Abstract
目的
检测咳嗽变异性哮喘(cough variant asthma,CVA)患儿外周血中微小RNA-138(microRNA-138,miR-138)、Runt相关转录因子3(Runt-related transcription factor 3,RUNX3)的表达水平及其对Th1/Th2平衡的调节作用。
方法
选取CVA患儿65例纳入CVA组,健康儿童30例纳入健康对照组,采集两组儿童外周静脉血,分离培养CD4+T细胞。ELISA法检测CD4+T细胞分泌的干扰素-γ(interferon-γ,IFN-γ)、白细胞介素(interleukin,IL)-2、IL-4、IL-5和IL-13的水平;流式细胞仪检测Th1、Th2细胞比例;实时荧光定量PCR检测CD4+T细胞中RUNX3 mRNA水平和外周血中miR-138水平;Western blot检测CD4+T细胞中RUNX3蛋白表达水平。双荧光素酶报告基因实验检测miR-138和RUNX3的靶向作用;RUNX3-mimic质粒转染至CD4+T细胞中,检测增加RUNX3的表达水平对IFN-γ、IL-2、IL-4、IL-5、IL-13水平和Th1、Th2细胞比例的影响。
结果
CVA组与健康对照组相比,CD4+T细胞分泌IFN-γ、IL-2水平降低,IL-4、IL-5和IL-13水平增加,Th1细胞比例和Th1/Th2比值降低,Th2细胞比例增加(P<0.05)。CVA组外周血中CD4+T细胞RUNX3 mRNA及其蛋白相对表达水平低于健康对照组(P<0.001),miR-138的相对表达水平高于健康对照组(P<0.001)。miR-138可靶向调节RUNX3的表达。CD4+T细胞中RUNX3的表达增加后,IFN-γ和IL-2水平升高,IL-4、IL-5和IL-13水平降低,Th1细胞比例和Th1/Th2比值增加,Th2细胞比例降低(P<0.05)。
结论
miR-138通过靶向RUNX3调节CVA患儿Th1/Th2平衡,为CVA的治疗提供了新方向。
Keywords: 咳嗽变异性哮喘, 微小RNA-138, Runt相关转录因子3, Th1/Th2平衡, 儿童
Abstract
Objective
To study the expression levels of microRNA-138 (miR-138) and Runt-related transcription factor 3 (RUNX3) in peripheral blood of children with cough variant asthma (CVA) and their regulatory effects on Th1/Th2 balance.
Methods
Sixty-five children with CVA (CVA group) and 30 healthy children (control group) were enrolled. Peripheral venous blood samples were collected for both groups, and CD4+ T cells were isolated and cultured. Enzyme-linked immunosorbent assay was used to measure the levels of interferon (IFN)-γ, interleukin (IL)-2, IL-4, IL-5, and IL-13 that were secreted by CD4+ T cells. Flow cytometry was used to determine the percentages of Th1 and Th2 cells. Quantitative real-time PCR was used to measure the level of RUNX3 mRNA in CD4+ T cells and the level of miR-138 in peripheral blood. Western blot was used to determine the protein expression of RUNX3 in CD4+ T cells. The dual-luciferase reporter assay was used to determine the targeting effects of miR-138 and RUNX3. The RUNX3-mimic plasmid was transfected into CD4+ T cells, and the effects on the levels of IFN-γ, IL-2, IL-4, IL-5, and IL-13 and the percentages of Th1 and Th2 cells were measured.
Results
Compared with the control group, the CVA group showed significantly decreased levels of IFN-γ and IL-2 from CD4+ T cells, significantly increased levels of IL-4, IL-5, and IL-13 from CD4+ T cells, significantly decreased Th1 cell percentage and Th1/Th2 ratio, and a significantly increased Th2 cell percentage (P<0.05). The CVA group showed significantly lower relative expression levels of RUNX3 mRNA and protein in CD4+ T cells in peripheral blood than the control group (P<0.001). The relative expression level of miR-138 was significantly higher in the CVA group than in the control group (P<0.001). MiR-138 could target the expression of RUNX3. Upregulating the expression of RUNX3 in CD4+ T cells induced significantly increased levels of IFN-γ and IL-2, significantly decreased levels of IL-4, IL-5, and IL-13, significantly increased Th1 cell percentage and Th1/Th2 ratio, and a significantly decreased Th2 cell percentage (P<0.05).
Conclusions
MiR-138 regulates Th1/Th2 balance by targeting RUNX3 in children with CVA, providing a new direction for the treatment of CVA.
Keywords: Cough variant asthma, MicroRNA-138, Runt-related transcription factor 3, Th1/Th2 balance, Child
咳嗽变异性哮喘(cough variant asthma,CVA)是一种特殊类型的哮喘,临床上以咳嗽为主要症状甚至是唯一症状。在学龄前期和学龄期儿童中常见,以反复发作和迁延不愈为特点。因此探索CVA的发病机制,合理治疗对避免CVA发展为典型哮喘具有一定帮助。近年来,T辅助细胞反应的不平衡一直受到关注,T辅助细胞分为两大类,Th1和Th2[1]。Th1细胞主要分泌白细胞介素(interleukin,IL)-2、干扰素-γ(interferon-γ,IFN-γ)和肿瘤坏死因子(tumor necrosis factor,TNF),它们参与细胞感染的防御机制[2]。Th2细胞主要分泌的细胞因子有IL-4、IL-5、IL-6、IL-9、IL-10和IL-13,参与过敏性炎症反应[3]。然而,在哮喘患者中观察到Th1/Th2失衡,Th2过度反应使其分泌的细胞因子增多,引发一连串免疫激活反应,导致IgE产生增加,促进嗜酸性粒细胞的生长和分化及黏液分泌增加,诱发气道炎症[4]。有研究显示,叶乙醇提取物通过抑制过敏性哮喘小鼠模型中的核转录因子-κB信号转导和组胺分泌,调节Th1/Th2失衡,从而减轻小鼠气道炎症反应[5]。间充质干细胞条件培养基通过调节CD4+T细胞向Th1/Th2效应细胞分化,可缓解卵清蛋白诱导的小鼠哮喘反应[6]。这些研究表明调节Th1/Th2 平衡可能是治疗CVA的一种新颖策略。
Runt相关转录因子3(Runt-related transcrip-tion factor 3,RUNX3)由高度保守且结构相似的RUNX基因家族编码,分布于外周血和免疫系统中,RUNX3蛋白表达失衡与多种疾病有关[7-9]。RUNX3在T细胞发育中具有关键功能,参与T辅助细胞分化,具有增强IFN-γ分泌和抑制IL-4分泌的双重作用,还可作用于T细胞的分化,影响Th1/Th2的平衡[10]。因此RUNX3对Th1/Th2平衡具有一定调节作用,有可能成为治疗哮喘的潜在靶点。微小RNA (microRNA,miR)是18~22个核苷酸组成的非编码RNA,是基因表达的微妙调控因子,在多种疾病中发挥作用,尤其是在慢性多因素疾病如哮喘[11]。有研究显示,miR-138在哮喘中可调节树突状细胞介导的Th2型免疫反应,参与哮喘的发病[12]。另有研究显示在银屑病中miR-138可通过靶向调节RUNX3的表达影响Th1/Th2的平衡[13]。因此本文探讨miR-138和RUNX3对CVA Th1/Th2平衡的影响,并进一步分析二者间是否具有靶向作用,为CVA的临床治疗提供方向。
1. 资料与方法
1.1. 一般资料
前瞻性选取2019年1月至2021年1月在我院接受治疗的CVA患儿65例纳入CVA组,其中男35例,女30例,年龄(8.7±2.6)岁。纳入标准:(1)CVA患儿的诊断符合《中国儿童慢性咳嗽诊断与治疗指南(2013年修订)》[14];(2)均为首次确诊患儿。排除标准:(1)合并肺结核或肺部感染;(2)合并免疫性疾病或过敏性疾病;(3)近期服用过免疫抑制剂或抗生素;(4)合并血液系统疾病;(5)合并肿瘤。同期选取健康受试者30例纳入健康对照组,排除近3个月有呼吸道感染史或有哮喘史的儿童,其中男18例,女12例,年龄(7.3±2.0)岁。两组儿童性别构成比和年龄比较差异无统计学意义(P>0.05),具有可比性。本研究经过医院伦理委员会批准(20181576),家属知情同意。
1.2. 外周血中CD4+T细胞的分离培养
收集CVA组和健康对照组儿童外周静脉血样本,乙二胺四乙酸抗凝后保存待用。通过Ficoll分离液溶液(北京索莱宝科技有限公司)梯度离心分离外周血单核细胞(peripheral blood mononuclear cells,PBMC),利用RPMI培养基洗涤并重悬于RPMI培养基中。然后将PBMC与抗CD4+单克隆抗体的磁珠包被,分离CD4+T细胞,采用完全T细胞培养基(RPMI1640培养基,10%胎牛血清,1%青霉素-链霉素,50 μmol/L β-巯基乙醇,100 U/mL IL-2)培养CD4+T细胞。
1.3. ELISA法检测IFN-γ、IL-2、IL-4、IL-5和IL-13水平
收集CVA组和健康对照组儿童CD4+T细胞的培养上清液,根据ELISA试剂盒说明书检测IFN-γ、IL-2、IL-4、IL-5和IL-13的浓度。
1.4. 流式细胞仪检测Th1和Th2细胞所占比例
收集CVA组和健康对照组CD4+T细胞,加入10 μL IFN-γ或IL-4细胞因子,促进T细胞向Th1细胞和Th2细胞分化,放置恒温箱中孵育4 h。加入10 μL CD3-PC5和CD8-PC7单克隆抗体,室温避光孵育15 min。加入固定液,再次放置15 min。加入3 mL PBS溶液,3 000 r/min离心5 min,弃上清。加入10 μL IL-4-藻红蛋白单克隆抗体、IFN-γ-异硫氰酸荧光素单克隆抗体避光孵育15 min。各管加入3 mL PBS,3 000 r/min 离心5 min,弃上清。0.5 mL PBS溶液重悬细胞,流式细胞仪检测Th1、Th2细胞百分比。
1.5. 实时荧光定量PCR检测CD4+T细胞中RUNX3 mRNA的表达水平
利用TRIzol试剂(上海联迈生物工程有限公司)提取CD4+T细胞的总RNA。根据TaKaRa RNA反转录试剂盒(宝生物工程大连有限公司)说明书将总RNA反转录为cDNA,反转录条件:37℃ 15 min,85℃ 5 s。以cDNA为模板,RUNX3上游引物序列5'-UACCGACCGAGCACAUCCAU-3',下游引物序列5'-AGCCGCGAGGACUCGAUCCU-3';以管家基因U6 mRNA水平作为内参照,U6上游引物序列5'-UCAAGCACGAUCAUGAU-3',下游引物序列5'-GUCAAGUUAGGUCGGUA-3'。按照TaKaRa实时荧光定量PCR试剂盒(宝生物工程大连有限公司)说明书进行PCR扩增。PCR扩增反应条件:95℃预变性5 min;95℃变性30 s,60℃退火30 s,72℃延伸30 s,共40个循环。采用2-ΔΔCt法计算目的基因相对表达量。
1.6. Western blot检测CD4+T细胞中RUNX3蛋白表达水平
提取CD4+T细胞的总蛋白,经BCA蛋白定量,取30 μg蛋白,经SDS-PAGE分离。将蛋白电泳产物转印至PVDF膜上。1%BSA室温封闭2 h,加入anti-RUNX3或anti-β-actin抗体(1∶1 000稀释),4℃孵育过夜。次日,加入相对应的二抗(1∶4 000稀释),室温孵育1 h。滴加化学发光剂,在Amersham Imager 600凝胶成像系统中拍照曝光。RUNX3蛋白表达水平以RUNX3蛋白灰度值/β-actin蛋白灰度值表示。
1.7. 双荧光素酶报告基因实验
Starbase数据库显示,miR-138基因序列中GUGGUCG碱基与RUNX3 3'UTR野生型(wild type,WT)基因序列中CACCAGC碱基配对,形成沉默复合体,阻碍RUNX3基因转录翻译,降低RUNX3蛋白表达水平;RUNX3 3'UTR突变型(mutation type,MUT)基因序列中与miR-138基因序列互补配对的碱基突变为CGUAUCG(图1)。根据RUNX3突变位点设计WT和MUT荧光素酶报告载体,分别设置RUNX3 WT组、RUNX3 WT+miR-138 mimic组、RUNX3 MUT组和RUNX3 MUT+miR-138 mimic组,各组对应加入RUNX3 WT荧光素酶报告载体、RUNX3 WT荧光素酶报告载体+miR-138 mimic、RUNX3 MUT荧光素酶报告载体、RUNX3 MUT荧光素酶报告载体+miR-138 mimic。采用LipofectamineTM 2000转染试剂辅助共转染至CD4+T细胞中,转染48 h后,PBS清洗1次,每孔加入100 μL裂解液,室温震荡10 min,收集细胞裂解液。每组取20 μL细胞裂解液与10 μL荧光素酶检测试剂快速混匀后,用生物发光检测仪检测各组荧光值。
图1. miR-138靶向调节RUNX3 .

1.8. RUNX3-mimic质粒转染CVA组CD4+T细胞
将CD4+T细胞接种于6孔板中,每孔40万个细胞,培养24 h后,随机分为对照组、NC-mimic组和RUNX3-mimic组,每组设3个复孔,参照Lipofectamine 2000说明书,NC-mimic组和RUNX3-mimic组分别转染NC-mimic(对照质粒)、RUNX3-mimic质粒,对照组不予转染(质粒均由湖南丰晖生物科技有限公司合成)。转染6 h后,更换新的培养基,继续培养48 h,收集各组细胞,采用Western blot检测转染效率。然后检测对照组、NC-mimic和RUNX3-mimic组间IFN-γ、IL-2、IL-4、IL-5、IL-13水平及Th1、Th2细胞比例。
1.9. 统计学分析
采用SPSS 21.0统计软件对数据进行统计分析,计量资料以均数±标准差()表示,两组间比较采用两样本t检验;多组间比较采用单因素方差分析,组间两两比较采用LSD-t检验。P<0.05为差异有统计学意义。
2. 结果
2.1. 两组间Th1、Th2细胞比例及其分泌主要细胞因子水平变化
CVA组与健康对照组相比,Th1分泌的主要细胞因子IFN-γ和IL-2水平降低,Th2分泌的主要细胞因子IL-4、IL-5和IL-13水平增加(P<0.001)。CVA组Th1细胞比例和Th1/Th2比值低于健康对照组,Th2细胞比例高于健康对照组(P<0.05)。见表1。
表1.
两组间Th1、Th2细胞比例及其分泌主要细胞因子水平比较 ()
| 组别 | n | IFN-γ (ng/L) | IL-2 (ng/L) | IL-4 (ng/L) | IL-5 (ng/L) | IL-13 (ng/L) | Th1 (%) | Th2 (%) | Th1/Th2 |
|---|---|---|---|---|---|---|---|---|---|
| 健康对照组 | 30 | 564±62 | 305±32 | 136±14 | 126±16 | 176±15 | 8.4±1.6 | 1.5±0.4 | 4.2±1.2 |
| CVA组 | 65 | 322±24 | 174±21 | 252±18 | 284±21 | 326±28 | 3.8±0.5 | 2.3±0.8 | 1.2±0.3 |
| t值 | 23.652 | 32.145 | 15.214 | 18.325 | 20.625 | 8.201 | 3.652 | 4.263 | |
| P值 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.026 | 0.005 |
注:[IFN-γ]干扰素-γ;[IL]白细胞介素。
2.2. 两组外周血CD4+T细胞RUNX3 mRNA及其蛋白表达水平变化
CVA组外周血CD4+T细胞RUNX3 mRNA相对表达水平为0.42±0.10,低于健康对照组(3.14±0.16,t=8.251,P<0.001)。CVA组外周血中CD4+T细胞RUNX3蛋白相对表达水平为0.14±0.03,低于健康对照组(1.16±0.12,t=5.965,P<0.001),见图2。
图2. Western blot检测两组外周血CD4+T细胞RUNX3蛋白表达电泳图.

2.3. CVA组miR-138的表达水平及与RUNX3的靶向调节作用
CVA组miR-138的相对表达水平为2.35±0.21,高于健康对照组(0.41±0.07,t=6.458,P<0.001)。RUNX3 WT组、RUNX3 WT+miR-138 mimic组、RUNX3 MUT组和RUNX3 MUT+miR-138 mimic组间相对荧光素酶活性分别为1.02±0.23、0.42±0.09、1.10±0.12、1.07±0.18,4组比较差异有统计学意义(F=9.251,P<0.001)。RUNX3 WT+miR-138 mimic组相对荧光素酶活性低于RUNX3 WT组(P=0.008),而RUNX3 MUT组和RUNX3 MUT+miR-138 mimic组相对荧光素酶活性与RUNX3 WT组相比差异无统计学意义(P>0.05)。
2.4. 增加CD4+T细胞中RUNX3的表达对Th1/Th2及其分泌的主要细胞因子的影响
对照组、NC-mimic组和RUNX3-mimic组的RUNX3蛋白灰度值分别为0.56±0.12、0.62±0.14和1.52±0.17,三组比较差异有统计学意义(F=8.634,P<0.001),且RUNX3-mimic组RUNX3蛋白灰度值高于对照组和NC-mimic组(P<0.001)(图3)。与对照组和NC-mimic组相比,RUNX3-mimic组中IFN-γ和IL-2水平升高,IL-4、IL-5和IL-13水平降低,Th1细胞比例和Th1/Th2比值增加,Th2比值细胞比例降低(P<0.05),见表2。
图3. Western blot法检测各组CD4+T细胞中RUNX3蛋白表达电泳图.
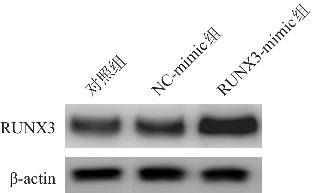
表2.
增加CD4+T细胞中RUNX3的表达对Th1/Th2及其分泌主要因子的影响 (n=3,)
| 组别 | IFN-γ (mg/L) | IL-2 (mg/L) | IL-4 (mg/L) | IL-5 (mg/L) | IL-13 (mg/L) | Th1 (%) | Th2 (%) | Th1/Th2 |
|---|---|---|---|---|---|---|---|---|
| 对照组 | 307±17 | 186±15 | 236±18 | 282±15 | 275±17 | 3.2±0.8 | 2.1±0.4 | 1.2±0.5 |
| NC-mimic组 | 315±19 | 180±18 | 248±16 | 276±22 | 270±18 | 3.5±0.9 | 2.3±0.6 | 1.2±0.4 |
| RUNX3-mimic组 | 378±25a | 284±19a | 124±14a | 140±19a | 147±13a | 7.3±1.8a | 1.7±0.4a | 3.8±1.0a |
| F值 | 11.251 | 15.654 | 16.632 | 17.625 | 18.142 | 6.625 | 3.854 | 4.210 |
| P值 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.010 | 0.006 |
注:[IFN-γ]干扰素-γ;[IL]白细胞介素。a示与对照组和NC-mimic组相比,P<0.05。
3. 讨论
众所周知,Th1/Th2细胞失衡及其分泌的细胞因子与哮喘的发生密切相关。在生理条件下,Th1和Th2细胞之间存在动态平衡,当这种平衡被打破时,会诱发哮喘发生[15]。Th2细胞主要产生IL-4、IL-5和IL-13,它们可增加血清中的IgE水平并募集嗜酸性粒细胞,诱导气道高反应[16]。Th1细胞分泌IFN-γ和IL-2可拮抗Th2诱导的免疫反应并抑制哮喘的发展[17]。本研究发现CVA组的IL-2和IFN-γ水平低于健康对照组,而IL-4、IL-5和IL-13水平高于健康对照组,表明CVA患儿存在Th1/Th2细胞失衡。通过提高Th1细胞水平同时抑制Th2细胞水平以恢复Th1/Th2平衡,应该是治疗哮喘的有效策略。已有研究显示,调节Th1/Th2细胞因子失衡可以减轻哮喘的炎症反应[15-18]。目前调节Th1/Th2平衡的主要为化学药物制剂,在本研究中主要探讨miRNA对Th1/Th2平衡的调节作用。因为miRNAs具有独特的特性,且易于合成和操作,在治疗疾病方面具有一定优势,另外其在哮喘中起着不可或缺的作用。
本研究中,CVA组miR-138的表达水平高于健康对照组,推测其可能参与哮喘的发病。以往有研究显示在银屑病中,miR-138可靶向调节RUNX3,缓解Th1/Th2的失衡[13]。在本研究中通过荧光素酶活性试验检测结果显示miR-138亦可直接靶向调节RUNX3。通常miRNAs通过调节相关靶基因来发挥作用,因此值得寻找miRNA相关的靶基因,并分析其对Th1、Th2的影响,为CVA的治疗提供方向。以往有研究显示miRNAs参与气道重塑诱发支气管哮喘发病[19-22]。然而miR-138靶向调节RUNX3后对Th1/Th2平衡是否存在影响,本研究通过转染实验增加RUNX3的表达水平后可改善Th1/Th2失衡,表明RUNX3对Th1/Th2平衡有一定调节作用。RUNX3对Th1/Th2平衡调节可能的作用机制如下:在Th1细胞分化过程中,RUNX3在CD4+T细胞中的表达增加,并以正反馈方式作用于T-bet转录因子[23]。T-bet是Th1细胞分化和IFN-γ分泌的启动子,同时抑制Th2相关的免疫事件[24]。另有研究表明RUNX3可以减弱Th2相关转录因子,后者是Th2分化的主要调节因子,并且是Th2介导的免疫反应所必需的[13]。
综上所述,CVA患儿存在Th1/Th2失衡、miR-138表达水平增加,RUNX3表达水平降低,miR-138可直接靶向调节RUNX3。增加RUNX3的表达水平后可改善Th1/Th2失衡,为CVA的治疗提供新的方向。
参考文献
- 1. Seumois G, Ramírez-Suástegui C, Schmiedel BJ, et al. Single-cell transcriptomic analysis of allergen-specific T cells in allergy and asthma[J].Sci Immunol, 2020, 5(48): eaba6087. PMID: . PMCID: . DOI: 10.1126/sciimmunol.aba6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. 吴献青, 方小玲, 聂妹芳, 等. IL-1β或TNF-α干预的子宫蜕膜细胞对母胎界面Th1/Th2及Th17/Treg平衡的影响[J].中南大学学报(医学版), 2017, 42(1): 66-71. PMID: . DOI: 10.11817/j.issn.1672-7347.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 3. Kwak EJ, Hong JY, Kim MN, et al. Chitinase 3-like 1 drives allergic skin inflammation via Th2 immunity and M2 macrophage activation[J].Clin Exp Allergy, 2019, 49(11): 1464-1474. PMID: . DOI: 10.1111/cea.13478. [DOI] [PubMed] [Google Scholar]
- 4. Lee SY, Kang B, Bok SH, et al. Macmoondongtang modulates Th1-/Th2-related cytokines and alleviates asthma in a murine model[J].PLoS One, 2019, 14(12): e0224517. PMID: . PMCID: . DOI: 10.1371/journal.pone.0224517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bui TT, Piao CH, Kim SM, et al. Citrus tachibanaleaves ethanol extract alleviates airway inflammation by the modulation of Th1/Th2 imbalance via inhibiting NF-κB signaling and histamine secretion in a mouse model of allergic asthma[J].J Med Food, 2017, 20(7): 676-684. PMID: . DOI: 10.1089/jmf.2016.3853. [DOI] [PubMed] [Google Scholar]
- 6. Keyhanmanesh R, Rahbarghazi R, Aslani MR, et al. Systemic delivery of mesenchymal stem cells condition media in repeated doses acts as magic bullets in restoring IFN-γ/IL-4 balance in asthmatic rats[J].Life Sci, 2018, 212: 30-36. PMID: . DOI: 10.1016/j.lfs.2018.09.049. [DOI] [PubMed] [Google Scholar]
- 7. Yu YY, Wang LL, Gu GX. The correlation between Runx3 and bronchial asthma[J].Clin Chim Acta, 2018, 487: 75-79. PMID: . DOI: 10.1016/j.cca.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 8. Boto P, Csuth TI, Szatmari I. RUNX3-mediated immune cell development and maturation[J].Crit Rev Immunol, 2018, 38(1): 63-78. PMID: . DOI: 10.1615/CritRevImmunol.2018025488. [DOI] [PubMed] [Google Scholar]
- 9. Song Q, Shang J, Zhang CF, et al. Transcription factor RUNX3 promotes CD8+ T cell recruitment by CCL3 and CCL20 in lung adenocarcinoma immune microenvironment[J].J Cell Biochem, 2020, 121(5-6): 3208-3220. PMID: . DOI: 10.1002/jcb.29587. [DOI] [PubMed] [Google Scholar]
- 10. Men S, Yu YY, Zhang YH, et al. Methylation landscape of RUNX3 promoter region as a predictive marker for Th1/Th2 imbalance in bronchiolitis[J].Med Sci Monit, 2019, 25: 7795-7807. PMID: . PMCID: . DOI: 10.12659/MSM.917196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van den Berge M, Tasena H. Role of microRNAs and exosomes in asthma[J].Curr Opin Pulm Med, 2019, 25(1): 87-93. PMID: . DOI: 10.1097/MCP.0000000000000532. [DOI] [PubMed] [Google Scholar]
- 12. Huang L, Wang MJ, Chen ZR, et al. MiR-138 regulates dendritic cells mediated Th2-type immune response by regulating the OX40L expression in asthma[J].Int J Clin Exp Pathol, 2017, 10(11): 10979-10988. PMID: . PMCID: . [PMC free article] [PubMed] [Google Scholar]
- 13. Fu DD, Yu WF, Li M, et al. MicroRNA-138 regulates the balance of Th1/Th2 via targeting RUNX3 in psoriasis[J].Immunol Lett, 2015, 166(1): 55-62. PMID: . DOI: 10.1016/j.imlet.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 14. 中华医学会儿科学分会呼吸学组慢性咳嗽协作组, 《中华儿科杂志》编辑委员会 . 中国儿童慢性咳嗽诊断与治疗指南(2013年修订)[J].中华儿科杂志, 2014, 52(3): 184-188. DOI: 10.3760/cma.j.issn.0578-1310.2014.03.005. [DOI] [Google Scholar]
- 15. Liang P, Peng S, Zhang M, et al. Huai Qi Huang corrects the balance of Th1/Th2 and Treg/Th17 in an ovalbumin-induced asthma mouse model[J].Biosci Rep, 2017, 37(6): BSR20171071. PMID: . PMCID: . DOI: 10.1042/BSR20171071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rahimi RA, Nepal K, Cetinbas M, et al. Distinct functions of tissue-resident and circulating memory Th2 cells in allergic airway disease[J].J Exp Med, 2020, 217(9): e20190865. PMID: . PMCID: . DOI: 10.1084/jem.20190865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matthews NC, Pfeffer PE, Mann EH, et al. Urban particulate matter-activated human dendritic cells induce the expansion of potent inflammatory Th1, Th2, and Th17 effector cells[J].Am J Respir Cell Mol Biol, 2016, 54(2): 250-262. PMID: . PMCID: . DOI: 10.1165/rcmb.2015-0084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boskabady MH, Neamati A. Improvement of tracheal responsiveness and Th1/Th2 balance in a rat model of asthma, treated with portulaca oleracea[J].Altern Ther Health Med, 2020, 26(6): 34-42. PMID: . [PubMed] [Google Scholar]
- 19. Lou LL, Tian MY, Chang JX, et al. MiRNA-192-5p attenuates airway remodeling and autophagy in asthma by targeting MMP-16 and ATG7[J].Biomed Pharmacother, 2020, 122: 109692. PMID: . DOI: 10.1016/j.biopha.2019.109692. [DOI] [PubMed] [Google Scholar]
- 20. Zhao MZ, Li YP, Geng XR, et al. Expression level of miRNA-126 in serum exosomes of allergic asthma patients and lung tissues of asthmatic mice[J].Curr Drug Metab, 2019, 20(10): 799-803. PMID: . DOI: 10.2174/1389200220666191011114452. [DOI] [PubMed] [Google Scholar]
- 21. Ji WT, Zhang QY, Shi HF, et al. The mediatory role of Majie cataplasm on inflammation of allergic asthma through transcription factors related to Th1 and Th2[J].Chin Med, 2020, 15(1): 53. PMID: . PMCID: . DOI: 10.1186/s13020-020-00334-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fan LX, Wang XJ, Fan LL, et al. MicroRNA-145 influences the balance of Th1/Th2 via regulating RUNX3 in asthma patients[J].Exp Lung Res, 2016, 42(8/10): 417-424. PMID: . DOI: 10.1080/01902148.2016.1256452. [DOI] [PubMed] [Google Scholar]
- 23. Vecellio M, Cohen CJ, Roberts AR, et al. RUNX3 and T-bet in immunopathogenesis of ankylosing spondylitis-novel targets for therapy?[J].Front Immunol, 2019, 9: 3132. PMID: . PMCID: . DOI: 10.3389/fimmu.2018.03132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Levine AG, Mendoza A, Hemmers S, et al. Stability and function of regulatory T cells expressing the transcription factor T-bet[J].Nature, 2017, 546(7658): 421-425. PMID: . PMCID: . DOI: 10.1038/nature22360. [DOI] [PMC free article] [PubMed] [Google Scholar]


