Abstract
Objectives
To evaluate the effects of orthodontic force on histomorphology and tissue factor expression in the dental pulp.
Materials and Methods
Two reviewers comprehensively and systematically searched the literature in the following databases: Latin American and Caribbean Health Sciences, Embase, Cochrane, PubMed, Scopus, Web of Science, and Grey literature (Google Scholar, OpenGrey, and ProQuest) up to September 2020. According to the Population, Intervention, Comparison, Outcomes, Studies criteria, randomized clinical trials (RCTs) and observational studies that evaluated the effects of orthodontic force on dental pulp were included. Case series/reports, laboratory-based or animal studies, reviews, and studies that did not investigate the association between orthodontic force and pulpal changes were excluded. Newcastle-Ottawa Scale and Cochrane risk-of-bias tool were used to assess the risk of bias. The overall certainty level was evaluated with the Grading of Recommendations Assessment, Development and Evaluation tool.
Results
26 observational studies and five RCTs were included. A detailed qualitative analysis of articles showed a wide range of samples and applied methodologies concerning impact of orthodontic force on the dental pulp. The application of orthodontic force seems to promote several pulpal histomorphological changes, including tissue architecture, cell pattern, angiogenesis, hard tissue deposition, inflammation, and alteration of the expression levels of 14 tissue factors.
Conclusions
Although the included articles suggest that orthodontic forces may promote histomorphological changes in the dental pulp, due to the very low-level of evidence obtained, there could be no well-supported conclusion that these effects are actually due to orthodontic movement. Further studies with larger samples and improved methods are needed to support more robust conclusions.
Keywords: Dental pulp, Orthodontics, Systematic review
INTRODUCTION
Orthodontic movement results from applying a force vector to a tooth for some time.1,2 The establishment of pressure and tension zones along the periodontal ligament is one of the theories that explain orthodontic movement.1,2 On the pressure side, the periodontal ligament undergoes disorganization and decreased production of fibers, in addition to a decrease in blood flow and cell replication.2,3 Simultaneously, on the tension side, the stimuli produced by stretching of the fiber bundles increase the cell replication rate and, consequently, enhance periodontal fiber production.2,4
The orthodontic force triggers a sequence of biological responses, which is a consequence of interferences in the physiological balance of the dental complex.1,2 Tooth-supporting structures and dental pulp may undergo extensive microscopic changes when exposed to different degrees of mechanical loading.2,5 There is still controversy regarding the optimal force level during orthodontic movement. However, it is commonly accepted that the application of inadequate or excessive forces results in unnecessary tissue damage.2,5 Several factors can influence pulpal changes while orthodontic treatment occurs, such as the type or direction of movement, distribution, intensity, and force duration.2,5,6
Several previous studies have reported changes in the dental pulp tissue after orthodontic force. Adequate knowledge related to pulp integrity preservation during orthodontic treatment is key for clinicians when applying orthodontic mechanics. Thus, this systematic review aimed to answer the following question: In permanent teeth, does orthodontic force promote changes in the histomorphology or the expression of tissue factors in dental pulp?
MATERIALS AND METHODS
Protocol and Registration
Reporting followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).7 A protocol was developed based on the PRISMA Protocols8 and registered (CRD42020180542) at the International Prospective Register of Systematic Reviews (PROSPERO).
Eligibility Criteria
The question of this systematic review was formulated using the acronym PICOS:
Population: vital permanent teeth with complete root formation;
Intervention: any type of orthodontic force;
Comparison: none, or studies comparing different orthodontic forces;
Outcomes: changes in the histomorphology or expression of tissue factors in the dental pulp after orthodontic movement;
Studies: randomized clinical trials (RCTs), cohort, case-control, or non-randomized clinical trials.
Exclusion Criteria
The following exclusion criteria were applied: case series/reports, laboratory-based or animal studies, reviews, and studies that did not investigate the association between orthodontic force and pulpal changes.
Information Sources and Search Strategy
Individual search strategies were developed for the following databases (until September 23, 2020): Latin American and Caribbean Health Sciences, Embase, Cochrane Library, PubMed, Scopus, Web of Science, and Grey literature (Google Scholar, OpenGrey, and ProQuest theses and dissertations). Additionally, two reviewers examined the selected studies' reference lists to identify potentially relevant articles.
Study Selection
Study selection was performed in two phases. First, two reviewers blindly assessed the titles and abstracts of identified records. Then, the same reviewers separately applied eligibility criteria to the full-text studies. Information was cross-checked in a consensus meeting in which disagreements were solved between them. If there was no consensus, a third reviewer was consulted to make a final decision.
Data Collection Process and Data Items
Two reviewers collected key data from the selected studies, and any disagreements were solved between them. Data collection consisted of study characteristics (authors, year, country, and study design), sample characteristics (experimental and control groups: sample size, gender, and age of participants; teeth on which the force was applied), methodology details (type, value, and duration of the force; follow-up period; and evaluation method), outcome assessment, and main results.
Summary Measures
Main outcomes were histomorphological changes (tissue architecture, cell pattern, angiogenesis, deposition of hard tissues, and inflammation) or tissue factor levels expressed by dental pulp after orthodontic movement. Data were collected as reported by primary studies, including absolute or relative frequencies, summary measures (eg, mean and median), and statistical analyses.
Risk of Bias in Individual Studies
The risk of bias (RoB) evaluation was carried out independently by three reviewers and the information was cross-checked in a consensus meeting. For observational studies, the Newcastle-Ottawa Scale (NOS) was used.9 NOS assigns a maximum of four points for selection, two points for comparability, and three points for exposure/outcome. Studies that reached up to four points were classified as high RoB, from five to six as moderate RoB, and more than seven as low RoB. For RCTs, the Cochrane risk-of-bias tool 2.0 was used.10 The domains evaluated were as follows: bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in the measurement of the outcome, bias in the selection of the reported result, and overall bias. Each domain was rated as low RoB, high RoB, or some concerns. Studies judged to be low RoB for all domains were classified as low RoB, studies judged to have some concerns in at least one domain were classified as some concerns, and studies judged to be at high RoB in at least one domain or was judged to have some concerns for multiple domains were classified as high RoB.
Quality of Evidence Assessment
The certainty of evidence was assessed by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) using the following parameters: RoB, inconsistency, indirectness, and imprecision. RoB referred to judgments about the quality of individual studies. Inconsistency was assessed based on the presence of heterogeneity between the studies and on the quality of the studies to produce consistent results. Indirectness was assessed for differences in population, intervention, and outcome measures. Imprecision was assessed based on the presentation and broad extent of the confidence interval and whether the sample size and characteristics were sufficient to be matched to the target population. The overall quality of evidence was rated very low, low, moderate, and high using the GRADE tool software (McMaster University, Hamilton, Canada).
RESULTS
Study Selection and Characteristics
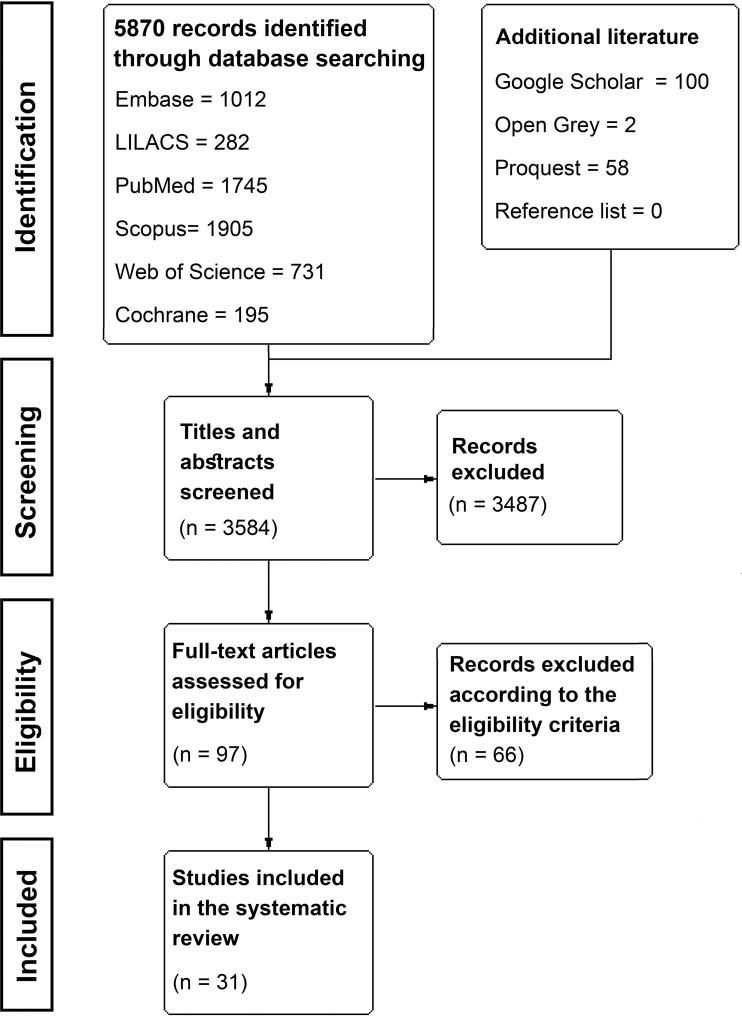
The initial search resulted in 3584 references, from which 97 studies were included for full-text assessment. Then, 66 studies were excluded, resulting in 31 included studies (Figure 1). Of these, 26 were observational studies, and five were RCTs.
Figure 1.
PRISMA flow diagram.
Eighteen studies evaluated the occurrence of histomorphological changes in the pulp after different forces: intrusion;4,11–14 extrusion;13,15–18 tipping movement;19 conventional orthodontic movement;3,20–23 or rapid maxillary expansion (RME).24–26 Fourteen studies evaluated the expression of tissue factors after orthodontic force application, including methionine-enkephalin (ME),27–29 calcitonin gene-related peptide (CGRP),27,30 substance P (SP),27,28 matrix metalloproteinase (MMP) 2 and 9,6,31 beta-Endorphin,27 c-Fos,6 caspases 3 and 9,20 proliferating cell nuclear antigen (PCNA),20 heat shock protein-60 (HSP60),20 macrophageal nitric oxide synthase (iNOS),31,32 neural nitric oxide synthase (nNOS),32 aspartate aminotransferase (AST),33–37 and alkaline phosphatase (AP).34
Risk of Bias Within Studies and Quality of Evidence
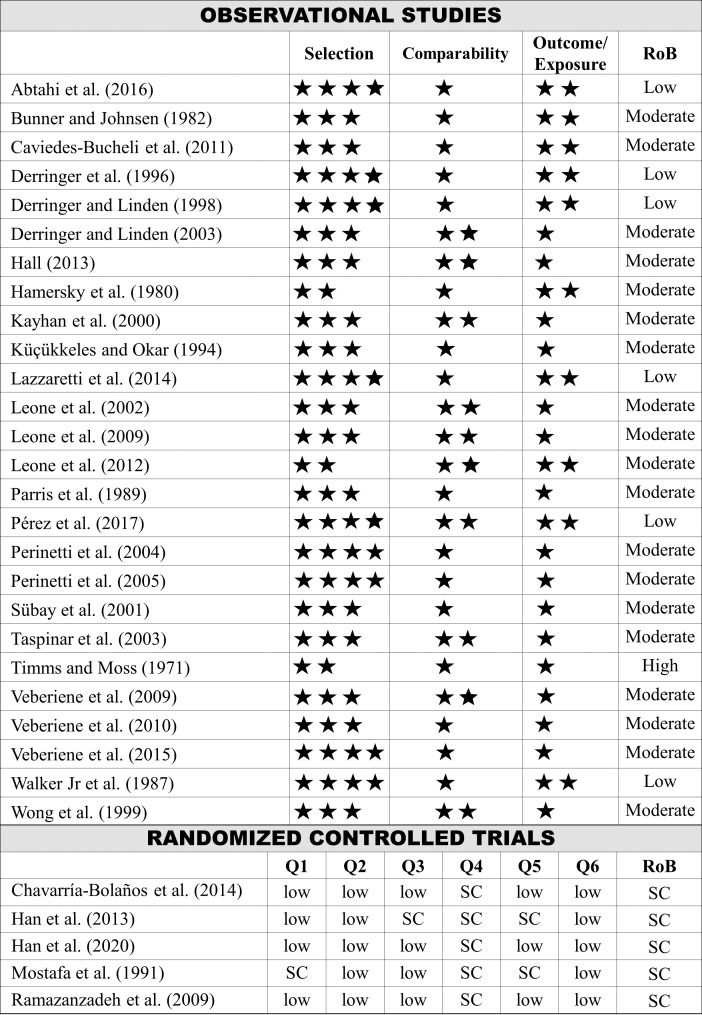
Among the observational studies, six were classified as low RoB,3,11,12,21,29,33 nineteen as moderate RoB,4,15,17–20,22–25,28,30–32,34–38 and one as high RoB.26 All RCTs6,13,14,16,27 were classified as some concerns (Figure 2).
Figure 2.
Risk of bias assessment. Newcastle-Ottawa Scale: maximum 4 points for selection, 2 points for comparability, and 3 points for exposure/outcome. Cochrane-tool 2.0: (Q1) bias arising from the randomization process; (Q2) bias due to deviations from intended interventions; (Q3) bias due to missing outcome data; (Q4) bias in measurement of the outcome; (Q5) bias in selection of the reported result; (Q6) overall bias.
GRADE analysis of the quality of the evidence was classified as very low for all outcomes (Table 1). Very low-quality evidence indicates that the real effect probably differed from the estimated effect.39 Therefore, there was very little confidence that the articles' effect was due to the application of orthodontic force. The high number of observational studies classified as moderate RoB and the fact that all RCTs were classified as some concerns, contributed to the RoB parameter being classified as serious or very serious. Inconsistency was classified as very serious for all outcomes due to the methodological heterogeneity existing among the included studies. Imprecision was classified as serious in observational studies due to the absence of presentation or the broad confidence interval for some results.
Table 1.
Analysis of the Quality of Evidence: GRADE* Assessment
| Outcome |
Quality Assessment |
GRADE Quality |
||||
| Studies (n) |
Risk of Bias |
Inconsistency |
Indirectness |
Imprecision |
||
| Histomorphological changes | 3 randomized trials | seriousa | very seriousb | not serious | not serious | ⊕○○○ Very low |
| 15 observational studies | very seriousc | very seriousb | not serious | seriousd | ⊕○○○ Very low | |
| Tissue factor expression | 2 randomized trials | seriousa | very seriousb | not serious | not serious | ⊕○○○ Very low |
| 12 observational studies | seriouse | very seriousb | not serious | seriousd | ⊕○○○ Very low | |
All studies were classified as some concerns. b the methodological heterogeneity between the included studies was considerably high; c 10 studies were classified as moderate and one as high risk of bias; d absence or extended confidence interval; e 10 studies were classified as moderate risk of bias.
GRADE Working Group grades of evidence.
Results of Individual Studies and Data Synthesis
Table 2 shows detailed information on the results of the included studies. Histomorphological changes were observed in the dental pulp of teeth moved orthodontically, such as odontoblastic aspiration,11 vacuolization, and odontoblastic layer disruption,13–16,20 disruption of the cell-free zone and predentin layer,14,15 adipose degeneration,14 fibrous tissue formation,12,13,16,24 cementum and dentin resorption,4,11,14,15,26 pulp stone formation,12,14,15,26 morphological changes of myelinated axons,18 respiration rate depression,23 and necrosis areas.13 Among the vascular alterations, blood vessel congestion and dilatation,12,14,16,19,25 vascular degeneration,4 hemorrhagic foci,16,25 growth and increase in the area of microvessels,3,15,19,21,24 reduction in the number of microvessels,22 and infiltration of chronic inflammatory cells11,25 were observed. Changes in the levels of 14 tissue factors expressed by the dental pulp were observed after orthodontic force: increased levels of AST,34,37 CGRP,30 SP,27 c-Fos,6 MMP-9,6 HSP60,20 caspases-3 and -9,20 PCNA,20 ME,28 nNOS and iNOS32, and decreased levels of MMP-2 and -9,31 ME,28,29 and AP.38
Table 2.
Characteristics of the Included Studies
| Authors (Year), Country |
Test Group (n) Patients, Teeth Age Range (Age Mean ± SD) |
Control Group |
Evaluated Teeth |
Force Type, Value (g), Duration |
| Outcome: Histomorphological Changes | ||||
| Abtahi et al. (2016), Iran | n = 22 patients 13–35 y | Contralateral teeth No treatment | Maxillary first premolars | Intrusion 15 g 7 to 30 d |
| Bunner and Johnsen (1982), USA | n = 2 patients, 4 teeth 22–23 y | n = 49 teeth No treatment | Mandibular first molars | Tipping and extrusion 90 to 100 g 10 d (short) to 171 d (long time) |
| Derringer et al. (1996), England | n = 15 teeth 11–14 y | n = 15 teeth 11–14 y No treatment | Maxillary and mandibular premolars | COM 51 to 102 g 2 wk |
| Derringer and Linden (1998), England | n = 14 patients, 14 teeth 11–14 y | Contralateral teeth No treatment | Maxillary second premolars | COM 51 to 102 g 2 wk |
| Derringer and Linden (2003), England | n = 10 patients, 10 teeth 11–14 y | n = 8 patients, 4 teeth 11–14 y No treatment | Maxillary second premolars | COM 51 to 102 g 2 wk |
| Hall (2013), Australia | n = 16 patients, 16 teeth 11–18 y | Contralateral teeth No treatment | Maxillary first premolars | Extrusion 50 g 14 d |
| Hamersky et al. (1980), USA | n = 17 patients, 34 teeth 11.8–25.8 y (15 y) | Contralateral teeth No treatment | Maxillary and mandibular first premolars | COM 170 g 72h ± 2 h |
| Han et al. (2013), China | n = 24 patients, 48 teeth 14–24y (17.9 y) | n = 3 patients, 6 teeth 14–24 y (17.9 y) No treatment | Maxillary first premolars | Intrusion 50 to 300 g 1 to 12 wk |
| Kayhan et al. (2000), Turkey | n = 34 teeth 15–17 y | n = 11 teeth 15–17 y No treatment | Maxillary premolars | RME 3 wk |
| Küçükkeles and Okar (1994), Turkey | n = 2 patients, 2 teeth | Contralateral teeth No treatment | Maxillary first premolars | Intrusion 150 g 3 mo |
| Lazzaretti et al. (2014), Brazil | n = 17 patients, 17 teeth 12–19 y | Contralateral teeth No treatment | Maxillary first premolars | Intrusion 60g 21 d |
| Leone et al. (2012), Italy | n = 20 patients, 20 teeth 10–14 y | No control group | Maxillary premolars | COM 3 to 6 mo |
| Mostafa et al. (1991), Egypt | n = 18 patients, 18 teeth 16–21 y (18 y) | Contralateral teeth No treatment | Maxillary first premolars | Extrusion 57g 1 wk to 4 wk |
| Ramazanzadeh et al. (2009), Iran | n = 26 patients, 40 teeth 14–24 y (16.8 y ± 3.2 y) | Contralateral teeth No treatment | Maxillary first premolars | Extrusion or intrusion 25 to 75 g 3 d to 3 wk |
| Sübay et al. (2001), Turkey | n = 15 patients, 40 teeth 15–18 y | No control group | Maxillary and mandibular first premolars | Extrusion 75g 10 d to 40 d |
| Taspinar et al. (2003), Turkey | n = 20 teeth 13–17 y | n = 8 teeth 13–17 y No treatment | Maxillary premolars | RME 22 d |
| Timms and Moss (1971), England | n = 8 patients 11–17 y | No control group | Maxillary premolars | RME 2 to 4 wk |
| Wong et al. (1999), Australia | n = 12 patients, 12 teeth | Contralateral teeth No treatment | Maxillary first premolars | Tipping movement 3 wk |
| Outcome: Tissue Factors Expression | ||||
| Caviedes-Bucheli et al. (2011), Colombia | n = 20 patients, 20 teeth 18–37 y | n = 10 teeth 18–37 y No treatment | Maxillary first premolars | Tipping and extrusion 56 g (moderate) to 224 g (severe) 24h |
| Chavarria-Bolanos et al. (2014), Mexico | n = 8 patients, 8 teeth 12–16 y | n = 8 patients, 8 teeth No treatment | Maxillary first premolars | Intrusion 150 to 200 g 24 h |
| Han et al. (2020), China | n = 12 patients, 24 teeth 14–24 y (17.9 y) | n = 3 patients, 6 teeth 14–24 y (17.9 y) No treatment | Maxillary first premolars | Intrusion 300 g 1 to 12 wk |
| Leone et al. (2002), Italy | 11–13 y | 11–13 y No treatment | Maxillary and mandibular premolars | COM |
| Leone et al. (2009), Italy | n = 6 patients, 6 teeth 11–14 y | n = 4 patients, 4 teeth 11–14 y No treatment | Maxillary premolars | COM |
| Leone et al. (2012), Italy | n = 20 patients, 20 teeth 10–14y | No control group | Maxillary premolars | COM 3 to 6 mo |
| Parris et al. (1989), USA | n = 11 patients, 22 teeth (15.1 y ± 4.9 y) | n = 9 patients, 18 teeth (13.9 y ± 1.7 y) No treatment | Maxillary and mandibular premolars | Tipping 120 to 245 g 21 to 72 min |
| Pérez et al. (2017), Spain | n = 20 patients, 20 teeth 14–21 y (17.35 y ± 2 y) | Contralateral teeth No treatment | Maxillary first premolars | Intrusion 75 g 48 h |
| Perinetti et al. (2004), Italy | n = 17 patients, 17 teeth 14.5–19.6 y (16.8 y ± 1.6y) | Contralateral teeth No treatment | Maxillary first premolars | COM 30 to 90 g 7 d |
| Perinetti et al. (2005), Italy | n = 16 patients, 16 teeth 15–19.6 y (17 y ± 1.6 y) | Contralateral teeth No treatment | Maxillary first premolars | COM 30 to 90 g 7 d |
| Veberiene et al. (2009), Lithuania | n = 21 patients, 21 teeth 11–21 y (15.5 ± 0.5 y) | Contralateral teeth No treatment | Maxillary and mandibular premolars | Intrusion 61 g 7 d |
| Veberiene et al. (2010), Lithuania | n = 13 patients, 13 teeth 14–22 y (16.5 ± 2.7 y) | No control group | Maxillary premolars | Intrusion 82 to 97 g 7 to 14 d |
| Veberiene et al. (2015), Lithuania | n = 10 patients, 20 teeth 16–34 y | n = 6 patients, 11 teeth 16–34 y (25.7 y ± 4.3 y) No treatment | Maxillary second premolars | COM 6m (±0.8 mo) |
| Walker Jr. et al. (1987), USA | n = 10 patients, 40 teeth | n = 6 patients, 20 teeth No treatment | Maxillary and mandibular premolars | Tipping 41 to 174 g 20 to 150 min |
AP indicates alkaline phosphatase; AST, aspartate aminotransferase; CGRP, calcitonin gene-related peptide; COM, conventional orthodontic movement; d, days; F, female; g, gram; h, hour; HSP60, heat shock protein 60; iNOS, macrophageal nitric oxide synthase; nNOS, neural nitric oxide synthase; M, male; mg, milligram; mo, months; ME, methionine-enkephalin; min, minute; mm, millimeter; MMP, matrix metalloproteinase; PCNA, proliferating cell nuclear antigen; PCR, polymerase chain reaction; RME, rapid maxillary expansion; SD, standard deviation; T, time; TEM, transmission electron microscope; w, week; y, years old.
Table 2.
Extended
| Assessment Method |
Main Results |
| Outcome: Histomorphological Changes | |
| Light microscope Evaluated changes: degree and type of inflammation, fibrous tissue formation, necrosis, disruption of odontoblastic layer, vascular dilation and resorption of cementum or dentin Groups: 13–18 y (n = 11)—adolescents, 25–35y (n = 11)—adults | 1. No significant differences of the histologic parameters between the test and control teeth during the 7 d and 1 m test period in adolescents and adults, P > .05 2. Significant differences between adolescents and adults after 1m in type and degree of inflammation in the test teeth, P = .032 3. Odontoblastic aspiration and cementum or dentin resorption were observed in adolescents-group after 7 d and in adult group after 30 days |
| Light microscope Times: 10 d (short-time) and 171 d (long time) Evaluated changes: intrapulpal axons | 1. No significant differences were found in intrapulpal axons (either qualitatively or quantitatively) 2. Alteration in axons myelinated were found in teeth with short time (1.5% to 3.5%) 3. No inflammation was evident |
| Light microscope and sequential video prints Times: 1 and 5 d of culture Evaluated changes: growth of microvessels | 1. Significantly greater growth in 5 d and 10 d in the pulps of orthodontically moved teeth, P < .05 2. Significantly greater growth in the orthodontic group in all areas at 5 d but only in the crown and root areas at 10 d, P < .05 3. Increase in microvessel length after 8 d and thickness was greater after 14 d 4. Vessel degeneration began after 14 to 21 days and after 28 d had occurred in most of pulp |
| Inverted microscope and sequential video prints Times: 5, 10, and 14 d of culture Evaluated changes: growth of microvessels | 1. Significantly greater growth in 5, 10, and 14d in the pulps of orthodontically moved teeth, P < .05 |
| Inverted microscope and sequential video prints Times: 1 and 5 d of culture Evaluated changes: growth of microvessels | 1. No significant difference in the control teeth at 5 or 10 d of culture, P > .05 2. Significant reduction of pulpal microvessels (5 and 10 d) of test teeth group, P < .05 |
| Light microscope, immunohistochemistry and assessment with spectrophotometer Evaluated changes: odontoblastic and predentine layer, blood vessels and general pulp morphology; fibroblasts number; blood vessel analysis | 1. Vacuolation and degeneration of the odontoblast layer 2. The area of blood vessels increased after force 3. Blood vessel congestion and/or hemorrhage were not observed 4. Pulp stones were also observed occasionally 5. Inconsistencies in the pre-dentine layer were observed: with some sections having a regular predentine layer, while the pre-dentinal layer in other sections was highly irregular |
| Immunohistochemistry and assessment with spectrophotometer Evaluated changes: pulpal respiration rate | 1. Between all control and experimental premolars, there was a 27.4% depression in the respiration rate. 2. Between maxillary and mandibular premolars, there was a 31.52% and 23.28% depression in the respiration rate, respectively. |
| Light microscope Times: immediately before placement of the lingual button (T1–baseline), 1 wk after force (T2), 4 wk after force (T3), 8 wk after force (T4), 12 wk after force (T5) | 1. T2: observed moderate vascular congestion and the structure of the cell-free zone was disrupted in 300 g-force group 2. T3: presence of vacuolization in coronal pulp and resorption zones with cementum deposition in 300 g-force group 3. T4: vascular congestion and dilatation were more than T3. Presence of pulp stones 4. T5: presence of odontoblastic and adipose degeneration, vacuole formation in the coronal pulp of 300 g-force group |
| Light microscope Times: 1 and 3m of treatment time Evaluated changes: number of vessels, vessel areas, minimum vessel diameters, maximum vessel diameters, and predentine widths | 1. Vessel area and minimum and maximum vessel diameters showed significant differences among the groups after 1 mo, P < .05 2. Presence of pulp fibrosis zones after 3 mo |
| TEM | 1. Vascular degeneration was the main change in the pulps of experimental group 2. Presence of resorption areas in cementum of experimental group |
| Light microscope Evaluated changes: inflammatory response, soft and hard tissue response, and count of the number of blood vessels and pulp calcifications | 1. A significant increase of fibrous tissue, number of pulpal nodules and vasodilation in the experimental group were observed, P < .05 2. No significant differences in the number of blood vessels between the groups, P > .05 |
| Light microscope Times: before treatment (T0), 3 mo after (T1), 6 mo after (T2) | 1. In T0 and T1 the pulpal tissue did not show any significant morphological alteration 2. In T2, odontoblastic vacuolization was observed |
| Light microscope Times: 1 wk after force (T1), 2 wk after force (T2), 4 wk after force (T3) | 1. Certain characteristic pulpal reactions arise from orthodontic extrusion, these reactions involve: circulatory disturbances with congested and dilated blood vessels, odontoblastic degeneration, vacuolization and oedema of the pulp tissues, and (by the fourth week) manifestation of fibrotic changes |
| Light microscope Times: 3 d after force (T1), 3 wk after force (T2) Evaluated changes: degree and type of inflammation, fibrotic tissue formation, pulp stones and dentin formation, necrosis, disruption and vacuole formation in the odontoblastic layer, aspiration into the dentin tubules, cementum or dentin resorption, and vasodilation | 1. The extrusive and intrusive forces promoted disruption of odontoblastic layer and vacuolization after 3 d and 3 wk in comparison with control group, P < .05 2. The extrusive force promoted more fibrous tissue formation in comparison with control group and intrusive force after 3 wk, P = .01 3. Incomplete necrosis was observed in one tooth in extrusive group after 3 wk |
| Light microscope Times: 10 d after force (T1), 40 d after force (T2); Evaluated changes: inflammatory response, soft and hard tissue responses | 1. None of the teeth in the groups showed any inflammatory reactions or reparative dentin formation at the test periods. |
| Light microscope Times: 3 mo after RME (T1), 6 mo after RME (T2), 18 mo after RME (T3) Evaluated changes: number of vessels, vessel diameter, hemorrhage, vascular congestion, inflammatory cell infiltration, and vacuoles. | 1. Vessel diameter, hemorrhage, congestion and inflammatory cell infiltration varied between groups, and the differences between the control and 3 mo groups, and the 3 mo and 18 mo groups were most significant |
| Light microscope | 1. Deposition of secondary dentin in pulpal floor and down on the inner walls of the root canals after 4 mo 2. Presence of pulp stones in the root canals after 4 mo |
| Light microscope Evaluated changes: blood vessels, odontoblast layer, and presence of disruption or inflammation | 1. In the orthodontic group, there are increase in blood vessel area and vessels appeared to be dilated, congested when compared with the control group, P < .01 |
| Outcome: Tissue Factors Expression | |
| Radioimmunoassay Tissue factor: CGRP | 1. CGRP levels were higher in the severe force-group (0.1380 ± 0.0278 pmol/mg) than in the moderate force group (0.0609 ± 0.0236 pmol/mg), P < .0001 2. Significant statistical differences were found between control group and the severe force group, but not with the moderate force group and control group |
| Radioimmunoassay Tissue factors: Substance P, CGRP, b-Endorphin, and ME | 1. Significant differences in substance P levels between the control-group (83.51 ± 11.35 pmol/mg) and orthodontic-group (131.91 ± 26.32 pmol/mg), P < .05 |
| Immunohistochemical and assessment with spectrophotometer, real-time PCR Times: control (T0), 1 wk after (T1), 4 wk after (T2), 8 wk after (T3), 12 wk after force (T4) Tissue factors: c-Fos and MMP-9 | 1. C-Fos and MMP-9 PCR analysis showed that protein expression increased after 4 wk of treatment, with statistical difference significant between T0 and T2, T3, T4, P < .05 2. There was appositive correlation between c-Fos and MMP-9 expression, P < .001 |
| Immunohistochemical and assessment with spectrophotometer Times: after 7 d (T1), after 14 d (T2), after 3 mo (T3), after 6 mo (T4), after 14 mo (T5) Tissue factors: nNOS and iNOS | 1. The results suggest a close correlation between the duration of the orthodontic force and the expression of NOS. 2. The presence of nNOS in the vessels and parenchymal tissue was observed after 6 mo of treatment, and the presence of iNOS was detected in the vessel walls after 3 mo, in the nerve fibers after 6 mo and in the odontoblasts after 14 mo of treatment. |
| Immunohistochemical and assessment with spectrophotometer Times: before treatment (T1), after 14 mo (T2), after 24 mo (T3) Tissue factors: MMP-2, MMP-9 and iNOS | 1. A reduction of MMP-2 and MMP-9 expression occurred in treated samples after 14 and 24 mo of treatment, P < .05 2. No significant differences were observed in the iNOS levels, P > .05 |
| Immunohistochemical and assessment with spectrophotometer Times: before treatment (T1), after 3 mo (T2), after 6 mo (T3) Tissue factors: HSP60, caspases 3 and 9, and PCNA. | 1. The levels of caspases, PCNA and HSP60 increased after T3, compared to T1 and T2, P < .05 |
| Radioimmunoassay Tissue factors: ME and substance P | 1. The substance P levels were not affected by the application force, P > .05 2. The levels of ME showed response sex-specific: in males the mean concentration decreases (control-group: 20 ± 26.7 pg/mg, ortho group: 12 ±9.4 pg/mg, P < .05) and in females increased (control-group: 26 ± 68.1 pg/mg, ortho group: 139 ± 346.4 pg/mg, P < .05) in response to orthodontic force |
| Immunohistochemical and assessment with spectrophotometer Tissue factors: AST activity | 1. No significant differences in AST levels between the control-group (1.787 ± 1.133 U/mL) and orthodontic group (1.942 ± 1.133), P > .05 |
| Immunohistochemical and assessment with spectrophotometer Tissue factors: AST activity | 1. Significant differences in AST levels between the control-group (3.6 ± 1.4 U/mL) and orthodontic group (6.7 ± 1.9 U/mL), P < .01 |
| Immunohistochemical and assessment with spectrophotometer Tissue factors: AP activity | 1. Significant differences in AP levels between the control-group (142 ± 33 U/mL) and orthodontic-group (89 ± 0.26), P < .01 |
| Immunohistochemical and assessment with spectrophotometer Tissue factors: AST activity | 1. Significant differences in AST levels between the control group (0.35 ± 0.24 U/mL) and orthodontic group (0.57 ± 0.44), P < .01 |
| Immunohistochemical and assessment with spectrophotometer Tissue factors: AST activity | 1. No significant differences in AST levels between T1 (0.27 ± 0.15 U/mL) and T2 (0.21 ± 0.15 U/mL), P = .32 |
| Immunohistochemical and assessment with spectrophotometer Tissue factors: AST activity | 1. No significant differences in AST levels between the control-group (25.29 ± 9.95 U/mL) and orthodontic group (25.54 ± 31.81), P = .312 |
| Reversed phase high-performance liquid chromatography (RP-HPLC), radioreceptor assay (RRA), radioimmunoassay (RIA), and mass spectrometry (MS) Tissue factors: ME | 1. Significant differences in ME levels by RIA analysis between the control-group (43.1 ng/g) and orthodontic-group (20.9 ng/g), P < .05 2. ME concentrations of the teeth extracted decreased in proportion to increasing forces |
DISCUSSION
It is widely accepted that orthodontic force often results in undesirable effects on the dentoalveolar complex; the dental pulp is one of the affected tissues. This systematic review included 31 articles describing dental pulp changes after orthodontic mechanotherapy. Maintaining pulp vitality increases the mechanical resistance of the teeth and long-term survival.40 Therefore, the clinician must be aware of the histomorphological aspects that the pulp may undergo due to orthodontic therapy to use adequate force and minimize damage to the tissue.
The phenomenon of movement of odontoblasts or their nuclei, known as “odontoblast aspiration”, was observed after intrusion.11 Several procedures can induce this alteration, characterized as a defense mechanism of the dentin-pulp complex against an applied external force.41 Disruption of the odontoblastic layer,13–16,20 cell-free zone, and predentin layer14,15 were also observed. These changes were commonly observed in studies that assessed dental material biocompatibility, in which the rupture of these tissue zones' integrity characterized an adverse event to their use.42,43 Based on this information, these changes in the pulp architecture could be explained as a non-physiological reaction of the tissue to orthodontic force.
Atrophic changes in tissues, such as fibrosis, are usually asymptomatic44 and were observed in the pulp of teeth subjected to RME,24 intrusion,12 and extrusion.13,16 Fibrosis is usually the result of a chronic condition resulting from a low-intensity stimulus that persists for a prolonged time, as seen in teeth affected by occlusal trauma or functioning as an abutment.44,45 Such results are controversial since pulp fibrosis was observed after orthodontic force for a short period (one week). Like other connective tissues, the pulp creates an inflammatory reaction as a defense mechanism when an external agent acts in a harmful way.46,47 The severity of inflammation is decisive in maintaining pulp vitality.48 In teeth subjected to orthodontic force, vascular events were mild and did not promote pulp degeneration. Congestion and dilation of blood vessels,12,14,16,19,25 small hemorrhagic foci,16,25 and infiltration of chronic inflammatory cells11,25 were observed, however, not affecting pulp integrity.
Angiogenesis refers to the formation of new blood vessels from pre-existing capillaries, which is of great importance in pulp regeneration.49 It is responsible for most blood vessels formed in the pulp under pathological conditions.49 Angiogenesis is initiated by a drop in the oxygen rate and decreased supply of nutrients for tissues.49 A drop in blood flow may occur due to orthodontic movement, especially in the first hours of force application.2,50 Angiogenesis was observed by an increased number of microvessels and their area in the pulp in teeth after orthodontic movement.3,15,19,21,24
Increased levels of some tissue factors have been observed in response to orthodontic force. SP and CGRP are neuropeptides released by pulp neurons due to various types of noxious stimuli.51 Increases in the levels of these neuropeptides have been observed after orthodontic force, suggesting that it can be harmful to the pulp if not controlled, as the elevation of the rates of these factors can trigger vasodilation, edema, activation of the immune system, and recruitment of inflammatory cells to the pulp.30,51 C-Fos is a transcription factor involved in the proliferation and differentiation of pulp cells52 and MMP-9 is an enzyme that degrades substances in the extracellular matrix during pathological processes. Therefore, increased levels of these biomarkers indicate damage to the pulp tissue due to orthodontic force.52 AST is an intracellular enzyme released extracellularly after cell death; its increased levels can be considered a marker of tissue damage.52 The AST levels reported by the included studies34,37 were comparable with levels in teeth with reversible pulpitis.53 Although a threshold AST level associated with pulp inflammation has not been defined, a significant increase in its levels in response to orthodontic force indicated that certain reactive mechanisms were occurring in the pulp.
The included studies showed a moderate RoB and in no case did the study's quality checklists meet all the parameters evaluated. Most studies showed moderate RoB mainly due to bias in the selection of the size and characteristics of the sample and heterogeneity between the exposed and non-exposed subjects. The method of assessment outcomes, the insufficient follow-up time for the results to occur, and the bias in measuring and interpreting the outcome contributed to these results. Quality of evidence was assessed using the GRADE-tool and demonstrated very-low strength overall performance. The imprecision of the results was mainly due to the broader confidence interval presented by the studies or the lack of presentation of these data. The included studies showed high heterogeneity; therefore, the inconsistency was assessed as very serious.
This systematic review had limitations that need to be discussed. The methodological heterogeneity of the studies due to the lack of standardization of the orthodontic force and assessment method of the results, and the limited number of studies, made it difficult to compare the results. Therefore, a combined quantitative synthesis was not considered adequate.54 Many studies had small sample sizes and samples with heterogeneous characteristics. Therefore, the results may have less statistical power and should be interpreted with caution by the clinician. Although limited by the very low quality of the studies, this SR had important strengths to highlight. The wide data collection approach, through multiple databases, allowed the inclusion of a large number of articles related to the review topic. The inclusion of studies of all languages and the use of no filter also reinforced the search process. These characteristics allowed a wide scope in the selection of articles, which increased the possibility of making a qualitative analysis of the data available in the literature within this field of research to synthesize the evidence and to set the baseline for future studies that, hopefully, will benefit from the critical analysis of the current published data.
The main objective of this review was to assess whether teeth moved orthodontically displayed pulp histomorphological changes to contribute to the diagnosis of dental pulp status during orthodontic movement. Based on the very low strength of evidence of the included articles, it was not possible to infer that regulary used orthodontic force was able to promote meaningful dental pulp changes. However, the studies seemed to suggest that the application of orthodontic force could promote, in an unidentified group of patients, several morphological changes as well as the expression of pulp tissue factors. Future research that investigates the effects of orthodontic force on pulp tissue requires RCTs, with larger samples and appropriate length of follow-up, along with standardized evaluation methods to obtain results with greater power of scientific evidence.
CONCLUSIONS
Although the included articles suggest that orthodontic forces may promote changes in pulp histomorphology and in the expression of tissue factors, the very low level evidence produced by the included articles does not support any categorical conclusion that these changes are consistently generated by orthodontic movement.
REFERENCES
- 1.Cattaneo PM, Dalstra M, Melsen B. Strains in periodontal ligament and alveolar bone associated with orthodontic tooth movement analyzed by finite element. Orthod Craniofac Res. 2009;12(2):120–128. doi: 10.1111/j.1601-6343.2009.01445.x. [DOI] [PubMed] [Google Scholar]
- 2.Krishnan V, Davidovitch Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop. 2006;129(4):e1–32. doi: 10.1016/j.ajodo.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Derringer KA, Jaggers DC, Linden RW. Angiogenesis in human dental pulp following orthodontic tooth movement. J Dent Res. 1996;75(10):1761–1766. doi: 10.1177/00220345960750100901. [DOI] [PubMed] [Google Scholar]
- 4.Küçükkeleş N, Okar I. Root resorption and pulpal changes due to intrusive force. J Marmara Univ Dent Fac. 1994;2(1):404–408. [PubMed] [Google Scholar]
- 5.Meikle MC. The tissue, cellular, and molecular regulation of orthodontic tooth movement: 100-years after Carl Sandstedt. Eur J Orthod. 2006;28(3):221–240. doi: 10.1093/ejo/cjl001. [DOI] [PubMed] [Google Scholar]
- 6.Han G, Liu W, Jiang H, et al. Extreme intrusive force affects the expression of c-Fos and MMP-9 in human dental pulp tissues. Medicine. 2020;99(9):e19394. doi: 10.1097/MD.0000000000019394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: PRISMA statement. Int J Surg. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols 2015: elaboration and explanation. BMJ. 2015;350 doi: 10.1136/bmj.g7647. g7647. [DOI] [PubMed] [Google Scholar]
- 9.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 10.Sterne JAC, Savović J, Page MJ, et al. RoB-2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 11.Abtahi M, Eslami N, Abadi RZ, Rezaei SP. The effect of intrusive orthodontic force on dental pulp of adults versus adolescents. Dent Res J. 2016;13(4):367–372. doi: 10.4103/1735-3327.187877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazzaretti DN, Bortoluzzi GS, Fernandes LFT, et al. Histologic evaluation of human pulp tissue after orthodontic intrusion. J Endod. 2014;40(10):1537–1540. doi: 10.1016/j.joen.2013.10.039. [DOI] [PubMed] [Google Scholar]
- 13.Ramazanzadeh BA, Sahhafian AA, Mohtasham N, et al. Histological changes in human dental pulp following application of intrusive and extrusive orthodontic forces. J Oral Scienc. 2009;51(1):109–115. doi: 10.2334/josnusd.51.109. [DOI] [PubMed] [Google Scholar]
- 14.Han G, Hu M, Zhang Y, Jiang H. Pulp vitality and histologic changes in human dental pulp after the application of moderate and severe intrusive orthodontic forces. Am J Orthod Dentofacial Orthop. 2013;144(4):518–522. doi: 10.1016/j.ajodo.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Hall E. The Effects of Orthodontic Force Application Upon the Tissues of the Human Dental Pulp [thesis] Birmingham, Ala: University of Birmingham; 2013. [Google Scholar]
- 16.Mostafa YA, Iskander KG, El-Mangoury NH. Iatrogenic pulpal reactions to orthodontic extrusion. Am J Orthod Dentofacial Orthop. 1991;99(1):30–34. doi: 10.1016/S0889-5406(05)81677-4. [DOI] [PubMed] [Google Scholar]
- 17.Sübay RK, Kaya H, Tarim B, et al. Response of human pulpal tissue to orthodontic extrusive applications. J Endod. 2001;27(8):508–511. doi: 10.1097/00004770-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Bunner M, Johnsen D. Quantitative assessment of intrapulpal axon response to orthodontic movement. Am J Orthod Dentofacial Orthop. 1982;82(3):244–250. doi: 10.1016/0002-9416(82)90145-2. [DOI] [PubMed] [Google Scholar]
- 19.Wong VS, Freer TJ, Joseph BK, Daley TJ. Tooth movement and vascularity of the dental pulp: a pilot study. Aust Dent J. 1999;15(4):246–250. [PubMed] [Google Scholar]
- 20.Leone A, Volponi AA, Campanella C, et al. Human dental pulp cell apoptosis: immunohistochemical study after applying orthodontic traction. J Biol Regul Homeost Agents. 2012;26(4):713–720. [PubMed] [Google Scholar]
- 21.Derringer KA, Linden RW. Enhanced angiogenesis induced by diffusible angiogenic growth factors released from human dental pulp explants of orthodontically moved teeth. Eur J Orthod. 1998;20(4):357–367. doi: 10.1093/ejo/20.4.357. [DOI] [PubMed] [Google Scholar]
- 22.Derringer KA, Linden RW. Angiogenic growth factors released in human dental pulp following orthodontic force. Arch Oral Biol. 2003;48(4):285–291. doi: 10.1016/s0003-9969(03)00008-6. [DOI] [PubMed] [Google Scholar]
- 23.Hamersky PA, Weimer AD, Taintor JF. The effect of orthodontic force application on the pulpal tissue respiration rate in the human premolar. Am J Orthod Dentofacial Orthop. 1980;77(4):368–378. doi: 10.1016/0002-9416(80)90103-7. [DOI] [PubMed] [Google Scholar]
- 24.Kayhan F, Küçükkeleş N, Demirel D. A histologic and histomorphometric evaluation of pulpal reactions following rapid palatal expansion. Am J Orthod Dentofacial Orthop. 2000;117(4):465–473. doi: 10.1016/s0889-5406(00)70167-3. [DOI] [PubMed] [Google Scholar]
- 25.Taşpinar F, Akgül N, Simşek G, et al. The histopathological investigation of pulpal tissue following heavy orthopaedic forces produced by rapid maxillary expansion. J Int Med Res. 2003;31(3):197–201. doi: 10.1177/147323000303100305. [DOI] [PubMed] [Google Scholar]
- 26.Timms DJ, Moss JP. An histological investigation into the effects of rapid maxillary expansion on the teeth and their supporting tissues. Eur Orthod Soc. 1971. pp. 263–271. [PubMed]
- 27.Chavarría-Bolaños D, Martinez-Zumaran A, Lombana N, et al. Expression of substance-P, calcitonin gene-related peptide, β-endorphin and methionine-enkephalin in human dental pulp tissue after orthodontic intrusion: a pilot study. Angle Orthod. 2014;84(3):521–526. doi: 10.2319/060313-423.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parris WG, Tanzer FS, Fridland GH, et al. Effects of orthodontic force on methionine enkephalin and substance-P concentrations in human pulpal tissue. Am J Orthod Dentofacial Orthop. 1989;95(6):479–489. doi: 10.1016/0889-5406(89)90411-3. [DOI] [PubMed] [Google Scholar]
- 29.Walker JA, Jr, Tanzer FS, Harris EF, et al. The enkephalin response in human tooth pulp to orthodontic force. Am J Orthod Dentofacial Orthop. 1987;92(1):9–16. doi: 10.1016/0889-5406(87)90290-3. [DOI] [PubMed] [Google Scholar]
- 30.Caviedes-Bucheli J, Moreno JO, Ardila-Pinto J, et al. The effect of orthodontic forces on calcitonin gene-related peptide expression in human dental pulp. J Endod. 2011;37(7):934–937. doi: 10.1016/j.joen.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 31.Leone A, Mauro A, Spatola GF, et al. MMP-2, MMP-9, and iNOS expression in human dental pulp subjected to orthodontic traction. Angle Orthod. 2009;79(6):1119–1125. doi: 10.2319/110308-557R.1. [DOI] [PubMed] [Google Scholar]
- 32.Leone A, Patel M, Uzzo ML, et al. Expression and modification of NO synthase in human dental pulps during orthodontic treatment. Bull Group Int Rech Sci Stomatol Odontol. 2002;44(2):57–60. [PubMed] [Google Scholar]
- 33.Pérez V, Marrugo SP, Pretell CM, et al. Concentración de la enzima AST en dientes sometidos a fuerzas ortodónticas intrusivas. Odontoestomatol. 2017;33(1):19–24. [Google Scholar]
- 34.Perinetti G, Varvara G, Festa F, Esposito P. Aspartate aminotransferase activity in pulp of orthodontically treated teeth. Am J Orthod Dentofacial Orthop. 2004;125(1):88–92. doi: 10.1016/j.ajodo.2003.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Veberiene R, Latkauskiene D, Racinskaite V, et al. Aspartate aminotransferase activity in the pulp of teeth treated for 6-months with fixed orthodontic appliances. Korean J Orthod. 2015;45(5):261–267. doi: 10.4041/kjod.2015.45.5.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veberiene R, Smailiene D, Baseviciene N, et al. Change in dental pulp parameters in response to different modes of orthodontic force application. Angle Orthod. 2010;80(6):1018–1022. doi: 10.2319/111309-641.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veberiene R, Smailiene D, Danielyte J, et al. Effects of intrusive force on selected determinants of pulp vitality. Angle Orthod. 2009;79(6):1114–1118. doi: 10.2319/110408-563R.1. [DOI] [PubMed] [Google Scholar]
- 38.Perinetti G, Varvara G, Salini L, Tetè S. AP activity in dental pulp of orthodontically treated teeth. Am J Orthod Dentofacial Orthop. 2005;128(4):492–496. doi: 10.1016/j.ajodo.2004.07.042. [DOI] [PubMed] [Google Scholar]
- 39.Schünemann HBJ, Guyatt G, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendations. GRADE Working Group. 2013.
- 40.Reeh ES, Messer HH, Douglas WH. Reduction in tooth stiffness as a result of endodontic and restorative procedures. J Endod. 1989;15(11):512–516. doi: 10.1016/S0099-2399(89)80191-8. [DOI] [PubMed] [Google Scholar]
- 41.Nemeth L, Erman A, Stiblar-Martincic D. Early odontoblastic layer response to cavity preparation and acid etching in rats. Folia Biol. 2006;54(1-2):23–30. [PubMed] [Google Scholar]
- 42.Costa CA, Ribeiro AP, Giro EM, et al. Pulp response after application of two resin modified glass ionomer cements in deep cavities of prepared human teeth. Dent Mat J. 2011;27(7):e158–170. doi: 10.1016/j.dental.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Garcés-Ortíz M, Ledesma-Montes C. Cytotoxicity of Ketac Silver cement. J Endod. 1997;23(6):371–373. doi: 10.1016/S0099-2399(97)80185-9. [DOI] [PubMed] [Google Scholar]
- 44.Aguiar TR, Tristao GC, Mandarino D, et al. Histopathologic changes in dental pulp of teeth with chronic periodontitis. Compend Contin Educ Dent. 2014;35(5):344–351. [PubMed] [Google Scholar]
- 45.Gautam S, Galgali SR, Sheethal HS, Priya NS. Pulpal changes associated with advanced periodontal disease: A histopathological study. J Oral Maxillofac Surg Med Pathol 2017; 21(1):58–63. doi: 10.4103/0973-029X.203795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shroff FR. Thoughts on the physiologic pathology of regressive and reparative changes in the dentine and dental pulp. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1952;5(1):51–58. doi: 10.1016/0030-4220(52)90073-x. [DOI] [PubMed] [Google Scholar]
- 47.Cooper PR, Chicca IJ, Holder MJ, Milward MR. Inflammation and regeneration in the dentin-pulp complex: net gain or net loss? J Endod. 2017;43(9s):S87–94. doi: 10.1016/j.joen.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 48.Bender IB. Reversible and irreversible painful pulpitides: diagnosis and treatment. Aust Endod J. 2000;26(1):10–14. doi: 10.1111/j.1747-4477.2000.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 49.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sabuncuoglu FA, Ersahan S. Changes in maxillary molar pulp blood flow during orthodontic intrusion. Aust Orthod J. 2014;30(2):152–160. [PubMed] [Google Scholar]
- 51.Caviedes-Bucheli J, Muñoz HR, Azuero-Holguín MM, Ulate E. Neuropeptides in dental pulp: the silent protagonists. J Endod. 2008;34(7):773–788. doi: 10.1016/j.joen.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 52.Belluardo N, Mudò G, Dell'Albani P, et al. NMDA receptor-dependent and -independent immediate early gene expression induced by focal mechanical brain injury. Neurochem Int. 1995;26(5):443–453. doi: 10.1016/0197-0186(94)00155-n. [DOI] [PubMed] [Google Scholar]
- 53.Spoto G, Fioroni M, Rubini C, et al. Aspartate aminotransferase activity in human healthy and inflamed dental pulps. J Endod. 2001;27(6):394–395. doi: 10.1097/00004770-200106000-00005. [DOI] [PubMed] [Google Scholar]
- 54.Ioannidis JP, Patsopoulos NA, Rothstein HR. Reasons or excuses for avoiding meta-analysis in forest plots. BMJ. 2008;336(7658):1413–1415. doi: 10.1136/bmj.a117. [DOI] [PMC free article] [PubMed] [Google Scholar]