Abstract
Although elevated estradiol levels facilitate chronic pelvic pain in animal models, it remains to be determined whether sex steroid levels are altered in a cross-section of women with chronic pelvic pain (CPP) and those at-risk for developing CPP. We sought to determine if sex steroid levels are increased in women with menstrual pain and whether those changes were more extreme in two groups of women with worsened pelvic pain profiles: a) dysmenorrhea plus evidence of bladder pain sensitivity and b) bladder pain syndrome. Serum samples were collected during the mid-luteal phase to measure estradiol, progesterone, testosterone, and sex hormone-binding globulin. We also compared quantitative sensory testing profiles to evaluate how sex steroid differences influence proposed pain sensitivity mechanisms. Women with combined dysmenorrhea and bladder sensitivity had higher estradiol concentrations than controls (487 [IQR 390 – 641] vs 404 [336 – 467] pmol/L, p = 0.042). Bladder pain syndrome participants had greater sex hormone-binding globulin than controls (83 [71 – 108] vs 55 [42 – 76 nmol/L; p = 0.027). Levels of pain sensitivity and mood were different across the groups, but the only significant relationship to sex steroids was that sex hormone-binding globulin was correlated to somatic symptoms (r = 0.26, p = 0.03). These findings show women potentially at-risk for CPP and women with diagnosed CPP exhibit altered circulating levels of sex steroids. Because these hormonal differences appear to be independent of mood or pain sensitivity, the role of sex steroids in the emergence of CPP may be via sensitization of visceral afferents.
Keywords: Dysmenorrhea, estradiol, quantitative sensory testing, sex hormone-binding globulin
Introduction
The role of sex steroids in dysmenorrhea or chronic pelvic pain conditions such as bladder pain syndrome is unknown, even though hormonal suppression is frequently prescribed to treat these conditions.1,2 Sex steroids are among the most potent transcriptional modulators of synaptic plasticity, with enormous potential to modulate pain signaling. 3 Estradiol can increase synaptic activity and facilitate pelvic pain by inducing signal cascades that phosphorylate NMDA synaptic receptors in experimental induction models of pelvic cross-organ sensitization.4,5 These results are consistent with many other studies showing that sex steroids affect pelvic nerve and visceral sensitivity.6–11 Conversely, reduced levels of estrogen (which often occurs during perimenopause) could exacerbate pain sensitivity by increasing levels of neuropeptides, and inflammatory molecules known to evoke pain sensitization. For example, animal models have demonstrated that ovariectomy-induced reductions in estradiol increase pain sensitivity and Substance P, with those changes normalized by administering supplementary estradiol.12,13 Likewise, estradiol can also increase COX-2 activity and prostaglandin synthesis systemically response to inflammation. 14 Thus, there are complex bidirectional effects of estradiol on pain sensitivity. Because women with dysmenorrhea are at a greater risk of developing chronic pelvic pain, 15 identifying sex steroid differences could help identify women at increased risk of developing chronic pelvic pain and suggest potential therapeutic targets.
Despite the potential importance of sex steroids in pain conditions, few studies have characterized the differences in women with episodic menstrual pain or chronic pelvic pain. Whereas one study reported higher estradiol concentrations in women with dysmenorrhea compared to pain-free controls, another reported no significant differences.16,17 The two studies' discrepancies could be due to an escalating association with dysmenorrhea and pelvic pain intensity. For example, participants with milder dysmenorrhea may have lower estradiol levels than those with more severe forms of both menstrual and chronic pelvic pain. Although there is a correlation between endogenous serum estradiol content and pain sensitivity,18,19 it remains to be determined whether endogenous levels are altered in chronic pain conditions.
Additionally, there are likely bidirectional effects of sex steroids and mood. 20 Indeed, studies in women with perimenstrual mood symptoms have often reported elevated serum estradiol concentrations during the luteal phase.21–23 Thus, any study establishing cross-sectional differences in sex steroids in episodic and chronic pain conditions should also evaluate the potential confounding effects of psychological factors. Because ∼40% of estradiol is sequestered by sex hormone-binding globulin (SHBG) and has been reported to correlate with pain severity24,25 and depression, 26 SHBG may be an essential molecule to evaluate simultaneously.
Given the bidirectional influences on sex steroids in pelvic pain modulation, these effects may be hard to untangle once chronic pain is established. Logically, assessing changing serum levels in patients during the transition into chronic pain may yield insight into the role of sex steroids and pain emergence. We hypothesized that increased circulating estradiol levels might be responsible for the transition to chronic pelvic pain. Specifically, in our data set, we hypothesized that elevated estradiol would be observed in a specific subset of women with dysmenorrhea and increased bladder sensitivity (DYSB). Our previous findings indicate that women with DYSB have decreased body and pelvic pain pressure thresholds compared to control participants.27,28 This group's experimental profile and presence of mild clinical symptoms (occasional mild symptoms below the threshold for diagnosis of chronic pain conditions) suggest an increased risk of developing chronic pelvic pain.27,28
In this study, we compare serum sex steroid levels and sex hormone-binding globulin (SHBG) from women with DYSB to healthy pain-free controls (HC), women with dysmenorrhea (DYS = dysmenorrhea without bladder sensitivity, or other chronic pain conditions), and those formally diagnosed with bladder pain syndrome (BPS). We also examined the relationship of sex steroids with psychological profiles and experimental pain sensitivity to understand whether their role on pain experience is related to these common potentially contributing factors.
Materials and methods
Participant recruitment
This prospective observational study was designed to characterize uterine cross-organ influences on experimental bladder pain sensitivity and was approved by the NorthShore University HealthSystem Institutional Review Board. Female participants, ages 18 to 45, were recruited using flyers posted in the community, the Illinois Women’s Health Registry, and referrals from gynecology clinics in our health system. Participants included in this study were recruited and enrolled from August 2014 to May 2018. Before enrollment, all potential participants were instructed to contact our team to complete a phone screen. If they were considered eligible for the study, participants were scheduled for an initial screening visit.
Screening visit
At the start of the screening visit, participants provided their written informed consent. Next, participants completed questionnaires disclosing their medical, surgical, psychological, and gynecological history using REDCap. 29 A gynecologist trained in pelvic pain evaluation performed a standardized pelvic exam on most participants with dysmenorrhea to confirm the absence of potential secondary dysmenorrhea. After confirming that occult pathology was rarely observed (3 times in the first 98 participants), we discontinued pelvic exams. The pelvic exam included systematic palpation of the perineum, vagina, pelvic floor, fornices, uterus, cervix, urethra, and bladder with explicit capture of areas of tenderness, presence of any uterovaginal prolapse, and identification of any palpable pelvic masses such as ovarian cysts, or leiomyoma. 30 A simplified version of the noninvasive sonographic bladder test (detailed below under Assessment Visit) was conducted at the screening visit for dysmenorrheic participants. They were asked to drink 20 ounces (591 mL) of water within 5 minutes and report when they felt the first sensation (i.e., when they first felt capable of urinating) and the first urge (i.e., when they would ordinarily void) to urinate. At each time point, participants recorded their bladder urgency and bladder pain on a 0–100 Visual Analogue Scale (VAS; 0: no pain or urgency; 100: worst pain or urgency imaginable).
All participants were provided luteinizing hormone urinary assay kits (Wondfo USA Co., Willowbrook, IL) to accurately time participation for the subsequent mid-luteal phase assessment visit (approximately 17–25 days post-onset of menses). Participants completed web-based daily diaries for a full menstrual cycle and rated the severity of their menstrual and bladder pain on a 0–10 numerical rating scale (NRS; 0: no pain, 10: worst pain imaginable).
To be enrolled as a healthy pain-free control, a participant had to rate their menstrual pain less than or equal to 3 on an NRS and have no concurrent chronic pain diagnoses. Questionnaires and diaries were used to confirm the lack of concurrent pain conditions. Enrolled dysmenorrheic participants needed to report menstrual pain greater than or equal to 5 on an NRS and have no concurrent chronic pain diagnoses. Participants in the dysmenorrhea with bladder sensitivity (DYSB) group met the criteria for dysmenorrhea and also reported pain (>15 on a 0–100 VAS) during the bladder pain test described below. The rationale for this threshold was established in an earlier publication. 31 Enrolled BPS participants needed a prior diagnosis of BPS/interstitial cystitis as defined by American Urological Association 32 for more than three months and reported pelvic pain greater than or equal to 3 on an NRS.
Participants were excluded from the study for the presence of active pelvic or abdominal malignancies, the absence of regular menses, active genitourinary infection in the last four weeks, the inability to read or comprehend the informed consent in English, the refusal to undergo pelvic examination/testing, BMI > 40, and hypertension. In order to focus on effects associated with primary dysmenorrhea, participants with known cases of secondary dysmenorrhea (e.g., fibroids, endometriosis) were excluded from all groups except the BPS group. Six participants in the BPS group had a prior history of endometriosis. Similarly, dysmenorrhea and healthy control participants with comorbid pain conditions were excluded. Participants with BPS were previously diagnosed with IBS (n = 5), endometriosis (n = 6), arthritis (n = 1), low back pain (n = 6), and migraine (n = 5). All participants were required to wash out of oral contraceptive pills or any other hormonal implants and complete daily diaries for an entire menstrual cycle before the assessment visit.
Assessment visit
All visits were scheduled for the participant’s expected luteal phase. Participants were asked to avoid taking short-acting, over-the-counter analgesics (i.e., ibuprofen, acetaminophen), opioids, and caffeine for at least six hours before the visit. We also prohibited the use of longer-acting NSAIDs (i.e., Aleve) for at least twelve hours before the study visit.
All participants performed a noninvasive bladder test, a mimic of clinical retrograde cystometry. 33 Before the test, participants emptied their bladders and were asked to drink 20 ounces (591 mL) of water within 5 minutes. Participants reported when they reached three standard cystometric urgency thresholds: first sensation, first urge, and maximum tolerance. 34 Participants also rated their bladder pain and urgency at each urgency threshold on a 0–100 VAS. 33 During the test, participants were provided an additional 10 ounces (296 mL) of water at 45 minutes and again at 60 minutes if they had not reached maximum tolerance. Participants were given a total of 120 minutes to complete the test to prevent unnecessary harm and injury. If a participant reached the time limit, she was instructed to report her bladder pain and urgency and then void, regardless of reaching maximum tolerance.
During the bladder test, participants completed questionnaires. On average, participants finished questionnaires 13 ± 5 minutes before rating “first urge” pain on the bladder task. The NIH Patient Reported Outcomes Measurement Information System (PROMIS) anxiety, depression, pain behavior, and pain interference scales 35 were administered to evaluate psychological and pain-related profiles. We characterized physical presentation of somatization using the Brief Symptom Inventory – Somatization subscale (BSI). 36 Participants also completed interstitial cystitis symptom index questionnaires to characterize their BPS symptoms. 37
After completion of the bladder test and questionnaires, we measured pain sensitivity using QST. For complete methods and prior results of QST (without correlation to hormone data), see our earlier study. 27 Because this sex steroid data set only examines a subset of our previous participants, we only included QST parameters which we were adequately powered to detect a difference. Pelvic pain pressure thresholds (PPTs) were assessed transvaginally at four sites (right and left iliococcygeus, anteriorly against the bladder, and posterior anorectal raphe) using a 1 cm2 diameter contact area, with a force-sensing resistor (Trossen Robotics, Downers Grove, IL) mounted inside the examiner's glove. A computer-generated visual guide was used to ramp the stimulus at 0.5 Newtons (N)/s. Body PPTs were evaluated with a digital algometer (Wagner Instruments, Greenwich, CT) with a 1 cm2 rubber tip applied at a ramp rate of 4 N/s with a computer-generated visual guide. Body PPTs were assessed at the right trapezius, the right medial knee fat pad, and the right greater trochanter. These sites correspond to the American College of Rheumatology guidelines for fibromyalgia tender point sites. 38 As a control, we also included a mid-forehead site. Each site was measured in the same order with a 10-second break between each internal site, 15 seconds between each external site, and a two-minute break between repeat measurements. Two trials were performed at each site, and the average threshold was used for analyses. We verified that we had interexaminer reliability of QST measurements (Cronbach α’s ≥ 0.9) before beginning testing and achieved high consistency (Cronbach α’s ≥ 0.9) across the body and pelvic sites. We also evaluated sensitivity to the cold pressor task by having participants immerse their right hand, up to the wrist, into a bucket of ice water (mean temperature = 1.7° C, standard deviation = 1.0° C) with a high-speed water circulation pump (7.4 L/min). After 10 seconds of immersion, participants were asked to rate their hand pain on a 0–10 NRS scale.
Blood collection and serum retrieval
Blood samples, collected by a trained research nurse using BD Vacutainer serum tubes (BD and Co., Franklin Lakes, NJ), remained at room temperature for 30–60 minutes to clot. Samples were then centrifuged at 1200 rpm for 10 minutes at room temperature to retrieve the serum supernatant. Serum samples were immediately aliquoted into cryogenic vials (Corning Inc., Corning, NY) and stored at -80°C until needed.
Serum hormone analyses
Hormone and SHBG assays were performed at the University of Virginia Ligand Assay & Analysis Core of the Center for Research in Reproduction. Radioimmunoassays were used to measure serum estradiol (sensitivity range: 10–1000 pg/mL; MP Biomedicals, Solon, OH). Enzyme immunosorbent assays (Immulite 2000, Siemens Healthcare, Los Angeles, CA) were used to measure serum progesterone (sensitivity range: 0.2 – 40 ng/mL), sex hormone-binding globulin (SHBG; sensitivity range: 2 – 180 nmol/L), and testosterone (sensitivity range: 10 – 1600 ng/dL). Participants with serum testosterone concentrations below the assay’s sensitivity range were assigned a default concentration of 10 ng/dL. All assays were run in duplicate to accommodate any potential errors in pipetting, analytes, contamination, or measurement. Coefficients of variation (Intra-assay, inter-assay) for each assay are as follows: estradiol (6.7%, 9.8%); progesterone (4.2%, 5.8%); SHBG (3.2%, 7.2%), and testosterone (4.9%, 7.1%). Any measurement with a coefficient of variation greater than or equal to 20% was excluded. This level of variation is a standard threshold for excluding potentially invalid data, particularly in hormonal ELISA measurements. 39
Power analysis
Our primary power analysis governing the planned sample size (http://clinicaltrials.gov/ct2/show/NCT02214550) was based on the primary objective for a longitudinal study on the experimental effects of hormonal suppression for menstrual pain and resulted in uneven group allocation. For this observational substudy, we performed a separate power analysis and used an ANOVA power analysis to provide a conservative estimate of the sample size needed. Our calculations suggested a minimum sample size of 76 participants for an omnibus fixed-effects one-way ANOVA (α = 0.05; β = 1 – 0.8) across the 4 groups assuming a large effect size (f = 0.4) using G-power. 40 Notably, sample sizes are somewhat similar for Kruskal-Wallis tests 41 that were needed because the data was not normally distributed. Additional participants (total n = 130) samples were drawn to account for potential analytic failures. Hormone concentrations in 124 participants were measurable, but high coefficients of variation (≥ 20%) resulted in excluding 9 SHBG, 4 estradiol, 7 testosterone, and 7 progesterone measurements. Excluded measurements were not included in analyses. There was no other missing data.
Data and statistical analyses
Data were analyzed in R 4.0 (R Core Team, 2020). p < 0.05 was considered the threshold for significance.
Group differences in clinical characteristics, demographic characteristics, and serum hormone concentrations were evaluated using a Kruskal-Wallis test accompanied by a Dunn’s test. Reported significant statistics were adjusted for multiple comparisons using the Benjamini-Hochberg procedure. 42 Because age is a known confounder of SHBG 43 and the BPS participants were older, we performed an additional sensitivity analysis including age as a covariate with ordered logistic regression. To evaluate if potential confounders (anxiety, menstrual pain, somatic symptoms) could have influenced group differences in sex steroid levels, we calculated Spearman’s correlation coefficient. As an exploratory analysis to further examine the relationship between all metrics of bladder sensitivity, QST and sex steroid levels, we calculated partial correlation coefficients accounting for age (similar to Andersson 44 ).
The data that support the findings of this study are available from the corresponding author, K.M.H., upon reasonable request.
Results
Participants’ demographic, menstrual, psychological, and sensory characteristics
There were no notable differences in race or BMI across the four participant groups (Table 1). BPS participants were about 7 years older than healthy controls, DYS participants, and DYSB participants.
Table 1.
Participants’ demographic information.
| HC | DYS | DYSB | BPS | P value | |
|---|---|---|---|---|---|
| n | 29 | 40 | 37 | 18 | |
| Age | 23.0 [18.0, 28.0] | 22.5 [20.0, 29.2] | 22.0 [20.0, 26.0] | 30.5 [27.2, 34.8] a | 0.008 |
| BMI kg/cm2 | 21.4 [21.0, 22.3] | 22.7 [20.7, 24.4] | 22.2 [20.2, 25.3] | 23.7 [19.8, 27.5] | 0.553 |
| Native American or Pacific Islander | 1 (3.4%) | 1 (2.5%) | 0 (0.0%) | 0 (0.0%) | 0.548 |
| Asian | 8 (27.6%) | 6 (15.0%) | 8 (21.6%) | 0 (0.0%) | 0.094 |
| Black | 2 (6.9%) | 14 (35.0%) | 8 (21.6%) | 4 (22.2%) | 0.054 |
| White | 18 (62.1%) | 24 (60.0%) | 23 (62.2%) | 15 (83.3%) | 0.347 |
| Hispanic | 2 (6.9 %) | 4 (10.0%) | 8 (21.6%) | 0 (0%) | 0.077 |
Note: BMI = Body Mass Index; HC = Healthy Control; DYS = Dysmenorrhea only; DYSB = Dysmenorrhea with Bladder Sensitivity; BPS = Bladder Pain Syndrome. Data are represented as median [25th, 75th percentile] or as number (percentage). Some participants selected multiple racial designations. P values are from Kruskal Wallis tests or X2 tests.
Bold numbers and superscript “a” indicate p < 0.05 different than HC after correction for multiple comparisons.
There were no group differences in menstrual characteristics other than menstrual pain rating. DYS, DYSB, and BPS participants reported severe menstrual pain (medians 70 – 73 on a 0–100 VAS), unlike pain-free controls (median 10, p’s < 0.05; Table 2).
Table 2.
Participants’ menstrual, psychological, and QST characteristics.
| HC | DYS | DYSB | BPS | P value | |
|---|---|---|---|---|---|
| Menstrual characteristics | |||||
| Hormonal contraceptive use in cycle prior to study washout | 2 (6.9%) | 4 (10.0%) | 5 (13.5%) | 4 (22.2%) | 0.439 |
| Hormonal contraceptive use in last 6 months | 3 (10.3%) | 8 (20.0%) | 6 (16.2%) | 6 (33.3%) | 0.252 |
| Cycle length | 28.0 [25.0, 32.0] | 29.0 [26.1, 33.4] | 29.0 [26.0, 33.0] | 28.0 [25.0, 30.8] | 0.499 |
| Visit cycle day | 19 [17 23] | 20 [18 24] | 20 [17 22] | 20 [17 24] | 0.853 |
| Menstrual pain 0–100 VAS | 10.0 [4.0, 22.0] | 73.0 [65.0, 81.2] a | 70.0 [64.5, 85.0] a | 72.0 [60.0, 98.0] a | <0.001 |
| Other pain symptom and psychological characteristics | |||||
| Pain behavior | 50.1 [36.7, 55.6] | 52.5 [36.7, 56.6] | 56.4 [53.9, 59.2] a | 58.9 [56.4, 61.7] a | <0.001 |
| Pain interference | 41.0 [41.0, 50.8] | 48.5 [41.0, 52.5] | 55.0 [50.8, 58.1] a | 62.2 [54.4, 65.2] a | <0.001 |
| Somatic symptoms | 1.0 [0.0, 3.0] | 1.0 [0.0, 4.0] | 3.0 [2.0, 6.0] a | 6.5 [3.0, 9.8] a | <0.001 |
| Anxiety | 53.8 [48.4, 56.3] | 56.3 [46.7, 61.3] | 56.3 [53.8, 61.3] a | 60.0 [55.4, 65.1] a | 0.006 |
| Depression | 53.4 [43.3, 57.1] | 51.2 [43.3, 55.3] | 54.3 [51.2, 61.6] | 57.1 [50.1, 62.5] | 0.036 |
| ICSI | 2 [2 4] | 2 [1 6] | 6 [4 9]a | 11 [9 14] | <0.001 |
| Quantitative sensory testing | |||||
| Bladder pain at first urge | 3.0 [1.0, 3.0] | 2.0 [1.0, 4.0] | 32.0 [24.0, 48.0] a | 42.5 [34.2, 55.0] a | <0.001 |
| Vaginal PPT (Newtons) | 11.9 [7.6, 14.1] | 7.6 [5.8, 12.8] | 6.3 [4.6, 10.7] a | 7.2 [4.5, 10.0] a | 0.010 |
| Body PPT (Newtons) | 23.9 [18.4, 34.1] | 18.1 [14.0, 27.2] | 18.5 [15.2, 22.5] a | 21.9 [15.1, 30.5] | 0.022 |
| Cold pressor (NRS) | 4.5 [3.0, 6.0] | 7.0 [5.0, 9.2] a | 6.0 [5.0, 7.0] a | 6.5 [4.8, 7.2] a | 0.001 |
Note: HC = Healthy pain free Control; DYS = Dysmenorrhea only; DYSB = Dysmenorrhea with Bladder Sensitivity; BPS = Bladder Pain Syndrome; VAS = Visual Analogue Scale; ICSI = Interstitial Cystitis Symptom Index Score NRS = Numerical Rating Scale. Data are represented as median [25th, 75th percentile] or as number (percentage). P values are from Chi-square or Kruskal Wallis tests.
Bold numbers and superscript “a” indicate p < 0.05 different from HC after correction for multiple comparisons.
Pain characteristics were also characterized by PROMIS pain behavior, pain interference scales, and the Interstitial Cystitis Symptom Index. Only DYSB and BPS participants had significantly worse PROMIS pain behavior and pain interference scores than pain-free controls after corrections for multiple comparisons (Table 2). DYSB and BPS participants also had more somatic symptoms compared to pain-free controls (Table 2). DYSB and BPS participants had higher anxiety scores than pain-free controls, but depression scores were not significantly different (Table 2). DYSB and BPS participants also had higher Interstitial Cystitis Symptom Index Scores than pain-free controls (Table 2).
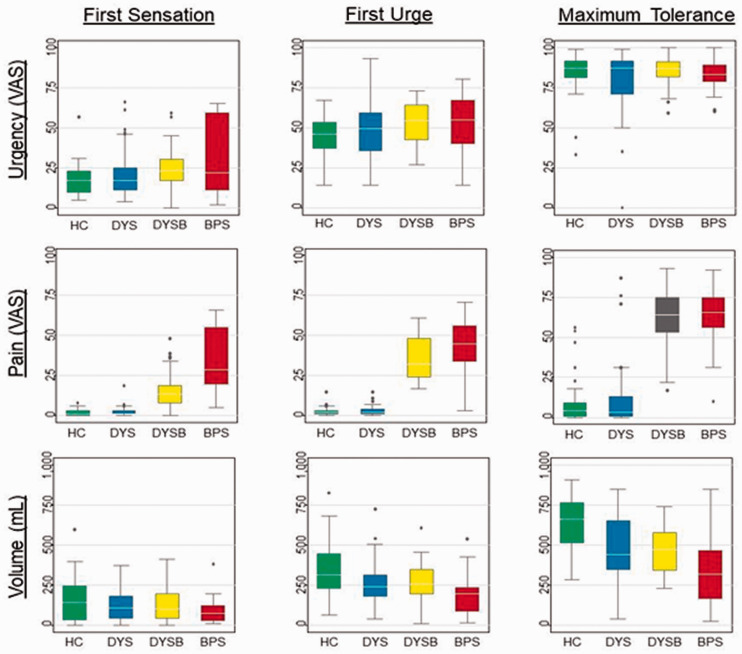
By definition, 31 DYSB participants reported experimental bladder pain greater than 15 on a 0–100 VAS at first urge, and they had pain levels similar to BPS participants (Table 2). Participants with DYSB reported nearly as much pain at first urge (32 [24, 48]) as BPS participants (43 [34, 55]; p = 0.091), but had significantly more bladder capacity (256 [1,94,345] vs. 192 [89,235]; p = 0.018). Notably, both DYSB and BPS participants had significantly more pain at first urge than control participants (Table 2). Similar results were obtained at first sensation and maximum tolerance (Figure 1). Significant increases in pain sensitivity were observed for vaginal PPT, body PPT, and cold pressor pain in DYSB and BPS participants compared to pain-free controls (Table 2: p’s < 0.05). Although DYS participants reported worse pain on the cold pressor task than healthy controls, they showed no significant differences for PPTs.
Figure 1.
Bladder urgency, pain, and volume measurements across the groups at cystometric thresholds. Box and whisker plots show median, 25th–75th percentiles, range, and outliers. HC = Healthy Control; DYS = Dysmenorrhea only; DYSB = Dysmenorrhea with Bladder Sensitivity; BPS = Bladder Pain Syndrome.
Serum hormone differences in complex pain groups
We next assessed whether DYS, DYSB, or BPS participants had differences in progesterone, estradiol, SHBG, and testosterone compared to controls (Table 3). There was no significant difference in progesterone or testosterone across groups and no significant group contrasts (p’s > 0.3). However, DYSB participants (487 [390 – 641] pg/mL) had a 20% higher serum estradiol concentration than pain-free controls (404 [336 – 467] pmol/L) even after corrections for multiple comparisons (p = 0.042). Serum SHBG concentrations were also different across groups, with BPS participants having 50% higher SHBG concentrations (83 [71 – 108] nmol/L) than controls (55 [42 – 76] nmol/L; p = 0.027). Because age is a known confounder, we confirmed that BPS participants had higher levels of SHBG (odds ratio: 2.7 ± 1.3; p = 0.045) accounting for age (odds ratio: 1.07 ± 0.03/year; p = 0.009) as a covariate in an ordered logistic regression model.
Table 3.
Participants sex steroid and SHBG concentrations.
| HC | DYS | DYSB | BPS | P value | |
|---|---|---|---|---|---|
| Progesterone nmol/L | 8.3 [1.5, 15.5] | 13.2 [3.6, 19.7] | 11.7 [1.8, 22.9] | 9.7 0.8, 19.1] | 0.463 |
| Estradiol pmol/L | 404.4 [336.2, 467.2] | 420.9 [337.5, 537.7] | 487.4 [389.9, 640.8] a | 384.6 [301.1, 436.0] | 0.054 |
| Testosterone nmol/L | 0.5 [0.3, 0.9] | 0.4 [0.3, 0.7] | 0.6 [0.3, 1.0] | 0.3 [0.3, 0.6] | 0.183 |
| SHBG nmol/L | 55.3 [41.8, 76.4] | 60.3 [38.5, 74.8] | 57.6 [39.6, 86.5] | 83.3 [70.5, 108.0] a | 0.050 |
Note: Data are represented as median [25th, 75th percentile] or as number (percentage). P values are from Kruskal Wallis tests.
Bold numbers and superscript “a” indicate p < 0.05 different than HC after correction for multiple comparisons.
The relationship between hormones, psychological factors, QST, and bladder pain
We next examined if there were any other meaningful associations between sex steroid levels and other pain variables or psychological factors that could alternatively be responsible for differences in bladder sensitivity. We calculated Spearman correlations in both the entire cohort and adjusted for multiple comparisons. Initially, we focused on critical variables that are different in DYSB (menstrual pain, somatic symptoms, and anxiety) and hormones that were different among the groups (estradiol and SHBG). Among all of these factors, only the correlation between SHBG and somatic symptoms was significant (r = 0.26, p = 0.03; Table 4). We conducted a sensitivity analysis excluding BPS participants to limit bias purely associated with group differences, and the correlation between SHBG and somatic symptoms was still significant (r = 0.20, p = 0.04).
Table 4.
Relationships of symptoms and pain sensitivity with circulating estradiol and SHBG.
| Parameter | Estradiol | SHBG |
|---|---|---|
| Menstrual pain | 0.07 | 0.03 |
| Somatic symptoms | −0.09 | 0.26a |
| Anxiety | 0.11 | 0.12 |
Note: Spearman’s correlation coefficients are reported for key parameters that differentiate participants with dysmenorrhea and bladder pain sensitivity. Bold numbers and superscript “a” indicate coefficients that are significant after correction for multiple comparisons.
Finally, partial correlation coefficients were calculated to explore any meaningful associations between sex steroid levels and bladder or somatic pain sensitivity (Table 5). Although SHBG was correlated to bladder pain at first sensation and first urge even after adjusting for age (p’s < 0.05), results were not significant after corrections for multiple comparisons. Correlations between sex steroid levels and other metrics of QST were not significant.
Table 5.
Relationships between sex steroids, bladder pain, and QST.
| Parameter | Progesterone | Estradiol | Testosterone | SHBG |
|---|---|---|---|---|
| Bladder pain first sensation | 0.10 | −0.13 | 0.07 | 0.25a |
| Bladder pain first urge | −0.01 | −0.03 | 0.11 | 0.21a |
| Bladder pain maximum tolerance | 0.09 | −0.03 | 0.14 | 0.12 |
| Body PPT | 0.06 | −0.05 | −0.07 | −0.02 |
| Vaginal PPT | 0.11 | −0.05 | −0.13 | 0.05 |
| Cold pressor | −0.01 | −0.15 | 0.15 | 0.16 |
Note: Partial correlation coefficients of all measured sex steroids adjusting for age are reported for all bladder and quantitative sensory tests. Bold numbers and superscript “a” indicate significant coefficients (p < 0.05).
Discussion
This cross-sectional study sought to examine if sex steroids were related to dysmenorrhea and experimental bladder pain sensitivity. Our results showed that women with bladder pain sensitivity (DYSB) had greater serum estradiol. In comparison, those with diagnosed bladder pain syndrome (BPS) had greater SHBG serum concentrations. Although SHBG was correlated with somatic symptoms, overall differences in hormone concentrations appear to be independent of mood or pain sensitivity.
It is intriguing that DYSB participants, whom we have shown exhibit some phenotypic aspects of chronic pain, yet are free of chronic pain symptoms, had significantly greater serum estradiol concentrations than pain-free control participants. The differences we detected occurred after ovulation and during the luteal phase, a time at which estradiol should be decreasing. Previous studies have found that serum estradiol concentrations during the luteal phase were higher in women who report the worst premenstrual symptoms.21–23 The elevated BSI sub-scores in women with DYSB suggest that they have more widespread somatic symptoms during the luteal phase than HC or DYS participants. Because differences in progesterone and testosterone were not significant, the increased estradiol in DYSB appears to be specific and is consistent with prior work in premenstrual syndrome.45,46 High levels of estradiol could impact multiple nociceptive circuits. Animal models have shown that estradiol can increase visceral sensitivity by affecting pelvic nerves.6–11 In women undergoing in vitro fertilization, supraphysiological levels of estradiol levels increase pain ratings with QST, suggesting that estradiol could affect widespread pain modulation. 18 Our prior finding of impaired conditioned pain modulation in women with dysmenorrhea and bladder sensitivity is consistent with the hypothesis that estradiol mediated alterations in descending modulation may play an important role in widespread somatic and visceral sensitivity. 27
Given its role in regulating estradiol, we anticipated that altered serum SHBG concentrations would be associated with menstrual or bladder pain. Although it has not been established whether women with dysmenorrhea have altered levels of SHBG, a vegan diet can simultaneously increase SHBG and reduce menstrual pain. 24 Optimal SHBG balance is relevant for other disease processes; prior work has shown that low SHBG is associated with metabolic dysfunction and inflammation. 15 However, in other studies, elevated SHBG is associated with adverse pain and psychological outcomes. For example, elevated serum concentrations of SHBG are reported in premenstrual syndrome during the luteal phase.45,46 After menopause, increased levels of SHBG are associated with more painful sex 25 and depression. 26 Conversely, the use of topical estradiol and testosterone in contraceptive induced vestibulodynia is associated with simultaneous reductions of pain and SHBG. 47 The presence of elevated estradiol in DYSB participants and SHBG in BPS participants suggests that complex hormonal alterations may occur over time due to many factors. The increased estradiol levels in DYSB could be due to many potential factors: environmental toxins, genetic susceptibility, or inflammation. 48 However, the older BPS participants are suffering from worse pain for a longer period of time than the DYSB participants and may be undergoing stress-induced gonadotropic suppression similar to what has been observed in animal models.49,50 Many other factors could also explain the shift from elevated estradiol to elevated SHBG. There is tight feedback control between SHBG and estradiol because SHBG sequesters estradiol, but required for transport. 51 Within hepatocytes (the primary source for systemic SHBG), there is a positive dose response curve between estradiol and increased synthesis of SHBG. 52 Previous studies have shown that oral contraceptives (which include synthetic estrogens) simultaneously decrease endogenous estradiol and increase SHBG levels.53,54 However, there were no significant group differences in recent usage of hormonal contraceptives. Further studies are needed to establish whether historical use of prior use or elevated estradiol at an earlier time point could affect SHBG at a later time point. It has been hypothesized that increases in SHBG result in androgen sequestration, increasing pain sensitivity, but research on the trajectory of estradiol and SHBG over time is needed to clarify whether increased SHBG is compensatory or contributes to worsening pain trajectories. 55
A handful of small studies have attempted to explore the relationship between sex steroids and pain sensitivity explicitly but have obtained different results from our study. A study of 11 healthy participants 56 reported that the only relationship between pain sensitivity and sex steroids was estradiol and thermal pain (r = 0.33, p = 0.06). Another slightly larger study (n = 19) failed to detect any correlations between sex steroids and thermal sensitivity. 57 A more recent study (n = 15) using a comprehensive QST protocol before and after induced hyperalgesia in healthy participants found a significant correlation between pinprick pain sensitivity and progesterone, but not estradiol. Although one study (n = 40) identified an association between testosterone and ischemia tolerance, analyses were restricted to healthy controls. 58 Our larger sample size (n = 124) controlling for chronic pain status allowed for a rigorous evaluation of the relationship between hormones and pain sensitivity, particularly in at-risk participants. It is plausible that prior studies of dysmenorrhea may have had confounding mixtures of both DYS and DYSB participants. As a result of this mixture of phenotypes, it may be difficult to compare them directly to determine the impact of hormones. Indeed, we observed group differences in sex steroids, but those differences were unrelated to experimental pain sensitivity results. Rather, alterations in sex steroids and pain sensitivity appear to represent two independent components that contribute to pelvic pain expression.
This study's key strengths include the experimental evaluation of pain sensitivity and serum quantification of multiple sex steroids, while accounting for psychological variables and menstrual cycle timing. Future studies should investigate sex steroids and experimental bladder pain sensitivity across the menstrual cycle, as our findings are limited to the mid-luteal phase. Also, because some women had testosterone serum concentrations below the sensitivity threshold, future studies should confirm findings with a more sensitive assay. When estradiol or testosterone is bound SHBG, they are not bioactive. 59 Because there are group differences in SHBG, future studies should also specifically analyze free estradiol and testosterone levels to examine bioavailability.
Overall, this cross-sectional study shows distinct hormonal profiles for dysmenorrhea with bladder pain sensitivity (i.e., DYSB) and BPS. Increased serum estradiol was associated with subclinical bladder pain, while increased serum SHBG was associated with more severe chronic bladder pain. The increased serum estradiol concentration supports our hypothesis that DYSB represents a distinct at-risk phenotype of dysmenorrhea. More extensive studies and interventions examining these hormones across time in dysmenorrheic women could clarify whether increased luteal estradiol or SHBG conveys additional risk for pelvic afferent sensitivity, and thus, risk of developing chronic pelvic pain.
Acknowledgments
The authors thank G.F. Gebhart for his scientific advice and editorial assistance. We also thank the NorthShore Biorepository for the serum retrieval and storage and the Center for Research in Reproduction’s Ligand Assay and Analysis Core for the hormone quantitation.
Footnotes
Authors’ Contributions: KMH, FFT and FAO conceived, designed and obtained funding for this project. EFG, GER and KED collected the data. FAO and KMH analyzed and interpreted the data. FAO and KMH drafted the manuscript. All authors read, edited and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Tu reports personal fees from AbbVie, Myovant, UroShape, and Wolters Kluwer, outside the submitted work; the remaining authors report no conflicts in interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by NICHD HD098193, NIDDK DK100368 and NorthShore University Health System.
ORCID iD: Kevin M Hellman https://orcid.org/0000-0001-9420-5602
References
- 1.Golobof A, Kiley J. The current status of oral contraceptives: progress and recent innovations. Semin Reprod Med 2016; 34: 145–151. [DOI] [PubMed] [Google Scholar]
- 2.Lentz GM, Bavendam T, Stenchever MA, Miller JL, Smalldridge J. Hormonal manipulation in women with chronic, cyclic irritable bladder symptoms and pelvic pain. Am J Obstet Gynecol 2002; 186: 1268–1271; discussion 1271–1273. [DOI] [PubMed] [Google Scholar]
- 3.Brinton RD. Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol Sci 2009; 30: 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng H-Y, Chen G-D, Lai C-Y, Hsieh M-C, Hsu H-H, Wu H-C, Lin T-B. PI3K modulates estrogen-dependent facilitation of colon-to-urethra cross-organ reflex sensitization in ovariectomized female rats. J Neurochem 2010; 113: 54–66. [DOI] [PubMed] [Google Scholar]
- 5.Ji Y, Tang B, Traub RJ. Modulatory effects of estrogen and progesterone on colorectal hyperalgesia in the rat. Pain 2005; 117: 433–442. [DOI] [PubMed] [Google Scholar]
- 6.Ji Y, Tang B, Traub RJ. Spinal estrogen receptor alpha mediates estradiol-induced pronociception in a visceral pain model in the rat. Pain 2011; 152: 1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khariv V, Acioglu C, Ni L, Ratnayake A, Li L, Tao Y-X, Heary RF, Elkabes S. A link between plasma membrane calcium ATPase 2 (PMCA2), estrogen and estrogen receptor α signaling in mechanical pain. Sci Rep 2018; 8: 17260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu B, Eisenach JC, Tong C. Chronic estrogen sensitizes a subset of mechanosensitive afferents innervating the uterine cervix. J Neurophysiol 2005; 93: 2167–2173. [DOI] [PubMed] [Google Scholar]
- 9.Myers B, Schulkin J, Greenwood-Van Meerveld B. Sex steroids localized to the amygdala increase pain responses to visceral stimulation in rats. J Pain 2011; 12: 486–494. [DOI] [PubMed] [Google Scholar]
- 10.Payrits M, Sághy É, Cseko K, Pohóczky K, Bölcskei K, Ernszt D, Barabás K, Szolcsányi J, Ábrahám IM, Helyes Z, Szoke É. Estradiol sensitizes the transient receptor potential vanilloid 1 receptor in pain responses. Endocrinology 2017; 158: 3249–3258. [DOI] [PubMed] [Google Scholar]
- 11.Tang B, Ji Y, Traub RJ. Estrogen alters spinal NMDA receptor activity via a PKA signaling pathway in a visceral pain model in the rat. Pain 2008; 137: 540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarajari S, Oblinger MM. Estrogen effects on pain sensitivity and neuropeptide expression in rat sensory neurons. Exp Neurol 2010; 224: 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cetinkaya A, Kilinc E, Camsari C, Ogun MN. Effects of estrogen and progesterone on the neurogenic inflammatory neuropeptides: implications for gender differences in migraine. Exp Brain Res 2020; 238: 2625–2639. [DOI] [PubMed] [Google Scholar]
- 14.Iwasa T, Matsuzaki T, Tungalagsuvd A, Munkhzaya M, Kawami T, Kato T, Kuwahara A, Yasui T, Irahara M. Effects of ovariectomy on the inflammatory responses of female rats to the central injection of lipopolysaccharide. J Neuroimmunol 2014; 277: 50–56. [DOI] [PubMed] [Google Scholar]
- 15.Simó R, Sáez-López C, Barbosa-Desongles A, Hernández C, Selva DM. Novel insights in SHBG regulation and clinical implications. Trends Endocrinol Metab TEM 2015; 26: 376–383. [DOI] [PubMed] [Google Scholar]
- 16.Liedman R, Hansson SR, Howe D, Igidbashian S, McLeod A, Russell RJ, Akerlund M. Reproductive hormones in plasma over the menstrual cycle in primary dysmenorrhea compared with healthy subjects. Gynecol Endocrinol off Endocrinol 2008; 24: 508–513. [DOI] [PubMed] [Google Scholar]
- 17.Wei S-Y, Chao H-T, Tu C-H, Li W-C, Low I, Chuang C-Y, Chen L-F, Hsieh J-C. Changes in functional connectivity of pain modulatory systems in women with primary dysmenorrhea. Pain 2016; 157: 92–102. [DOI] [PubMed] [Google Scholar]
- 18.Nisenblat V, Engel-Yeger B, Ohel G, Aronson D, Granot M. The association between supra-physiological levels of estradiol and response patterns to experimental pain. Eur J Pain Lond Pain 2010; 14: 840–846. [DOI] [PubMed] [Google Scholar]
- 19.Rhudy JL, Bartley EJ, Palit S, Kerr KL, Kuhn BL, Martin SL, Delventura JL, Terry EL. Do sex hormones influence emotional modulation of pain and nociception in healthy women? Biol Psychol 2013; 94: 534–544. [DOI] [PubMed] [Google Scholar]
- 20.Balzer BWR, Duke S-A, Hawke CI, Steinbeck KS. The effects of estradiol on mood and behavior in human female adolescents: a systematic review. Eur J Pediatr 2015; 174: 289–298. [DOI] [PubMed] [Google Scholar]
- 21.Bäckström T, Wide L, Södergård R, Carstensen H. FSH, LH, TeBG-capacity, estrogen and progesterone in women with premenstrual tension during the luteal phase. J Steroid Biochem 1976; 7: 473–476. [DOI] [PubMed] [Google Scholar]
- 22.Munday MR, Brush MG, Taylor RW. Correlations between progesterone, oestradiol and aldosterone levels in the premenstrual syndrome. Clin Endocrinol (Oxf) 1981; 14: 1–9. [DOI] [PubMed] [Google Scholar]
- 23.Seippel L, Bäckström T. Luteal-phase estradiol relates to symptom severity in patients with premenstrual syndrome. J Clin Endocrinol Metab 1998; 83: 1988–1992. [DOI] [PubMed] [Google Scholar]
- 24.Barnard ND, Scialli AR, Hurlock D, Bertron P. Diet and sex-hormone binding globulin, dysmenorrhea, and premenstrual symptoms. Obstet Gynecol 2000; 95: 245–250. [DOI] [PubMed] [Google Scholar]
- 25.Peixoto C, Carrilho CG, Ribeiro TTDSB, da Silva LM, Gonçalves EA, Fernandes L, Nardi AE, Cardoso A, Veras AB. Relationship between sexual hormones, quality of life and postmenopausal sexual function. Trends Psychiatry Psychother 2019; 41: 136–143. [DOI] [PubMed] [Google Scholar]
- 26.Colangelo LA, Craft LL, Ouyang P, Liu K, Schreiner PJ, Michos ED, Gapstur SM. Association of sex hormones and sex hormone-binding globulin with depressive symptoms in postmenopausal women: the multiethnic study of atherosclerosis. Menopause N Menopause 2012; 19: 877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hellman KM, Roth GE, Dillane KE, Garrison EF, Oladosu FA, Clauw DJ, Tu FF. Dysmenorrhea subtypes exhibit differential quantitative sensory assessment profiles. Pain 2020; 161: 1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tu FF, Datta A, Atashroo D, Senapati S, Roth G, Clauw DJ, Hellman KM. Clinical profile of comorbid dysmenorrhea and bladder sensitivity: a cross-sectional analysis. Am J Obstet Gynecol 2020; 222: 594.e1–594.e11. e1- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hellman KM, Patanwala IY, Pozolo KE, Tu FF. Multimodal nociceptive mechanisms underlying chronic pelvic pain. Am J Obstet Gynecol 2015; 213: 827.e1–827.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hellman KM, Datta A, Steiner ND, Kane Morlock JN, Garrison EF, Clauw DJ, Tu FF. Identification of experimental bladder sensitivity among dysmenorrhea sufferers. Am J Obstet Gynecol 2018; 219: 84.e1–84.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanno PM, Erickson D, Moldwin R, Faraday MM. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol 2015; 193: 1545–1553. [DOI] [PubMed] [Google Scholar]
- 33.Tu FF, Epstein AE, Pozolo KE, Sexton DL, Melnyk AI, Hellman KM. A noninvasive bladder sensory test supports a role for dysmenorrhea increasing bladder noxious mechanosensitivity. Clin J Pain 2013; 29: 883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, van Kerrebroeck P, Victor A, Wein A. The standardisation of terminology of lower urinary tract function: report from the standardisation Sub-committee of the international continence society. Am J Obstet Gynecol 2002; 187: 116–126. [DOI] [PubMed] [Google Scholar]
- 35.Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, Ader D, Fries JF, Bruce B, Rose M. The patient-reported outcomes measurement information system (PROMIS): progress of an NIH roadmap cooperative group during its first two years. Med Care 2007; 45: S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Derogatis LR, Melisaratos N. The brief symptom inventory: an introductory report. Psychol Med 1983; 13: 595–605. [PubMed] [Google Scholar]
- 37.O'Leary MP, Sant GR, Fowler FJ, Whitmore KE, Spolarich-Kroll J. The interstitial cystitis symptom index and problem index. Urology 1997; 49: 58–63. [DOI] [PubMed] [Google Scholar]
- 38.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum 1990; 33: 160–172. [DOI] [PubMed] [Google Scholar]
- 39.Reed GF, Lynn F, Meade BD. Use of coefficient of variation in assessing variability of quantitative assays. Clin Diagn Lab Immunol 2002; 9: 1235–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faul F, Erdfelder E, Lang A-G, Buchner A. G*power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007; 39: 175–191. [DOI] [PubMed] [Google Scholar]
- 41.Hecke TV. Power study of anova versus Kruskal-Wallis test. J Stat Manag Syst 2012; 15: 241–247. [Google Scholar]
- 42.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol 1995; 57: 289–300. [Google Scholar]
- 43.Maggio M, Lauretani F, Basaria S, Ceda GP, Bandinelli S, Metter EJ, Bos AJ, Ruggiero C, Ceresini G, Paolisso G, Artoni A, Valenti G, Guralnik JM, Ferrucci L. Sex hormone binding globulin levels across the adult lifespan in women – the role of body mass index and fasting insulin. J Endocrinol Invest 2008; 31: 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andersson AM, Juul A, Petersen JH, Müller J, Groome NP, Skakkebaek NE. Serum inhibin B in healthy pubertal and adolescent boys: relation to age, stage of puberty, and follicle-stimulating hormone, luteinizing hormone, testosterone, and estradiol levels. J Clin Endocrinol Metab 1997; 82: 3976–3981. [DOI] [PubMed] [Google Scholar]
- 45.Massil HY, Thomas M, O'Brien PM. Sex hormone binding globulin concentrations in premenstrual syndrome. Br J Obstet Gynaecol 1993; 100: 697–698. [DOI] [PubMed] [Google Scholar]
- 46.Thys-Jacobs S, McMahon D, Bilezikian JP. Differences in free estradiol and sex hormone-binding globulin in women with and without premenstrual dysphoric disorder. J Clin Endocrinol Metab 2008; 93: 96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burrows LJ, Goldstein AT. The treatment of vestibulodynia with topical estradiol and testosterone. Sex Med 2013; 1: 30–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huhtinen K, Ståhle M, Perheentupa A, Poutanen M. Estrogen biosynthesis and signaling in endometriosis. Mol Cell Endocrinol 2012; 358: 146–154. [DOI] [PubMed] [Google Scholar]
- 49.Roney JR, Simmons ZL. Elevated psychological stress predicts reduced estradiol concentrations in young women. Adapt Hum Behav Physiol 2015; 1: 30–40. [Google Scholar]
- 50.Breen KM, Billings HJ, Wagenmaker ER, Wessinger EW, Karsch FJ. Endocrine basis for disruptive effects of cortisol on preovulatory events. Endocrinology 2005; 146: 2107–2115. [DOI] [PubMed] [Google Scholar]
- 51.Thaler MA, Seifert-Klauss V, Luppa PB. The biomarker sex hormone-binding globulin - from established applications to emerging trends in clinical medicine. Best Pract Res Clin Endocrinol Metab 2015; 29: 749–760. [DOI] [PubMed] [Google Scholar]
- 52.Nader N, Raverot G, Emptoz-Bonneton A, Déchaud H, Bonnay M, Baudin E, Pugeat M. Mitotane has an estrogenic effect on sex hormone-binding globulin and corticosteroid-binding globulin in humans. J Clin Endocrinol Metab 2006; 91: 2165–2170. [DOI] [PubMed] [Google Scholar]
- 53.Key TJ, Pike MC, Moore JW, Bulbrook RD, Clark GM, Allen DS, Wang DY. The relationships of SHBG with current and previous use of oral contraceptives and oestrogen replacement therapy. Contraception 1989; 39: 179–186. [DOI] [PubMed] [Google Scholar]
- 54.Palatsi R, Hirvensalo E, Liukko P, Malmiharju T, Mattila L, Riihiluoma P, Ylöstalo P. Serum total and unbound testosterone and sex hormone binding globulin (SHBG) in female acne patients treated with two different oral contraceptives. Acta Derm Venereol 1984; 64: 517–523. [PubMed] [Google Scholar]
- 55.Evans SF, Kwok Y, Solterbeck A, Pyragius C, Hull ML, Hutchinson MR, Rolan P. The relationship between androgens and days per month of period pain, pelvic pain, headache, and TLR4 responsiveness of peripheral blood mononuclear cells in young women with dysmenorrhoea. J Pain Res 2021; 14: 585–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fillingim RB, Maixner W, Girdler SS, Light KC, Harris MB, Sheps DS. Ischemic but not thermal pain sensitivity varies across the menstrual cycle. Psychosom Med 1997; 59: 512–520. [DOI] [PubMed] [Google Scholar]
- 57.Söderberg K, Sundström Poromaa I, Nyberg S, Bäckström T, Nordh E. Psychophysically determined thresholds for thermal perception and pain perception in healthy women across the menstrual cycle. Clin J Pain 2006; 22: 610–616. [DOI] [PubMed] [Google Scholar]
- 58.Bartley EJ, Palit S, Kuhn BL, Kerr KL, Terry EL, DelVentura JL, Rhudy JL. Nociceptive processing in women with premenstrual dysphoric disorder (PMDD): the role of menstrual phase and sex hormones. Clin J Pain 2015; 31: 304–314. [DOI] [PubMed] [Google Scholar]
- 59.Mendel CM. The free hormone hypothesis: a physiologically based mathematical model. Endocr Rev 1989; 10: 232–274. [DOI] [PubMed] [Google Scholar]