Abstract
Objectives
To evaluate the ability of different esthetic archwires to retain oral biofilms in vitro.
Materials and Methods
Seven different brands of coated orthodontic archwires were tested: two epoxy coated, two polytetrafluoroethylene coated, two rhodium coated, and one silver plus polymer coated. Conventional uncoated metallic archwires were used as controls. Streptococus mutans adherence to archwires was quantified by colony count following 24 hours of biolfilm growth, and total wire-associated biofilm was measured using a crystal violet staining assay. For both tests, two conditions were used: 0% sucrose and 3% sucrose. For statistical analysis, P < .05 was considered as statistically significant.
Results
For S. mutans colony forming units per biofilm, there were no statistically significant differences among the various archwires (P = .795 for 0% sucrose; P = .905 for 3% sucrose). Regarding total biofilm formed on archwires in the 3% sucrose condition, there were statistically significant differences in crystal violet staining only for the comparison between Niti Micro Dental White and Copper Ni-Ti wires (P < .05).
Conclusions
The clinical use of esthetic-coated orthodontic wires may be considered to have similar risks as uncoated archwires for biofilm retention.
Keywords: Orthodontics, Esthetic archwires, Biofilm, Orthodontic wire, Streptococcus mutans, Bacterial adhesion
INTRODUCTION
Traditional orthodontic archwires are manufactured from stainless steel, cobalt-chromium-nickel alloy, nickel-titanium (NiTi), or titanium alloys.1 Because of increased demand for better esthetics, coated-metallic and fiber-reinforced archwires were introduced to complement esthetic brackets in orthodontics.2 Frequently used coatings include epoxy resin and polytetrafluoroethylene (PTFE), which improve esthetics, but can modify the surface in a way that can adversely affect several properties including biomechanics, mechanical durability, corrosion behavior, and plaque accumulation.3,4,5 Epoxy resin is a synthetic resin composed of the combination of epoxy and other polymers. Useful properties include its adhesion to metal, resistance to solubility, electrical insulation, and dimensional stability.1 However, studies have demonstrated higher cytotoxicity in human gingival fibroblasts after 30 days of exposure to epoxy-coated NiTi esthetic archwires compared with their uncoated pairs.5 PTFE is a synthetic polymer consisting primarily of strong carbon and fluorine bonds. PTFE has the third lowest coefficient of friction of any known solid that is heat resistant and hydrophobic.6 To increase esthetics, wires can be coated with biopolymers, and there are also rhodium-plated wires that present a “white appearance.”7,8
Orthodontic treatment in general can lead to an increase in biofilm accumulation attributed to the retention of plaque on orthodontic appliances involved in the treatment (brackets, elastomers, powerchains, elastic bands, etc).9 The combination of increased biofilm accumulation along with poor oral hygiene can lead to periodontal disease10 and enamel decalcification.11 Although the secondary effects of orthodontic treatment on periodontal tissues are transitory, enamel alterations attributed to orthodontic treatment are frequently permanent.11 Therefore, it is important to determine whether coated archwires pose an added level of risk when compared with their conventional counterparts. Ongoing research in the field of esthetic archwires has focused on the color, coating stability, mechanical properties, and surface characteristics.2,7,12 Although numerous studies have investigated bacterial adhesion and surface biofilm accumulation on coated wires, the risk of increased bacterial accumulation remains uncertain.13–15
Streptococcus mutans has a well documented role in the initiation of dental caries and enamel decalcification and is found in areas of white-spot lesions associated with orthodontic appliances.16 Although not always the predominant member in caries-associated dental plaque, S. mutans can produce extracellular polysaccharides (EPS) when in the presence of sucrose, which can facilitate further colonization of S. mutans and other cariogenic organisms to enamel and other surfaces.17 Because the accumulation of EPS may alter the binding of other cariogenic organisms, it is important to not only assess bacterial adhesion to esthetic archwires in the absence of dietary sugars but also in an environment that facilitates the production of EPS. Therefore, in vitro S. mutans adhesion in the absence of sucrose and biofilm accumulation in the presence of sucrose on different esthetic archwires was evaluated.
MATERIALS AND METHODS
Experimental Design and Sample Preparation
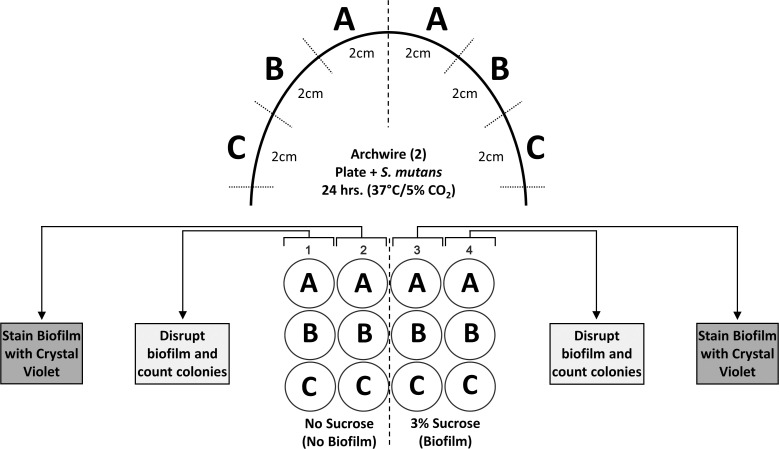
Table 1 describes the characteristics of the different coated orthodontic archwires used in this study. Experimental methodology is illustrated in Figure 1. Orthodontic archwires with cross-sectional dimensions of 0.016 × 0.022 inches were removed from the supplier's packaging material and bisected. The 1-cm distal ends of each half of the wire were cut and discarded. Then, each half of the wire was cut into three 2-cm sections, placed into the wells of a 12-well polystyrene tissue culture plate, and sterilized using ultraviolet light for 30 minutes. The following two conditions were used: (1) 0% sucrose, which results in bacterial adhesion but little biofilm formation, and (2) 3% sucrose, which allows S. mutans to form extracellular insoluble glucans that enable binding and biofilm accumulation on the wire surfaces. Three wire sections (A, B, C) from each condition (−/+ sucrose) were used for the quantitative biofilm measurement determined by colony count and for total biofilm accumulation by crystal violet staining. Two separate experiments were performed (n = 6 for each condition for each biofilm assessment method).
Table 1.
Investigated Orthodontic Archwires
| Group Code |
Product |
Coating Type |
Manufacturer |
Lot No. |
| CG1 | NT3 SE | No coating (control) | American Orthodontics (Sheboygan, Wisc) | G74366 149464 152960 |
| CG2 | Copper Ni-Ti | No coating (control) | Ormco Corporation (Orange, Calif) | 51768825 |
| EX1 | Tooth Tone Coated Archwire | Epoxy | Ortho Technology (Tampa, Fla) | PO19425 |
| EX2 | Nitanium Super Elastic | Epoxy | Ortho Organizers, Inc. (Carlsbad, Calif) | 183877 |
| PT1 | FLI Wire | PTFE | RMO Inc. (Denver, Colo) | WO-786152 |
| PT2 | Niti Micro Dental White | PTFE | Acme Monaco Co (New Britain, Conn) | WO-727181 |
| RH1 | Bio-active RC | Rhodium | Gc Orthodontics Europe GmbH (Breckerfeld, France) | A346 |
| RH2 | NiTi Dental White S | Rhodium | Acme Monaco Co (New Britain, Conn) | |
| SP1 | Dany Coated Archwire | Silver + Polymer | Dany BMT Co. Ltd. (Gwanyang-Dong, South Korea) | 362412 |
Figure 1.
Experimental design to assess S. mutans biofilm accumulation on various commercial orthodontic archwires.
Strains and Culture Methods
S. mutans (American Type Culture Collection - ATCC® 25175™) was used in all adhesion studies. Bacterial stock was kept at −80°C in Brain Heart Infusion (BHI) broth + 20% glycerol. Before assays, S. mutans was grown from glycerol stock on BHI agar for 48 hours at 37°C/5% CO2. Overnight cultures were made in 5 mL of BHI broth and grown statically for 16 hours at 37°C/5% CO2.
Bacterial Biofilm Growth on Orthodontic Wires
Biofilms were inoculated using overnight cultures of S. mutans that were normalized to optical density at 600 nm (OD600) = 1.0 in fresh BHI broth. First, 500 μL of the normalized culture (∼1.5 × 108 colony forming units [CFU]) were added to each well containing sterilized wires plus an additional 440 μL of BHI broth. For bacterial adhesion and background staining of wires, 60 μL of sterile deionized water were added (0% sucrose). Previous studies demonstrated that 3% sucrose biofilms were more resistant to inhibitors; therefore, a final concentration of 3% sucrose was used to monitor biofilm accumulation by facilitating production of extracellular glucans by S. mutans.18–20 Plates were then incubated statically for 24 hours at 37°C/5% CO2. All wires were simultaneously analyzed in two separate experiments (n = 6 sections, two half wires total) to control for variability in biofilm growth between experiments. After incubation for 24 hours, all supernatants were carefully removed, and wells were washed once with 1 mL of sterile phosphate-buffered saline (PBS) before subsequent analysis.
Colony Count Assessment of Bacterial Adhesion
Orthodontic wires were aseptically removed from the well using forceps and placed into a microcentrifuge tube and washed 3 times in PBS to remove nonadherent bacteria. Next, an additional 1 mL of PBS was added to the tube and gently shaken. Immediately, a 100 μL sample was taken for serial dilution and plating on BHI agar for a baseline of free/unbound bacteria remaining after washes. Samples were then vortexed at max speed for 10 seconds followed by sonication in an ultrasonic bath for 5 minutes and another 10-second vortex just before serial dilution. Serial dilution was performed and plated on BHI agar for biolfilm-associated S. mutans quantification. After incubation for 48 hours at 37°C/5% CO2, bacterial colonies were counted from each plate, and the colony forming units/biofilm on each segment of wire was calculated.
Crystal Violet Biofilm Staining
Orthodontic wires were aseptically removed from the well and placed into a microcentrifuge tube and washed with 1 mL of PBS, followed by heat fixation at 80°C for 30 minutes. Wires were then stained with 1 mL of 0.5% crystal violet for 30 minutes. Afterward, the crystal violet solution was aspirated, and the wires were washed three times with distilled water or until no stain was found in the wash. Biofilm-associated stain was then solubilized by the addition of 1 mL of 33% acetic acid solution and shaken for 10 minutes. Afterward, 100 μL of the solubilized crystal violet solution from each specimen was added into a well of a 96-well plate in duplicate, and the absorbance was measured at 570 nm. Average of the duplicates was used as the absorbance for each section of wire. Three wells of the plate without containing a wire, in each condition (−/+ sucrose), were stained in the same way, and absorbance readings of these wells were used as the normalization factor for biofilm growth variation between experiments. Plate biofilm was normalized to A570 = 6.0, and the normalization factor was applied to the biofilms formed on archwires.
Statistical Analysis
Statistical analysis was performed using Sigma Plot 14.0 Software (Systat Software Inc. GmbH, Erkrath, Germany). The data were analyzed to test the assumption of normal distribution and homogeneity of variance. CFU were log10 transformed before analysis using a Kruskal-Wallis test. Absorbance values from crystal violet biofilm staining test were analyzed using Kruskal-Wallis (0% sucrose condition) or one-way analysis of variance (ANOVA; 3% sucrose condition) and Tukey honestly significant difference post hoc tests. The comparison between 0% sucrose and 3% sucrose conditions within each group was evaluated through different Student's t-tests or Mann-Whitney U-tests. A P value lower than .05 was considered statistically significant.
RESULTS
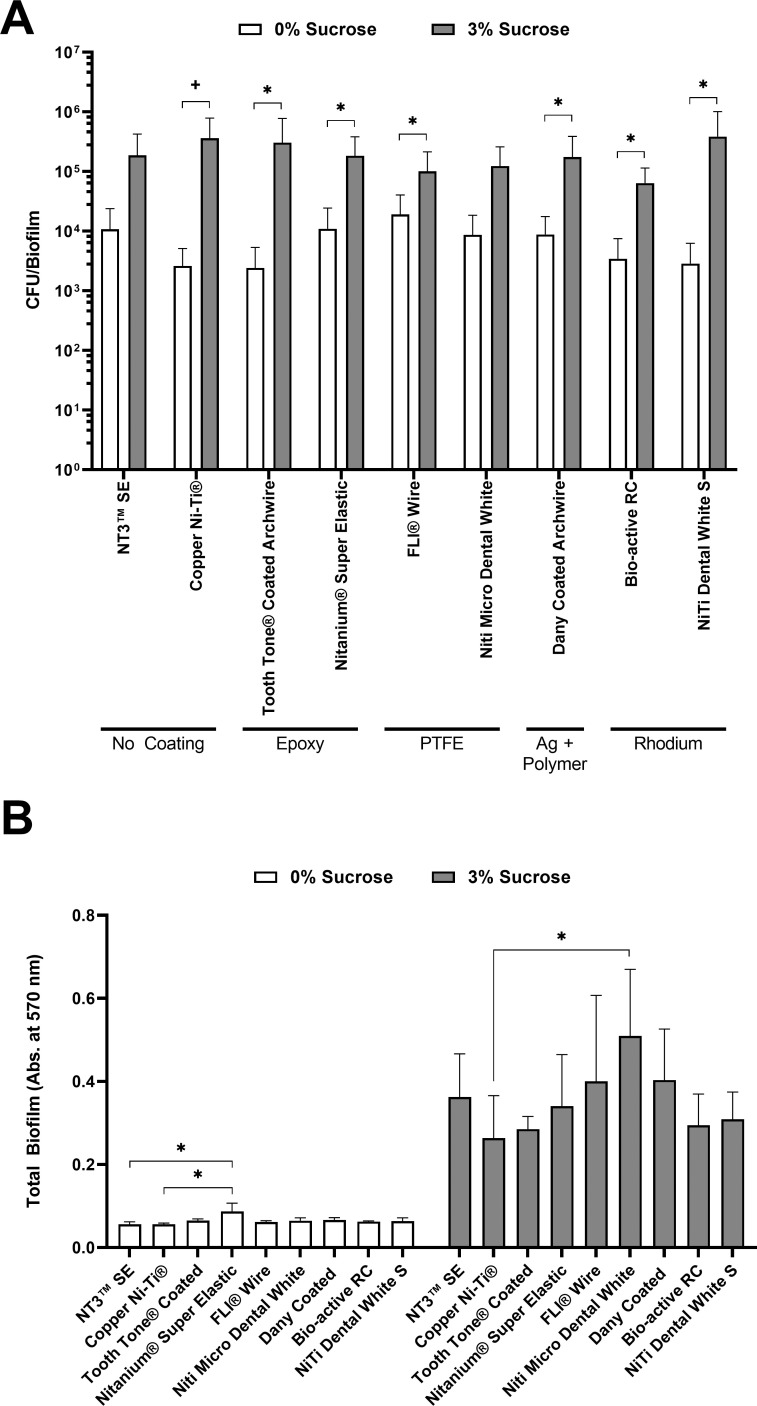
Arch wire-adherent S. mutans counts were determined by dispersal from the wires and subsequent colony counting. Mean colony counts in both conditions are shown in Figure 2A. Table 2 shows the median and interquartile range of S. mutans CFU/biofilm per section of orthodontic archwire when incubated in 0% sucrose or 3% of sucrose. Within each condition, there were no statistically significant differences among the archwires (P = .795 for 0% sucrose; P = .905 for 3% sucrose). There was a statistically significant increase in CFU/biofilm between the 0% sucrose and 3% sucrose conditions for each wire except for NT3 SE and Niti Micro Dental White (P ≤ .132). When comparing the fold increase in CFU/biofilm from 0% sucrose to 3% sucrose, Copper Ni-Ti, Tooth Tone Coated Archwire, and NiTi Dental White S archwires achieved the highest values, demonstrated by an observed fold increase higher than 100.
Figure 2.
Following growth of S. mutans biofilms on wire sections for 24 hours at 37°C/5% CO2, wires were assessed for bacterial adhesion by (A) colony counting and (B) crystal violet staining. Bars represent the means, and error bars represent plus one standard deviation. (A) Colony counts were not significantly different among wires in the same growth conditions. Statistically significant changes (P < .05) in colony counts between 0% sucrose and 3% sucrose on the same wires were evaluated using the Student's t-test (*) or Mann-Whitney U-test (+). (B) For each orthodontic wire, statistically significant increases in crystal violet staining were found between the 0% and 3% sucrose conditions. Statistical differences between different wires were calculated using Kruskal-Wallis (0% sucrose) and one-way ANOVA (3% sucrose), with significant differences indicated (*P < .05). Ag indicates silver; Abs., absorbance.
Table 2.
Median and Interquartile Range of CFU/Biofilm Formed on the Orthodontic Archwire Sectionsa
| Group Name |
CFU/Biofilm (Log10) 0% Sucrose |
CFU/Biofilm (Log10) 3% Sucrose |
Fold Change 0% to 3% Sucroseb |
| NT3 SE | 3.33 (2.00–4.37) | 4.65 (2.25–5.58)c | 17.44 |
| Copper Ni-Tid | 3.15 (2.53–3.72) | 5.11 (4.27–5.90)c | 138.09 |
| Tooth Tone Coated Archwire | 3.11 (2.89–3.49) | 4.93 (4.24–5.63)c | 126.07 |
| Nitanium Super Elastic | 3.75 (2.89–4.23) | 4.83 (4.00–5.61)c | 16.67 |
| FLI Wire | 3.64 (2.45–4.56) | 4.56 (3.72–5.36)c | 5.31 |
| Niti Micro Dental Whited | 3.59 (2.78–4.26) | 4.64 (3.57–5.37) | 14.24 |
| Dany Coated Archwire | 3.77 (3.05–4.25) | 4.75 (3.70–5.57)c | 19.83 |
| Bio-active RC | 3.07 (2.85–3.87) | 4.77 (4.36–4.93)c | 18.5 |
| NiTi Dental White S | 2.98 (2.73–3.80) | 4.68 (3.58–5.72)c | 135.56 |
There were no statistically significant differences between wires within each condition (P > .05) using the Kruskal-Wallis test.
Calculated from the mean of the samples without log10 conversion.
Statistically significant difference between 0% sucrose and 3% sucrose (P < .05). CFU comparisons between row means were analyzed using Student's t-test.
Statistically significant difference between 0% sucrose and 3% sucrose (P < .05). CFU comparisons between row means were analyzed using the Mann-Whitney U-test.
Total biofilm accumulation was measured by crystal violet staining (Figure 2B). Table 3 shows the means and standard deviations of absorbance at 570 nm after crystal violet biofilm staining. Nitanium Super Elastic showed the highest levels of staining in the 0% sucrose condition; however, statistical significance was only found when compared with the NT3 SE and Copper Ni-Ti archwires (P ≤ .002). For the 3% sucrose biofilm condition, there was a statistically significant difference between Niti Micro Dental White and Copper Ni-Ti (P = .025). When comparing crystal violet staining from 0% sucrose to 3% sucrose, the largest increase in biofilm accumulation was calculated for the Niti Micro Dental White archwire, but this increase was not statistically significant.
Table 3.
Mean (Standard Deviation) of Absorbance at 570 nm Using Crystal Violet Biofilm Staininga
| Group Name |
Total Biofilm in 0% Sucrose (Absorbance at 570 nm) |
Total Biofilm in 3% Sucrose (Absorbance at 570 nm)b |
Fold Increase in Total Biofilm (0% to 3% sucrose)c |
| NT3 SEd | 0.056 (0.006)* | 0.36 (0.10)ठ| 6.45 |
| Copper Ni-Tid | 0.056 (0.003)* | 0.26 (0.10)§ | 4.69 |
| Tooth Tone Coated Archwire | 0.065 (0.003)*† | 0.29 (0.03)‡§ | 4.39 |
| Nitanium Super Elastic | 0.087 (0.019)† | 0.34 (0.12)‡§ | 3.91 |
| FLI Wire | 0.061 (0.003)*† | 0.40 (0.21)‡§ | 6.48 |
| Niti Micro Dental White | 0.065 (0.007)*† | 0.51 (0.16)‡ | 7.90 |
| Dany Coated Archwired | 0.066 (0.005)*† | 0.40 (0.12)‡§ | 6.08 |
| Bio-active RC | 0.062 (0.002)*† | 0.30 (0.08)‡§ | 4.72 |
| NiTi Dental White S | 0.064 (0.007)*† | 0.31 (0.07)‡§ | 4.82 |
For each column, one-way independent tests, Kruskal-Wallis (0% sucrose) and one-way ANOVA (3% sucrose) with Tukey honestly significant difference post hoc tests, were used based on tests for both normality and equal variance. Different superscript symbols indicate statistically significant differences in biofilm staining between wires in the same condition (P < .05).
Crystal violet staining of biofilm in the 3% sucrose condition was significantly increased for each wire when compared with the 0% sucrose condition (P < .05). These comparisons were analyzed using Student's t-test.
Calculated from the mean of each column.
Crystal violet staining of biofilm in the 3% sucrose condition was significantly increased for each wire when compared with the 0% sucrose condition (P < .05). These comparisons were analyzed using the Mann-Whitney U-test.
DISCUSSION
In this study, the in vitro ability of different esthetic archwires to retain oral biofilms was evaluated through a quantitative biofilm measurement and a biofilm staining test. No statistically significant differences in surface-associated S. mutans CFU were found between esthetic and conventional orthodontic wires. Significantly increased crystal violet biofilm staining in adhesion studies in the absence of sucrose was observed for Nitanium Super Elastic (epoxy) when compared with the uncoated NT3 SE and Copper Ni-Ti archwires. In the 3% sucrose condition, only Niti Micro Dental White (PTFE coated) achieved statistically significant higher overall biofilm accumulation when compared with Copper Ni-Ti. Considering this, the hypothesis tested here was partially accepted.
Viable S. mutans were found on all types of wires evaluated for both 0% and 3% sucrose medium using a colony count assay (Figure 2A, Table 2). There were no statistically significant differences in CFU between wires within either culture condition, indicating that the esthetic coatings in this study did not have an influence on the in vitro adherence of viable bacteria regardless of exogenous sucrose. It was previously demonstrated that surface accumulation of bacteria to biomaterials, including orthodontic wires, is influenced by their surface roughness and surface energy.21,22 In the case of orthodontic wires, these properties have been extensively studied both in vitro and in vivo.23–26 The type of coating material and its surface characteristics, particularly surface roughness and apparent surface energy, play decisive roles in the extent of bacterial adhesion.2 Previous studies found significant positive correlations between the surface energy and S. mutans adhesion to both esthetic and metallic-base orthodontic wires and concluded that materials with high surface energy attracted more bacteria to its surface.14,27 By comparing the surface topography of coated and uncoated orthodontic wires, some studies concluded that coating might not affect the surfaces' parameters considerably.28,29 This feature could be the reason why no statistically significant differences in bacterial count were detected among the materials evaluated in this study. The results obtained in this study differed from those reported by other authors.14,30,31 It is worth mentioning that these referenced studies used different conditions for the bacterial adhesion studies and different brands of wire. Also, none of them evaluated both 0% and 3% sucrose conditions, making direct comparisons difficult.
The fold increase in S. mutans CFU associated with archwires from 0% to 3% sucrose was also calculated. The results from this calculation suggested that the Copper Ni-Ti (138.09-fold), Tooth Tone Coated Archwire (126.07-fold), and NiTi Dental White S archwires (135.56-fold) trended toward the largest increases in biofilm formation when S. mutans produced extracellular glucans in the presence of sucrose, approaching 10 times the level of the remaining groups (Table 2). This slight increase could have been due to the presence of a TiO2 oxide passive layer within the surface of NiTi-based alloys, which has been associated with a higher surface energy and higher bacterial adhesion.14
S. mutans total biofilm was also measured using a crystal violet staining assay (Figure 2B, Table 3). This quantification method has proven extremely useful for estimation of overall biofilm accumulation on surfaces, including bacteria and biofilm extracellular matrix.32 In the absence of sucrose, statistically significant differences were only detected for the comparison between the Nitanium Super Elastic and NT3 SE or Copper Ni-Ti wires. The differences observed could have been associated with the composition of the coating for Nitanium Super Elastic, which was made of epoxy resin. The hydroxyl groups of epoxy resins tend to absorb water molecules that, consequently, turn the surface hydrophilic.33 The high hydrophilicity could have been the reason for the increase in background staining seen with this wire rather than staining of overall biofilm.34 It is possible, yet not likely, that this difference was attributed to increased bacterial attachment in this condition where biofilm formation was minimum, as there was no significant difference in bacterial count between these two wires (Table 2).
Conversely, for the 3% sucrose condition, significantly higher absorbance values were observed for the Niti Micro Dental White compared with the Copper Ni-Ti wires. This result was surprising because this orthodontic wire is fabricated using PFTE coating, which has been previously demonstrated to reduce biofilm formation.35,36 Despite this, potentially, under the test conditions used for this study, the surface coating material from this orthodontic wire may have slightly degraded, increasing its roughness and consequently its ability to retain biofilm.37 It is also possible that the increase in absorbance could have been a consequence of increased stain absorption by the wire coating. Although higher biofilm accumulation on this PTFE coating could indicate an increased risk to develop dental caries, future in vivo studies with these wires are needed to further assess the attraction of pathogens and biofilm accumulation differences as surface changes during placement can have significant changes in biofilm adhesion.38,39
Limitations of this study included that biofilm formation was only performed in vitro with a monospecies biofilm of S. mutans in conditions with and without sucrose. S. mutans is a commonly used model organism for bacterial adhesion/biofilm accumulation and should demonstrate differences in biofilm accumulation attributed to variations in surface characteristics of archwires, yet it does not fully model the physiological environment in the oral cavity. Another limitation was that the assays performed to measure biofilm may lack precision needed for measurement of minute differences in biofilm formation observed on small pieces of wire; however, many segments were measured to account for variability in the system.
CONCLUSIONS
Within the limitations of this study, it was possible to conclude the following:
No significant difference in bacterial adhesion (0% sucrose) or biofilm accumulation (3% sucrose) was found among the tested wires when assessed by colony counting.
Significantly increased crystal violet staining of biofilm at 3% sucrose was only observed in Niti Micro Dental White when compared with Copper Ni-Ti wires.
Clinical use of esthetic-coated orthodontic wires may be considered to have similar risks for biofilm accumulation compared with uncoated archwires.
ACKNOWLEDGMENTS
The current study was carried out with the support of the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. This research was also supported by the University of Detroit Mercy School of Dentistry. The authors of this study have no conflicts of interest to disclose.
REFERENCES
- 1.Mikulewicz M, Gronostajski Z, Wielgus A, Chojnacka K. Transparent orthodontic archwires: a systematic literature review. Arch Civ Mech Eng. 2017;17(3):651–657. [Google Scholar]
- 2.Haryani J, Ranabhatt R. Contemporary esthetic orthodontic archwires–a review. J Dent Mater Tech. 2016;5(3):125–130. [Google Scholar]
- 3.Dittmer MP, Hellemann CF, Grade S, et al. Comparative three-dimensional analysis of initial biofilm formation on three orthodontic bracket materials. Head Face Med. 2015;11(10):1–6. doi: 10.1186/s13005-015-0062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alobeid A, Dirk C, Reimann S, El-Bialy T, Jäger A, Bourauel C. Mechanical properties of different esthetic and conventional orthodontic wires in bending tests: an in vitro study. J Orofac Orthop. 2017;78(3):241–252. doi: 10.1007/s00056-016-0078-5. [DOI] [PubMed] [Google Scholar]
- 5.Rongo R, Valletta R, Bucci R, et al. In vitro biocompatibility of nickel-titanium esthetic orthodontic archwires. Angle Orthod. 2016;86(5):789–795. doi: 10.2319/100415-663.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasipek S, Senisik NE, Çetin ES. An examination of bacterial colonisation on nickel-titanium arch-wires with different surface properties. J Clin Diagnostic Res. 2019;13(6):ZC01–ZC06. [Google Scholar]
- 7.Muguruma T, Iijima M, Yuasa T, Kawaguchi K, Mizoguchi I. Characterization of the coatings covering esthetic orthodontic archwires and their influence on the bending and frictional properties. Angle Orthod. 2017;87(4):610–617. doi: 10.2319/022416-161.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi S, Park D-J, Kim K-A, Park K-H, Park H-K, Park Y-G. In vitro sliding-driven morphological changes in representative esthetic NiTi archwire surfaces. Microsc Res Tech. 2015;78(10):926–934. doi: 10.1002/jemt.22557. [DOI] [PubMed] [Google Scholar]
- 9.Mei L, Chieng J, Wong C, Benic G, Farella M. Factors affecting dental biofilm in patients wearing fixed orthodontic appliances. Prog Orthod. 2017;18(1):1–6. doi: 10.1186/s40510-016-0158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorbunkova A, Pagni G, Brizhak A, Farronato G, Rasperini G. Impact of orthodontic treatment on periodontal tissues: a narrative review of multidisciplinary literature. Int J Dent. 2016;2016:4723589. doi: 10.1155/2016/4723589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khoroushi M, Kachuie M. Prevention and treatment of white spot lesions in orthodontic patients. Contemp Clin Dent. 2017;8(1):11–19. doi: 10.4103/ccd.ccd_216_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Da Silva DL, Mattos CT, Simão RA, Ruellas ACDO. Coating stability and surface characteristics of esthetic orthodontic coated archwires. Angle Orthod. 2013;83(6):994–1001. doi: 10.2319/111112-866.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mousavi SM, Shamohammadi M, Rastegaar Z, Skini M, Rakhshan V. Effect of esthetic coating on surface roughness of orthodontic archwires. Int Orthod. 2017;15(3):312–321. doi: 10.1016/j.ortho.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Kim IH, Park HS, Kim YK, Kim KH, Kwon TY. Comparative short-term in vitro analysis of mutans streptococci adhesion on esthetic, nickel-titanium, and stainless-steel arch wires. Angle Orthod. 2014;84(4):680–686. doi: 10.2319/061713-456.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raji SH, Shojaei H, Ghorani PS, Rafiei E. Bacterial colonization on coated and uncoated orthodontic wires: a prospective clinical trial. Dent Res J. 2014;11(6):680–683. [PMC free article] [PubMed] [Google Scholar]
- 16.Tanner ACR, Sonis AL, Lif Holgerson P, et al. White-spot lesions and gingivitis microbiotas in orthodontic patients. J Dent Res. 2012;91(9):853–858. doi: 10.1177/0022034512455031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowen WH, Koo H. Biology of Streptococcus mutansderived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011;45(1):69–86. doi: 10.1159/000324598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishra S, Routray S, Kumar Sahu S, Bhusan Nanda S, Charan Sahu K. The role and efficacy of herbal antimicrobial agents in orthodontic treatment. J Clin Diagn Res. 2014. 8(6):ZC12–ZC14. [DOI] [PMC free article] [PubMed]
- 19.Lee D-H, Seo B-R, Kim H-Y, et al. Inhibitory effect of Aralia continentalis on the cariogenic properties of Streptococcus mutans. J Ethnopharmacol. 2011;137(2):979–984. doi: 10.1016/j.jep.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Ogawa A, Furukawa S, Fujita S, et al. Inhibition of Streptococcus mutans biofilm formation by Streptococcus salivarius FruA. Appl Environ Microbiol. 2011;77(5):1572–1580. doi: 10.1128/AEM.02066-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quirynen M, Marechal M, Busscher HJ, Weerkamp AH, Darius PL, van Steenberghe D. The influence of surface free energy and surface roughness on early plaque formation. An in vivo study in man. J Clin Periodontol. 1990;17(3):138–144. doi: 10.1111/j.1600-051x.1990.tb01077.x. [DOI] [PubMed] [Google Scholar]
- 22.Quirynen M, Bollen CML. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man: a review of the literature. J Clin Periodontol. 1995;22(1):1–14. doi: 10.1111/j.1600-051x.1995.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 23.Rudge P, Sherriff M, Bister D. A comparison of roughness parameters and friction coefficients of aesthetic archwires. Eur J Orthod. 2015;37(1):49–55. doi: 10.1093/ejo/cju004. [DOI] [PubMed] [Google Scholar]
- 24.Elayyan F, Silikas N, Bearn D. Ex vivo surface and mechanical properties of coated orthodontic archwires. Eur J Orthod. 2008;30(6):661–667. doi: 10.1093/ejo/cjn057. [DOI] [PubMed] [Google Scholar]
- 25.Wichelhaus A, Geserick M, Hibst R, Sander FG. The effect of surface treatment and clinical use on friction in NiTi orthodontic wires. Dent Mater. 2005;21(10):938–945. doi: 10.1016/j.dental.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Bourauel C, Fries T, Drescher D, Plietsch R. Surface roughness of orthodontic wires via atomic force microscopy, laser specular reflectance, and profilometry. Eur J Orthod. 1998;20(1):79–92. doi: 10.1093/ejo/20.1.79. [DOI] [PubMed] [Google Scholar]
- 27.Abraham KS, Jagdish N, Kailasam V, Padmanabhan S. Streptococcus mutans adhesion on nickel titanium (NiTi) and copper-NiTi archwires: a comparative prospective clinical study. Angle Orthod. 2017;87(3):448–454. doi: 10.2319/040516-270.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shamohammadi M, Hormozi E, Moradinezhad M, Moradi M, Skini M, Rakhshan V. Surface topography of plain nickel-titanium (NiTi), as-received aesthetic (coated) NiTi, and aesthetic NiTi archwires sterilized by autoclaving or glutaraldehyde immersion: a profilometry/SEM/AFM study. Int Orthod. 2019;17(1):60–72. doi: 10.1016/j.ortho.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Dokku A, Peddu R, Prakash A, Padhmanabhan J, Kalyani M, Devikanth L. Surface and mechanical properties of different coated orthodontic archwires. J Indian Orthod Soc. 2018;52:238. [Google Scholar]
- 30.Zhang M, Liu X, Shang H, Lin J. Comparison of TiN and CN coatings on orthodontic stainless steel: tribological and biological evaluation. Surf Coatings Technol. 2019;362:381–387. [Google Scholar]
- 31.Asiry MA, AlShahrani I, Almoammar S, Durgesh BH, Al Kheraif AA, Hashem MI. Influence of epoxy, polytetrafluoroethylene (PTFE) and rhodium surface coatings on surface roughness, nano-mechanical properties and biofilm adhesion of nickel titanium (Ni-Ti) archwires. Mater Res Express. 2018;5(2):026511. [Google Scholar]
- 32.Wilson C, Lukowicz R, Merchant S, et al. Quantitative and qualitative assessment methods for biofilm growth: a mini-review. Res Rev J Eng Technol. 2017;6(4):1–25. [PMC free article] [PubMed] [Google Scholar]
- 33.Han SO, Drzal LT. Water absorption effects on hydrophilic polymer matrix of carboxyl functionalized glucose resin and epoxy resin. Eur Polym J. 2003;39(9):1791–1799. [Google Scholar]
- 34.Busscher HJ, Rinastiti M, Siswomihardjo W, van der Mei HC. Biofilm formation on dental restorative and implant materials. J Dent Res. 2010;89(7):657–665. doi: 10.1177/0022034510368644. [DOI] [PubMed] [Google Scholar]
- 35.Arango S, Peláez-Vargas A, García C. Coating and surface treatments on orthodontic metallic materials. Coatings. 2013;3(1):1–15. [Google Scholar]
- 36.Demling A, Elter C, Heidenblut T, et al. Reduction of biofilm on orthodontic brackets with the use of a polytetrafluoroethylene coating. Eur J Orthod. 2010;32(4):414–418. doi: 10.1093/ejo/cjp142. [DOI] [PubMed] [Google Scholar]
- 37.Abdulkader YC, Kamaruddin AF, Mydin RBSMN. Effects of salivary pH on coating durability of two different aesthetic archwire coatings under a simulated intraoral environment. Saudi Dent J. 2020;32(6):306–313. doi: 10.1016/j.sdentj.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolff MS, Larson C. The cariogenic dental biofilm: good, bad or just something to control? Braz Oral Res. 2009;23(suppl 1):31–38. doi: 10.1590/s1806-83242009000500006. [DOI] [PubMed] [Google Scholar]
- 39.Taha M, El-Fallal A, Degla H. In vitro and in vivo biofilm adhesion to esthetic coated arch wires and its correlation with surface roughness. Angle Orthod. 2016;86(2):285–291. doi: 10.2319/122814-947.1. [DOI] [PMC free article] [PubMed] [Google Scholar]