Abstract
Calvarial critical-size defect has been used to assess techniques and materials in the bone regeneration field. Previous studies utilized young adult rats with 3 months of age, which might not reflect the geriatric conditions. This study aimed to assess the dimensions of the calvarial critical-size defect in aged rats.
Seventy-two rats in a randomized block design were allocated into a control young adult (11–12 weeks), and a test old group (22–24 months). Both groups were divided according to bone defect's size: 3 mm, 5 mm, and 7 mm defects, which were surgically created and followed for 4 and 8 weeks. Radiographic and histologic analyses were performed. Based on the results, additional groups with 4 mm defect size were added following the same protocols. Young groups yielded higher bone volumes, defect closure percentages, and density of newly formed bone. Closure of cranial defects was only observed in 3 mm defects in both age groups after 8 weeks; however, the 4 mm defect group demonstrated bony bridging after 8 weeks in young but not old rats. Results confirmed that 5-mm defect is considered a critical size for calvarial bone defects in young adult rats; however, 4 mm defect might be considered critical size for the aged rats after 8 weeks.
Keywords: Aged rats, animal study, calvarial bone, critical size defect, rodents
Alveolar bone loss remains a challenge in the oral rehabilitation field.1 Several systemic and local factors might contribute to and influence the severity of bone loss, including inflammatory periodontal diseases, trauma, malignancy, and a wider range of pathological lesions.2 Also, following tooth loss, continuous bone resorption might take place as a result of the postextraction remodeling.3 However, adequate alveolar bone dimensions is required for implant placement and around natural teeth to ensure long-term predictable outcomes of rehabilitation procedures.4 Therefore, when inadequate bone dimensions present, regenerative procedures might be indicated to increase the available bone width or hight.4 Several regenerative materials and techniques have been established in this filed,5,6 however, before their clinical application, a thourogh in vitro and in vivo evaluations of these materials and techniques usually performed.7,8
Rat calvarial critical size defect (CSD) has been considered as one of the most predictable and widely used in vivo model in the field of bone regeneration,7,8 it has been extensively used in the development and establishment of a wide range of regenerative materials and techniques.8
Several reports have investigated the effectiveness of numerous synthetic scaffolds and grafting/bone substitute materials including apatitites, calcium phosphates, and calcium sulphate among others.9,10 Natural materials also have been widely studied including dried friezed allografts, xenografts, and corals by CSD model.9,11,12
The investigations have been extended to include resorpable and nonresorbable barrier membraes in the guided bone and tissue regeneration techniques including collagen, and chitosan membranes.12,13 On the other hand, several bioactive molecules have been also evaluated with the same model.14
Critical size defect has been defined as the defect that does not heal within the duration of the study.15 Controversy continues in the literature regarding the diameter of the rat calvarial CSD with reports ranged between 4 mm and 8 mm, reflecting the need for a control group in each study.8 However, many researchers adopted the 5 mm diameter as a critical-size calvarial defect in healthy rats.16
Several systemic and local factors might influence the regenerative capacity of the bone, including diseases, medications, and the individual's age. Previous reports investigated the calvarial CSD dimensions in the neonate and juvenile mice (4–6 days), and young adult rats (10–12 weeks).8,17,18 However, CSD dimensions have never been evaluated in geriatric rats compared to young adult rats.
In fact, with the improved health-care services worldwide, the number of elderly patients is progressively increasing, and the number of older patients who require bone regeneration procedure is also growing. Additionally, several reports demonstrated that old individuals have a higher rate of bone fracture,19,20 a decline in bone healing potential, and compromised wound healing.21–23 This might reflect the need for a well-established model for CSD in geriatric rats.
As most previous animal studies utilized 3-months-old young adult rats with 3 months of age, which might not represent the actual geriatric conditions, this study aimed to assess dimensions of the calvarial bone CSD in aged rats compared to young adult ones.
MATERIALS AND METHODS
Animals
Seventy-two male Sprague–Dawley (SD) healthy rats were obtained from the Animal House at Jordan University of Science and Technology. Rats were allocated according to their age into a control young adult group (Y) with 11 to 12 weeks of age (weight, mean ± SD: 312 ± 28 g) (n = 36), and a test old-age group (O) with 23 to 24 months of age (weight, mean ± SD: 556 ± 262 g) (n = 36). The animals were housed and kept separately in cages under veterinary supervision at the animal house in the university campus 1 week before surgical procedures in a room with a 12-hour light/dark cycle and 22°C to 24°C. Food (standard laboratory diet) and drinking water were available ad libitum. All experimental procedures were approved by the Animal Care and Use Committee at the university before starting the study.
The test and the control groups were subdivided according to the planned calvarial defect's size as following: Young with 3 mm defects (Y3), young with 5 mm defects (Y5), young with 7 mm defects (Y7). Old-age with 3 mm defects (O3), old-age with 5 mm defects (O5), and old-age with 7 mm defects (O7).
Surgical Procedure
Rats were anesthetized with a combination of ketamine (10% Ketasol; Richter Pharma AG, Wels, Austria), and xylazine (Rompuns, Bayer, Leverkusen, Germany) with 55 and 6 mg/kg, respectively. After general anesthesia, the scalp was shaved and disinfected with povidone-iodine. Local anesthetic solution (Ubistesine forte, 3M, Germany) with epinephrine was injected at the midline. A 2 cm-long midline incision was made along the sagittal suture, and the skin, subcutaneous tissue, and periosteum were reflected, exposing the parietal bones.
For the 3 and 5 mm defects, 2 full-thickness nonsuture associated bone defects were trephined under constant normal saline irrigation in the dorsal part of the parietal bone lateral to the sagittal suture. Care was taken during the surgical procedure to prevent damage to the dura mater. A small periosteal elevator was used to elevate the trephined bone segment carefully. For the 7 mm group, bone defects were made in the mid-sagittal area. Full-thickness bone drilling was avoided here, in order to prevent brain and dura matter injury. Alternatively, a trephine bur with an outer diameter of 7 mm was used to mark the defect boundary lines; then, a small round diamond bur was used under continuous saline irrigation to deepen these lines. After making the defects, a small periosteal elevator was used carefully to elevate the bone segment.
The periosteum first was sutured in place with 4–0 polyglycolic acid suture, (PGA, ACUFIRM, Germany), then the skin was repositioned and sutured by 4–0 silk (SILK, Atramat, Mexico). The sutured area was disinfected with povidone-iodine solution again after the surgery. Then, rats were housed at room temperature and observed until they did not show any visible signs of distress. After that, they were transferred to their cages and were given access to water and food ad libidum. Finally, rats were monitored for signs of complications related to surgery or illness.
Tissue Harvest and Radiological Analyses
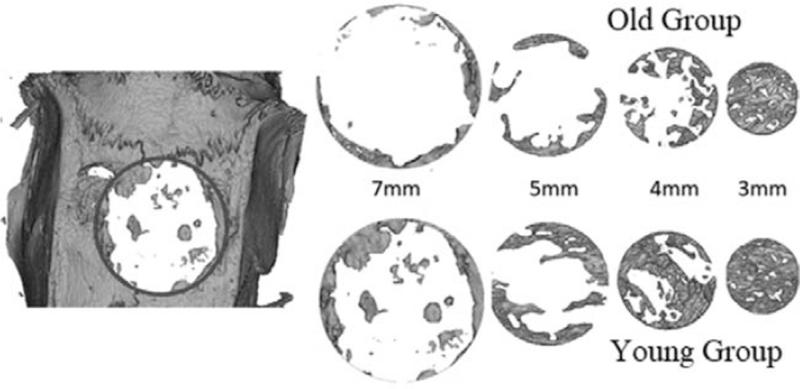
Rats were sacrificed at 4 or 8 weeks after the surgery. The skin was dissected, and defect sites were removed along with surrounding bone and soft tissues. X-ray imaging was performed by a micro-CT scanner (InspeXio; Shimadzu Science East Corporation, Tokyo, Japan) with a voxel size of 70 mm/pixel. Dicom files were then imported to Mimics.12 software (Materialize, Leuven, Belgium) to make the 3D reconstruction. The settings were adjusted for the slices distance and orientation first and Mimics Basic Module was used to generate the first mask representing the whole harvested cranial bone. Region growing function was then used to segment the area of interest with 3 mm, 5 mm, and 7 mm, corresponding to the size of the defects in this study, with the full thickness of the cranial bone for the analysis. The areas of interest were placed where the original defects were located as the margins were visually detectable (Fig. 1).
FIGURE 1.
Micro-computed tomography images reconstructed for young and adult groups at 8 weeks. The newly formed bone in the region of interest is shown.
The 3D volume was then generated from the area of interest Mask work, the newly formed bone volume was measured, and the percentage of defect closure was also calculated according to micro-CT images.
Histological Evaluation
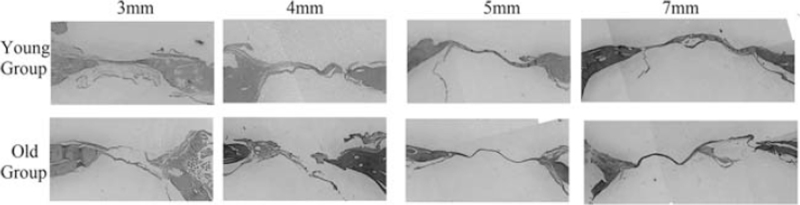
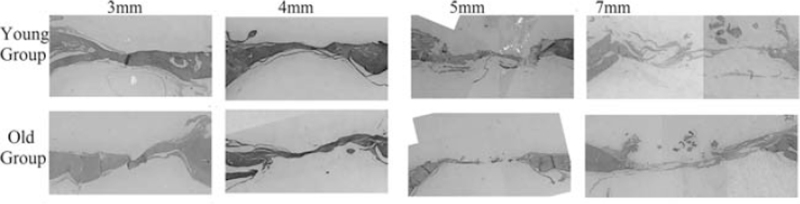
All specimens were fixed in 10% formalin and decalcified in 10% formic acid for 14 days. Samples were then dehydrated by gradually increasing the ethanol concentration, and placed then in xylene for 3 hours. After completion of the decalcification process, the specimens were sectioned using a microtome (MICROM HM 315, Thermo Fisher Scientific Inc, Germany) in a coronal section through the middle of the defects. The samples were then embedded in paraffin, sectioned at 5 μm thickness, and stained with hematoxylin and eosin stain. The samples were then evaluated by light microscopy (Optika, B- 150DB, Italy). Images of the histologic sections were captured by a digital camera connected to a light microscope (Optika, B-190TB, Italy) with an original magnification 4× and loaded to a computer. The slides were studied for the detection of new bone formation (Figs. 2 and 3).
FIGURE 2.
Photomicrographs of the cranial defects (coronal section) after 4 weeks representing the young and old groups. (Hematoxylin and eosin stain).
FIGURE 3.
Photomicrographs of the cranial defects (coronal section) after 8 weeks representing the young and old groups. (Hematoxylin and eosin stain).
Additional Test and Control Groups
Following the radiographic assessment of the preliminary results, an additional test and control groups were added with 4 mm defect size (Y4 and O4). The above mentioned surgical, radiographic, and histological protocols were also followed for the additional groups.
Statistical Analysis
The analyses were performed using the Statistical Analysis System (SAS version 14.3, SAS Institute, Cary, NC). Means in supplemental tables and texts are expressed as mean (SD); differences were considered significant at P ≤ 0.05 (Supplementary Digital Content, Table 1).
The random block design follows that data were collected from 16 groups (blocks or experimental units).24 In each block, 6 rats were randomly assigned, and measurements for defect size, age (young versus old), week (week 4 versus week 8), bone volume (outcome), and closure proportions (outcome) were obtained from different rats for each block.
We modeled the new bone volume as linear, and bone defect closure proportion (bounded between 0 and 1) as beta regression model (logit = linear predictor). For the outcome measure of bone density, data were collected only from 8 blocks (at week 8). The density measurements from different rats within each block were also modeled as linear. The effect of defect size on outcome variables was analyzed using mixed-effects models with defect size (4 categories), week and age variables, treated as fixed effects, and group (blocking variable) as a random intercept. This allowed us to account for the correlations between the multiple variables observations within each block. The denominator degrees of freedom were calculated using the method of Kenward–Roger correction and analyses were written with a mixed procedure for linear models and generalized linear mixed models procedure for beta regression.
RESULTS
Mortality Rate and Complications
A total of 96 male SD rats were utilized in this study. Three animals died in the young adult groups as a result of intraoperative injury of the dura matter due to the convexity of the calvarial bone compared to the straight cutting level of the trephine bur; 1 rat from Y5 group and the other 2 rats were in Y7 group. In the old groups, 4 animals died, 2 were in the O3 group during the surgery, and 2 from O7 (one during the surgery, the other after 1 day). As a result, a total of 45 young and 44 old rats completed the study; they recovered well after surgery, and soft tissue wounds healed without showing clinical signs of inflammation within 2 weeks.
Radiological Observation
After 4 weeks, only a slight amount of new bone was formed in some areas along the margins of calvarial defects in the young group. At 8 weeks, more new bone formation was observed with the thin layer of bone at the margins extending toward the center in Y7 and Y5. However, a relatively complete defect closure was only observed in the Y3 group. Bone defect bridging with lateral residual defects was observed at 8 weeks in Y4 group (Fig. 1).
Less amounts of new bone formation were observed in the old group generally. At 8 weeks, more new bone formation was observed compared to 4 weeks results. However, complete closure of the bone defect was only observed in O3 group after 8 weeks. Statistically significant results were observed in 3 mm (P < 0.001) and 4 mm (P = 0.001) defects compared to 5 mm defect.
Radiographic Defect Closure, New Bone Volume, and Density
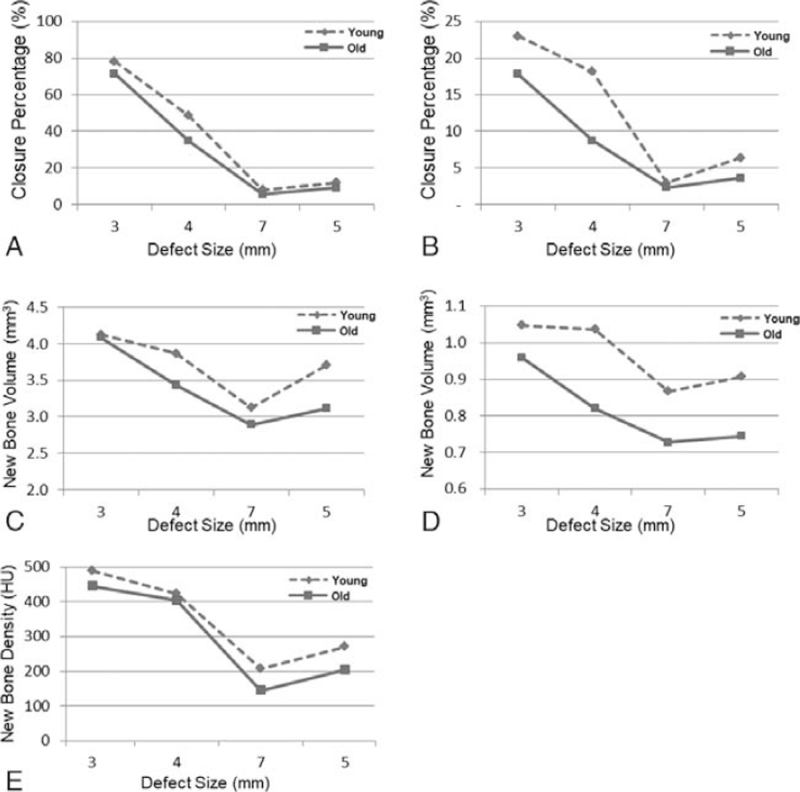
Figure 4 shows the percentage of defect closure, newly formed bone volume, and density, for young and old groups. In the young group, after 4 weeks, the mean newly formed bone volume ranged between 0.87 mm3 to 1.05 mm3 in Y7 and Y3 groups, respectively. On the other hand, the old group showed less new bone formation with a range of 0.73 mm3 to 0.96 mm3 in O7 and O3, respectively. However, after 8 weeks, a larger newly formed bone was observed in all groups compared to 4 weeks findings with the largest volume of 4.12 and 4.08 mm3 were observed in Y3 and O3 groups, respectively (Supplementary Digital Content, Table 1, Fig. 4).
FIGURE 4.
Mean results affected by the defect size for young and old groups after 4 and 8 weeks: (A) Closure proportion at week 8. (B) Closure proportion at week 4. (C) Bone volume at week 8. (D) Bone volume at week 4. (E) Bone density at week 8.
Young adult groups demonstrated higher defect closure percentage after 4 and 8 weeks compared to the old groups. After 4 weeks, mean closure proportion in the young group ranged between 2.94% and 23% in Y7 and Y3, respectively, and between 2.36% and 17.8% in the old group, respectively. However, after 8 weeks, closure proportion increased in both young and old groups ranging between 8.02% in Y7 and 78.12% in Y3 and in the old group ranging between 5.68% in O7 and 71.5% in O3 group.
The density of newly formed bone (measured at 8 weeks) was larger in the young adult group compared to the old group. Among all the variables evaluated in this study, the rat's age significantly influences the newly formed bone density (P = 0.019). In 3 mm defect, density ranged between 489.17 hounsfield unit (HU) and 444.20 HU in the Y3 and O3, respectively; however, density in 7 mm defect ranged between 207.80 in young and 144.80 in old groups, respectively.
Histological Evaluation
After 4 weeks, the new bone failed to progress toward the center of the defect, and only a thin layer of new bone was seen at the defect margins. The central portion of the defect was filled with compressed fibrous connective tissue (Fig. 2). On the other hand, after 8 weeks, more bone was evident at the margins extending toward the center of the defect; however, complete fill of the defect was only observed in O3 and Y3 groups (Fig. 3).
DISCUSSION
Numerous studies in the literature have utilized the rat calvarial CSD model to evaluate the effectiveness of materials and techniques in the field of bone regeneration.8 However, young adult rats were mostly utilized, which might not reflect the in vivo conditions of older individuals. The current study might be the first one that evaluates the dimensions of calvarial CSD in old rats compared to the young adult ones in the same experiment.
Three cranial defect sizes were utilized firstly in this study, including 3, 5, and 7 mm. The radiographic evaluation, which was confirmed histologically, revealed that both 5 and 7 mm defects failed to bridge spontaneously in the old and young adult groups after 4 and 8 weeks.
Complete bone fill was observed in the 3 mm defects of the old and young adult groups after 8 weeks. Accordingly, an additional old and young groups with 4 mm calvarial defects have been evaluated as a second step in this study.
Bony bridging of the 4 mm defects was observed after 8 weeks in the young adult group, which was not observed in the 5 mm defect, on the other hand, 4 mm defects failed to bridge in the old group after 8 weeks, sustaining a residual central bony defect. These results might indicate that after 8 weeks, 5 mm defect is the CSD for the young adult group, while 4 mm defect might be considered as CSD for old adult rats. This was in agreement with previous studies that utilized the 5 mm as a critical size for young adult rats,16,25,26 therefore, the results of the 5 mm defect in this study have been utilized as a reference variable to which, results of other defect sizes were compared.
Unfortunately, establishing the CSD dimensions in aged rats might be complicated by a lack of an agreement for CSD dimensions for young adult rats in the literature.27 The controversy of this issue might reflect the need for standardization every time by incorporating a control group to demonstrate the difference in the bone healing capacity, which was followed in the current study.
The differences in the amount of healing and so, the CSD determination could be attributed to several factors, including the species and strains of the animals utilized along with the anatomic location,28 defect size,29 periosteum intactness,30 dura matter,31 and animal age.18 In this study, all factors have been standardized to highlight the effect of age.
The surgical technique might also influence the healing of bone defect,27 a modification of the surgical technique in 7 mm defects has been used in this study due to the discrepancy between the flat-end trephine bur and the curved parietal bone.
Critical size defect was defined previously as the smallest bone defect that does not heal spontaneously through the lifetime of the animal without intervention.32 The concept has been revised to be the defect dimension that does not heal spontaneously through the duration of the study.33 The latter definition might reflect the increasing needs for shorter healing periods sought by researchers, physicians/dentists as well as patients in the bone regeneration field. Accordingly, in this study, the follow-up periods were limited to 4 and 8 weeks only. In addition, the critical time between the 4th and 8th week after injury in rodent models was found to be sufficient to estimate the total healing that will occur.27 This has been confirmed in this study, as the results of 4 weeks demonstrated a dense connective tissue at the surgical site in both young and old groups, which might indicate that 4 weeks period was inadequate for the spontaneous healing process to be achieved.
At 8 weeks, the bone defects with 3 mm diameter regenerate spontaneously until a relatively complete fill of the defect in young and old rats, which was in agreement with previous studies involved 3 mm defects in young age rats, showed complete fill.26
The HU has been used in this study to evaluate the density of newly formed bone. The gray value/HU is correlated to the degree of mineralization represented by the intercellular matrix deposition. In this study, significantly higher newly formed bone density has been found in young rat groups compared to corresponding older groups. This might be attributed to the slower late mineralization rate34 accompanied by calcium stores depletion noted in older individuals.35
In fact, age-related changes are expected to suppress the bone healing capacity36 by affecting bone cells, bone architecture, hormones, growth factors, and cell signaling molecules. Also, with aging, mineral composition is affected by the rate of revascularization, and wound healing also decreases, resulting in impaired bone function.37 Delayed bone formation in elderly individuals38 might be attributed in part to the prolonged inflammatory response that affects the differentiation of bone-forming cells along with the deficiency of progenitor cells.39 Previous studies demonstrated that mesenchymal stem cells explanted from geriatric mice were found to be reduced in number with decreased in vitro proliferation.40 Moreover, aged animals demonstrated reduced vascular density, which might delay angiogenesis during the fracture healing.22
Despite these differences in the volume and density of newly formed bone, the overall cellular and molecular mechanisms of the subcritical defect (3 mm) healing appeared to be maintained in the old rat group and were parallel to the process observed in the young adult group.
Although it is hard to apply precisely the results of animal studies on clinical trials, in vivo study model is considered a crucial part in developing new biomaterials and techniques in the field of bone regeneration. Animal models, particularly the rat calvarial bone defect model have been widely used, linking in vitro and clinical trials.8
The precise correlation between laboratory rats age and human is still a subject of debate41 compared rat and human ages at different phases of their life and indicated that rats are considered to be aged when they are 24 months of age, which corresponds to 60 years old in humans. However, Flurkey et al42 gave a range of 18–24 months of age for rats that correspond to 56 to 69 years in humans. In this study, rats with 24 months old have been utilized to represent the aged group.
Older patients represent an increasing percentage of the population seeking periodontal treatment and oral rehabilitation procedures, including bone grafting and dental implants. The results of this study demonstrated that rat cranial CSD dimensions of 4 mm might be used in future studies to investigate materials and techniques to improve the clinical care of elderly patients.
Limitations of this study might include that only 2 age groups have been evaluated. Inclusion of a middle-aged group would clearly evaluate the changes of bone healing with age.
Aged rats showed significantly less new bone formation with lower density compared to young adult rats. However, the results of this study confirmed that the 5 mm defect is considered a critical size for calvarial bone defects in young adult and 4 mm defects for the aged SD rats after 8 weeks.
Supplementary Material
Acknowledgments
The authors thank Dr Abdul-Rahman Hudieb- Monash University, for the statistical analysis.
Footnotes
This research has been supported by the Deanship of Scientific Research at Jordan University of Science and Technology (Research grant no. 592–2016).
The authors report no conflicts of interest.
Supplemental digital contents are available for this article.
REFERENCES
- 1.Troeltzsch M, Troeltzsch M, Kauffmann P, et al. Clinical efficacy of grafting materials in alveolar ridge augmentation: a systematic review. J Cranio-Maxillofacial Surg 2016; 44:1618–1629. [DOI] [PubMed] [Google Scholar]
- 2.Di Benedetto A, Gigante I, Colucci S, et al. Periodontal disease: linking the primary inflammation to bone loss. Clin Dev Immunol 2013; 2013:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kingsmill VJ. Post-extraction remodeling of the adult mandible. Crit Rev Oral Biol Med 1999; 10:384–404. [DOI] [PubMed] [Google Scholar]
- 4.Misch CE. Available bone and dental implant treatment plans. Dental Implant Prosthetics 2015; Philadelphia: Elsevier Mosby, 315–339. [Google Scholar]
- 5.Elnayef B, Monje A, Gargallo-Albiol J, et al. Vertical ridge augmentation in the atrophic mandible: a systematic review and meta-analysis. Int J Oral Maxillofac Implants 2017; 32:291–312. [DOI] [PubMed] [Google Scholar]
- 6.Aghaloo TL, Misch C, Lin G-H, et al. Bone augmentation of the edentulous maxilla for implant placement: a systematic review. Int J Oral Maxillofac Implants 2016; 31:s19–30. [DOI] [PubMed] [Google Scholar]
- 7. da Silva Morais A, Oliveira JM, Reis RL. Small animal models. Osteochondral Tissue Engineering. 2018:1059:423–439. [DOI] [PubMed] [Google Scholar]
- 8.Gomes PS, Fernandes MH. Rodent models in bone-related research: the relevance of calvarial defects in the assessment of bone regeneration strategies. Lab Anim 2011; 45:14–24. [DOI] [PubMed] [Google Scholar]
- 9.Gavazzoni A, Filho LI, Hernandes L. Analysis of bone formation andmembrane resorption in guided bone regeneration using deproteinized bovine bone mineral versus calcium sulfate. J Mater Sci Mater Med 2018; 29:167–174. [DOI] [PubMed] [Google Scholar]
- 10.Rojbani H, Nyan M, Ohya K, et al. Evaluation of the osteoconductivity of α-tricalcium phosphate, β-tricalcium phosphate, and hydroxyapatite combined with or without simvastatin in rat calvarial defect. J Biomed Mater Res A 2011; 98:488–498. [DOI] [PubMed] [Google Scholar]
- 11.Naaman NB, Ouhayoun JP. Bone formation with discs or particles of natural coral skeleton plus polyglactin 910 mesh: histologic evaluation in rat calvaria. Int J Oral Maxillofac Implants 1998; 13:115–120. [PubMed] [Google Scholar]
- 12.Mokbel N, Bou Serhal C, Matni G, et al. Healing patterns of critical size bony defects in rat following bone graft comparative study. Oral Maxillofac Surg 2008; 12:73–78. [DOI] [PubMed] [Google Scholar]
- 13.Song JM, Shin SH, Kim YD. Comparative study of chitosan/fibroin-hydroxyapatite and collagen membranes for guided bone regeneration in rat calvarial defects: micro-computed tomography analysis. Int J Oral Sci 2014; 6:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kizilaslan S, Karabuda Z, Olgac V. The effect of concentrated growth factor on calvarial bone in diabetic healing. J Craniofac Surg 2020; 31:158–161. [DOI] [PubMed] [Google Scholar]
- 15.Gosain AK, Santoro TD, Song L-S, et al. Osteogenesis in calvarial defects: contribution of the dura, the pericranium, and the surrounding bone in adult versus infant animals. Plast Reconstr Surg 2003; 112:515–527. [DOI] [PubMed] [Google Scholar]
- 16.Vajgel A, Mardas N, Farias BC, et al. A systematic review on the critical size defect model. Clin Oral Implants Res 2013; 25:879–893. [DOI] [PubMed] [Google Scholar]
- 17.Wu X, Downes S, Watts DC. Evaluation of critical size defects of mouse calvarial bone: an organ culture study. Microsc Res Tech 2010; 73:540–547. [DOI] [PubMed] [Google Scholar]
- 18.Aalami OO, Nacamuli RP, Lenton KA, et al. Applications of a mouse model of calvarial healing: differences in regenerative abilities of juveniles and adults. Plast Reconstr Surg 2004; 114:713–720. [DOI] [PubMed] [Google Scholar]
- 19.Cauley JA, Thompson DE, Ensrud KC, et al. Risk of mortality following clinical fractures. Osteoporos Int 2000; 11:556–561. [DOI] [PubMed] [Google Scholar]
- 20.Rose S, Maffulli N. Hip fractures. An epidemiological review. Bull Hosp Jt Dis 1999; 58:197–201. [PubMed] [Google Scholar]
- 21.Yao B, Huang S, Gao D, et al. Age-associated changes in regenerative capabilities of mesenchymal stem cell: impact on chronic wounds repair. Int Wound J 2015; 13:1252–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark D, Nakamura M, Miclau T, et al. Effects of aging on fracture healing. Curr Osteoporos Rep 2017; 15:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burr DB. Changes in bone matrix properties with aging. Bone 2019; 120:85–93. [DOI] [PubMed] [Google Scholar]
- 24.Festing MFW. Randomized block experimental designs can increase the power and reproducibility of laboratory animal experiments. ILAR J 2014; 55:472–476. [DOI] [PubMed] [Google Scholar]
- 25.Artzi Z, Weinreb M, Tal H, et al. Experimental intrabony and periodontal defects treated with natural mineral combined with a synthetic cell-binding peptide in the canine: morphometric evaluations. J Periodontol 2006; 77:1658–1664. [DOI] [PubMed] [Google Scholar]
- 26.Aybar Odstrcil A, Territoriale E, Missana L. An experimental model in calvaria to evaluate bone therapies. Acta Odontol Latinoam 2005; 18:63–67. [PubMed] [Google Scholar]
- 27.Cooper GM, Mooney MP, Gosain AK, et al. Testing the critical size in calvarial bone defects: revisiting the concept of a critical-size defect. Plast Reconstr Surg 2010; 125:1685–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Najjar TA, Kahn D. Comparative study of healing and remodeling in various bones. J Oral Surg 1977; 35:375–379. [PubMed] [Google Scholar]
- 29.Bosch C, Melsen B, Vargervik K. Importance of the critical-size bone defect in testing bone-regenerating materials. J Craniofac Surg 1998; 9:310–316. [DOI] [PubMed] [Google Scholar]
- 30.Huh J-Y, Choi B-H, Kim B-Y, et al. Critical size defect in the canine mandible. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005; 100:296–301. [DOI] [PubMed] [Google Scholar]
- 31.Gosain AK, Gosain SA, Sweeney WM, et al. Regulation of osteogenesis and survival within bone grafts to the calvaria: the effect of the dura versus the pericranium. Plast Reconstr Surg 2011; 128:85–94. [DOI] [PubMed] [Google Scholar]
- 32.Schmitz JP, Hollinger JO. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res 1986; NA:299–308. [PubMed] [Google Scholar]
- 33.Gosain AK, Song L, Yu P, et al. Osteogenesis in cranial defects: reassessment of the concept of critical size and the expression of TGF-beta isoforms. Plast Reconstr Surg 2000; 106:360–371. 372. [DOI] [PubMed] [Google Scholar]
- 34.Lopas LA, Belkin NS, Mutyaba PL, et al. Fractures in geriatric mice show decreased callus expansion and bone volume. Clin Orthop Relat Res 2014; 472:3523–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan GK, Duque G. Age-related bone loss: old bone, new facts. Gerontology 2002; 48:62–71. [DOI] [PubMed] [Google Scholar]
- 36.Boskey AL, Coleman R. Aging and Bone. J Dent Res 2010; 89:1333–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu C, Miclau T, Hu D, et al. Cellular basis for age-related changes in fracture repair. J Orthop Res 2005; 23:1300–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferretti C, Lucarini G, Andreoni C, et al. Human periosteal derived stem cell potential: the impact of age. Stem Cell Rev Rep 2014; 11:487–500. [DOI] [PubMed] [Google Scholar]
- 39.O’Driscoll SWM, Saris DBF, Ito Y, et al. The chondrogenic potential of periosteum decreases with age. J Orthop Res 2001; 19:95–103. [DOI] [PubMed] [Google Scholar]
- 40.Mutyaba PL, Belkin NS, Lopas L, et al. Notch signaling in mesenchymal stem cells harvested from geriatric mice. J Orthop Trauma 2014; 28:S20–S23. [DOI] [PubMed] [Google Scholar]
- 41.Sengupta P. The laboratory rat: relating its age with human's. Int J Prev Med 2013; 4:624–630. [PMC free article] [PubMed] [Google Scholar]
- 42.Flurkey K, Mcurrer J, Harrison D. Mouse models in aging research. The Mouse in Biomedical Research 2007; New York: Elsevier, 637–672. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.