above in an in vivo labeling experiment. 293 cells were transfected with the various TTF-1 mutants, cultured for 24 h in the presence of 10% calf serum, and starved in 0.2% calf serum for 18 h. Subsequently, cells were left untreated or were treated with the MEK inhibitor U0126 for 4 h and then stimulated with PMA for 45 min in the presence of 32Pi. Phosphorylation of mutants ΔS1-24 and ΔS64 was significantly induced by PMA in an ERK-dependent manner (Fig. 5C). In contrast, phosphorylation of the S18A/ΔS64 TTF-1 mutant was no longer induced by PMA, and its residual phosphorylation could not be inhibited by U0126 (Fig. 5D). Equal amounts of TTF-1 were monitored by immunoblotting (Fig. 5C and D).
Taken together, these data demonstrate that TTF-1 can be directly phosphorylated in vitro and in vivo by ERK at residues S18, S328, and S337. Thus, TTF-1 is likely to belong to the family of transcription factors that are directly phosphorylated by ERK, although the functional significance of ERK-mediated phosphorylation could not be directly tested (see Discussion).
V12N38 Ras affects TTF-1 transcriptional activity without inducing ERK activation.
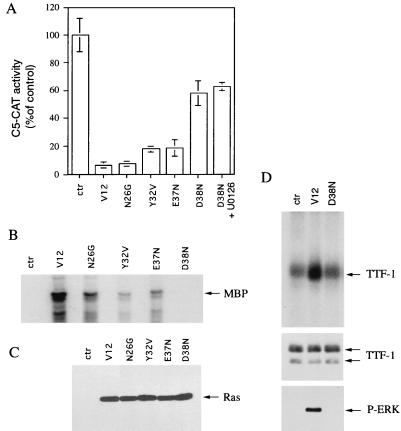
To further investigate the mechanisms through which Ras represses TTF-1 function, we tested other V12 Ras mutants, each carrying a different amino acid substitution in the effector domain (1, 52, 60, 65). We reasoned that if other signaling pathways in addition to the Raf/MEK/ERK cascade are involved in repression of TTF-1 activity, it should be possible to identify mutants unable to activate ERK but still able to interfere with C5-CAT activity. FRTL-5 cells were transiently transfected with the various Ras mutants and with C5-CAT, and their ability to inhibit expression of the reporter gene was measured (Fig. 6A). Each mutant was also transfected into 293 cells together with a plasmid encoding an epitope-tagged version of MAP kinase (HA-ERK2), to measure their ability to activate ERK (Fig. 6B). Transiently expressed HA-ERK2 was isolated by immunoprecipitation, and its activity was measured in an immunocomplex kinase assay with MBP as the substrate. Mutants N26G, Y32V, and E37N displayed readily detectable ERK2 activation and efficient C5-CAT inhibition. In contrast, the mutant D38N did not show any detectable MAP kinase activation, even after long gel exposure (Fig. 6B and data not shown), but was still able to inhibit C5-CAT transcription (Fig. 6A). Interestingly, the partial inhibition obtained with the D38N mutant was not affected by treatment with the MEK inhibitor U0126, further suggesting that V12N38 activity is not elicited through the ERK pathway (Fig. 6A). Similar expression of the V12 Ras mutants was demonstrated by immunoblotting in transfected 293 cells (Fig. 6C). To test whether V12N38 Ras (D38N) inhibition was associated with TTF-1 phosphorylation, 293 cells were cotransfected with wild-type TTF-1 in the presence of the V12 Ras and V12N38 Ras mutants, and an in vivo labeling experiment was performed as described above. In contrast to V12 Ras, V12N38 expression was unable to induce TTF-1 phosphorylation, suggesting that its inhibition occurs through a phosphorylation-independent mechanism (Fig. 6D).
FIG. 6.
V12N38 Ras mutant inhibits TTF-1 function without inducing MAP kinase activation. (A) FRTL-5 cells were transfected with C5-CAT and pCMV expressing V12 Ras (0.2 μg), V12N26G Ras (1 μg), V12Y32V Ras (1 μg), V12E37N Ras (1 μg), V12D38N Ras (1 μg), or βGAL as a control (ctr). In the case of V12D38N Ras, samples were either untreated (D38N) or treated with 50 μM U0126 for 48 h before the end of the experiment (D38N+U0126). The CAT assay was performed as described in the legend to Fig. 1. C5-CAT activity was expressed as a percentage of reporter activity in the absence of Ras. Error bars indicate the standard errors of the means of four independent experiments. (B) The Ras mutant ability to activate MAP kinase was evaluated by transient transfection in 293 cells in the presence of pCMV HA-ERK2. Lysates were immunoprecipitated with anti-Ha antibodies, and ERK activity was measured by an in vitro kinase assay with MBP as the substrate (see Materials and Methods). This experiment was repeated twice with similar results. (C) The 293 total cell lysates used for panel B were subjected to immunoblotting with anti-H-Ras antibodies to ensure equal amounts of Ras proteins in the extracts. (D) In vivo labeling experiment. 293 cells were transfected with pCMV encoding wild-type TTF-1 in the presence of V12 Ras (V12), V12D38N Ras (D38N), or βGAL as a control (ctr). After 24 h, cells were incubated with 0.2% calf serum for 18 h and then incubated for 2 h with 32Pi in serum- and phosphate-free DMEM. Cell lysates were subjected to immunoprecipitation with anti-TTF-1 antibodies, run on SDS–10% PAGE, and then blotted on a PVDF membrane as described in Materials and Methods. After transfer, the membrane was subjected to autoradiography (upper panel) and then used to perform immunoblotting with anti-TTF-1 antibodies (middle panel). In the lower panel, total cell extracts were run on SDS–4 to 15% PAGE gel gradient, and immunoblotting was performed with anti-phospho-specific-ERK antibodies.
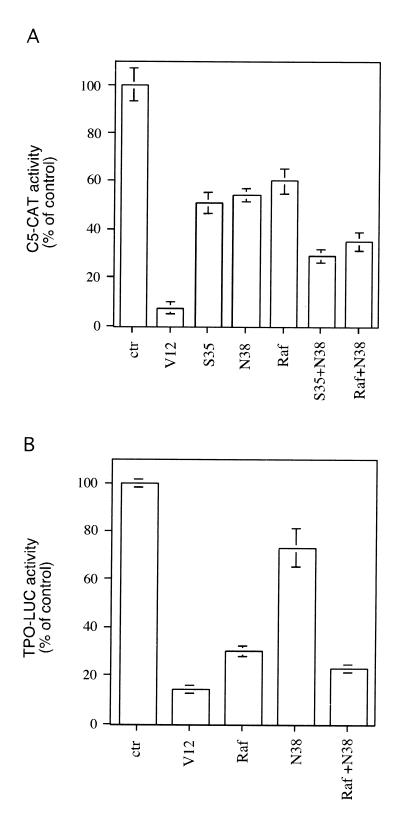
The V12N38 Ras could induce as-yet-uncharacterized signaling molecules that, in concert with the Raf/MEK/ERK pathway, could lead to TTF-1 inactivation and ultimately to inhibition of thyroid-specific gene expression. To test this possibility, thyroid cells were transfected with C5-CAT and expression vectors for V12S35 Ras, V12N38 Ras, Raf-BXB, or various combinations of these mutants (Fig. 7A). As shown above, expression of each construct alone resulted in a partial inhibition of the C5-CAT activity. Importantly, concomitant expression of both V12S35/Raf-BXB and V12N38 Ras resulted in a much stronger inhibition of C5-CAT activity (70 to 75%) (Fig. 7A).
FIG. 7.
Concomitant expression of V12N38 Ras and Raf-BXB inhibits thyroid-specific gene expression. (A) FRTL-5 cells were transfected with C5-CAT (2.5 μg), pCMV expressing various Ras constructs (V12 Ras [0.2 μg], V12S35 Ras [1 μg], and V12N38 Ras [1 μg]), Raf-BXB (1 μg), or βGAL as a control (ctr). CAT activity was measured as described in the legend to Fig. 1. (B) FRTL-5 cells were transfected as in A except that TPO-Luc (2.5 μg) was used as the reporter gene. Luciferase activity was measured as described in the legend to Fig. 1. The reporter activities were calculated as a percentage of the untreated controls. Error bars indicate the standard errors of the means of four independent experiments.
To evaluate the contribution of these pathways in the context of a naturally occurring promoter, the activity of the TPO promoter was assayed in the presence of Raf-BXB, V12N38 Ras, or both. As shown in Fig. 7B, Raf-BXB could strongly inhibit the TPO promoter, while V12N38 Ras was less efficient. Importantly, concomitant activation of Raf-BXB and pathways downstream of V12N38 could almost completely suppress the promoter activity, reproducing the Ras-mediated inhibitory effect.
Taken together, these data indicate that V12N38 Ras activates a Raf-independent pathway(s) that, in concert with Raf, suppresses TTF-1 function and may participate in repression of thyroid-specific gene transcription.
DISCUSSION
Mutations in the ras protooncogene are a common event in epithelial cell tumorigenesis and result in uncontrolled cell proliferation, morphological transformation, and loss of the differentiated phenotype. In thyroid cells, expression of activated Ras results in suppression of thyroid-specific gene expression and, as shown in the present work, in complete inhibition of thyroid-specific promoters. While the signaling pathways leading to cell cycle progression, anchorage-independent growth, and morphological transformation have been studied extensively, little is known about the signal transduction pathways involved in loss of the differentiated phenotype. We have investigated the effect of the ras oncogene on the activity of the transcription factor TTF-1, a crucial regulator of thyroid-specific gene expression. Previous studies in our laboratory have demonstrated that oncogenic H-Ras inhibits TTF-1 transcriptional activity in thyroid follicular cells without altering its expression or its ability to bind to DNA (18). In the present work, we have set up a transient-transfection assay to test the ability of Ras to acutely inhibit TTF-1 function, thus avoiding any potential effect due to long-term neoplastic transformation of these cells. Our results provide the first demonstration that in thyroid follicular cells, the acute effects of V12 Ras result in inactivation of TTF-1 transcriptional activity, as measured with a synthetic reporter gene containing five TTF-1 binding sites. Interestingly, this mechanism is distinct from the one elicited by Ras on skeletal myogenesis, where Ras inhibits the ability of the transcription factor MyoD to induce muscle fibers without affecting its ability to activate transcription of a synthetic promoter (32).
We have used Ras effector region mutants as well as activated forms of several signaling molecules to investigate the mechanisms of TTF-1 inactivation and loss of thyroid-specific gene expression mediated by Ras. We find that Ras interferes directly with TTF-1 activation through two complementary mechanisms, one involving activation of the Raf/MEK/ERK kinase cascade, and the other one involving an independent and as-yet-uncharacterized pathway triggered by the V12N38 Ras mutant.
In thyroid cells, Ras has been shown to activate efficiently both the ERK and the JNK pathway (10). Several lines of evidence suggest an involvement of the Raf/MEK/ERK cascade in Ras-mediated inhibition of TTF-1 activity. First, V12S35 Ras, a mutant known to specifically bind and activate Raf, represses TTF-1 function. Second, expression of an activated form of Raf (Raf-BXB) results in a similar inhibition of TTF-1 transcriptional activity, suggesting that the effect elicited by V12S35 Ras is not due to activation of Raf-independent pathways. Third, the MEK-specific inhibitors U0126 and PD98059 can partially rescue TTF-1 activity in V12 Ras-transfected cells, suggesting that Raf-mediated inactivation of TTF-1 is MEK dependent. By all these criteria, activation of the Raf/MEK/ERK pathway, although not sufficient to completely inhibit TTF-1 function, plays a significant role in reducing its activity. Importantly, Raf activation plays a significant inhibitory role also on the native TPO promoter, suggesting a general involvement of the Raf/MEK/ERK cascade in Ras suppression of thyroid-specific gene expression.
We have previously reported that stable expression of an activated form of MEK (MEKΔN3/S218E/S222) had no effect on thyroid-specific gene expression (10). However, stable expression of MEKΔN3/S218E/S222 results in inefficient activation of ERK in vivo compared with that elicited by the ras oncogene, possibly due to the lack of a nuclear export signal sequence in the MEK-activated mutant (24). Alternatively, compensatory mechanisms that may result upon chronic stimulation of this pathway could have been responsible for masking some of the acute effects observed in transient transfections.
Upon activation, ERKs translocate to the nucleus and phosphorylate several nuclear targets, among which are transcription factors such as Elk1, Ets1, and c-Myc (12, 20, 37, 59). Since ERK activation results in inhibition of TTF-1 activity, we have tested the possibility that TTF-1 may be a direct target of ERK. Indeed, we have found that TTF-1 is directly phosphorylated in vitro by ERK and becomes heavily phosphorylated in vivo by constitutively active forms of Ras and Raf. TTF-1 phosphorylation occurs also by stimulation of the endogenous ERK pathway, as shown by TPA treatment. Furthermore, Ras- and PMA-mediated phosphorylation is strictly dependent on the ERK pathway, since a MEK-specific inhibitor completely suppresses induction of phosphorylation. TTF-1 is not the first differentiation-specific transcription factor to be identified as an ERK target. Similarly, the adipogenic transcription factor PPARγ is also phosphorylated by ERK and its activity is inhibited by the Raf/MEK/ERK pathway (23). TTF-1 contains several S/TP sequences, the minimal consensus sequence for phosphorylation by ERKs. We have identified the potential sites for ERK phosphorylation and demonstrated that a triple mutant carrying three serine-to-alanine substitutions is no longer phosphorylated by ERK either in vitro or in vivo. Phosphorylation of some ERK nuclear targets results in changes in intracellular localization. However, TTF-1 is predominantly localized in the nucleus, as judged from immunofluorescence studies, and expression of V12 Ras is unable to alter TTF-1 nuclear staining in thyroid and nonthyroid cell lines (data not shown).
The functional activity of the mutated TTF-1 protein could be assessed neither in thyroid cells, since this assay is complicated by the presence of endogenous TTF-1, nor in nonthyroid cells, where Ras is unable to elicit a convincing inhibition of TTF-1 (data not shown). We tried to overcome these problems by testing GAL4 fusion proteins in thyroid cells in the presence and absence of Ras. Although both the N-terminal portion (amino acids 1 to 159) and the C-terminal portion (amino acids 295 to 372) of TTF-1 function as transcriptional activators when fused to GAL4 (13), their activity is not significantly altered in the presence of Ras, suggesting that the integrity of the entire molecule is required for Ras repression (data not shown). In contrast, full-length TTF-1 fused to GAL4 or portions of TTF-1 containing the homeodomain are unable to activate a GAL4-responsive promoter (data not shown; M. De Felice and R. Di Lauro, unpublished observations). Similar observations have been reported for other transcription factors, such as HoxA7, Msx-1, Nk-1, and Nk-4 (7, 51, 64). Thus, although ERK-mediated phosphorylation of TTF-1 is likely to play a role in its inactivation, we could not formally prove this point. However, it is conceivable that TTF-1 phosphorylation by ERKs may result in changes in its ability to interact with thyroid-specific accessory proteins, resulting in inefficient activation of the transcriptional machinery. We have recently isolated potential transcriptional regulators of TTF-1 by yeast two-hybrid screening (C. Missero et al., unpublished data). Such studies may reveal new insights not only into TTF-1 function but also into its inactivation in Ras-transformed cells.
Besides Raf, other signal transduction molecules such as PI3K and RalGDS play a crucial role as Ras downstream effectors. Interestingly, activation of PI3K and RalGDS is not only insufficient to inhibit TTF-1 function by itself but also unable to enhance Raf-mediated suppression of TTF-1 activity. These data may be somewhat surprising in view of the fact that, in fibroblasts, these regulators have been shown to cooperate with the Raf/MEK/ERK kinase cascade to result in uncontrolled cell proliferation and neoplastic transformation (29, 45, 46). Staurosporine, a broad-spectrum inhibitor of protein kinases, is sufficient to fully restore TTF-1 activity in the presence of oncogenic Ras, strongly supporting the hypothesis that activation of downstream kinases is a crucial component of Ras-mediated inhibition of TTF-1 function. However, of the several specific protein kinase inhibitors tested, only the MEK inhibitors U0126 and PD98059 could partially rescue Ras inhibition, further suggesting a role for this MAP kinase pathway in the inhibition of TTF-1 transcriptional activity.
To identify other pathways that may contribute to inhibition of TTF-1 function, we have used a number of less-characterized V12 Ras mutants. Interestingly, we have found that V12N38 Ras expression results in a significant inhibition of TTF-1 activity and acts in concert with the Raf/MEK/ERK cascade to further suppress TTF-1 function. Importantly, V12N38 Ras is unable to induce ERK activity in mammalian cells and was previously reported to display no transforming activity and to be unable to bind Raf (52, 65). In a yeast two-hybrid assay, V12N38 Ras selectively interacts with Byr2 (38, 56). Byr2 is a yeast homolog of MEKK1, a MAP kinase kinase kinase, ultimately responsible for JNK activation in response to stress stimuli (34, 41). Activation of the MEKK1/JNK pathway could cooperate with the Raf/MEK/ERK pathway to suppress TTF-1 function and thyroid differentiation. However, as mentioned above, V12 Rac, which activates JNK efficiently (11, 40), displays no ability to inhibit TTF-1 function. Similarly, the mutant V12G37 Ras, which has no activity on TTF-1 function in our assay, has been shown to efficiently interact with Byr2 in the yeast two-hybrid assay and to activate JNK in mammalian cells (57). Thus, V12N38 Ras is likely to activate as-yet-unidentified Ras downstream effectors, which may contribute to TTF-1 inactivation. Future identification of these pathways will be of general relevance in understanding the mechanisms underlying loss of the differentiated phenotype in transformed epithelial cells.
ACKNOWLEDGMENTS
We are grateful to Julian Downward, Alan Hall, and John Kyriakis for providing various constructs. We thank Enzo Calautti for helpful discussion and valuable advice. We appreciate critical reading of the manuscript by Gilda Cobellis. We also thank Elio Biffali and Raimondo Pannone at the Molecular Biology Service at the Stazione Zoologica for excellent technical help and suggestions.
This work was supported by the Associazione Italiana per la Ricerca sul Cancro. M.T.P. is a recipient of a fellowship from the Consiglio Nazionale delle Ricerche.
REFERENCES
- 1.Akasaka K, Tamada M, Wang F, Kariya K, Shima F, Kikuchi A, Yamamoto M, Shirouzu M, Yokoyama S, Kataoka T. Differential structural requirements for interaction of Ras protein with its distinct downstream effectors. J Biol Chem. 1996;271:5353–5360. doi: 10.1074/jbc.271.10.5353. [DOI] [PubMed] [Google Scholar]
- 2.Ambesi-Impiombato F S, Parks L A M, Coon H G. Culture of hormone-dependent functional epithelial cells from rat thyroids. Proc Natl Acad Sci USA. 1980;77:3455–3459. doi: 10.1073/pnas.77.6.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker S J, Markowitz S, Fearon E R, Willson J K, Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990;249:912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- 4.Bos J L. The ras gene family and human carcinogenesis. Mutat Res. 1988;195:255–271. doi: 10.1016/0165-1110(88)90004-8. [DOI] [PubMed] [Google Scholar]
- 5.Brasier A R, Tate J E, Habener J F. Optimized use of the firefly luciferase assay as a reporter gene in mammalian cell lines. Biotechniques. 1989;7:1116–1122. [PubMed] [Google Scholar]
- 6.Campbell S L, Khosravi-Far R, Rossman K L, Clark G J, Der C J. Increasing complexity of Ras signaling. Oncogene. 1998;17:1395–1413. doi: 10.1038/sj.onc.1202174. [DOI] [PubMed] [Google Scholar]
- 7.Choi C Y, Lee Y M, Kim Y H, Park T, Jeon B H, Schulz R A, Kim Y. The homeodomain transcription factor NK-4 acts as either a transcriptional activator or repressor and interacts with the p300 coactivator and the groucho corepressor. J Biol Chem. 1999;274:31543–31552. doi: 10.1074/jbc.274.44.31543. [DOI] [PubMed] [Google Scholar]
- 8.Civitareale D, Castelli M P, Falasca P, Saiardi A. Thyroid transcription factor 1 activates the promoter of the thyrotropin receptor gene. Mol Endocrinol. 1993;7:1589–1595. doi: 10.1210/mend.7.12.8145764. [DOI] [PubMed] [Google Scholar]
- 9.Civitareale D, Lonigro R, Sinclair A J, Di Lauro R. A thyroid-specific nuclear protein essential for tissue-specific expression of the thyroglobulin promoter. EMBO J. 1989;8:2537–2542. doi: 10.1002/j.1460-2075.1989.tb08391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cobellis G, Missero C, Di Lauro R. Concomitant activation of MEK-1 and Rac-1 increases the proliferative potential of thyroid epithelial cells, without affecting their differentiation. Oncogene. 1998;17:2047–2057. doi: 10.1038/sj.onc.1202130. [DOI] [PubMed] [Google Scholar]
- 11.Coso O A, Chiariello M, Yu J C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind J S. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 12.Davis R J. Transcriptional regulation by MAP kinases. Mol Reprod Dev. 1995;42:459–467. doi: 10.1002/mrd.1080420414. [DOI] [PubMed] [Google Scholar]
- 13.De Felice M, Damante G, Zannini M, Francis-Lang H, Di Lauro R. Redundant domains contribute to the transcriptional activity of the thyroid transcription factor 1. J Biol Chem. 1995;270:26649–26656. doi: 10.1074/jbc.270.44.26649. [DOI] [PubMed] [Google Scholar]
- 14.Downward J. Control of ras activation. Cancer Surv. 1996;27:87–100. [PubMed] [Google Scholar]
- 15.Endo T, Kaneshige M, Nakazato M, Ohmori M, Harii N, Onaya T. Thyroid transcription factor-1 activates the promoter activity of rat thyroid Na+/I− symporter gene. Mol Endocrinol. 1997;11:1747–1755. doi: 10.1210/mend.11.11.0012. [DOI] [PubMed] [Google Scholar]
- 16.Farid N R, Shi Y, Zou M. Molecular basis of thyroid cancer. Endocr Rev. 1994;15:202–232. doi: 10.1210/edrv-15-2-202. [DOI] [PubMed] [Google Scholar]
- 17.Francis-Lang H, Price M, Polycarpou-Schwarz M, Di Lauro R. Cell-type-specific expression of the rat thyroperoxidase promoter indicates common mechanisms for thyroid-specific gene expression. Mol Cell Biol. 1992;12:576–588. doi: 10.1128/mcb.12.2.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Francis-Lang H, Zannini M, De Felice M, Berlingieri M T, Fusco A, Di Lauro R. Multiple mechanisms of interference between transformation and differentiation in thyroid cells. Mol Cell Biol. 1992;12:5793–5800. doi: 10.1128/mcb.12.12.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fusco A, Berlingieri M T, Di Fiore P P, Portella G, Grieco M, Vecchio G. One- and two-step transformations of rat thyroid epithelial cells by retroviral oncogenes. Mol Cell Biol. 1987;7:3365–3370. doi: 10.1128/mcb.7.9.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gille H, Kortenjann M, Thomae O, Moomaw C, Slaughter C, Cobb M H, Shaw P E. ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. EMBO J. 1995;14:951–962. doi: 10.1002/j.1460-2075.1995.tb07076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guazzi S, Price M, De Felice M, Damante G, Mattei M G, Di Lauro R. Thyroid nuclear factor 1 (TTF-1) contains a homeodomain and displays a novel DNA binding specificity. EMBO J. 1990;9:3631–3639. doi: 10.1002/j.1460-2075.1990.tb07574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofer F, Fields S, Schneider C, Martin G S. Activated Ras interacts with the Ral guanine nucleotide dissociation stimulator. Proc Natl Acad Sci USA. 1994;91:11089–11093. doi: 10.1073/pnas.91.23.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu E, Kim J B, Sarraf P, Spiegelman B M. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARγ. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- 24.Jaaro H, Rubinfeld H, Hanoch T, Seger R. Nuclear translocation of mitogen-activated protein kinase kinase (MEK1) in response to mitogenic stimulation. Proc Natl Acad Sci USA. 1997;94:3742–3747. doi: 10.1073/pnas.94.8.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joneson T, Bar-Sagi D. Ras effectors and their role in mitogenesis and oncogenesis. J Mol Med. 1997;75:587–593. doi: 10.1007/s001090050143. [DOI] [PubMed] [Google Scholar]
- 26.Joneson T, White M A, Wigler M H, Bar-Sagi D. Stimulation of membrane ruffling and MAP kinase activation by distinct effectors of RAS. Science. 1996;271:810–812. doi: 10.1126/science.271.5250.810. [DOI] [PubMed] [Google Scholar]
- 27.Karga H, Lee J K, Vickery A L, Jr, Thor A, Gaz R D, Jameson J L. Ras oncogene mutations in benign and malignant thyroid neoplasms. J Clin Endocrinol Metab. 1991;73:832–836. doi: 10.1210/jcem-73-4-832. [DOI] [PubMed] [Google Scholar]
- 28.Katz M E, McCormick F. Signal transduction from multiple Ras effectors. Curr Opin Genet Dev. 1997;7:75–79. doi: 10.1016/s0959-437x(97)80112-8. [DOI] [PubMed] [Google Scholar]
- 29.Khosravi-Far R, White M A, Westwick J K, Solski P A, Chrzanowska-Wodnicka M, Van Aelst L, Wigler M H, Der C J. Oncogenic Ras activation of Raf/mitogen-activated protein kinase-independent pathways is sufficient to cause tumorigenic transformation. Mol Cell Biol. 1996;16:3923–3933. doi: 10.1128/mcb.16.7.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kikuchi A, Demo S D, Ye Z-H, Chen Y-W, Williams L T. ralGDS family members interact with the effector loop of ras p21. Mol Cell Biol. 1994;14:7483–7491. doi: 10.1128/mcb.14.11.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox C H, Ward J M, Gonzalez F J. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- 32.Kong Y, Johnson S E, Taparowsky E J, Konieczny S F. Ras p21Val inhibits myogenesis without altering the DNA binding or transcriptional activities of the myogenic basic helix-loop-helix factors. Mol Cell Biol. 1995;15:5205–5213. doi: 10.1128/mcb.15.10.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kupperman E, Wofford D, Wen W, Meinkoth J L. Ras inhibits thyroglobulin expression but not cyclic adenosine monophosphate-mediated signaling in Wistar rat thyrocytes. Endocrinology. 1996;137:96–104. doi: 10.1210/endo.137.1.8536648. [DOI] [PubMed] [Google Scholar]
- 34.Lange-Carter C A, Pleiman C M, Gardner A M, Blumer K J, Johnson G L. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science. 1993;260:315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- 35.Lazzaro D, Price M, de Felice M, Di Lauro R. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development. 1991;113:1093–1104. doi: 10.1242/dev.113.4.1093. [DOI] [PubMed] [Google Scholar]
- 36.Lowy D R, Willumsen B M. Function and regulation of ras. Annu Rev Biochem. 1993;62:851–891. doi: 10.1146/annurev.bi.62.070193.004223. [DOI] [PubMed] [Google Scholar]
- 37.Marais R, Marshall C J. Control of the ERK MAP kinase cascade by Ras and Raf. Cancer Surv. 1996;27:101–125. [PubMed] [Google Scholar]
- 38.Masuda T, Kariya K, Shinkai M, Okada T, Kataoka T. Protein kinase Byr2 is a target of Ras1 in the fission yeast Schizosaccharomyces pombe. J Biol Chem. 1995;270:1979–1982. doi: 10.1074/jbc.270.5.1979. [DOI] [PubMed] [Google Scholar]
- 39.Miller M J, Prigent S, Kupperman E, Rioux L, Park S H, Feramisco J R, White M A, Rutkowski J L, Meinkoth J L. RalGDS functions in Ras- and cAMP-mediated growth stimulation. J Biol Chem. 1997;272:5600–5605. doi: 10.1074/jbc.272.9.5600. [DOI] [PubMed] [Google Scholar]
- 40.Minden A, Lin A, Claret F X, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 41.Minden A, Lin A, McMahon M, Lange-Carter C, Derijard B, Davis R J, Johnson G L, Karin M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 42.Missero C, Cobellis G, De Felice M, Di Lauro R. Molecular events involved in differentiation of thyroid follicular cells. Mol Cell Endocrinol. 1998;140:37–43. doi: 10.1016/s0303-7207(98)00027-6. [DOI] [PubMed] [Google Scholar]
- 43.Morrison D K, Cutler R E. The complexity of Raf-1 regulation. Curr Opin Cell Biol. 1997;9:174–179. doi: 10.1016/s0955-0674(97)80060-9. [DOI] [PubMed] [Google Scholar]
- 44.Ohno M, Zannini M, Levy O, Carrasco N, Di Lauro R. The paired-domain transcription factor Pax8 binds to the upstream enhancer of the rat sodium/iodide symporter gene and participates in both thyroid-specific and cyclic-AMP-dependent transcription. Mol Cell Biol. 1999;19:2051–2060. doi: 10.1128/mcb.19.3.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiu R G, Chen J, Kirn D, McCormick F, Symons M. An essential role for Rac in Ras transformation. Nature. 1995;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- 46.Qiu R G, Chen J, McCormick F, Symons M. A role for Rho in Ras transformation. Proc Natl Acad Sci USA. 1995;92:11781–11785. doi: 10.1073/pnas.92.25.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rameh L E, Cantley L C. The role of phosphoinositide 3-kinase lipid products in cell function. J Biol Chem. 1999;274:8347–8350. doi: 10.1074/jbc.274.13.8347. [DOI] [PubMed] [Google Scholar]
- 48.Ridley A J, Paterson H F, Johnston C L, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 49.Rodriguez-Viciana P, Warne P H, Dhand R, Vanhaesebroeck B, Gout I, Fry M J, Waterfield M D, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez-Viciana P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 51.Schnabel C A, Abate-Shen C. Repression by HoxA7 is mediated by the homeodomain and the modulatory action of its N-terminal-arm residues. Mol Cell Biol. 1996;16:2678–2688. doi: 10.1128/mcb.16.6.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shirouzu M, Koide H, Fujita-Yoshigaki J, Oshio H, Toyama Y, Yamasaki K, Fuhrman S A, Villafranca E, Kaziro Y, Yokoyama S. Mutations that abolish the ability of Ha-Ras to associate with Raf-1. Oncogene. 1994;9:2153–2157. [PubMed] [Google Scholar]
- 53.Songyang Z, Lu K P, Kwon Y T, Tsai L H, Filhol O, Cochet C, Brickey D A, Soderling T R, Bartleson C, Graves D J, DeMaggio A J, Hoekstra M F, Blenis J, Hunter T, Cantley L C. A structural basis for substrate specificities of protein Ser/Thr kinases: primary sequence preference of casein kinases I and II, NIMA, phosphorylase kinase, calmodulin-dependent kinase II, CDK5, and Erk1. Mol Cell Biol. 1996;16:6486–6493. doi: 10.1128/mcb.16.11.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suarez H G. Genetic alterations in human epithelial thyroid tumours. Clin Endocrinol. 1998;48:531–546. doi: 10.1046/j.1365-2265.1998.00443.x. [DOI] [PubMed] [Google Scholar]
- 55.Urano T, Emkey R, Feig L A. Ral-GTPases mediate a distinct downstream signaling pathway from Ras that facilitates cellular transformation. EMBO J. 1996;15:810–816. [PMC free article] [PubMed] [Google Scholar]
- 56.Van Aelst L, Barr M, Marcus S, Polverino A, Wigler M. Complex formation between RAS and RAF and other protein kinases. Proc Natl Acad Sci USA. 1993;90:6213–6217. doi: 10.1073/pnas.90.13.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.White M A, Nicolette C, Minden A, Polverino A, Van Aelst L, Karin M, Wigler M H. Multiple Ras functions can contribute to mammalian cell transformation. Cell. 1995;80:533–541. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- 58.White M A, Vale T, Camonis J H, Schaefer E, Wigler M H. A role for the Ral guanine nucleotide dissociation stimulator in mediating Ras-induced transformation. J Biol Chem. 1996;271:16439–16442. doi: 10.1074/jbc.271.28.16439. [DOI] [PubMed] [Google Scholar]
- 59.Whitmarsh A J, Davis R J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 60.Winkler D G, Johnson J C, Cooper J A, Vojtek A B. Identification and characterization of mutations in Ha-Ras that selectively decrease binding to cRaf-1. J Biol Chem. 1997;272:24402–24409. doi: 10.1074/jbc.272.39.24402. [DOI] [PubMed] [Google Scholar]
- 61.Wolthuis R M, Bauer B, van't Veer L J, de Vries-Smits A M, Cool R H, Spaargaren M, Wittinghofer A, Burgering B M, Bos J L. RalGDS-like factor (Rlf) is a novel Ras and Rap 1A-associating protein. Oncogene. 1996;13:353–362. [PubMed] [Google Scholar]
- 62.Yuryev A, Wennogle L P. The RAF family: an expanding network of post-translational controls and protein-protein interactions. Cell Res. 1998;8:81–98. doi: 10.1038/cr.1998.9. [DOI] [PubMed] [Google Scholar]
- 63.Zannini M, Acebron A, De Felice M, Arnone M I, Martin-Perez J, Santisteban P, Di Lauro R. Mapping and functional role of phosphorylation sites in the thyroid transcription factor-1 (TTF-1) J Biol Chem. 1996;271:2249–2254. doi: 10.1074/jbc.271.4.2249. [DOI] [PubMed] [Google Scholar]
- 64.Zhang H, Catron K M, Abate-Shen C. A role for the Msx-1 homeodomain in transcriptional regulation: residues in the N-terminal arm mediate TATA binding protein interaction and transcriptional repression. Proc Natl Acad Sci USA. 1996;93:1764–1769. doi: 10.1073/pnas.93.5.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang X F, Settleman J, Kyriakis J M, Takeuchi-Suzuki E, Elledge S J, Marshall M S, Bruder J T, Rapp U R, Avruch J. Normal and oncogenic p21ras proteins bind to the amino-terminal regulatory domain of c-Raf-1. Nature. 1993;364:308–313. doi: 10.1038/364308a0. [DOI] [PubMed] [Google Scholar]