Abstract
Ultrasound has become indispensable for identification of thyroid and parathyroid pathology, but normal parathyroid glands have historically been considered too subtle to accurately detect. Inability to identify and protect parathyroid glands can result in hypoparathyroidism and hypocalcemia during thyroidectomy surgery as well as misinterpretation of central neck structures in the postoperative neck. Advances in ultrasound resolution have opened the door to novel applications for this technology. In this study, we report the first surgeon-performed ultrasound identification of normal parathyroid glands in a series of 6 patients, confirmed by parathyroid tissue aspirate or parathyroid autofluorescence. Recognition of normal parathyroid glands using ultrasound can be valuable for preventing postoperative hypoparathyroidism and in increasing the accuracy of postsurgical ultrasound surveillance.
Keywords: parathyroid, ultrasound
Ultrasound has become indispensable for identification of thyroid and parathyroid pathology. Surgeon-performed ultrasound is commonly used for identification of hyperfunctioning parathyroid glands (sensitivity/specificity 87/95% 1 ), and the feasibility of ultrasound-guided fine-needle aspiration (FNA) of parathyroid lesions for parathyroid hormone (PTH) washout is documented. 2 Normal parathyroid glands, however, have long been considered too subtle to accurately detect with noninvasive imaging. 3 Inability to protect parathyroid glands during thyroidectomy can result in hypoparathyroidism with resultant hypocalcemia. Accepted strategies to reduce hypoparathyroidism risk involve preserving blood supply and autotransplanting ischemic glands. Despite these precautions, temporary and permanent hypoparathyroidism remain common complications, with rates estimated at 6% to 30% and 1% to 4%, respectively.4-6 Preoperative identification of parathyroid glands could be useful in surgical planning and decrease iatrogenic hypoparathyroidism. In this study, we report the first surgeon-performed ultrasound identification of normal parathyroid glands, confirmed by tissue aspirate or autofluorescence.
Methods
This study was approved by the Stanford Institutional Review Board. Patient demographics are outlined in Table 1 . Normal parathyroid gland candidates were identified during preoperative clinic visits and/or routine surgeon-performed preincision ultrasonography using a Logiq S8 ultrasound machine and 50-mm 4- to 15-MHz transducer (GE Healthcare). Bilateral thyroid lobes, isthmus, and neck compartments were examined in transverse and sagittal orientations. In 2 patients with a clearly accessible candidate normal parathyroid gland, ultrasound-guided FNA was performed via capillary technique after anesthesia induction and prior to incision. This sample was rinsed through the 25-gauge needle with 0.5 cc saline and assayed for PTH level. Serum PTH was evaluated concurrently. In 4 other patients, candidate gland autofluorescence was measured using either PTeye (Medtronic; gland/background ratio >2.0 interpreted as parathyroid tissue) or FLUOBEAM LX (Fluoptics; subjective fluorescence above background interpreted as parathyroid tissue).
Table 1.
Summary of identified normal parathyroid glands
| Case No. | Age, y | Sex | Diagnosis a | PTG location | PTH aspiration, pg/mL | Serum PTH, pg/mL | Autofluorescence |
|---|---|---|---|---|---|---|---|
| 1 | 54 | M | PTC | Left inferior | 408.0 | 57.6 | — |
| 2 | 57 | M | PTC | Left inferior | 117.6 | 47.4 | Yes (PTEye) |
| 3 | 57 | F | PTC | Left inferior | — | — | Yes (FLUOBEAM LX) |
| 4 | 42 | F | Graves’ disease | Left inferior | — | — | Yes (PTeye) |
| 5 | 38 | F | AI thyroiditis | Left superior | — | — | Yes (FLUOBEAM LX) |
| 6 | 43 | M | PTC | Left inferior | — | — | Yes (FLUOBEAM LX) |
Abbreviations: AI, autoimmune; PTC, papillary thyroid carcinoma; PTG, parathyroid gland; PTH, parathyroid hormone; ——, Characteristics of normal parathyroid glands identified by ultrasound and verified by tissue aspirate and/or autofluorescence.
Diagnosis = main diagnosis associated with patient encounter, which was also the indication for surgery.
Results
We identified normal parathyroid gland candidates via ultrasound in 31 patients. These appeared as oval, hyperechoic structures in the perithyroidal region, particularly inferior to the thyroid gland in the low central neck ( Figure 1 ). Preincision ultrasound-guided FNA of 2 parathyroid candidate resulted in values of 408.0 and 117.6 pg/mL, whereas serum PTH levels at the same time were 57.6 and 47.4 pg/mL (reference, 15.0-65.0 pg/mL; Table 1 ). These values suggested parathyroid tissue in our aspirate. Normal-appearing parathyroid glands were later visually identified intraoperatively at locations corresponding to the preoperative ultrasound ( Figure 2A ) and preserved with their blood supply intact. Both patients had normal PTH levels at the conclusion of surgery.
Figure 1.
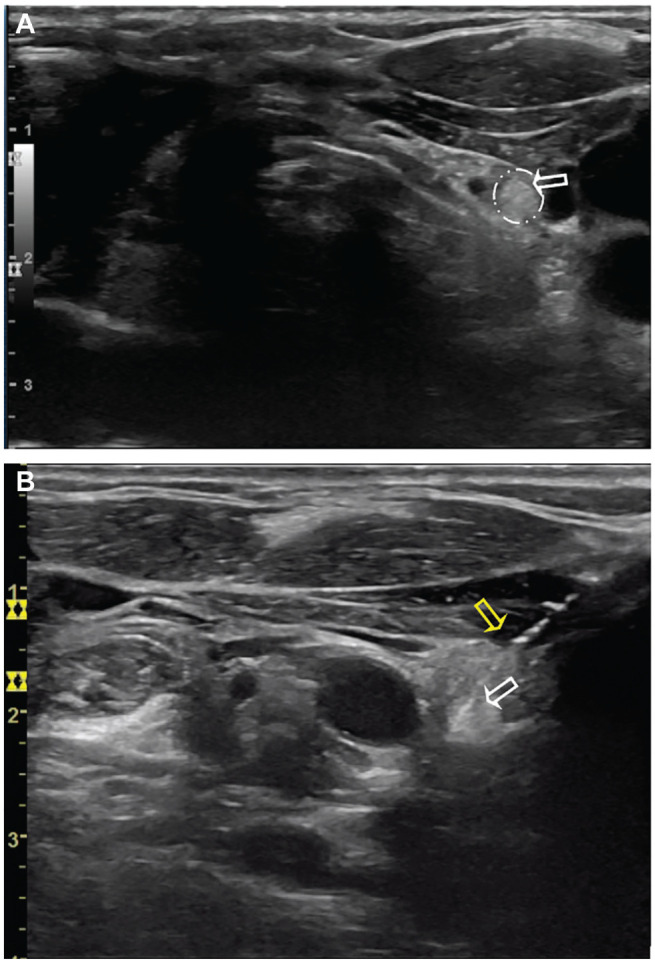
Preincision ultrasound of patient 2. (A) Left side transverse. (B) Left side transverse but inverted (craniocaudal view) during fine-needle aspiration. White arrows: hyperechoic parathyroid gland candidate; yellow arrow: needle used for parathyroid tissue aspiration.
Figure 2.
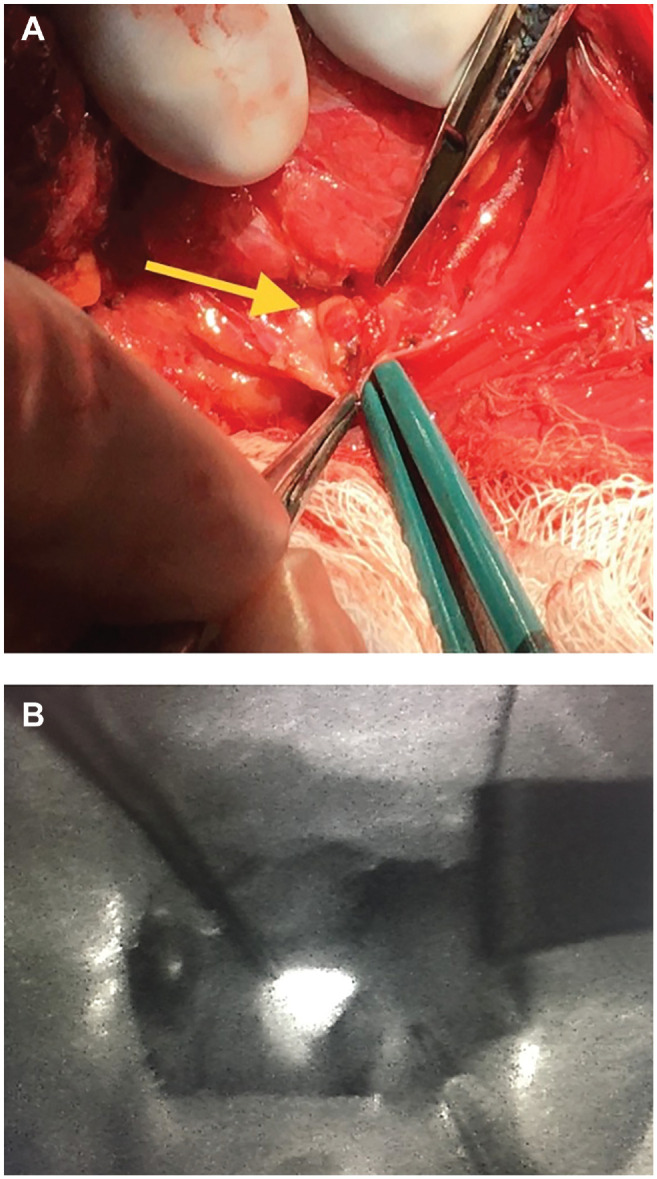
(A) Normal left inferior parathyroid gland (yellow arrow) from patient 2. (B) Autofluorescence from normal left superior parathyroid gland, seen using the FLUOBEAM LX camera, from patient 5. Both glands corresponded with location on preoperative ultrasound.
In 4 other patients, preoperative ultrasound but not FNA was performed, and autofluorescence was measured intraoperatively at locations corresponding to the ultrasound. Candidate parathyroid glands were identified and preserved in each of these instances ( Figure 2B ).
Discussion
The use of ultrasound in diagnosing and treating neck pathology has expanded in recent years with technological improvements. High-resolution images allow more precise identification of smaller lesions; to date, this has been applied toward thyroid cancer.7,8 Increased use and familiarity among surgeons heralded a new era of preoperative preparation and postoperative evaluation in thyroid and parathyroid disease. Meanwhile, numerous new technologies have been developed for parathyroid identification, notably choline positron emission tomography–computed tomography, 9 assays of parathyroid autofluorescence, 10 and gamma probe-aided identification of Tc99m sestamibi-labeled parathyroid glands. 11
Previous work suggested normal parathyroid glands may be hyperechoic and homogeneous on ultrasound, although gland identity was never confirmed by histopathological, biochemical, or fluorescence review. 12 Multiple other studies have concluded ultrasonography to be unreliable for identification of normal parathyroid glands.3,13
Here, however, we report successful identification of normal parathyroid glands using ultrasound, in which they appear as distinct, homogeneously hyperechoic structures in the perithyroidal central neck. Although histologic confirmation of normal parathyroid tissue is impractical, FNA of candidate glands in 2 patients yielded a PTH level far in excess of the simultaneous serum measurement, strongly suggesting sampling of parathyroid tissue. In these and other patients, we have also used intraoperative autofluorescence to confirm the expected location of normal parathyroid glands as seen on preoperative ultrasound. Notably, inferior glands comprised the majority of those identified, perhaps due to their relatively superficial location.
Three potential explanations for the hyperechoic appearance of normal parathyroid glands relate to (1) fat prevalence, (2) oxyphil/chief cell ratio, and (3) mitochondrial abundance. Normal glands contain 10% to 50% fat, which may appear hyperechoic. Accordingly, hyperplastic parathyroid tissue is both hypoechoic and with reduced fat content.14,15 Proliferation of densely arranged chief cells in parathyroid adenomas has been associated with hypoechoic appearance. 16 A normal proportion of oxyphil cells may account for normal gland hyperechogenicity, but no data support this. A third consideration relates to mitochondria, which are abundant in parathyroid glands and other hyperechoic tissues, including Hurthle cell lesions. The substance that autofluoresces in parathyroid glands has yet to be identified but is postulated to be the calcium sensing receptor, possibly the same substance that contributes to hyperechogenicity.
While normal parathyroid glands cannot be readily identified in every patient, surgeon-performed preoperative ultrasound remains a useful tool to assess for the potential location of normal parathyroid gland candidates. This not only facilitates identification and preservation during thyroid surgery but also reduces misinterpretation of central neck structures in the postoperative neck.
Conclusions
We hereby demonstrate that normal parathyroid glands can be identified in select patients using ultrasound, typically appearing as oval and homogenously hyperechoic structures in the perithyroidal region. Recognition and subsequent protection of normal parathyroid glands is important in preventing postoperative hypoparathyroidism. Moreover, ability to recognize normal parathyroid glands reduce false positives on surveillance. As with many findings on ultrasonography, recognition of structures is highly related to knowledge and anticipation of anatomy.
Author Contributions
Samuel M. Cohen, collected patient data, analyzed data, wrote manuscript; Julia E. Noel, devised study outline, collected patient data, wrote manuscript; Cassandra L. Puccinelli, collected patient data, revised manuscript; Lisa A. Orloff, devised study outline, collected patient data, wrote manuscript.
Disclosures
Competing interests: None.
Sponsorships: None.
Funding source: None.
Footnotes
ORCID iD: Samuel M. Cohen  https://orcid.org/0000-0003-4277-6184
https://orcid.org/0000-0003-4277-6184
References
- 1. Steward DL, Danielson GP, Afman CE, Welge JA. Parathyroid adenoma localization: surgeon-performed ultrasound versus sestamibi. Laryngoscope. 2006;116(8):1380-1384. [DOI] [PubMed] [Google Scholar]
- 2. Abati A, Skarulis MC, Shawker T, Solomon D. Ultrasound-guided fine-needle aspiration of parathyroid lesions: a morphological and immunocytochemical approach. Hum Pathol. 1995;26(3):338-343. [DOI] [PubMed] [Google Scholar]
- 3. Ha TK, Kim DW, Jung SJ. Ultrasound detection of normal parathyroid glands: a preliminary study. Radiol Med. 2017;122(11):866-870. [DOI] [PubMed] [Google Scholar]
- 4. Zambudio AR, Rodríguez J, Riquelme J, Soria T, Canteras M, Parrilla P. Prospective study of postoperative complications after total thyroidectomy for multinodular goiters by surgeons with experience in endocrine surgery. Ann Surg. 2004;240(1):18-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thomusch O, Machens A, Sekulla C, et al. Multivariate analysis of risk factors for postoperative complications in benign goiter surgery: prospective multicenter study in Germany. World J Surg. 2000;24(11):1335-1341. [DOI] [PubMed] [Google Scholar]
- 6. Christou N, Mathonnet M. Complications after total thyroidectomy. J Visc Surg. 2013;150(4):249-256. [DOI] [PubMed] [Google Scholar]
- 7. Sanabria A, Kowalski LP, Shah JP, et al. Growing incidence of thyroid carcinoma in recent years: factors underlying overdiagnosis. Head Neck. 2018;40(4):855-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davies L, Hoang JK. Thyroid cancer in the USA: current trends and outstanding questions. Lancet Diabetes Endocrinol. 2021;9(1):11-12. [DOI] [PubMed] [Google Scholar]
- 9. Lee SW, Shim SR, Jeong SY, Kim SJ. Direct comparison of preoperative imaging modalities for localization of primary hyperparathyroidism: a systematic review and network meta-analysis. JAMA Otolaryngol Head Neck Surg. 2021;147(8):692-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McWade MA, Paras C, White LM, Phay JE, Mahadevan-Jansen A, Broome JT. A novel optical approach to intraoperative detection of parathyroid glands. Surgery. 2013;154(6):1371-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grubbs EG, Mittendorf EA, Perrier ND, Lee JE. Gamma probe identification of normal parathyroid glands during central neck surgery can facilitate parathyroid preservation. Am J Surg. 2008;196(6):931-936. [DOI] [PubMed] [Google Scholar]
- 12. Xia C, Zhu Q, Li Z, et al. Study of the ultrasound appearance of the normal parathyroid using an intraoperative procedure. J Ultrasound Med. 2019;38(2):321-327. [DOI] [PubMed] [Google Scholar]
- 13. Ghervan C. Thyroid and parathyroid ultrasound. Med Ultrasound. 2011;13(1):80-84. [PubMed] [Google Scholar]
- 14. Dufour DR, Wilkerson SY. The normal parathyroid revisited: percentage of stromal fat. Hum Pathol. 1982;13(8):717-721. [DOI] [PubMed] [Google Scholar]
- 15. Huppert BJ, Reading CC. Parathyroid sonography: imaging and intervention. J Clin Ultrasound. 2007;35(3):144-155. [DOI] [PubMed] [Google Scholar]
- 16. Wieneke JA, Smith A. Parathyroid adenoma. Head Neck Pathol. 2008;2(4):305-308. [DOI] [PMC free article] [PubMed] [Google Scholar]


