Abstract
Objective
The current coronavirus disease 2019 pandemic has increased interest in the use of high-flow nasal cannula (HFNC) in the transport setting. The purpose of this report was to outline the clinical workflow of using HFNC in transport and the results of a retrospective chart review of patients undergoing interhospital transfer on HFNC.
Methods
We conducted a retrospective chart review of all patient transfers using HFNC between January 2018 and June 2019. The primary data abstracted from patient charts included patient demographics, transport distance, HFNC settings including flow rate in liters per minute and fraction of inspired oxygen (Fio2), and vital signs.
Results
There was a total of 220 patients, 148 pediatric and 72 adult patients. Both pediatric groups experienced statistically significant reductions in heart rate, systolic blood pressure, and diastolic blood pressure. The most common flow rate for both pediatric groups was 10 L/min and 50 L/min for adults. For pediatrics, the most common settings ranged between 30% and 50% Fio2, with the most common setting being 30% Fio2. The adult Fio2 settings ranged from 30% to 100% Fio2, with the 2 most common settings being 50% Fio2 and 80% Fio2. No patients were intubated during the transport encounter.
Conclusion
Our study provides evidence that HFNC is feasible and tolerated by patients and is an additional option for noninvasive ventilation in transport across the age continuum. Future studies are needed to compare HFNC with other noninvasive modalities that include assessing patient tolerance and comfort as contributing factors and to identify indications and contraindications for use in the transport setting.
High-flow nasal cannula (HFNC) has been used in the inpatient setting for almost 20 years with the first reported study in pediatrics in 2002.1 Initial studies focused on the use of HFNC in neonatal and pediatric patients2 , 3 and then transitioned into adult applications in the 2010s.4, 5, 6 The trend of pediatric use is also replicated in the transport literature with initial reports of feasibility of use in neonates and noninferiority to nasal cannula reported in 2014,7 , 8 with 3 additional studies reporting safety and efficacy to present day.9, 10, 11
The evidence for the effectiveness of HFNC in adult applications is mixed. In the emergency department setting, individual studies reported improved dyspnea and comfort in patients presenting with acute dyspnea and hypoxemia5 , 12 and a reduction in the need for escalation of oxygen therapy within the first 24 hours of admission.12 , 13 Three meta-analyses support that HFNC decreases the rate of intubation.12 , 14 , 15 However, evidence is mixed on the influence of the use of HFNC on clinical outcomes, with 1 review supporting a shortened length of stay14 and no difference in mortality between those receiving HFNC versus conventional oxygen therapy.14 , 15 In the intensive care unit setting, for postextubation patients, HFNC decreased reintubation and postextubation respiratory failure but had no effect on mortality.16 In postoperative patients, the use of HFNC significantly reduced hospital length of stay and provided evidence of reducing reintubation rates but had no effect on overall mortality.17 Despite unclear positive patient outcomes, treatment with HFNC does not appear to pose any significant risk to patients.6
The current coronavirus disease 2019 (COVID-19) pandemic has increased interest in the use of HFNC in the transport setting. There are multiple considerations that range from the risk of aerosol dispersion to the benefit of reducing the need for mechanical ventilation.18 , 19 To our knowledge, there are no reports of the use of HFNC in the adult population during interhospital transfer. The purpose of this report is 2-fold. First, we outline the clinical workflow of using HFNC in transport, including the setup and settings considerations. We then present the results of a retrospective chart review of patients undergoing interhospital transfer on HFNC.
Part 1: Clinical Workflow
Oxygen therapy provided through conventional means is initiated via a nasal cannula or face mask. With these therapies, oxygen is delivered up to 15 L/min, although limiting factors may include dilution of oxygen via mixing with room air and mismatched to patient flow rates.20 Although some patients require escalation of oxygen therapy through noninvasive ventilation (NIV) or mechanical ventilation, consideration for management might also include the initiation of humidified HFNC. Patients who present with acute respiratory failure and tachypnea often have increased peak inspiratory flow rates, which can exceed the oxygen flow delivered by conventional oxygen delivery systems.21 When initiating humidified HFNC therapy, an air-oxygen blend is used with a heated humidifier connected to a nasal cannula through a single-limb heated inspiratory circuit. The device is able to deliver a fraction of inspired oxygen (Fio 2) ranging from 21% to 100% with flow rates up to 60 L/min. A primary advantage when compared with conventional oxygen therapy includes the ability to adjust flow rates above the patient's maximum inspiratory flow rate, which ensures increased accuracy with regard to the Fio 2 delivered.22 In addition, humidified HFNC allows for washout of physiologic dead space while also supplementing ventilation via the creation of positive end-expiratory pressure.20 Although the positive end-expiratory pressure effect is varied and based on flow rate, breath initiated through the mouth or nose, geometry of the upper airway, and the fit of the cannula in the nostrils, mean values can range between 2.7 and 7.4 cm H20.21
Another benefit of HFNC is due to the ability to humidify and warm the gas that is administered. Administering humidified oxygen can help relieve dryness within the upper airways, preserve mucosal integrity, and facilitate secretion clearance. Warmed and humidified gas can also aid in the reduction of the work of breathing. All of this can result in improved patient comfort.
When managing patients with acute respiratory failure during critical care transportation, much consideration is placed on the selection of the delivery mechanism for supplemental oxygen therapy during transport. This is based off of multiple factors including the etiology of the respiratory failure, the severity of hypoxemia and hypercapnia, and overall patient comfort and tolerance of the intervention.22
When transitioning from conventional oxygen therapy to heated HFNC therapy, initial settings are typically a 50-L/min flow rate, 50% Fio 2, and a dew point temperature of 37°C, which is otherwise known as “50/50.” Cannula positioning is an important consideration because the prongs should sit well in the nostrils yet prevent complete occlusion. Oxygen is titrated for adequate oxygen saturation while the flow rate is adjusted to meet the patient's inspiratory flow rate demand. After the initiation of therapy, clinical status should be monitored and arterial blood gas performed within 1 to 2 hours. Indications of respiratory failure, including increased work of breathing, worsening gas exchange, and tachypnea, indicate failure of HFNC and the potential need for escalation to NIV or intubation as appropriate.
During the early stages of the COVID-19 pandemic, given the presumed aerosol-generating capabilities, many clinicians were hesitant to initiate or had strict limitations on flow when using heated HFNC during transport. However, as time progressed, it was found that the aerosol-generating effects were minimal,18 and crew safety was addressed through the use of respiratory precautions while in the patient's room and during transportation. Flow restrictions were lifted, and keen attention to detail was placed on ensuring placement of a well-fitting nasal cannula with a surgical mask to cover the patient's nose and mouth during transport. Additional COVID-19 considerations include having the patient wear a face mask while the transport crew don N95 masks and eye protection during all phases of transport. Ferno (Brendale, Queensland, Australia) offers a patient shield that attaches over the cot, which is effective in containing water droplets but may have some disadvantages related to patient comfort.
Pretransport Considerations
Before transport, the transport crew should identify the patient's response to therapy, current status and settings, and response to the initiation of humidified HFNC. Further considerations for implementation during transport include the ease of implementation and overall system management, ability to communicate, maintenance of a nasogastric or orogastric tube, minimization of skin breakdown, and overall ability to calculate oxygen use during transport. Limitations specific to NIV to consider include the inability to cooperate with a mask, anxiety due to movement during transport nausea/vomiting, agitation, and high aspiration risk among others.
The primary consideration pretransport is calculating oxygen consumption/usage with priority given to calculating both the current consumption and maximum consumption for the maximum anticipated duration of the trip between oxygen sources. Oxygen consumption calculations tend to be more reliable with HFNC than with the use of NIV or bilevel positive airway pressure because leaks around the face mask are common and patient oxygen consumption (through increases in tidal volume or respiratory rate) can be variable and rapidly change with NIV.
Specific settings for the Airvo2 (Fisher & Paykel Healthcare; Auckland, New Zealand) in junior mode ranges from 2 to 25 L/min, with a fixed dew point temperature of 34°C. The adult mode ranges from 10 to 60 L/min, with dew point options of 31°, 34°, and 37°C. As an example, oxygen consumption can be up to 3,600 L/h (60 L/min at 100% Fio 2 × 60 minutes) with the standard “M” tank capacity of 3,120 L, providing approximately 53 minutes of supply. High-capacity oxygen storage methods such as liquid oxygen (LOX) provides increased oxygen capacity. For instance, our rotor wing LOX provides 8,000 gaseous liters, whereas our mobile ICU LOX has a capacity of 17,000 gaseous liters.
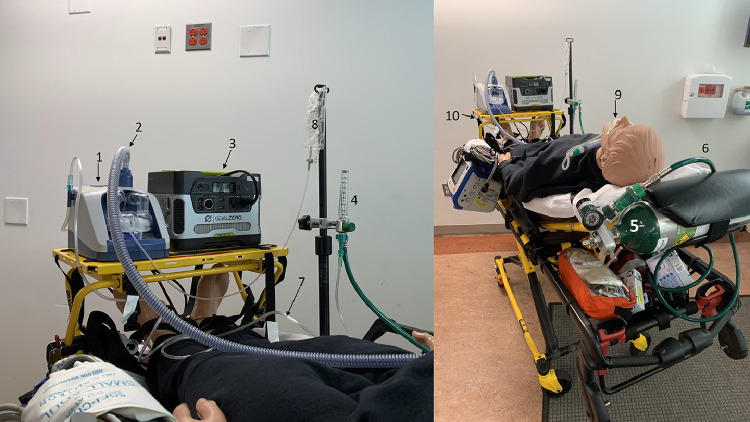
Another primary consideration is Fio 2 management based on the mode of transport. Depending on your local geography, mission planning should account for elevation changes, particularly for those programs flying rotorcraft at higher altitudes. Because the delivered Fio 2 is passively achieved by setting the oxygen flow rate, varying Fio 2 results will be achieved. For example, when mission planning for fixed wing missions, you can plan on pressurizing the cabin to lower altitudes or sea level, whereas in rotor wing aircraft flying at higher altitudes, changing cabin pressurization is not an option so rerouting may need to be considered. Although 100% Fio 2 is not always achievable depending on the pressure altitude and oxygen flow rate, the primary advantage of high flow rates beyond a nonrebreather, even in combination with a nasal cannula, provide the greatest benefit. Table 1 provides a list of necessary equipment, and Figure 1 displays a typical setup of the equipment on the stretcher. Additional resources for preplanning can be accessed via an app provided by the manufacturer that includes a simulation platform, a quick reference setup, and a troubleshooting guide.
Table 1.
The Minimum Equipment Necessary to Use Stand-alone High-Flow Nasal Cannula
|
The numbers in the table correspond with the numbered labels in Figure 1A and B.
Figure 1.
(A and B) HFNC equipment setup on a stretcher. The numbers in the figure correspond with Table 1. This is the minimum equipment necessary to use stand-alone HFNC.
En Route Considerations
The primary considerations during transport are specifically related to resource management. Like many devices not originally intended for use during transport, power management can be complicated. The Airvo2 does not have an integrated power supply and thus relies on a “pluggable” power source. We use the Goal Zero Yeti 400 (Goal Zero; South Bluffdale, UT) as an external power source when transitioning between clinical units and the transport vehicle that warrants the following considerations:
-
•
Setting the Airov2 dew point temperature at the highest setting can result in a power consumption rate that is greater than the battery recharge rate. This can reduce the overall life of the battery by limiting the effectiveness of the battery charger even though it is plugged in during transport. However, power consumption can be reduced by decreasing the dew point temperature to the lowest setting.
-
•
The dew point target may not be achieved based on ambient temperatures, particularly if you cannot maintain the highest dew point temperature setting due to power consumption considerations (see Table 1 for maximum battery life).
Post-transport Considerations
After use, the unit requires cleaning and a sanitation cycle that takes 55 minutes to complete. This may be completed during the return trip to the base depending on the drive time and/or ability to maintain power supply if the trip is shorter than the cycle time. We also have additional units at the base that can be swapped out if needing to complete another transfer.
Part 2: Clinical Results
Methods
We conducted a retrospective chart review of all patient transfers using HFNC between January 2018 and June 2019 before the initial appearance of COVID-19 in the United States. Electronic health record data were abstracted by trained data collectors using a standardized data collection sheet. Data collectors completed initial training using the data collection sheet and codebook, with the first 5 charts of each abstractor double reviewed and assessed for discrepancies. Identified discrepancies were adjudicated between the 2 reviewers for agreement. Then, random chart audits were conducted to assess data abstraction fidelity. The primary data abstracted from patient charts included patient demographics, transport distance, HFNC settings including flow rate in L/min and Fio 2, and the first and last set of vital signs. Measures of central tendency are reported for patient demographics and HFNC settings. A review of the histogram and analysis via the Shapiro-Wilk test indicated that the vital sign data were non-normally distributed; therefore, we used the Wilcoxon matched pairs signed rank test to assess for differences between pre- and post-transport vital signs. We compared the first set of vital signs obtained by the transport crew before transport and the last set of vital signs obtained at the transfer of care. A P value < .05 was considered statistically significant. All analyses were performed using R studio 1.4.1 (RStudio; Boston, MA). This study was approved by the Cleveland Clinic Institutional Review Board (#14-1556).
Results
There was a total of 220 patients, 148 pediatric and 72 adult patients. Table 2 presents the demographics and summary statistics by group. The majority of patients in all groups were male. Adult patients tended to be older with a mean age of 63 years and with greater transport distances. Table 3 presents the pre- and post-transport vital sign comparisons, with the only significant difference in adults being an improvement in oxygen saturations via pulse oximetry measurement. There was a trend toward improvement, or lowering in systolic blood pressure, but this difference was not statistically significant. Both pediatric groups experienced statistically significant reductions in heart rate, systolic blood pressure, and diastolic blood pressure. Among the pediatric groups, both respiratory rate and pulse oximetry exhibited the smallest change between pre- and post-transport and were not statistically significant. No patients were intubated during the transport encounter.
Table 2.
Patient Characteristics by Group
| Pediatric |
Adult | ||
|---|---|---|---|
| < 12 Months | 1-18 Years | >18 Years | |
| Total sample | 70 | 74 | 71 |
| Age mean (SD) | 4.7 (3.3) | 3.3 (3.4) | 63 (14.3) |
| Range | 0-11 | 1-14 | 25-88 |
| Male n (%) | 48 (69) | 42 (57) | 40 (56) |
| Transport distance | 12.9 | 12.2 | 32.7 |
| Miles (range) | (4.4-58.5) | (3-20.6) | (1-176) |
SD = standard deviation.
Table 3.
Pre- and Post-transport Vital Signs
| Pediatric |
Adult |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| < 12 Months |
1-18 Years |
> 18 Years |
|||||||
| Transport Phase | Pre | Post | P Value(95% CI) | Pre | Post | P Value(95% CI) | Pre | Post | P Value(95% CI) |
| Heart rate | |||||||||
| Mean (SD) | 153 (24) | 142 (20) | <.001 (−13.9 to −5.0) |
155 (55) | 148 (22) | <.001 (12.5 to −4.9) |
93 (20) | 93 (17) | .91 (−1.9 to 1.5) |
| Systolic BP | |||||||||
| Mean (SD) | 105 (15) | 102 (16) | .02 (−5.9 to −0.5) |
106 (15) | 101 (17) | .001 (−9.9 to −2.5) |
128 (22) | 126 (21) | .58 (−5.0 to 2.5) |
| Diastolic BP | |||||||||
| Mean (SD) | 65 (12) | 60 (11) | .002 (−8.9 to −2.0) |
65 (11) | 60 (12) | .004 (−8.0 to −1.5) |
76 (13) | 73 (12) | .08 (−4.5 to 0.49) |
| Respiratory rate | |||||||||
| Mean (SD) | 39 (12) | 40 (12) | .19 (−0.5 to 3.5) |
41 (14) | 39 (10) | .20 (−4.0 to 1.0) |
22 (6) | 23 (6) | .99 (−1.5 to 1.5) |
| Pulse oximetry | |||||||||
| Mean (SD) | 97 (6) | 97 (6) | .26 (−.0003 to 1.5) |
97 (4) | 98 (3) | .30 (−0.5 to 1.5) |
94 (5) | 95 (4) | .04 (.0004–1.5) |
BP = blood pressure; CI = confidence interval; SD = standard deviation.
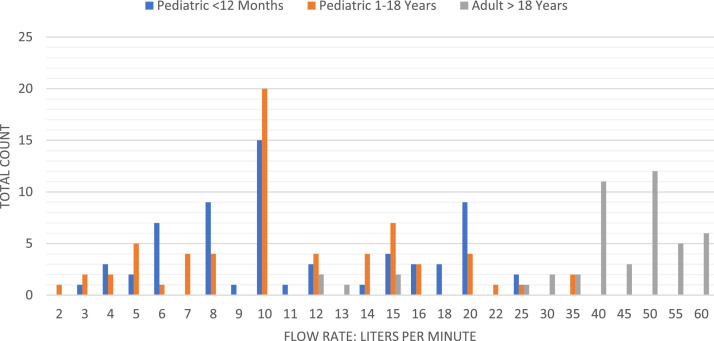
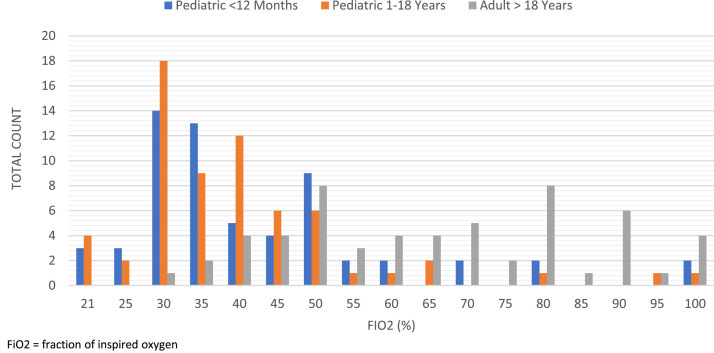
Figure 2 presents the flow rates by patient subgroup. Both pediatric groups tended toward lower flow rates. The most common flow rate for both pediatric groups was 10 L/min. Alternatively, the adult population tended toward higher flow rates, with the most common setting of 50 L/min. Figure 3 presents the Fio 2. Although both pediatric patient groups covered the entire range from 21% to 100% Fio 2, the most common settings ranged between 30% to 50% Fio 2, with the most common setting being 30% Fio 2. The adult Fio 2 settings ranged from 30% to 100% Fio 2, with the 2 most common settings being 50% Fio 2 and 80% Fio 2.
Figure 2.
HFNC Flow Rates
Figure 3.
HFNC Fio2
Discussion
We set out to describe the use of HFNC in the transport setting across the age continuum. Our results indicate that the use of HFNC is feasible and provided evidence of safe transport with statistically significant improvement in pre- and post-transport heart rate and blood pressure in both pediatric groups, a statistically significant increase in adult pulse oximetry although not clinically significant, and no evidence of physiologic decompensation.
The results from this study provide the first report of settings used in the transport setting in adult patients.8 , 11 Adult Fio 2 settings most often reflected the 50/50 manufacturer-recommended starting settings, or 50% Fio 2 and 50 L/min. There was limited variation in settings between pediatric groups. While not a direct comparison to Muniyappa et al.9 as their patient population was specifically neonates, the <12 months pediatric group initial FiO2 settings averaged between 30-40%, however our average flow rate was higher at 10 lpm versus their 3.8 lpm. Benefits of using HFNC on low settings instead of a nasal cannula in pediatric patients are supported by the primary mechanisms of action that decrease nasopharyngeal resistance, wash out pulmonary dead space, reduce inflow of ambient air, and increase airway pressure. Thus, recent evidence supports using HFNC with low Fio 2 settings in pediatric patients as an effective treatment in cases of moderate to severe bronchiolitis during initial low-flow oxygen failure states and as initial noninvasive respiratory support after birth.23 Together, these data provide insight on the use of HFNC in transport.
There are several other considerations for the use of HFNC in the transport setting. The first is the potential cost of acquiring the equipment and additional costs in consumables for each trip. Supply cost depends on whether you can continue the patient from the referring provider's setup or need a new setup; the costs can be significant compared with other ventilation support modalities. Furthermore, the HFNC setup and use are more cumbersome than using a transport ventilator that is confined to 1 package with internal battery power. Conversely, our crews have reported antidotally that patients tolerated HFNC well during transport. Additionally, crews noted improved tolerance when patients were changed to HFNC from another NIV method, including when they have used NIV methods via the transport ventilator. The provided reasons for increased patient comfort and tolerance included temperature-controlled humidified air delivery, a nasal cannula instead of a face mask, and pressure supplied via the unit compared to a transport ventilator administering bilevel positive pressure compared to a beside unit. Although these are only anecdotal reports and not specifically measured in this study, future studies should investigate the subjective experience of both patients and transport personnel on the use and tolerance of HFNC in the transport setting compared with other NIV modalities because this may identify additional benefits to the implementation of HFNC in the transport environment.
There were several limitations to our work. First, this was a single health system transport team's experience and may not be externalized to other settings. Second, there could have been unmeasured factors that may have accounted for the pre- to post-transport difference in vital signs that were not accounted for in the matched pairs analysis such as medication administration. This was only a descriptive secondary review; future work should assess for the presence and impact of other interventions during HFNC use. Lastly, we did not compare HFNC with other methods of NIV. Future studies are necessary to assess for the equivalence or noninferiority/superiority of HFNC in transport.
Conclusion
Our study provides evidence that HFNC is feasible and tolerated by patients and is an additional option for NIV in transport across the age continuum. Future studies are needed to compare HFNC with other noninvasive modalities that include assessing patient tolerance and comfort as contributing factors and to identify indications and contraindications for use in the transport setting.
References
- 1.Dutta S. High-flow nasal cannula versus nasal continuous positive airway pressure in the management of apnea of prematurity. Pediatrics. 2002;109:718–719. doi: 10.1542/peds.109.4.718. author reply 718-719. [DOI] [PubMed] [Google Scholar]
- 2.Saslow JG, Aghai ZH, Nakhla TA, et al. Work of breathing using high-flow nasal cannula in preterm infants. J Perinatol. 2006;26:476–480. doi: 10.1038/sj.jp.7211530. [DOI] [PubMed] [Google Scholar]
- 3.Shoemaker MT, Pierce MR, Yoder BA, DiGeronimo RJ. High flow nasal cannula versus nasal CPAP for neonatal respiratory disease: a retrospective study. J Perinatol. 2007;27:85–91. doi: 10.1038/sj.jp.7211647. [DOI] [PubMed] [Google Scholar]
- 4.Roca O, Perez-Teran P, Masclans JR, et al. Patients with New York Heart Association class III heart failure may benefit with high flow nasal cannula supportive therapy: high flow nasal cannula in heart failure. J Crit Care. 2013;28:741–746. doi: 10.1016/j.jcrc.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Rittayamai N, Tscheikuna J, Praphruetkit N, Kijpinyochai S. Use of high-flow nasal cannula for acute dyspnea and hypoxemia in the emergency department. Respir Care. 2015;60:1377–1382. doi: 10.4187/respcare.03837. [DOI] [PubMed] [Google Scholar]
- 6.Rochwerg B, Granton D, Wang DX, et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Med. 2019;45:563–572. doi: 10.1007/s00134-019-05590-5. [DOI] [PubMed] [Google Scholar]
- 7.Boyle MA, Dhar A, Broster S. Introducing high-flow nasal cannula to the neonatal transport environment. Acta Paediatr. 2017;106:1363. doi: 10.1111/apa.13910. [DOI] [PubMed] [Google Scholar]
- 8.Schlapbach LJ, Schaefer J, Brady AM, Mayfield S, Schibler A. High-flow nasal cannula (HFNC) support in interhospital transport of critically ill children. Intensive Care Med. 2014;40:592–599. doi: 10.1007/s00134-014-3226-7. [DOI] [PubMed] [Google Scholar]
- 9.Muniyappa B, Honey G, Yoder BA. Efficacy and safety of nasal high-flow therapy for neonatal transport. Air Med J. 2019;38:298–301. doi: 10.1016/j.amj.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Cheema B, Welzel T, Rossouw B. Noninvasive ventilation during pediatric and neonatal critical care transport: a systematic review. Pediatr Crit Care Med. 2019;20:9–18. doi: 10.1097/PCC.0000000000001781. [DOI] [PubMed] [Google Scholar]
- 11.Holbird S, Holt T, Shaw A, Hansen G. Noninvasive ventilation for pediatric interfacility transports: a retrospective study. World J Pediatr. 2020;16:422–425. doi: 10.1007/s12519-020-00363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang CC, Lan HM, Li CJ, et al. Use high-flow nasal cannula for acute respiratory failure patients in the emergency department: a meta-analysis study. Emerg Med Int. 2019;2019 doi: 10.1155/2019/2130935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones PG, Kamona S, Doran O, Sawtell F, Wilsher M. Randomized controlled trial of humidified high-flow nasal oxygen for acute respiratory distress in the emergency department: the HOT-ER study. Respir Care. 2016;61:291–299. doi: 10.4187/respcare.04252. [DOI] [PubMed] [Google Scholar]
- 14.Wang K, Zhao W, Li J, Shu W, Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10:37. doi: 10.1186/s13613-020-00653-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortegiani A, Crimi C, Sanfilippo F, et al. High flow nasal therapy in immunocompromised patients with acute respiratory failure: a systematic review and meta-analysis. J Crit Care. 2019;50:250–256. doi: 10.1016/j.jcrc.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Granton D, Chaudhuri D, Wang D, et al. High-flow nasal cannula compared with conventional oxygen therapy or noninvasive ventilation immediately postextubation: a systematic review and meta-analysis. Crit Care Med. 2020;48:e1129–e1136. doi: 10.1097/CCM.0000000000004576. [DOI] [PubMed] [Google Scholar]
- 17.Lu Z, Chang W, Meng S, et al. The effect of high-flow nasal oxygen therapy on postoperative pulmonary complications and hospital length of stay in postoperative patients: a systematic review and meta-analysis. J Intensive Care Med. 2020;35:1129–1140. doi: 10.1177/0885066618817718. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal A, Basmaji J, Muttalib F, et al. High-flow nasal cannula for acute hypoxemic respiratory failure in patients with COVID-19: systematic reviews of effectiveness and its risks of aerosolization, dispersion, and infection transmission. Can J Anaesth. 2020;67:1217–1248. doi: 10.1007/s12630-020-01740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Fink JB, Ehrmann S. High-flow nasal cannula for COVID-19 patients: risk of bio-aerosol dispersion. Eur Respir J. 2020;56 doi: 10.1183/13993003.03136-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee CC, Mankodi D, Shaharyar S, et al. High flow nasal cannula versus conventional oxygen therapy and non-invasive ventilation in adults with acute hypoxemic respiratory failure: a systematic review. Respir Med. 2016;121:100–108. doi: 10.1016/j.rmed.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Renda T, Corrado A, Iskandar G, Pelaia G, Abdalla K, Navalesi P. High-flow nasal oxygen therapy in intensive care and anaesthesia. Br J Anaesth. 2018;120:18–27. doi: 10.1016/j.bja.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Ischaki E, Pantazopoulos I, Zakynthinos S. Nasal high flow therapy: a novel treatment rather than a more expensive oxygen device. Eur Respir Rev. 2017;26 doi: 10.1183/16000617.0028-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon JW. High-flow nasal cannula oxygen therapy in children: a clinical review. Clin Exp Pediatr. 2020;63:3–7. doi: 10.3345/kjp.2019.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]