Abstract
Background
This single-center study aimed to investigate the effects of repetitive transcranial magnetic stimulation (rTMS) on modulation of thyroid hormone levels and cognition in the recovery stage of patients with cognitive dysfunction following stroke.
Material/Methods
Seventy post-stroke patients who had cognitive impairment were randomly assigned to either the rTMS group or the control (sham) group. Both groups were administered basic treatment, with the rTMS group receiving rTMS (1 Hz, 90% MT, 1000 pulse/20 min, once a day for 5 days, for a total of 20 times), the stimulation site was the contralateral dorsolateral prefrontal cortex (DLPFC), and the sham group receiving sham stimulation which had the same stimulation parameters and site, except that the coil plane was placed perpendicular to the surface of the scalp. Cognitive function assessment and thyroid function tests were performed before and after 4 weeks of treatment.
Results
Serum levels of triiodothyronine (T3), free triiodothyronine (FT3), and thyroid stimulating hormone (TSH) showed a positive correlation with Montreal Cognitive Assessment (MoCA) scale score of stroke patients in the recovery phase. The post-treatment change in the scores of MoCA and Modified Barthel Index (MBI) and scores of 3 cognitive domains (visuospatial function, memory, and attention), as well as serum T3, FT3, and TSH levels, were improved more significantly in the rTMS group, and T3 and FT3 levels significantly affected the MoCA scores within the reference range.
Conclusions
Serum T3, FT3, and TSH levels of stroke patients in the recovery phase were positively correlated with MoCA score. rTMS increased T3, FT3, and TSH levels and also improved MoCA and MBI of patients in the recovery phase of stroke.
Keywords: Cognition, Stroke, Thyroid Dysgenesis, TMS 42
Background
Stroke often causes a variety of dysfunctions affecting movement, sensation, cognition, speech, and emotion. Post-stroke cognitive impairment has a high incidence rate (20–80%) [1]. It is associated with poor functional outcomes, which affect the recovery process and the capacity of patients to live independently. Cognitive dysfunction, if not treated at an early stage, eventually progresses to dementia [2]. Stroke patients often have abnormal thyroid hormones metabolism, typically characterized by decreased serum levels of triiodothyronine (T3) and free triiodothyronine (FT3) [3]. In a study by Alevizaki et al, 56% of patients with first-ever stroke (n=737) had low-T3 syndrome in the acute phase; in addition, low-T3 syndrome was found to be an independent risk factor in patients with acute stroke [4].
Decreased thyroid hormone (TH) levels affect the cerebral blood flow and the metabolism of proteins and nucleic acids, leading to diffuse neurological dysfunction [5]; this results in cognitive decline and eventually affects the ability to independently perform activities of daily living [6]. Studies have shown that lower total serum thyroxine (T4) levels are associated with higher probability of cognitive impairment. In addition, abnormal TH levels in patients with vascular and non-vascular dementia were closely related to their cognitive levels; the decreased serum T3 and FT3 levels were the main factors causing cognitive decline [7]. Chronic low levels of TH aggravate post-stroke cognitive impairment [8]; therefore, identification of safe and effective interventions for these patients at an early stage is imperative.
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive cortical stimulation method that is easy to operate, safe, and reliable. According to current evidence-based medical data, rTMS treatment can have significant benefits for motor dysfunction, aphasia, and lateral neglect after stroke [9]. Meanwhile, rTMS is gradually being used in clinical treatment of cognitive impairment; in addition, basic research on synaptic plasticity, neuroelectrophysiology, and metabolomics has also been carried out to study the mechanism of action of rTMS [10,11]. Ren et al [12] found that rTMS can increase T4 and thyroid stimulating hormone (TSH) levels and reduce cholesterol and blood lipid levels. In a study by Szuba et al [13], application of 10 Hz high-frequency rTMS to the left prefrontal cortex (PFC) of patients with major depression significantly improved the patient’s mood and increased serum TSH levels after 20 treatment sessions. Trojak et al [14] observed a significant increase in serum TSH level after low-frequency rTMS on the left dorsolateral prefrontal cortex (DLPFC) of patients with depression; these findings suggested that the increase in serum TSH levels may be attributable to the effect of rTMS on DLPFC, which affects the hypothalamic-pituitary-thyroid (HPT) axis [14].
Several studies have investigated thyroid hormone levels and functional outcomes in post-stroke patients; however, most of these studies focused mainly on the acute phase of stroke. These findings suggest that changes in thyroid hormones levels can reflect the severity of disease in patients with different types of cerebrovascular diseases [5,6], which is usually shown by a low level of T3, and there is a negative correlation between T3 levels and the prognosis of stroke [15]. The relationship between thyroid hormones levels and cognitive dysfunction in the recovery phase of stroke is not well characterized. Low-frequency rTMS reduces the excitability of the healthy brain hemisphere, reduces inhibition of the affected brain hemisphere, and reconstructs a new competitive inhibition balance pattern between the left and right brain hemispheres, which is beneficial to the recovery of stroke patients, and the low frequency is safer and almost never causes epilepsy [16]. Therefore, the present single-center study investigated the effects of repetitive transcranial magnetic stimulation (rTMS) on the modulation of thyroid hormone levels and cognition in the recovery stage of patients with cognitive dysfunction following stroke, and also investigated the correlation between the two. We proposed the hypothesis that rTMS on DLPFC indirectly affects HPT axis activity and regulates thyroid hormones levels, thereby improving cognitive function. This may also provide new insights for clinical intervention in patients with post-stroke cognitive impairment and low levels of thyroid hormones.
Material and Methods
Participants
In this prospective study, patients who were recovering from stroke and had cognitive impairment were recruited and screened between July 2019 and December 2020 at the Department of Rehabilitation Medicine, Shijiazhuang People’s Hospital, third ward. The inclusion criteria were: (1) age >50 years; (2) first-ever stroke with unilateral hemispheric lesions diagnosed by CT or MRI (diagnostic criteria of the revised 4th National Cerebrovascular Disease conference, 1995); (3) disease course: 14 days to 2 months with stable vital signs; (4) years of education ≥8 years; (5) right-handed; (6) ability to complete cognitive tests and clinical examinations; (7) Mini-Mental State Examination (MMSE) score <26; (8) passed rTMS safety screening and met the safety standards for participating in the rTMS intervention [17].
The exclusion criteria were: (1) severe organ dysfunction such as heart, lung, liver, and kidney damage; (2) severe mental, psychological, visual, auditory, or speech (aphasia) disorder; (3) history of thyroid disease; (4) coma, lethargy, unconsciousness, dementia, or cognitive impairment before stroke (assessed by interview and review of medical history); (5) severe infection; (6) history of epilepsy; (7) long-term use of corticosteroids or amiodarone.
The drop-out criteria were: (1) patients who were uncooperative in completing the rTMS or sham stimulation treatment; (2) patients who were uncooperative in completing the cognitive assessment; (3) patients who withdrew from the treatment due to other reasons.
The formula for sample size was n1=n2=[2(uα+uβ)2σ2]/δ2, set α=0.05, uα=1.96. We set β as=0.1 and uβ as=1.645. Literature review for estimate was δ=2.90, σ=3.18, then n1=n2=31. Due to a reduced sample size during the test, the sample size was increased by 10%, with 35 patients in each group, for a total of 70 patients (n: sample size; uα: the U value is checked corresponding to level α; uβ: the U value corresponds to type II error probability β; δ: difference between the mean of 2 populations(u2-u1); σ: population standard deviation).
Seventy patients met the study-selection criteria and were randomly assigned to either the rTMS group or the control (sham) group using a random number table. After treatment, 2 and 3 patients dropped out from the rTMS and sham groups, respectively, leaving 33 patients (n=33) in the rTMS group and 32 patients (n=32) in the sham group. Written informed consent was obtained from all patients prior to enrollment. This study was approved by the Ethics Committee of Shijiazhuang People’s Hospital (approval number: 2020034).
Procedure
All enrolled patients underwent assessment of cognitive function and blood tests 1 day before treatment. Subjects in both groups received basic treatments, including conventional medication, conventional rehabilitation, and cognitive training. The rTMS group received basic treatment and rTMS treatment, while the sham group received basic treatment and sham stimulation treatment. After 4 weeks of treatment, patients in both groups underwent cognitive function assessment and blood tests. Patients were kept blinded to the study hypothesis.
Conventional Rehabilitation
Conventional rehabilitation included use of neurodevelopmental treatment, motor relearning program, and activities of daily living training to carry out rehabilitation training of hemiplegic limbs. Roll over, sit up, stand up, weight-bearing exercise on the affected limbs, walking exercise, balance training, and occupational therapy were also conducted.
Cognitive Training Protocol
A professional occupational therapist recommended the following trainings based on the patient’s cognitive and functional status at admission: (1) Attention training – visual tracking and PAD game; (2) memory training – recognize, retell, and recall pictures; (3) orientation training – answer the location and arrangement of related items; (4) visuospatial ability training – puzzles and identification of objects; (5) execution training – crafting and completing tasks independently; (6) judgment and reasoning ability – pad “maze” puzzle games. The trainings were conducted once a day (30 min per session) 5 days a week for a total of 4 weeks.
rTMS Protocol
In the rTMS group, the “8” coil of the M100 Ultimate transcranial magnetic stimulator (Shenzhen Yingchi Technology Co. Ltd.) was positioned horizontally over the patient’s scalp with the center of the coil oriented tangentially to the scalp. The sham group received the same stimulation parameters, except that the coil plane was placed perpendicular (90o) to the surface of the scalp [18]. Before the start of treatment, a surface electrode was used to determine the motor threshold (MT). The recording and reference electrodes were placed on the patient’s contralateral abductor pollicis brevis, the ground wire was placed on the contralateral wrist, and the magnetic stimulation coil was positioned over the brain motor area contralateral to the abductor pollicis brevis for single-pulse magnetic stimulation. The recorded motor evoked potentials (MEPs) were analyzed and the stimulation intensity adjusted. The minimum stimulus intensity required to achieve 5 out of 10 stimuli greater than 50 μV was used as the motor threshold [19–21]. The stimulation parameters were as follows: the contralateral DLPFC was the stimulation site, TMS coil was placed at positions F3 and F4 according to the international 10–20 system for EEG electrode placement, stimulation frequency was 1 Hz, stimulation intensity was 90% of the motor threshold, stimulation time was 10s with 3s intervals, and daily stimulation of 20 mins for a total of 1000 pulse a day, 5 days per week for 4 weeks (total 20 times) [17]. During treatment, the patients were in supine position, relaxed, and refrained from moving; the stimulation sites were marked for each stimulation to enhance the accuracy of the stimulation site. One or 2 professionally trained rehabilitation therapists closely observed the facial expressions of the patient from a separate room. If the patient was found to have signs of discomfort, the treatment was stopped immediately and necessary measures were taken.
Outcome Measures
Cognition Assessment
Cognitive function was assessed using MMSE, Montreal Cognitive Assessment (MoCA) scale, and Modified Barthel Index (MBI). MMSE is the most widely used tool to assess cognitive function; it mainly assesses time and space orientation, immediate memory, attention and calculation, delayed memory, language, and visuospatial skills. The scale assesses a total of 6 aspects using 30 questions; each question is awarded 1 point, with a maximum total score of 30 points. Higher scores indicate better cognitive function [22].
The MoCA scale consists of 11 items measuring 8 cognitive domains (attention and concentration, execution functions, memory, language, visuospatial skills, abstraction, calculation, and orientation) with a maximum score of 30 points. Patients with ≤12 years of education are awarded an additional point. A score ≥26 indicates normal cognitive function, while a score <26 indicates impaired cognitive function [23].
MBI consists of 10 items such as feeding, personal hygiene, bowel control, bathing, and dressing, with a maximum total score of 100 points. According to the degree of dependency, each activity is rated from level 1 to 5; level 1 indicates the lowest independency and level 5 indicates the highest independency. Total score > 60 points indicates slight dependency, 40–60 points indicate moderate dependency, 20–40 points indicate severe dependency, and < 20 points indicate total dependency [24].
Serum levels of thyroid hormones and thyroid stimulating hormone
Blood samples (10 mL) were obtained from the contralateral elbow vein after overnight fasting at 07: 00 AM on the day before the treatment and on the day after the completion of treatment. The blood sample was transferred into a vacuum tube (with coagulant), allowed to stand for 1 h, and centrifuged using the LDZ4-1.2 medical centrifuge (Beijing Shining Sun Technology Co. Ltd.) at 3000 rpm for 10 min, to separate the serum. The serum was stored at −80°C until further processing. Electrochemiluminescence immunoassay (Roche, Cobas 601, fully automated analyzer) was used to determine serum levels of T3, T4, FT3, free thyroxine (FT4), and TSH. All reagents were stored in an upright position at 2–8°C, sealed, and protected from light.
Data Analysis
SPSS statistical software (version 25.0) was used to perform statistical analysis. P-P Plots was used to test the normality of all continuous variables. Measurement data with normal distribution are presented as mean±standard deviation, and the measurement data with non-normal distribution are presented as median (P25, P75). A scatter plot of pre-treatment thyroid hormones levels and MoCA scores in the 2 groups was prepared to show the correlation trend. Spearman correlation analysis with multiple linear regression was used to analyze the effects of thyroid hormones levels on the MoCA scores (MoCA scores=dependent variable and T3, T4, FT3, FT4, and TSH levels=independent variables). Age, disease course, and years of education in the 2 groups were compared using the 2-sample independent t test, while sex, lesion side, and type of stroke were compared using the chi-squared test. Pre- and post-treatment cognitive function scores and thyroid hormones levels were compared within groups using the paired samples t test. Post-treatment between-group change with respect to cognitive function scores and the thyroid hormones indicators were assessed using the Wilcoxon rank-sum test for 2 independent samples. Spearman correlation analysis with multiple linear regression was performed to assess the correlation between the post-treatment change in thyroid hormones levels and cognitive function scores (post-test–pre-test) in the rTMS group. P values <0.05 were considered indicative of statistical significance.
Results
Comparison of General Data Between the 2 Groups
There were no significant differences between the rTMS and sham groups with respect to sex, age, disease course, side of lesion, type of stroke, and years of education (P>0.05) (Table 1).
Table 1.
Comparison of general data between the 2 groups.
| Group | Sex (n) | Age (years) | Disease course (days) | Side of lesion (n) | Type of stroke (n) | Years of education | |||
|---|---|---|---|---|---|---|---|---|---|
| M | F | Left | Right | Ischemic | Hemorrhagic | ||||
| rTMS (n=33) | 21 | 12 | 61.79±5.51 | 28.64±12.60 | 15 | 18 | 22 | 11 | 10.21±1.60 |
| Sham (n=32) | 19 | 13 | 59.47±6.75 | 27.78±11.01 | 13 | 19 | 18 | 14 | 10.47±1.92 |
| Statistic | 0.125 | 1.519 | 0.291 | 0.155 | 0.745 | −0.587 | |||
| P | 0.724 | 0.134 | 0.772 | 0.694 | 0.388 | 0.559 | |||
Data presented as mean±standard deviation or frequency. rTMS – repetitive transcranial magnetic stimulation.
Correlation Between Serum Thyroid Hormones Levels and Cognition
Relationship of Serum T3, T4, FT3, FT4, and TSH Levels with MoCA Score
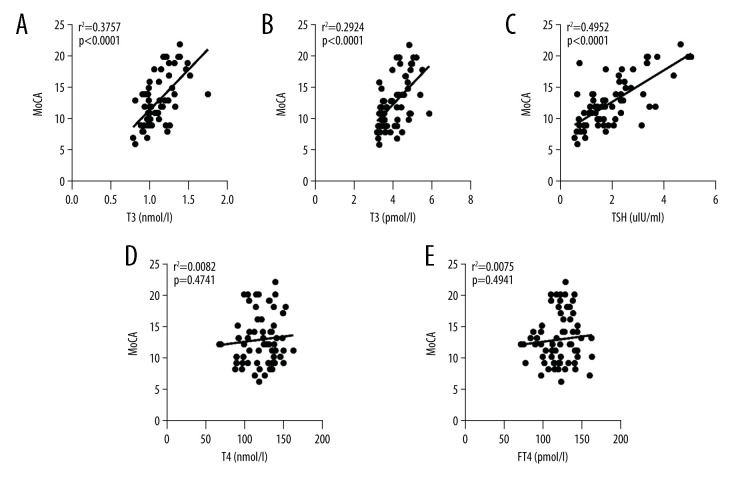
The trends were determined by preparing the scatter plot of serum T3, T4, FT3, FT4, and TSH levels against MoCA scores. Spearman correlation analysis revealed a positive correlation of T3, FT3, and TSH with MoCA scores (PT3 <0.001, correlation coefficient, rT3=0.612, coefficient of determination, R2T3=0.376; PFT3 <0.001, correlation coefficient, rFT3=0.544, coefficient of determination, R2FT3=0.292; PTSH <0.001, correlation coefficient, rTSH=0.658, coefficient of determination, R2TSH=0.495; n=65). This suggested that within a certain range, the lower the levels of serum T3, FT3, and TSH, the lower were the MoCA scores and the more severe was the cognitive impairment (Figure 1A–1C). T4 and FT4 showed no correlation with MoCA scores (PT4=0.560 >0.05, correlation coefficient, rT4=0.074, coefficient of determination, R2T4=0.008; PFT4=0.490 >0.05, correlation coefficient, rFT4=0.087, coefficient of determination, R2FT4=0.007; n=65) (Figure 1D, 1E).
Figure 1.
Scatter plots of serum T3, FT3, TSH, T4, and FT4 levels against MoCA score: (A) Serum triiodothyronine (T3) against MoCA score. (B) Free triiodothyronine (FT3) against MoCA score. (C) Thyroid stimulating hormone (TSH) against MoCA score. (D) Serum thyroxine (T4) against MoCA score. (E) Free thyroxine (FT4) against MoCA score. r2 stands for coefficient of determination, P stands for P value.
Multiple Linear Regression Analysis of MoCA Scores with Serum T3, T4, FT3, FT4, and TSH Levels
Multiple linear regression analysis was used to assess the relationship of MoCA scores with serum T3, T4, FT3, FT4, and TSH levels. The results showed that T3, FT3, and TSH levels significantly affected the MoCA scores (PT3 <0.05, PFT3 <0.05, PTSH <0.05; F=24.670, degree of freedom=64; dependent variable=MoCA scores; independent variables=T3, T4, FT3, FT4, and TSH levels) (Table 2).
Table 2.
Multiple linear regression analysis of MoCA scores with serum T3, T4, FT3, FT4, and TSH levels.
| Model | Unstandardized coefficient | Standardized coefficient | t | Significance | Collinearity | |||
|---|---|---|---|---|---|---|---|---|
| B | Standard error | Beta | Tolerance | VIF | ||||
| 1 | (Constant) | −6.599 | 2.811 | −2.347 | 0.022 | |||
| T3 (nmol/L) | 5.682 | 1.902 | 0.268 | 2.987 | 0.004 | 0.681 | 1.469 | |
| T4 (nmol/L) | 0.008 | 0.015 | 0.043 | 0.528 | 0.599 | 0.809 | 1.236 | |
| FT3 (pmol/L) | 1.707 | 0.495 | 0.279 | 3.446 | 0.001 | 0.837 | 1.195 | |
| FT4 (pmol/L) | 0.136 | 0.164 | 0.069 | 0.826 | 0.412 | 0.783 | 1.277 | |
| TSH (uIU/ml) | 1.811 | 0.318 | 0.499 | 5.693 | <0.001 | 0.713 | 1.403 | |
Dependent variable: MoCA scores; independent variables: T3, T4, FT3, FT4, and TSH levels. MoCA – Montreal Cognitive Assessment scale; VIF – variance inflation factor.
Pre- and Post-treatment Cognitive Function Scores in the 2 Groups
There was no significant between-group difference with respect to pre-treatment MoCA scores, MBI, and the scores for the 4 cognitive domains (visuospatial function, memory, language, and attention) (P>0.05 for all). However, post-treatment MoCA scores, MBI, and scores for the 4 cognitive domains in both groups were significantly higher than the respective pre-treatment scores (P <0.05 for all) (Table 3). In addition, we compared the post-treatment change in cognitive function scores (post-test–pre-test) in the 2 groups; the results showed significant differences in the MoCA scores, MBI, and scores for 3 cognitive domains (visuospatial function, memory and attention) (P <0.05 for all), and these indicators were improved more significantly in the rTMS group (Table 4).
Table 3.
Pre- and post-treatment cognitive function scores in the 2 groups.
| Group | Time | MoCA | Visuospatial function | Memory | Language | Attention | MBI |
|---|---|---|---|---|---|---|---|
| rTMS (n=33) | Pre | 12.64±4.15 | 2.09±1.10 | 2.15±0.87 | 1.24±0.44 | 2.94±1.12 | 47.91±12.99 |
| Post | 18.09±4.57 | 3.18±1.10 | 3.48±1.06 | 1.64±0.65 | 4.30±1.10 | 62.21±16.58 | |
| t | 11.891 | 7.129 | 8.288 | 4.073 | 8.429 | 8.352 | |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Sham (n=32) | Pre | 12.94±3.95 | 2.38±1.16 | 2.56±0.98 | 1.44±0.50 | 3.16±1.25 | 47.91±12.63 |
| Post | 14.81±5.13 | 2.91±1.09 | 3.03±1.20 | 1.75±0.72 | 3.75±1.20 | 53.41±18.71 | |
| t | 3.865 | 3.947 | 3.150 | 3.304 | 3.221 | 3.005 | |
| P | 0.001 | <0.001 | 0.004 | 0.002 | 0.003 | 0.005 |
Data presented as mean±standard deviation. rTMS – repetitive transcranial magnetic stimulation; MoCA – Montreal Cognitive Assessment scale; MBI – Modified Barthel Index.
Table 4.
Comparison of post-treatment change (post-test–pre-test) in cognitive function scores between the 2 groups.
| Group | MoCA | Visuospatial function | Memory | Language | Attention | MBI |
|---|---|---|---|---|---|---|
| rTMS (n=33) | 6.00 (5.00, 6.50) | 1.00 (0.50, 2.00) | 1.00 (1.00, 2.00) | 0.00 (0.00, 1.00) | 1.00 (1.00, 2.00) | 14.00 (9.00, 20.50) |
| Sham (n=32) | 2.00 (0.25, 3.00) | 1.00 (0.00, 1.00) | 1.00 (0.00, 1.00) | 0.00 (0.00, 1.00) | 1.00 (0.00, 1.00) | 5.00 (−1.50, 11.25) |
| U | −4.581 | −2.531 | −3.648 | −0.413 | −2.918 | −3.369 |
| P | <0.001 | 0.011 | <0.001 | 0.680 | <0.004 | 0.001 |
Data presented as median (P25, P75). rTMS – repetitive transcranial magnetic stimulation; MoCA – Montreal Cognitive Assessment scale; MBI – Modified Barthel Index.
Pre- and Post-treatment Levels of Thyroid Hormones and TSH in the 2 Groups
There was no significant between-group difference with respect to pre-treatment thyroid hormones indicators (P>0.05 for all). In the rTMS group, post-treatment serum levels of T3, FT3, and TSH were significantly higher than the pre-treatment levels (P<0.05 for all). Similarly, in the sham group, the post-treatment levels of T3 and FT3 were significantly higher than the pre-treatment levels (Table 5). In addition, we assessed the post-treatment change in thyroid hormones index (post-test–pre-test) of the 2 groups; the results showed significant differences in the levels of T3, FT3, and TSH (P<0.05), and these indicators were improved more significantly in the rTMS group (Table 6).
Table 5.
Comparison of pre- and post-treatment levels of thyroid hormones and TSH in the 2 groups.
| Group | Time | T3 (nmol/L) | T4 (nmol/L) | FT3 (pmol/L) | FT4 (pmol/L) | TSH (uIU/mL) |
|---|---|---|---|---|---|---|
| rTMS (n=33) | Pre | 1.11±0.25 | 116.92±22.97 | 4.01±0.67 | 12.29±1.94 | 2.02±1.05 |
| Post | 1.31±0.18 | 116.62±21.18 | 4.54±0.66 | 11.93±2.38 | 2.91±1.16 | |
| t | 6.114 | −0.072 | 4.295 | −0.887 | 5.324 | |
| P | <0.001 | 0.943 | <0.001 | 0.382 | <0.001 | |
| Sham (n=32) | Pre | 1.11±0.19 | 124.19±20.29 | 4.02±0.66 | 11.94±2.17 | 2.02±1.18 |
| Post | 1.18±0.24 | 119.02±20.84 | 4.18±0.68 | 11.32±2.02 | 2.25±1.25 | |
| t | 2.066 | −1.838 | 2.058 | −1.677 | 1.920 | |
| P | 0.047 | 0.076 | 0.048 | 0.104 | 0.064 |
Data presented as mean±standard deviation. rTMS – repetitive transcranial magnetic stimulation.
Table 6.
Comparison of post-treatment change (post-test–pre-test) in thyroid hormones and TSH levels in the 2 groups.
| Group | T3 (nmol/L) | T4 (nmol/L) | FT3 (pmol/L) | FT4 (pmol/L) | TSH (uIU/mL) |
|---|---|---|---|---|---|
| rTMS (n=33) | 0.21 (0.10, 0.36) | −0.92 (−16.47, 18.40) | 0.57 (0.07, 1.04) | −0.40 (−1.62, 0.60) | 0.98 (0.29, 1.55) |
| Sham (n=32) | 0.11 (−0.07, 0.11) | −9.63 (−18.22, 1.25) | 0.08 (−0.09, 0.43) | −1.00 (−2.36, 0.92) | 0.07 (−0.17, 0.47) |
| U | −2.324 | −1.319 | −2.625 | −0.761 | −3.438 |
| P | 0.020 | 0.187 | 0.009 | 0.447 | 0.001 |
Data presented as median (P25, P75). rTMS – repetitive transcranial magnetic stimulation.
Correlation Between Thyroid Hormones Indicators and Changes in the Cognitive Function Scores (Posttest–Pretest) in the rTMS Group
Correlation analysis of post-treatment change showed T3 was positively correlated with MoCA scores, scores for 2 cognitive domains (visuospatial function and memory), and MBI. Within the reference range, the higher the T3 level, the higher were the MoCA scores, scores for the 2 cognitive domains, and the MBI. FT3 showed a positive correlation with attention score, and within the reference range, the higher the FT3 level, the higher was the attention score. TSH showed a positive correlation with visuospatial function and attention scores, and within the reference range, the higher the TSH level, the higher were the visuospatial function and attention scores (Table 7).
Table 7.
Correlation analysis of thyroid hormones levels and changes in the cognitive function scores (post-test–pre-test) in the rTMS group (r, p).
| MoCA | Visuospatial function | Memory | Language | Attention | MBI | |
|---|---|---|---|---|---|---|
| T3 (nmol/L) | 0.350, 0.046* | 0.414, 0.017* | 0.445, 0.010* | 0.149, 0.409 | 0.156, 0.386 | 0.378, 0.030* |
| T4 (nmol/L) | 0.138, 0.444 | 0.146, 0.416 | 0.081, 0.654 | 0.268, 0.131 | 0.088, 0.626 | 0.080, 0.656 |
| FT3 (pmol/L) | 0.334, 0.058 | 0.220, 0.219 | 0.152, 0.398 | 0.067, 0.712 | 0.435, 0.011* | 0.009, 0.962 |
| FT4 (pmol/L) | −0.100, 0.581 | 0.028, 0.876 | 0.261, 0.142 | −0.061, 0.735 | 0.037, 0.839 | 0.104, 0.566 |
| TSH (uIU/mL) | 0.156, 0.387 | 0.364, 0.037* | 0.208, 0.245 | 0.132, 0.462 | 0.387, 0.026* | 0.272, 0.126 |
r: correlation coefficient,
p<0.05.
rTMS – repetitive transcranial magnetic stimulation; MoCA – Montreal Cognitive Assessment scale; MBI – Modified Barthel Index; MoCA – Montreal Cognitive Assessment scale.
Multiple Linear Stepwise Regression Analysis of Cognitive Function Scores and with Serum T3, T4, FT3, FT4, and TSH Levels (Post-test–Pre-test) in the rTMS Group
Multiple linear regression analysis of post-treatment change was used to assess the relationship of cognitive function scores with serum T3, T4, FT3, FT4, and TSH levels (post-test–pre-test), showing that T3 and FT3 levels significantly affected the MoCA scores (PT3 <0.05, PFT3 <0.05, F=5.585, degree of freedom=32; dependent variable=MoCA scores; independent variables=T3 and FT3 levels (Table 8).
Table 8.
Multiple linear stepwise regression analysis of cognitive function scores with serum T3, T4, FT3, FT4, and TSH levels (post-test–pre-test) in the rTMS group.
| Model | Unstandardized coefficient | Standardized coefficient | t | Significance | Collinearity | |||
|---|---|---|---|---|---|---|---|---|
| B | Standard error | Beta | Tolerance | VIF | ||||
| 1 | (Constant) | 3.734 | 0.673 | 5.550 | <0.001 | |||
| T3 (nmol/L) | 4.372 | 2.106 | 0.324 | 2.076 | 0.047 | 1.000 | 1.000 | |
| FT3 (pmol/L) | 1.536 | 0.580 | 0.413 | 2.648 | 0.013 | 1.000 | 1.000 | |
Dependent variable: MoCA scores (posttest–pretest); independent variables: T3, FT3 levels (post-test–pre-test) MoCA – Montreal Cognitive Assessment scale.
Discussion
Studies have shown that in addition to impairing nerve function and cognitive function, stroke also causes disorders of the HPT axis, which leads to abnormal levels of thyroid hormones [15,25]. Changes in thyroid hormones levels were suggested to reflect disease severity in patients with various cardiovascular diseases [5,6]. A study found low T3 levels and high T4 levels in patients with acute ischemic stroke [15]. In another study, low level of T3 in patients with stroke was correlated with poor prognosis [26]. Quinlan et al [8] found a negative correlation between serum FT3 level and the risk of progression to Alzheimer disease. Patients with vascular disease were also found to have abnormal serum thyroid hormones levels, mainly manifested as low T3 syndrome; in addition, changes in T3 level were closely associated with the degree of dementia [27]. Most contemporary studies on thyroid hormones and post-stroke functional outcomes have focused on the acute phase of stroke. Therefore, in the present study, we investigated the relationship between thyroid hormones levels and cognitive dysfunction during the recovery phase of stroke.
As a clinical treatment for cognitive impairment, rTMS is widely used to treat aphasia and to improve memory function and hemispatial neglect following stroke [28,29]. The DLPFC is a brain region that has been targeted for the application of TMS in many studies [30]. DLPFC is closely associated with the process of cognitive control and is involved in many cognitive tasks. The cumulative effect of rTMS becomes evident with increased stimulation time and frequency. Therefore, in this study, patients were administered rTMS treatment for 4 weeks (contralateral dorsolateral prefrontal lobe, 1 Hz); the results showed that the post-treatment MoCA scores, MBI, and scores for 4 cognitive domains (visuospatial function, memory, language and attention) were significantly higher than the pre-treatment levels. The same results were seen in the sham group; however, there were significant differences between the rTMS and sham groups with respect to post-treatment MoCA scores, MBI, and scores for 3 cognitive domains (visuospatial function, memory, and attention). It showed that basic treatments such as conventional medication, conventional rehabilitation, and cognitive training were useful for improving cognition, and additional rTMS was associated with greater cognitive improvement. The potential mechanisms by which rTMS improves cognitive function include improving blood flow in the stimulated region and other interacting brain areas, improving metabolism in brain cells, promoting white matter repair and growth, repairing cognitive circuits [31,32], regulating calcium channels [33], enhancing synaptic plasticity [34], and regulating functional connectivity of the neural networks [35].
Only a few studies have investigated the effect of rTMS on thyroid hormones levels, and the specific mechanism is still unclear. George et al [36] found that rTMS (5 Hz, intensity of 120% motor threshold) of the prefrontal lobe in healthy elderly people increased serum TSH level. A study by Kitos et al [37] found that 1-Hz low-frequency rTMS applied to patients with major depression for 3 weeks significantly improved mood and increased serum TSH level. In addition, low-frequency (1-Hz) rTMS when applied to the left DLPFC of patients with depression was found to increase serum TSH levels [14]; these findings suggested that rTMS at various frequencies (high and low) and sites (left and right) can increase TSH levels, and suggested that the changes in serum TSH level may be attributable to the effect of rTMS on the DLPFC, which in turn affects the HPT axis. The purpose of the study was to observe the effect of 1-Hz rTMS on the thyroid hormone levels of patients in the recovery phase of stroke.
In the present study, post-treatment serum T3, FT3, and TSH levels in the rTMS group were significantly higher than the pre-treatment levels; in addition, there were significant differences between the 2 groups with respect to the post-treatment change in serum T3, FT3, and TSH levels (post-test–pre-test). Serum T3, FT3, and TSH levels showed a positive correlation with MoCA scores; within the reference range, the lower the serum levels of T3, FT3, and TSH, the lower were the MoCA scores, and the more severe was the cognitive impairment. Specifically, within reference range, the higher the T3 level, the higher the MoCA score, scores for the 2 cognitive domains (visuospatial function and memory), and MBI; the higher the FT3 level, the higher the attention score; and the higher the TSH level, the higher the visuospatial function and attention scores. Thus, it is important to monitor the serum levels of T3, FT3, TSH, and cognitive function during the recovery phase of stroke, and to actively explore safe and effective non-pharmacological treatments to increase thyroid hormones levels and improve cognitive function.
The increase in T3, FT3, and TSH levels is likely attributable to the fact that the selected stimulation site (DLPFC) is a part of the brain that is easily targeted by the magnetic fields from TMS; in addition, upon stimulation, DLPFC regulates the interaction between different brain regions [38]. For example, Bilek et al [39] found that application of rTMS to DLPFC regulates the interaction between the prefrontal lobe and the hippocampus. In addition, Zikopoulos et al [40] used fluorescence histochemistry and electron microscopy to show the connection between the frontal cortex and hypothalamus. They injected fluorescent tracer into the prefrontal cortex of rhesus monkeys and observed the tracer in the ventral anterior nucleus of the thalamus. Conversely, the tracer was injected retrogradely into the ventral anterior nucleus of rhesus monkeys, and the tracer was observed in the prefrontal cortex. These findings demonstrated a close connection between the frontal cortex and hypothalamus. Stimulation of the cerebral cortex by rTMS generates current that excites nerve cells at the stimulation site as well as in the remote areas, thus promoting the connection between anatomical functional areas of the brain [9]. The PFC and thalamus are interconnected, and increasing the excitability of the mediodorsal thalamus was shown to enhance task performance [41].
However, we observed no significant changes in serum T4 and FT4 levels after rTMS; in addition, serum T4 and FT4 levels showed no correlation with MoCA scores, which is consistent with previous reports [7,8]. The lack of increase in T4 and FT4 levels after rTMS treatment may be explained by the fact that the post-stroke changes in central regulation and thyroid hormones are different from the changes in thyroid hormones and cognitive degeneration in healthy elderly people. The mechanism for the changes in thyroid hormones after rTMS treatment may also be different.
Our findings suggest that 4 weeks of rTMS (1-Hz, contralateral DLPFC) can improve the cognitive function of patients in the recovery phase of stroke, and also increase serum T3, FT3, and TSH levels. In addition, within the reference range, the higher the levels of serum T3, FT3, and TSH, the higher the cognitive function and partial cognitive domains scores. Therefore, we hypothesize that while rTMS acts on the contralateral DLPFC to improve cognitive function, it can enhance the activity of nerve cells in the thalamus, which in turn has an effect on the hypothalamus; thus, by affecting the HPT axis, it leads to increased serum levels of T3, FT3, and TSH.
Conclusions
rTMS (contralateral DLPFC, 1-Hz) treatment increases T3, FT3, and TSH levels and also improves MoCA scores, MBI, and scores for 4 cognitive domains (visuospatial function, memory, language, and attention) in patients in the recovery phase of stroke. We hypothesize that rTMS acts on the contralateral DLPFC and affects the HPT axis, thereby causing changes in thyroid hormones. Thus, the higher the thyroid hormones levels, the greater was the improvement in cognitive function. However, further research is needed to verify these results.
Footnotes
conflicts of interest.
None declared.
Declaration of Figures Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: This work was supported by Hebei Province 333 Talent Project (A202002023), and the Hebei Province Key Research and Development Project (20377727D)
References
- 1.Sun JH, Tan L, Yu JT. Post-stroke cognitive impairment: Epidemiology, mechanisms and management. Ann Transl Med. 2014;2(8):80. doi: 10.3978/j.issn.2305-5839.2014.08.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jokinen H, Melkas S, Ylikoski R, et al. Post-stroke cognitive impairment is common even after successful clinical recovery. Eur J Neurol. 2015;22(9):1288–94. doi: 10.1111/ene.12743. [DOI] [PubMed] [Google Scholar]
- 3.Bunevicius A, Smith T, Laws ER. Low tri-iodothyronine syndrome in neurosurgical patients: A systematic review of literature. World Neurosurg. 2016;95:197–207. doi: 10.1016/j.wneu.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 4.Alevizaki M, Synetou M, Xynos K, et al. Low triiodothyronine: A strong predictor of outcome in acute stroke patients. Eur J Clin Invest. 2007;37(8):651–57. doi: 10.1111/j.1365-2362.2007.01839.x. [DOI] [PubMed] [Google Scholar]
- 5.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–99. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Duan L, Han Y, et al. Predictors for vascular cognitive impairment in stroke patients. BMC Neurol. 2016;16:115. doi: 10.1186/s12883-016-0638-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gussekloo J, van Exel E, de Craen AJ, et al. Thyroid status, disability and cognitive function, and survival in old age. JAMA. 2004;292(21):2591–99. doi: 10.1001/jama.292.21.2591. [DOI] [PubMed] [Google Scholar]
- 8.Quinlan P, Horvath A, Wallin A, et al. Low serum concentration of free triiodothyronine (FT3) is associated with increased risk of Alzheimer’s disease. Psychoneuroendocrinology. 2019;99:112–19. doi: 10.1016/j.psyneuen.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Lefaucheur J-P, Aleman A, Baeken C, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018) Clin Neurophysiol. 2020;131(2):474–528. doi: 10.1016/j.clinph.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Ma Q, Qiang J, Gu P, et al. Age-related autophagy alterations in the brain of senescence accelerated mouse prone 8 (SAMP8) mice. Exp Gerontol. 2011;46(7):533–41. doi: 10.1016/j.exger.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Ma J, Zhang Z, Kang L, et al. Repetitive transcranial magnetic stimulation (rTMS) influences spatial cognition and modulates hippocampal structural synaptic plasticity in aging mice. ExpGerontol. 2014;58:256–68. doi: 10.1016/j.exger.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Ren W, Ma J, Li J, et al. Repetitive transcranial magnetic stimulation (rTMS) modulates lipid metabolism in aging adults. Front Aging Neurosci. 2017;9:334–40. doi: 10.3389/fnagi.2017.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szuba MP, O’Reardon JP, Rai AS, et al. Acute mood and thyroid stimulating hormone effects of transcranial magnetic stimulation in major depression. Biol Psychiatry. 2001;50(1):22–27. doi: 10.1016/s0006-3223(00)01118-5. [DOI] [PubMed] [Google Scholar]
- 14.Trojak B, Chauvet-Gelinier JC, Verges B, et al. Significant increase in plasma thyroid-stimulating hormone during low-frequency repetitive transcranial magnetic stimulation. J Neuropsychiatry Clin Neurosci. 2011;23(1):E12. doi: 10.1176/jnp.23.1.jnpe12. [DOI] [PubMed] [Google Scholar]
- 15.Jiang X, Xing H, Wu J, et al. Prognostic value of thyroid hormones in acute ischemic stroke – a meta-analysis. Sci Rep. 2017;7(1):16256. doi: 10.1038/s41598-017-16564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pino GD, Pellegrino G, Assenza G, et al. Modulation of brain plasticity in stroke: A novel model for neurorehabilitation. Nat Rev Neurol. 2014;10(10):597–608. doi: 10.1038/nrneurol.2014.162. [DOI] [PubMed] [Google Scholar]
- 17.Rossi S, Hallett M, Rossini PM, et al. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–39. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SH, Han HJ, Ahn HM, et al. Effects of five daily high-frequency rTMS on Stroop task performance in aging individuals. Neurosci Res. 2012;74(3–4):256–60. doi: 10.1016/j.neures.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Moscatelli F, Messina G, Valenzano A, et al. Functional assessment of corticospinal system excitability in karate athletes. PLoS One. 2016;11(5):e0155998. doi: 10.1371/journal.pone.0155998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moscatelli F, Messina G, Valenzano A, et al. Differences in corticospinal system activity and reaction response between karate athletes and non-athletes. Neurol Sci. 2016;37(12):1947–53. doi: 10.1007/s10072-016-2693-8. [DOI] [PubMed] [Google Scholar]
- 21.Moscatelli F, Valenzano A, Petito A, et al. Relationship between blood lactate and cortical excitability between taekwondo athletes and non-athletes after hand-grip exercise. Somatosens Mot Res. 2016;33(2):137–44. doi: 10.1080/08990220.2016.1203305. [DOI] [PubMed] [Google Scholar]
- 22.Ou C, Li C, An X, et al. Assessment of cognitive impairment in patients with cerebral infarction by MMSE and MoCA scales. J Coll Physicians Surg Pak. 2020;30(3):342–43. doi: 10.29271/jcpsp.2020.03.342. [DOI] [PubMed] [Google Scholar]
- 23.Yu J, Li J, Huang X. The Beijing version of the Montreal Cognitive Assessment as a brief screening tool for mild cognitive impairment: A community-based study. BMC Psychiatry. 2012;12:156. doi: 10.1186/1471-244X-12-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohura T, Ishizaki T, Higashi T, et al. Reliability and validity tests of an evaluation tool based on the modified Barthel Index. International Journal of Therapy & Rehabilitation. 2011;18(8):422–28. [Google Scholar]
- 25.Lamba N, Liu C, Zaidi H, et al. A prognostic role for Low tri-iodothyronine syndrome in acute stroke patients: A systematic review and meta-analysis. Clin Neurol Neurosurg. 2018;169:55–63. doi: 10.1016/j.clineuro.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 26.Dhital R, Poudel DR, Tachamo N, et al. Ischemic stroke and impact of thyroid profile at presentation: A systematic review and meta-analysis of observational studies. J Stroke Cerebrovasc Dis. 2017;26(12):2926–34. doi: 10.1016/j.jstrokecerebrovasdis.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Chen Z, Liang X, Zhang C, et al. Correlation of thyroid dysfunction and cognitive impairments induced by subcortical ischemic vascular disease. Brain Behav. 2016;6(4):452. doi: 10.1002/brb3.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turriziani P, Smirni D, Zappala G, et al. Enhancing memory performance with rTMS in healthy subjects and individuals with Mild Cognitive Impairment: The role of the right dorsolateral prefrontal cortex. Front Hum Neurosci. 2012;6:62. doi: 10.3389/fnhum.2012.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manenti R, Cotelli M, Calabria M, et al. The role of the dorsolateral prefrontal cortex in retrieval from long-term memory depends on strategies: A repetitive transcranial magnetic stimulation study. Neuroscience. 2010;166(2):501–7. doi: 10.1016/j.neuroscience.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 30.Bush G. Cingulate, frontal, and parietal cortical dysfunction in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69(12):1160–67. doi: 10.1016/j.biopsych.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kozel FA, Johnson KA, Nahas Z, et al. Fractional anisotropy changes after several weeks of daily left high-frequency repetitive transcranial magnetic stimulation of the prefrontal cortex to treat major depression. J ECT. 2011;27(1):5–10. doi: 10.1097/YCT.0b013e3181e6317d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu H, Wu H, Cheng H, et al. Improvement of white matter and functional connectivity abnormalities by repetitive transcranial magnetic stimulation in crossed aphasia in dextral. Int J Clin Exp Med. 2014;7(10):3659–68. [PMC free article] [PubMed] [Google Scholar]
- 33.Wang HL, Xian XH, Wang YY, et al. Chronic high-frequency repetitive transcranial magnetic stimulation improves age-related cognitive impairment in parallel with alterations in neuronal excitability and the voltage-dependent Ca2+ current in female mice. Neurobiol Learn Mem. 2015;118:1–7. doi: 10.1016/j.nlm.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Yang HY, Liu Y, Xie JC, et al. Effects of repetitive transcranial magnetic stimulation on synaptic plasticity and apoptosis in vascular dementia rats. Behav Brain Res. 2015;281:149–55. doi: 10.1016/j.bbr.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 35.Wang JX, Voss JL. Long-lasting enhancements of memory and hippocampal-cortical functional connectivity following multiple-day targeted noninvasive stimulation. Hippocampus. 2015;25(8):877–83. doi: 10.1002/hipo.22416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.George MS, Wassermann EM, Williams WA, et al. Changes in mood and hormone levels after rapid-rate transcranial magnetic stimulation (rTMS) of the prefrontal cortex. J Neuropsychiatry Clin Neurosci. 1996;8(2):172–80. doi: 10.1176/jnp.8.2.172. [DOI] [PubMed] [Google Scholar]
- 37.Kito S, Hasegawa T, Fujita K, et al. Changes in hypothalamic-pituitary-thyroid axis following successful treatment with low-frequency right prefrontal transcranial magnetic stimulation in treatment-resistant depression. Psychiatry Res. 2010;175(1–2):74–77. doi: 10.1016/j.psychres.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Chen AC, Oathes DJ, Chang C, et al. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proc Natl Acad Sci USA. 2013;110(49):19944–49. doi: 10.1073/pnas.1311772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bilek E, Schafer A, Ochs E, et al. Application of high-frequency repetitive transcranial magnetic stimulation to the DLPFC alters human prefrontal-hippocampal functional interaction. J Neurosci. 2013;33(16):7050–56. doi: 10.1523/JNEUROSCI.3081-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zikopoulos B, Barbas H. Parallel driving and modulatory pathways link the prefrontal cortex and thalamus. PLoS One. 2007;2(9):e848. doi: 10.1371/journal.pone.0000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bolkan SS, Stujenske JM, Parnaudeau S, et al. Thalamic projections sustain prefrontal activity during working memory maintenance. Nat Neurosci. 2017;20(7):987–96. doi: 10.1038/nn.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]