Abstract
The evolution of AMPK and its homologues enabled exquisite responsivity and control of cellular energetic homeostasis. Recent work has been critical in establishing the mechanisms that determine AMPK activity, novel targets of AMPK action, and the distribution of AMPK-mediated control networks across the cellular landscape. The role of AMPK as a hub of metabolic control has led to intense interest in pharmacologic activation as a therapeutic avenue for a number of disease states including obesity, diabetes, and cancer. As such, critical work on the compartmentalization of AMPK, its downstream targets, and the systems it influences have progressed in recent years. The variegated distribution of AMPK-mediated control of metabolic homeostasis has revealed key insights on AMPK in normal biology and future directions for AMPK-based therapeutic strategies.
Trefts & Shaw eTOC
Trefts and Shaw review the latest advances in studies of the AMPK signaling pathway, a central energy-sensing kinase that promotes catabolism and inhibits anabolism to limit cell growth and promote cell survival under conditions of low nutrients and low cellular energy. Highlights include new intersections with mTOR, lysosomes, and mitochondria.
Introduction
Cellular life exists as an open system that relies on extraction of chemical energy to oppose tendencies towards disorganization at the molecular level (von Bertalanffy, 1950). As such, cells have evolved an array of specialized biochemical processes to extract, repurpose, and store chemical energy in the form of ATP. Mitochondria within eukaryotic cells are a hub for harnessing chemical energy by linking catabolism of organic macronutrients, such as glucose or lipids, with oxidative phosphorylation (oxphos) for production of ATP. The chemical energy of ATP is stored in its phosphate bonds. Utilization of ATP across the cell results in its breakdown to ADP, which can progressively breakdown to AMP as well. Constant cellular catabolism of macronutrients maintains an elevated ATP to ADP ratio, which allows for the adenine nucleotide pool to serve as a reservoir of chemical energy. To maintain appropriate ATP levels cells must constantly sense and adapt to turnover of energetic content in the adenine nucleotide pool. This requires balanced modulation of energetically demanding anabolic and catabolic processes for ATP production. AMP-activated protein kinase (AMPK) is the primary sensor of cellular energy through adenine nucleotide levels that evolved and is maintained across all eukaryotic organisms. AMPK senses the energetic content of the cell through direct interactions with ATP, ADP, and AMP. Decreases in the energetic content of this pool, such as an elevated AMP:ATP ratio, lead to AMPK activation. In turn, activated AMPK regulates the activity of a network of downstream substrates, including metabolic enzymes, signaling complexes, and transcriptional regulators, which impart both acute and chronic adaptations to conditions of reduced energy. In addition to its regulation of cellular metabolism, in higher eukaryotes, AMPK plays roles in organismal metabolism, and is activated in response to caloric restriction as well as exercise
The AMPK heterotrimeric complex and its functional implications
AMPK is an obligate heterotrimeric complex consisting of an α, β, and γ subunit. The catalytic domain is contained within the α subunit, while the β and γ subunits serve regulatory functions (Figure 1). Mammals express multiple isoforms of each subunit including two α (α1 and α2), two β (β1 and β2), and three γ (γ1, γ2, and γ3) isoforms each expressed from independent genes. All isoforms of each subunit class can interact in a 1:1:1 heterotrimeric ratio leading to 12 possible AMPK complexes. The expression of each isoform varies across tissues and cells, though the α1, β1, and γ1 subunits are expressed across a number of tissues and cell types in humans. In contrast, AMPKα2 and AMPKβ2 are the dominant isoforms of the skeletal and cardiac muscle in humans. The subunit expression profiles and tissue specific expression of dominant isoforms has noted variability across species (Stephenne et al., 2011; J. Wu et al., 2013). Differences in hepatocyte expression of AMPKβ subunits between human and other pre-clinical model species noted in these studies may influence species dependent effects of pharmacologic AMPK activators, which has important ramifications in the design of therapeutic molecules and trials focusing on AMPK activation. Of all of the subunit isoforms, AMPKγ3 expression is the most restricted, being primarily isolated to the skeletal and cardiac tissues. Complexes comprised of different isoform combinations are typically regarded as functionally redundant, however, isoform specific biochemical properties and regulation of AMPK activity have been reported. While substrates or downstream effectors specific to one particular heterotrimeric combination are yet to be identified, there is great interest in understanding isoform specific effects on AMPK function and the potential for leveraging these differences therapeutically through small molecule allosteric activators.
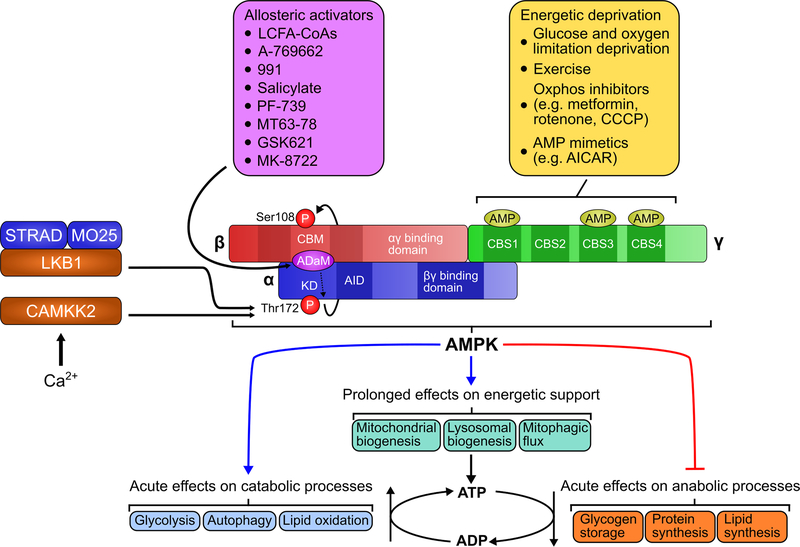
Figure 1. AMPK Domain Structure, Upstream Inputs and Downstream Outputs.
AMPK is a heterotrimeric complex consisting of a catalytic (α) and two regulatory (β and γ) domains. Within these domains are several structural elements that enable the dynamic regulation of AMPK activity and responsivity. Phosphorylation of Thr172 of the α subunit kinase domain by upstream kinases serves as a primary AMPK activating event. This is canonically responsive to structural shifts induced by altered adenine nucleotide energy content. This nuecleotide-dependent signaling is involved in responses to physiologic and pharmacologic manipulations of cellular energy. Additionally, Thr172 phosphorylation can be induced independent of cellular energy changes, which occurs during calcium induced activation of AMPK by CAMKK2. Upon activation AMPK acts on multiple targets to acutely restore cellular energy while promoting prolonged support of energetic sufficiency.
AMPKα subunits contain the kinase domain (KD) at the N terminus, an autoinhibitory domain (AID) coupled to a linker region, and a βγ subunit binding domain at the C terminus. Within the KD is a conserved threonine residue (Thr172) in the activation loop that is phosphorylated by upstream kinases, liver kinase B1 (LKB1) and calcium calmodulin dependent protein kinase kinase β (CaMKK2). Thr172 phosphorylation is required for full activation of AMPK (Willows et al, 2017). AMPK β subunits contain an N terminus myristoylation site as well as a conserved carbohydrate binding module (CBM) that facilitates interactions with glycogen, and an αγ binding domain at the C terminus. Recent work has assessed the physiologic implications of AMPKβ subunit CBM glycogen binding (Hoffman et al., 2020). These studies indicate a role for glycogen binding in stabilizing AMPK complex formation, AMPK activation, and lipid metabolism across multiple tissues. AMPKγ subunits provide the energy sensing functionality of AMPK through their cystathionine-β-synthase (CBS) domains that bind adenine nucleotides. While each γ subunit contains 4 CBS domains capable of binding adenine nucleotides CBS1 and CBS3 appear to be the primary sites responsible for energetic sensing of AMPK (Hardie et al., 2016). AMPKγ subunit isoforms can also vary in the length of their N termini with AMPKγ2 and AMPKγ3 possessing an extended N terminus relative to AMPKγ1. Variations in this N terminus region appear to modulate responses of AMPKγ subunits to pharmacologic activation via 991 (Willows et al., 2017) and may play a role in tissue specific responses to other synthetic AMPK activators.
Consistent interest in the therapeutic potential of AMPK activation has also driven a renaissance of structural insights and functional implications of the quaternary structure of AMPK. Specifically, multiple studies detailing the crystal structure of AMPK have now been undertaken that invoke a variety of conditions to determine the influence of nucleotides (Calabrese et al., 2014; Chen et al., 2013; Li et al., 2015; Xiao et al., 2011; Xin et al., 2013; Yan et al., 2018) and synthetic ligands (Calabrese et al., 2014; Ngoei et al., 2018; Xiao et al., 2013; Yan et al., 2019). Critical aspects of AMPK activation at the structural level revealed by these studies include the involvement of regulatory subunit-interacting motifs (RIMs) located within the α-linker of the α subunit, as structural relays between the α subunit and γ subunit CBS domains. This arrangement creates a conformational link between nucleotide binding and promotion of AMPK activation. An additional feature of the AMPK quaternary structure occurs at the interface of the α subunit KD and the β subunit CBM where a distinct interactive pocket is formed. This has been termed the Allosteric Drug and Metabolite (ADaM) site as it has been identified as the primary location for binding of a host of small molecule activators of AMPK (Calabrese et al., 2014; Ngoei et al., 2018; Xiao et al., 2013). This site has garnered considerable interest for its role in activation of AMPK by synthetic ligands such as A-769662 (Cool et al., 2006) and compound 991 (Xiao et al., 2013) Multiple pre-clinical programs leveraged this structural motif to develop pan-AMPK activators as potential metabolic therapies for type II diabetes (Cokorinos et al., 2017; Myers et al., 2017; Steneberg et al., 2018). Pan-AMPK activators 991, PF739, MK8722, and O304 all were found to enhance skeletal muscle glucose uptake and lower blood glucose in a number of preclinical models, including obese rodents, dogs, and non-human primates (Steinberg and Carling, 2019). Despite these encouraging effects, the mice and non-human primates treated with the potent MK-8722 compound also developed cardiac hypertrophy with cardiac glycogen accumulation, though without cardiac dysfunction (Myers et al 2017). It is known that individuals undertaking chronic endurance exercise also develop a similar cardiac phenotype, though whether this is a barrier to the further development of pan-AMPK activators for metabolic diseases remains to be seen. Importantly, MK-8722 related compounds with reduced half-life in the body may provide a balance between beneficial effects and the cardiac glycogen phenotype (Muise et al., 2019). Interestingly, use of such compounds revealed a common transcriptional signature in a number of tissues between synthetic AMPK activation and exercise training. Additionally, recent work detailing the chemical characteristics that determine isoform selectivity of A-769662 and a novel “glucose importagog” SC4 may offer a road forward for improved design of next generation AMPK-based therapeutics (Ngoei et al., 2018).
The majority of work detailing the ADaM site has focused on its capacity for mediating activation of AMPK by synthetic ligands. In contrast, no endogenous ligand or resultant regulatory functions had been described until very recently. A study has now demonstrated interaction of long chain fatty acid (LCFA) CoA esters with this site (Pinkosky et al., 2020). This interaction is capable of stimulating AMPK activation. As AMPK has numerous targets involved, directly and indirectly, in the regulation of lipid metabolism this interaction likely offers a direct feedback loop for lipids on AMPK signaling. Interestingly, this novel endogenous interaction appears to be unique to complexes containing the AMPKβ1 isoform. This is reminiscent of the selectivity of A-769662 for AMPKβ1 containing complexes (Scott et al., 2008). Whether an analogous endogenous ligand also exists for AMPKβ2 containing complexes or if this site only exists in β2 complexes as an evolutionary remnant remains an important question for future studies.
Subcellular localization of AMPK: Distributing control of a master metabolic regulator
With such a broad array of direct targets and processes influenced by AMPK (Garcia and Shaw, 2017; Hardie et al., 2016; Herzig and Shaw, 2018; Steinberg and Carling, 2019) interest has evolved on the inputs that regulate AMPK and how these may determine its variegated effects across the cell. This has led to the advancement of several concepts in the field of AMPK research that are critical to progressing overall understanding of AMPK and its expansive role across cell biology. One particular area of interest has been resolving the subcellular localization of AMPK. Early studies indicated the capacity for regulation of membrane and cytoplasmic pools of AMPK through posttranslational modifications as well as the capacity for AMPK to localize to the nucleus (Kodiha et al., 2007; Mitchelhill et al., 1997; Salt et al., 1998). These studies set the precedent for existence of subcellular pools of AMPK, which paralleled studies of subcellular pools of the AMPK ortholog Snf1p in budding yeast (Hedbacker and Carlson, 2008). More recently, the design and implementation of genetically encoded fluorescent indicators of AMPK activity have enabled visualization of AMPK activity within living cells (Tsou et al., 2011). This technique has also been modified to resolve AMPK activation across multiple organelles (Miyamoto et al., 2015). From these initial works and technical breakthroughs an expansive set of studies have assessed differential localization of AMPK as well as the role that this plays in AMPK activation, downstream signaling, and functional output (Chauhan et al., 2020). In addition to the abundance of AMPK in the cytosol, these studies have established AMPK localization at the mitochondria, which is a classic metabolic hub. Interestingly, they have also revealed the presence of AMPK at additional sites including lysosomes and the endoplasmic reticulum (ER). Presence and translocation of AMPK to the nucleus has been noted across multiple studies in the past 25 years, however, functional outputs of nuclear AMPK have been detailed in only a limited number of studies. Advances in the study of AMPK in different subcellular compartments has helped provide a framework for understanding how AMPK rapidly reprograms cell metabolism to engender cell survival under low energy conditions.
Classic modes of AMPK signaling within the cytoplasm
Early studies demonstrated the presence of AMPK within the cytoplasm and the capacity for altered localization downstream of specific stimuli (Mitchelhill et al., 1997; Salt et al., 1998). Despite potential for localization and complex formation across diverse cell compartments a significant pool of AMPK is maintained within the cytoplasm (Salt et al., 1998; Tsou et al., 2011). In line with this, the localization and activation profile of LKB1, the primary upstream activating kinase of AMPK, is dependent on its complexing with STE20 related adaptor (STRAD) and mouse protein 25 (MO25) isoforms (Boudeau et al., 2003; Lizcano et al., 2004). Interactions with STRAD-MO25 promote cytoplasmic localization and activation of LKB1, which creates a platform for cytoplasmic activation of AMPK and a set of AMPK related kinases (AMPKRs). This cytoplasmic signaling cassette is thought to be the primary mediator for AMPK activation by energetic stress to promote phosphorylation of AMPK substrates (González et al., 2020). AMPK can phosphorylate metabolic enzymes within the cytoplasm, such as acetyl-CoA carboxylase (ACC) 1 at ser79. This event inhibits energetically intensive lipid synthesis and indirectly promotes mitochondrial oxidative metabolism (Fullerton et al., 2013; Munday et al., 1988; Ruderman et al., 2003). Interestingly, AMPK inhibition of ACC was also connected recently to the well-established ability of AMPK to promote cell survival in cancer cells, as AMPK was shown to inhibit ferroptosis following energetic stress, and this effect required AMPK phosphorylation of ACC (Lee et al., 2020). Of note, the same regulatory of ACC by AMPK was shown to inhibit ferroptosis and promote neuroprotective effects of two natural product-derived Alzheimer’s disease drug candidates that therapeutically increase acetyl-CoA levels in cell culture of brains of aging mice (Currais et al., 2019).
A second major classic function of AMPK in the cytoplasm is to inhibit one of major anabolic pro-growth machines in the cell – the mammalian target of rapamycin complex 1 (mTORC1) (González et al., 2020; Liu and Sabatini, 2020). AMPK inhibits mTORC1 in a two-pronged fashion, via phosphorylation and activation of the tuberous sclerosis complex 2 (TSC2) tumor suppressor and the phosphorylation of two inhibitory serines in the critical mTORC1 scaffold Raptor to limit overall anabolic drive of the cell (Van Nostrand et al., 2020). This creates a widely distributed network of energetic responsivity that can influence a broad array of cell processes to maintain or restore energy balance of the cell. The phosphorylation of a Raptor ortholog by an AMPK ortholog appears conserved through both plants and budding yeast (Hughes Hallett et al., 2015; Wu et al., 2019), and represents one of the best appreciated molecular mechanisms to couple low nutrients to direct inhibition of cellular growth.
A third major well-established function of AMPK is in the activation of autophagy, which is triggered under low energy conditions from AMPK-dependent phosphorylation of theUnc-51 Like Autophagy Activating Kinase 1 (ULK1) (Egan et al., 2011; Kim et al., 2011). ULK1 and its homolog ULK2 represent the only protein kinases in the canonical autophagy cascade. ULK1 catalytic activity is regulated in a two-pronged mechanism by AMPK: by direct phosphorylation of at least 4 different sites and via AMPK-dependent suppression of mTORC1 inhibitory phosphorylation of ULK1. Once activated, both ULK1 and AMPK appear to directly phosphorylate distinct sites in the downstream PI3-kinase related VPS34 and the subunit Beclin1, part of the Autophagy Initiation Complex (Egan et al., 2015; Kim et al., 2013; Russell et al., 2013) (see Figure 2). Interestingly, the ULK1 phosphorylation sites in VPS34 and Beclin appear to lie in front of LC3-interaction (LIR) motifs, providing an additional mechanic basis for how ULK1 may contribute to regulation of this complex (Birgisdottir et al., 2019). In addition to both targeting components of the Beclin/VPS34 complex, AMPK and ULK1 have also been reported to phosphorylate the sole transmembrane protein in the core autophagy cascade – ATG9 (Mack et al., 2012; Weerasekara et al., 2014), which is another rate-limiting autophagy protein involved in autophagosome formation. In the interest of brevity, additional substrates are detailed in Figure 2, including key targets involved in glycogen metabolism which may relate to cardiac effects observed with chronic genetic or pharmacological activation. A recent chemical genetic screen for new AMPK substrates also identified an understudied protein involved in glycogen binding, STBD1, so additional insights into how AMPK balances lipid and carbohydrate metabolism remain to be discovered (Ducomm et al., 2019). Finally, cytoplasmic AMPK may serve as a pool to draw from for more localized complexing and signaling actions at differentially regulated organelle sites. In this regard, understanding the mechanisms that determine where AMPK is localized and its translocation between compartments is a very active area of research currently.
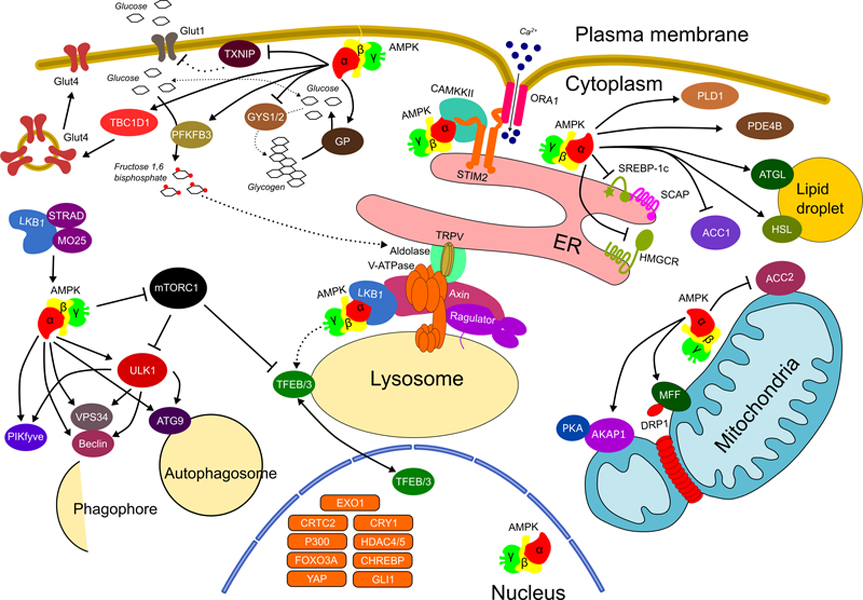
Figure 2. AMPK substrates act in specific subcellular locales to rewire metabolism.
AMPK is a master of distributed metabolic control, acting within specific compartments of the cell to maintain local metabolic homeostasis, which combine to maintain overall cellular metabolic homeostasis. Phosphorylation and activation of AMPK within discrete locales is differentially controlled by its upstream kinases LKB1 and CAMKK2, which are responsive to a variety of stimuli across these compartments. AMPK directly influences the balance of macronutrient metabolism and handling through its effects on cellular glucose uptake (TXNIP/Glut1, TBC1D1/Glut4), glycolysis (PFKFB3), glycogen storage (GS and GP), lipid and cholesterol synthesis (SREBP1C, HMGCR, and ACC1/2), lipolysis (ATGL and HSL), and lipid oxidation. AMPK also supports the overall maintenance of metabolically active organellar systems including mitochondria (MFF) and multiple aspects of the endolysosomal compartment (PIKfyve, ULK1, VPS34, Beclin, ATG9, TFEB/3). AMPK can also influence the activity of other master metabolic switches (mTORC1) enabling communication between the overall anabolic and catabolic drive of cells.
Mitochondrial AMPK as a multimodal regulator of organelle function
AMPK exists as a critical hub of energy sensing and metabolic regulation (Garcia and Shaw, 2017). Mitochondria are the primary source of ATP production and house numerous metabolic processes to support overall cell function (Martínez-Reyes and Chandel, 2020). Classical targets of AMPK are known to alter metabolic inputs that determine mitochondrial metabolism (e.g. acetyl CoA carboxylase or 6-phosphofructo-2-kinase) (Fullerton et al., 2013; Marsin et al., 2000; Munday et al., 1988). AMPK has been known for more than 20 years to promote mitochondrial biogenesis (Bergeron et al., 2001; Winder et al., 2000), which was in part connected to AMPK-dependent activation of PGC1α, a master transcriptional regulator of mitochondrial biogenesis (Cantó et al., 2010, 2009; Jäger et al., 2007; Reznick and Shulman, 2006).
Despite the influential role of AMPK on mitochondrial function and homeostasis until recently the existence and implications for mitochondrially localized AMPK had not been fully realized. Indeed, localization of AMPK to the mitochondria would enable efficient energetic sensing and rapid modification of mitochondrial proteins to control functional outputs. In support of this hypothesis, mitochondrial localization of AMPK activity has been demonstrated (Miyamoto et al., 2015). Also critical to understanding functional implications of mitochondrial AMPK are demonstrations of differential responsivity to nutrient deprivation (Miyamoto et al., 2015; Zong et al., 2019) or mitochondrial electron transport chain (ETC) inhibitors (Hung et al., 2021). Whereas glucose deprivation produces a somewhat delayed activation of mitochondrial AMPK (2 – 4 hours), direct inhibition of mitochondrial oxidative phosphorylation function stabilizes and activates AMPK in the cytoplasm and at the mitochondria in a rapid manner (~2 mins). This dichotomy likely represents a mechanism for the distributed control of localized pools of AMPK activation based on the type of cellular stress that is encountered. Unlike the uniform depictions in textbooks, mitochondria are now well-appreciated to exist as a dynamically regulated network. To maintain this network the production (biogenesis) and incorporation (fusion) of healthy mitochondria must be balanced with fragmentation (fission) to enable pruning and turnover (autophagy/mitophagy) of dysfunctional components (Mishra and Chan, 2016). As mitochondria are dynamically regulated to undergo fusion and fission events based on the requirements of the cell and environmental conditions, the capacity to initiate structural modifications during times of apparent dysfunction or elevated demand are imperative. Though mitochondrial fusion is thought to bolster oxidative phosphorylation capacity which many associate with AMPK, in fact the most acute role of AMPK across cell types has been revealed as a requirement for AMPK to promote mitochondrial fission following mitochondrial poisons (Herzig and Shaw, 2018). In line with this, multiple AMPK substrates relating to mitochondrial fission have been identified using unbiased phospho-proteomic pulldowns with the AMPK substrate motif antibody, including mitochondrial fission factor (MFF) (Ducommun et al., 2015) and ARMC10 (Chen et al., 2019). MFF is an outer mitochondrial membrane protein that was identified as the long-sought receptor for the conserved dynamin-related protein 1 (DRP1) that is the critical protein in mitochondrial fission across all eukaryotes. MFF was demonstrated to be necessary and sufficient for AMPK dependent effects on mitochondrial fragmentation elicited by ETC inhibitors (rotenone, antimycin A) and small molecule AMPK activators (Toyama et al., 2016). Importantly, mutation of the two conserved canonical AMPK sites in MFF to alanine prevented endogenous DRP1 recruitment to mitochondria following AICAR or Rotenone, and conversely, expression of an MFF cDNA with substitution of these serines to phospo-mimetic residues in MFF KO cells resulted in cells with chronically short mitochondria, a remarkable effect on mitochondrial morphology from two specific amino acids in the MFF protein (Toyama et al., 2016). Metformin induction of AMPK-dependent MFF phosphorylation and mitochondrial fission was reported to improve mitochondrial respiration and restore the mitochondrial life cycle, and HFD-fed-mice with liver-specific knockout of AMPKα1/2 exhibited higher blood glucose levels when treated with metformin (Wang et al., 2019). Notably, a very recent careful quantitative analysis of mitochondrial divisions examining the functional engagement of the different mitochondrial DRP1 receptors revealed that MFF is involved in ER-constriction dependent midzone mitochondrial scission events that results in successful proliferation of mitochondria, as opposed to scission at the mitochondrial periphery which involved the DRP1 receptor Fis1 and often led to mitochondrial degradation (Kleele et al., 2021). Another piece of the puzzle comes from findings that AMPK directly phosphorylates the outer mitochondrial membrane protein AKAP1(Hoffman et al., 2015), which anchors PKA near the mitochondria, where it in turn can directly phosphorylate DRP1 (Gomes et al., 2011). The other recently indicated AMPK substrate with a role in mitochondria is ARMC10, a reported interactor of the mitochondrial bound Rho GTPase Miro1 (López-Doménech et al., 2012), which controls mitochondrial trafficking (López-Doménech et al., 2018). ARMC10 overexpression was shown to promote mitochondrial fission and ARMC10 KO prevented AMPK-promoted mitochondrial fission (Chen et al., 2019). Interestingly, Miro1 has also been reported to regulate ER-mitochondrial contact sites (Modi et al., 2019) so it will be interesting to examine whether AMPK or ARMC10 play roles in those processes as well.
One hypothetical function for the fragmentation of elongated mitochondrial networks into hundreds of individual mitochondria following mitochondrial poisons is to allow for quality control assessment of mitochondrial fragments in which mitochondrial membrane potential and other metabolic features can be better examined to ensure that only dysfunctional daughter mitochondria are removed without whole-scale removal of bulk mitochondria mass which may be functionally intact. The removal and destruction of specific cellular organelles and protein aggregates via specific autophagy receptors has led to a revolution in understanding of cellular homeostasis processes (Birgisdottir and Johansen, 2020; Kirkin, 2020). The specific removal of defective mitochondria occurs via a specialized form of autophagy termed mitophagy, which in many though not all circumstances involves the E3 ubiquitin ligase Parkin, which is encoded by PARK2, the most frequent genetic cause of early onset Parkinson’s disease (Pickles et al., 2018). Though the initial connections of AMPK to the activation of ULK1 was suggestive of a defect in mitophagy due to a specific and notable accumulation of defective mitochondria in cells lacking AMPK, or ULK1, or bearing ULK1 mutated in all AMPK phosphorylation sites (Egan et al., 2011), it remained unclear whether AMPK or ULK1 controlled any specific components of the mitophagy pathway or simply enabled mitophagy via general control of macroautophagy as mentioned above. Subsequent studies demonstrated that one of the best conserved AMPK phosphorylation sites in ULK1Ser555, is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy (Laker et al., 2017), though a precise role for AMPK in canonical PINK1-Parkin mitophagy has remained unclear (Seabright et al., 2020; Vargas et al., 2019) until very recently (Hung et al., 2021; Laker et al., 2017; Seabright et al., 2020). ULK1 was found to directly phosphorylate conserved ParkinSer108, within a recently identified ten amino acid domain required for full-Parkin activation termed the ACT domain (Swatek et al., 2019). Prior to this discovery, Parkin was known to be activated by its recruitment from the cytoplasm following treatment of cells with mitochondrial depolarizing agents, but not following ETC inhibition. Mitochondrial recruitment of Parkin was shown to require phosphorylation of ParkinSer65 by the mitochondrial transmembrane kinase PINK1, whose levels are only stabilized in depolarized mitochondria (Harper et al., 2018; Lazarou et al., 2015). Notably, we found that treatment of cells with ETC inhibitors or the mitochondrial depolarizing agent CCCP resulted in maximal AMPK activation by 5 minutes with similar maximal phosphorylation of Parkin in the cytoplasm but not mitochondria on Ser108. Biochemical association of Parkin with the mitochondria did not occur appreciably until 20–30 minutes after mitochondrial insults, with PINK1 phosphorylation of Parkin and Ubiquitin at the mitochondria occurring at and after that time, when Parkin Ser108 phosphorylation was rapidly decreasing. This represents an unexpected early step in Parkin activation, which was prevented with AMPK KO, ULK1 inhibition, or mutation of Ser108 and surrounding serines (Hung et al., 2021). These new findings indicate that Parkin is rapidly phosphorylated by AMPK/ULK1 signals under many conditions that do not involve full mitochondrial depolarization and PINK1 activation. Given that AMPK is promoting full mitochondrial fission within 20 minutes of ETC inhibition, this provides a molecular mechanism by which mitochondrial networks are fragmented and Parkin may be partially activated by AMPK/ULK1, induced in some way to efficiently translocate to mitochondria to survey for mitochondria lacking proper mitochondrial membrane potential, hence where PINK1 will be stabilized to promote full Parkin activation and marking of mitochondria for destruction. Only those minority of fragmented mitochondria with depolarized mitochondria would have stabilized PINK1, and hence would receive additional Parkin Ser65 phosphorylation and subsequent downstream biochemical steps in mitophagy (recruitment of mitophagy receptors, etc) (Figure 3). The centrality of AMPK in the acute response to mitochondrial damage was also observed in a recent study performing a genomic CRISPR screen for factors mediating inhibition of mTORC1 after the ETC inhibitor oligomycin (Condon et al., 2021). The authors found that AMPK was required for an early 0–3h suppression of mTORC1 after oligomycin whereas an HRI-EIF2A dependent induction of mRNAs encoding the mTORC1 inhibitors REDD1/DDIT4 and Sestrin2 were required for a second wave of mTORC1 inhibition. Cells knocked out for both AMPK and HRI were largely unable to inhibit mTORC1 after oligomycin (Condon et al., 2021).
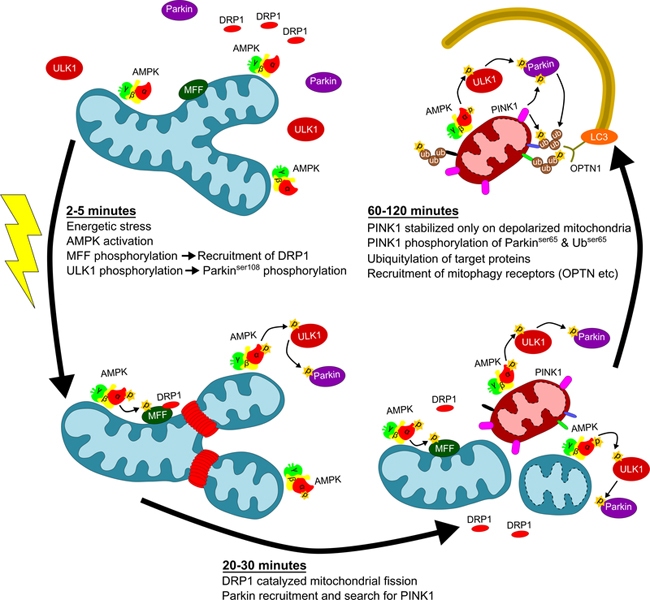
Figure 3. AMPK rapidly promotes fission and mitophagy following mitochondrial energetic stress.
One of the key organelles supported by AMPK for the maintenance of cellular metabolic homeostasis are the mitochondria. AMPK acutely senses energetic stress through levels of adenine nucleotides and glycolytic intermediates that can shift under physiologic or pathologic conditions. Following initial stimulation of AMPK, MFF undergoes activating phosphorylation enabling recruitment of DRP1 for mitochondrial fission. ULK1 is activated downstream of AMPK while the E3 ubiquitin ligase, Parkin, is recruited to the mitochondria and activated under these conditions. Upon activation, Parkin ubiquitylates target proteins on the outer mitochondrial membrane (OMM). PINK1 is stabilized on the outer mitochondrial membrane of depolarized mitochondria phosphorylating Parkin and ubiquitin groups attached to OMM proteins. Phosphorylated ubiquitin acts as a tag for identification of mitophagy receptors, such as optineurin (OPTN), that mediate later steps of mitochondrial turnover.
Lysosomal AMPK as a primary glucose sensor and regulator of the endolysosomal compartment.
One more newly coveted locale that has arisen as a hub of AMPK function is the lysosome. Over the past decade several studies have elucidated key roles of the lysosome in metabolism (Abu-Remaileh et al., 2017) and cellular differentiation (Young et al., 2016). Identification of the lysosome as a center of amino acid sensing and regulation of mTOR signaling (Saxton and Sabatini, 2017) promoted the idea of lysosomes as a hub of cellular metabolic regulation (Lamming and Bar-Peled, 2019). This dogmatic shift resulted in a host of studies detailing novel regulatory mechanisms for metabolic pathways occurring at the lysosome, including AMPK. These studies have progressively outlined the localization, functional implications, and mechanistic regulation of lysosomal AMPK (M. Li et al., 2019; Zhang et al., 2014, 2017; Zong et al., 2019).
Analogous to the relationship of amino acids and mTOR activation, glucose is often regarded as a primary nutrient input that is determinant of AMPK activity. Historically, this relationship was hypothesized to depend on an increased AMP:ATP ratio secondary to limited glucose supply. However, recent work has shown the capacity for glucose deprivation, independent of gross changes in cellular ATP levels, to activate lysosomal AMPK (M. Li et al., 2019). This activation of lysosomal AMPK has been linked to the glycolytic intermediate, fructose-1,6- bisphosphate (FBP). Specifically, decreased FBP levels were shown to promote formation of an LKB1-AMPK complex at the lysosomal surface and the glycolytic enzyme Aldolase was shown to be required (M. Li et al., 2019), as well as the Wnt pathway scaffold protein Axin (Zhang et al., 2014, 2017). Specifically, a role for axin in acute AMPK activation by glucose starvation (≤ 20 minutes) (Zhang et al., 2017) has been demonstrated. However, longer glucose starvation (≥ 1 hour) does not appear to require Axin (Orozco et al., 2020; Zhang et al., 2017). The data overall supports a model in which FBP-unoccupied Aldolase binds and inhibits ER-localized transient receptor potential channel subfamily V (TRPV), inhibiting calcium release from the ER in low glucose conditions. Decreased calcium at ER lysosome contact sites renders inhibited TRPV accessible to bind the lysosomal v-ATPase that then recruits AXIN:LKB1 to activate AMPK independently of AMP. This mode of AMPK activation appears to hinge on v-ATPase-Ragulator complexes (Zhang et al., 2014) - that also have demonstrated roles in the activation of mTOR (Bar-Peled et al., 2012). Recently, investigators used AMPK-KO cells to try to define whether mTOR could sense glucose independently of AMPK. mTOR was found to sense a metabolite downstream of aldolase and upstream of the GAPDH-catalysed steps of glycolysis (Orozco et al., 2020). Utilizing cells lacking AMPK and expressing triose kinase the authors identified dihydroxyacetone phosphate (DHAP) as the key metabolite in this mode of mTOR activation. DHAP is a precursor for lipid synthesis, a process mTORC1 is well-established to control, and sensing of DHAP by mTORC1 provides a measure by which mTORC1 can sense the flux in the pathway. More recently, use of point mutations in Aldolase appear to separate some of the AMPK and mTOR control, reinforcing this as a key node for glycolytic flux to directly connect to these master regulators of catabolism and anabolism(Li et al., 2021). Overall, these studies posit vATPase-Ragulator complexes as critical switches in nutrient metabolism, which integrate multiple nutritional inputs with their appropriate downstream signaling pathways at the lysosome.
Similar to its broad functions at the mitochondria, AMPK not only localizes to the endolysosomal compartment, but also mediates the underlying biogenesis and processing of these organelles. One critical function of this metabolic signaling hub at the lysosome is the regulation of transcription factors EB and E3 (TFEB/TFE3) and their downstream network of genes (Carroll and Dunlop, 2017). This network is linked by a common genetic motif denoted the “coordinated lysosomal expression and regulation” (CLEAR) motif (Sardiello et al., 2009). This motif is targeted by members of the MITF and TFE transcription factor family to stimulate lysosomal biogenesis. Lysosomal biogenesis and maintenance are critical for appropriate autophagic processing at the cellular level, which is a known functional output of AMPK activation. mTOR was demonstrated to directly phosphorylate multiple conserved serines in TFEB/TFE3, resulting in their exclusion from the nucleus (Martina et al., 2012; Roczniak-Ferguson et al., 2012; Settembre et al., 2012). More recently evidence has arisen for regulation of TFEB/TFE3 by AMPK through direct phosphorylation (Paquette et al., 2021) though mutation of the proposed AMPK sites did not impact nuclear shuttling of TFEB by AMPK, nor did the proposed AMPK sites in TFEB match the well-established AMPK substrate consensus motif, so additional mechanisms of AMPK control of TFEB must exist.
As AMPK is a well-documented inhibitor of mTOR activity, effects of AMPK on TFEB/TFE3 were hypothesized to be due to inhibition of mTOR. In line with this, AMPK was demonstrated as a critical determinant of the phosphorylation status and nuclear localization of TFEB/TFE3 (Eichner et al., 2019; Young et al., 2016). These studies demonstrated a genetic requirement of AMPK for the expression of CLEAR network genes during embryonic stem cell differentiation (Young et al., 2016) or in lung cancer cells under conditions of glucose deprivation, where AMPK provides a survival signal (Eichner et al., 2019). Indeed Kras mutant genetic engineered lung tumors lacking AMPK exhibited loss of lysosome content and an inability to grow past a certain metabolic restriction, consistent with early studies showing AMPK is required for cell survival under low energy conditions (Shaw et al., 2004b, 2004a), including tumor cells (Jeon et al., 2012; Laderoute et al., 2006; Saito et al., 2015). While AMPK was oft hypothesized as a tumor suppressor, due to its anti-catabolic effects, complete removal of AMPK at the time of tumor initiation in solid tumors prevented proper tumor establishment and progression (Eichner et al., 2019). These findings are in line with increased awareness of autophagy as a critical process in oncogenic development, progression, and resistance to treatment (Amaravadi et al., 2019). Therefore, targeting pathways critical to lysosomal biogenesis and/or autophagy may provide therapeutic inroads for previously underserved cancers (Limpert et al., 2018).
In concert with transcriptional promotion of lysosomal biogenesis, AMPK activation has also been indicated in the initiation, maturation, and processing at multiple sites within the endolysosomal compartment. Phosphatidylinositol processing appears as a common link for this functional output of AMPK. AMPK has demonstrated effects on this pathway through phosphorylation of several substrates including progestin and adipoQ receptor 3 (PAQR3) and 1-phosphatidylinositol 3-phosphate 5-kinase (PIKfyve). Phosphorylation of PAQR3 by AMPK appears important for the assembly of protein complexes containing autophagy related gene 14 like (ATG14L) and Phosphatidylinositol 3-kinase VPS34 (VPS34). This can promote autophagosome formation (Xu et al., 2016). AMPK can also directly phosphorylate the phosphoinositide phosphate kinase, 1-phosphatidylinositol 3-phosphate 5-kinase (PIKfyve), which is responsible for the synthesis of PI(5)P. Originally, PIKfyve activation by AMPK was identified as a control point for exercise stimulated translocation of glucose transporter 4 (GLUT4) to the plasma membrane from intracellular vesicles (Liu et al., 2013) but more recently AMPK-mediated activation of PIKfyve was shown to coordinate with the Biogenesis of lysosome-related organelles complex-1 (BORC1) to promote lysosomal reformation (Yordanov et al., 2019). Finally, it was recently demonstrated that in addition to AMPK, ULK1 also directly phosphorylates the lipid kinase PIKfyve on a distinct serine - Ser1548 - which is essential for glucose starvation-dependent autophagy. AMPK-dependent activated ULK1 was shown to stimulate synthesis of PI(5)P which was shown to directly contribute to autophagosomes, and like PI(3)P synthesized by VPS34, may serve to recruit lipid-binding autophagy receptors to the nascent phagophore (Karabiyik et al., 2021).
Beyond nutrient and energetic stress, direct lysosomal damage has been shown to be one of the strongest inducers of autophagy, and recent studies discovered a new signaling mechanism in which cytoplasmic galectin family proteins were found to bind and control mTOR and AMPK activity after this stress (Jia et al., 2018). Gal8 inhibited mTOR activity through its Ragulator-Rag signaling machinery, whereas galectin-9 activated AMPK in response to lysosomal injury, via a novel mechanism involving the deubiqutinating enzyme USP9X and the kinase TAK1 (Jia et al., 2020). Collectively the data suggest galectin and ubiquitin systems converge to activate AMPK and autophagy during endomembrane homeostasis. With such broad indications for the action of AMPK at the lysosome and its capacity for regulating the overall function of this organelle system it will be critical to understand the specific physiological and pathologicala contexts that rely on AMPK-mediated events.
Linking diverse activating inputs of AMPK at the endoplasmic reticulum
As is the case with many cellular functions and organelles, ideas surrounding the endoplasmic reticulum (ER) have evolved considerably in recent years. Classically regarded as a hub of protein processing and secretion, the functions of the ER have been expanded to include intercellular calcium signaling and lipid homeostasis among other functions (Sicari et al., 2020). Upon insufficiency in any of these ER functions a critical set of responses is undertaken to limit cellular damage and restore sufficient function (Almanza et al., 2019). As a hub of lipid metabolism and processing AMPK has identified actions on key components of lipid homeostasis at the ER including sterol regulatory-element binding protein 1c (SREBP-1c) and 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGCR). AMPK phosphorylates SREBP-1c directly. This acts as an inhibitory switch that maintains SREBP-1c at the ER, which negates its capacity for translocation to the nucleus where it acts as a master promoter of lipid synthetic programming. Additionally, phosphorylation of HMGCR by AMPK limits the synthesis of cholesterol. This likely minimizes energy utilization in the short term, but may also influence membrane dynamics through altered cholesterol homeostasis. These interactions contribute to the overarching functions of AMPK as a master regulator of lipid metabolism, but other aspects of ER function, such as calcium homeostasis, have been increasingly recognized for their influence on AMPK activity.
While LKB1 was identified as being the kinase upstream of AMPK, and required for AMPK activation in conditions of low energy, a second upstream kinase, CaMKK2, was also identified for AMPK (Hawley et al., 2005; Hurley et al., 2005; Woods et al., 2005). CaMKK2 responds to intracellular calcium levels downstream of hormonal cues and shifts in ion concentrations. Indeed, regulation of AMPK by CaMKK2 appears to be critical for specific effects on cellular and physiologic functions. As a critical mediator of cellular signaling events the concentrations of intracellular and intraorganellar calcium are tightly regulated. As previously mentioned, the endoplasmic reticulum maintains critical aspects of cellular calcium homeostasis. This indicates the ER as a potential base for alternative activation of AMPK by CAMKK2 through effects on calcium levels. In line with this, multiple studies have now demonstrated a relationship between AMPK and the ER-localized protein Stromal interaction molecule 2 (STIM2) (Chauhan et al., 2019; Nelson et al., 2019; Stein et al., 2019). From these studies STIM2 appears to complex with AMPK and CAMKK2 at the ER. This interaction is promoted by release of calcium from the ER lumen. Additionally, AMPK promotes aspects of calcium homeostasis via phosphorylation of STIM1 and 2, which was demonstrated to occur in murine liver following a therapeutic dose of metformin (Stein et al., 2019).
The enigmatic role of nuclear AMPK
The ability of a fraction of the total cellular AMPK pool to shuttle into and out of the nucleus has been reported numerous times. Despite, this the functional significance of nuclear AMPK has yet to be fully determined. Hypotheses have varied from regulation of metabolic processes in the nucleus to direct phosphorylation of transcription factors to control expression of metabolism and growth-related genes. The functional significance of nuclear AMPK remains an active area of interest without a unified theory. Both AMPKα1 and α2 contain well-defined nuclear export sequences (Kazgan et al., 2010). Both also contain a short conserved basic sequence that was proposed to serve as a nuclear localization sequence in AMPKα2 (Suzuki et al., 2007) though this has not been corroborated in other studies (Kazgan et al., 2010) so further work may clarify the signals governing AMPK nuclear shuttling. Despite earlier focus on nuclear AMPKa2 in muscle (McGee et al., 2003; Steinberg et al., 2006), it is now clear that endogenous AMPKa1 can be found in the nucleus under certain circumstances (Lamia et al., 2009; Vara-Ciruelos et al., 2018). AMPK has been suggested to phosphorylate a number of transcriptional regulators (see bottom of Figure 2), though notably many of them shuttle in and out of the nucleus (CRY1, CRTC2, HDACs, YAP, FOXO3A, GLI1) and it remains unclear which of these are bona fide substrates of AMPK in the nucleus. Whether modification of these, or other, transcriptional regulators is specific to nuclear AMPK is maintained as an active area of research.
Another new area for targets of AMPK lies in the control epigenetic modifications, including reports that AMPK phosphorylates and stabilizes the epigenetic enzyme TET2, which catalyzes the demethylation of 5-methylcytosine on DNA (Kundu et al., 2020; Wu et al., 2018; Zhang et al., 2019). AMPK has also been connected to EZH2 (Wan et al., 2018) and other epigenetic regulators (Marin et al., 2017; Tanaka et al., 2019, 2015), though much work remains to validate these proteins as direct substrates of AMPK in vivo and to decode the functional consequences of these specific phosphorylation events under glucose- and energy-limited conditions. Interestingly, these enzymes rely on a host of carbon-based metabolites (e.g. acetyl-CoA, S-adenosyl methionine, etc) as substrates for the modifications they produce on histones and DNA. Therefore, the regulation of these enzymes by AMPK represents control points of genetic programming and metabolism. This adds to the growing body of work detailing the direct impact of metabolism on the epigenetic landscape (Campbell and Wellen, 2018).
More recently, AMPK has been shown to specifically be activated in the nucleus in a calcium and CAMKK2-dependent manner following DNA damage (S. Li et al., 2019; Vara-Ciruelos et al., 2018) and the exonuclease Exo1 involved in stressed replication forks was a proposed target. AMPK has been proposed to play a role in UVB response previously (C. L. Wu et al., 2013) and a recent careful analysis of genetic ablation of AMPK in the mouse skin showed defects in UVB response and general growth, though mTORC1 was a key target of AMPK in that process (Crane et al., 2021). Artemis, a protein involved in non-homologous end-joining DNA repairs has been reported in multiple independent studies as a nuclear AMPK interactor (Chen et al., 2020; Nakagawa et al., 2012), which may relate to a recent finding that Kras LKB1-deficient lung cancers show specific defects in double strand repair when compared to Kras tumors with other coincident mutations (Deng et al., 2021).
Building a unified model for AMPK function following different stimuli
While localization certainly implies potential for differential regulation of downstream substrates it also appears critical for specific input effectors of AMPK activation as well. Activation of lysosomal AMPK by glucose deprivation appears to precede activation of mitochondrial AMPK under the same condition (Zong et al., 2019). Recent work has shown the capacity for glucose deprivation, independent of gross changes in ATP levels, to activate AMPK. This effect was shown to be dependent on a relationship between voltage channels and glycolytic enzymes located on the lysosomal membrane surface (M. Li et al., 2019). While this study demonstrated AMPK activation independent of large-scale changes to energetic content of the cell it remains to be determined whether “pockets” of energetic deficiency can arise and influence AMPK activation based on localized cellular functions.
Recent studies have elucidated a hierarchy of glucose-dependent AMPK activation that starts at the lysosome. AMPK activation within this compartment appears to be independent of wholesale changes to the energetic status of the cell. In contrast, rapid activation of AMPK at the lysosome by glucose deprivation occurs as a result of its existence in complex with aldolase, a metabolic enzyme that converts fructose 1,6-bisphosphate, into dihydroxyacetone phosphate (DHAP) and glyceraldehyde 3-phosphate (G3P). The wave of glucose-dependent AMPK activation progresses to cytosolic and mitochondrial AMPK over time (Zong et al., 2019). These effects are likely dependent on the delayed impacts of glucose deprivation on energetic status, which is in line with the classic model of AMPK activation. While the lysosome-first model has been demonstrated as critical for responses to glucose levels, other models of AMPK activation do not appear to follow this paradigm. Specifically, inhibitors of mitochondrial ATP production trigger acute activation of AMPK and downstream signals at the mitochondria within minutes of treatment, which correlated with loss of ATP and increases in mitochondrial ROS (Hung et al., 2021; Toyama et al., 2016).
Adding to the complexity of AMPK activation is recent work highlighting the capacity for LCFA-CoAs to act as activators as well (Pinkosky et al., 2020). This work establishes a direct effect of lipid-based metabolites on AMPK and it identified endogenous modes of engaging the AMPK ADaM site that is the primary site of action for direct activation of AMPK by pharmaceuticals. AMPK has indirect connections to other metabolites such as amino acids, through mTOR, and NAD+/NADH, through sirtuin signaling. However, there likely remain other metabolic inputs that influence AMPK activation directly, which may operate in organelle specific manners following the trend of glucose deprivation (Carling, 2019).
Conclusion and perspectives
Since its earliest study, AMPK has been noted to coordinately regulate different metabolic pathways (Carling et al., 1987). The prevailing dogma often indicated AMPK activation as a monolith of binary function that was inactive in energy replete conditions and active during limited energy supply. A plethora of new discoveries have provided resolution for AMPK activation as it coordinates with multiple metabolic cues and protein interactions in a localized manner to invoke targeted signaling programs. In turn, these loci of AMPK signaling operate in concert to govern cellular behaviors. Over the past decade it has become increasingly apparent that AMPK phosphorylates a limited number of substrates at specific linchpin points in different cellular processes, all united in the effect to restore energetic and metabolic homeostasis to a cell in nutrient-deprived conditions.
Acknowledgements
E.F.T. is funded by F32DK126418 postdoctoral fellowship. R.J.S. holds the William R. Brody Chair and is a professor in the Molecular and Cell Biology Laboratory at the Salk Institute of Biological Studies. The work from our laboratory described in this review was supported by grants from the National Institutes of Health (R35CA172229, P01CA120964) and by an AHA-Allen Initiative in Brain Health and Cognitive Impairment award made jointly through the American Heart Association and The Paul G. Allen Frontiers Group: 19PABH134610000. Research also supported by funding to the Salk Cancer Center Support Grant P30CA014195. We apologize to the many authors and many studies which could not be cited due to space limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Remaileh M, Wyant GA, Kim C, Laqtom NN, Abbasi M, Chan SH, Freinkman E, Sabatini DM, 2017. Lysosomal metabolomics reveals V-ATPase- and mTOR-dependent regulation of amino acid efflux from lysosomes. Science 358, 807–813. 10.1126/science.aan6298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almanza A, Carlesso A, Chintha C, Creedican S, Doultsinos D, Leuzzi B, Luís A, McCarthy N, Montibeller L, More S, Papaioannou A, Püschel F, Sassano ML, Skoko J, Agostinis P, de Belleroche J, Eriksson LA, Fulda S, Gorman AM, Healy S, Kozlov A, Muñoz-Pinedo C, Rehm M, Chevet E, Samali A, 2019. Endoplasmic reticulum stress signalling - from basic mechanisms to clinical applications. FEBS J. 286, 241–278. 10.1111/febs.14608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaravadi RK, Kimmelman AC, Debnath J, 2019. Targeting Autophagy in Cancer: Recent Advances and Future Directions. Cancer Discov. 9, 1167–1181. 10.1158/2159-8290.CD-19-0292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Peled L, Schweitzer LD, Zoncu R, Sabatini DM, 2012. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 150, 1196–208. 10.1016/j.cell.2012.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron R, Ren JM, Cadman KS, Moore IK, Perret P, Pypaert M, Young LH, Semenkovich CF, Shulman GI, 2001. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am. J. Physiol. Endocrinol. Metab. 281, E1340–6. 10.1152/ajpendo.2001.281.6.E1340 [DOI] [PubMed] [Google Scholar]
- Birgisdottir ÅB, Johansen T, 2020. Autophagy and endocytosis - interconnections and interdependencies. J. Cell Sci. 133. 10.1242/jcs.228114 [DOI] [PubMed] [Google Scholar]
- Birgisdottir ÅB, Mouilleron S, Bhujabal Z, Wirth M, Sjøttem E, Evjen G, Zhang W, Lee R, O’Reilly N, Tooze SA, Lamark T, Johansen T, 2019. Members of the autophagy class III phosphatidylinositol 3-kinase complex I interact with GABARAP and GABARAPL1 via LIR motifs. Autophagy 15, 1333–1355. 10.1080/15548627.2019.1581009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudeau J, Baas AF, Deak M, Morrice NA, Kieloch A, Schutkowski M, Prescott AR, Clevers HC, Alessi DR, 2003. MO25α/β interact with STRADα/β enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. EMBO J. 22, 5102–5114. 10.1093/emboj/cdg490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese MF, Rajamohan F, Harris MS, Caspers NL, Magyar R, Withka JM, Wang H, Borzilleri KA, Sahasrabudhe PV, Hoth LR, Geoghegan KF, Han S, Brown J, Subashi TA, Reyes AR, Frisbie RK, Ward J, Miller RA, Landro JA, Londregan AT, Carpino PA, Cabral S, Smith AC, Conn EL, Cameron KO, Qiu X, Kurumbail RG, 2014. Structural basis for AMPK activation: natural and synthetic ligands regulate kinase activity from opposite poles by different molecular mechanisms. Structure 22, 1161–1172. 10.1016/j.str.2014.06.009 [DOI] [PubMed] [Google Scholar]
- Campbell SL, Wellen KE, 2018. Metabolic Signaling to the Nucleus in Cancer. Mol. Cell 71, 398–408. 10.1016/j.molcel.2018.07.015 [DOI] [PubMed] [Google Scholar]
- Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J, 2009. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458, 1056–60. 10.1038/nature07813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J, 2010. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 11, 213–9. 10.1016/j.cmet.2010.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carling D, 2019. AMPK hierarchy: a matter of space and time. Cell Res. 29, 425–426. 10.1038/s41422-019-0171-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carling D, Zammit VA, Hardie DG, 1987. A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett. 223, 217–22. 10.1016/0014-5793(87)80292-2 [DOI] [PubMed] [Google Scholar]
- Carroll B, Dunlop EA, 2017. The lysosome: A crucial hub for AMPK and mTORC1 signalling. Biochem. J. 474, 1453–1466. 10.1042/BCJ20160780 [DOI] [PubMed] [Google Scholar]
- Chauhan AS, Liu X, Jing J, Lee H, Yadav RK, Liu J, Zhou Y, Gan B, 2019. STIM2 interacts with AMPK and regulates calcium-induced AMPK activation. FASEB J. 33, 2957–2970. 10.1096/fj.201801225R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan AS, Zhuang L, Gan B, 2020. Spatial control of AMPK signaling at subcellular compartments. Crit. Rev. Biochem. Mol. Biol. 55, 17–32. 10.1080/10409238.2020.1727840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Xin FJ, Wang J, Hu J, Zhang YY, Wan S, Cao LS, Lu C, Li P, Yan SF, Neumann D, Schlattner U, Xia B, Wang ZX, Wu JW, 2013. Conserved regulatory elements in AMPK. Nature 498, E8–E10. 10.1038/nature12189 [DOI] [PubMed] [Google Scholar]
- Chen Z, Lei C, Wang C, Li N, Srivastava M, Tang M, Zhang H, Choi JM, Jung SY, Qin J, Chen J, 2019. Global phosphoproteomic analysis reveals ARMC10 as an AMPK substrate that regulates mitochondrial dynamics. Nat. Commun. 10, 104. 10.1038/s41467-018-08004-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Wang C, Jain A, Srivastava M, Tang M, Zhang H, Feng X, Nie L, Su D, Xiong Y, Jung SY, Qin J, Chen J, 2020. AMPK Interactome Reveals New Function in Non-homologous End Joining DNA Repair. Mol. Cell. Proteomics 19, 467–477. 10.1074/mcp.RA119.001794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cokorinos EC, Delmore J, Reyes AR, Albuquerque B, Kjøbsted R, Jørgensen NO, Tran JL, Jatkar A, Cialdea K, Esquejo RM, Meissen J, Calabrese MF, Cordes J, Moccia R, Tess D, Salatto CT, Coskran TM, Opsahl AC, Flynn D, Blatnik M, Li W, Kindt E, Foretz M, Viollet B, Ward J, Kurumbail RG, Kalgutkar AS, Wojtaszewski JFP, Cameron KO, Miller RA, 2017. Activation of Skeletal Muscle AMPK Promotes Glucose Disposal and Glucose Lowering in Non-human Primates and Mice. Cell Metab. 25, 1147–1159.e10. 10.1016/j.cmet.2017.04.010 [DOI] [PubMed] [Google Scholar]
- Condon KJ, Orozco JM, Adelmann CH, Spinelli JB, van der Helm PW, Roberts JM, Kunchok T, Sabatini DM, 2021. Genome-wide CRISPR screens reveal multitiered mechanisms through which mTORC1 senses mitochondrial dysfunction. Proc. Natl. Acad. Sci. U. S. A. 118. 10.1073/pnas.2022120118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, Dickinson R, Adler A, Gagne G, Iyengar R, Zhao G, Marsh K, Kym P, Jung P, Camp HS, Frevert E, 2006. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 3, 403–16. 10.1016/j.cmet.2006.05.005 [DOI] [PubMed] [Google Scholar]
- Crane ED, Wong W, Zhang H, O’Neil G, Crane JD, 2021. AMPK Inhibits mTOR-Driven Keratinocyte Proliferation after Skin Damage and Stress. J. Invest. Dermatol. 10.1016/j.jid.2020.12.036 [DOI] [PubMed] [Google Scholar]
- Currais A, Huang L, Goldberg J, Petrascheck M, Ates G, Pinto-Duarte A, Shokhirev MN, Schubert D, Maher P, 2019. Elevating acetyl-CoA levels reduces aspects of brain aging. Elife 8, 1–21. 10.7554/eLife.47866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Thennavan A, Dolgalev I, Chen T, Li J, Marzio A, Poirier JT, Peng DH, Bulatovic M, Mukhopadhyay S, Silver H, Papadopoulos E, Pyon V, Thakurdin C, Han H, Li F, Li S, Ding H, Hu H, Pan Y, Weerasekara V, Jiang B, Wang ES, Ahearn I, Philips M, Papagiannakopoulos T, Tsirigos A, Rothenberg E, Gainor J, Freeman GJ, Rudin CM, Gray NS, Hammerman PS, Pagano M, Heymach JV, Perou CM, Bardeesy N, Wong K-K, 2021. ULK1 inhibition overcomes compromised antigen presentation and restores antitumor immunity in LKB1-mutant lung cancer. Nat. Cancer 2, 503–514. 10.1038/s43018-021-00208-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducommun S, Deak M, Sumpton D, Ford RJ, Núñez Galindo A, Kussmann M, Viollet B, Steinberg GR, Foretz M, Dayon L, Morrice NA, Sakamoto K, 2015. Motif affinity and mass spectrometry proteomic approach for the discovery of cellular AMPK targets: identification of mitochondrial fission factor as a new AMPK substrate. Cell. Signal. 27, 978–88. 10.1016/j.cellsig.2015.02.008 [DOI] [PubMed] [Google Scholar]
- Ducommun S, Deak M, Zeigerer A, Göransson O, Seitz S, Collodet C, Madsen AB, Jensen TE, Viollet B, Foretz M, Gut P, Sumpton D, Sakamoto K Chemical genetic screen identifies Gapex-5/GAPVD1 and STBD1 as novel AMPK substrates. Cell Signal. 2019. May;57:45–57. doi: 10.1016/j.cellsig.2019.02.001 [DOI] [PubMed] [Google Scholar]
- Egan DF, Chun MGH, Vamos M, Zou H, Rong J, Miller CJ, Lou HJ, Raveendra-Panickar D, Yang C-C, Sheffler DJ, Teriete P, Asara JM, Turk BE, Cosford NDP, Shaw RJ, 2015. Small Molecule Inhibition of the Autophagy Kinase ULK1 and Identification of ULK1 Substrates. Mol. Cell 59, 285–97. 10.1016/j.molcel.2015.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ, 2011. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331, 456–61. 10.1126/science.1196371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner LJ, Brun SN, Herzig S, Young NP, Curtis SD, Shackelford DB, Shokhirev MN, Leblanc M, Vera LI, Hutchins A, Ross DS, Shaw RJ, Svensson RU, 2019. Genetic Analysis Reveals AMPK Is Required to Support Tumor Growth in Murine Kras-Dependent Lung Cancer Models. Cell Metab. 29, 285–302.e7. 10.1016/j.cmet.2018.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullerton MD, Galic S, Marcinko K, Sikkema S, Pulinilkunnil T, Chen ZP, O’Neill HM, Ford RJ, Palanivel R, O’Brien M, Hardie DG, Macaulay SL, Schertzer JD, Dyck JRB, van Denderen BJ, Kemp BE, Steinberg GR, 2013. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 19, 1649–1654. 10.1038/nm.3372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia D, Shaw RJ, 2017. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 66, 789–800. 10.1016/j.molcel.2017.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes LC, Benedetto G. Di, Scorrano L, 2011. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat. Cell Biol. 13, 589–598. 10.1038/ncb2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González A, Hall MN, Lin S-C, Hardie DG, 2020. AMPK and TOR: The Yin and Yang of Cellular Nutrient Sensing and Growth Control. Cell Metab. 31, 472–492. 10.1016/j.cmet.2020.01.015 [DOI] [PubMed] [Google Scholar]
- Hardie DG, Schaffer BE, Brunet A, 2016. AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol. 26, 190–201. 10.1016/j.tcb.2015.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JW, Ordureau A, Heo J-M, 2018. Building and decoding ubiquitin chains for mitophagy. Nat. Rev. Mol. Cell Biol. 19, 93–108. 10.1038/nrm.2017.129 [DOI] [PubMed] [Google Scholar]
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG, 2005. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2, 9–19. 10.1016/j.cmet.2005.05.009 [DOI] [PubMed] [Google Scholar]
- Hedbacker K, Carlson M, 2008. SNF1/AMPK pathways in yeast. Front. Biosci. 13, 2408–20. 10.2741/2854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig S, Shaw RJ, 2018. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 19, 121–135. 10.1038/nrm.2017.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman NJ, Parker BL, Chaudhuri R, Fisher-Wellman KH, Kleinert M, Humphrey SJ, Yang P, Holliday M, Trefely S, Fazakerley DJ, Stöckli J, Burchfield JG, Jensen TE, Jothi R, Kiens B, Wojtaszewski JFP, Richter EA, James DE, 2015. Global Phosphoproteomic Analysis of Human Skeletal Muscle Reveals a Network of Exercise-Regulated Kinases and AMPK Substrates. Cell Metab. 22, 922–35. 10.1016/j.cmet.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman NJ, Whitfield J, Janzen NR, Belhaj MR, Galic S, Murray-Segal L, Smiles WJ, Ling NXY, Dite TA, Scott JW, Oakhill JS, Brink R, Kemp BE, Hawley JA, 2020. Genetic loss of AMPK-glycogen binding destabilises AMPK and disrupts metabolism. Mol. Metab. 41, 101048. 10.1016/j.molmet.2020.101048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes Hallett JE, Luo X, Capaldi AP, 2015. Snf1/AMPK promotes the formation of Kog1/Raptor-bodies to increase the activation threshold of TORC1 in budding yeast. Elife 4, 1–19. 10.7554/eLife.09181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung C, Lombardo PS, Malik N, Brun SN, Hellberg K, Van Nostrand JL, Garcia D, Baumgart J, Diffenderfer K, Asara JM, Shaw RJ, 2021. AMPK/ULK1-mediated phosphorylation of Parkin ACT domain mediates an early step in mitophagy. Sci. Adv. 7, 1–15. 10.1126/sciadv.abg4544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA, 2005. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J. Biol. Chem. 280, 29060–29066. 10.1074/jbc.M503824200 [DOI] [PubMed] [Google Scholar]
- Jäger S, Handschin C, St-Pierre J, Spiegelman BM, 2007. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. U. S. A. 104, 12017–22. 10.1073/pnas.0705070104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon S-M, Chandel NS, Hay N, 2012. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 485, 661–665. 10.1038/nature11066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Abudu YP, Claude-Taupin A, Gu Y, Kumar S, Choi SW, Peters R, Mudd MH, Allers L, Salemi M, Phinney B, Johansen T, Deretic V, 2018. Galectins Control mTOR in Response to Endomembrane Damage. Mol. Cell 70, 120–135.e8. 10.1016/j.molcel.2018.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Bissa B, Brecht L, Allers L, Choi SW, Gu Y, Zbinden M, Burge MR, Timmins G, Hallows K, Behrends C, Deretic V, 2020. AMPK, a Regulator of Metabolism and Autophagy, Is Activated by Lysosomal Damage via a Novel Galectin-Directed Ubiquitin Signal Transduction System. Mol. Cell 77, 951–969.e9. 10.1016/j.molcel.2019.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabiyik C, Vicinanza M, Son SM, Rubinsztein DC, 2021. Glucose starvation induces autophagy via ULK1-mediated activation of PIKfyve in an AMPK-dependent manner. Dev. Cell. 10.1016/j.devcel.2021.05.010 [DOI] [PubMed] [Google Scholar]
- Kazgan N, Williams T, Forsberg LJ, Brenman JE, 2010. Identification of a nuclear export signal in the catalytic subunit of AMP-activated protein kinase. Mol. Biol. Cell 21, 3433–3442. 10.1091/mbc.E10-04-0347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kim YC, Fang C, Russell RC, Kim JH, Fan W, Liu R, Zhong Q, Guan K-L, 2013. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell 152, 290–303. 10.1016/j.cell.2012.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kundu M, Viollet B, Guan K-L, 2011. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13, 132–41. 10.1038/ncb2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkin V, 2020. History of the Selective Autophagy Research: How Did It Begin and Where Does It Stand Today? J. Mol. Biol. 432, 3–27. 10.1016/j.jmb.2019.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleele T, Rey T, Winter J, Zaganelli S, Mahecic D, Perreten Lambert H, Ruberto FP, Nemir M, Wai T, Pedrazzini T, Manley S, 2021. Distinct fission signatures predict mitochondrial degradation or biogenesis. Nature 593, 435–439. 10.1038/s41586-021-03510-6 [DOI] [PubMed] [Google Scholar]
- Kodiha M, Rassi JG, Brown CM, Stochaj U, 2007. Localization of AMP kinase is regulated by stress, cell density, and signaling through the MEK-->ERK1/2 pathway. Am. J. Physiol. Cell Physiol. 293, C1427–36. 10.1152/ajpcell.00176.2007 [DOI] [PubMed] [Google Scholar]
- Kundu A, Shelar S, Ghosh AP, Ballestas M, Kirkman R, Nam H, Brinkley GJ, Karki S, Mobley JA, Bae S, Varambally S, Sudarshan S, 2020. 14–3-3 proteins protect AMPK-phosphorylated ten-eleven translocation-2 (TET2) from PP2A-mediated dephosphorylation. J. Biol. Chem. 295, 1754–1766. 10.1074/jbc.RA119.011089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laderoute KR, Amin K, Calaoagan JM, Knapp M, Le T, Orduna J, Foretz M, Viollet B, 2006. 5’-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol. Cell. Biol. 26, 5336–5347. 10.1128/MCB.00166-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laker RC, Drake JC, Wilson RJ, Lira VA, Lewellen BM, Ryall KA, Fisher CC, Zhang M, Saucerman JJ, Goodyear LJ, Kundu M, Yan Z, 2017. Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat. Commun. 8, 548. 10.1038/s41467-017-00520-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, Thompson CB, Evans RM, 2009. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 326, 437–440. 10.1126/science.1172156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming DW, Bar-Peled L, 2019. Lysosome: The metabolic signaling hub. Traffic 20, 27–38. 10.1111/tra.12617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, Sideris DP, Fogel AI, Youle RJ, 2015. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524, 309–314. 10.1038/nature14893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Zandkarimi F, Zhang Y, Meena JK, Kim J, Zhuang L, Tyagi S, Ma L, Westbrook TF, Steinberg GR, Nakada D, Stockwell BR, Gan B, 2020. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 22, 225–234. 10.1038/s41556-020-0461-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Zhang C-S, Feng J-W, Wei X, Zhang C, Xie C, Wu Y, Hawley SA, Atrih A, Lamont DJ, Wang Z, Piao H-L, Hardie DG, Lin S-C, 2021. Aldolase is a sensor for both low and high glucose, linking to AMPK and mTORC1. Cell Res. 31, 478–481. 10.1038/s41422-020-00456-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Zhang C-S, Zong Y, Feng J-W, Ma T, Hu M, Lin Z, Li X, Xie C, Wu Y, Jiang D, Li Y, Zhang C, Tian X, Wang W, Yang Y, Chen J, Cui J, Wu Y-Q, Chen X, Liu Q-F, Wu J, Lin S-Y, Ye Z, Liu Y, Piao H-L, Yu L, Zhou Z, Xie X-S, Hardie DG, Lin S-C, 2019. Transient Receptor Potential V Channels Are Essential for Glucose Sensing by Aldolase and AMPK. Cell Metab. 30, 508–524.e12. 10.1016/j.cmet.2019.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Lavagnino Z, Lemacon D, Kong L, Ustione A, Ng X, Zhang Y, Wang Y, Zheng B, Piwnica-Worms H, Vindigni A, Piston DW, You Z, 2019. Ca(2+)-Stimulated AMPK-Dependent Phosphorylation of Exo1 Protects Stressed Replication Forks from Aberrant Resection. Mol. Cell 74, 1123–1137.e6. 10.1016/j.molcel.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang L, Zhou XE, Ke J, de Waal PW, Gu X, Tan MHE, Wang D, Wu D, Xu HE, Melcher K, 2015. Structural basis of AMPK regulation by adenine nucleotides and glycogen. Cell Res. 25, 50–66. 10.1038/cr.2014.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limpert AS, Lambert LJ, Bakas NA, Bata N, Brun SN, Shaw RJ, Cosford NDP, 2018. Autophagy in Cancer: Regulation by Small Molecules. Trends Pharmacol. Sci. 39, 1021–1032. 10.1016/j.tips.2018.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GY, Sabatini DM, 2020. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 21, 183–203. 10.1038/s41580-019-0199-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lai Y-C, Hill EV, Tyteca D, Carpentier S, Ingvaldsen A, Vertommen D, Lantier L, Foretz M, Dequiedt F, Courtoy PJ, Erneux C, Viollet B, Shepherd PR, Tavaré JM, Jensen J, Rider MH, 2013. Phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) is an AMPK target participating in contraction-stimulated glucose uptake in skeletal muscle. Biochem. J. 455, 195–206. 10.1042/BJ20130644 [DOI] [PubMed] [Google Scholar]
- Lizcano JM, Göransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Mäkelä TP, Hardie DG, Alessi DR, 2004. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 23, 833–43. 10.1038/sj.emboj.7600110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Doménech G, Covill-Cooke C, Ivankovic D, Halff EF, Sheehan DF, Norkett R, Birsa N, Kittler JT, 2018. Miro proteins coordinate microtubule- and actin-dependent mitochondrial transport and distribution. EMBO J. 37, 321–336. 10.15252/embj.201696380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Doménech G, Serrat R, Mirra S, D’Aniello S, Somorjai I, Abad A, Vitureira N, García-Arumí E, Alonso MT, Rodriguez-Prados M, Burgaya F, Andreu AL, García-Sancho J, Trullas R, Garcia-Fernàndez J, Soriano E, 2012. The Eutherian Armcx genes regulate mitochondrial trafficking in neurons and interact with Miro and Trak2. Nat. Commun. 3, 814. 10.1038/ncomms1829 [DOI] [PubMed] [Google Scholar]
- Mack HID, Zheng B, Asara JM, Thomas SM, 2012. AMPK-dependent phosphorylation of ULK1 regulates ATG9 localization. Autophagy 8, 1197–214. 10.4161/auto.20586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin TL, Gongol B, Zhang F, Martin M, Johnson DA, Xiao H, Wang Y, Subramaniam S, Chien S, Shyy JYJ, 2017. AMPK promotes mitochondrial biogenesis and function by phosphorylating the epigenetic factors DNMT1, RBBP7, and HAT1. Sci. Signal. 10, 1–12. 10.1126/scisignal.aaf7478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den Berghe G, Carling D, Hue L, 2000. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr. Biol. 10, 1247–1255. 10.1016/S0960-9822(00)00742-9 [DOI] [PubMed] [Google Scholar]
- Martina JA, Chen Y, Gucek M, Puertollano R, 2012. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy 8, 903–14. 10.4161/auto.19653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Reyes I, Chandel NS, 2020. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 11, 102. 10.1038/s41467-019-13668-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee SL, Howlett KF, Starkie RL, Cameron-Smith D, Kemp BE, Hargreaves M, 2003. Exercise increases nuclear AMPK alpha2 in human skeletal muscle. Diabetes 52, 926–928. 10.2337/diabetes.52.4.926 [DOI] [PubMed] [Google Scholar]
- Mishra P, Chan DC, 2016. Metabolic regulation of mitochondrial dynamics. J. Cell Biol. 212, 379–87. 10.1083/jcb.201511036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchelhill KI, Michell BJ, House CM, Stapleton D, Dyck J, Gamble J, Ullrich C, Witters LA, Kemp BE, 1997. Posttranslational modifications of the 5’-AMP-activated protein kinase beta1 subunit. J. Biol. Chem. 272, 24475–9. 10.1074/jbc.272.39.24475 [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Rho E, Sample V, Akano H, Magari M, Ueno T, Gorshkov K, Chen M, Tokumitsu H, Zhang J, Inoue T, 2015. Compartmentalized AMPK signaling illuminated by genetically encoded molecular sensors and actuators. Cell Rep. 11, 657–70. 10.1016/j.celrep.2015.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi S, López-Doménech G, Halff EF, Covill-Cooke C, Ivankovic D, Melandri D, Arancibia-Cárcamo IL, Burden JJ, Lowe AR, Kittler JT, 2019. Miro clusters regulate ER-mitochondria contact sites and link cristae organization to the mitochondrial transport machinery. Nat. Commun. 10, 17–19. 10.1038/s41467-019-12382-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muise ES, Guan H-P, Liu J, Nawrocki AR, Yang X, Wang C, Rodríguez CG, Zhou D, Gorski JN, Kurtz MM, Feng D, Leavitt KJ, Wei L, Wilkening RR, Apgar JM, Xu S, Lu K, Feng W, Li Y, He H, Previs SF, Shen X, van Heek M, Souza SC, Rosenbach MJ, Biftu T, Erion MD, Kelley DE, Kemp DM, Myers RW, Sebhat IK, 2019. Pharmacological AMPK activation induces transcriptional responses congruent to exercise in skeletal and cardiac muscle, adipose tissues and liver. PLoS One 14, e0211568. 10.1371/journal.pone.0211568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday MR, Campbell DG, Carling D, Hardie DG, 1988. Identification by amino acid sequencing of three major regulatory phosphorylation sites on rat acetyl-CoA carboxylase. Eur. J. Biochem. 175, 331–8. 10.1111/j.1432-1033.1988.tb14201.x [DOI] [PubMed] [Google Scholar]
- Myers RW, Guan HP, Ehrhart J, Petrov A, Prahalada S, Tozzo E, Yang X, Kurtz MM, Trujillo M, Trotter DG, Feng D, Xu S, Eiermann G, Holahan MA, Rubins D, Conarello S, Niu X, Souza SC, Miller C, Liu J, Lu K, Feng W, Li Y, Painter RE, Milligan JA, He H, Liu F, Ogawa A, Wisniewski D, Rohm RJ, Wang L, Bunzel M, Qian Y, Zhu W, Wang H, Bennet B, Scheuch LLF, Fernandez GE, Li C, Klimas M, Zhou G, Van Heek M, Biftu T, Weber A, Kelley DE, Thornberry N, Erion MD, Kemp DM, Sebhat IK, 2017. Systemic pan-AMPK activator MK-8722 improves glucose homeostasis but induces cardiac hypertrophy. Science (80-. ). 357, 507–511. 10.1126/science.aah5582 [DOI] [PubMed] [Google Scholar]
- Nakagawa K, Uehata Y, Natsuizaka M, Kohara T, Darmanin S, Asaka M, Takeda H, Kobayashi M, 2012. The nuclear protein Artemis promotes AMPK activation by stabilizing the LKB1-AMPK complex. Biochem. Biophys. Res. Commun. 427, 790–795. 10.1016/j.bbrc.2012.09.140 [DOI] [PubMed] [Google Scholar]
- Nelson ME, Parker BL, Burchfield JG, Hoffman NJ, Needham EJ, Cooke KC, Naim T, Sylow L, Ling NX, Francis D, Norris DM, Chaudhuri R, Oakhill JS, Richter EA, Lynch GS, Stöckli J, James DE, 2019. Phosphoproteomics reveals conserved exercise-stimulated signaling and AMPK regulation of store-operated calcium entry. EMBO J. 38, e102578. 10.15252/embj.2019102578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngoei KRW, Langendorf CG, Ling NXY, Hoque A, Varghese S, Camerino MA, Walker SR, Bozikis YE, Dite TA, Ovens AJ, Smiles WJ, Jacobs R, Huang H, Parker MW, Scott JW, Rider MH, Foitzik RC, Kemp BE, Baell JB, Oakhill JS, 2018. Structural Determinants for Small-Molecule Activation of Skeletal Muscle AMPK α2β2γ1 by the Glucose Importagog SC4. Cell Chem. Biol. 25, 728–737.e9. 10.1016/j.chembiol.2018.03.008 [DOI] [PubMed] [Google Scholar]
- Orozco JM, Krawczyk PA, Scaria SM, Cangelosi AL, Chan SH, Kunchok T, Lewis CA, Sabatini DM, 2020. Dihydroxyacetone phosphate signals glucose availability to mTORC1. Nat. Metab. 2, 893–901. 10.1038/s42255-020-0250-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette M, El-Houjeiri L, C Zirden L, Puustinen P, Blanchette P, Jeong H, Dejgaard K, Siegel PM, Pause A, 2021. AMPK-dependent phosphorylation is required for transcriptional activation of TFEB and TFE3. Autophagy 1–19. 10.1080/15548627.2021.1898748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles S, Vigié P, Youle RJ, 2018. Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Curr. Biol. 28, R170–R185. 10.1016/j.cub.2018.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkosky SL, Scott JW, Desjardins EM, Smith BK, Day EA, Ford RJ, Langendorf CG, Ling NXY, Nero TL, Loh K, Galic S, Hoque A, Smiles WJ, Ngoei KRW, Parker MW, Yan Y, Melcher K, Kemp BE, Oakhill JS, Steinberg GR, 2020. Long-chain fatty acyl-CoA esters regulate metabolism via allosteric control of AMPK β1 isoforms. Nat. Metab. 2, 873–881. 10.1038/s42255-020-0245-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick RM, Shulman GI, 2006. The role of AMP-activated protein kinase in mitochondrial biogenesis. J. Physiol. 574, 33–9. 10.1113/jphysiol.2006.109512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roczniak-Ferguson A, Petit CS, Froehlich F, Qian S, Ky J, Angarola B, Walther TC, Ferguson SM, 2012. The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Sci. Signal. 5, ra42. 10.1126/scisignal.2002790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman NB, Saha AK, Kraegen EW, 2003. Minireview: malonyl CoA, AMP-activated protein kinase, and adiposity. Endocrinology 144, 5166–71. 10.1210/en.2003-0849 [DOI] [PubMed] [Google Scholar]
- Russell RC, Tian Y, Yuan H, Park HW, Chang YY, Kim J, Kim H, Neufeld TP, Dillin A, Guan KL, 2013. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol. 15, 741–750. 10.1038/ncb2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Chapple RH, Lin A, Kitano A, Nakada D, 2015. AMPK Protects Leukemia-Initiating Cells in Myeloid Leukemias from Metabolic Stress in the Bone Marrow. Cell Stem Cell 17, 585–596. 10.1016/j.stem.2015.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt I, Celler JW, Hawley SA, Prescott A, Woods A, Carling D, Hardie DG, 1998. AMP-activated protein kinase: greater AMP dependence, and preferential nuclear localization, of complexes containing the alpha2 isoform. Biochem. J. 334 ( Pt 1, 177–87. 10.1042/bj3340177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, Di Malta C, Donaudy F, Embrione V, Polishchuk RS, Banfi S, Parenti G, Cattaneo E, Ballabio A, 2009. A gene network regulating lysosomal biogenesis and function. Science 325, 473–7. 10.1126/science.1174447 [DOI] [PubMed] [Google Scholar]
- Saxton RA, Sabatini DM, 2017. mTOR Signaling in Growth, Metabolism, and Disease. Cell 168, 960–976. 10.1016/j.cell.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JW, van Denderen BJW, Jorgensen SB, Honeyman JE, Steinberg GR, Oakhill JS, Iseli TJ, Koay A, Gooley PR, Stapleton D, Kemp BE, 2008. Thienopyridone drugs are selective activators of AMP-activated protein kinase beta1-containing complexes. Chem. Biol. 15, 1220–30. 10.1016/j.chembiol.2008.10.005 [DOI] [PubMed] [Google Scholar]
- Seabright AP, Fine NHF, Barlow JP, Lord SO, Musa I, Gray A, Bryant JA, Banzhaf M, Lavery GG, Hardie DG, Hodson DJ, Philp A, Lai Y-C, 2020. AMPK activation induces mitophagy and promotes mitochondrial fission while activating TBK1 in a PINK1-Parkin independent manner. FASEB J. 34, 6284–6301. 10.1096/fj.201903051R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Zoncu R, Medina DL, Vetrini F, Erdin Serkan, Erdin Serpiluckac, Huynh T, Ferron M, Karsenty G, Vellard MC, Facchinetti V, Sabatini DM, Ballabio A, 2012. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 31, 1095–108. 10.1038/emboj.2012.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, DePinho RA, Cantley LC, 2004a. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell 6, 91–99. 10.1016/j.ccr.2004.06.007 [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC, 2004b. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. U. S. A. 101, 3329–35. 10.1073/pnas.0308061100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicari D, Delaunay-Moisan A, Combettes L, Chevet E, Igbaria A, 2020. A guide to assessing endoplasmic reticulum homeostasis and stress in mammalian systems. FEBS J. 287, 27–42. 10.1111/febs.15107 [DOI] [PubMed] [Google Scholar]
- Stein BD, Calzolari D, Hellberg K, Hu YS, He L, Hung C-M, Toyama EQ, Ross DS, Lillemeier BF, Cantley LC, Yates JR, Shaw RJ, 2019. Quantitative In Vivo Proteomics of Metformin Response in Liver Reveals AMPK-Dependent and -Independent Signaling Networks. Cell Rep. 29, 3331–3348.e7. 10.1016/j.celrep.2019.10.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg GR, Carling D, 2019. AMP-activated protein kinase: the current landscape for drug development. Nat. Rev. Drug Discov. 18, 527–551. 10.1038/s41573-019-0019-2 [DOI] [PubMed] [Google Scholar]
- Steinberg GR, Watt MJ, McGee SL, Chan S, Hargreaves M, Febbraio MA, Stapleton D, Kemp BE, 2006. Reduced glycogen availability is associated with increased AMPKalpha2 activity, nuclear AMPKalpha2 protein abundance, and GLUT4 mRNA expression in contracting human skeletal muscle. Appl. Physiol. Nutr. Metab. 31, 302–12. 10.1139/h06-003 [DOI] [PubMed] [Google Scholar]
- Steneberg P, Lindahl E, Dahl U, Lidh E, Straseviciene J, Backlund F, Kjellkvist E, Berggren E, Lundberg I, Bergqvist I, Ericsson M, Eriksson B, Linde K, Westman J, Edlund T, Edlund H, 2018. PAN-AMPK activator O304 improves glucose homeostasis and microvascular perfusion in mice and type 2 diabetes patients. JCI insight 3. 10.1172/jci.insight.99114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenne X, Foretz M, Taleux N, Van Der Zon GC, Sokal E, Hue L, Viollet B, Guigas B, 2011. Metformin activates AMP-activated protein kinase in primary human hepatocytes by decreasing cellular energy status. Diabetologia 54, 3101–3110. 10.1007/s00125-011-2311-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Okamoto S, Lee S, Saito K, Shiuchi T, Minokoshi Y, 2007. Leptin stimulates fatty acid oxidation and peroxisome proliferator-activated receptor alpha gene expression in mouse C2C12 myoblasts by changing the subcellular localization of the alpha2 form of AMP-activated protein kinase. Mol. Cell. Biol. 27, 4317–27. 10.1128/MCB.02222-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swatek KN, Usher JL, Kueck AF, Gladkova C, Mevissen TET, Pruneda JN, Skern T, Komander D, 2019. Insights into ubiquitin chain architecture using Ub-clipping. Nature 572, 533–537. 10.1038/s41586-019-1482-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Konishi A, Obinata H, Tsuneoka M, 2019. Metformin activates KDM2A to reduce rRNA transcription and cell proliferation by dual regulation of AMPK activity and intracellular succinate level. Sci. Rep. 9, 18694. 10.1038/s41598-019-55075-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Yano H, Ogasawara S, Yoshioka S-I, Imamura H, Okamoto K, Tsuneoka M, 2015. Mild Glucose Starvation Induces KDM2A-Mediated H3K36me2 Demethylation through AMPK To Reduce rRNA Transcription and Cell Proliferation. Mol. Cell. Biol. 35, 4170–4184. 10.1128/MCB.00579-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama EQ, Herzig S, Courchet J, Lewis TL Jr., Losón OC, Hellberg K, Young NP, Chen H, Polleux F, Chan DC, Shaw RJ, 2016. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science (80-. ). 351, 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou P, Zheng B, Hsu C-H, Sasaki AT, Cantley LC, 2011. A fluorescent reporter of AMPK activity and cellular energy stress. Cell Metab. 13, 476–486. 10.1016/j.cmet.2011.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nostrand JL, Hellberg K, Luo E-C, Van Nostrand EL, Dayn A, Yu J, Shokhirev MN, Dayn Y, Yeo GW, Shaw RJ, 2020. AMPK regulation of Raptor and TSC2 mediate metformin effects on transcriptional control of anabolism and inflammation. Genes Dev. 34, 1330–1344. 10.1101/gad.339895.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vara-Ciruelos D, Dandapani M, Gray A, Egbani EO, Evans AM, Hardie DG, 2018. Genotoxic Damage Activates the AMPK-α1 Isoform in the Nucleus via Ca(2+)/CaMKK2 Signaling to Enhance Tumor Cell Survival. Mol. Cancer Res. 16, 345–357. 10.1158/1541-7786.MCR-17-0323 [DOI] [PubMed] [Google Scholar]
- Vargas JNS, Wang C, Bunker E, Hao L, Maric D, Schiavo G, Randow F, Youle RJ, 2019. Spatiotemporal Control of ULK1 Activation by NDP52 and TBK1 during Selective Autophagy. Mol. Cell 74, 347–362.e6. 10.1016/j.molcel.2019.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bertalanffy L, 1950. The theory of open systems in physics and biology. Science 111, 23–9. 10.1126/science.111.2872.23 [DOI] [PubMed] [Google Scholar]
- Wan L, Xu K, Wei Y, Zhang J, Han T, Fry C, Zhang Z, Wang YV, Huang L, Yuan M, Xia W, Chang W-C, Huang W-C, Liu C-L, Chang Y-C, Liu Jinsong, Wu Y, Jin VX, Dai X, Guo J, Liu Jia, Jiang S, Li J, Asara JM, Brown M, Hung M-C, Wei W, 2018. Phosphorylation of EZH2 by AMPK Suppresses PRC2 Methyltransferase Activity and Oncogenic Function. Mol. Cell 69, 279–291.e5. 10.1016/j.molcel.2017.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang Y, An H, Liu T, Qin C, Sesaki H, Guo S, Radovick S, Hussain M, Maheshwari A, Wondisford FE, O’Rourke B, He L, 2019. Metformin Improves Mitochondrial Respiratory Activity through Activation of AMPK. Cell Rep. 29, 1511–1523.e5. 10.1016/j.celrep.2019.09.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerasekara VK, Panek DJ, Broadbent DG, Mortenson JB, Mathis AD, Logan GN, Prince JT, Thomson DM, Thompson JW, Andersen JL, 2014. Metabolic-stress-induced rearrangement of the 14–3-3ζ interactome promotes autophagy via a ULK1- and AMPK-regulated 14–3-3ζ interaction with phosphorylated Atg9. Mol. Cell. Biol. 34, 4379–88. 10.1128/MCB.00740-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willows R, Navaratnam N, Lima A, Read J, Carling D, 2017. Effect of different γ-subunit isoforms on the regulation of AMPK. Biochem. J. 474, 1741–1754. 10.1042/BCJ20170046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO, 2000. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J. Appl. Physiol. 88, 2219–26. 10.1152/jappl.2000.88.6.2219 [DOI] [PubMed] [Google Scholar]
- Woods A, Dickerson K, Heath R, Hong S-P, Momcilovic M, Johnstone SR, Carlson M, Carling D, 2005. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2, 21–33. 10.1016/j.cmet.2005.06.005 [DOI] [PubMed] [Google Scholar]
- Wu CL, Qiang L, Han W, Ming M, Viollet B, He YY, 2013. Role of AMPK in UVB-induced DNA damage repair and growth control. Oncogene 32, 2682–2689. 10.1038/onc.2012.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Hu D, Chen H, Shi G, Fetahu IS, Wu F, Rabidou K, Fang R, Tan L, Xu S, Liu H, Argueta C, Zhang L, Mao F, Yan G, Chen J, Dong Z, Lv R, Xu Y, Wang M, Ye Y, Zhang S, Duquette D, Geng S, Yin C, Lian CG, Murphy GF, Adler GK, Garg R, Lynch L, Yang P, Li Y, Lan F, Fan J, Shi Y, Shi YG, 2018. Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature 559, 637–641. 10.1038/s41586-018-0350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Puppala D, Feng X, Monetti M, Lapworth AL, Geoghegan KF, 2013. Chemoproteomic analysis of intertissue and interspecies isoform diversity of AMP-activated protein kinase (AMPK). J. Biol. Chem. 288, 35904–35912. 10.1074/jbc.M113.508747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Shi L, Li L, Fu L, Liu Y, Xiong Y, Sheen J, 2019. Integration of nutrient, energy, light, and hormone signalling via TOR in plants. J. Exp. Bot. 70, 2227–2238. 10.1093/jxb/erz028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Sanders MJ, Carmena D, Bright NJ, Haire LF, Underwood E, Patel BR, Heath RB, Walker PA, Hallen S, Giordanetto F, Martin SR, Carling D, Gamblin SJ, 2013. Structural basis of AMPK regulation by small molecule activators. Nat. Commun. 4, 1–10. 10.1038/ncomms4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, Jing C, Walker PA, Eccleston JF, Haire LF, Saiu P, Howell SA, Aasland R, Martin SR, Carling D, Gamblin SJ, 2011. Structure of mammalian AMPK and its regulation by ADP. Nature 472, 230–3. 10.1038/nature09932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin F-J, Wang J, Zhao R-Q, Wang Z-X, Wu J-W, 2013. Coordinated regulation of AMPK activity by multiple elements in the α-subunit. Cell Res. 23, 1237–40. 10.1038/cr.2013.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D-Q, Wang Z, Wang C-Y, Zhang D-Y, Wan H-D, Zhao Z-L, Gu J, Zhang Y-X, Li Z-G, Man K-Y, Pan Y, Wang Z-F, Ke Z-J, Liu Z-X, Liao L-J, Chen Y, 2016. PAQR3 controls autophagy by integrating AMPK signaling to enhance ATG14L-associated PI3K activity. EMBO J. 35, 496–514. 10.15252/embj.201592864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Zhou XE, Novick SJ, Shaw SJ, Li Y, Brunzelle JS, Hitoshi Y, Griffin PR, Xu HE, Melcher K, 2019. Structures of AMP-activated protein kinase bound to novel pharmacological activators in phosphorylated, non-phosphorylated, and nucleotide-free states. J. Biol. Chem. 294, 953–967. 10.1074/jbc.RA118.004883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Zhou XE, Xu HE, Melcher K, 2018. Structure and Physiological Regulation of AMPK. Int. J. Mol. Sci. 19. 10.3390/ijms19113534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanov TE, Hipolito VEB, Liebscher G, Vogel GF, Stasyk T, Herrmann C, Geley S, Teis D, Botelho RJ, Hess MW, Huber LA, 2019. Biogenesis of lysosome-related organelles complex-1 (BORC) regulates late endosomal/lysosomal size through PIKfyve-dependent phosphatidylinositol-3,5-bisphosphate. Traffic 20, 674–696. 10.1111/tra.12679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young NP, Kamireddy A, Van Nostrand JL, Eichner LJ, Shokhirev MN, Dayn Y, Shaw RJ, 2016. AMPK governs lineage specification through Tfeb-dependent regulation of lysosomes. Genes Dev. 30, 535–552. 10.1101/gad.274142.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C-S, Jiang B, Li M, Zhu M, Peng Y, Zhang Y-L, Wu Y-Q, Li TY, Liang Y, Lu Z, Lian G, Liu Q, Guo H, Yin Z, Ye Z, Han J, Wu J-W, Yin H, Lin S-Y, Lin S-C, 2014. The lysosomal v-ATPase-Ragulator complex is a common activator for AMPK and mTORC1, acting as a switch between catabolism and anabolism. Cell Metab. 20, 526–40. 10.1016/j.cmet.2014.06.014 [DOI] [PubMed] [Google Scholar]
- Zhang CS, Hawley SA, Zong Y, Li M, Wang Z, Gray A, Ma T, Cui J, Feng JW, Zhu M, Wu YQ, Li TY, Ye Z, Lin SY, Yin H, Piao HL, Hardie DG, Lin SC, 2017. Fructose-1,6-bisphosphate and aldolase mediate glucose sensing by AMPK. Nature 548, 112–116. 10.1038/nature23275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Guan X, Choi UL, Dong Q, Lam MMT, Zeng J, Xiong J, Wang X, Poon TCW, Zhang H, Zhang X, Wang H, Xie R, Zhu B, Li G, 2019. Phosphorylation of TET2 by AMPK is indispensable in myogenic differentiation. Epigenetics Chromatin 12, 32. 10.1186/s13072-019-0281-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong Y, Zhang CS, Li M, Wang W, Wang Z, Hawley SA, Ma T, Feng JW, Tian X, Qi Q, Wu YQ, Zhang C, Ye Z, Lin SY, Piao HL, Hardie DG, Lin SC, 2019. Hierarchical activation of compartmentalized pools of AMPK depends on severity of nutrient or energy stress. Cell Res. 29, 460–473. 10.1038/s41422-019-0163-6 [DOI] [PMC free article] [PubMed] [Google Scholar]