Abstract
BACKGROUND:
Patients with GATA2 deficiency present with nontuberculous mycobacterial infections, severe viral infections (particularly refractory human papillomavirus disease), lymphedema, myelodysplastic syndrome (MDS), and acute myeloid leukemia. Patients with GATA2 deficiency who undergo allogeneic hematopoietic stem cell transplantation prior to the development of life-threatening infections or cytogenetic abnormalities may have optimal clinical outcomes.
OBJECTIVES:
The aim of this article is to determine ways in which oncology nurses can identify GATA2 deficiency in patients early and optimize treatment decisions.
METHODS:
A case study is presented of a 33-year-old man with recurrent infections and MDS and his two sons, all of whom were found to have the same GATA2 mutation.
FINDINGS:
Oncology nurses play an important role in early detection and identification by interviewing patients and obtaining a complete and thorough family history.
Keywords: GATA2 deficiency, myelodysplastic syndrome, acute myeloid leukemia
INDIVIDUALS WHO HAVE GATA2 DEFICIENCY experience severe, life-threatening infections, respiratory problems, deafness, lymphedema, and leukemia. As reviewed by Spinner et al. (2014), GATA2 deficiency was first described by four different research groups, resulting in the following four different names: monocytopenia and mycobacterial infections, or MonoMAC (Hsu et al., 2011; Vinh et al., 2010); deficiencies in dendritic, monocyte B, and natural killer lymphoid cells (Bigley et al., 2011; Dickinson et al., 2011); familial myelodysplastic syndrome (MDS)/acute myeloid leukemia (AML) (Hahn et al., 2011); and Emberger syndrome (Mansour et al., 2010; Ostergaard et al., 2011). Signs and symptoms of GATA2 deficiency first appear in childhood or early adulthood, but patients and family members can have variable presentations. The incidence of this disease is higher than originally thought; as many as 72% of adolescents with MDS and monosomy 7 (a single copy of chromosome 7) have an underlying GATA2 deficiency (Wlodarski et al., 2016).
Allogeneic hematopoietic stem cell transplantation (HSCT) is currently the only definitive therapy for GATA2 deficiency (Cuellar-Rodriguez et al., 2011; Dickinson et al., 2014; Donadieu et al., 2018; Spinner et al., 2014; Wlodarski et al., 2016). HSCT is a treatment option for patients with recurrent, treatment-refractory, severe infections (Cuellar-Rodriguez et al., 2011). Allogeneic HSCT replaces the defective immune and hematopoietic cells in a patient with stem cells from a healthy donor, thereby restoring normal immune and marrow function. However, HSCT has little impact on some manifestations, such as lymphedema. GATA2 deficiency is familial in about half of patients with the disease and de novo in the remainder (Spinner et al., 2014). The GATA2 mutation in the familial cases is inherited as Mendelian dominant, and it functions by loss of activity, or haploinsufficiency.
Healthcare providers can influence the clinical outcomes of these patients by obtaining a detailed family history. This is particularly important for patients with newly diagnosed MDS or AML for whom a pattern of inheritance and whether other family members are potentially affected need to be identified to explore the possibility of a GATA2 mutation. Patients with a GATA2 mutation who undergo HSCT prior to developing life-threatening infections or cytogenetic abnormalities have considerably better disease-free outcomes.
GATA2
The syndrome of monocytopenia and mycobacterial infections, typically Mycobacterium avium, termed MonoMAC, was first recognized at the National Institutes of Health (NIH) (Vinh et al., 2010). It was shown that mutations in the zinc-finger transcription factor GATA2 lead to GATA2 deficiency and explain the MonoMAC syndrome (Hsu et al., 2011). GATA2 regulates gene transcription for hematopoietic cell lineage development and proliferation. As a master regulator of hundreds of genes, GATA2 deficiency results in a constellation of disease manifestations (Genetics Home Reference, 2018). Wlodarski, Collin, and Horwitz (2017) reported that the loss of function of GATA2 will lead to aberrant hematopoiesis with myeloid neoplasia, which can be complicated by acquisition of abnormal cytogenetic results. Király et al. (2018) reported that in familial MDS/AML, several other somatic genetic mutations may further add to and affect GATA2 deficiency.
Nursing Interventions
An accurate family history is a valuable, inexpensive, and often underused tool to manage GATA2 deficiency. Family history provides an opportunity to identify individuals with a predisposition to cancer. By eliciting a detailed three-generation pedigree (family history), oncology nurses can address whether an underlying genetic mutation caused the disease and if screening is indicated for individual patients and family members.
Nurses should be aware of common and standard pedigree symbols. Bennett et al. (1995) defined standardized human pedigree nomenclature. An accurate and detailed family history is essential for the recognition of trends and familial patterns. A standard pedigree is an excellent way to deliver visual representation of a family cancer history and genetic transmission (Mahon, 2016). Although obtaining a family history may come naturally to nurses, putting together a family pedigree requires skills and may take practice and time to develop.
“There is a spectrum of GATA2 mutation, with some family members being severely affected and others harboring it but remaining asymptomatic.”
One approach to genetic counseling is when a practice includes a genetic nurse practitioner (Mahon, 2012). Genetic counseling associated with GATA2 mutation includes genetic education (inheritance, recurrence risk, specific mutation) and assessment of psychosocial aspects, including the possibility of having affected family members, which can create challenges. This is particularly true when children or potential future offspring may be affected with the GATA2 mutation. The possibility of raising a child with special needs, family stress, and psychological stress related to the diagnosis must be considered (Krulik et al., 1999).
Tools and Guidelines for Nurses
Providing additional educational opportunities and resources will help nurses become more proficient and comfortable in the specialized field of genetics and genomics (Montgomery, 2017). Nurses are competent healthcare providers who have the ability to obtain comprehensive and extensive family histories. In addition, nurses could identify family members at risk, help with informed decision making, and assist in interpretation of genetic and genomic test and therapy results. Nurses also can make referrals (to genetic counselors or specialists) for at-risk individuals and family members as deemed appropriate (Calzone et al., 2010; Montgomery, 2017). Although nurses are not expected to become experts or specialists in genetics, they should have a basic knowledge and foundation. Nurses are in the position to provide education, resources, and referrals to patients and their family members.
Nurses are perceived by patients and their family members as educated and trusted healthcare providers. At times, patients may feel comfortable asking, discussing with, and disclosing to nurses concerns related to their personal and family histories that may have previously gone intentionally or unintentionally undisclosed because they were thought to be unremarkable or unrelated. Nurses play a vital role in patient education and advocacy by being sensitive to the needs of their patients and family members (Jenkins, Grady, & Collins, 2005).
Ethical Considerations
Several ethical concerns are related to genetics and genomics. Nurses should be aware of potential ethical dilemmas. Nurses also should be cognizant of the resources available to them, including consultation services and experts trained in this area (e.g., genetic counselors). Specific ethical dilemmas related to patients with a GATA2 mutation and their family members include the possibility of the GATA2 mutation affecting other family members (who may or may not want to be tested) and future offspring. There is a spectrum of GATA2 mutation, with some family members being severely affected and others harboring it but remaining asymptomatic.
Case Study
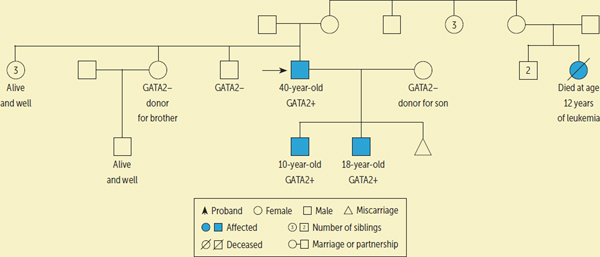
A 33-year-old man had recurrent, disseminated mycobacterial infections, anal condylomata, pulmonary alveolar proteinosis, and MDS. His peripheral blood showed monocytopenia and B and NK cell lymphopenia. GATA2 testing confirmed a heterozygous p.T354M mutation. His family history revealed four asymptomatic siblings and a cousin who died of leukemia. In addition, his younger son had nose bleeds and warts, and his older son was neutropenic with MDS. Both children were subsequently found to have the GATA2 mutation.
The 33-year-old proband received a 10/10 human leukocyte antigen (HLA) matched peripheral blood stem cell transplantation from his 31-year-old sister. Prior to transplantation, his sister was confirmed to not harbor the GATA2 mutation. Following successful HSCT and engraftment, the patient developed steroid-responsive graft-versus-host disease (GVHD) (skin, liver, gastrointestinal) and cryptogenic organizing pneumonia (which has since resolved). The patient is currently alive and well. He is 40 years old, is 7 years post-transplantation, is off immunosuppression without GVHD, and has complete resolution of GATA2-related hematologic deficiencies (see Figure 1).
FIGURE 1.:
CASE STUDY PEDIGREE FOR PROBAND WITH GATA2 DEFICIENCY
At the time of his father’s HSCT, the 4-year-old son was found to have GATA2 deficiency as well. Because he has remained clinically stable, HSCT is not indicated at this time. He has mildly hypocellular bone marrow, but his peripheral hematologic laboratory values are normal. Although HSCT is a curative therapy for GATA2 deficiency, HSCT is not without risk. At NIH, a major criterion for a patient to receive HSCT is a clinical history of at least one life-threating infection or intermediate- or high-risk MDS. Neither of these conditions was met at this time point, so the younger son is being monitored closely at the discretion of his physician, with routine complete blood counts and bone marrow biopsies. He is currently 10 years old.
About 5.5 years after the father’s transplantation, his 16-year-old son underwent HSCT because of persistent neutropenia, MDS, and confirmed GATA2 mutation. He received an HLA-related haploidentical bone marrow HSCT from his 38-year-old mother. Prior to transplantation, the mother was tested to confirm that she did not have the GATA2 mutation. Although GATA2 mutation is autosomal dominant, which in this case was clearly inherited from the father’s side, all related donors were tested. His transplantation course was complicated by cytomegalovirus and varicella zoster reactivation, both of which responded to therapy. He had liver and oral GVHD, which also responded to therapy. He is currently alive and well at 18 years old, at 19 months post-transplantation, and in complete hematologic remission for GATA2 deficiency.
History of GATA2
Alternative treatment options for patients with GATA2 deficiency include receiving supportive care for the complications of GATA2 deficiency (cancer surveillance; viral, mycobacterial, fungal, and bacterial infections) (Cuellar-Rodriguez et al., 2011). Although patients with GATA2 deficiency are frequently healthy in childhood, they have a 90% risk of clinical complications, including MDS by the age of 60 years (Spinner et al., 2014). Patients with GATA2 deficiency have a median survival of 60 years (Collin, Dickinson, & Bigley, 2015). Given the lethal outcome of untreated GATA2 deficiency, the only option for long-term, disease-free survival is the reconstruction of the hematopoietic compartments using allogeneic HSCT (Cuellar-Rodriguez et al., 2011). Patients with GATA2 deficiency live with infections and recurrent health problems, including serious, life-threatening illnesses, that often do not respond to therapy (Dickinson et al., 2011).
HSCT as Treatment for GATA2 Deficiency
Providers caring for bone marrow failure diseases, including GATA2 deficiency, confront a challenge in preparing patients for HSCT. Lad et al. (2016) reported that allogeneic HSCT can offer a long-term resolution for patients with GATA2 deficiency, given their numerous comorbidities (marrow failure, immunodeficiency, and pulmonary disease). In addition, lung pathologic abnormalities can improve after HSCT. Many patients have preexisting organ dysfunction, which places them at higher risk for complications. A risk of morbidity and mortality also exists from the conditioning chemotherapy used to facilitate donor engraftment. These factors justify the need to develop less toxic myeloablative regimens (Burroughs et al., 2017).
Diseases such as GATA2 deficiency can result in more than one family member undergoing HSCT. Shah et al. (2017) discussed their experience with monozygotic twins with GATA2 deficiency who received marrow from the same haploidentical donor but had different transplantation outcomes in terms of GVHD. This report demonstrated that variability in outcomes can be expected, depending on prior infectious exposures.
When searching for available donors among family members, the related donors must be screened to ensure they are not affected with GATA2 deficiency. Family members who are being screened to be a bone marrow donor should not have a clinical history suggesting GATA2 deficiency. However, some members of the family may harbor a GATA2 mutation and be completely asymptomatic. The use of matched unrelated donors carries a slightly higher risk because of increased incidence of GVHD and delayed immune reconstitution (Wilke Shapiro, 2016).
Given the limited understanding of these diseases, they provide unique challenges for healthcare providers. Additional manifestations of the disease may be discovered during the transplantation period. The clinical course of underlying disease can significantly improve with aggressive supportive care. More research on the non-immunologic and immune effects of the mutations needs to be done to determine if HSCT is appropriate for all patients (Connelly, 2017).
Post-Transplantation Complications
Many factors increase the risk of complications occurring during transplantation, including previous cancer therapies, underlying organ disease (lung, liver, kidney, or cardiac dysfunction), previous infection history, age (older patients have more transplantation-related complications), and possible psychological dysfunction. Assessment of a patient’s caregiver’s ability to respond to and care for the patient after HSCT is also important. Community involvement, insurance coverage, employment, and financial resources are an essential part of a social evaluation (Wilke Shapiro, 2016). Therefore, obtaining this information is vital during initial intake and ongoing assessments.
Immediate post-transplantation complications include hepatic sinusoidal obstruction syndrome, acute GVHD, idiopathic pulmonary interstitial pneumonitis, and infections (viral or bacterial). Long-term post-transplantation complications include fatigue, weight loss, chronic GVHD, fertility issues, organ dysfunction (pulmonary, renal, cardiac, and adrenal), infection, and psychological disorders (Wilke Shapiro, 2016).
Discussion
Patients with GATA2 deficiency who undergo HSCT prior to the development of life-threatening infections, myeloid transformation, or unfavorable cytogenetic abnormalities have improved clinical outcomes compared to patients already harboring these signs of clinical progression (Parta et al., 2018). The information presented in this article can guide nurses to develop better nursing care plans for patients with MDS who could have GATA2 deficiency, including those who may benefit from HSCT.
Implications for Practice
Once patients have been identified or referred for transplantation, no standard template of protocol-driven questions exists to elicit a family history related to GATA2 deficiency for patients undergoing HSCT. Therefore, the interview process for transplantation nurses is dependent on their training and experience (this is excluding the initial work and intake done by genetic counselors at the time of diagnosis and screening). The authors plan to develop and implement such a tool to improve consistency and provide guidance to the oncology community. In addition, more formalized education and competency validation for genetic and genomic training and education for research and bedside nurses taking care of patients with GATA2 deficiency undergoing HSCT would be useful.
Conclusion
Oncology nurses, armed with knowledge of MDS and GATA2 deficiency, can identify patients and their family members who may need genetic testing by obtaining extensive three-generation family pedigrees. GATA2 deficiency is morbid and may be fatal; HSCT represents the only definitive treatment. Information regarding GATA2 deficiency can be used to provide support and help to oncology nurses and providers caring for these patients; this could help to identify health issues before and after HSCT and to guide the educational needs of patients with GATA2 deficiency. In addition, providers can assist in determining the risk factors for family members, anticipate their needs, and assist in obtaining genetic counseling and testing for patients and their family members. Nurses can refer patients with MDS who have signs or symptoms consistent with GATA2 deficiency to genetic counseling prior to GATA2 testing; this can be done using genetic testing for bone marrow failure diseases.
IMPLICATIONS FOR PRACTICE.
Consider GATA2 deficiency as a potential cause of myelodysplastic syndrome (MDS) in young adults, and perform a multigeneration family pedigree to identify family members who may need genetic testing for GATA2 mutations.
Detect GATA2 deficiency early; patients who undergo stem cell transplantation (SCT) prior to development of life-threatening infections or cytogenetic abnormalities have markedly improved clinical outcomes.
Obtain detailed family histories to optimize treatment decisions, particularly for patients with MDS or acute myeloid leukemia, and to identify potential affected family members with GATA2 deficiency who need evaluation for allogeneic hematopoietic SCT.
Acknowledgments
The authors take full responsibility for this content. This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases and National Cancer Institute, National Institutes of Health. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the U.S. Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This article has been reviewed by independent peer reviewers to ensure that it is objective and free from bias.
Contributor Information
Kristen Cole, Center for Cancer Research at the National Cancer Institute, National Institutes of Health in Bethesda, MD..
Daniele N. Avila, Center for Cancer Research at the National Cancer Institute, National Institutes of Health in Bethesda, MD..
Mark Parta, Clinical Monitoring Research Program Directorate of the Frederick National Laboratory for Cancer Research sponsored by the National Cancer Institute, National Institutes of Health in Bethesda, MD..
Bazetta Blacklock Schuver, Office of the Clinical Director at the Center for Cancer Research at the National Cancer Institute, National Institutes of Health in Bethesda, MD..
Steven M. Holland, Division of Intramural Research at the National Institute of Allergy and Infectious Diseases, National Institutes of Health in Bethesda, MD..
Nirali Shah, Pediatric Oncology Branch of the Center for Cancer Research, National Institutes of Health in Bethesda, MD..
Dennis Hickstein, Experimental Transplantation and Immunology Branch of the Center for Cancer Research, National Institutes of Health in Bethesda, MD..
REFERENCES
- Bennett RL, Steinhaus KA, Uhrich SB, O’Sullivan CK, Resta RG, Lochner-Doyle, … Hamanishi J (1995). Recommendations for standardized human pedigree nomenclature. Pedigree Standardization Task Force of the National Society of Genetic Counselors. American Journal of Human Genetics, 56, 745–752. [PMC free article] [PubMed] [Google Scholar]
- Bigley V, Haniffa M, Doulatov S, Wang XN, Dickinson R, McGovern N, … Collin M (2011). The human syndrome of dendritic cell, monocyte, B and NK lymphoid deficiency. Journal of Experimental Medicine, 208, 227–234. 10.1084/jem.20101459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burroughs LM, Shimamura A, Talano JA, Domm JA, Baker KK, Delaney C, … Woolfrey AE (2017). Allogeneic hematopoietic cell transplantation using treosulfan-based conditioning for treatment of marrow failure disorders. Biology of Blood and Marrow Transplantation, 23, 1669–1677. 10.1016/j.bbmt.2017.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzone KA, Cashion A, Feethman S, Jenkins J, Prows CA, Williams JK, & Wung SF (2010). Nurses transforming health care using genetics and genomics. Nursing Outlook, 58, 26–35. 10.1016/j.outlook.2009.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin M, Dickinson R, & Bigley V (2015). Haematopoietic and immune defects associated with GATA2 mutation. British Journal of Haematology, 169, 173–187. 10.1111/bjh.13317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly JA (2017). Hematopoietic stem cell transplant for a new primary immunodeficiency disorder: A voyage where no transplant physician has gone before. Biology of Blood and Marrow Transplantation, 23, 863–864. 10.1016/j.bbmt.2017.04.008 [DOI] [PubMed] [Google Scholar]
- Cuellar-Rodriguez J, Gea-Banacloche J, Freeman AF, Hsu AP, Zerbe CS, Calvo KR, … Hickstein DD (2011). Successful allogeneic hematopoietic stem cell transplantation for GATA2 deficiency. Blood, 118, 3715–3720. 10.1182/blood-2011-06-365049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson RE, Griffin H, Bigley V, Reynard LN, Hussain R, Haniffa M, … Collin M (2011). Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood, 118, 2656–2658. 10.1182/blood-2011-06-360313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson RE, Milne P, Jardine L, Zandi S, Swierczek SI, McGovern N, … Collin M (2014). The evolution of cellular deficiency in GATA2 mutation. Blood, 123, 863–874. 10.1182/blood-2013-07-517151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donadieu J, Lamant M, Fieschi C, de Fontbrune FS, Caye A, Ouachee M, … Pasquet M (2018). Natural history of GATA2 deficiency in a survey of 79 French and Belgian patients. Haematologica, 103, 1278–1287. 10.3324/haematol.2017.181909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genetics Home Reference. (2018). GATA2 gene. Retrieved from https://ghr.nlm.nih.gov/gene/GATA2#synonyms
- Hahn CN, Chong CE, Carmichael CL, Wilkins EJ, Brautigan PJ, Li XC, … Scott HS (2011). Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nature Genetics, 43, 1012–1017. 10.1038/ng.913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu AP, Sampaio EP, Khan J, Calvo KR, Lemieux JE, Patel SY, … Holland SM (2011). Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood, 118, 2653–2655. 10.1182/blood-2011-05-356352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins J, Grady PA, & Collins FS (2005). Nurses and the genomic revolution. Journal of Nursing Scholarship, 37, 98–101. 10.1111/j.1547-5069.2005.00020.x [DOI] [PubMed] [Google Scholar]
- Király AP, Kállay K, Gángó A, Kellner Á, Egyed M, Szőke A, … Bödör C (2018). Familial acute myeloid leukemia and myelodysplasia in Hungary. Pathology Oncology Research, 24, 83–88. 10.1007/s12253-017-0216-4 [DOI] [PubMed] [Google Scholar]
- Krulik T, Turner-Henson A, Kanematsu Y, al-Ma’aitah R, Swan J, & Holaday B (1999). Parenting stress and mothers of young children with chronic illness: A cross-cultural study. Journal of Pediatric Nursing, 14, 130–140. 10.1016/S0882-5963(99)80051-7 [DOI] [PubMed] [Google Scholar]
- Lad DP, Zypchen L, Ryan F, Elwood K, Holland S, Hickstein D, & Nantel SH (2016). Successful allogeneic hematopoietic cell transplantation using Flu-Bu-ATG conditioning for “Mono-MAC syndrome” [GATA2 deficiency]. Biology of Blood and Marrow Transplantation, 22(3, Suppl.), S333. 10.1016/j.bbmt.2015.11.815 [DOI] [Google Scholar]
- Mahon SM (2012). Complexities of genetic care: Implications for advanced practice nurses. Journal for Nurse Practitioners, 8, e23–e27. 10.1016/j.nurpra.2012.04.020 [DOI] [Google Scholar]
- Mahon SM (2016). The three-generation pedigree: A critical tool in cancer genetics care. Oncology Nursing Forum, 43, 655–660. 10.1188/16.ONF.655-660 [DOI] [PubMed] [Google Scholar]
- Mansour S, Connell F, Steward C, Ostergaard P, Brice G, Smithson S, … Murday V (2010.). Emberger syndrome—Primary lymphedema with myelodysplasia: Report of seven new cases. American Journal of Medical Genetics Part A, 152A, 2287–2296. 10.1002/ajmg.a.33445 [DOI] [PubMed] [Google Scholar]
- Montgomery S (2017). Genetics in the clinical setting. American Nurse Today, 12(10), 10–17. [Google Scholar]
- Ostergaard P, Simpson MA, Connell FC, Steward CG, Brice G, Woollard WJ, … Mansour S (2011). Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nature Genetics, 43, 929–931. 10.1038/ng.923 [DOI] [PubMed] [Google Scholar]
- Parta M, Shah NN, Baird K, Rafei H, Calvo KR, Cole K, … Hickstein DD (2018). Allogeneic hematopoietic stem cell transplant for GATA2 deficiency using a busulfan-based regimen. Biology of Blood and Bone Marrow Transplantation, 24, 1250–1259. 10.1016/j.bbmt.2018.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NN, Parta M, Baird K, Rafei H, Cole K, Holland SM, & Hickstein DD (2017). Monozygotic twins with GATA2 deficiency: Same haploidentical-related donor, different severity of GvHD. Bone Marrow Transplantation, 52, 1580–1582. 10.1038/bmt.2017.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinner MA, Sanchez LA, Hsu AP, Shaw PA, Zerbe CS, Calvo KR, … Holland SM (2014). GATA2 deficiency: A protean disorder of hematopoiesis, lymphatics, and immunity. Blood, 123, 809–821. 10.1182/blood-2013-07-515528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinh DC, Patel SY, Uzel G, Anderson VL, Freeman AF, Olivier KN, … Holland SM (2010). Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood, 115, 1519–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke Shapiro T (2016). Nursing implications of blood and marrow transplantation. In Itano JK, Brant JM, Conde FA, & Saria MG (Eds.), Core curriculum for oncology nursing (pp. 212–225). St. Louis, MO: Elsevier/Saunders. [Google Scholar]
- Wlodarski MW, Collin M, & Horowitz MS (2017). GATA2 deficiency and related myeloid neoplasms. Seminars in Hematology, 54, 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarski MW, Hirabayashi S, Pastor V, Starý J, Hasle H, Masetti R, … Niemeyer CM (2016). Prevalence, clinical characteristics, and prognosis of GATA2-related myelodysplastic syndromes in children and adolescents. Blood, 127, 1387–1397. 10.1182/blood-2015-09-669937 [DOI] [PubMed] [Google Scholar]