Abstract
Assessment of the Ki67 index is critical for grading well differentiated neuroendocrine tumors (WD-NETs), which can show a broad range of labeling that defines the WHO grade (G1-G3). Poorly differentiated neuroendocrine carcinomas (PD-NECs) have a relatively high Ki67 index, greater than 20% in all cases and commonly exceeding 50%. After anecdotally observing PD-NECs with an unexpectedly low and heterogeneous Ki67 index following chemotherapy in 5 cases, we identified 15 additional cases of treated high-grade neuroendocrine neoplasms (HG-NENs). The study cohort comprised 20 cases; 11 PD-NECs, 8 mixed adenoneuroendocrine carcinomas (MANECs), and 1 WD-NET, G3 from various anatomic sites (gastrointestinal tract, pancreas, larynx, lung, and breast). The Ki67 index was evaluated on pre-treatment (when available) and post-treatment samples. Topographic heterogeneity in the Ki67 index was expressed using a semi-quantitative score of 0 (no heterogeneity to 5 (>80% difference between maximal Ki67 and minimal Ki67 indices). Relative to the pre-treatment group (n=9, mean Ki67 of 86.3%, range 80–100%), the neoplasms in the post-treatment group (n=20, mean Ki67 of 47.7%, range 1–90%) showed a significantly lower Ki67 index (18/20 cases). Of the 18 cases with a relatively low Ki67 index, 15 showed heterogeneous labeling (mean heterogeneity score of 2.3, range 1–5) and in 3 cases it was a homogeneously low. This phenomenon was observed in all subtypes of HG-NENs. In 6 cases, the alterations in Ki67 index following treatment were sufficient to place these HG-NENs in the WHO G1 or G2 grade, erroneously suggesting a diagnosis of WD-NET, and in 9 cases there was sufficient heterogeneity in the Ki67 index to suggest that a limited biopsy may sample an area of low Ki67, even though hotspot regions with a Ki67 index of >20% persisted. In 7 cases, the alterations in the Ki67 index were accompanied by morphologic features resembling a WD-NET. These observations suggest that there is a potential for misinterpretation of previously treated PD-NECs as WD-NETs, or for assigning a lower grade in G3 WD-NETs. While the prognostic significance of treatment-associated alterations in Ki67 index is unknown, awareness of this phenomenon is important to avoid this diagnostic pitfall when evaluating treated NENs.
Keywords: neuroendocrine, high grade, carcinoma, proliferation index, Ki67, post-treatment
Introduction:
As our understanding of neuroendocrine neoplasms (NENs) continues to evolve, their classification has undergone several revisions over recent years. The 2019 WHO classification of gastrointestinal and pancreatobiliary NENs separates well differentiated neuroendocrine tumors (WD-NETs) from poorly differentiated neuroendocrine carcinomas (PD-NECs), as these two types of neuroendocrine neoplasms differ substantially in their clinical behavior, treatment, and molecular underpinnings (1). WD-NETs are graded as G1, G2, or G3 based on the mitotic rate and Ki67 labeling index (2). Poorly differentiated neuroendocrine carcinomas (PD-NECs) have a relatively high Ki67 index, greater than 20% in essentially all cases and commonly in excess of 50% in the large cell variant (LCNEC) and close to 90% in the small cell variant (SCNEC). It is important to emphasize that the Ki67 index should be used for tumor grading and cannot by itself be used to separate NET from NEC in high grade neoplasms. It has been recognized that the Ki67 index for WD-NETs can reach well beyond the 20% threshold previously thought to define the poorly differentiated category. This is the basis for the relatively new category of WD-NET G3. In fact, WD-NETs can have Ki67 indices in excess of 50%, yet they remain well differentiated at the histological and molecular levels. The distinction between WD-NET G3 and PD-NEC can be particularly challenging in biopsy specimens, and immunohistochemical labeling for surrogate markers of molecular alterations in TP53 and RB1, and DAXX and ATRX in pancreatic primaries, may be helpful (3). Although WD-NETs can progress from lower grade (G1 or G2) to high grade (G3), it is generally believed that WD-NETs do not progress to PD-NECs, and once a diagnosis of a particular neuroendocrine neoplasm is established, recurrences or metastases represent the same tumor type as the primary (4).
Neuroendocrine neoplasms can occur in conjunction with a non-neuroendocrine component, and these mixed neoplasms are classified as mixed neuroendocrine-non-neuroendocrine neoplasms (MiNENs) by the WHO (1). The most common combination is mixed adenoneuroendocrine carcinoma (MANEC), which includes an adenocarcinoma component along with a PD-NEC component (5). Because the PD-NEC component is thought to drive the behavior of MANECs, it is common for patients to be treated with platinum-based chemotherapy, as are pure PD-NECs.
Patients who present with metastatic disease generally receive chemotherapy with or without radiation therapy as the primary mode of treatment. Some locoregional-stage PD-NECs also receive primary (neoadjuvant) chemotherapy, with subsequent consideration of resection. In cases where pathologic specimens are obtained following therapy, treatment-related changes may be recognizable in the neoplasm (6). In addition to classically described morphologic alterations such as inflammation, necrosis or fibrosis, we have anecdotally observed cases of PD-NECs in which the proliferative rate of the tumor had significantly decreased following treatment. In this study we examine selected cases of high grade NENs for which pathologic specimens were available following chemotherapy to assess alterations in the Ki67 index and morphology that could cause challenges in diagnosis.
Materials and methods:
The study was approved by the institutional review board. The pathology database was reviewed for any cases of high-grade neuroendocrine neoplasms (including WHO G3 WD-NETs, PD-NECs or MANECs) that had received chemotherapy treatment, with or without radiation therapy, before resection or biopsy. Five cases (including gastrointestinal, head and neck, breast, and bronchopulmonary) were identified prospectively, and a search for additional cases was performed using the above criteria. We identified 172 cases of HG-NENs of the gastrointestinal/pancreaticobiliary tract of which 20 cases had a history of treatment with chemotherapy with or without radiation and pre- and post-treatment samples were available. Five cases were excluded due to material inadequacy. The final cohort comprised of 20 cases (5 index cases and 15 retrospectively collected).
The Ki67 labeling index was assessed on the post-treatment and, when paraffin-embedded blocks were available, pre-treatment samples (n = 9) for comparison. Immunohistochemistry was performed using the MIB-1 antibody (Dako; Carpinteria, CA) at 1:100 dilution on the BenchMark XT automated equipment (Ventana Medical System Inc., Tucson, AZ). The tumor morphology and Ki67 index were assessed by three pathologists (MV, LT and DK). The Ki67 index was calculated as the percentage of neoplastic cells with nuclear labeling in the areas of maximum (hotspot) and minimum (coldspot) staining (each hotspot and coldspot was defined to correspond to a 20x microscopic field), using printed photographs to facilitate accurate counting. The resulting Ki67 indices for each region were recorded. When heterogeneous, and the case was assigned a heterogeneity score based on the difference of labeling index between the hotspot and the coldspot; score 0 = no difference, score 1 = <20% difference, score 2 = 20–40%, score 3 = 40–60 %, score 4 = 60–80%, score 5 = >80 %. A mitotic count was performed in the areas of highest and lowest Ki67 labeling. At least 10 high power fields (2 mm2) were assessed when adequate tissue was available. For MANECs, the proliferative rate was determined in the neuroendocrine carcinoma component only.
When present, morphological alterations in the post treatment samples, as compared to the pre-treatment ones, were recorded as well. Details of the specific chemotherapy regimens used in each case were recorded from the clinical notes. Statistical analysis was performed using (two tailed), unpaired t-test (using Graphpad prism 8.2.1).
Results:
The previously treated cases (n = 20) included 10 primary tumors and 10 metastases. Pre-treatment samples were available for comparison of morphology in all cases and the pre-treatment Ki67 index was available in 9 cases. In the other 11 cases, no material was available to perform a Ki67 stain for study purposes. The primary sites included the gastrointestinal tract (esophagus, stomach, colon and rectum), pancreas, larynx, lung, and breast. The final diagnosis in 19 cases was PD-NEC or MANEC and one case was a WD-NET G3 of the pancreas. In 13/20 cases, patients were treated with platinum-based chemotherapy and 5/20 with 5-Fluorouracil (5-FU) based chemotherapy; in the one case of metastatic mammary NE carcinoma, the patient received hormonal therapy. The details of the treatment regimen were not available in one case. In 3 cases, radiation therapy was included in addition to chemotherapy. Table 1 summarizes the details of the primary site, diagnosis and treatment for each case.
Table 1:
Location of the primary neoplasm with diagnosis and subsequent treatment:
| Case # | Primary site | Diagnosis | Location of the pre/post treatment specimens | Treatment |
|---|---|---|---|---|
| 1 | Esophagus | PD-NEC, LC | Esophagus/esophagus | Cisplatin +irinotecan |
| 2 | Stomach | MANEC | Liver/stomach | Cisplatin + CPT11 |
| 3 | Pancreas | PD-NEC, SC | Pancreas/liver | Cisplatin + etoposide |
| 4 | Stomach | PD-NEC, LC | Stomach/stomach | Carboplatin + etoposide + RT |
| 5 | Rectum | MANEC | Rectum/rectum | Folfox + RT |
| 6 | Larynx | PD-NEC, SC | Larynx/lymph node | carboplatin |
| 7 | Stomach | MANEC | Abdomen/Liver and stomach | Folfox |
| 8 | Stomach | MANEC | Stomach/stomach | Carboplatin + Paclitaxel |
| 9 | Pancreas | WD-NET, G3 | Pancreas/pancreas | Folfirinox |
| 10 | GEJ | MANEC | GEJ/GEJ | Carboplatin + Irinotecan |
| 11 | Stomach | PD-NEC, mixed SC & LC | Stomach/stomach | Cisplatin + etoposide |
| 12 | Esophagus | PD-NEC, LC | Esophagus/esophagus | Cisplatin + etoposide |
| 13 | Rectum | MANEC | Rectum/liver | Cepcetabine + Folfox |
| 14 | Esophagus | MANEC | Esophagus/esophagus | Chemotherapy + RT, details unknown |
| 15 | GEJ | PD-NEC, SC | GEJ/GEJ | Carboplatin + Paclitaxel |
| 16 | Cecum | MANEC | Cecum/cecum | Folfox |
| 17 | Stomach | PD-NEC, SC | Stomach/stomach | Carboplatin + etoposide + Folfiri |
| 18 | GEJ | PD-NEC, mixed SC & LC | GEJ/GEJ | Carboplatin + paclitaxel |
| 19 | Lung | PD-NEC, SC | Lung/adrenal | Cisplatin + Etoposide |
| 20 | Breast | PD-NEC, SC | Breast/liver | Faslodex+ Lupron + Ibrace + Leterozole |
Abbreviations: PD-NEC – poorly differentiated neuroendocrine carcinoma, WD-NET – well differentiated neuroendocrine tumor, MANEC – mixed adenocarcinoma neuroendocrine carcinoma, SC- small cell carcinoma, LC – large cell neuroendocrine carcinoma, GEJ – gastroesophageal junction, RT- radiation therapy, Folfox 5-FU and oxaliplatin, Folfiri – 5-FU and Irinotecan, Folfirinox-5-FU, Irinotecan and Oxaliplatin
In 15/20 post-treatment samples, we observed a Ki67 index of <20%, at least focally (i.e., in a coldspot), and in 6 cases (30%) the overall Ki67 index based on the hotspot was <20% (see table 2, Fig 1–4). In 3 cases, the highest Ki67 index in the entire sample was ≤1%. Thus, there were 6 cases in which the post-treatment Ki67 index falsely suggested a diagnosis of WD-NET G2 (3 cases) or G1 (3 cases). The degree of heterogeneity in the post-treatment samples was marked, with 15/20 cases showing some degree of heterogeneity, 12 of which had a heterogeneity score >1 (mean score = 2.4). In the 9 cases with pre-treatment Ki67 data, the overall Ki67 index was consistently 70–100% (mean = 84.4%), and there was much less heterogeneity. Seven cases had no heterogeneity and the other two had a heterogeneity score of 1. Comparing the pre- and post-treatment Ki67 indices in these 9 cases, in 5 there was a reduction in the overall Ki67 index based on the hotspots, and in two addition cases there were regions within the treated samples (coldspots) with greatly reduced Ki67 labeling; in the remaining two cases there was no change in Ki67 following treatment.
Table 2:
Results of Ki67 staining on pre-treatment and post-treatment samples with morphologic comparison
| Case # | Pre-treatment Ki 67 index (%) | Pre-treatment Ki67 range | Pre-treatment Ki67 heterogeneity score | Post-treatment Ki67 index | Post-treatment Ki67 range (%) | Post-treatment Ki67 heterogeneity score | Post-treatment Morphology alteration treatment |
|---|---|---|---|---|---|---|---|
| 1 | 80 | none | 0 | 80 | 50–80 | 2 | same |
| 2 | 80 | 70–80 | 1 | 80 | 80 | 0 | same |
| 3 | 90 | none | 0 | 90 | 90 | 0 | same |
| 4 | 70 | none | 0 | 90 | 1–90 | 5 | More pleomorphic |
| 5 | NA | NA | NA | 60 | 1–60 | 3 | More glandular and pleomorphic |
| 6 | 100 | none | 0 | 10 | 3–10 | 1 | Better differentiated |
| 7 | 80 | 70–80 | 1 | 40 | 1–40 | 2 | Better differentiated |
| 8 | NA | NA | NA | 70 | 1–70 | 4 | same |
| 9 | 90 | none | 0 | 10 | 1–10 | 1 | More pleomorphic |
| 10 | NA | NA | NA | 70 | 1–70 | 4 | same |
| 11 | NA | NA | NA | 50 | 1–50 | 3 | same |
| 12 | NA | NA | NA | 10 | 1–10 | 1 | More pleomorphic |
| 13 | NA | NA | NA | <1 | <1 | 0 | Better differentiated |
| 14 | NA | NA | NA | <1 | <1 | 0 | Same |
| 15 | NA | NA | NA | 60 | 1–60 | 3 | Better differentiated |
| 16 | NA | NA | NA | 70 | 1–70 | 4 | same |
| 17 | NA | NA | NA | 50 | 20–50 | 2 | Better differentiated |
| 18 | NA | NA | NA | 70 | 40–70 | 2 | same |
| 19 | 90 | none | 0 | 1 | 1 | 0 | Better differentiated |
| 20 | 80 | none | 0 | 40 | 1–40 | 2 | Better differentiated |
Abbreviations: NA – not applicable
Figure 1:
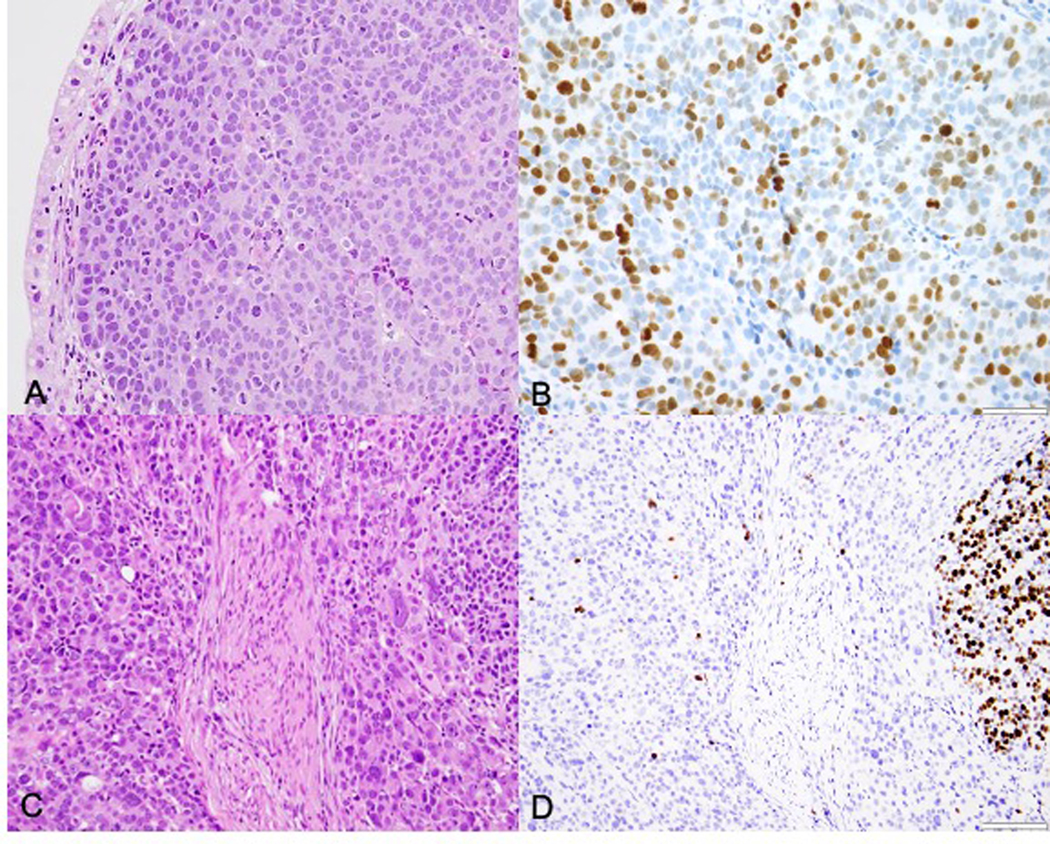
Case 4: Pre-treatment gastric biopsy shows a PD-NEC, LC (A, H&E) with a proliferation index of 70% (B, Ki67). Post-treatment H&E sections of the stomach show a more pleomorphic tumor morphology (C) and heterogeneous labeling of Ki67 with some areas showing <1% labeling (D).
Figure 4:
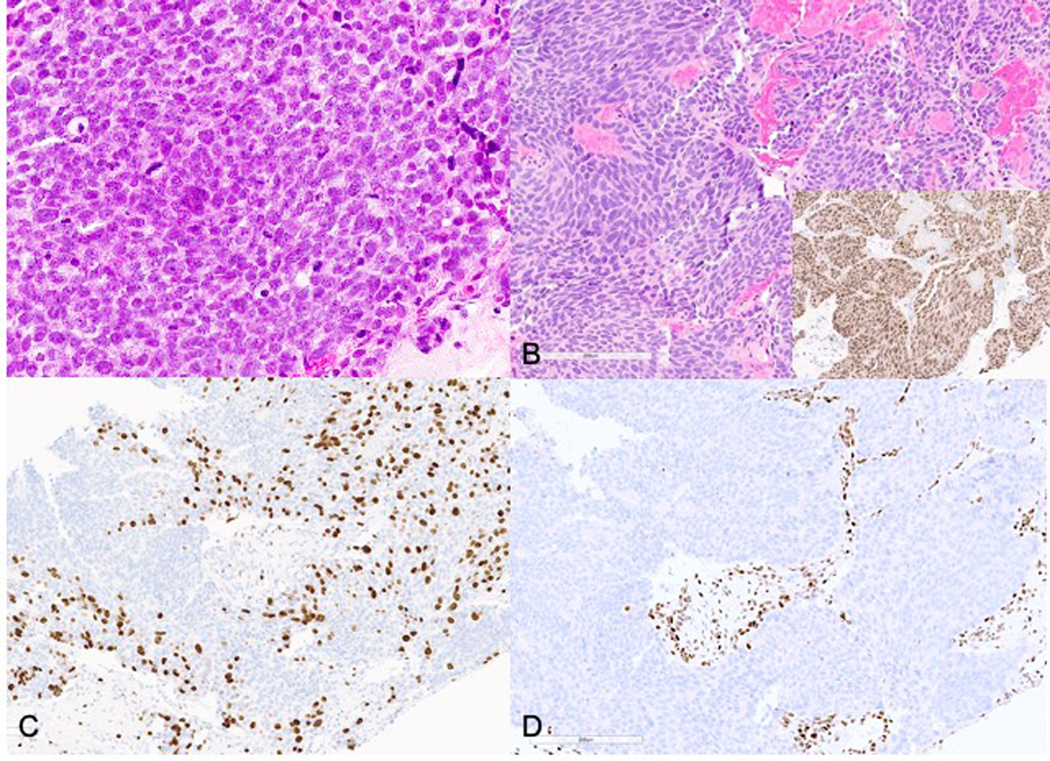
Case 9: Pancreas biopsy showing a HG-NEN (A, H&E) with proliferation index of 90% (B, Ki67). Sections of pancreatic resection after therapy show a neoplasm with a well differentiated morphology (C, H&E, inset: ATRX immunohistochemical stain demonstrating loss of nuclear staining in neoplastic cells) and low Ki-67 (D), supporting a WD-NET.
Thus, in 18/20 cases (90%), there were at least regions within the post-treatment samples with a Ki67 index that was either reduced significantly compared to the matched pre-treatment or fell within a range (<20%) not qualifying for high grade based on the WHO grading system. The Ki67 index based on the hotspot areas averaged 84.4% (range 80–100%) in pre-treatment samples and 47.7% (range 1–90%) in post-treatment samples (p=0.002).
16/20 cases had sufficient material in the post-treatment samples for the evaluation of mitotic activity. In 13/16 cases (81%), the mitotic rate in the areas corresponding to the decreased Ki67 index was <20/2 mm2 (Mean Ki67 – 16.17 %) while in 3 cases it was >20/2 mm2 (mean Ki67– 37%). In 5 cases, we were able to perform separate mitotic counts in the Ki67 hotspot and coldspot regions and observed a lower mitotic rate in the low Ki67 areas (mitotic rate range of 1–80/2 mm2), as compared to the high Ki67 areas (range of 21–133/2 mm2). In 5 cases, the mitotic rate in the hotspot and cold spot regions was same (uniformly low) while in 3 cases, no mitoses were identified in the post-treatment specimen.
Comparing the morphology of the pre-treatment and post-treatment samples, the morphologic features fell in two categories. In the first category (n = 9), the post-treatment carcinoma showed persistent tumor cellularity, cytologic atypia, and nuclear pleomorphism similar to the pre-treatment samples (Fig 1). In the second category (n = 11), there was morphologic transformation of the neoplastic cells. Four of these cases (2 MANEC and 2 PD-NEC, LC) showed more nuclear pleomorphism in the post-treatment samples (Fig 2), and 7 (2 MANEC and 5 PD-NEC, SC) (64%) showed a more monotonous, cytologically bland appearance with an associated reduction of the proliferative index and apoptosis, features that resembled a well-differentiated neuroendocrine tumor (Fig 3).
Figure 2:
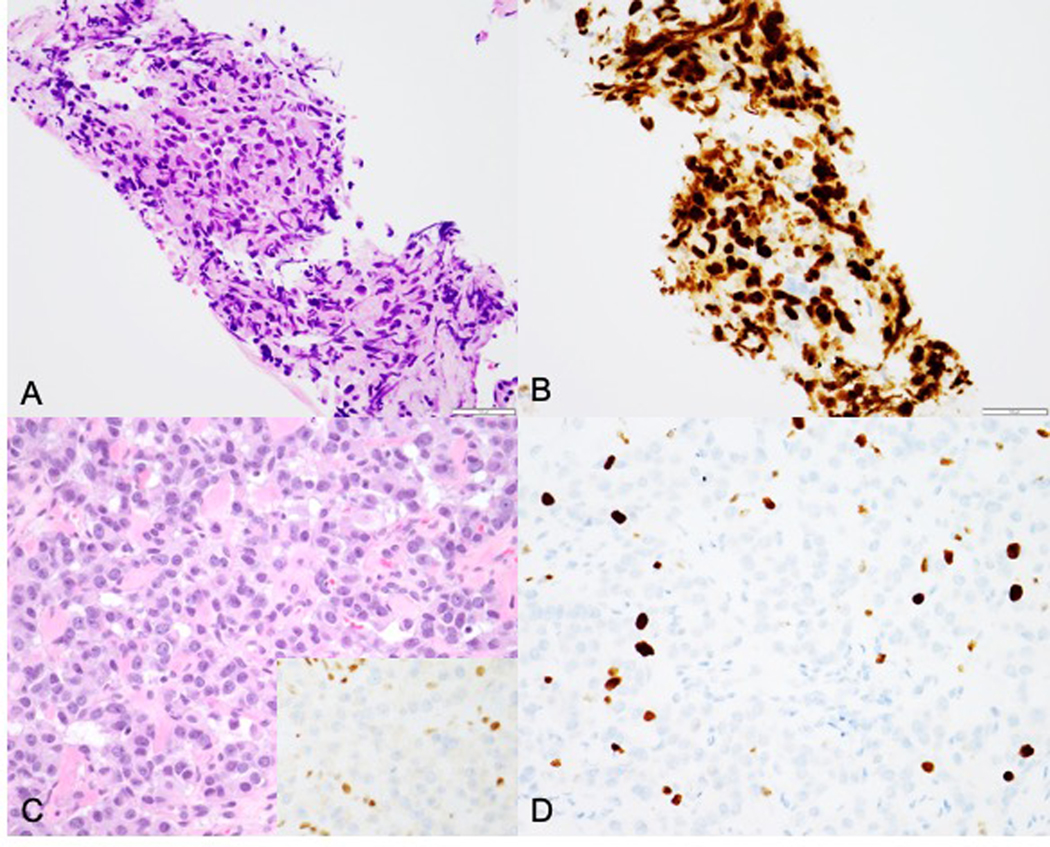
Case 12: Biopsy of distal esophagus showing a mixed adenoneuroendocrine carcinoma (A, H&E) with diffuse synaptophysin positivity in the neuroendocrine component (B). Post-treatment resection specimen shows residual viable neuroendocrine carcinoma component (C) with corresponding low Ki67 (D).
Figure 3:
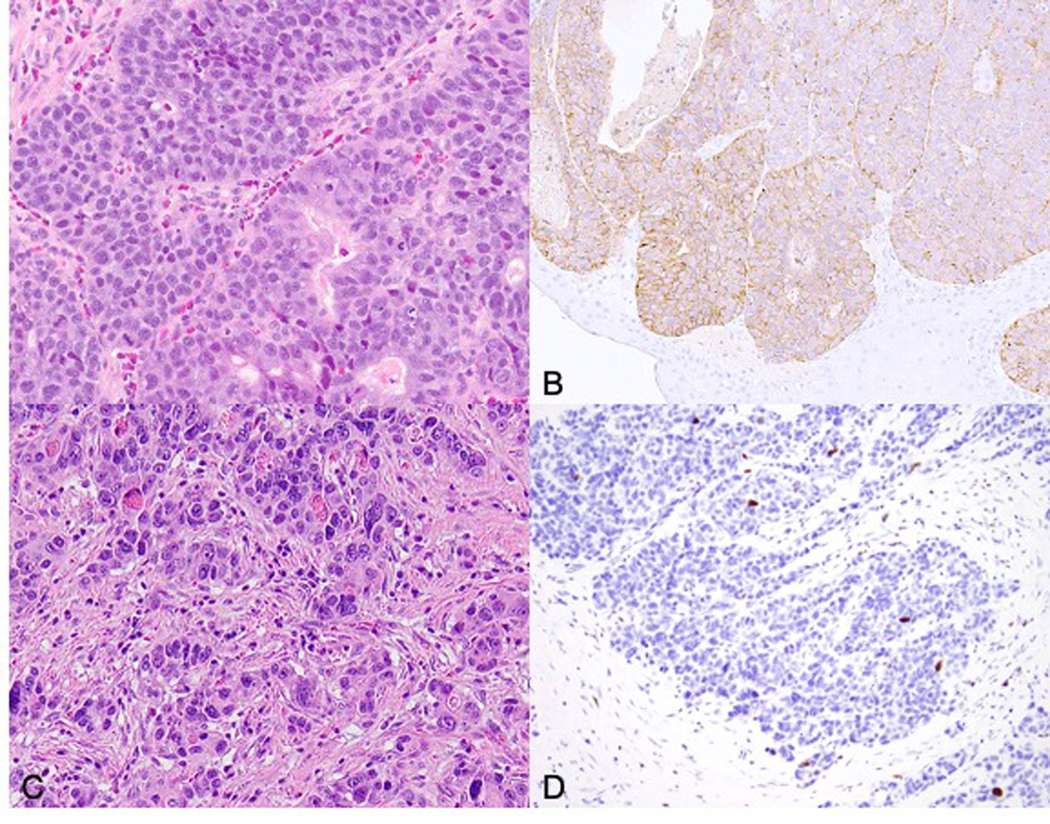
Case 20: Breast biopsy showing small cell carcinoma prior to treatment (A, H&E), biopsy of liver metastasis post-treatment showing more nested, round to spindled bland neuroendocrine cells, resembling a well differentiated neuroendocrine tumor (B, H&E, inset: ER immunohistochemical stain). Ki-67 labeling was heterogeneous ranging from 1–40% (C). This neoplasm shows loss of Rb (D), supporting the diagnosis.
Case 9 in our study was diagnosed as a HG-NEN based on a limited biopsy sample (pre-treatment Ki67 – 90%, Fig 4, A–B)) and received neoadjuvant chemotherapy with Folfirinox. The resection specimen showed a neuroendocrine neoplasm with more well differentiated morphology with focal areas of nuclear pleomorphism, Ki67 labeling in the range of 1–10% and loss of staining for ATRX by immunohistochemistry (Fig 4, C–D). Extensive sampling of the resection specimen and careful review of histology failed to demonstrate a high grade component matching the biopsy. A mutation in ATRX was later confirmed by molecular studies, supporting a final classification as a WD-NET, G3.
Discussion:
Role of Ki67 in grading neuroendocrine neoplasms, with an emphasis on WD-NETs, G3 vs PD-NECs
Proliferative rate, especially the Ki67 index, is very important for the prognostic grading of WD-NETs, which has been shown to stratify outcome in both the primary and metastatic settings (7). Equally critical is the distinction of WD-NETs from PD-NECs, given their striking difference in genomics, prognosis, and response to specific chemotherapies (4). Although PD-NECs are no longer formally graded (WHO 2019), as they are high grade be definition, they are believed to show a Ki67 index uniformly >20%, and often greater than 50%. This range overlaps with that of WD-NETs, G3, and distinction between these entities often requires genomic analysis or immunolabeling for distinguishing gene products such as p53, Rb, or (for pancreatic primaries) DAXX and ATRX (4). Within the PD-NEC group there has not been much emphasis on the specific Ki67 index as a prognostic factor, although some data suggest that PD-NECs in the lower end of the Ki67 range are less aggressive than those with higher Ki67 indices (8). Small cell carcinoma in particular tends to have a very high Ki67 index (in excess of 70%), whereas large cell neuroendocrine carcinomas are more variable (9).
The importance of proliferative rate in all NE neoplasms has led to the common practice of reassessing the Ki67 index whenever a tissue sample is obtained (10, 11).
WD-NETs may show grade progression over time or in different sites of disease, and as the current study demonstrates, PD-NECs may show a reduction in the Ki67 index, either focally or diffusely, following treatment with chemotherapy. According to our data, 30% of PD-NECs can have an overall post-therapy Ki67 in the G1 or G2 range, based upon the hot-spot regions, and another 45% of cases show heterogeneity of Ki67 labeling, with cold-spot regions that fall within the G1 or G2 range. Given the size we defined for the cold-spots (at least one 20X microscopic field = 0.8 mm2), it appears possible that biopsies taken from these heterogeneous cases could reveal only the regions with a low Ki67 index. Thus, our data have demonstrated the significant risk to misclassify previously treated PD-NECs as WD-NETs based on the Ki67 index. Furthermore, some cases also showed morphologic alterations in the neoplastic cells, with a more monotonous, cytologically bland appearance, that can also simulate the morphology of a WD-NET. While in the 4/20 cases, the post treatment specimens showed more nuclear pleomorphism, in 7/20 cases (including 5 PD-NEC, SC), the morphologic transition suggested a better differentiated neoplasm. This can also be a diagnostic pitfall if prior clinical and pathologic information are not known.
Despite these similarities, there is evidence that true transformation between WD-NETs and PD-NECs is extremely unusual (9, 12). Combined neoplasms with both well and poorly differentiated components are nearly non-existent. In contrast, PD-NECs are commonly combined with non-neuroendocrine carcinoma elements, such as the adenocarcinoma components in the MANECs in the present study. WD-NETs can show grade progression, including to WD-NET, G3, but despite the overlap in Ki67 index with PD-NECs, WD-NETs, G3 remain well differentiated and retain the same distinctive spectrum of genomic alterations as their lower grade counterparts. WD-NETs, G3 also respond less well to platinum-based therapy than PD-NECs yet have a more protracted clinical course (12). All of these considerations emphasize the importance of accurately diagnosing PD-NECs, even after prior chemotherapy has induced alterations in morphology and proliferative index.
Post-treatment reduction and heterogeneity in Ki67 labeling
In our study, the most important observation was a significant decrease in the Ki67 proliferation index in treated NENs, PD-NECs in particular. Additionally, the Ki67 labeling was heterogeneous, ranging from nil to as high as 90% in post-treatment samples. Also, decreased and variable proliferative activity in post-treatment PD-NECs may be seen in primaries of any organ. The only other study that documents similar observations is by Brambilla et al., who described morphologic changes after chemotherapy in 20 small cell carcinomas of the lung, including a better differentiated phenotype with a lower Ki67 index in 10% of cases following treatment (17). Recently, Blesl et al. described a case of single case pancreatic WD-NET, G3 that was downgraded to a G2 neoplasm after adjuvant chemotherapy (cisplatin, etoposide and temozolomide) (18). We observed this phenomenon in case 9 (Fig 4) of this series, where the initial diagnosis based on a biopsy was a HG-NEN of uncertain differentiation and the patient was treated with Folfirinox. Subsequently, the patient underwent surgery; in addition to morphologic changes the tumor also showed a marked decrease in Ki67 index. The definitive classification of this neoplasm as a WD-NET was supported by loss of ATRX by immunohistochemistry and ATRX mutation demonstrated by subsequent next generation sequencing studies. Although this and the reported cases are only anecdotes, they demonstrate the potential that chemotherapy may alter the proliferative rate of WD-NETs, in addition to PD-NECs, although the potential to misdiagnose such cases is low compared with PD-NECs that show a Ki67 index in the G1 or G2 range.
The mechanism for decreased proliferation and alterations in morphology after chemotherapy is unclear. It is plausible that a component of less proliferative cells, unapparent in PD-NECs prior to treatment, is resistant to chemotherapy and persists after treatment. This phenomenon has been demonstrated in irradiated rectal adenocarcinomas, which interestingly can demonstrate predominantly neuroendocrine differentiation after neoadjuvant therapy (6). The neuroendocrine elements appear non-proliferative, and when they are the only residual component after treatment, there is an excellent prognosis (6). Additionally, chemotherapy can induce novel gene mutations and other alterations that could affect tumor cell differentiation. Chemotherapy induced cell differentiation is also well recognized in a variety of other neoplasms, including carcinomas of various sites and neuroblastic tumors (6, 19–21). Rasbridge et al noted that in cases of mammary carcinoma, the post-therapy Ki67 index may be higher or lower than the pre-therapy sample. In our study, the Ki67 index in the adenocarcinoma component of MANEC was not as significantly altered as that in the neuroendocrine component. It is possible that the neuroendocrine component is more responsive to therapy than the adenocarcinoma component, especially in cases where the chemotherapy regimens were largely directed towards the PD-NEC component. We also attempted to correlate the mitotic rate with the decrease in Ki67 index. However, the assessment of mitotic rate was much more challenging in these cases due to small size and suboptimal preparation of the specimens. Nevertheless, we could establish a concomitant decrease in mitotic rate in 13 cases in the same areas which also showed a lower Ki67 index. Hence, it is likely that heterogeneity in mitotic rate and proliferation in general exists in HG-NENs that have been treated with chemotherapy.
Implications of post-treatment reduction in Ki67 in diagnosis of NENs
The observations in this study are important for several reasons. First, this study shows that grading of treated NENs can be unreliable, especially for PD-NECs in small biopsy specimens. A significant number of our cases had sufficient reduction in the Ki67 index post-treatment to suggest a diagnosis of a lower grade and better differentiated neoplasm, i.e., WHO G1 or G2 WD-NETs. If a definitive diagnosis (WD-NET vs. PD-NEC) has been established prior to treatment, it should always be referenced, since the post-treatment diagnosis is likely to be the same entity, even if the proliferation rate appears to be altered. Ki67 staining performed on post-treatment samples needs to be interpreted with caution. When the Ki67 is <20% or heterogeneous, it is important to acquire clinical history and review pre-treatment specimens. It is important to emphasize that proliferative index alone is not sufficient for neuroendocrine neoplasm classification. Thus, a pre-treatment diagnosis of PD-NEC or WD-NET should be consistent throughout the disease course even with post-treatment Ki67 index decrease or increase and morphologic alterations in the tumor.
Limitations of this study
While this is the first attempt to study post-treatment alterations in Ki67 labeling of high grade NENs, we acknowledge, our study has several limitations. The sample cohort is small and biased. In addition, not all the pre-treatment material and corresponding Ki67 staining was available for review. Our cohort was composed of highly selected index cases (n=5) and the search for additional cases was limited by availability of both pre-and post-treatment samples for Ki67 staining. Many of the patients with an initial diagnosis of PD-NEC did not receive resection or repeat biopsies.
We recognize that the phenomenon of tumor grade alteration is common in neoplasms of the gastrointestinal tract and pancreatobilliary system, but extrapolation of the frequency to other organs systems would be an overgeneralization. Systematic studies of pre-treatment and post-treatment specimens are required to establish definitive correlation and frequency. The clinical implications of reduction in Ki67 index or morphologic alterations on the clinical outcome are not clear at this time.
Conclusions:
Our study demonstrates that chemotherapy can significantly alter the Ki67 index in HG-NENs. Whenever the discordance between the morphology and proliferation index in cases of NENs occurs, further acquisition of clinical history and evaluation of pre-treatment pathology can facilitate the correct pathologic interpretation. Until evidence emerges to suggest a change in prognosis based on these treatment-associated alterations, post-treatment PD-NECs should retain their originally established diagnostic classification regardless of the Ki67 index and morphologic features. Post-treatment decrease in tumor proliferation and Ki67 labeling heterogeneity may become a more recognized phenomenon as the chemotherapeutic options broaden with a need for repeated sampling of the neoplasm during the treatment course for assessment of tumor progression and treatment response. Further studies are needed to determine whether treatment-induced changes in the Ki67 index alter the prognosis of these highly aggressive carcinomas.
References:
- 1.Klimstra DS, Kloppel G, La Rosa S. Classification of neuroendocrine neoplasms of the digestive system. In: Arends MJ, Fukayama M, Klimstra DS, editor. WHO Classifcation of Tumors editorial Board Digestive System tumors. 1. 5th ed. Lyon (France): IARC Press; 2019. p. 16–9. [Google Scholar]
- 2.Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018;31(12):1770–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang LH, Basturk O, Sue JJ, Klimstra DS. A Practical Approach to the Classification of WHO Grade 3 (G3) Well-differentiated Neuroendocrine Tumor (WD-NET) and Poorly Differentiated Neuroendocrine Carcinoma (PD-NEC) of the Pancreas. Am J Surg Pathol. 2016;40(9):1192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang LH, Untch BR, Reidy DL, O’Reilly E, Dhall D, Jih L, et al. Well-Differentiated Neuroendocrine Tumors with a Morphologically Apparent High-Grade Component: A Pathway Distinct from Poorly Differentiated Neuroendocrine Carcinomas. Clin Cancer Res. 2016;22(4):1011–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.La Rosa S, Sessa F, Uccella S. Mixed Neuroendocrine-Nonneuroendocrine Neoplasms (MiNENs): Unifying the Concept of a Heterogeneous Group of Neoplasms. Endocr Pathol. 2016;27(4):284–311. [DOI] [PubMed] [Google Scholar]
- 6.Shia J, Guillem JG, Moore HG, Tickoo SK, Qin J, Ruo L, et al. Patterns of morphologic alteration in residual rectal carcinoma following preoperative chemoradiation and their association with long-term outcome. Am J Surg Pathol. 2004;28(2):215–23. [DOI] [PubMed] [Google Scholar]
- 7.Kloppel G. Neuroendocrine Neoplasms: Dichotomy, Origin and Classifications. Visc Med. 2017;33(5):324–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freis P, Graillot E, Rousset P, Hervieu V, Chardon L, Lombard-Bohas C, et al. Prognostic factors in neuroendocrine carcinoma: biological markers are more useful than histomorphological markers. Sci Rep. 2017;7:40609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yachida S, Vakiani E, White CM, Zhong Y, Saunders T, Morgan R, et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol. 2012;36(2):173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi H, Zhang Q, Han C, Zhen D, Lin R. Variability of the Ki-67 proliferation index in gastroenteropancreatic neuroendocrine neoplasms - a single-center retrospective study. BMC Endocr Disord. 2018;18(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh S, Hallet J, Rowsell C, Law CH. Variability of Ki67 labeling index in multiple neuroendocrine tumors specimens over the course of the disease. Eur J Surg Oncol. 2014;40(11):1517–22. [DOI] [PubMed] [Google Scholar]
- 12.Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24(1):152–60. [DOI] [PubMed] [Google Scholar]
- 13.Coriat R, Walter T, Terris B, Couvelard A, Ruszniewski P. Gastroenteropancreatic Well-Differentiated Grade 3 Neuroendocrine Tumors: Review and Position Statement. Oncologist. 2016;21(10):1191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavalcanti MS, Gonen M, Klimstra DS. The ENETS/WHO grading system for neuroendocrine neoplasms of the gastroenteropancreatic system: a review of the current state, limitations and proposals for modifications. Int J Endocr Oncol. 2016;3(3):203–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basturk O, Yang Z, Tang LH, Hruban RH, Adsay V, McCall CM, et al. The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol. 2015;39(5):683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Z, Tang LH, Klimstra DS. Effect of tumor heterogeneity on the assessment of Ki67 labeling index in well-differentiated neuroendocrine tumors metastatic to the liver: implications for prognostic stratification. Am J Surg Pathol. 2011;35(6):853–60. [DOI] [PubMed] [Google Scholar]
- 17.Brambilla E, Moro D, Gazzeri S, Brichon PY, Nagy-Mignotte H, Morel F, et al. Cytotoxic chemotherapy induces cell differentiation in small-cell lung carcinoma. J Clin Oncol. 1991;9(1):50–61. [DOI] [PubMed] [Google Scholar]
- 18.Blesl A, Krones E, Pollheimer MJ, Haybaeck J, Wiesspeiner U, Lipp RW, et al. Downgrading of a G3 Neuroendocrine Tumor to a G2 Tumor: Can First-Line Cytotoxic Chemotherapy Change the Tumor Biology? Case Rep Oncol. 2017;10(3):1121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moll UM, Chumas J. Morphologic effects of neoadjuvant chemotherapy in locally advanced breast cancer. Pathol Res Pract. 1997;193(3):187–96. [DOI] [PubMed] [Google Scholar]
- 20.McCluggage WG, Lyness RW, Atkinson RJ, Dobbs SP, Harley I, McClelland HR, et al. Morphological effects of chemotherapy on ovarian carcinoma. J Clin Pathol. 2002;55(1):27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raaf JH, Cangir A, Luna M. Induction of neuroblastoma maturation by a new chemotherapy protocol. Med Pediatr Oncol. 1982;10(3):275–82. [DOI] [PubMed] [Google Scholar]