Abstract
Background/Objective:
Exposure to air pollution may contribute to both increasing depressive symptoms and decreasing episodic memory in older adulthood, but few studies have examined this hypothesis in a longitudinal context. Accordingly, we examined the association between air pollution and changes in depressive symptoms and episodic memory and their interrelationship in oldest-old (aged ≥ 80 years) women.
Design:
Prospective cohort data from the Women’s Health Initiative Memory Study-Epidemiology of Cognitive Health Outcomes (WHIMS-ECHO).
Setting:
Geographically-diverse community-dwelling population.
Participants:
1,583 dementia-free women aged ≥ 80.
Measurements:
Women completed up to 6-annual memory assessments (latent composite of East Boston Memory Test and Telephone Interview for Cognitive Status) and the 15-item Geriatric Depression Scale. We estimated 3-year average exposures to regional PM2.5 (particulate matter with aerodynamic diameter <2.5 μm; interquartile range [IQR] = 3.35μg/m3) and NO2 (IQR = 9.55 ppb) at baseline and during a remote period 10 years earlier, using regionalized national universal kriging.
Results:
Latent change structural equation models examined whether residing in areas with higher pollutant levels was associated with annual changes in standardized episodic memory and depressive symptoms while adjusting for potential confounders. Remote NO2 (β=.287 per IQR; p=.002) and PM2.5 (β=.170 per IQR; p=.019) exposure was significantly associated with larger increases in standardized depressive symptoms, although the magnitude of the difference, less than 1 point on the GDS-15, is of questionable clinical significance. Higher depressive symptoms were associated with accelerated episodic memory declines (β=−.372; p=.001), with a significant indirect effect of remote NO2 and PM2.5 exposure on episodic memory declines mediated by depressive symptoms. There were no other significant indirect exposure effects.
Conclusions:
These findings in oldest-old women point to potential adverse effects of late-life exposure to air pollution on subsequent interplay between depressive symptoms and episodic memory, highlighting air pollution as an environmental health risk factor for older women.
Keywords: Depressive symptoms, episodic memory, air pollution, oldest-old
1. Introduction
People age 80 year and older, referred to hereafter as the oldest-old, represent the fastest growing segment of the US population1. A u-shaped curve of depressive symptoms (DS) exists across older adulthood, with late-life DS decreasing during the early period of older adulthood with an uptick in the oldest-old2. Declines in episodic memory (EM)3, become more pronounced after age 80 and typically co-occur with DS4. There is considerable environmental influence on the etiology of both DS5 and EM6, 7; however, in the oldest-old, the role of the physical environments has been understudied.
Exposure to air pollutants, such as ambient PM2.5 (particulate matter with aerodynamic diameter <2.5 μm) and gaseous NO2 (nitrogen dioxide), may represent novel environmental risk factors of accelerated brain aging8. Longitudinal studies have examined PM2.5 exposure as a risk factor for DS, EM decline, and dementia in older adulthood9–13. No longitudinal studies have examined associations between exposure to traffic-related pollutants with DS or depression in late life. Previous work on has also focused on associations with exposures measured in recent years immediately prior to the emotional or cognitive health assessment. It is unknown how remote exposure to air pollution is associated with trajectories of DS and EM performance in the oldest-old.
Research examining longitudinal interrelationships among PM2.5 exposure, DS, and EM suggests that air pollution-related memory decline may precede increases in DS14. Specifically, PM2.5 exposure averaged 3-years prior to annual neuropsychological assessments was associated with declining performance on EM tests, which were then associated with subsequent increases in DS. Although this report was the first demonstrating the temporal dynamics between DS and EM affected by air pollution exposure in late life, the average baseline age was 73 years old, making it difficult to generalize the observed associations to the oldest-old. This previous work was further limited by studying only PM2.5, whereas other studies had reported the associations of EM decline with NO215, 16.
The purpose of this longitudinal study was to examine the associations of exposures to NO2 and PM2.5 with changes in EM and DS over a 5-year period in a geographically-diverse community-dwelling cohort of women aged 80 years and older. We also examined whether the observed associations, might vary by pollutants (NO2 vs. PM2.5) and exposure time period (in recent vs. remote years).
2. Materials and Methods
2.1. Study Population
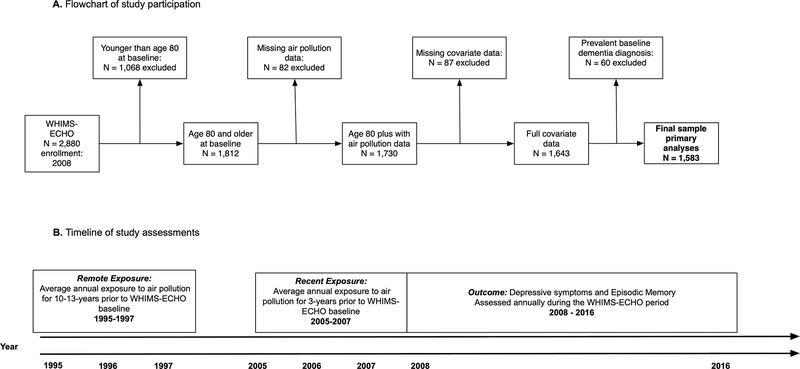
This longitudinal cohort study included 1,583 community-dwelling older women age 80 years or older enrolled in the Women’s Health Initiative Memory Study of the Epidemiology of Cognitive Health Outcomes (WHIMS-ECHO)17 who were dementia-free (see Supplemental Methods for description of dementia ascertainment) at study baseline (aged 80–93 years old). The WHIMS-ECHO began in 2008, and was an extension study to the Women’s Health Initiative Memory Study (WHIMS)18 which itself was an ancillary study to the larger Women’s Health Initiative (WHI) trial of postmenopausal hormone therapy19 (see Supplementary Figure 1 for a panel A for a flowchart of this study sample while panel B presents a timeline of study assessments). Participants completed annual phone-based neuropsychological assessments (up to 6 assessments), including measures of DS and EM. A more detailed description of the study population is included in the Supplemental methods.
2.2. Assessment of DS
DS were assessed at WHIMS-ECHO baseline and at each annual follow-up using the 15-item Geriatric Depression Scale (GDS-15)20. GDS-15 scores were positively skewed, so scores were transformed using a 3-quantile spline transformation applied in our previous work11. Transformed scores were standardized on a T-score metric (Mean = 50; SD = 10), based on the baseline mean and standard deviation. Higher scores reflect greater depression symptoms.
2.3. Assessment of verbal EM
Verbal EM was assessed by the immediate (IR) and delayed recall (DR) measures from both the East Boston Memory Test (EBMT)21 and word-list items of the Telephone Interview for Cognitive Status-modified (TICSm)22. A higher score represents better performance on these four measures of EM. Performance on each measure was also standardized on a T-score metric based on the baseline mean and standard deviation. In order to minimize the number of models that were fit, we combined the four measures of EM (EBMT-IR; EBMT-DR; TICSm-IR; and TICSm-DR) into a latent factor that captures the overall performance of EM (see Supplemental methods for additional details).
2.4. Assessment of ambient PM2.5 and NO2
Participants’ addresses were prospectively collected at each WHI assessment and geocoded using standardized procedures23, 24. Ambient annual mean concentrations of PM2.5 in ug/m3 and NO2 in ppb at each location were estimated using regionalized universal kriging models, which were based on US Environmental Protection Agency (EPA) monitoring data25–27. Given the annual estimates, we calculated 3-year average exposures for each of these two pollutants both at WHIMS-ECHO baseline (referred to as recent exposures) and during the remote period 10 years earlier (referred to as remote exposures). Remote exposure corresponds to average annual exposure 10–13 years prior to the WHIMS-ECHO baseline (see Figure 1 panel B). Both exposure variables were scaled to the interquartile range (IQR) based on the remote exposure time period (remote PM2.5 IQR = 3.35 μg/m3; remote NO2 IQR = 9.55 ppb).
Figure 1.
(A).Flowchart of study participation. (B). Timeline of study assessments
WHIMS-EHCHO = Women’s Health Initiative Memory Study of the Epidemiology of Cognitive Health Outcomes
2.5. Relevant Covariate Data
A structured questionnaire was administered at WHIMS baseline to gather information on time-independent covariates: demographics (age, race/ethnicity), geographic region of residence (Northeast, South, Midwest, and West); socioeconomic status (education; family income; employment status); lifestyle factors (smoking; alcohol use; physical activities); and clinical characteristics, including past or present self-reported postmenopausal hormone treatment, history of cardiovascular disease (including previous coronary heart, stroke, or transient ischemic attack), hypertension (defined as elevated blood pressure or use of antihypertensive medication), hypercholesterolemia, and diabetes mellitus (defined as physician diagnosis plus oral medications, or insulin therapy). Reliability and validity of these self-reported medical histories and the physical measures have been previously documented28. Neighborhood socioeconomic characteristics (nSES) were characterized using standard methods29.
2.6. Statistical Analysis
Structural equation models (SEMs) for latent change scores (LCSs)30, 31 were constructed to characterize associations between air pollution exposure and temporal changes in the two inter-related neuropsychological processes (EM; DS) over the first five years of the WHIMS-ECHO study period. We examined change over one-year intervals because women completed annual assessments of both EM and DS. The Supplemental methods provide a more detailed description of the analytic approach.
We first constructed univariate LCS models to examine the association between exposure and annual change in each of these two neuropsychological processes, separately for DS (Supplemental Figure S1) and EM (Supplemental Figure S2). For DS, the equation to estimate annual individual-specific change in DS for individual i at timepoint t (Δdepi,t) was written as:
| (Equation 1) |
In the above equation, Δdepi,t denotes the estimated individual-specific annual change in DS and is a function of the following effects: individual-specific constant linear change (denoted by slpdepi), non-linear proportional change capturing the extent to which magnitude of change is dependent on previous estimate of DS (βdep); and the effect of exposure (γexposure on Δdep). Exposure effects, and individual-specific estimates of initial symptoms and linear change were adjusted for the following covariates: age at the WHIMS-ECHO baseline, race/ethnicity, employment status, geographic region of residence, education, household income, lifestyle factors (smoking; alcohol use; physical activities), nSES, and clinical characteristics (any prior hormone use ever, hypercholesterolemia, hypertension, diabetes, and history of cardiovascular disease). Analogous equations can be written for univariate SEM to estimate exposure effects on change in the EM latent factor. In univariate models for EM, a latent factor of EM performance consisting of the four measures of EM (EBMT-IR, EBMT-DR, TICSm-IR, and TICSm-DR) was created at each timepoint (see Supplemental methods). Separate models were run to examine how the defined neuropsychological process was influenced by ambient PM2.5 or NO2, each including remote and recent exposures effects.
2.6.3. Bivariate latent change score models.
Figures S3 and S4 in Supplemental materials present the full bivariate models that were estimated. In the bivariate models change in DS (Δdepi,t) was again a function of linear systematic change (slpdepi), proportional change (βdep), and the effect of exposure (γexposure on Δdep). The equation to estimate change in DS also contains a coupling parameter linking EM performance with subsequent change in DS (γlem on Δdep).
The specific indirect effect of exposure on changes in DS was estimated by multiplying the two estimated coupling parameters while deriving estimates of 95% confidence intervals (95% CI) via Monte Carlo simulation32. All bivariate LCS models were adjusted for the same set of covariates as described in the univariate LCS models. Analogous equations can be written to examine whether there was an indirect effect of exposure on changes in EM mediated by exposure-related changes in DS. Again, separate models were run to examine effects of remote and recent exposure to both PM2.5 and NO2.
We conducted three sensitivity analyses to examine the robustness of our study findings. We first excluded women (n=137) with either prevalent stroke at the beginning of the study period or incident stroke by 2017 and re-ran these analyses to examine whether our findings could be explained by stroke risk. Second, we excluded women (n=289) who developed dementia (see Supplemental methods for dementia ascertainment) by June 2018, to explore whether any observed associations remain among the oldest-old who were cognitively-intact during the entire study period. Last, we excluded women (n=127) who self-reported a history of depression prior to the WHIMS baseline, to explore whether findings could be explained by prior history of depression. All LCS models were conducted using the SEM program MPLUS version 833 which was run via the MPLUS Automation package34 in R.
3. Results
On average, participants completed nearly five (mean±S.D.= 4.25±1.81) assessments of EM and DS. Table 1 compares the distribution of 3-year average exposures to regional PM2.5 and NO2 at the remote period 10 years before the WHIMS-ECHO baseline assessment by population characteristics. Participants exposed to higher concentrations of remote PM2.5 were more likely to be racial/ethnic minorities (African-American or Hispanic White), residing in the South, and have hypercholesterolemia. Participants with higher concentrations of remote NO2 exposure were more likely to have either more or less than a high school education, to reside in the West, and to be racial/ethnic minorities, current smokers, and non-drinkers (less than one drink per day).
Table 1.
Comparison of Estimated Remote PM2.5 and NO2 Exposures by Population Characteristics at Baseline (N = 1,583).
| Distribution of remote 3-year averagea PM2.5 exposure(µg/m3) |
Distribution of remote 3-year averagea NO2 exposure (ppb) |
||||||
|---|---|---|---|---|---|---|---|
| Population Characteristics | N | Mean ± SD | (25th, Median, 75th) | pb | Mean ± SD | (25th, Median, 75th) | pb |
| Overall | 1,583 | 13.34 ± 2.80 | (11.7, 13.4, 15.0) | 16.22 ± 7.28 | (10.9, 15.3, 20.4) | ||
| Region of Residence | <.01 | <.01 | |||||
| Northeast | 498 | 12.95 ± 1.87 | (11.7, 13.0, 14.0) | 17.42 ± 7.75 | (11.2, 16.0, 21.6) | ||
| South | 302 | 14.07 ± 2.04 | (12.9, 14.3, 15.5) | 13.95 ± 6.46 | (8.7, 12.4, 18.7) | ||
| Midwest | 351 | 13.49 ± 2.13 | (11.9, 13.4, 15.1) | 13.98 ± 4.82 | (10.3, 14.0, 17.6) | ||
| West | 432 | 13.17 ± 4.17 | (9.6, 13.3, 15.8) | 18.25 ± 7.97 | (12.9, 17.8, 22.5) | ||
| Race/Ethnicity | <.01 | <.01 | |||||
| African-American | 68 | 15.16 ± 2.16 | (14.0, 15.2, 16.0) | 21.09 ± 8.43 | (13.9, 21.1, 26.2) | ||
| Hispanic White | 18 | 14.59 ± 2.91 | (13.1, 14.1, 16.4) | 22.08 ± 7.06 | (17.9, 21.9, 25.9) | ||
| Non-Hispanic White | 1455 | 13.23 ± 2.78 | (11.5, 13.3, 14.9) | 15.82 ± 7.04 | (10.6, 15.0, 19.7) | ||
| Other or Missing | 42 | 13.89 ± 3.19 | (12.3, 13.7, 15.4) | 19.69 ± 8.71 | (13.8, 19.1, 24.9) | ||
| Education | .22 | .02 | |||||
| Less than high school | 70 | 13.72 ± 3.00 | (11.9, 13.9, 15.7) | 17.32 ± 8.57 | (11.4, 16.4, 22.1) | ||
| High school | 330 | 13.14 ± 2.62 | (11.4, 13.0, 14.8) | 15.26 ± 6.69 | (10.4, 14.5, 19.0) | ||
| More than high school | 1,183 | 13.38 ± 2.83 | (11.8, 13.4, 15.1) | 16.43 ± 7.33 | (10.9, 15.6, 20.5) | ||
| Employment | .56 | <.01 | |||||
| Currently working | 179 | 13.51 ± 2.86 | (12.1, 13.4, 15.0) | 17.83 ± 8.42 | (11.2, 17.1, 22.7) | ||
| Not working | 151 | 13.18 ± 2.81 | (11.7, 13.5, 14.9) | 15.29 ± 6.37 | (10.7, 14.2, 19.0) | ||
| Retired | 1253 | 13.34 ± 2.78 | (11.6, 13.4, 15.0) | 16.11 ± 7.17 | (10.7, 15.2, 20.2) | ||
| Income (in USD) | .23 | .20 | |||||
| < 9,999 | 47 | 13.15 ± 3.56 | (10.8, 13.7, 15.3) | 16.01 ± 8.46 | (9.7, 15.2, 20.1) | ||
| 10,000–34,999 | 232 | 13.22 ± 2.88 | (11.6, 13.0, 15.0) | 15.54 ± 7.31 | (9.4, 15.1, 19.0) | ||
| 35,000–49,999 | 425 | 13.20 ± 2.87 | (11.4, 13.3, 14.9) | 15.96 ± 7.32 | (10.6, 15.0, 19.8) | ||
| 50,000–74,999 | 296 | 13.48 ± 2.82 | (11.7, 13.5, 15.4) | 16.30 ± 7.21 | (10.6, 15.5, 21.0) | ||
| 75,000 or more | 357 | 13.46 ± 2.48 | (12.0, 13.5, 14.8) | 16.86 ± 7.27 | (11.4, 15.9, 21.0) | ||
| Don’t know | 72 | 12.99 ± 2.50 | (11.5, 13.0, 14.4) | 15.72 ± 6.88 | (11.3, 13.9, 19.3) | ||
| Lifestyle | |||||||
| Smoking status | .23 | <.01 | |||||
| Never smoked | 920 | 13.4 ± 2.79 | (11.7, 13.4, 14.9) | 15.74 ± 6.96 | (10.4, 14.8, 19.7) | ||
| Past smoker | 600 | 13.26 ± 2.73 | (11.6, 13.3, 15.1) | 16.74 ± 7.50 | (11.2, 16.2, 20.8) | ||
| Current Smoker | 63 | 13.90 ± 3.38 | (11.3, 14.2, 15.9) | 18.29 ± 8.80 | (11.2, 18.1, 23.0) | ||
| Alcohol use | .23 | <.01 | |||||
| Non-drinker | 190 | 13.40 ± 2.97 | (11.7, 13.4,15.1) | 14.51 ± 7.93 | (8.6, 12.3, 18.1) | ||
| Past drinker | 271 | 13.44 ± 2.96 | (11.5, 13.5, 15.5) | 15.80 ± 7.20 | (10.5, 14.5, 19.8) | ||
| Less than 1 drink/ day | 916 | 13.39 ± 2.66 | (11.7, 13.4, 14.9) | 16.75 ± 7.14 | (11.6, 16.1, 20.9) | ||
| More than 1 drink/ day | 206 | 12.97 ± 2.96 | (11.3, 13.2, 15.1) | 16.00 ± 7.10 | (10.7, 15.2, 20.2) | ||
| Moderate or strenuous activities ≥ 20 minutes | .18 | .10 | |||||
| No activity | 852 | 13.38 ± 2.79 | (11.8, 13.5, 15.0) | 16.09 ± 7.40 | (10.6, 15.3, 20.2) | ||
| Some activity | 84 | 13.80 ± 2.42 | (12.2, 14.0, 15.5) | 17.68 ± 6.73 | (13.5, 17.9, 22.2) | ||
| 2–4 episodes/week | 347 | 13.36 ± 2.82 | (11.7, 13.2, 15.0) | 16.65 ± 7.31 | (11.6, 15.3, 20.9) | ||
| ≥4 episodes/week | 300 | 13.09 ± 2.86 | (11.3, 13.1, 14.9) | 15.70 ± 7.00 | (10.6, 14.5, 19.7) | ||
| Physical Health | |||||||
| Hypertension | .71 | .43 | |||||
| No | 1025 | 13.32 ± 2.84 | (11.6, 13.4, 15.0) | 16.12 ± 7.24 | (10.7, 15.2, 20.1) | ||
| Yes | 558 | 13.38 ± 2.72 | (11.8, 13.5, 15.0) | 16.42 ± 7.33 | (11.2, 15.6, 20.9) | ||
| Treated hypercholesterolemia | .03 | .90 | |||||
| No | 1302 | 13.27 ± 2.81 | (11.6, 13.3, 15.0) | 16.21 ± 7.24 | (10.7, 15.4, 20.3) | ||
| Yes | 281 | 13.68 ± 2.71 | (11.9, 13.7, 15.4) | 16.27 ± 7.47 | (11.2, 14.8, 20.7) | ||
| Diabetes Mellitus | .92 | .77 | |||||
| No | 1513 | 13.34 ± 2.81 | (11.7, 13.4, 15.0) | 16.23 ± 7.30 | (10.9, 15.4, 20.4) | ||
| Yes | 70 | 13.38 ± 2.48 | (11.8, 13.7, 15.0) | 15.97 ± 6.86 | (10.8, 15.0, 19.7) | ||
| Cardiovascular disease | .51 | .26 | |||||
| No | 1343 | 13.36 ± 2.82 | (11.7, 13.4, 15.0) | 16.31 ± 7.30 | (10.9, 15.4, 20.5) | ||
| Yes | 240 | 13.23 ± 2.64 | (11.7, 13.4, 14.9) | 15.74 ± 7.11 | (10.5, 15.1, 19.8) | ||
| Prior hormone therapy | .10 | .06 | |||||
| No | 886 | 13.45 ± 2.56 | (11.9, 13.5, 14.9) | 16.53 ± 7.37 | (10.9, 15.6, 20.9) | ||
| Yes | 697 | 13.21 ± 3.07 | (11.3, 13.3, 15.1) | 15.83 ± 7.14 | (10.7, 15.2, 19.7) | ||
| Hormone therapy assignment | .11 | .55 | |||||
| E-alone intervention | 269 | 15.71 ± 7.47 | (10.1, 14.2, 19.5) | 13.23 ± 2.86 | (11.5, 13.2, 15.3) | ||
| E-alone control | 295 | 16.74 ± 7.07 | (11.7, 16.2, 20.6) | 13.54 ± 3.02 | (11.8, 13.7, 15.4) | ||
| E+P intervention | 486 | 15.78 ± 7.56 | (10.3, 14.8, 19.7) | 13.29 ± 2.81 | (11.6, 13.2, 15.0) | ||
| E+P control | 553 | 16.60 ± 7.00 | (11.3, 15.9, 20.9) | 13.34 ± 2.62 | (11.9, 13.5, 14.8) | ||
Note
3-year average of the annual exposure estimated before the remote period of 10-years prior to the WHIMS-ECHO baseline at each participant’s location using the national spatiotemporal model
p values estimated from ANOVA F-tests or t-tests comparing the mean exposures.
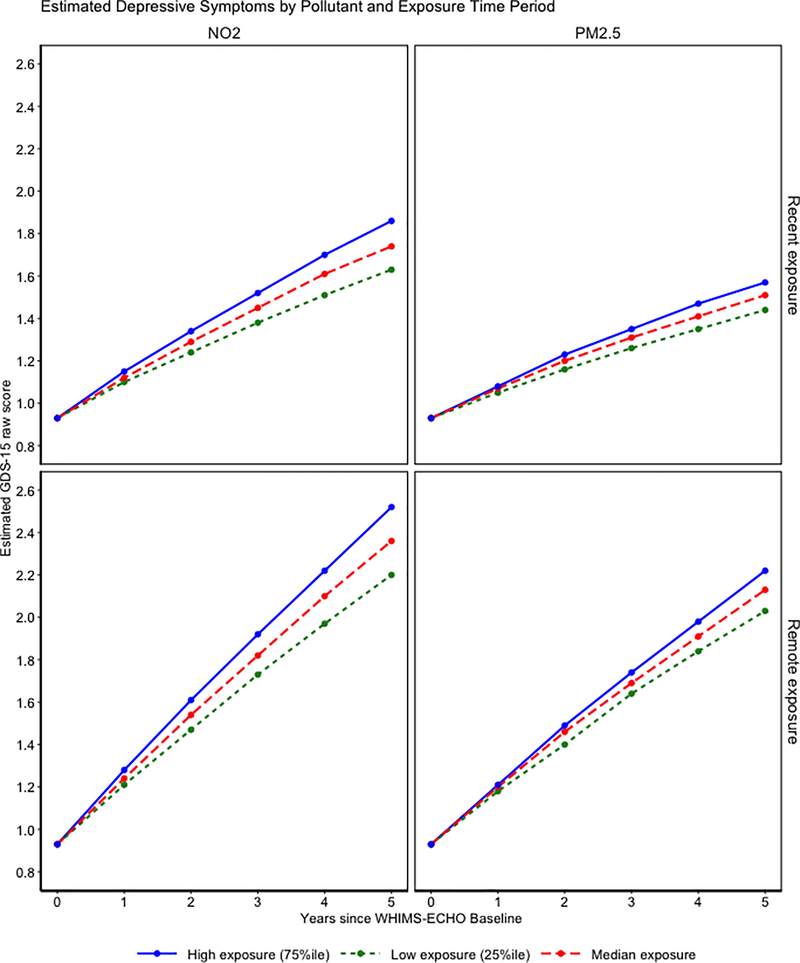
All univariate LCS models fit data acceptably (Supplemental Table S1 for all model fit indices). Increased remote exposures were associated with greater annual increases in DS during the WHIMS-ECHO follow-up (Supplemental Table S2). For example, one inter-quartile increment increase in PM2.5 (3.35 μg/m3) exposure was associated with a .170 larger annual increase in T-score standardized DS (95% confidence interval (CI) = .027-.312). A similar putative adverse exposure effect was observed for NO2 (γremote NO2 on Δdep = .287; 95% CI = .105-.470; per inter-quartile increment of 9.55 ppb). Although similar patterns of increased DS were also found in those residing in locations with higher exposures during the recent 3 years before WHIMS-ECHO baseline, the associations did not reach statistical significance (p=.090 for NO2; p=.126 for PM2.5). In contrast, there was no statistically significant association of EM decline with either PM2.5 or NO2, regardless of exposure time periods. Figure 2 presents the exposure effect parameter estimates and depicts estimated trajectories of EM and DS (transformed back to GDS-15 units to aid in clinical interpretation) associated with each pollutant at either relatively low (25th percentile), average (median), or relatively high (75th percentile) exposure concentrations among women with the average performance of EM or DS at WHIMS-ECHO baseline and average individual-specific linear change. Women residing in areas with high exposure to NO2 experienced 24% larger increases in DS compared to women with low exposure while higher PM2.5 exposure was associated with 17% larger increases. Applying recently published guidelines for effect size interpretation our observed effect sizes of exposure on changes in depressive symptoms are considered small yet potentially consequential35.
Figure 2.
Estimated depressive symptoms as measured by the 15-item Geriatric Depression Scale by pollutants and exposure time period for low (25th percentile), median, and high (75th percentile) average 3-year exposure. The exposure effects are portrayed in a grid with the first row representing recent exposure and the second row representing remote exposure. The first column represents the NO2 exposure effect while the second column is the PM2.5 exposure effect. Therefore, the graph illustrated in the first row and first column represents the effect of recent NO2 exposure.
* Denotes p <.05
** Denotes p<.01
In Table 2, the results of bivariate LCS models examining the direct and indirect effect of exposure on changes in DS are presented. All models exhibited acceptable model fit (see Supplemental Table S1). Consistent with univariate models, neither NO2 nor PM2.5 exposure over the remote or recent period was associated with EM declines. Additionally, EM performance in the oldest-old women was not associated with subsequent changes in DS.
Table 2.
Bivariate latent change score structural equation models examining the direct effect of recent and remote exposure to NO2 and PM2.5 on changes in depressive symptoms as well as the respective indirect exposure effect mediated by episodic memory (N = 1,583).
| Exposure: NO2 | ||
| Remotea Exposure | Recentb Exposure | |
| Estd (95% CI) |
Estd (95% CI) |
|
| Estimates of Direct Effectc | ||
| Effect of NO2 on annual change in depressive symptoms (γNO2 on Δdep) | .221 (−.093; .535) | .169 (−.185; .523) |
| Estimates of Indirect Effectc | ||
| Effects of NO2 on annual changes in episodic memory (γNO2on Δem) | −.074 (−.264; .116) | −.163 (−.441; .114) |
| Effects of episodic memory performance on annual change in depressive symptoms (γLem on Δdep) | .112 (−.276; .500) | .094 (−.294; .483) |
| Indirect effect of NO2 on annual change in depressive symptoms (γNO2on Δem * γLem on Δdep) | −.008 (−.078; .035)e | −.015 (−.140; .059)e |
| Exposure: PM2.5 | ||
| Remotea Exposure | Recentb Exposure | |
| Estd (95% CI) | Estd (95% CI) | |
| Estimates of Direct Effectc | ||
| Effect of PM2.5 on annual change in depressive symptoms (γPM2.5 on Δdep) | .108 (−.158; .373) | .115 (−.194; .424) |
| Estimates of Indirect Effectc | ||
| Effects of PM2.5 on annual changes in episodic memory (γPM2.5 on Δem) | −.095 (−.272; .082) | −.026 (−.260; .209) |
| Effects of episodic memory performance on annual change in depressive symptoms (γLem on Δdep) | .113 (−.278; .503) | .085 (−.296; .466) |
| Indirect effect of PM2.5 on annual change in depressive symptoms (γPM2.5 on Δem * γLem on Δdep) | −.011 (−.088; .041)e | −.002 (−.079; .050)e |
Abbreviations: NO2 = nitrogen dioxide; PM2.5 = particulate matter with <2.5 μm; episodic memory = latent factor consisting of the immediate and delayed recall from the East Boston Memory Test and the world list memory from the Telephone Interview for Cognitive Status; depressive symptoms = 15-item Geriatric Depression Scale, CI = Confidence interval, Est = parameter estimate
Estimates bolded if statistically significant at p<0.05
Remote represents the 3-year average exposures to regional PM2.5 and NO2 10 years prior to study baseline
Recent represents the 3-year average exposures to regional PM2.5 and NO2 for the 3 years prior to study baseline
All effects below were derived from the bivariate structural equation models (SEM) as depicted in figure 2 panel A, with exposure scaled by interquartile range from the remote period ( PM2.5 scaled by 3.35 μg/m3 and NO2 scaled by 9.55 ppb).
In all models, the effects were adjusted for initial age at WHIMS-ECHO, race/ethnicity, geographic region of residence, employment status, education, household income, lifestyle factors (smoking, alcohol use, physical activities), clinical characteristics (use of hormone treatment; hypercholesterolemia, hypertension, diabetes, and history of cardiovascular disease), and neighborhood socioeconomic characteristics.
95% confidence interval for the indirect effect is asymmetric and estimated via Monte Carlo Simulation
Results for the direct and indirect effects of exposure on changes in EM performance are presented in Table 3. Remote NO2 and PM2.5 exposures were both associated with increased DS over the follow-up period. For example, a one inter-quartile increment in remote NO2 exposure was associated with a .255 larger annual increases in T-score standardized DS. Oldest-old women with higher DS tended to have accelerated declines in EM during the subsequent year, with a one T-score increase in DS being associated with .377-.380 larger annual declines in EM performance. The resulting indirect effect of remote exposure on accelerating EM declines mediated by DS was present for both pollutants, with a larger effect estimate per interquartile range for NO2 exposure. The magnitude of effects of recent exposures during the 3 years before the WHIMS-ECHO baseline were of similar magnitude as remote exposure although not statistically significant.
Table 3.
Bivariate latent change score structural equation models examining the direct effect of recent and remote exposure to NO2 and PM2.5 on changes in episodic memory as well as the respective indirect effect mediated by depressive symptoms (N = 1,583).
| Exposure: NO2 | ||
| Remotea Exposure | Recentb Exposure | |
| Estd (95% CI) | Estd (95% CI) | |
| Estimates of Direct Effectc | ||
| Effect of NO2 on annual change in episodic memory (γNO2 on Δem) | .179 (−.119; .477) | −.024 (−.425; .377) |
| Estimates of Indirect Effectc | ||
| Effects of NO2 on annual changes in GDS-15 (γNO2 on Δdep) | .255 (.074; .436) | .203 (−.042; .447) |
| Effects of GDS-15 performance on annual change in episodic memory (γLdep on Δem) | −.372 (−.594; −.150) | −.384 (−.615; −.154) |
| Indirect effect of NO2 on annual change in episodic memory (γNO2 on Δdep * γLdep on Δem) | −.095 (−.171; −.026) e | −.078 (−.184; .018)e |
| Exposure: PM2.5 | ||
| Remotea Exposure | Recentb Exposure | |
| Estd (95% CI) | Estd (95% CI) | |
| Estimates of Direct Effectc | ||
| Effect of PM2.5 on annual change in episodic memory (γPM2.5 on Δem) | .114 (−.149; .377) | .154 (−.183; .491) |
| Estimates of Indirect Effectc | ||
| Effects of PM2.5 on annual changes in GDS-15 (γPM2.5 on Δdep) | .138 (−.004; .280) | .123 (−.081; .328) |
| Effects of GDS-15 performance on annual change in episodic memory (γLdep on Δem) | −.377 (−.608; −.146) | −.380 (−.612; −.148) |
| Indirect effect of PM2.5 on annual change in episodic memory (γPM2.5 on Δdep * γLdep on Δem) | −.052 (−.112; −.001) e | −.047 (−.130; .035)e |
Abbreviations: NO2 = nitrogen dioxide; PM2.5 = particulate matter with <2.5 μm; episodic memory = latent factor consisting of the immediate and delayed recall from the East Boston Memory Test and the world list memory from the Telephone Interview for Cognitive Status; depressive symptoms = 15-item Geriatric Depression Scale, CI = Confidence interval, Est = parameter estimate
Estimates bolded if statistically significant at p<0.05
Remote represents the 3-year average exposures to regional PM2.5 and NO2 10 years prior to study baseline
Recent represents the 3-year average exposures to regional PM2.5 and NO2 for the 3 years prior to study baseline
All effects below were derived from the bivariate structural equation models (SEM) as depicted in figure 2 panel A, with exposure scaled by interquartile range from the remote period ( PM2.5 scaled by 3.35 μg/m3 and NO2 scaled by 9.55 ppb).
In all models, the effects were adjusted for initial age at WHIMS-ECHO, race/ethnicity, geographic region of residence, employment status, education, household income, lifestyle factors (smoking, alcohol use, physical activities), clinical characteristics (use of hormone treatment; hypercholesterolemia, hypertension, diabetes, and history of cardiovascular disease), and neighborhood socioeconomic characteristics.
95% confidence interval for the indirect effect is asymmetric and estimated via Monte Carlo Simulation
In our sensitivity analyses, the observed direct associations between exposures and increased DS (Table S3), as well as the resulting indirect effects on EM decline mediated by increased DS (Table S4), were largely the same after excluding women who experienced a stroke. After excluding oldest-old women who developed dementia by 2018, the associations of increased DS with remote exposures to NO2 and PM2.5 (Supplement Table S5), as well as the indirect effects on EM decline (Supplement Table S6), were substantially attenuated. The corresponding indirect effect of exposure on declines in EM mediated by DS was no longer statistically significant. After excluding oldest-old women who self-reported a history of depression prior to the WHIMS baseline, the associations between exposure to higher concentrations of PM2.5 and NO2 and annual changes in DS were significantly attenuated (Supplement Table S7). The corresponding indirect effect of exposure to PM2.5 on declines in EM mediated by DS was no longer statistically significant (Supplement Table S8). The corresponding indirect effect of exposure to NO2 on declines in EM mediated by DS was attenuated but remained statistically significant.
4. Discussion
In this geographically-diverse cohort of women aged 80 years and older, we found that living in locations with higher exposures to ambient air pollution was associated with increased DS and this putatively adverse exposure effect may differ by pollutants. In univariate analyses, long-term exposure to ambient NO2 or PM2.5 was associated with increased DS, of small effect size, over the follow-up. Associations between exposure and changes in DS were slightly stronger and statistically significant for exposures of 10–13 years before the neuropsychological assessment. These observed associations were also stronger with NO2, as compared to PM2.5 exposure. The magnitude of these exposure effects on increases in DS were of questionable clinical significance corresponding to less than a one-unit increase in raw GDS-15 score per IQR increment of the exposure. We did not find any statistically significant direct association of EM declines with remote or recent exposure to NO2 and PM2.5. In bivariate models, women with increasing DS tended to have larger declines in EM one year later, whereas EM was not associated with subsequent changes in DS. A statistically significant indirect effect of remote exposure to either NO2 or PM2.5 on declining EM via increased DS was present. This suggests DS might serve as a neuropsychological mediator of the association between long-term exposure and EM decline in the oldest-old women. Findings could not be explained by socio-demographic factors, lifestyle, individual SES, neighborhood socioeconomic characteristics, or clinical characteristics. To our knowledge, this is the first study to examine how air pollution exposures are associated with DS as well as the interrelationship between DS and EM in the oldest-old population.
Our study demonstrates evidence that living in areas with higher levels of ambient NO2 and PM2.5 are associated with increases in DS, of small magnitude, in the oldest-old. Previous longitudinal studies examining the association between air pollution and DS or major depression in late-life have produced mixed results. For instance, PM2.5 exposure might increase the risk of clinically significant depression across adulthood36, but such association was less consistent among middle-aged and older women37. Long-term PM2.5 exposure has also been associated with more severe DS in some studies11, 38 , but no direct associations in the others14, 39, 40. However, none of the prior studies focused on the oldest-old populations. The magnitudes of the effect size of exposure on increases in DS were small and of questionable clinical significance. The modest-sized effect highlights the need for a larger sample to have adequate statistical power to detect effects of public health significance. Additionally, although we adjusted for important demographic, clinical, and lifestyle factors in our analyses, air quality might be a surrogate for other unmeasured social determinants of health. The statistically significant increases in DS associated with remote exposures to PM2.5 and NO2, our data suggest that the neurotoxic insults resulting from air pollution exposures may accumulate over time, and the resulting neuropathological processes may continue up to 10 years or longer before the increases in DS become measurable in the oldest-old women. In our sensitivity analyses excluding older women with dementia, the observed exposure effect on increased in DS as well as its neurocognitive consequences was attenuated and no longer statistically significant. This suggests that the observed exposure effects on increased DS may coincide with neuropathological processes leading to dementia.
Findings of this study expand the results of our previous work14, suggesting the effect of living in areas with higher air pollution on the dynamic relation of DS and EM may vary by age in late life, although the exact reasons are unclear. In the present study on oldest-old, we found that DS were a neuropsychological mediator of the association between exposure and declining EM. In our previous study among older women with an average age of 73 (SD=3.8 years), we found EM was a neuropsychological mediator of the association between exposure and increased DS. Furthermore, we found no direct associations between time-varying PM2.5 exposure on increased DS, nor indirect effects on EM declines. Age differences in the perception of cognitive changes may be present. Declining EM may be more distressing in earlier older adulthood compared to later older adulthood. It is possible that worse memory may be more distressing at younger ages. Therefore, we may see increases in DS being a reaction to worse EM at younger ages. During later older adulthood worse memory may not be as distressing therefore there is no significant association between worse memory and subsequent increases in DS. Future studies should examine possible contributions to age differences in the association between DS and memory across older adulthood.
In the oldest-old women, the air pollution exposure effect on neuropsychological processes of brain aging may vary by pollutants. Specifically, we found that NO2 exposure had a stronger association with changes in DS compared to PM2.5 and DS. In the sensitivity analyses excluding dementia, the association between NO2 exposure and DS was attenuated and no longer statistically significant. Previous studies have shown that exposure to both PM2.5 and NO2 may increase risk of dementia41. The attenuation of the exposure effect after excluding women with incident dementia suggests that exposure to NO2 and PM2.5 may be contributing to a common neuropathological process contributing to both dementia risk and DS. Similarly, excluding prior depression attenuated the association between PM2.5 and NO2 on increases in DS. However, NO2 was attenuated less than PM2.5 after excluding women with a prior history of depression. This observation suggests that PM2.5 may impact areas of the brain contributing to DS earlier in life. The observed differential exposure effects suggest that traffic-related air pollution, as compared to particulate matter from regional sources, may exert a greater neurotoxic effect on the brain areas important for emotional regulation and health of older people. Further research with animals and humans is needed to compare the adverse physiological effects of late-life exposures to NO2 and PM2.5.
We recognize several limitations of our study. First, exposure estimates are still subject to measurement error, and we did not measure personal exposures directly. We compared average annual remote (10–13 years prior to WHIMS-ECHO baseline) and recent (0–3 years prior to WHIMS-ECHO baseline) exposure while not examining the magnitude or change in exposure for the years in between remote and recent exposure periods. Future studies need to examine how changes in exposure over time impact trajectories of DS and EM in the oldest-old. Second, although our data did not support the hypothesis that EM is a neuropsychological mediator of brain aging associated with exposure, we could not rule out the possibility that increased exposure may impact other cognitive abilities implicated with DS, specifically executive functioning. Third, the oldest-old women included in these analyses were mostly Caucasian well educated and generally in good health, whereas those excluded due to missing data on air pollution exposure or relevant covariates tended to have fewer years of and were more likely be African-American and of lower socioeconomic status, and in poorer health. Although these factors were all accounted for in our analyses, differences in these population characteristics may limit the generalizability of our study findings. Lastly, our study did not include men or younger women.
Our study provides epidemiologic evidence that living in areas with higher levels of PM2.5 and NO2 in late life may contribute directly to increased DS, of small magnitude, and indirectly to declines in EM of the oldest-old women. These findings highlight that the adverse effect of air pollution on the interplay between DS and EM is heterogenous, likely varying by pollutants and age.
Supplementary Material
Figure S1. Univariate Latent Change Score Structural Equation Model to estimate the effect of exposure to air pollution on changes in depressive symptoms (as measured by the Geriatric Depression Scale −15).
Figure S2. Univariate Latent Change Score Structural Equation Model to estimate the effect of exposure to air pollution on changes in episodic memory (as measured by a latent factor consisting of immediate and delayed recall from the East Boston Memory Test and the word list memory from the Telephone Interview of Cognitive Status -modified).
Figure S3. Depiction of the full bivariate model constructed to estimate the indirect effect of exposure on changes in depressive symptoms.
Figure S4. Depiction of the full bivariate model constructed to estimate the indirect effect of exposure on declines of episodic memory.
Table S1. Model fit statistics of all univariate and bivariate latent change score models
Table S2. Univariate structural equation models examining the associations between remote and recent exposure to PM2.5 and NO2 on change in depressive symptoms and episodic memory (N = 1,583).
Table S3. Sensitivity analyses excluding women with incident stroke during the WHIMS -ECHO follow -up period (n=137): to examine whether observed univariate a associations between air pollution exposures and change in emotional distress could be confounded by stroke (N = 1,446).
Table S4. Sensitivity analyses excluding women who experienced a stroke during the WHIMS - ECHO follow -up period (n=137): to examine whether bivariate latent change score structural equation models examining the direct effect of remote exposure to NO2 and PM2.5 on changes in episodic memory as well as the respective indirect effect mediated by depressive symptoms were explained by stroke (N = 1,446).
Table S5. Sensitivity analyses excluding women with dementia during the WHIM S -ECHO follow -up period (n=289): to examine whether observed univariate a associations between air pollution exposures and change in emotional distress could be confounded by dementia risk (N = 1,294).
Table S6. Sensitivity analyses excluding women with dementia during the WHIMS -ECHO follow -up period (n=289): to examine whether bivariate latent change score structural equation models examining the direct effect of remote exposure to NO2 and PM2.5 on changes in episodic memory as well as the respective indirect effect mediated by depressive symptoms were explained by dementia risk (N = 1,294).
Table S7. Sensitivity analyses excluding women with self-reported prior history of depression prior to the WHIMS baseline (n=127): to examine whether observed univariate a associations between air pollution exposures and change in depressive symptoms could be confounded by earlier life depression (N = 1,456).
Table S8. Sensitivity analyses excluding women with self-reported prior history of depression prior to the WHIMS baseline (n=127): to examine whether bivariate latent change score structural equation models examining the direct effect of remote exposure to NO2 and PM2.5 on changes in episodic memory as well as the respective indirect effect mediated by depressive symptoms were explained by early life depression (N = 1,456).
5. Acknowledgments
The corresponding authors, Chen and Petkus, affirm that all of the authors have contributed significantly to the work and has obtained consent from all contributors.
Funding sources
The WHIMS was funded by Wyeth Pharmaceuticals, St Davids, PA,USA, and Wake Forest University. This study and related research are supported by the National Institute of Environmental Health Sciences (R01ES025888; 5P30ES007048), the National Institute on Aging (R01AG033078) and The Alzheimer’s Disease Research Center at USC (P50AG005142). Petkus and Chen are supported in part by the RF1AG054068. Younan and Chen are also supported by the P01AG055367. The Women’s Health Initiative Study of Cognitive Aging was supported by the Department of Health and Human Services and the National Institute on Aging (N01-AG-1-2106), The WHI program is funded by the National Heart, Lung, and Blood Institute (NIH) through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C and HHSN271201100004C. The air pollution models were developed under a STAR research assistance agreement, No. RD831697 (MESA Air) and RD-83830001 (MESA Air Next Stage), awarded by the US Environmental Protection Agency (EPA).
Financial Disclosure: This study and related research are supported by the National Institute of Environmental Health Sciences (R01ES025888; 5P30ES007048), the National Institute on Aging (R01AG033078) and The Alzheimer’s Disease Research Center at USC (P50AG005142). Petkus and Chen are supported in part by the RF1AG054068. Younan and Chen are also supported by the P01AG055367. The WHIMS was funded by Wyeth Pharmaceuticals, St Davids, PA,USA, and Wake Forest University. The Women’s Health Initiative Study of Cognitive Aging was supported by the Department of Health and Human Services and the National Institute on Aging (N01-AG-1-2106), The WHI program is funded by the National Heart, Lung, and Blood Institute (NIH) through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C and HHSN271201100004C. The air pollution models were developed under a STAR research assistance agreement, No. RD831697 (MESA Air) and RD-83830001 (MESA Air Next Stage), awarded by the US Environmental Protection Agency (EPA)
Sponsors Role: Representatives from the NIH serving on the WHI Publications and Presentations Committee reviewed this manuscript before submission but had no direct role in its development and final submission.
Footnotes
Conflicts of Interest: The authors have no conflicts
Academic conferences
This manuscript was submitted for presentation at the 2020 Annual Meeting of the International Society of Environmental Epidemiology in Washington D.C.
References
- [1].Ortman JM, Velkoff VA, Hogan H. An aging nation: The older population in the United States population estimates and projections. 2014, pp. 1140. [Google Scholar]
- [2].Sutin AR, Terracciano A, Milaneschi Y, An Y, Ferrucci L, Zonderman AB. The trajectory of depressive symptoms across the adult life span. JAMA Psychiatry. 2013;70: 803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tulving E Episodic memory: from mind to brain. Annu Rev Psychol. 2002;53: 1–25. [DOI] [PubMed] [Google Scholar]
- [4].Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7: 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Petkus AJ, Beam CR, Johnson W, et al. Gene-environment interplay in depressive symptoms: moderation by age, sex, and physical illness. Psychol Med. 2017;47: 1836–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Swan GE, Reed T, Jack LM, et al. Differential genetic influence for components of memory in aging adult twins. Arch Neurol. 1999;56: 1127–1132. [DOI] [PubMed] [Google Scholar]
- [7].McClearn GE, Johansson B, Berg S, et al. Substantial genetic influence on cognitive abilities in twins 80 or more years old. Science. 1997;276: 1560–1563. [DOI] [PubMed] [Google Scholar]
- [8].The Lancet N Air pollution and brain health: an emerging issue. Lancet Neurol. 2018;17: 103. [DOI] [PubMed] [Google Scholar]
- [9].Cacciottolo M, Wang X, Driscoll I, et al. Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models. Transl Psychiatry. 2017;7: e1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Younan D, Petkus AJ, Widaman KF, et al. Particulate matter and episodic memory decline mediated by early neuroanatomic biomarkers of Alzheimer’s disease. Brain. 2020;143: 289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Petkus AJ, Younan D, Wang X, et al. Particulate Air Pollutants and Trajectories of Depressive Symptoms in Older Women. Am J Geriatr Psychiatry. 2019;27: 1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lim YH, Kim H, Kim JH, Bae S, Park HY, Hong YC. Air pollution and symptoms of depression in elderly adults. Environ Health Perspect. 2012;120: 1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chen H, Kwong JC, Copes R, et al. Exposure to ambient air pollution and the incidence of dementia: A population-based cohort study. Environment international. 2017;108: 271–277. [DOI] [PubMed] [Google Scholar]
- [14].Petkus AJ, Younan D, Widaman K, et al. Exposure to fine particulate matter and temporal dynamics of episodic memory and depressive symptoms in older women. Environ Int. 2020;135: 105196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kulick ER, Wellenius GA, Boehme AK, et al. Long-term exposure to air pollution and trajectories of cognitive decline among older adults. Neurology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kulick ER, Elkind MSV, Boehme AK, et al. Long-term exposure to ambient air pollution, APOE-ε4 status, and cognitive decline in a cohort of older adults in northern Manhattan. Environ Int. 2020;136: 105440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Espeland MA, Rapp SR, Manson JE, et al. Long-term Effects on Cognitive Trajectories of Postmenopausal Hormone Therapy in Two Age Groups. J Gerontol A Biol Sci Med Sci. 2017;72: 838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shumaker SA, Reboussin BA, Espeland MA, et al. The Women’s Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Control Clin Trials. 1998;19: 604–621. [DOI] [PubMed] [Google Scholar]
- [19].Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19: 61–109. [DOI] [PubMed] [Google Scholar]
- [20].Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17: 37–49. [DOI] [PubMed] [Google Scholar]
- [21].Scherr PA, Albert MS, Funkenstein HH, et al. Correlates of cognitive function in an elderly community population. Am J Epidemiol. 1988;128: 1084–1101. [DOI] [PubMed] [Google Scholar]
- [22].Welsh KA, Breitner JC, Magruder-Habib KM. Detection of dementia in the elderly using telephone screening of cognitive status. Neuropsychiatry, Neuropsychology, & Behavioral Neurology. 1993;6: 103–110. [Google Scholar]
- [23].Whitsel EA, Rose KM, Wood JL, Henley AC, Liao D, Heiss G. Accuracy and repeatability of commercial geocoding. Am J Epidemiol. 2004;160: 1023–1029. [DOI] [PubMed] [Google Scholar]
- [24].Whitsel EA, Quibrera PM, Smith RL, et al. Accuracy of commercial geocoding: assessment and implications. Epidemiol Perspect Innov. 2006;3: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bergen S, Sheppard L, Sampson PD, et al. A national prediction model for PM2.5 component exposures and measurement error-corrected health effect inference. Environ Health Perspect. 2013;121: 1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sampson PD, Richards M, Szpiro AA, et al. A regionalized national universal kriging model using Partial Least Squares regression for estimating annual PM2.5 concentrations in epidemiology. Atmos Environ (1994). 2013;75: 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Young MT, Bechle MJ, Sampson PD, et al. Satellite-Based NO2 and Model Validation in a National Prediction Model Based on Universal Kriging and Land-Use Regression. Environ Sci Technol. 2016;50: 3686–3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Heckbert SR, Kooperberg C, Safford MM, et al. Comparison of self-report, hospital discharge codes, and adjudication of cardiovascular events in the Women’s Health Initiative. Am J Epidemiol. 2004;160: 1152–1158. [DOI] [PubMed] [Google Scholar]
- [29].Chi GC, Hajat A, Bird CE, et al. Individual and Neighborhood Socioeconomic Status and the Association between Air Pollution and Cardiovascular Disease. Environ Health Perspect. 2016;124: 1840–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McArdle JJ. A latent difference score approach to longitudinal dynamic structural analyses. Structural Equation Model: Present Future, 2001, pp. 342–380. [Google Scholar]
- [31].Kievit RA, Brandmaier AM, Ziegler G, et al. Developmental cognitive neuroscience using latent change score models: A tutorial and applications. Dev Cogn Neurosci. 2018;33: 99–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Selig JP, Preacher KJ. Monte Carlo method for assessing mediation: An interactive tool for creating confidence intervals for indirect effets. http://quantpsy.org, 2008.
- [33].Muthen LK, Muthen BO. Mplus User’s Guide. Sixth Edition ed. Los Angeles, CA, 2010. [Google Scholar]
- [34].Hallquist MN, Wiley JF. MplusAutomation: An R Package for Facilitating Large-Scale Latent Variable Analyses in Mplus. Struct Equ Modeling. 2018;25: 621–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Funder DC, Ozer DJ. Evaluating effect size in psychological research: Sense and nonsense. Advances in Methods and Practices in Psychological Science. 2019;2: 156–168. [Google Scholar]
- [36].Kim KN, Lim YH, Bae HJ, Kim M, Jung K, Hong YC. Long-Term Fine Particulate Matter Exposure and Major Depressive Disorder in a Community-Based Urban Cohort. Environ Health Perspect. 2016;124: 1547–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kioumourtzoglou MA, Power MC, Hart JE, et al. The Association Between Air Pollution and Onset of Depression Among Middle-Aged and Older Women. Am J Epidemiol. 2017;185: 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wang R, Yang B, Liu P, et al. The longitudinal relationship between exposure to air pollution and depression in older adults. Int J Geriatr Psychiatry. 2020. [DOI] [PubMed] [Google Scholar]
- [39].Wang Y, Eliot MN, Koutrakis P, et al. Ambient air pollution and depressive symptoms in older adults: results from the MOBILIZE Boston study. Environ Health Perspect. 2014;122: 553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pun VC, Manjourides J, Suh H. Association of Ambient Air Pollution with Depressive and Anxiety Symptoms in Older Adults: Results from the NSHAP Study. Environ Health Perspect. 2017;125: 342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Peters R, Ee N, Peters J, Booth A, Mudway I, Anstey KJ. Air Pollution and Dementia: A Systematic Review. J Alzheimers Dis. 2019;70: S145–S163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Univariate Latent Change Score Structural Equation Model to estimate the effect of exposure to air pollution on changes in depressive symptoms (as measured by the Geriatric Depression Scale −15).
Figure S2. Univariate Latent Change Score Structural Equation Model to estimate the effect of exposure to air pollution on changes in episodic memory (as measured by a latent factor consisting of immediate and delayed recall from the East Boston Memory Test and the word list memory from the Telephone Interview of Cognitive Status -modified).
Figure S3. Depiction of the full bivariate model constructed to estimate the indirect effect of exposure on changes in depressive symptoms.
Figure S4. Depiction of the full bivariate model constructed to estimate the indirect effect of exposure on declines of episodic memory.
Table S1. Model fit statistics of all univariate and bivariate latent change score models
Table S2. Univariate structural equation models examining the associations between remote and recent exposure to PM2.5 and NO2 on change in depressive symptoms and episodic memory (N = 1,583).
Table S3. Sensitivity analyses excluding women with incident stroke during the WHIMS -ECHO follow -up period (n=137): to examine whether observed univariate a associations between air pollution exposures and change in emotional distress could be confounded by stroke (N = 1,446).
Table S4. Sensitivity analyses excluding women who experienced a stroke during the WHIMS - ECHO follow -up period (n=137): to examine whether bivariate latent change score structural equation models examining the direct effect of remote exposure to NO2 and PM2.5 on changes in episodic memory as well as the respective indirect effect mediated by depressive symptoms were explained by stroke (N = 1,446).
Table S5. Sensitivity analyses excluding women with dementia during the WHIM S -ECHO follow -up period (n=289): to examine whether observed univariate a associations between air pollution exposures and change in emotional distress could be confounded by dementia risk (N = 1,294).
Table S6. Sensitivity analyses excluding women with dementia during the WHIMS -ECHO follow -up period (n=289): to examine whether bivariate latent change score structural equation models examining the direct effect of remote exposure to NO2 and PM2.5 on changes in episodic memory as well as the respective indirect effect mediated by depressive symptoms were explained by dementia risk (N = 1,294).
Table S7. Sensitivity analyses excluding women with self-reported prior history of depression prior to the WHIMS baseline (n=127): to examine whether observed univariate a associations between air pollution exposures and change in depressive symptoms could be confounded by earlier life depression (N = 1,456).
Table S8. Sensitivity analyses excluding women with self-reported prior history of depression prior to the WHIMS baseline (n=127): to examine whether bivariate latent change score structural equation models examining the direct effect of remote exposure to NO2 and PM2.5 on changes in episodic memory as well as the respective indirect effect mediated by depressive symptoms were explained by early life depression (N = 1,456).