Introduction
Metabolic acidosis is a chronic condition that many people in the Western world have but do not realise it.1–3 It occurs when there is retention of acid in the body, which leads to a depletion in the bicarbonate stores of the body. The term metabolic acidosis is typically used when referring to low blood pH or acidemia due to a metabolic abnormality. However, this is inappropriate as most cases of metabolic acidosis do not have acidemia. In fact, a low blood pH is typically one of the last surrogate markers to become abnormal in those with low-grade metabolic acidosis.2 This is because the body maintains a normal blood pH at the expense of bicarbonate reserves. Metabolic acidosis primarily occurs inside the cell and in the fluid that surrounds our tissues (interstitial fluid).4 When checking for metabolic acidosis the clinician should look at fasting serum bicarbonate, urinary pH (with a measurement at least 4 hours separated from the last ingested meal) and 24-hour urinary citrate levels. While there is not a universally accepted way to diagnose low-grade metabolic acidosis, this paper will help to give the clinician insights into checking for this condition in their patients.
Defining metabolic acidosis
Acidemia, or too much acid in the blood, only occurs when the body’s buffering capacity can no longer maintain a normal pH level. A normal blood pH is considered to be 7.35–7.45. However, even at a normal blood pH metabolic acidosis can occur. In fact, once the blood pH falls below 7.4, there is usually acid retention in the body and low-grade metabolic acidosis.2 However, the blood pH does not drop below the normal range until metabolic acidosis has become severe. Once this occurs it is usually referred to as ‘metabolic acidosis’ by the clinician. However, this is actually acidemia (or too much acid in the blood). Thus, someone with a low blood pH has likely had low-grade metabolic acidosis for years or more likely decades.
Low-grade metabolic acidosis is something that many people in the Western world have.2 Low-grade means there are no apparent or noticeable harms but the body is retaining acid, depleting bicarbonate stores and damage is occurring in numerous tissues in the body. Typically, with low-grade metabolic acidosis, the blood pH drops slightly, as does the bicarbonate levels, but they will still be in the ‘normal’ range. Thus, if blood pH and/or bicarbonate levels are at the lower end of normal this is highly suggestive that someone has metabolic acidosis.2
With low-grade metabolic acidosis, the total blood buffering capacity is reduced and thus a greater reliance on muscle, bone and connective tissue will be required for the elimination of additional acid. The harms of low-grade metabolic acidosis increase with age and decreasing kidney function as the kidneys ability to excrete acid goes down. The increase in the acid load in the body worsens kidney function over the long run. For example, chronic metabolic acidosis leads to nephron hypertrophy in the kidneys of animals.5 This is likely due to the toxic effects that ammonia has on the kidneys. Metabolic acidosis can also lead to an increased loss of sodium and potassium in the urine, as well as an increased loss of water increasing the risk of dehydration.6 In addition, metabolic acidosis can cause magnesium and calcium loss out the urine.7 8
Regulation of acid-base homoeostasis
‘One characteristic of the contemporary human diet for which no quantitative comparison has been made with the inferred ancestral preagricultural diet is its imbalance of nutrient precursors of hydrogen and bicarbonate ions, resulting in the body’s net production of non-carbonic acid, ranging over an order of magnitude from 10 to 150 mEq/day among diets.’9
A normal healthy body has numerous buffering systems to combat acid accumulation. However, if your buffering capacity is reduced or cannot meet the acid load, then harms can ensue such as a breakdown of muscle, connective tissue and bone.2 Mild acidosis can increase the removal of minerals from the bone and increase the activity of osteoclasts while reducing the activity of osteoblasts causing the release of calcium from bone and increasing the risk of osteoporosis and kidney stone formation.2 These harms are more likely to occur in those with decreased kidney function.
It is the slow depletion in our body’s buffering systems that keeps our blood pH normal despite acid overload. Thus, some individuals can have a normal blood pH level and a fully functioning buffering system in the body, whereas others can have a normal blood pH but their buffering systems are deficient. An accumulated of acid in the cell and/or the interstitial fluid can lead to cellular damage, pain and insulin resistance.4 10
One buffering system in the body is bicarbonate. This system is the primary buffering system in the blood, and it helps to maintain a normal blood pH. Typically, serum bicarbonate levels drop prior to blood pH. Thus, measuring fasting serum bicarbonate levels is important when checking for low-grade metabolic acidosis. However, it should still be paired with several other tests. A normal serum bicarbonate level is considered 23–30 mEq/L. However, optimal serum bicarbonate levels are more around 25–30 mEq/L (preferably 27–30 mEq/L).2 11 12 A serum bicarbonate level at or below 24 mEq/L should be considered suboptimal and suggests low-grade metabolic acidosis, especially if paired with low urinary citrate or high urinary ammonium.2 A urinary pH of <5.3 in adults or <5.6 in children almost certainly indicates metabolic acidosis and is found in a large portion of the population.13 However, a urinary pH <6.5 indicates a net acid excretion of >40 mEq, which can lead to acid retention and metabolic acidosis in approximately 50% of the population.14 15 Thus, even a urinary pH of 6.5 or less can suggest low-grade metabolic acidosis. For most individuals, an optimal urinary pH is ~6.8–7.0, which signifies a net acid excretion of approximately zero.14 Box 1 summarises the ways to test for low-grade metabolic acidosis.
Box 1. Ways to test for low-grade metabolic acidosis2 12.
Suboptimal fasting serum bicarbonate: <27 mEq/L.28 29
Optimal bicarbonate level: 27–32 mEq/L.
Optimal level prior to exercise: 31–38 mEq/L.
Suboptimal blood pH: <7.42.
Optimal blood pH 7.42–7.45.
Optimal blood pH prior to exercise: 7.45–7.50.
-
High urinary ammonium (NH4+):
Normal range is 15–45 ug/dL.
Optimal <40 mmol/day.
-
Urinary ammonium starts to fall when glomerular filtration rate (GFR) drops below 40 mL/min.
Thus, 24-hour urinary ammonium levels are not an appropriate way to look for low-grade metabolic acidosis once the GFR is <40 mL/min.
-
High 24-hour urinary calcium (compared with calcium intake)
-
~16.67% of dietary calcium gets excreted in the urine, so for a dietary calcium intake of 900 mg, 150 mg will typically come out in the urine.
If more than 16.67% of dietary calcium is coming out in the urine this suggests calcium loss, which may be due to metabolic acidosis.
-
-
Low 24-hour urinary citrate
<320 mg/24 hours.30
Optimal urinary citrate level: 600–800 mg/24 hours.
-
Low urinary pH
A urinary pH of <6.0 is formed from a diet that produces a net acid excretion of 70 mEq/day or higher, which for most people will lead to acid retention.14
-
Optimal urinary pH (net acid excretion of zero): ~6.8–7.5.14
Urine pH should not be taken first thing in the morning or less than 4 hours from eating. Spot urinary pH should be taken 4 hours or longer after eating but not first thing in the morning. The best time to take a urinary spot pH would be before dinner (at least 4 hours after eating lunch).
-
Low partial pressure of carbon dioxide
-
< 35 mm Hg.
Suboptimal <38 mm Hg.
-
Four main mechanisms that compensate for chronic latent metabolic acidosis
When proteins get broken down in the body, they release H+ions (hydrogen ions or protons). Once the organic anions are used up (bicarbonate, citrate, lactate, acetate, gluconate, malate, etc) the main buffering system that eliminates acid in the body are the kidneys. The kidneys produce ammonia (NH3) to bind to the acid (H+) to form ammonium (NH4+).2 Phosphate can also move with hydrogen ions for their elimination out in the urine. However, the main pathway that acid gets excreted by the kidneys is from the increased formation of ammonia which is directly toxic to the kidneys.16 Importantly, the buffering system that allows for this to occur is our muscle. Indeed, the production ammonia, which occurs in the kidney’s tubular cells, comes primarily from the breakdown of the nitrogenous amino acid glutamine. Thus, skeletal muscle gets broken down and provides the nitrogen from glutamine for the excretion of acid out the body. This muscle breakdown may be compensated for by lifting weights for example, which signals the body to grow muscle, but muscle protein synthesis and recovery will be reduced in a state of acidosis.17 Even if the muscle breakdown is offset by muscle protein synthesis, the kidneys will slowly become damaged from having to chronically excrete high acid loads.2
The second pathway that helps to eliminate acid in the body is from an increased secretion of hydrogen ions in the renal tubules. Even with mild acidosis, the quantity and activity of the Na+/H+ ion exchanger in the kidneys is increased, leading to H+ elimination but concomitant sodium reabsorption.2 The overall effects on urinary sodium are still not conclusive, however, as data suggest that metabolic acidosis increases the loss of sodium out in the urine, potentially due to tubulointerstitial damage, and loss in the capacity of the kidneys to reabsorb sodium.6 16
The third elimination pathway for acid out the body is combining citrate to hydrogen ions. When we accumulate acid, citrate excretion in the urine goes down because it is reabsorbed back into the body by the tubular cells of the kidney. Thus, in states of low-grade metabolic acidosis the urine will contain less citrate. Citrate (citrate3−) can accept three protons forming uncharged citric acid. This eventually gets broken down into water and carbon dioxide eliminating 3H+ ions. However, the reduction in citrate in the urine increases the risk of kidney stones.2 The reduction in urinary citrate leads to less citrate to bind to urinary calcium ions, which form more soluble calcium-citrate-complexes compared with oxalic acid. Thus, calcium-oxalate kidney stones can be caused by low-grade metabolic acidosis, which may be improved with increasing dietary bicarbonate or citrate.
The fourth and final buffering system is the release of minerals (and their organic anions, like phosphate and carbonate) from the bones and cellular compartments. Studies from in the 1960s confirmed that high dietary acid loads increase the breakdown of bone.18 In fact, mild acidosis increases osteoclast activity and decreases osteoblast activity leading to increased bone breakdown and decreased bone building, respectively.2 This can cause an increase in the release of calcium and phosphorus from bone, higher calcium level in the urine and an increased risk of calcium-oxalate kidney stones.
Endogenous sources of acid
-
H+ (protons)
Exists as H3O+ (hydronium cations), the acid is bound to water.
-
Sulfuric acid
Sulphate anions+hydrogen ions.
-
Phosphoric acid
Phosphate anions+hydrogen ions.
-
Uric acid
Urate anions+hydrogen ions.
-
Lactic acid
Lactate anions+hydrogen ions.
-
Ketoacids (acetoacetic acid and beta-hydroxybutyric acid)
Ketone body (acetoacetate and beta-hydroxybutyrate) anions plus the hydrogen ions.
Exogenous sources of acid
Animal foods (especially sharp/processed cheese, eggs and meat).
Grains.
-
Ketogenic/low-carb diets.
This generally only increases acid load until the body adapts to utilising ketones.
Anaerobic exercise.
-
Prolonged fasting.
~48 hours or longer.
Endogenous base buffers
Bicarbonate.
Citrate.
Bone.
Protein.
Creatine.
Phosphate.
Carnosine.
Haemoglobin.
Albumin.
Exogenous base buffers
Lactate, acetate, malate, gluconate, citrate, bicarbonate.19
Fruits.
-
Vegetables.
Particularly spinach, dates, raisins, prunes, black currants and plums.
Coffee and tea.
Why do humans accumulate acid in the body?
In the body, acid is defined as hydrogen ions (H+) and pH, stands for ‘power of hydrogen’, which is a measurement of hydrogen ions in the blood on a logarithmic scale. Base is defined as a hydrogen ion acceptor, which, includes bicarbonate (HCO3−) and citrate in the body. Most diets lead to a positive excretion of acid out in the urine each day.20 21 Around 35 mEq of bicarbonate, or potential bicarbonate, is lost in the stool each day, even in people eating diets with excess acid ingestion.20 22 During periods of metabolic acidosis, there is a fall in alkali loss out the stool, but not a complete shut off.22 The kidney is also important for reabsorbing large amounts of bicarbonate, which decreases with age and kidney damage. Thus, humans can be thought of as acid producing, alkali losing organisms, which is partly why so many people in the Western world are thought to have low-grade metabolic acidosis.
In healthy people with normal kidney function, once the amount of acid that gets excreted out in the urine is >0.4–1 mEq/kg of body weight per day (eg, 40–70 mEq/day for a 70 kg adult), there is acid retention in the body.23 24 Thus, the kidneys can only excrete a set amount of acid each day before there is retention. A typical Western diet leads to a net endogenous acid production of around 50–100 mEq of acid per day.21 Thus, most people are already near their threshold for retaining acid.21 As kidney function declines, which typically occurs at a rate of 1% starting at the age of 20 years old, the kidneys’ ability to excrete acid and prevent acid retention in the body goes down.3 Indeed, the acid excretion capacity of the kidneys in those 55–75 years old is about 8 mEq less per day vs young people.25 This is why people who are 55 or older are particularly susceptible to acid retention. Additionally, as we age, we tend to select foods that are more acid-producing versus base-forming, which further contributes to acid retention in the body.
Where does dietary acid come from?
Animal protein is the largest source of dietary acid as it is high in the sulfur-containing amino acids methionine and cysteine, which leads to the formation of sulfuric acid and hydrogen ions in the body. As mentioned previously, hydrogen ions, which are technically bound to water as H3O+ and are called hydronium ions, is the acid in the body. Hydrogen ions are also provided in the diet from the metabolism of dietary phosphate. Animal meat and eggs are also high phosphoproteins and phospholipids that contain high amounts of phosphoserine, lecithin and ammonium, which also form hydrogen ions in the body. Thus, animal protein, particularly, meat, eggs and cheese, is what leads to the formation of large amounts of acid in the body. Fruits and vegetables are high in organic anions like citrate, malate and gluconate, which get converted to bicarbonate in the body.20 Bicarbonate is the base in our body that neutralises the acid. Thus, animal foods are a positive potential renal acid load (PRAL), whereas plant foods have a negative PRAL. A list of PRAL of various foods is listed in table 1.
Table 1.
The potential renal acid load (PRAL) of foods15 31 (mEq of acid/3.5 oz.)
| Food | PRAL |
| Parmesan cheese | 34.2 |
| Processed cheese | 28.7 |
| Cheddar cheese | 26.4 |
| Egg yolks | 23.4 |
| Hard cheeses | 19.2 |
| Gouda cheese | 18.6 |
| Corned beef | 13.2 |
| Brown rice | 12.5 |
| Salami | 11.6 |
| Trout | 10.8 |
| Liver sausage | 10.6 |
| Luncheon meat | 10.2 |
| Chicken meat | 8.7 |
| Pork | 7.9 |
| Beef | 7.8 |
| Spaghetti, white | 6.5 |
| Cornflakes | 6.0 |
| White bread | 3.7 |
| Yoghurt, plain | 1.5 |
| Whole milk | 0.7 |
| Coca Cola | 0.4 |
| Tea | −0.3 |
| Grape juice | −1.0 |
| White wine | −1.2 |
| Broccoli | −1.2 |
| Coffee | −1.4 |
| Apples | −2.2 |
| Red wine | −2.4 |
| Lemon juice | −2.5 |
| Potatoes | −4.0 |
| Cauliflower | −4.0 |
| Zucchini | −4.6 |
| Carrots | −4.9 |
| Celery | −5.0 |
| Bananas | −5.5 |
| Spinach | −14 |
| Raisins | −21 |
The lungs cannot affect acid-base status in the long-run because one bicarbonate gets neutralised to eliminate one hydrogen ion via this pathway. Thus, when there is a lack of dietary base to neutralise excess acid, the body must call on the kidneys to eliminate the acid by increasing the production of ammonia and/or the intracellular phosphate release and/or breakdown bone for additional bicarbonate and alkaline minerals.
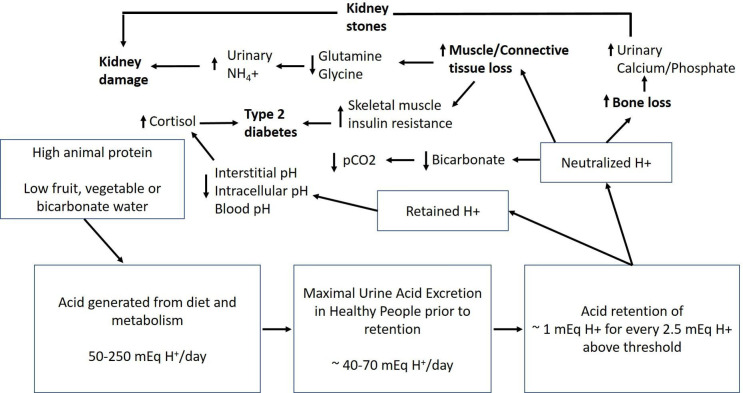
As mentioned previously producing ammonia to eliminate excess acid is harmful to the kidneys if elevated over the long run. Additionally, muscles and connective tissues get broken down to provide nitrogen for the formation of the ammonia. Thus, constantly producing high amounts of ammonia to excrete large amounts of acid can have long-term health consequences. This system is not perfect, and the kidneys can only excrete a certain amount of acid before there is retention. This is why the easiest solution to handle a diet that provides large amounts of acid is to either consume bicarbonate supplements (sodium or potassium bicarbonate), bicarbonate mineral waters (making sure the water is low in sulfate) or fruits and vegetables to offset high dietary acid loads. For example, 3 g of potassium citrate inhibits 30 mEq of acid and 5 g of sodium citrate inhibits 60 mEq of acid. Potassium and sodium citrate should be consumed after a meal and with plenty of water and people who have high potassium levels in their blood or have kidney disease need to be careful with potassium supplements. Strategies for suppressing the dietary acid load are listed in table 2. Figure 1, box 2 summarise the harms of low-grade metabolic acidosis.
Table 2.
Strategies for suppressing the dietary acid load
| Acid suppressor | Comments |
| Fruits and vegetables | See PRAL in table 1 |
| Sodium citrate | 5 g suppresses 60 mEq of acid. Should be taken with food. |
| Potassium citrate | 3 g suppresses 30 mEq of acid. Typically, no more than 3 g is taken with each meal. |
| Sodium or potassium bicarbonate | This can suppress stomach acid and thus sodium or potassium citrate is the better option. |
| Bicarbonate mineral waters (low in sulfate) | 1 mEq of bicarbonate inhibits 1 mEq of acid. Typically, the bicarbonate levels are fairly low and should not affect stomach pH. There is a slow accumulation of bicarbonate in the body when drinking bicarbonate mineral waters and this is a better option than sodium or potassium bicarbonate supplements. |
PRAL, potential renal acid load.
Figure 1.
The harms of low-grade metabolic acidosis: adapted from Passey. Reducing the dietary acid load: how a more alkaline diet benefits patients with chronic kidney disease 2016.21 pCO2, partial pressure of carbon dioxide.
Box 2. The harms of low-grade metabolic acidosis2 32 33.
Type 2 diabetes.
Insulin resistance.
Increased gluconeogenesis.
Hypertension.
Bone loss.
-
Osteoporosis/osteopenia/sarcopenia
Mineral loss from bone matrix.
Increased osteoclast activity (more bone breakdown).
Reduced osteoblast activity (less bone building).
Muscle loss and reduce muscle strength.
Connective tissue loss.
Fibromyalgia.
-
Hyperuriceamia
Gout.
-
Kidney function decline
Tubulointerstitial damage.
-
Kidney stones
Less citrate to bind to calcium and more calcium to oxalic acid increasing calcium oxalate stone formation.
Reduced urine pH increasing uric acid stone formation.
-
Salt loss out the urine
Negative sodium and chloride balance.
-
Other mineral deficiencies
Increased loss of sodium, chloride, potassium, calcium, magnesium, sulfate and phosphate out the urine.
The sodium and potassium loss are due to a decrease in the reabsorption of these minerals by the kidneys,6 34 35 which likely reduces the reabsorption of taurine
The loss of calcium, magnesium and phosphate are from bone losses.7
Taurine loss
-
Increased water loss out the urine.
Dehydration.
Decreased exercise performance.
Summary: how acid-base status is maintained in the body and why low-grade acidosis is so common
The average diet in the Western world leads to a net acid excretion of 50–100 mEq/day.
The body loses 35 mEq of bicarbonate or bicarbonate forming substances per day.
The kidneys must be relied on to prevent low-grade acidosis as the lungs cannot affect acid-base status over the long-run (one bicarbonate is neutralised to eliminate one hydrogen ion via the lungs).
The kidneys of a healthy person can only excrete 40–70 mEq of acid per day before acid is retained in the body. Most Americans are consuming diets that produce this much acid or more per day.
Animal-based or carnivore diets typically provide 150–250 mEq of acid per day, which means that these types of diets lead to significant acid retention unless exogenous bicarbonate forming substances are being consumed (bicarbonate mineral waters or supplements, fruits or vegetables).
Once the kidneys reach their threshold (40–70 mEq of acid per day), approximately 1 mEq of acid is retained per 2.5 mEq of acid above the threshold.
-
If the diet does not contain enough bicarbonate (bicarbonate-forming substances or citrate) and minerals (sodium, potassium, magnesium and calcium) to neutralise the excess acid then negative consequences to numerous bodily systems take place:
Bone will breakdown to increase bicarbonate buffering as well as alkaline minerals for sulfate excretion, which leads to mineral loss and weak bones.
Muscle and connective tissue will breakdown to eliminate hydrogen ions along with ammonium, which taxes glutamine and glycine status.
The kidneys will slowly become damaged from the high production of ammonia.
Kidney stones can form due to the increased reabsorption of citrate and the increased calcium out in the urine.
The increase in acid in the cell can reduce the function of numerous enzymes and processes and has harmful effects on all tissues.
Table 2 summarises the harms of low-grade metabolic acidosis.
Metabolic acidosis did not appear to be a problem during Paleolithic times
The estimated average net endogenous acid production of a 21st century hunter gatherer diet is −88 mEq/day, which is on the alkaline side.9 In other words, the majority of these diets (87% to be exact) are retaining base and net alkaline. However, certain Palaeolithic diets did lead to a net endogenous acid production of up to ~100 mEq/day. Thus, the dietary acid load could have been fairly high, however, this was determined by food availability and geographical location. The goal during evolutionary times was not to prevent metabolic acidosis and chronic disease but to consume enough food to keep us alive. Today, most people in the Western world consume a diet that leads to a net endogenous acid production of 50–100 mEq/day21 26 whereas humans following a more carnivorous type of diet produce around 150–250 mEq/day.23 26
Other factors that increase acid load
When going on a low-carb or ketogenic diet, ammonium (NH4+) is produced to excrete the negatively charged ketone bodies (the positively charged ammonium gets eliminated with the negatively charged ketone bodies). This usually occurs after about 1 week on a ketogenic diet as sodium is the initial positively charged molecule that gets lost in the urine. Thus, being on a low-carb or ketogenic diet, at least acutely, increases acid load due to the increased production of the acidic ketone bodies. Prolonged fasting is also another condition that increases the body’s acid load.27 After just 48 hours of fasting, the body goes into a state of mild metabolic acidosis due to the increased production of ketone bodies. Other factors that increase the acid load of the body include consuming a diet high in animal foods and anaerobic exercise, which increases the production of acid in the cell and further increases the acid load. Someone on a carnivore or animal-based diet that performs frequent high-intensity anaerobic exercise, is in a constant state of ketosis, and also performs prolonged fasting is certainly excreting, and likely retaining, large amounts of acid.
For example, a typically carnivore diet of 2 pounds of meat and six eggs per day provides a net acid excretion of around 200–220 mEq of acid and a net acid accumulation of around 50–60 mEq/day.23 26 For every 2.5 mEq of acid produced over 0.4–1 mEq/kg of body weight, approximately 1 mEq of acid is retained. For example, in an average adult weighing 70 kg (155 pounds), if the diet leads to 100 mEq of net acid excretion per day, this is 30–60 mEq of acid over the 0.4–1 mEq/kg threshold leading to retention of acid in the body. These calculations are based on data from healthy people, whereas in individuals with kidney disease acid retention typically occurs at a net acid excretion of just 20 mEq/day.21 For an average 70 kg adult who consumes a typical carnivore diet that leads to a net acid excretion of 200–220 mEq/day, there will be an approximately 52–72 mEq of acid retained per day.23 26 However, optimal acid excretion out the kidneys is 0 mEq/day. For athletic performance, being slightly alkaline, especially prior to performance is optimal.
Conclusion
Low-grade metabolic acidosis is a common phenomenon in the Western world. We have provided several ways for clinicians to test for metabolic acidosis and strategies to neutralise a high dietary acid load. A greater emphasis on low-grade metabolic acidosis should be provided during medical school so that clinicians are more aware of this condition and how to treat it.
Footnotes
Contributors: All authors contributed to the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Alpern RJ, Sakhaee K. The clinical spectrum of chronic metabolic acidosis: homeostatic mechanisms produce significant morbidity. Am J Kidney Dis 1997;29:291–302. 10.1016/S0272-6386(97)90045-7 [DOI] [PubMed] [Google Scholar]
- 2.Vormann J, Goedecke T. Acid-base homeostasis: latent acidosis as a cause of chronic diseases. Schweizerische Zeitschrift fur GanzheitsMedizin 2006;18:255–66. [Google Scholar]
- 3.Frassetto LA, Morris RC, Sebastian A. Effect of age on blood acid-base composition in adult humans: role of age-related renal functional decline. Am J Physiol 1996;271:F1114–22. 10.1152/ajprenal.1996.271.6.F1114 [DOI] [PubMed] [Google Scholar]
- 4.Marunaka Y. The proposal of molecular mechanisms of weak organic acids intake-induced improvement of insulin resistance in diabetes mellitus via elevation of interstitial fluid pH. Int J Mol Sci 2018;19. 10.3390/ijms19103244. [Epub ahead of print: 19 Oct 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fine L. The biology of renal hypertrophy. Kidney Int 1986;29:619–34. 10.1038/ki.1986.45 [DOI] [PubMed] [Google Scholar]
- 6.Menegon LF, Figueiredo JF, Gontijo JA. Effect of metabolic acidosis on renal tubular sodium handling in rats as determined by lithium clearance. Braz J Med Biol Res 1998;31:1269–73. 10.1590/S0100-879X1998001000006 [DOI] [PubMed] [Google Scholar]
- 7.Rylander R, Remer T, Berkemeyer S, et al. Acid-base status affects renal magnesium losses in healthy, elderly persons. J Nutr 2006;136:2374–7. 10.1093/jn/136.9.2374 [DOI] [PubMed] [Google Scholar]
- 8.Rylander R, Tallheden T, Vormann J. Acid-base conditions regulate calcium and magnesium homeostasis. Magnes Res 2009;22:262–5. 10.1684/mrh.2009.0182 [DOI] [PubMed] [Google Scholar]
- 9.Sebastian A, Frassetto LA, Sellmeyer DE, et al. Estimation of the net acid load of the diet of ancestral preagricultural Homo sapiens and their hominid ancestors. Am J Clin Nutr 2002;76:1308–16. 10.1093/ajcn/76.6.1308 [DOI] [PubMed] [Google Scholar]
- 10.Vormann J, Worlitschek M, Goedecke T, et al. Supplementation with alkaline minerals reduces symptoms in patients with chronic low back pain. J Trace Elem Med Biol 2001;15:179–83. 10.1016/S0946-672X(01)80064-X [DOI] [PubMed] [Google Scholar]
- 11.Kanda E, Ai M, Yoshida M, et al. High serum bicarbonate level within the normal range prevents the progression of chronic kidney disease in elderly chronic kidney disease patients. BMC Nephrol 2013;14:4. 10.1186/1471-2369-14-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pizzorno J, Frassetto LA, Katzinger J. Diet-Induced acidosis: is it real and clinically relevant? Br J Nutr 2010;103:1185–94. 10.1017/S0007114509993047 [DOI] [PubMed] [Google Scholar]
- 13.Visveswaran K. Essentials of nephrology. 2nd ed. BI Publications Pvt Ltd, 2009. [Google Scholar]
- 14.Remer T. Influence of diet on acid-base balance. Semin Dial 2000;13:221–6. 10.1046/j.1525-139x.2000.00062.x [DOI] [PubMed] [Google Scholar]
- 15.Remer T, Manz F. Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc 1995;95:791–7. 10.1016/S0002-8223(95)00219-7 [DOI] [PubMed] [Google Scholar]
- 16.Chen W, Levy DS, Abramowitz MK. Acid base balance and progression of kidney disease. Semin Nephrol 2019;39:406–17. 10.1016/j.semnephrol.2019.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gough LA, Brown D, Deb SK, et al. The influence of alkalosis on repeated high-intensity exercise performance and acid-base balance recovery in acute moderate hypoxic conditions. Eur J Appl Physiol 2018;118:2489–98. 10.1007/s00421-018-3975-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemann J, Litzow JR, Lennon EJ. The effects of chronic acid loads in normal man: further evidence for the participation of bone mineral in the defense against chronic metabolic acidosis. J Clin Invest 1966;45:1608–14. 10.1172/JCI105467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naka T, Bellomo R. Bench-to-bedside review: treating acid-base abnormalities in the intensive care unit--the role of renal replacement therapy. Crit Care 2004;8:108–14. 10.1186/cc2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrington JT, Lemann J. The metabolic production and disposal of acid and alkali. Med Clin North Am 1970;54:1543–54. 10.1016/S0025-7125(16)32570-6 [DOI] [PubMed] [Google Scholar]
- 21.Passey C. Reducing the dietary acid load: how a more alkaline diet benefits patients with chronic kidney disease. J Ren Nutr 2017;27:151–60. 10.1053/j.jrn.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 22.Lennon EJ, Lemann J. Influence of diet composition on endogenous fixed acid production. Am J Clin Nutr 1968;21:451–6. 10.1093/ajcn/21.5.451 [DOI] [PubMed] [Google Scholar]
- 23.Lennon EJ, Lemann J, Litzow JR. The effects of diet and stool composition on the net external acid balance of normal subjects. J Clin Invest 1966;45:1601–7. 10.1172/JCI105466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurtz I, Maher T, Hulter HN, et al. Effect of diet on plasma acid-base composition in normal humans. Kidney Int 1983;24:670–80. 10.1038/ki.1983.210 [DOI] [PubMed] [Google Scholar]
- 25.Berkemeyer S, Vormann J, Günther ALB, et al. Renal net acid excretion capacity is comparable in prepubescence, adolescence, and young adulthood but falls with aging. J Am Geriatr Soc 2008;56:1442–8. 10.1111/j.1532-5415.2008.01799.x [DOI] [PubMed] [Google Scholar]
- 26.Sebastian A, Frassetto LA, Morris RC. The acid-base effects of the contemporary Western diet: An evolutionary perspective. In: Alpern RJ, Hebert SC, eds. The kidney: physiology and pathophysiology, 2006. [Google Scholar]
- 27.Drenick EJ. The effects of acute and prolonged fasting and refeeding on water, electrolyte, and acid-base metabolism. In: Maxwell MH, Kleeman CR, Co M-H, et al., eds. Clinical disorders of fluid and electrolyte metabolism, 1972. [Google Scholar]
- 28.Tabatabai LS, Cummings SR, Tylavsky FA, et al. Arterialized venous bicarbonate is associated with lower bone mineral density and an increased rate of bone loss in older men and women. J Clin Endocrinol Metab 2015;100:1343–9. 10.1210/jc.2014-4166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frassetto LA, Morris RC, Sebastian A. Dietary sodium chloride intake independently predicts the degree of hyperchloremic metabolic acidosis in healthy humans consuming a net acid-producing diet. Am J Physiol Renal Physiol 2007;293:F521–5. 10.1152/ajprenal.00048.2007 [DOI] [PubMed] [Google Scholar]
- 30.Medscape . Hypocitraturia overview of potassium citrate and calcium citrate. Available: https://emedicine.medscape.com/article/444968-overview
- 31.Lanham-New SA. The balance of bone health: tipping the scales in favor of potassium-rich, bicarbonate-rich foods. J Nutr 2008;138:172S–7. 10.1093/jn/138.1.172S [DOI] [PubMed] [Google Scholar]
- 32.Adeva MM, Souto G. Diet-Induced metabolic acidosis. 30. Edinburgh, Scotland: Clinical nutrition, 2011: 416–21. [DOI] [PubMed] [Google Scholar]
- 33.Barzel US, Massey LK. Excess dietary protein can adversely affect bone. J Nutr 1998;128:1051–3. 10.1093/jn/128.6.1051 [DOI] [PubMed] [Google Scholar]
- 34.Fang Y-W, Yang S-S, Cheng C-J, et al. Chronic metabolic acidosis activates renal tubular sodium chloride cotransporter through angiotension II-dependent WNK4-SPAK phosphorylation pathway. Sci Rep 2016;6:18360. 10.1038/srep18360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sartorius OW, Roemmelt JC, Pitts RF, et al. The renal regulation of acid-base balance in man. IV. The nature of the renal compensations in ammonium chloride acidosis. J Clin Invest 1949;28:423–39. 10.1172/JCI102087 [DOI] [PMC free article] [PubMed] [Google Scholar]