Abstract
Introduction:
The objective of this study was to identify county-level characteristics that may be high impact targets for opioid and antibiotic interventions to improve dental prescribing.
Methods:
Prescriptions during 2012–2017 were extracted from IQVIA LRx. Primary outcomes were yearly county-level antibiotic and opioid prescribing rates. Multivariable negative binomial regression identified associations between prescribing rates and county-level characteristics. All analyses occurred in 2020.
Results:
Over time, dental opioid prescribing rates decreased by 20% (4.02 to 3.22/100 persons), while antibiotic rates increased by 5% (6.85 to 7.19/100 persons). Higher number of dentists per capita, higher proportion of female residents, and higher proportion of residents less than 65 years old were associated with increased opioid rates. Relative to the West, location in the Northeast (59%, 95% CI, 52%−65%) and Midwest (64%, 95% CI, 60%−70%) was associated with lower opioid prescribing rates. Higher clinician density, median household income, proportion female, and proportion white were all independently associated with higher antibiotic rates. Location in the Northeast (149%, 95% CI, 137%−162%) and Midwest (118%, 95% CI, 111%−125%) was associated with higher antibiotic rates. Opioid and antibiotic prescribing rates were positively associated.
Conclusions:
Dental prescribing of opioids are decreasing while dental antibiotic prescribing are increasing. High prescribing of antibiotics was associated with high prescribing of opioids. Strategies focused on optimizing dental antibiotics and opioids is needed given their impact on population health.
Keywords: Antibiotics, Opioids, Prescribing patterns, Dentists
Introduction
The appropriate and inappropriate use of antibiotics and opioids can have significant risks to patient safety: bacterial resistance, Clostridioides difficile infection (CDI), opioid overdose, drug dependence and drug diversion. In 2017, 47,600 Americans died of an opioid overdose, 12,800 Americans died of CDI, and 35,900 Americans died of infections caused by antibiotic-resistant pathogens.1–3 Thus, the overprescribing of antibiotics and opioids is a public health crisis.4,5
Dentists prescribe one out of 10 outpatient prescriptions for both antibiotics and opioids in the U.S. In fact, opioids and antibiotics are the primary medication classes prescribed by dentists.6–8 Compared to guidelines published by the American Dental Association (ADA), American Heart Association, and American Association of Orthopedic Surgeons, prescribing practices of dentists have shown frequent overprescribing of antibiotics, particularly for infection prophylaxis.9–12 More than 80% of antibiotics prescribed for prophylaxis before dental visits are not indicated per guidelines.13 Despite evidence demonstrating that non-opioid analgesics are more effective than opioids for acute dental pain, research has demonstrated that dentists overprescribe opioids, contributing to the opioid epidemic and cases of opioid overdose.14–19 It is estimated that between 29–50% of opioids prescribed to adult dental patients are overprescribed.20 In 2016, dentists in the U.S. prescribed opioids far more frequently than English dentists, including opioids with a high potential for abuse.21 However, knowledge regarding patterns of dentists’ medication prescribing by county and geographic factors are limited.
Given the high volume of prescribing of both medication classes by dentists, and a high frequency of inappropriate prescribing among dentists, efforts to further characterize the geographic pattern of prescribing practices may identify where interventions should be targeted in order to modify dental antibiotic and opioid prescribing. Thus, whether opioid prescribing is associated with antibiotic prescribing was examined, and trends over time and geographic and county factors associated with opioid and antibiotic prescribing by dentists were described.
Methods
Study Design and Setting
The study was a retrospective observational analysis using the IQVIA Longitudinal Prescription (“LRx”) database to identify opioid and antibiotic prescriptions written by general dentists from 2012 through 2017. General dentists were defined with the ADA classification of general practice, pediatric and public health. The LRx database contains 92% of all dispensed prescriptions, and represent all outpatient prescriptions, across all payers, including community pharmacies and nongovernmental mail service pharmacies.22 Prescriptions dispensed from Veterans Affairs pharmacies are not included in IQVIA datasets. Prescriptions from dentists who were not actively practicing were excluded. A dentist was defined as actively practicing if they had at least 20 prescriptions for any medication filled per year.23
Outcomes
The original units of analysis were actively prescribing dentists in counties in the U.S., excluding U.S. territories. Prescription and provider data were aggregated by county. Three outcomes on prescribing by dentists were measured: 1) an overall number of prescriptions, 2) the proportion of all prescriptions that were antibiotics and opioids, and 3) prescribing rates by each medication class. Rates were adjusted for county population size (per 100) as reported by the U.S. Census Bureau for each year. Prescription counts were summarized by class (antibiotics, opioids, other) and the county where the prescriber was located. Systemic antibiotics and opioids were defined according to the American Hospital Formulary Service. County-level trends in prescribing rates were assessed. A sensitivity analysis using days’ supply of each class as alternative outcomes was performed. Mean days’ supply for each county for each calendar year was calculated using the total number of prescriptions of each medication class and total days’ supply for each class.
Measures
County-level data were linked from the American Community Survey (proportion female, proportion white, proportion ≥ 65 years of age), the U.S. Census Bureau’s Small Area Income and Poverty Estimates program (median household income, proportion living in poverty). Counties were classified as metropolitan, urban, or rural as defined by the Rural-Urban Continuum Codes categorization schema, published by the Economic Research Service of the U.S. Department of Agriculture. County clinician density was defined as the number of actively prescribing dentists per 100,000 county residents.
Statistical analysis
Separate multivariable regression models were fit for prescribing rates and days’ supply for each drug category as the dependent variable and county-level characteristics and the prescribing rate of the complementary drug category as independent variables (e.g., with county-level antibiotic prescribing rate as the dependent variable, county-level socioeconomic characteristics and county-level opioid prescribing rate were treated as independent variables). County-level independent variables were categorized as follows: Prescribers per capita, median household income, proportion female, proportion white, proportion ≥ 65 years, and proportion living in poverty were categorized into tertiles with the bottom tertile as the referent. For county Census Region and county rurality classification, the West census region and metro areas were treated as referents, respectively. To account for the longitudinal nature of the prescribing rates, a generalized linear mixed model approach with negative binomial response distribution was employed.24 Fixed effect terms for time and time-squared and random county and random trend terms were employed in each model. All analyses were conducted using SAS software (SAS, version 9.4) in 2020.
Results
The final dataset consisted of 2900 U.S. counties. From 2012 to 2017, dentists prescribed a total of 71.4 million opioids and 136 million antibiotics. Across this time period, the rate of dental opioid prescribing decreased 20% (from 4.02 to 3.22/100 persons) and the rate of antibiotic prescribing increased 5% (from 6.85 to 7.19/100 persons; Figure 1a). There was considerable regional variability in prescribing patterns for opioids but less so for antibiotics (Figures 1b and 1c). In 2017, the Southern Census region had the twice the opioid prescribing rate as the Northeast (4.0/100 persons vs. 1.7/100 persons, respectively). This pattern in opioid prescribing rates persisted across the study period. There was less regional variability in antibiotic prescribing rate; however, the regional pattern of antibiotic prescribing rates changed over the study period.
Figure 1:
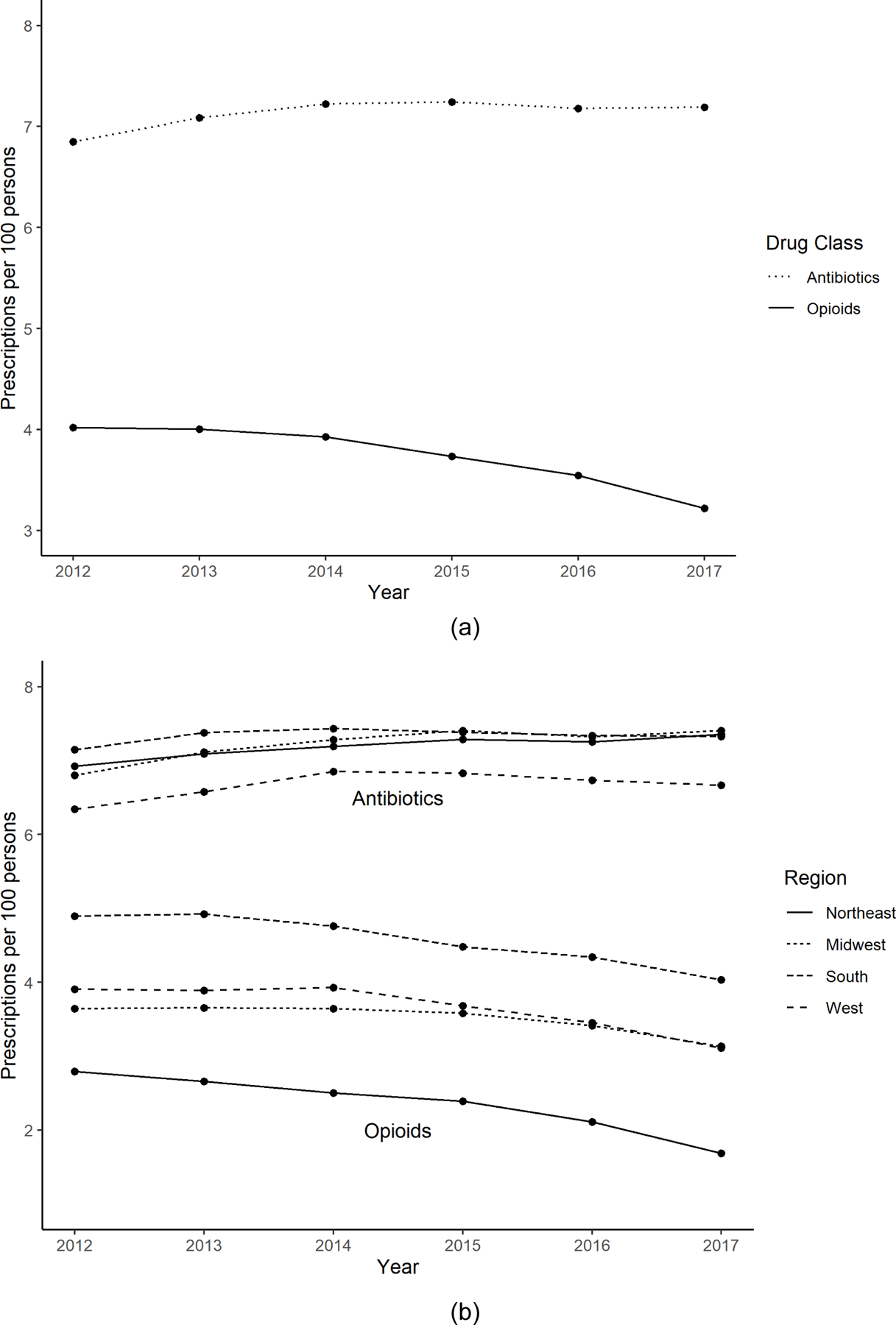
Opioid and Antibiotic Prescribing Rates by Dentists, 2012 to 2017
Figures 2 shows the percent change in opioid and antibiotic prescribing, respectively, by county between 2012 and 2017. The majority (64.6%) of counties showed a decrease of greater than 10% in dental opioid prescribing rates, while 20.8% of counties showed an increase of greater than 10%. Only 23.3% of counties showed a decrease of greater than 10% in the dental antibiotic prescribing rate, while 45.3% of counties showed an increase of greater than 10%.
Figure 2:
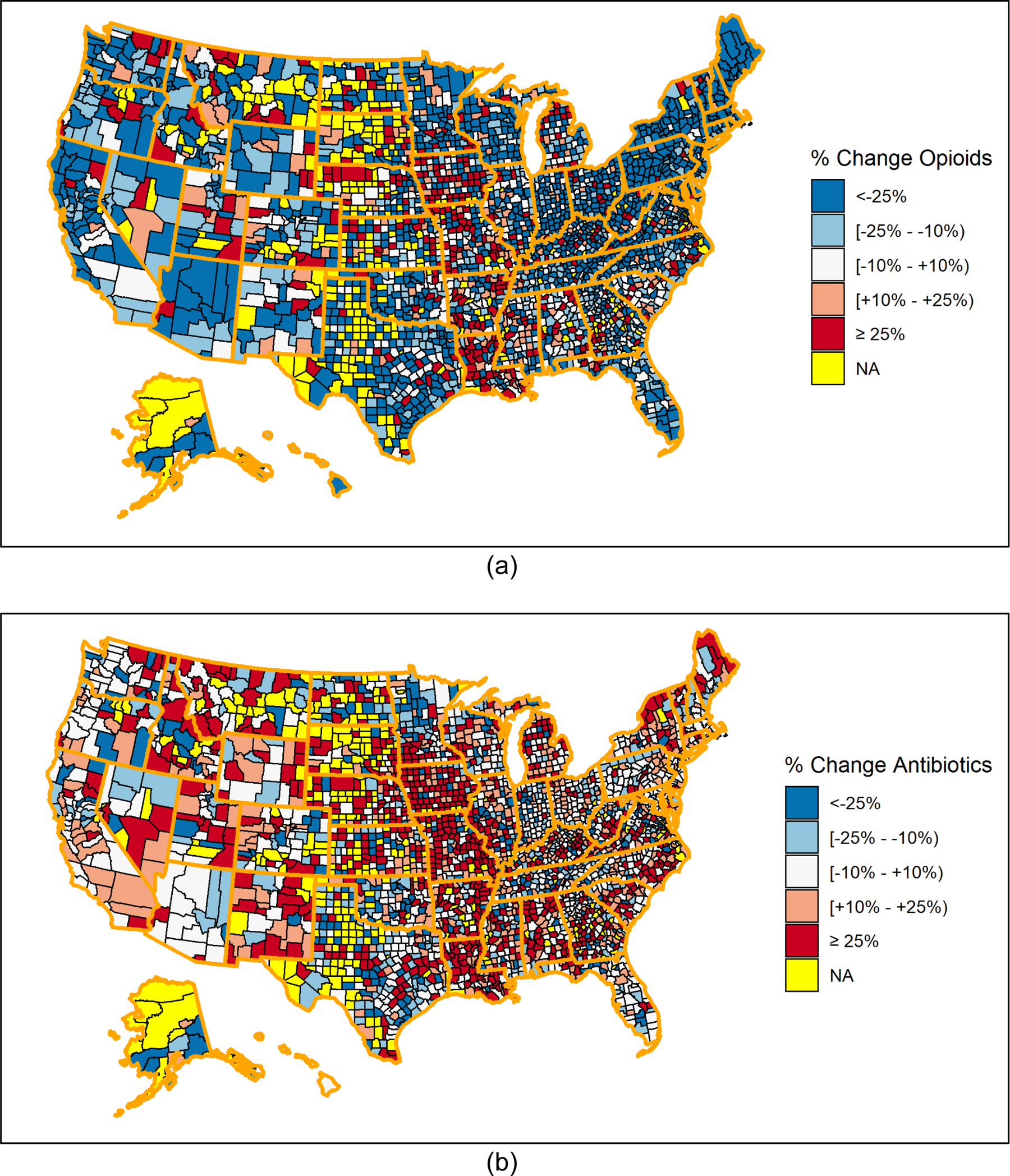
Percent change in dental opioid and antibiotic prescribing rates by county, 2012 to 2017
Unadjusted associations for county-level characteristics and opioid and antibiotic prescribing rates in 2017 are listed in Table 1. Counties with the highest clinician density (clinicians/100,000 persons) had an opioid prescribing rate (3.42 prescriptions/100 persons) of 1.65 times that of counties with the lowest clinician density (2.08/100 persons). Other factors associated with increased opioid prescribing included having a higher vs lower proportion of female residents (3.48 vs 2.76/100 persons) and a higher vs lower proportion of residents living in poverty (3.57 vs 2.94/100 persons). A higher proportion of the county being white, 65 years or older, and being in a rural county was associated with lower opioid prescribing. Similar factors and others were associated with increased antibiotic prescribing including a higher clinician density vs lower density (7.82 vs 4.05/100 persons), high vs low median household income (7.28 vs 6.27/100 persons), a high vs low proportion of females (7.88 vs 5.91/100 persons), and being in the middle tertile vs lowest tertile of poverty (7.59 vs 7.02/100 persons). Rural and urban settings, and larger proportion of white residents, were associated with lower antibiotic prescribing rates.
Table 1:
Opioid and antibiotic prescribing according to county-level characteristics, 2017
| Opioids | Antibiotics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range | Population | Total Opioids Rx’d | Rx per 100 Persons | Unadjusted RR (95% CI) | p-value | Total Antibiotics Rx’d | Rx per 100 Persons | Unadjusted RR (95% CI) | p- value | |
| Clinicians per 100K Persons | ||||||||||
| Lowest Tertile | 2.5 – 26.4 | 28,494,403 | 592,623 | 2.08 | Referent | 1,152,605 | 4.05 | Referent | ||
| Middle Tertile | 26.4 – 40.8 | 62,987,588 | 1,877,427 | 2.98 | 1.43 (1.27–1.62) | <.0001 | 3,960,227 | 6.29 | 1.55 (1.47–1.64) | <.0001 |
| Highest Tertile | 40.9 – 314.5 | 232,293,921 | 7,949,022 | 3.42 | 1.65 (1.49–1.82) | <.0001 | 18,173,931 | 7.82 | 1.93 (1.84–2.03) | <.0001 |
| Median Household Income, $ | ||||||||||
| Lowest Tertile | 24,783 – 41,819 | 20,556,142 | 740,562 | 3.60 | Referent | 1,288,177 | 6.27 | Referent | ||
| Middle Tertile | 41,824 – 50,940 | 59,070,506 | 2,067,852 | 3.50 | 0.97 (0.79–1.19) | 0.7851 | 4,218,926 | 7.14 | 1.14 (1.03–1.27) | 0.0150 |
| Highest Tertile | 50,943 – 136,191 | 244,149,264 | 7,610,658 | 3.12 | 0.87 (0.71–1.05) | 0.1380 | 17,779,660 | 7.28 | 1.16 (1.05–1.28) | 0.0029 |
| Proportion Female, % | ||||||||||
| Lowest Tertile | 32.0 – 50.0 | 49,710,887 | 1,370,700 | 2.76 | Referent | 2,938,058 | 5.91 | Referent | ||
| Middle Tertile | 50.0 – 50.9 | 120,527,738 | 3,704,782 | 3.07 | 1.11 (0.99–1.26) | 0.0852 | 8,247,109 | 6.84 | 1.16 (1.10–1.22) | <.0001 |
| Highest Tertile | 50.9 – 58.1 | 153,537,287 | 5,343,590 | 3.48 | 1.26 (1.13–1.41) | <.0001 | 12,101,596 | 7.88 | 1.33 (1.27–1.40) | <.0001 |
| Proportion White, % | ||||||||||
| Lowest Tertile | 0.6 – 73.6 | 213,798,064 | 7,126,510 | 3.33 | Referent | 15,716,244 | 7.35 | Referent | ||
| Middle Tertile | 73.8 – 91.1 | 83,205,734 | 2,707,742 | 3.25 | 0.98 (0.89–1.07) | 0.5991 | 5,943,711 | 7.14 | 0.97 (0.93–1.01) | 0.1944 |
| Highest Tertile | 91.2 – 100.0 | 26,772,114 | 584,820 | 2.18 | 0.66 (0.59–0.73) | <.0001 | 1,626,808 | 6.08 | 0.83 (0.79–0.87) | <.0001 |
| Proportion in Poverty, % | ||||||||||
| Lowest Tertile | 3.0 – 13.0 | 157,917,960 | 4,638,936 | 2.94 | Referent | 11,087,708 | 7.02 | Referent | ||
| Middle Tertile | 13.1 – 18.2 | 123,389,135 | 4,262,227 | 3.45 | 1.18 (1.05–1.32) | 0.0061 | 9,366,662 | 7.59 | 1.08 (1.04–1.13) | 0.0005 |
| Highest Tertile | 18.3 – 46.7 | 42,468,817 | 1,517,909 | 3.57 | 1.22 (1.03–1.44) | 0.0209 | 2,832,393 | 6.67 | 0.95 (0.88–1.03) | 0.2021 |
| Proportion ≥ 65 y.o. | ||||||||||
| Lowest Tertile | 6.3–14.8 | 184,959,300 | 13,084,734 | 3.50 | Referent | 13,084,734 | 6.40 | Referent | ||
| Middle Tertile | 14.9–18.0 | 90,702,718 | 6,919,396 | 3.20 | 0.92 (0.82–1.03) | 0.1331 | 6,919,396 | 6.40 | 1.08 (1.03–1.13) | 0.0033 |
| Highest Tertile | 18.1–54.2 | 47,727,346 | 3,276,531 | 2.40 | 0.83 (0.75–0.91) | 0.0002 | 3,276,531 | 5.90 | 0.97 (0.92–1.02) | 0.2742 |
| Census Region | ||||||||||
| West | 77,145,643 | 2,399,233 | 3.11 | Referent | 5,144,818 | 6.67 | Referent | |||
| Midwest | 67,739,622 | 2,121,140 | 3.13 | 1.01 (0.87–1.17) | 0.9292 | 5,015,736 | 7.40 | 1.11 (1.03–1.20) | 0.0074 | |
| Northeast | 56,060,884 | 943,994 | 1.68 | 0.54 (0.45–0.65) | <.0001 | 4,125,536 | 7.36 | 1.10 (1.01–1.20) | 0.0234 | |
| South | 122,829,763 | 4,954,705 | 4.03 | 1.30 (1.12–1.50) | 0.0005 | 9,000,673 | 7.33 | 1.10 (1.03–1.17) | 0.0045 | |
| Rural-Urban Continuum | ||||||||||
| Metro | 278,740,079 | 9,086,297 | 3.26 | Referent | 20,495,635 | 7.35 | Referent | |||
| Urban | 41,112,721 | 1,254,239 | 3.05 | 0.94 (0.87–1.01) | 0.0870 | 2,618,680 | 6.37 | 0.87 (0.83–0.90) | <.0001 | |
| Rural | 3,923,112 | 78,536 | 2.00 | 0.61 (0.54–0.70) | <.0001 | 172,448 | 4.40 | 0.60 (0.55–0.65) | <.0001 | |
Boldface indicates statistical significance (p<0.05). Abbreviations: CI, confidence interval; RR, risk ratio; Rx, prescriptions; Rx’d, prescribed
In the multivariable analysis of county-level opioid prescribing rates, associations were broadly consistent with the unadjusted analysis (Table 2). The county-level characteristic that accounted for the largest increase in opioid prescribing was census region. The adjusted opioid prescribing rate for the South Census region was 1.79 times that of the Northeast (95% CI, 1.62–1.97), 1.63 times that of the Midwest (95% CI, 1.53–1.72), but not statistically significantly greater than the West. A one-point increase in the rate of county-level antibiotic prescribing was associated with a 18% increase in the rate of opioid prescribing (95% CI, 18%−19%), controlling for other county-level characteristics. Middle and highest clinician density had a 7% (95% CI, 5%−8%) and 11% (95% CI, 9%−14%) increase, respectively, in the rate of opioid prescribing compared to the lowest clinician density. Counties with higher proportion female had an adjusted opioid prescribing rate 3% higher than the counties with the lowest proportion female (95% CI, 2%−5%, for middle tertile; 95% CI, 1%−5%, for highest tertile). Rural counties had lower adjusted rates of opioid prescribing compared to metropolitan areas (64%, 95% CI, 60%−69%). Proportion white and proportion living in poverty were county-level characteristics that were not related to opioid prescribing rate when adjusted for other county-level characteristics.
Table 2:
Multivariable regression models relating county-level socioeconomic characteristics with county-level prescription rates.
| Opioids Rx Rate as Outcome | Antibiotics Rx Rate as Outcome | ||||
|---|---|---|---|---|---|
| County-level Predictor | Level | Adjusted RR (95% CI) | p-value | Adjusted RR (95% CI) | p-value |
| Dentists per 100K persons | Lowest Tertile | Referent | Referent | ||
| Middle Tertile | 1.07 (1.05–1.08) | <.0001 | 1.12 (1.11–1.14) | <.0001 | |
| Highest Tertile | 1.11 (1.09–1.14) | <.0001 | 1.23 (1.21–1.25) | <.0001 | |
| Median Household Income | Lowest Tertile | Referent | Referent | ||
| Middle Tertile | 0.99 (0.97–1.00) | 0.0314 | 1.01 (1.00–1.02) | 0.0303 | |
| Highest Tertile | 0.99 (0.97–1.01) | 0.2695 | 1.02 (1.01–1.03) | 0.0076 | |
| Proportion Female | Lowest Tertile | Referent | Referent | ||
| Middle Tertile | 1.03 (1.02–1.05) | <.0001 | 1.02 (1.01–1.03) | 0.0032 | |
| Highest Tertile | 1.03 (1.01–1.05) | 0.0025 | 1.03 (1.01–1.04) | 0.0009 | |
| Proportion White | Lowest Tertile | Referent | Referent | ||
| Middle Tertile | 1.00 (0.97–1.04) | 0.9919 | 1.03 (1.00–1.06) | 0.0237 | |
| Highest Tertile | 0.96 (0.92–1.01) | 0.0935 | 1.05 (1.02–1.09) | 0.0018 | |
| Proportion in Poverty | Lowest Tertile | Referent | Referent | ||
| Middle Tertile | 1.00 (0.99–1.02) | 0.6316 | 1.01 (1.00–1.01) | 0.3215 | |
| Highest Tertile | 1.01 (0.99–1.02) | 0.5294 | 1.01 (0.99–1.02) | 0.3100 | |
| Proportion ≥ 65 y.o. | Lowest Tertile | Referent | Referent | ||
| Middle Tertile | 0.98 (0.96–1.01) | 0.1774 | 1.01 (0.99–1.03) | 0.1663 | |
| Highest Tertile | 0.92 (0.89–0.95) | <.0001 | 1.02 (1.00–1.05) | 0.0401 | |
| Region | West | Referent | Referent | ||
| Midwest | 0.64 (0.60–0.70) | <.0001 | 1.18 (1.11–1.25) | <.0001 | |
| Northeast | 0.59 (0.52–0.65) | <.0001 | 1.49 (1.37–1.62) | <.0001 | |
| South | 1.05 (0.97–1.13) | 0.2252 | 1.04 (0.99–1.10) | 0.1441 | |
| Rural/Urban/Metro | Metro | Referent | Referent | ||
| Rural | 0.64 (0.60–0.69) | <.0001 | 0.69 (0.65–0.73) | <.0001 | |
| Urban | 0.96 (0.91–1.01) | 0.1083 | 1.00 (0.96–1.04) | 0.8472 | |
| One Point Increase in | Antibiotics | Opioids | |||
| Prescription Rate of: | 1.18 (1.18–1.19) | <.0001 | 1.14 (1.14–1.14) | <.0001 | |
Boldface indicates statistical significance (p<0.05).
Abbreviations: CI, confidence interval; RR, risk ratio; Rx, prescriptions
In the multivariable analysis of county-level antibiotic prescribing rates, county-level characteristics independently associated with antibiotic prescribing rates included clinician density, median household income, proportion female, proportion white, proportion greater than 65 years old, census region and rurality (Table 2). Metropolitan and urban counties had an adjusted antibiotic prescribing rate that was 45% greater than rural counties (95% CI, 37%−54%, for metropolitan; 95% CI, 37%−53 for urban counties). High clinician density was associated with a 23% higher rate of antibiotic prescribing relative to low clinician density (95% CI, 21%−25%). Location in the Northeast census region was associated with a 49% higher rate of antibiotic prescribing relative to the West (95% CI, 37%−62%), and the Midwest was associated with a 18% higher rate relative to the West (95% CI, 11%−25%). Counties with higher proportion white (3–5% higher rate), higher income (1–2% higher rate), or higher proportion female (2–3% higher rate) had a higher adjusted rate of antibiotic prescribing relative to counties with the lowest proportion white, lowest median incomes, and lowest proportion of female residents, respectively. Finally, a one-point increase in the rate of opioid prescribing was associated with a 14% higher antibiotic prescribing rate (95% CI, 14%−14%), controlling for other county-level characteristics.
In a sensitivity analysis, days’ supply was used as an alternative measure of opioid and antibiotic prescribing. Days’ supply for opioids decreased over time from an average of 3.64 days to 3.41 days (Appendix Table 1). In multivariable analyses, few county-level characteristics were significantly related to days’ supply prescribed for either opioids or antibiotics. Neither clinician density, median household income, proportion female, proportion in poverty, nor urbanization were significantly related to mean days’ supply of opioids in the adjusted analysis. Location in the Northeast was related to an 8% increase in the opioid mean days’ supply (95% CI, 5%−11%), and location in the Midwest was associated with a 5% increase (95% CI, 3%−7%) as compared to the West. Location in the South did not have a significant effect on opioid mean days’ supply relative to the West. Higher proportion white was related to a decrease in the opioid mean days’ supply (range, 3% – 5% decrease). A one-day increase in the mean days’ supply of antibiotics at the county-level was related to 2% increase in the mean days’ supply of opioids (95% CI, 2–3%), controlling for other county-level characteristics.
Days’ supply for antibiotics had a very small decrease between 2012 and 2017 from an average of 7.66 days to 7.51 days. Similar to opioid days’ supply, few county-level characteristics were significantly related to antibiotic mean days’ supply in multivariable analysis. Location in the Midwest was associated with an 8% decrease in the mean days’ supply of antibiotics prescribed (95% CI, −6%– −8%) when compared to the West. Urban counties had a 2% lower mean days’ supply of antibiotics when compared to metropolitan counties (95% CI, −4% to −1%). The proportion of white residents was significantly related to antibiotic mean days’ supply. Counties with the highest proportion of white residents had a 1% decrease in mean days’ supply when compared to counties with the lowest proportion white (95% CI, −2% to 0%), whereas counties in the middle tertile had a 1% increase in mean days’ supply (95% CI, 1%−2%). Finally, there was a modest positive association between increased days’ supply of antibiotics and increased days’ supply of opioids (1%, 95% CI, 0%−1%).
Discussion
Between 2012 and 2017, opioid prescribing rates by dentists decreased in 72% of 2,885 counties with data available, while the antibiotic prescribing rate increased in 61% of counties. Consistent with national trends for all prescribers,25,26 these results demonstrate that opioid prescribing by dentists is decreasing by a small absolute amount. Although most of the associations were relatively small after multivariable adjustment, this study identified county-level factors jointly associated with opioid and antibiotic prescribing rates. Higher numbers of dentists per capita practicing in a county (clinician density) and metropolitan and urban practice location were associated with higher prescribing rates of antibiotic and opioids. Another study, which was not restricted to dental prescribers, found a similar increase in opioid prescribing in counties where there were more dentists and primary care physicians per capita.26 Interestingly, increased prescribing of opioids by dentists was associated with increased prescribing of antibiotics at the county-level, adjusting for other county-level characteristics. These results may indicate that geographic areas with more dentists per capita may have a culture of prescribing and/or have greater dental care availability.
Other characteristics associated with prescribing rates of each medication class differed. The Midwest and Northeast were associated with higher antibiotic prescribing, but lower opioid prescribing. In national prescribing trends, the highest prescribing was observed in the South for both classes.27,28 Dental antibiotic prescribing rates in the South remained relatively unchanged over the study period, while the Northeast and Midwest increased. By 2017, antibiotic prescribing rates in the Northeast and Midwest were equivalent to the South. The variation seen in prescribing for both classes by county, region, and county characteristics may indicate a lack of standardization in dental prescribing practices for opioids and antibiotics, although this variation may also be due to difference in the patient populations, such as the prevalence of severe dental pathology.
County-level demographics were significant in predicting prescription patterns: counties with higher proportion female and with a higher proportion of persons <65 years of age had higher adjusted opioid prescribing rates. Counties with higher income, higher proportion female, higher proportion white, and higher proportion persons ≥65 years had higher adjusted antibiotic prescribing rates. Explaining these patterns is complicated. National data consistently reveal that people of color and people living in poverty experience increased burden of untreated dental decay.29 As a result, one might hypothesize that these dental disparities could lead to increased prescriptions for opioids and antibiotics given the pain and infection often associated with dental root caries. However, published data are mixed, and the extent to which a higher prevalence of oral health problems accounts for patterns in opioid and antibiotic prescribing is unclear. In a Medicaid study, African Americans and non-Hispanic whites were twice as likely to receive an opioid at a dental visit than Hispanics, although dental diagnoses did not explain difference in prescribing.30 This result is in contrast to two studies that demonstrated decreased prescribing of opioids and antibiotics for people of color.26,31 A third study found that the odds of being prescribed opioids varied by race/ethnicity, after statistical adjustment for personal characteristics and procedure type.32
Decreased dental opioid prescribing rates are likely due to increasing literature on the impact of prescription opioids on opioid misuse and dependence, regulations limiting opioid supply, and increased availability of prescribing resources (e.g., pain management guidelines, prescription monitoring programs).33–36 While opioid prescribing is decreasing, antibiotic prescribing by dentists is increasing. These results are consistent with data reported by King, et al., which showed that while overall U.S. antibiotic prescribing was decreasing, antibiotics prescribed by dentists increased from 2011–2016.37 While many resources to guide antibiotic prescribing by primary medical clinicians are available, there are few resources for dentists. However, Centers for Disease Control and Prevention (CDC) guidance includes dentists and dental practices as a key audience in outpatient antibiotic stewardship guidance (among other outpatient prescribers/ health care settings).38 The implementation of the CDC antibiotic stewardship guidance in dentistry has been shown to be effective.39 With antibiotic prescribing by dentists increasing, there is an urgent need to test the effectiveness and feasibility of stewardship strategies in dental practices.
These results have limitations. Variables on individual patient co-morbidities, dental visit-characteristics, and indicators of oral health were not available. The statistical analyses relied on county-level data to describe associations between characteristics and prescribing, so these results may not reflect individual-level characteristics associated with prescribing. These analyses also utilized large population-based data of considerable sample size; therefore, it is important to interpret these findings in terms of clinical significance versus statistical significance alone. The multivariable models do not account for potential spatial dependence which may impact inferences from the models.
Without patient co-morbidities and specific visit characteristics, it was not possible to assess prescription appropriateness. In addition, the study data do not include information on county-level prevalence of dental pathology, edentulousness or other population-based measures of dental health. Nor does it contain information regarding the demand for dental services. However, recent publications indicate that greater than half of antibiotics and opioids prescribed by dentists are not indicated or are prescribed in excessive amounts, as measured by potency, metric quantity, or days’ supply.13,20,21 Together with this prior literature, this study’s findings demonstrate 1) an association in prescribing rates between classes and 2) increasing prescribing of antibiotics. Given the significant public health impact associated with prescribing of antibiotics and opioids, these results should be a call to action to improve evidence-based prescribing in dentistry.
Conclusion
In this study, prescribing of antibiotics and prescribing of opioids by dental providers were positively correlated. This suggests that implementation strategies focused on best practices in prescribing of both medication classes may be more impactful in modifying prescribing behaviors than those strategies focused on either class alone. Antibiotic and opioid stewardship interventions focused in communities categorized as metropolitan areas and with more dentists per capita practicing in a county may have a substantial impact on population health.
Supplementary Material
Acknowledgement
The content is solely the responsibility of the authors and does not necessarily represent the official views of Agency for Healthcare Research and Quality, the Department of Veterans Affairs, the US government, or of IQVIA or any of its affiliated entities. The statements, findings, conclusions, views, and opinions contained and expressed in this article are based in part on data obtained under license from IQVIA (source: LRx January 2011 to December 2017, IQVIA Inc.). All rights reserved.
Research reported in this publication was supported by the Agency for Healthcare Research and Quality under award number R01 HS25177 (PI: Suda). The sponsor had no role in the design or conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research or Quality.
Footnotes
Financial Disclosure:
Colin C. Hubbard has no financial disclosures.
Charlesnika T. Evans has no financial disclosures.
Gregory S. Calip is an employee of Flatiron Health.
Susan Rowan has no financial disclosures.
Walid F. Gellad has no financial disclosures.
Allen Campbell is an employee of IQVIA.
Alan E. Gross has no financial disclosures.
Ronald C. Hershow has no financial disclosures.
Jessina C. McGregor has no financial disclosures.
Lisa K. Sharp has no financial disclosures.
Katie J. Suda has no financial disclosures.
This work was presented in part at the International Conference on Health Policy Statistics. San Diego, California, Jan. 2020.
References
- 1.CDC. Antibiotic Resistance Threats in the United States, 2019. Atlanta, GA: U.S. Department of Health and Human Services, CDC; 2019. [Google Scholar]
- 2.Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G, Release E. Drug and Opioid-Involved Overdose Deaths — United States, 2013 – 2017. 2019;67:2013–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC. CDC Newsroom Releases: Nearly half a million Americans suffered from Clostridium difficile infections in a single year. http://www.cdc.gov/media/releases/2015/p0225-clostridium-difficile.html. Published 2015. Accessed Feb. 4, 2017.
- 4.National Action Plan for Combating Antibiotic-Resistant Bacteria. Washington, D.C.: The White House; 2015. [Google Scholar]
- 5.National Action Plan for Adverse Drug Event Prevention. Washington, D.C.: U. S. Department of Health Human Services Office of Disease, Prevention Health, Promotion; 2014. [Google Scholar]
- 6.Cope AL, Chestnutt IG. Inappropriate prescribing of antibiotics in primary dental care: reasons and resolutions. Prim Dent J. 2014;3(4):33–37. [DOI] [PubMed] [Google Scholar]
- 7.Ringwalt C, Gugelmann H, Garrettson M, et al. Differential prescribing of opioid analgesics according to physician specialty for Medicaid patients with chronic noncancer pain diagnoses. Pain Res Manag. 2014;19(4):179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SRB. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305(13):1299–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epstein JB, Chong S, Le ND. A survey of antibiotic use in dentistry. J Am Dent Assoc. 2000;131(11):1600–1609. [DOI] [PubMed] [Google Scholar]
- 10.Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116(15):1736–1754. [DOI] [PubMed] [Google Scholar]
- 11.Rethman MP, Watters W 3rd, Abt E, et al. The American Academy of Orthopaedic Surgeons and the American Dental Association clinical practice guideline on the prevention of orthopaedic implant infection in patients undergoing dental procedures. J Bone Joint Surg Am. 2013;95(8):745–747. [DOI] [PubMed] [Google Scholar]
- 12.American Dental A, American Academy of Orthopedic S. Antibiotic prophylaxis for dental patients with total joint replacements. J Am Dent Assoc. 2003;134(7):895–899. [DOI] [PubMed] [Google Scholar]
- 13.Suda KJ, Calip GS, Zhou J, et al. Assessment of the appropriateness of antibiotic prescriptions for infection prophylaxis before dental procedures, 2011 to 2015. JAMA Netw Open. 2019;2(5):e193909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yingling NM, Byrne BE, Hartwell GR. Antibiotic use by members of the American Association of Endodontists in the year 2000: report of a national survey. J Endod. 2002;28(5):396–404. [DOI] [PubMed] [Google Scholar]
- 15.Wright ER, Kooreman HE, Greene MS, Chambers RA, Banerjee A, Wilson J. The iatrogenic epidemic of prescription drug abuse: county-level determinants of opioid availability and abuse. Drug Alcohol Depend. 2014;138:209–215. [DOI] [PubMed] [Google Scholar]
- 16.McCauley JL, Leite RS, Melvin CL, Fillingim RB, Brady KT. Dental opioid prescribing practices and risk mitigation strategy implementation: Identification of potential targets for provider-level intervention. Subst Abuse. 2016;37(1):9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porucznik CA, Johnson EM, Rolfs RT, Sauer BC. Specialty of prescribers associated with prescription opioid fatalities in Utah, 2002–2010. Pain Med. 2014;15(1):73–78. [DOI] [PubMed] [Google Scholar]
- 18.Aldous JA, Engar RC. Analgesic prescribing patterns in a group of dentists. Gen Dent. 2000;48(5):586–590. http://www.ncbi.nlm.nih.gov/pubmed/11199639. [PubMed] [Google Scholar]
- 19.Baker JA, Avorn J, Levin R, Bateman BT. Opioid prescribing after surgical extraction of teeth in Medicaid patients, 2000–2010. JAMA. 2016;315(15):1653–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suda KJ, Zhou J, Rowan SA, et al. Overprescribing of opioids to adults by dentists in the U.S., 2011–2015. Am J Prev Med. 2020;58(4):473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suda KJ, Durkin MJ, Calip GS, et al. Comparison of opioid prescribing by dentists in the United States and England. JAMA Netw Open. 2019;2(5):e194303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boardman C, Inventor; IMS Health Inc., assignee. System and method for estimating product distribution using a product specific universe. US patent 7,174,304. February 6, 2007.
- 23.Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60(9):1308–1316. [DOI] [PubMed] [Google Scholar]
- 24.Stroup W Generalized linear mixed models. Boca Raton, FL: Taylor & Francis Group; 2013. [Google Scholar]
- 25.Schieber LZ, Guy GP Jr., Seth P, et al. Trends and patterns of geographic variation in opioid prescribing practices by state, United States, 2006–2017. JAMA Netw Open. 2019;2(3):e190665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guy GP Jr., Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arizpe A, Reveles KR, Aitken SL. Regional variation in antibiotic prescribing among Medicare part D enrollees, 2013. BMC Infect Dis. 2016;16(1):744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDonald DC, Carlson K, Izrael D. Geographic variation in opioid prescribing in the U.S. J Pain. 2012;13(10):988–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JK, Baker LA, Seirawan H, Crimmins EM. Prevalence of oral health problems in U.S. adults, NHANES 1999–2004: exploring differences by age, education, and race/ethnicity. Spec Care Dentist. 2012;32(6):234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janakiram C, Chalmers NI, Fontelo P, et al. Sex and race or ethnicity disparities in opioid prescriptions for dental diagnoses among patients receiving Medicaid. J Am Dent Assoc. 2018;149(4):246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung S, Sexton ME, Owens S, Spell N, Fridkin S. Variability of antibiotic prescribing in a large healthcare network despite adjusting for patient-mix: reconsidering targets for improved prescribing. Open Forum Infect Dis. 2019;6(2):ofz018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinmetz CN, Zheng C, Okunseri E, Szabo A, Okunseri C. Opioid Analgesic Prescribing Practices of Dental Professionals in the United States. JDR Clin Trans Res. 2017;2(3):241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rasubala L, Pernapati L, Velasquez X, Burk J, Ren YF. Impact of a mandatory prescription drug monitoring program on prescription of opioid analgesics by dentists. PLoS ONE. 2015;10(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bohnert ASB, Guy GP, Losby JL. Opioid prescribing in the United States before and after the Centers for Disease Control and Prevention’s 2016 opioid guideline. Ann Intern Med. 2018;169(6):367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soelberg CD, Brown RE, Du Vivier D, Meyer JE, Ramachandran BK. The US opioid crisis: current federal and state legal issues. Anesth Analg. 2017;125(5):1675–1681. [DOI] [PubMed] [Google Scholar]
- 36.Rutkow L, Chang H-Y, Daubresse M, Webster DW, Stuart EA, Alexander GC. Effect of Florida’s prescription drug monitoring program and pill mill laws on opioid prescribing and use. JAMA Intern Med. 2015;175(10):1642–1649. [DOI] [PubMed] [Google Scholar]
- 37.King LM, Bartoces M, Fleming-Dutra KE, Roberts RM, Hicks LA. Changes in US outpatient antibiotic prescriptions From 2011–2016. Clin Infect Dis. 2020;70(3):370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1–12. [DOI] [PubMed] [Google Scholar]
- 39.Gross AE, Hanna D, Rowan SA, Bleasdale SC, Suda KJ. Successful Implementation of an antibiotic stewardship program in an academic dental practice. Open Forum Infect Dis. 2019;6(3):ofz067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


