Structured Abstract
Objective:
To determine physician-reported use of and barriers to active surveillance for thyroid cancer.
Summary Background Data:
It isn’t clear if active surveillance for thyroid cancer is widely used.
Methods:
Surgeons and endocrinologists identified by thyroid cancer patients from the Surveillance, Epidemiology, and End Results (SEER) registries of Georgia and Los Angeles County were surveyed between 2018–2019. Multivariable weighted logistic regression analyses were conducted to determine physician acceptance and use of active surveillance.
Results:
Of the 654 eligible physicians identified, 448 responded to the survey (69% response rate). The majority (76%) believed that active surveillance was an appropriate management option, but only 44% used it in their practice. Characteristics of physicians who stated that active surveillance was appropriate management, but did not report using it included more years in practice (reference group < 10 years in practice): 10–19 years - OR 0.50 [CI 0.28–0.92]; 20–29 years - OR 0.31 [CI 0.15–0.62]; ≥30 years - OR 0.30 [CI 0.15–0.61] and higher patient volume 11–30 patients per year (OR 0.39 [CI 0.21–0.70]) and >50 patients per year (OR 0.33 [CI 0.16–0.71]) compared to ≤ 10, with no significant difference in those seeing 31–50 patients. Physicians reported multiple barriers to implementing active surveillance including patient does not want (80.3%), loss to follow-up concern (78.4%), more patient worry (57.6%) and malpractice lawsuit concern (50.9%).
Conclusion and Relevance:
Despite most physicians considering active surveillance to be appropriate management, more than half are not using it. Addressing existing barriers is key to improving uptake.
Mini-Abstract:
This survey of a diverse cohort of physicians providing care to thyroid cancer patients demonstrates that most providers consider active surveillance to be an appropriate management plan for some patients with low-risk thyroid cancer. However, less than half of the providers are currently using it in their practice.
INTRODUCTION:
There has been a rise in the incidence of papillary thyroid cancer in the United States with an estimated 52,890 cases in 2020.1 A major contributor to this increasing incidence is the detection of small low-risk cancers which are isolated to the thyroid, with no clinical evidence of cervical lymph node metastases.2–4 The death rates from all thyroid cancer have remained very low, with approximately 0.4 deaths per 100,000 patients, and are even lower for those with low risk cancers isolated to the thyroid gland.1 Historically the majority of thyroid cancers have been treated with thyroid surgery, however this treatment strategy carries with it a small, but significant, risk of surgical complications and the potential for over treatment.5–7
Active surveillance for small, low risk papillary thyroid cancers has been described as an alternative to surgical treatment, initially in Japan and subsequently in the United States.8, 9 These initial studies demonstrated that patients with primary tumors <1.0–1.5 cm in size and without evidence of local invasion or lymph node metastasis on cervical ultrasound could opt to forgo initial surgery as long as close and consistent surveillance follow-up with cervical ultrasound (i.e., every six months to two years) could be performed by a team experienced in the management of thyroid cancer.10, 11 Furthermore, this treatment option was safest in those ≥ 60 years of age as risk of progression was greater in younger patients.12, 13 Using these criteria for case selection, studies have shown that the rates of subsequent growth of the primary tumor or development of new lymph node metastases is low with approximately 15% of patients converting to surgical therapy during active surveillance.13–15 As a result of these findings, recent clinical care guidelines for the management of differentiated thyroid cancer have included active surveillance as an option for patients with thyroid cancer < 1 cm and without extrathyroidal extension or lymph node involvement.4, 11
Despite the growing acceptance of active surveillance as a suitable management option for patients with low risk papillary thyroid cancer, the rates of active surveillance use in the United States, outside of selected centers, remain unclear. There are multiple variables involved in the decision to pursue active surveillance for the management of thyroid cancer which include disease parameters (size, evidence of local invasion, cytopathologic diagnostic criteria, presence of lymph node metastases, concurrent thyroid pathology, etc.), patient factors (age, acceptance of a less aggressive treatment of a known malignancy, opinions about the need for thyroid hormone replacement following thyroidectomy, willingness to undergo prolonged surveillance, patient anxiety about living with cancer, among others) and physician factors (experience with active surveillance in the management of thyroid cancer, availability of resources to perform active surveillance follow-up, worry about subsequent progression of disease, etc.).10 This study’s objectives were to understand physicians’ attitudes toward use of active surveillance, how often it is being used, and the perceived barriers to its use in a diverse cohort of physicians who treat a population-based cohort of thyroid cancer patients.
METHODS:
Study participants:
To identify surgeons and endocrinologists involved in the management of thyroid cancer, we first surveyed patients affiliated with Surveillance, Epidemiology and End Results (SEER) registries of Georgia and Los Angeles county.7, 16, 17 Patients were asked to identify the specific surgeons and endocrinologists involved in their thyroid cancer management. There were 699 physicians identified, of which 45 were ineligible due to retirement or no longer in practice, unable to be located, deceased prior to the initial mailing, or did not meet study screening criteria after initial contact was made. (Figure 1) Between October 2018 to August 2019, all physicians identified by more than one patient (N=482) and a random sample of those identified by only one patient (N=172) were sent surveys for a total of 654 in the sample. In order to enhance response rates, an incentive was included in the initial mailing and non-responders were followed up with phone, fax, or email to encourage completion of the survey. Double data entry method was used to ensure a <1% error rate. There were 448 physicians who completed the survey for a response rate of 69% (448/654)18 Of the previously surveyed thyroid cancer patients in 2014–2015 SEER cohort, 75% were treated by physicians who responded to our physician survey.
Figure 1.
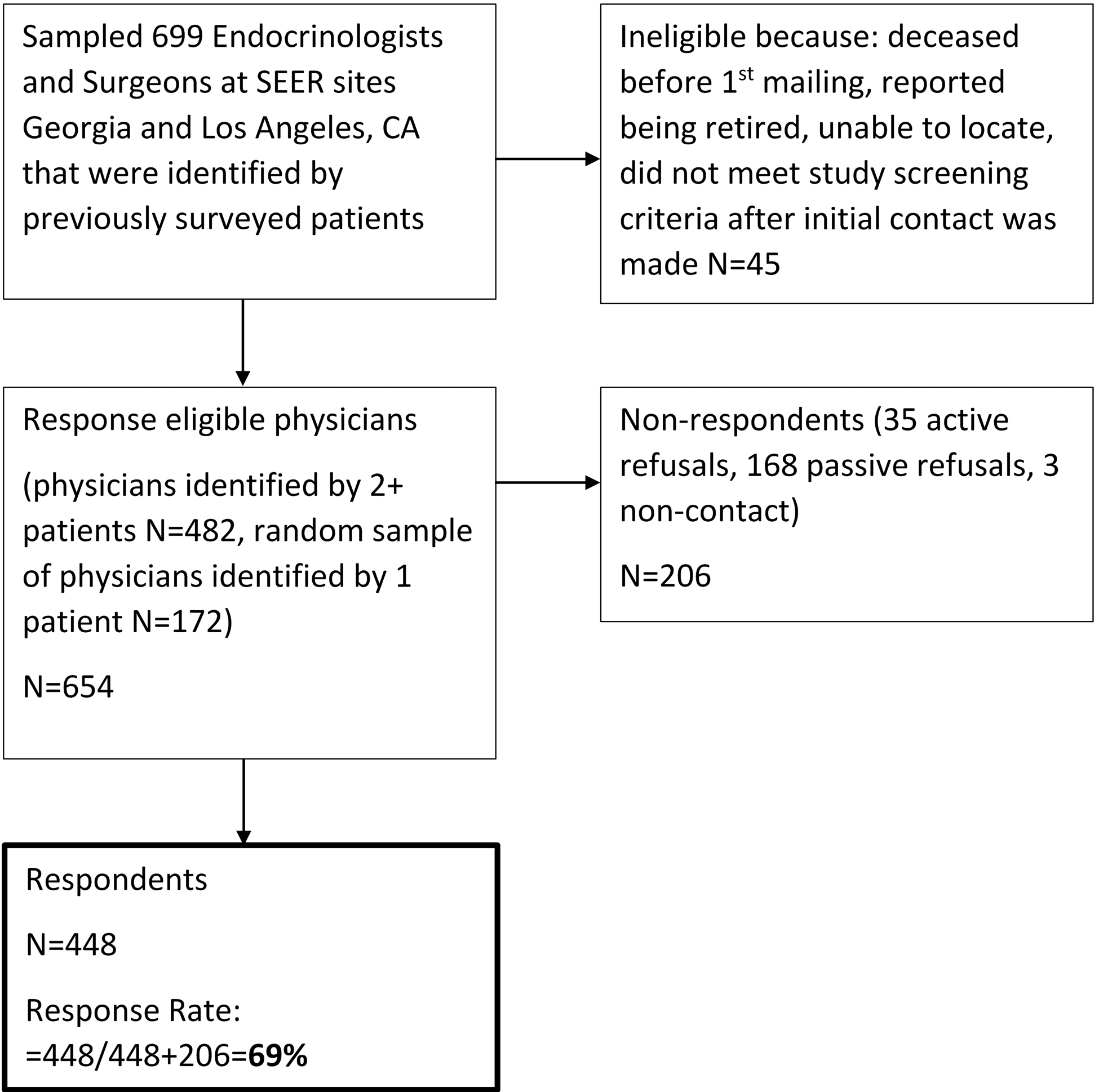
Flow diagram illustrating study cohort selection.
This study was approved by the Institutional Review Boards of the University of Michigan Emory University, and University of Southern California, as well as the Georgia Department of Public Health, and the Committee for the Protection of Human Subjects (California State Institutional Review Board). In addition, approval was received from the California Cancer Registry.
Survey Questionnaire Design and Content
The study investigators who were surgeons, endocrinologists, and survey methodologists designed the survey content based on prior literature, a conceptual framework, and prior experience caring for thyroid cancer patients.6, 7, 17, 19–21 The survey instrument included multiple topics related to thyroid cancer diagnosis and management, including items on the use of active surveillance. The survey instrument was piloted in a subset of 9 surgeons and endocrinologists who were not included in the sample. No questions were asked about specific patients. Physicians were asked general questions about active surveillance as well as about their practice (specialty, practice setting, years in practice, and volume of thyroid cancer patients). As shown in Table 1, physicians were asked about acceptance of active surveillance, use of active surveillance, and barriers to active surveillance.
Table 1.
Survey Measures
|
Acceptance of active surveillance as a management option
Is active surveillance an appropriate management option for some patients with thyroid cancer?
|
|
Use of active surveillance
In your practice, do you currently recommend active surveillance in the management of thyroid cancer patients?
|
|
Barriers to active surveillance
In patients for whom active surveillance is clinically appropriate, which of the following are barriers to implementing active surveillance for thyroid cancer? Please select ALL that apply.
|
Covariates
The physician survey included demographic questions on the specialty (general surgery, endocrinology, otolaryngology, other), practice setting (private practice or community health center, academic medical center, large medical group or staff-model HMO, other), years in practice (<10, 10–19, 20–29, ≥30), the number of patients with thyroid cancer they managed in the past year (≤10, 11–30, 31–50, >50), and SEER site (Georgia, Los Angeles).
Statistical analysis:
Univariate frequencies were examined. Univariate analysis significance was calculated with a Rao-Scott Chi-Square Test. Two multivariable regression analyses were performed. The first was to determine the factors associated with physician acceptance of active surveillance as an appropriate management for thyroid cancer. The second was to determine factors associated with physicians who believe active surveillance is appropriate but do not recommend it.
In order to account for representativeness we used response weights and estimated the weights using the following variables: survey response, SEER site/location, physician specialty (as identified by the patient), and patient volume (determine by the number of patients that identified each physician). This methodology accounts for possible difference between survey responders and non-responders and thus reduces non-response bias. Stata 15.1 and R version 3.6.1 were used for all statistical analyses and Wald 95% confidence interval (CI) was used to determine statistical significance.
RESULTS:
Of the 699 surgeons and endocrinologists identified by SEER registry patients who previously responded to our patient survey, 45 were ineligible because they were deceased prior to the initial mailing, retired or were no longer with the practice on record and could not be located. A total of 654 physicians were response-eligible and 448 responded to the survey (69% response rate). (Figure 1) The demographics of the 448 physician survey respondents are included in Table 2. The physician respondent specialty was general surgery in 30%, otolaryngology in 28%, and endocrinology in 42%. Just over half of physicians identified their practice setting as private practice or community health clinic (55%). Physician respondents reported their thyroid cancer patient volume in the past 12 months as: ≤10 (31%), 11–30 (34%), 31–50 (14%) and >50 (21%).
Table 2.
Demographics of physician survey respondents (N=448)
| Characteristics | N(%) |
|---|---|
| Specialty | |
| General Surgery | 134 (30%) |
| Otolaryngology | 130 (28%) |
| Endocrinology | 176 (42%) |
| Practice setting | |
| Private practice or community health clinic | 244 (55%) |
| Large medical group or staff-model HMO | 114 (25%) |
| Academic medical center | 83 (19%) |
| Other | 4 (1%) |
| Years in practice | |
| <10 years | 82 (19%) |
| 10–19 years | 144 (33%) |
| 20–29 years | 119 (27%) |
| ≥30 years | 94 (21%) |
| Thyroid cancer patient volume in past year | |
| None | |
| ≤10 | 137 (31%) |
| 11–30 | 154 (34%) |
| 31–50 | 62 (14%) |
| >50 | 90 (21%) |
| SEER Site | |
| Georgia | 205 (49%) |
| Los Angeles | 243 (51%) |
The physician respondents’ perspectives on the use of active surveillance in the management of thyroid cancer are presented in Table 3. When asked if active surveillance was an appropriate management option for some patients with thyroid cancer 76% responded “yes” and 24% responded “no”. When asked in their practice if they currently recommend active surveillance in the management of thyroid cancer patients 44% responded “yes” while 56% responded “no”. The 190 physicians who answered yes to this question were asked of which patients and thyroid cancer disease characteristics they would recommend active surveillance as a management option; and increasing age and decreasing tumor size were associated with increased use of active surveillance. (Table 2)
Table 3.
Physician opinions on the use of active surveillance in the management of thyroid cancer (N=448)
| Survey Question | N(%) |
|---|---|
| Is active surveillance an appropriate management options for some patients with thyroid cancer? | |
| Yes | 336 (76%) |
| No | 105 (24%) |
| In your practice, do you currently recommend active surveillance in the management of thyroid cancer patients? | |
| Yes | 190 (44%) |
| No | 247 (56%) |
| Which patients do you recommend management with active surveillance? Please select ALL that apply. (N=190) | |
| A 65-year-old female with a < 1 cm and no known lymph node involvement | 135 (71%) |
| A 40-year-old female with a < 1 cm and no known lymph node involvement | 94 (49%) |
| A 65-year-old female with a < 2 cm and no known lymph node involvement | 60 (32%) |
| A 40-year-old female with a < 2 cm and no known lymph node involvement | 56 (30%) |
The results of the multivariable logistic regression analyzing physician acceptance of active surveillance as an appropriate option for some patients with thyroid cancer are presented in Figure 2a. Odds ratios <1 were less accepting and odds ratios >1 were more accepting of active surveillance as a treatment option. Those physicians who identified their practice setting as large medical group or HMO (OR 0.35 [95% CI 0.13–0.93]) or private practice or community health clinic (OR 0.26 [95% CI 0.11–0.65]) were less likely to report that active surveillance is an appropriate management option as compared to those at academic medical centers. Number of years in practice, the number of thyroid cancer patients treated in the past year, and SEER registry site had no significant effect. In multivariable analysis, when the acceptance of active surveillance was compared between specialties using endocrinologists as the reference, there was a non-significant trend towards increased acceptance by otolaryngologists (OR 2.06) compared to general surgeons (OR 0.61). An additional univariate analysis was performed to assess whether acceptance of active surveillance differed by surgeon specialty. Otolaryngologists were significantly more likely to accept active surveillance as a management option than general surgeons (86% vs. 63%, p<0.001).
Figure 2a.
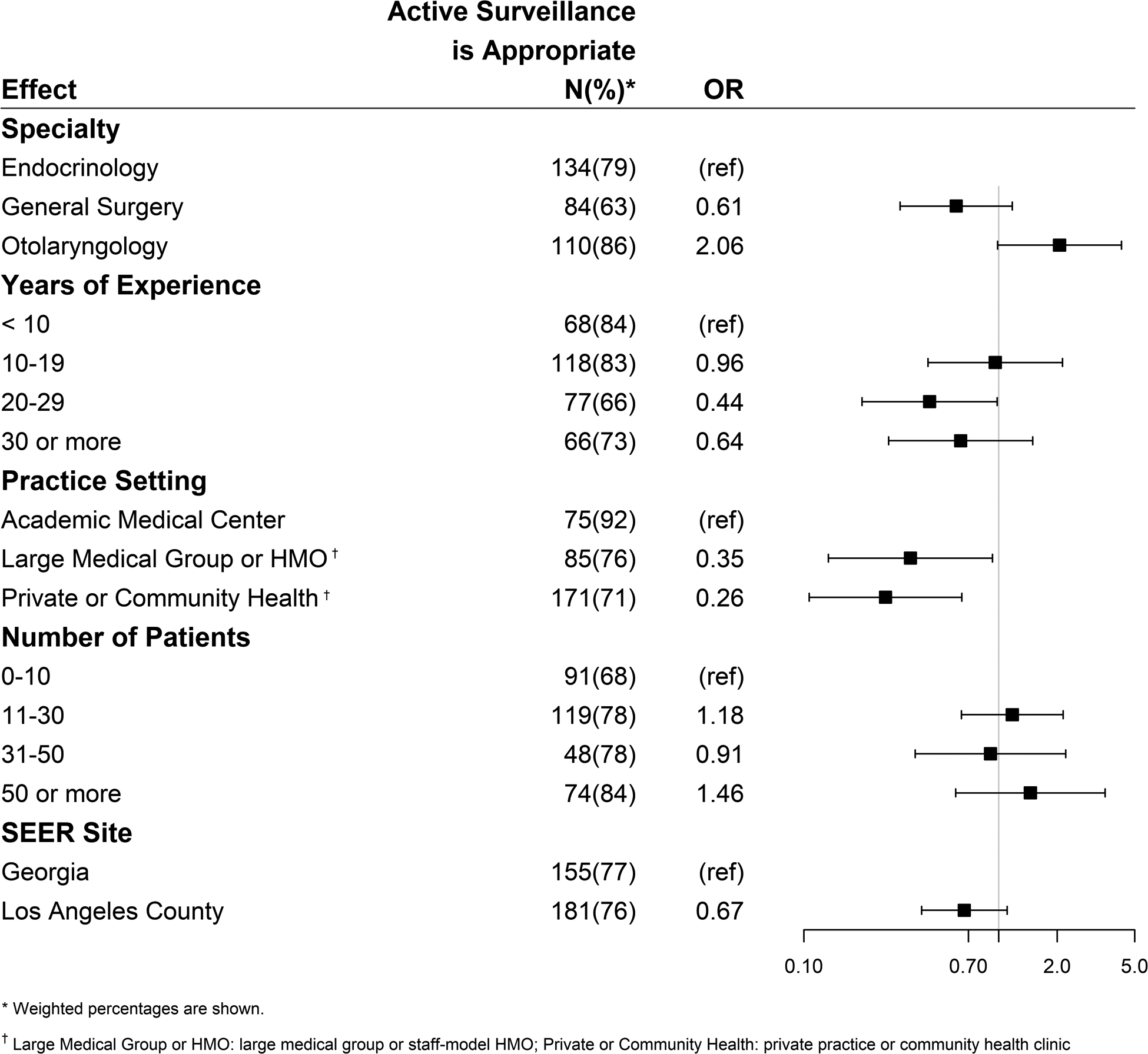
Factors associated with acceptance of active surveillance as an appropriate management option for thyroid cancer. (Odds ratios <1 are less accepting and odds ratios >1 are more accepting of active surveillance).
Figure 2b demonstrates characteristics associated with physicians who report that active surveillance is an appropriate management option for thyroid cancer, but who report that they do not use active surveillance in their practice. Odds ratios <1 were less likely and odds ratios >1 were more likely to recommend active surveillance in their practice. Physicians with more years in practice were less likely to use active surveillance (reference group < 10 years in practice): 10–19 years - OR 0.50 [95% CI 0.28–0.92]; 20–29 years - OR 0.31 [95% CI 0.15–0.62]; ≥30 years - OR 0.30 [95% CI 0.15–0.61]. A trend test was performed on the odds ratios for the categories of the years of practice and it was significance (p<0.001). Regarding volume, compared to physicians who reported seeing 10 or fewer thyroid cancer patients per year, those seeing 11–30 patients per year (OR 0.39 [95% CI 0.21–0.70]) and those seeing >50 patients (OR 0.33 [95% CI 0.16–0.71]) were less likely to use active surveillance, but there was no significant difference seen in those seeing 31–50 patients. Physician-reported specialty and practice type had no significant influence on use of active surveillance among those who endorsed it as an acceptable management option.
Figure 2b.
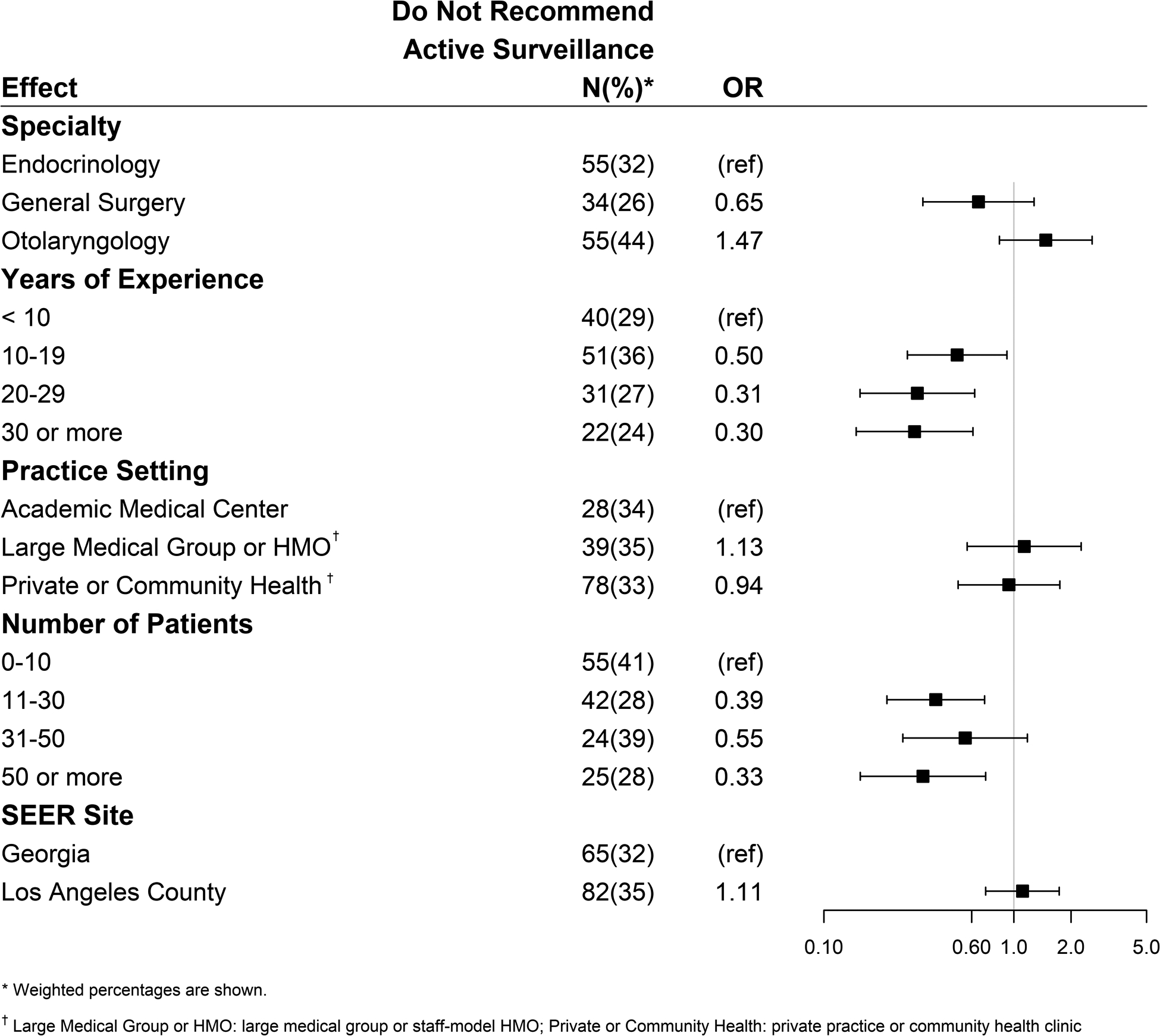
Factors associated with physicians who accept active surveillance as an appropriate management but do not report using it in their practice. (Odds ratios <1 are less likely and odds ratios >1 are more likely to recommend active surveillance).
All physicians were asked “In patients for whom active surveillance is clinically appropriate, which of the following are barriers to implementing active surveillance for thyroid cancer? Please select ALL that apply.” (Figure 3) The most commonly reported barriers to using active surveillance were related to patient factors including: Patient does not want (80%), loss to follow-up concern (78%), and will lead to more patient worry (58%). The most common barriers reported related to physician factors included: malpractice lawsuit concern (51%), concern risk misclassified (50%), unknown length of follow-up necessary (49%), colleagues not using it (47%), and no optimal surveillance strategy (45%). Only 5% reported that active surveillance was never an appropriate management option.
Figure 3.
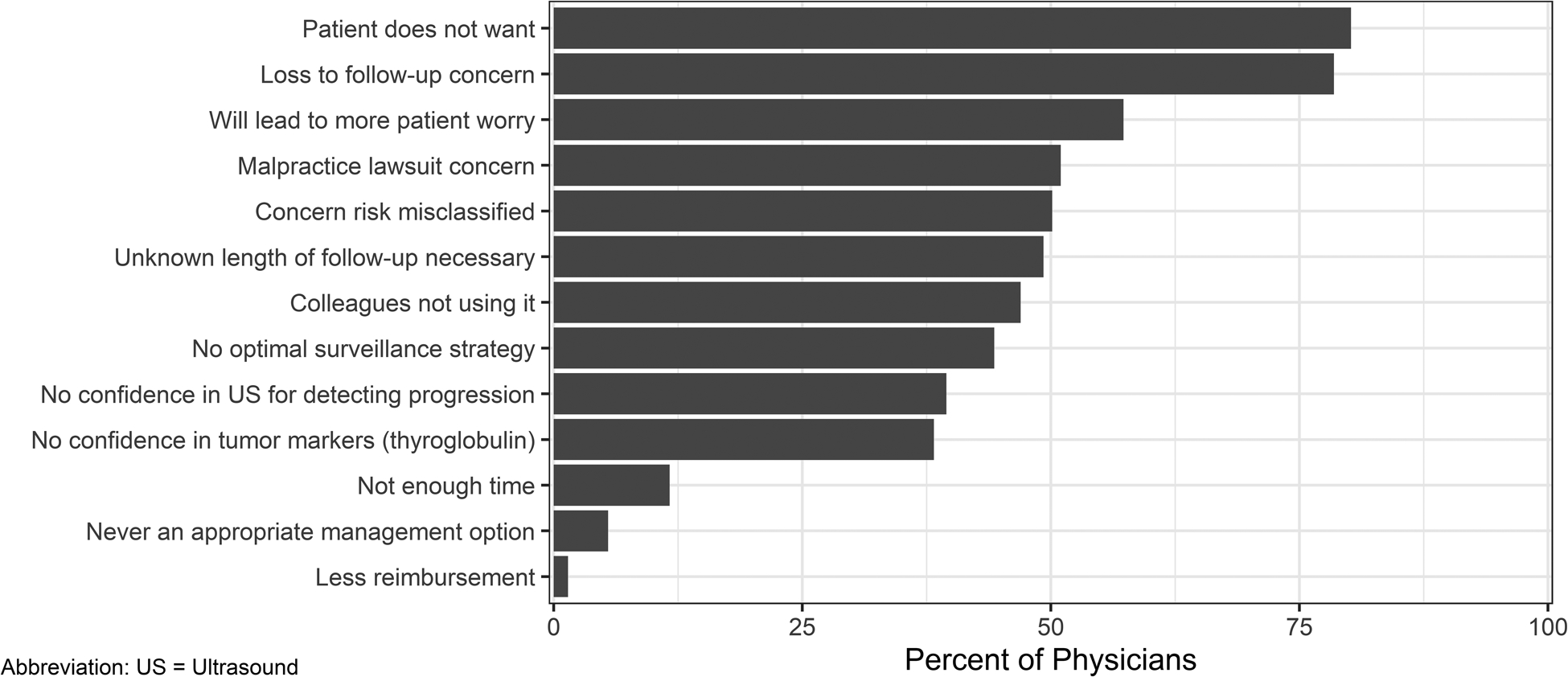
Percentage of surveyed physicians who reported each of the respective barriers to active surveillance implementation.
DISCUSSION:
The key findings of this study of a diverse cohort of physicians managing patients with thyroid cancer demonstrates that while more than three quarters of physicians agree that active surveillance is a valid option for some patients with low risk thyroid cancer, less than half actually use active surveillance in their practice. Physicians who practice in an academic setting were more likely to accept the use of active surveillance. Physicians who accepted that active surveillance was appropriate, but didn’t use it themselves, were more likely to have more years in practice and greater patient volume. When the acceptance of active surveillance was compared between general surgeons and otolaryngologists there was significantly higher acceptance by otolaryngologists, however when other variables were controlled for and endocrinologists were used as the reference group there was no longer a significant difference based on surgical specialty. The possible reasons for this difference is unclear, but speculation suggests this may be related to differences in training, variation in the dissemination of research supporting non-operative management, or differences in other variables such as practice type, patient volume or patient demographics between general surgeons and otolaryngologists. The majority of surveyed physicians did not report that the validity of active surveillance as a management option was a barrier to its use as only 5% of respondents indicated that active surveillance was “never” an appropriate management option for patients with thyroid cancer. However, this study did reveal that there are multiple barriers to the use of active surveillance, including perceived obstacles for both patients and physicians.
This study is innovative as it focuses on the influence of physician factors and physician perception of patient factors on the acceptance of and use of active surveillance for thyroid cancer. This study complements prior work by others that focused on patient perception of active surveillance. The patient factors which influence acceptance of active surveillance have been previously studied and include patient concerns about well-being, the risks of thyroid surgery, the disruptive nature of having surgery, and their confidence in the health care team.22 Conversely, similar to other cancers such as prostate, others studying thyroid cancer and active surveillance have noted that some patients choose surgery over active surveillance due to a desire for definitive treatment, to avoid worry during active surveillance follow-up, and to address the emotional aspects of being diagnosed with cancer.5, 23 We recognize that both the specific disease characteristics and patient-centered decision making are key components for proceeding with active surveillance or surgery for low risk thyroid cancer. However, the physician has to first offer and endorse active surveillance as a management option and physician perceptions of both their own barriers and their patients’ barriers can influence the shared decision-making process. In our study the most common barrier to active surveillance reported by physicians was their patients’ willingness to be managed with active surveillance over surgery. This concern is supported by a prior patient-centric study that evaluated the effects that a diagnosis of “cancer” can have on patient decision making. This prior study demonstrated a tendency for patients to pursue treatment with surgery rather than active surveillance once a diagnosis of cancer is made, even if the possibility of surgical complications outweighs the risk of significant harm with active surveillance.24 In addition to patient barriers, the other commonly reported barriers to implementing active surveillance identified by our study include the logistical issues of surveillance follow-up such as worry that patients would be lost to follow-up, that physicians would misclassify a patient’s risk, that the duration of follow-up needed with active surveillance was unclear, and that the optimal strategy for surveillance in active surveillance remained undefined.
The strengths of our study include the diverse cohort of physicians responding to the survey which included both high, medium and low volume providers who had varying lengths of time in practice and a diverse specialty mix. The limitations of this study include the fact that the survey asked physicians to recall their practice of recommending active surveillance for their general population of thyroid cancer patients. This could introduce recall bias. Additionally, the survey asked if active surveillance was currently used by the physician but did not quantify how often active surveillance was being recommended for appropriate low risk thyroid cancer patients. This may lead to an overestimation of the meaningful use of active surveillance.
As there has been a shift from more to less intensive management for thyroid cancer, the concept of active surveillance for thyroid cancer has gained attention. Active surveillance is an accepted management strategy for other low risk cancers, such as prostate, however, as demonstrated in this study, there are clear barriers to its use for thyroid cancer. Benefits of active surveillance include the absence of surgical risks and potential to tailor management to disease severity. But, prior to broad implementation, multiple obstacles need to be addressed including managing patient worry, creating the infrastructure necessary to avoid loss to follow-up, and improving patient and physician awareness of risks-benefits. Initiatives to address these obstacles could include national organization-sponsored patient education programs about the low risk nature of most thyroid cancer, development of national guidelines on the specifics of how to follow patients during active surveillance and development of national tracking systems to prevent patients being lost to follow-up.
As far as we know, this study is the first to evaluate physician-reported acceptance and use of active surveillance as well as perceived barriers to wide-spread implementation in a diverse cohort of physicians who treat a population-based cohort of thyroid cancer patients. The study’s findings highlight the obstacles to implementing active surveillance broadly and emphasize the need for clinical care guidelines to give specific recommendations on the how and when to use active surveillance.
In summary, physicians managing patients with thyroid cancer have widely accepted active surveillance as an appropriate management option for patients with low risk thyroid cancer, however less than half currently recommend active surveillance for their patients. Physicians report many obstacles to the use of active surveillance, including patient, physician, and logistical barriers. Developing approaches to address existing barriers is key to improving uptake of active surveillance.
ACKNOWLEDGMENTS:
This study is supported by the National Cancer Institute (NCI) Grant No. R01 CA201198 to Principal Investigator, Dr. Megan Haymart. Dr. Haymart also receives funding from R01 HS024512 from the Agency for Healthcare Research and Quality (AHRQ). The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; the Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under Cooperative Agreement No. 5NU58DP003862-04/DP003862; the NCI’s SEER Program under Contract No. HHSN261201000035C awarded to the University of Southern California. The collection of cancer incidence data in Georgia was supported by Contract No. HHSN261201800003I, Task Order No. HHSN26100001 from the NCI, and Cooperative Agreement No. 5NU58DP003875-04 from the CDC. The ideas and opinions expressed herein are those of the authors, and endorsement by the State of California and State of Georgia Departments of Public Health, the NCI, and the CDC or their contractors and subcontractors is not intended nor should be inferred.
Conflicts of Interest and Source of Funding:
This study is supported by the National Cancer Institute (NCI) Grant No. R01 CA201198 to Principal Investigator, Dr. Megan Haymart. Dr. Haymart also receives funding from R01 HS024512 from the Agency for Healthcare Research and Quality (AHRQ). For the remaining authors none were declared.
Contributor Information
David T. Hughes, Department of Surgery, Michigan Medicine, University of Michigan, Ann Arbor, MI.
David Reyes-Gastelum, Department of Internal Medicine, Michigan Medicine, University of Michigan, Ann Arbor, MI.
Kevin C. Ward, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, GA.
Ann S. Hamilton, Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA.
Megan R. Haymart, Department of Internal Medicine, Michigan Medicine, University of Michigan, Ann Arbor, MI.
REFERENCES:
- 1.American Cancer Society: Key Statistics for Thyroid Cancer. Available at: https://www.cancer.org/cancer/thyroid-cancer/about/key-statistics.html. Accessed March 4, 2020.
- 2.Lim H, Devesa SS, Sosa JA, et al. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974–2013. JAMA 2017; 317(13):1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes DT, Haymart MR, Miller BS, et al. The most commonly occurring papillary thyroid cancer in the United States is now a microcarcinoma in a patient older than 45 years. Thyroid 2011; 21(3):231–6. [DOI] [PubMed] [Google Scholar]
- 4.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016; 26(1):1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haymart MR, Miller DC, Hawley ST. Active Surveillance for Low-Risk Cancers - A Viable Solution to Overtreatment? N Engl J Med 2017; 377(3):203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papaleontiou M, Hughes DT, Guo C, et al. Population-Based Assessment of Complications Following Surgery for Thyroid Cancer. J Clin Endocrinol Metab 2017; 102(7):2543–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovatch KJ, Reyes-Gastelum D, Hughes DT, et al. Assessment of Voice Outcomes Following Surgery for Thyroid Cancer. JAMA Otolaryngol Head Neck Surg 2019; 145:823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito Y, Uruno T, Nakano K, et al. An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid 2003; 13(4):381–7. [DOI] [PubMed] [Google Scholar]
- 9.Tuttle RM, Fagin JA, Minkowitz G, et al. Natural History and Tumor Volume Kinetics of Papillary Thyroid Cancers During Active Surveillance. JAMA Otolaryngol Head Neck Surg 2017; 143(10):1015–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brito JP, Ito Y, Miyauchi A, et al. A Clinical Framework to Facilitate Risk Stratification When Considering an Active Surveillance Alternative to Immediate Biopsy and Surgery in Papillary Microcarcinoma. Thyroid 2016; 26(1):144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel KN, Yip L, Lubitz CC, et al. The American Association of Endocrine Surgeons Guidelines for the Definitive Surgical Management of Thyroid Disease in Adults. Ann Surg 2020; 271(3):e21–e93. [DOI] [PubMed] [Google Scholar]
- 12.Miyauchi A, Ito Y, Oda H. Insights into the Management of Papillary Microcarcinoma of the Thyroid. Thyroid 2018; 28(1):23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito Y, Miyauchi A, Kihara M, et al. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid 2014; 24(1):27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oda H, Miyauchi A, Ito Y, et al. Incidences of Unfavorable Events in the Management of Low-Risk Papillary Microcarcinoma of the Thyroid by Active Surveillance Versus Immediate Surgery. Thyroid 2016; 26(1):150–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho SJ, Suh CH, Baek JH, et al. Active Surveillance for Small Papillary Thyroid Cancer: A Systematic Review and Meta-Analysis. Thyroid 2019; 29(10):1399–1408. [DOI] [PubMed] [Google Scholar]
- 16.Surveillance, Epidemiology, and End Results (SEER) Program Populations (1969–2017) National Cancer Institute, DCCPS, Surveillance Research Program 2018. Available at: https://seer.cancer.gov/popdata/. Accessed March 2, 2020.
- 17.Hughes DT, Reyes-Gastelum D, Kovatch KJ, et al. Energy level and fatigue after surgery for thyroid cancer: A population-based study of patient-reported outcomes. Surgery 2020; 167(1):102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Association for Public Opinion Research- Response Rates. Available at: https://www.aapor.org/Education-Resources/For-Researchers/Poll-Survey-FAQ/Response-Rates-An-Overview.aspx. Accessed October 29, 2019.
- 19.Haymart MR, Banerjee M, Yang D, et al. Variation in the management of thyroid cancer. J Clin Endocrinol Metab 2013; 98(5):2001–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haymart MR, Banerjee M, Yang D, et al. Referral patterns for patients with high-risk thyroid cancer. Endocr Pract 2013; 19(4):638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esfandiari NH, Hughes DT, Reyes-Gastelum D, et al. Factors Associated With Diagnosis and Treatment of Thyroid Microcarcinomas. J Clin Endocrinol Metab 2019; 104(12):6060–6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawka AM, Ghai S, Yoannidis T, et al. A Prospective Mixed-Methods Study of Decision-Making on Surgery or Active Surveillance for Low-Risk Papillary Thyroid Cancer. Thyroid 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nickel B, Brito JP, Moynihan R, et al. Patients’ experiences of diagnosis and management of papillary thyroid microcarcinoma: a qualitative study. BMC Cancer 2018; 18(1):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dixon PR, Tomlinson G, Pasternak JD, et al. The Role of Disease Label in Patient Perceptions and Treatment Decisions in the Setting of Low-Risk Malignant Neoplasms. JAMA Oncol 2019; 5(6):817–823. [DOI] [PMC free article] [PubMed] [Google Scholar]


