ABSTRACT
Carbapenem-resistant hypervirulent Klebsiella pneumoniae (CR-hvKP) poses a significant public health challenge worldwide, but research on IMP-producing CR-hvKP and its tigecycline resistance is extremely scarce. We report herein the recovery of two IMP-4–producing, capsular serotype K2, sequence type 65 (K2-ST65), hypervirulent K. pneumoniae isolates (C1672 and C2051), which caused severe and fatal infections in ICU patients, after retrospectively screening 3,285 carbapenem-resistant K. pneumoniae isolates from 26 provinces in China. Notably, C2051 also demonstrated tigecycline nonsusceptibility, mediated by a frameshift mutation in the TetR/AcrR family transcriptional regulator. Both strains harbored blaIMP-4 and critical plasmid-borne virulence genes (rmpA/rmpA2, iucA, and iroN) and demonstrated high virulence in Galleria mellonella, indicating CR-hvKP. The blaIMP-4 gene was located on the IncU- and IncN-type plasmids, which showed high stability in C1672 and C2051 after serial passage for 5 days, with retention rates of 87% and 93.7%, respectively. No significant differences in growth rates were observed among the parental strains and the corresponding resistance plasmid-cured mutants (P = 0.5273), suggesting that strains carrying the blaIMP and virulence plasmids have the potential to exist for a long time without compromising fitness. The genetic environments of the blaIMP-4 gene in both strains were similar, and it has been inferred that the genetic regions of blaIMP-4 were inserted into different backbones. Several conjugal transfer genes, such as traO, traE, traN, and traBCD, were identified in the blaIMP-4-bearing plasmid of C2051, suggesting a higher ability for plasmid transmission. The convergence of IMP carbapenemase and tigecycline nonsusceptibility in a classic hypervirulent K. pneumoniae lineage highlights the need to enhance clinical awareness and epidemiologic surveillance.
IMPORTANCE To date, research on IMP-producing CR-hvKP is extremely scarce. Only one case of urinary tract infection caused by an IMP-6–producing K1-ST23 hypervirulent K. pneumoniae isolate in Japan was recorded, with a limited description of clinical information and genomic features. None of the published studies examined the virulence of the reported strains or the stability and fitness of resistance plasmids or presented a phylogenetic analysis. This dearth of data is notable because CR-hvKP infections are increasingly identified, but critical characteristics of the emerging resistance mediated by IMP carbapenemases in CR-hvKP remain unknown. Here, we report the emergence of two IMP-4 carbapenemase-producing K2-ST65 hypervirulent K. pneumoniae isolates that caused severe and fatal infections in clinical settings in China. Notably, one of them also demonstrated tigecycline nonsusceptibility. These strains carrying blaIMP and virulence plasmids had the potential to exist for a long time without compromising their fitness, highlighting the urgent need to enhance clinical awareness and epidemiologic surveillance to prevent their dissemination.
KEYWORDS: tigecycline nonsusceptibility, carbapenem-resistant hypervirulent Klebsiella pneumoniae, IMP carbapenemase, fitness
INTRODUCTION
The emergence of carbapenem resistance in hypervirulent Klebsiella pneumoniae strains poses a significant public health challenge worldwide (1). Carbapenem-resistant hypervirulent K. pneumoniae (CR-hvKP) is recognized as an important pathogen of clinical concern, with a propensity to cause severe and life-threatening infections (2). Although CR-hvKP cases have mainly been sporadically reported, a drift toward increasing prevalence has been observed (3), and such strains have disseminated rapidly in China (2–6).
Previous studies have elucidated that the CR-hvKP strains have evolved either from classic hypervirulent K. pneumoniae (e.g., capsular serotype K1, sequence type 23 [K1-ST23] and K2-ST65 clones) by acquiring resistance plasmids harboring carbapenemase genes or from carbapenem-resistant K. pneumoniae (e.g., K47-ST11 and K64-ST11 clones) by acquiring a pLVPK-like virulence plasmid (7). Furthermore, CR-hvKP has also emerged by acquiring a hybrid plasmid coharboring carbapenem resistance and virulence genes (8, 9). The most dominant carbapenem resistance genes detected in CR-hvKP are blaKPC and blaNDM (7). To date, research on IMP-producing CR-hvKP has been extremely scarce, with only one case recorded (10). Recently, Harada et al. described one case of urinary tract infection caused by an IMP-6–producing K1-ST23 hypervirulent K. pneumoniae isolate in Japan, underlining the importance of tracking such emerging resistance (10).
Carbapenem-resistant Enterobacteriaceae are often resistant to almost all available antibiotics, except tigecycline, colistin, and ceftazidime-avibactam (11). However, the clinical use of colistin is limited by toxicity and ceftazidime-avibactam is compromised by metallo-β-lactamase–producing bacteria, leaving tigecycline as the last therapeutic option (12). Studies on tigecycline resistance among CR-hvKP have been extremely limited until now. We searched the PubMed database using the terms “carbapenem-resistant hypervirulent K. pneumoniae” OR “carbapenemase-producing hypervirulent K. pneumoniae” AND “tigecycline” for articles published up to 1 June 2021 with no language restrictions. Only nine papers were found, four of which were on tigecycline nonsusceptible CR-hvKP, with the majority being identified in ST11 CR-hvKP strains (13–15). Here, we report the carriage of the carbapenemase gene blaIMP-4 in two K2-ST65 hypervirulent K. pneumoniae isolates in China, and notably, one of them demonstrated reduced tigecycline susceptibility mediated by an efflux pump. Importantly, to the best of our knowledge, this is the first known report of the emergence of tigecycline nonsusceptibility in CR-hvKP of the K2-ST65 lineage, highlighting the need for further surveillance.
RESULTS AND DISCUSSION
Clinical and microbiological characterization of strains C1672 and C2051.
Strain C2051 was hospital-acquired and recovered from a blood sample of a 74-year-old male patient with a history of appendectomy, pulmonary disease, and malignant lymphoma. The patient developed a liver abscess, endophthalmia, severe abdominal infection, intestinal obstruction, intestinal fistula, thrombocytopenia, bloodstream infection, and septic shock during hospitalization. He received treatment with imipenem plus central venous catheter, urinary catheter, mechanical ventilation, and gastric intubation; however, he finally died of multiple organ failure. A second strain, C1672, was recovered from the sputum of a 52-year-old man with ventilator-associated pneumonia in the intensive care unit. Combination antimicrobial therapy, including cefotaxime-sulbactam, moxifloxacin, and amikacin, was used for the treatment. The central venous catheter, urinary catheter, and tracheal cannula were inserted during hospitalization.
Both strains belonged to the K2-ST65 lineage and were resistant to third-generation cephalosporins and carbapenems due to their IMP-4 carbapenemase production. They were also intrinsically resistant to cefotaxime-avibactam. In addition, C2051 was not susceptible to tigecycline (MIC = 4 mg/liter). C1672 and C2051 presented as mucoid colonies on agar plates but tested negative for the string test, as the viscous strings formed were below 5 mm when stretching colonies using an inoculation loop. In addition, both strains exhibited intermediate resistance to serum killing in vitro (Fig. 1a). In the Galleria mellonella infection model, C1672 and C2051 demonstrated virulence higher than or similar to that of the positive-control strain NTUH-K2044 after 72 h of infection (Fig. 1b), suggesting that these isolates were hypervirulent. The patient demographics, clinical presentations, outcomes, and the microbiological features of these two strains are summarized in Tables 1 and 2.
FIG 1.
In vitro and in vivo virulence of C1672 and C2051. (a) Serum killing assay of C1672 and C2051. Strains from our previous study, C639 and C789, which were sensitive or resistant, respectively, to the serum killing, were used as negative and positive controls. Data are mean values ± standard errors of the means (SEM). (b) Virulence of C1672 and C2051 in the G. mellonella infection model. PBS, strain ZR2, and strain NTUH-K2044 were used as the blank, negative, and positive controls, respectively.
TABLE 1.
Clinical characteristics of two patients infected with IMP-4–producing CR-hvKP isolates in China
| Characteristic | Value or information for patient from whom indicated isolate was obtained |
|
|---|---|---|
| C1672 | C2051 | |
| Age (yr) | 52 | 74 |
| Sex | Male | Male |
| City | Xuzhou | Lanzhou |
| Hospital ward | ICU | ICU |
| Type of specimen | Sputum | Blood |
| Date of isolation | 17 April 2016 | 23 December 2015 |
| Clinical characteristic | ||
| Underlying disease(s) | Unknown | Appendectomy, pulmonary disease, malignant lymphoma |
| Clinical presentation(s) | Ventilator-associated pneumonia | Liver abscess, endophthalmia, severe abdominal infection, intestinal obstruction, intestinal fistula, thrombocytopenia, bloodstream infection, septic shock |
| Laboratory findings | ||
| Temp (°C) | 38.3 | 39.1 |
| White blood cell count/mm3 | 11.9 × 109 | 10.88 × 109 |
| Indwelling devices | Central venous catheter, urinary catheter, tracheal cannula | Central venous catheter, urinary catheter, mechanical ventilation, and gastric tube |
| Outcome | Discharged | Died |
TABLE 2.
Microbiological characteristics of IMP-4–producing CR-hvKP isolates from two patients in China
| Characteristic | ||
|---|---|---|
| C1672 | C2051 | |
| MIC (mg/liter) of: | ||
| Cefoxitin | >256 | >256 |
| Cefotaxime | 32 | 256 |
| Ceftriaxone | 128 | 256 |
| Ceftazidime | 256 | >256 |
| Cefepime | 16 | 64 |
| Piperacillin-tazobactam | 4 | 256 |
| Cefoperazone-sulbactam | 64 | 128 |
| Imipenem | 1 | 2 |
| Meropenem | 2 | 2 |
| Ertapenem | 4 | 8 |
| Amikacin | 8 | 1 |
| Ciprofloxacin | 0.5 | 16 |
| Levofloxacin | 0.5 | 8 |
| Minocycline | 2 | 128 |
| Chloramphenicol | >128 | 64 |
| Fosfomycin | 128 | 64 |
| Aztreonam | 256 | 256 |
| Tigecycline | 0.5 | 4 |
| Colistin | 0.25 | 0.5 |
| Ceftazidime-avibactama | >256 | >256 |
| Serotype and sequence type | K2-ST65 | K2-ST65 |
| String test | Negative | Negative |
| Resistance replicon; size (bp) | IncU; 328,921 | IncN; 54,214 |
| Resistance determinant | bla IMP-4 | bla IMP-4 |
| Virulence replicon; size (bp) | IncHI1B/IncFIB; 240,271 | IncHI1B/IncFIB; 236,472 |
| Major virulence genes | ||
| Capsule regulators | rmpA, rmpA2 | rmpA, rmpA2 |
| Siderophores | iroN, iutA, iucABCD | iroN, iutA, iucABCD |
| Serum killing | Intermediate | Intermediate |
| Virulence in G. mellonella model | Similar to NTUH-K2044 | Higher than NTUH-K2044 |
Intrinsic resistance.
The AcrAB-TolC efflux pump in tigecycline nonsusceptibility.
In the absence of tigecycline, the bacterial growth rates of strain C2051 with or without phenyl-arginine-β-naphthylamide (PAβN) were similar, suggesting that the presence of PAβN at a concentration of 25 mg/liter did not affect the growth of the bacteria. The addition of the efflux pump inhibitor PAβN reversed tigecycline nonsusceptibility, with a decrease in the MIC of tigecycline from 4 mg/liter to ≤0.5 mg/liter. PAβN is known to inhibit resistance-nodulation-division (RND) efflux pumps (16). Therefore, the nonsusceptibility to tigecycline in strain C2051 was likely related to the activity of efflux pumps.
The most-critical and best-studied efflux pump in members of the Enterobacteriaceae is AcrAB-TolC, which belongs to the RND family (17). It consists of the inner-membrane transport protein AcrB, outer-membrane channel TolC, and membrane fusion protein AcrA (17). The expression of AcrAB-TolC is mediated by a few local and global regulators, such as AcrR, MarAR, and SoxSR. Thus, we checked the mutations, insertions, or deletions in genes that mediate tigecycline resistance, namely, acrA, acrB, acrR, marA, oqxAB, oqxR, lon, ramA, ramR, rarA, and soxS. A frameshift mutation (2-bp deletion) in the TetR/AcrR family transcriptional regulator was identified in strain C2051. Furthermore, strain C2051 was negative for the plasmid-mediated tigecycline resistance mechanism tet(X), which encodes the tetracycline-inactivating enzyme, and the RND-type efflux pump gene cluster tmexCD1-toprJ1, which was recently reported to confer resistance to tigecycline (18, 19). Meanwhile, none of the novel mobile tigecycline resistance mechanisms were discovered, as tigecycline-nonsusceptible transconjugants were not acquired in the conjugation assays.
Stability of carbapenem resistance and its impact on fitness.
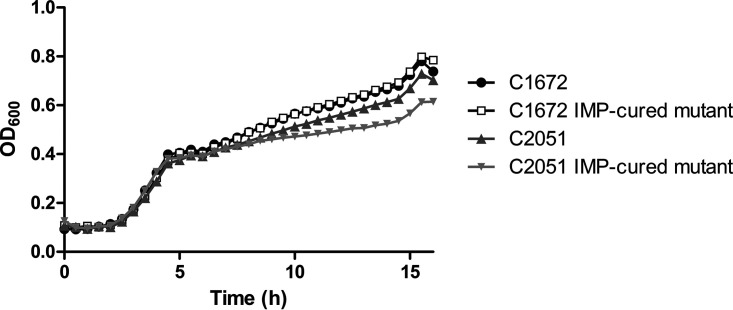
Infections caused by IMP-producing CR-hvKP have rarely been reported (10). We speculated that the cooccurrence of a blaIMP-bearing plasmid and a virulence plasmid may bring a high fitness cost to the host strain; therefore, resistance plasmid stability and strain fitness after acquiring resistance plasmids were evaluated. However, to our surprise, the resistance plasmid harboring blaIMP demonstrated high stability after serial passage for 5 days in C1672 and C2051, with retention rates of 87% and 93.7%, respectively. Furthermore, no significant differences in the growth rates were observed between the parental strain and the corresponding resistance plasmid-cured mutants (P = 0.5273) (Fig. 2). Taken together, these results indicate that strains carrying the blaIMP gene and virulence plasmid have the potential to exist for a long time without compromising fitness, raising our concern about this emerging resistance in clinical settings.
FIG 2.
Fitness of C1672/C2051 and their corresponding plasmid-cured mutants. There were no significant differences in growth between the parental strains and the corresponding resistance plasmid-cured mutants (P = 0.5273).
Genomic features.
The whole-genome sequences of C1672 and C2051 were obtained by hybrid de novo assembly of short- and long-read sequencing data. The complete genome of strain C1672 was assembled into three contigs, including one chromosome (5,345,742 bp), one virulence plasmid (240,271 bp, designated pVir-C1672), and one multidrug resistance plasmid (328,921 bp, designated pRes-C1672) harboring blaIMP-4. Several resistance genes that mediate resistance to β-lactams (blaSHV-12), sulfonamides (sul1 and sul2), aminoglycosides [aac(6′)-Ib-cr, aac(3)-IId, aph(3′')-Ib, aph(6)-Id, and armA], rifampin (arr3), quinolone (qnrS1), macrolides [msr(E) and mph(E)], and chloramphenicol (catA2) were also found in the resistance plasmid pRes-C1672. The genome assembly for C2051 was composed of a 5,280,186-bp chromosome and three plasmids, including one 236,472-bp virulence plasmid (designated pVir-C2051) and two resistance plasmids, one of which was a 54,214-bp resistance plasmid (designated pRes-C2051) bearing blaIMP-4 and qnrS1 and the other one a 131,632-bp IncFII plasmid carrying blaCTX-M-15, blaTEM-1, sul1, sul2, aph(3′')-Ib, aph(6)-Id, aph(3′)-Ia, aac(3)-IId, aac(6′)-Ib-cr, mph(A), qnrB, aadA16, dfrA27, and arr-3. Each isolate harbored a pLVPK-like virulence plasmid belonging to the IncHI1B/IncFIB replicon that was highly similar to plasmid pLVPK (GenBank accession no. AY378100), with more than 90% coverage and 99% identity (Fig. 3a). Another pLVPK-like plasmid, pTHC11-1 (accession no. AP019549), which was found in an IMP-6-producing K1-ST23 CR-hvKP isolate, was also used in the comparative analysis, and the results showed they all shared high similarity (Fig. 3a).
FIG 3.
Comparative genomic analysis of the virulence and resistance plasmids. (a) Alignment analysis of virulence plasmid sequences among pVir-C1672, pVir-C2051, and previously reported virulence plasmids, including pLVPK (accession no. AY378100) and pTHC11-1 (accession no. AP019549). (b) The region containing resistance genes in pRes-C1672 and pRes-C2051. (c) Comparison of pRes-C1672 and pRes-C2051 resistance plasmids from this study with 10 complete resistance plasmids bearing blaIMP that originated from K. pneumoniae strains and whose sequences were downloaded from the RefSeq database, as well as pTHC11-2 (accession no. AP019550). The maximum-likelihood phylogenetic tree was built by RaxML from an alignment generated by Roary and filtered to remove recombination using ClonalFrameML. The Interactive Tree Of Life (https://itol.embl.de) was used for visualization. The bootstraps are shown as blue circles on the branches. (d) Alignment analysis of resistance plasmid sequences among pRes-C1672, pRes-C2051, and previously reported resistance plasmids pTHC11-2 (accession no. AP019550) and GCF_008632115.1 (accession no. CM018324).
The blaIMP-bearing plasmids, pRes-C1672 and pRes-C2051, were not identical and belonged to the IncU and IncN replicons, respectively. The IncN replicon of C2051 was a canonical pIMP-4-like resistance plasmid that was highly similar to the plasmid bearing blaIMP-4 p13SP-IMP (GenBank accession no. MH909334.1), with more than 100% coverage and 99.95% identity, whereas blaIMP-4 was borne by a new ST65 IncU plasmid in the C1672 isolate. The blaIMP-4 gene was in a class 1 integron with the gene arrangement of the retron-type RNA-directed DNA polymerase-blaIMP-4-IntI1 (Fig. 3b). The genetic environments of the blaIMP-4 gene in pRes-C1672 and pRes-C2051 were very similar, except that the IS6 family transposase was inserted in the downstream region of the blaIMP-4 gene in pRes-C2051 (Fig. 3b). Thus, we inferred that the genetic regions of blaIMP-4 were inserted into different backbones.
Since the conventional hypervirulent K. pneumoniae lineage (K2-ST65) has been associated with the carriage of a pLVPK-like plasmid, we speculated that C1672 and C2051 originally carried the virulence plasmids (pVir-C1672 and pVir-C2051) and acquired the resistance plasmids. We analyzed the phylogenetic information of the resistance plasmids (pRes-C1672 and pRes-C2051) from this study, 10 complete resistance plasmids bearing blaIMP originating from K. pneumoniae whose sequences we downloaded from the RefSeq database, and pTHC11-2 (accession no. AP019550), which is an IncN plasmid harboring blaIMP-6 isolated from a K1-ST23 CR-hvKP strain in Japan, as mentioned above. The maximum-likelihood phylogenetic tree showed a link between pRes-C2051 and GCF_008632115.1 (accession no. CM018324), which was isolated from a K28-ST1873 carbapenem-resistant K. pneumoniae isolate from Hangzhou, China (Fig. 3c). To explain why pRes-C2051 could be transferred into the classic hypervirulent K. pneumoniae K2-ST65 lineage, we further compared pRes-C2051 with GCF_008632115.1 (accession no. CM018324) and pTHC11-2 (accession no. AP019550). Compared with GCF_008632115.1, several conjugal transfer genes, such as the traO, traE, traN, traB, traC, and traD genes, were identified in pRes-C2051, suggesting a higher ability for bacterial conjugation and transmission in C2051 (Fig. 3d).
In conclusion, we report the emergence of two IMP-4 carbapenemase-producing K2-ST65 hypervirulent K. pneumoniae isolates that caused severe and fatal infections in clinical settings in China. Notably, one of them also demonstrated tigecycline nonsusceptibility, most likely mediated by a frameshift mutation in the TetR/AcrR family transcriptional regulator. These strains, carrying blaIMP and virulence plasmids, have the potential to exist for a long time without compromising their fitness, highlighting the urgent need to enhance clinical awareness and epidemiologic surveillance to generate essential data for preventing their dissemination.
MATERIALS AND METHODS
Bacterial isolates and detection of resistance and virulence genes.
A total of 3,285 carbapenem-resistant K. pneumoniae isolates were retrospectively collected from 26 provinces across China between 2014 and 2019. Species identification was confirmed by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (Bruker Daltonics, Billerica, MA, USA), and isolates were stored at −80°C until use. Carbapenem resistance was defined as nonsusceptibility to at least one carbapenem agent according to the CLSI breakpoints (imipenem, ≥2 mg/liter; meropenem, ≥2 mg/liter; and ertapenem, ≥1 mg/liter) or strains with carbapenemase production (20, 21).
All isolates were screened for the cocarriage of both a blaIMP-bearing resistance plasmid and a pLVPK-like virulence plasmid by detecting the carbapenemase gene blaIMP and critical plasmid-borne virulence genes, including genes associated with hypermucoviscosity (rmpA and rmpA2), aerobactin (iucA), and salmochelin (iroN), using PCR and Sanger sequencing (3, 22). Positive results for all four of these virulence genes in a strain were considered an indicator of the full-length virulence plasmid according to previous studies (2). Two strains (C1672 and C2051) were enrolled in this study because they carried blaIMP and all four virulence genes. Clinical information on cases involving these two strains was reviewed and collected, including demographics, underlying diseases, specimen types, clinical manifestations, antibiotic exposure histories, use of invasive devices, antimicrobial treatments, and outcomes.
Antimicrobial susceptibility testing and microbiological features.
C1672 and C2051 were tested for antimicrobial susceptibility using the agar and broth microdilution method according to CLSI guidelines (23). Pseudomonas aeruginosa strain ATCC 27853 and Escherichia coli strain ATCC 25922 were used as controls. Data were interpreted using CLSI breakpoints, while the criteria for tigecycline followed those established by the U.S. Food and Drug Administration (21, 24). Multilocus sequence typing (MLST) was performed according to the protocol on the Pasteur Institute MLST website (https://bigsdb.pasteur.fr/klebsiella/klebsiella.html). K serotypes (K1, K2, K5, K20, K54, K57, K47, and K64) and hypermucoviscosity were also investigated as previously described (3).
Serum killing assay.
A serum killing assay was conducted to determine in vitro virulence according to previous studies (25). An exponential-phase culture was diluted to 106 CFU/ml. An inoculum of 50 μl of bacterial culture was added to 150 μl of pooled human sera obtained from 10 healthy individuals. Viable counts were checked at 0, 60, 120, and 180 min of incubation at 37°C and 200 rpm. The results were analyzed by comparing the CFU from each time point and expressed as either sensitive, intermediate, or resistant. Carbapenem-resistant K. pneumoniae strains from our previous study (3), C639 and C789, which were sensitive and resistant to serum killing, respectively, were used as negative and positive controls. Each strain was tested three times.
Galleria mellonella infection model.
To determine the in vivo virulence, 250 to 350 mg of pathogen-free G. mellonella larvae were obtained from the Huiyude Biotech Company (Tianjin, China). A mid-log-phase bacterial culture was diluted using phosphate-buffered saline (PBS), and 10 μl (with a concentration of 107 CFU/ml) was injected into the left proleg of each larvae using a microsample syringe (26). PBS, ST11 carbapenem-resistant K. pneumoniae strain ZR2 with low virulence, and K1-ST23 hypervirulent K. pneumoniae strain NTUH-K2044 were used as the blank, negative, and positive controls, respectively. After injection, larvae were kept at 37°C in the dark and mortality rates were observed for 72 h. Ten larvae were used as a sample population, and each strain was tested twice (27).
Efflux pump inhibitory test.
The PAβN inhibitory test was used to explore whether tigecycline nonsusceptibility was mediated by efflux pumps (16, 28). The MIC of tigecycline (Pfizer, NY, USA) was determined according to CLSI recommendations using the broth microdilution method. A stock solution of PAβN (Sigma-Aldrich, Shanghai, China) was prepared at a concentration of 5 mg/ml in sterile water, with its final concentration in Mueller-Hinton broth at 25 mg/liter. Bacterial growth in Mueller-Hinton broth containing tigecycline with and without PAβN was detected in parallel. A growth control with 25 mg/liter PAβN in Mueller-Hinton broth was also added to check the effect of PAβN alone on each strain.
Conjugation experiment.
To explore the presence of a plasmid-mediated resistance mechanism for tigecycline nonsusceptibility, conjugation experiments were performed using C2051 as the donor and E. coli strain J53 as the recipient, according to previous studies (18). The donor and recipient strains were mixed at a ratio of 1:1 for 24 h. The transconjugants were then selected on China blue lactose agar supplemented with tigecycline (1 mg/liter) and sodium azide (100 mg/liter). The transconjugants were confirmed by MALDI-TOF MS, and antimicrobial susceptibility was determined using the broth microdilution method.
Stability assay of carbapenem resistance plasmid and fitness cost analysis.
Resistance plasmid stability was assessed as previously described (29). Briefly, the isolates were cultured in LB broth at 37°C with shaking (200 rpm), and then serial passaging was performed for 5 days with 1:1,000 dilutions in antibiotic-free LB broth. After 5 days, cultures were serially diluted and plated on MH agar plates without antibiotics or with ertapenem (2 mg/liter). The retention rate of the IMP-bearing plasmid was expressed as the percentage of CFU on the MH plate containing ertapenem compared to the number on the antibiotic-free plate. Colonies were selected and subjected to PCR for validation of blaIMP-4 genes.
Furthermore, to determine the potential effects of resistance plasmids on fitness, the growth kinetics of C1672/C2051 and their corresponding plasmid-cured mutants were investigated (18). Overnight cultures were diluted to an optical density at 600 nm (OD600) of ∼0.1 and grown at 37°C with 200-rpm shaking. The culture cell density was determined every 30 min by measuring the OD600.
Whole-genome sequencing and bioinformatics analysis.
Genomic DNA of C1672 and C2051 was prepared using the TIANamp bacterial DNA kit (Tiangen Biotech, Beijing, China) and sequenced using the Illumina HiSeq platform and PacBio RS II system (Pacific Biosciences) to characterize the plasmid. The Unicycler pipeline version 0.4.7 was used for de novo assembly. The draft genome sequence was annotated using Rapid Annotation using Subsystem Technology (RAST) (http://rast.nmpdr.org/) and Prokka. Antimicrobial resistance genes, plasmid replicon types, virulence genes, serotypes, virulence scores, and insertion sequence (IS) elements were analyzed by using the Center for Genomic Epidemiology service (http://www.genomicepidemiology.org/) and several databases, including SerotypeFinder, PlasmidFinder, ResFinder, CARD, VirulenceFinder, VFDB, Kleborate (https://github.com/katholt/Kleborate), ICEberg, and ISFinder. BLAST Ring Image Generator (BRIG) and Easyfig tools were used to visualize the plasmid and genetic context comparisons.
Phylogenetic analysis.
We compared the sequences of resistance plasmids bearing blaIMP from our study with those of other complete resistance plasmids harboring blaIMP isolated from K. pneumoniae strains (Table S1 in the supplemental material) that were downloaded from the RefSeq database. The plasmid sequences were aligned using Roary software (version 3.11.2) (30), and the recombinant regions of the alignments were removed using ClonalFrameML software (version 1.2) before phylogenetic analysis (31). Maximum-likelihood phylogenetic trees were constructed using RAxML software (version 8.2.10) and visualized using the Interactive Tree Of Life (https://itol.embl.de) (32, 33).
Statistical analysis.
Statistical analysis was performed with one-way analysis of variance (ANOVA) using GraphPad Prism version 5. A P value of <0.05 was considered to be statistically significant.
Ethics.
This study was approved by the Ethics Committee of Peking University People’s Hospital (reference number 2019PHE001).
Data availability.
The complete sequences of the two strains in this study, C1672 and C2051, were deposited in the GenBank database (accession no. CP073917 to CP073919 and CP073920 to CP073923).
ACKNOWLEDGMENTS
This study was partly supported by the National Natural Science Foundation of China (grants no. 81961130396, 81991533, and 81625014), and Newton Advanced Fellowship award (grant/award no. NAF009\1002).
Control strains NTUH-K2044 and ZR2 were kindly donated by Dongsheng Zhou (State Key Laboratory of Pathogen and Biosecurity, Beijing Institute of Microbiology and Epidemiology, Beijing, China) and Rong Zhang (Department of Clinical Laboratory Medicine, Second Affiliated Hospital of Zhejiang University, Hangzhou, China).
We declare no conflicts of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Hui Wang, Email: wanghui@pkuph.edu.cn.
Gyanu Lamichhane, Johns Hopkins University School of Medicine.
REFERENCES
- 1.Russo TA, Marr CM. 2019. Hypervirulent Klebsiella pneumoniae. Clin Microbiol Rev 32:e00001-19. doi: 10.1128/CMR.00001-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gu D, Dong N, Zheng Z, Lin D, Huang M, Wang L, Chan EW, Shu L, Yu J, Zhang R, Chen S. 2018. A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: a molecular epidemiological study. Lancet Infect Dis 18:37–46. doi: 10.1016/S1473-3099(17)30489-9. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Jin L, Ouyang P, Wang Q, Wang R, Wang J, Gao H, Wang X, Wang H, China Carbapenem-Resistant Enterobacteriaceae (CRE) Network. 2020. Evolution of hypervirulence in carbapenem-resistant Klebsiella pneumoniae in China: a multicentre, molecular epidemiological analysis. J Antimicrob Chemother 75:327–336. doi: 10.1093/jac/dkz446. [DOI] [PubMed] [Google Scholar]
- 4.Feng Y, Lu Y, Yao Z, Zong Z. 2018. Carbapenem-resistant hypervirulent Klebsiella pneumoniae of sequence type 36. Antimicrob Agents Chemother 62:e02644-17. doi: 10.1128/AAC.02644-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang R, Lin D, Chan EW, Gu D, Chen GX, Chen S. 2016. Emergence of carbapenem-resistant serotype K1 hypervirulent Klebsiella pneumoniae strains in China. Antimicrob Agents Chemother 60:709–711. doi: 10.1128/AAC.02173-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan R, Lu Y, Zhu Y, Lan P, Jiang S, Lu J, Shen P, Yu Y, Zhou J, Jiang Y. 2021. A sequence type 23 hypervirulent Klebsiella pneumoniae strain Presenting carbapenem resistance by acquiring an IncP1 blaKPC-2 plasmid. Front Cell Infect Microbiol 11:641830. doi: 10.3389/fcimb.2021.641830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X, Dong N, Chan EW, Zhang R, Chen S. 2021. Carbapenem resistance-encoding and virulence-encoding conjugative plasmids in Klebsiella pneumoniae. Trends Microbiol 29:65–83. doi: 10.1016/j.tim.2020.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Dong N, Lin D, Zhang R, Chan EW, Chen S. 2018. Carriage of blaKPC-2 by a virulence plasmid in hypervirulent Klebsiella pneumoniae. J Antimicrob Chemother 73:3317–3321. [DOI] [PubMed] [Google Scholar]
- 9.Turton J, Davies F, Turton J, Perry C, Payne Z, Pike R. 2019. Hybrid resistance and virulence plasmids in “high-risk” clones of Klebsiella pneumoniae, including those carrying blaNDM-5. Microorganisms 7:326. doi: 10.3390/microorganisms7090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harada S, Aoki K, Ishii Y, Ohno Y, Nakamura A, Komatsu M, Tateda K. 2019. Emergence of IMP-producing hypervirulent Klebsiella pneumoniae carrying a pLVPK-like virulence plasmid. Int J Antimicrob Agents 53:873–875. doi: 10.1016/j.ijantimicag.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Rossolini GM, Stone GG. 2020. Assessment of the in vitro activity of ceftazidime/avibactam against a global collection of multidrug-resistant Klebsiella spp. from the INFORM surveillance programme (2015–2017). Int J Antimicrob Agents 56:106111. doi: 10.1016/j.ijantimicag.2020.106111. [DOI] [PubMed] [Google Scholar]
- 12.Poirel L, Jayol A, Nordmann P. 2017. Polymyxins: antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin Microbiol Rev 30:557–596. doi: 10.1128/CMR.00064-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang YH, Chou SH, Liang SW, Ni CE, Lin YT, Huang YW, Yang TC. 2018. Emergence of an XDR and carbapenemase-producing hypervirulent Klebsiella pneumoniae strain in Taiwan. J Antimicrob Chemother 73:2039–2046. doi: 10.1093/jac/dky164. [DOI] [PubMed] [Google Scholar]
- 14.Lin ZW, Zheng JX, Bai B, Xu GJ, Lin FJ, Chen Z, Sun X, Qu D, Yu ZJ, Deng QW. 2020. Characteristics of hypervirulent Klebsiella pneumoniae: does low expression of rmpA contribute to the absence of hypervirulence? Front Microbiol 11:436. doi: 10.3389/fmicb.2020.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin X, Chen Q, Shen F, Jiang Y, Wu X, Hua X, Fu Y, Yu Y. 2021. Resistance evolution of hypervirulent carbapenem-resistant Klebsiella pneumoniae ST11 during treatment with tigecycline and polymyxin. Emerg Microbes Infect 10:1129–1136. doi: 10.1080/22221751.2021.1937327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li XZ, Plésiat P, Nikaido H. 2015. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin Microbiol Rev 28:337–418. doi: 10.1128/CMR.00117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camp J, Schuster S, Vavra M, Schweigger T, Rossen JWA, Reuter S, Kern WV. 2021. Limited multidrug resistance efflux pump overexpression among multidrug-resistant Escherichia coli strains of ST131. Antimicrob Agents Chemother 65:e01735-20. doi: 10.1128/AAC.01735-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun S, Gao H, Liu Y, Jin L, Wang R, Wang X, Wang Q, Yin Y, Zhang Y, Wang H. 2020. Co-existence of a novel plasmid-mediated efflux pump with colistin resistance gene mcr in one plasmid confers transferable multidrug resistance in Klebsiella pneumoniae. Emerg Microbes Infect 9:1102–1113. doi: 10.1080/22221751.2020.1768805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohsin M, Hassan B, Martins WMBS, Li R, Abdullah S, Sands K, Walsh TR. 2021. Emergence of plasmid-mediated tigecycline resistance tet(X4) gene in Escherichia coli isolated from poultry, food and the environment in South Asia. Sci Total Environ 787:147613. doi: 10.1016/j.scitotenv.2021.147613. [DOI] [PubMed] [Google Scholar]
- 20.Chea N, Bulens SN, Kongphet-Tran T, Lynfield R, Shaw KM, Vagnone PS, Kainer MA, Muleta DB, Wilson L, Vaeth E, Dumyati G, Concannon C, Phipps EC, Culbreath K, Janelle SJ, Bamberg WM, Guh AY, Limbago B, Kallen AJ. 2015. Improved phenotype-based definition for identifying carbapenemase producers among carbapenem-resistant Enterobacteriaceae. Emerg Infect Dis 21:1611–1616. doi: 10.3201/eid2109.150198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CLSI. 2020. Performance standards for antimicrobial susceptibility testing, 30th ed. M100. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 22.Wang X, Xu X, Li Z, Chen H, Wang Q, Yang P, Zhao C, Ni M, Wang H. 2014. An outbreak of a nosocomial NDM-1-producing Klebsiella pneumoniae ST147 at a teaching hospital in mainland China. Microb Drug Resist 20:144–149. doi: 10.1089/mdr.2013.0100. [DOI] [PubMed] [Google Scholar]
- 23.CLSI. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 11th ed. M07. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 24.FDA. 2009. Tygacil (tigecycline) for injection. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021821s016lbl.pdf.
- 25.Abate G, Koh TH, Gardner M, Siu LK. 2012. Clinical and bacteriological characteristics of Klebsiella pneumoniae causing liver abscess with less frequently observed multi-locus sequences type, ST163, from Singapore and Missouri, US. J Microbiol Immunol Infect 45:31–36. doi: 10.1016/j.jmii.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Insua JL, Llobet E, Moranta D, Pérez-Gutiérrez C, Tomás A, Garmendia J, Bengoechea JA. 2013. Modeling Klebsiella pneumoniae pathogenesis by infection of the wax moth Galleria mellonella. Infect Immun 81:3552–3565. doi: 10.1128/IAI.00391-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Spiropoulos J, Cooley W, Khara JS, Gladstone CA, Asai M, Bossé JT, Robertson BD, Newton SM, Langford PR. 2018. Galleria mellonella—a novel infection model for the Mycobacterium tuberculosis complex. Virulence 9:1126–1137. doi: 10.1080/21505594.2018.1491255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ni W, Li Y, Guan J, Zhao J, Cui J, Wang R, Liu Y. 2016. Effects of efflux pump inhibitors on colistin resistance in multidrug-resistant Gram-negative bacteria. Antimicrob Agents Chemother 60:3215–3218. doi: 10.1128/AAC.00248-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao H, Liu Y, Wang R, Wang Q, Jin L, Wang H. 2020. The transferability and evolution of NDM-1 and KPC-2 co-producing Klebsiella pneumoniae from clinical settings. EBioMedicine 51:102599. doi: 10.1016/j.ebiom.2019.102599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, Holden MT, Fookes M, Falush D, Keane JA, Parkhill J. 2015. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 31:3691–3693. doi: 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Didelot X, Wilson DJ. 2015. ClonalFrameML: efficient inference of recombination in whole bacterial genomes. PLoS Comput Biol 11:e1004041. doi: 10.1371/journal.pcbi.1004041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Letunic I, Bork P. 2021. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res 49:W293–W296. doi: 10.1093/nar/gkab301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download SPECTRUM01305-21_Supp_1_seq2.pdf, PDF file, 0.03 MB (27.7KB, pdf)
Data Availability Statement
The complete sequences of the two strains in this study, C1672 and C2051, were deposited in the GenBank database (accession no. CP073917 to CP073919 and CP073920 to CP073923).