Abstract
Alcohol-induced cirrhosis results partially from the excessive production of collagen matrix proteins, which, predominantly αI(I) collagen, are produced and secreted by activated hepatic stellate cells (HSC). The accumulation of αI(I) collagen in HSC during cirrhosis is largely due to an increase in αI(I) collagen gene expression. Acetaldehyde, the major active metabolite of alcohol, is known to stimulate αI(I) collagen production in HSC. However, the mechanisms responsible for it remain unknown. The aim of this study was to elucidate the mechanisms by which αI(I) collagen gene expression is induced by acetaldehyde in rat HSC. In the present study, the acetaldehyde response element was located in a distal GC box, previously described as the UV response element, in the promoter of the αI(I) collagen gene (−1484 to −1476). The GC box was predominantly bound by the DNA binding transcription factor BTEB (basic transcription element binding protein), expression of which was acetaldehyde and UV inducible. Blocking BTEB protein expression significantly reduced the steady-state levels of the acetaldehyde-induced αI(I) collagen mRNA, suggesting that BTEB is required for this gene expression. Further studies found that acetaldehyde activated Jun N-terminal kinase (JNK) 1 and 2 and activator protein 1 (AP-1) transactivating activity. Inhibition of JNK activation resulted in the reduction of the acetaldehyde-induced BTEB protein abundance and αI(I) collagen mRNA levels, indicating that the expression of both genes is JNK dependent in HSC. Taken together, these studies demonstrate that BTEB mediates acetaldehyde-induced, JNK-dependent αI(I) collagen gene expression in HSC.
The sinusoidal hepatic stellate cells (HSC) are the major effectors during hepatic fibrogenesis and cirrhosis. During the early stages of hepatic injury associated with cirrhosis, the normally quiescent, vitamin A-storing HSC transform into actively proliferating, collagen-producing myofibroblast-like cells (34). Alcohol is one of the principal causes of cirrhosis. While the major clinical problem of alcohol-induced hepatic fibrogenesis has been the subject of numerous studies, the precise molecular mechanism(s) which leads to the increase in αI(I) collagen in HSC remains incompletely understood (2, 6, 24, 26, 35, 36). It was demonstrated that acetaldehyde, but not ethanol, induced the increase in the αI(I) collagen gene expression (up to 2.5-fold) measured by Northern blots in cultured 3T3 fibroblasts (2). Further studies indicated that acetaldehyde increased the αI(I) collagen gene transcription in cultured HSC demonstrated by transcription run-on assays (4). These previous observations clearly indicated that acetaldehyde induced the αI(I) collagen gene expression by transcription. However, their results did not exclude the possibility of other mechanisms being involved, such as posttranscriptional regulation. Stefanovic et al. identified a novel RNA-protein interaction targeted to the C-rich sequence in the αI(I) collagen mRNA untranslated region (UTR), which might play an important role in increasing αI(I) collagen mRNA stability in activated HSC (32). This result suggested that the increase in the αI(I) collagen gene expression in activated HSC may involve both transcriptional and posttranscriptional mechanisms. As acetaldehyde is the major initial and active metabolite of alcohol, most studies have used it in lieu of alcohol (2, 6, 24, 26, 35, 36). It has been recognized that acetaldehyde is at least partially responsible for causing the increase in αI(I) collagen gene expression in alcohol-induced fibrogenesis (2, 6, 24, 26, 35, 36). Acetaldehyde is associated with the production of acetate, adduct formation, lipid peroxidation, and changes in the redox state of cells, all of which may play contributory roles in the development of hepatic fibrogenesis by an as yet incompletely understood mechanism (2, 6, 24, 26, 35, 36).
Eukaryotic cells respond to and integrate the extracellular stimuli through specific signal transduction pathways. Three of them are simplified as follows: (i) Raf→MEK1,2→extracellular signal regulated kinase 1 and 2 (ERK1,2); (ii) MEKK1→SEK1→c-Jun N-terminal kinase 1 and 2 (JNK1,2)/SAPK; (iii) MEKK1→SEK1→p38 (34). In each of the cascades, an upstream kinase phosphorylates and activates an immediate downstream substrate kinase. Extracellular signals are, thereby, transduced through the cytoplasmic cascades to their nuclear target genes and regulate their expression in cells. JNK was identified as a kinase that bound and phosphorylated the proto-oncogene c-Jun on Ser-63 and Ser-73 within its NH2-terminal activation domain (13). The transcription factor activator protein 1 (AP-1) consists of either Jun-Jun homodimers or Fos-Jun heterodimeric complexes. AP-1 binds to the palindromic TPA-response element (TRE) sequence TGA(C/G)TCA in the promoter regions of many genes, including c-jun, and regulates gene expression (37). AP-1 DNA binding and transcriptional activities are generally correlated with an increase in the abundance of the AP-1 complex as well as with changes in the phosphorylation of the c-Jun protein (28, 31). AP-1 has been described as a major modulator of cell growth, differentiation, and apoptosis (12, 17, 28). It was found that acetaldehyde increased the steady state levels of c-fos and c-jun mRNA transcripts in HSC (5). In addition, inhibitors of protein kinase (PKC) activity blocked the stimulatory effects of acetaldehyde on the increases in fos and jun mRNA, as well as on the induction of αI(I) collagen gene expression in HSC (5).
We have previously reported that serum, via ERK and JNK pathways, stimulated and up-regulated αI(I) collagen gene expression in cultured HSC through different regions of the 5′-upstream promoter sequence (UPS) of the gene (7, 8, 11). While the ERK-stimulatory signal was mapped to the most proximal NF-1 and SP-1 binding domains of the 5′ UPS of the gene, a distal GC box (−1484 to −1476) in the 5′ UPS of the gene played a central role in receiving extracellular signals through the JNK pathway (8). A recent study also reported that fibronectin and inflammatory cytokines, such as interleukin 1α (IL-1α) and tumor necrosis factor alpha (TNF-α), activated JNK, ERK, and AP-1 in rat HSC (29). 4-Hydroxy-2,3-nonenal (HNE), an aldehydic product of lipid peroxidation, was found to interact directly with JNK in human HSC (27). Our recent studies also demonstrated that JNK and AP-1 activation were required for the UV-induced increase in αI(I) collagen gene expression in HSC (8). The UV response element was located in the distal GC box of the 5′ promoter of the αI(I) collagen gene, and the GC box was bound by a DNA binding protein, termed BTEB (basic transcription element binding protein) (8).
The present studies were designed to localize the acetaldehyde response element in the 5′ UPS of the αI(I) collagen gene and to elucidate the mechanisms by which acetaldehyde induces αI(I) collagen gene expression in rat HSC.
In the present report, the acetaldehyde response element was located in the distal GC box (−1484 to −1476) in the 5′ UPS of the αI(I) collagen gene. The same region was previously described as the UV response element (8). The GC box binding protein BTEB was acetaldehyde inducible. Blocking BTEB protein production resulted in a significant reduction in acetaldehyde-induced αI(I) collagen gene expression. Additional experiments indicated that acetaldehyde activated JNK1,2 and that the acetaldehyde-induced αI(I) collagen gene transcription was JNK dependent. Inhibition of JNK by curcumin, a JNK inhibitor at low doses, significantly reduced the acetaldehyde-induced BTEB protein abundance as well as the steady-state levels of endogenous αI(I) collagen mRNA in HSC. Taken together, activation of JNK by acetaldehyde results in the expression of BTEB and αI(I) collagen genes in HSC. These results indicate that the GC box binding protein BTEB mediates the acetaldehyde-induced αI(I) collagen gene expression in HSC via a JNK-dependent pathway. Our results suggest a complex model wherein JNK and AP-1 activations induced by acetaldehyde are intimately linked to stimulate the production of the recently described transcription factor BTEB. BTEB plays an as yet unappreciated role in coordinating the HSC response to extracellular stimulations and bringing about an increase in αI(I) collagen gene expression.
MATERIALS AND METHODS
Stellate cell isolation and culture.
HSC were isolated from male Sprague-Dawley rats as previously described (7, 8, 11). Cells were used for culture in tissue culture flasks precoated with type I collagen and maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum and 10% newborn calf serum (10/10 medium). Experiments were carried out with cells between passages 2 and 6. In most experiments, cells were serum starved for 48 h in DMEM with 0.4% fetal bovine serum (0.4% medium) before incubation in 0.4% medium with or without acetaldehyde (100 μM) for an additional 36 h. Acetaldehyde was replenished every 12 h in cultured cells in sealed flasks to minimize the effects of evaporation. The acetaldehyde concentration (100 μM) used in this study is in the lower range compared to prior acetaldehyde studies (150 to 200 μM) in HSC (2, 6, 24, 26, 35, 36). We considered that the lower dose of acetaldehyde in cell culture should be closer to the pathophysiological concentration found in the in vivo setting (39) if the dose could induce enough stimulation and effects for detection. In some experiments, curcumin (15 μM), a JNK inhibitor at low doses from Sigma, in the 0.4% medium was added to cells 3 h before the addition of acetaldehyde. Chemical inhibitors of acetaldehyde dehydrogenase were not used because previous experiments showed that they induced some degree of cytotoxicity (data not shown).
Suppression of BTEB expression.
BTEB translation was blocked by use of antisense oligonucleotides (38). In general, 60 to 80% confluent serum-starved HSC were incubated in DMEM with 0.4% serum and with the indicated concentrations of antisense or sense BTEB oligonucleotides for 3 h before the addition of acetaldehyde (100 μM). The medium was replaced next day with fresh DMEM containing acetaldehyde and antisense or sense BTEB oligonucleotides. The total treatment lasted for 48 h. The sequence coding for the first 7 amino acids (21 nucleotides) of BTEB cDNA was used as sense or antisense BTEB oligonucleotides (16). Phosphorothionate-modified oligonucleotides were synthesized by Life Technologies (Grand Island, N.Y.).
The sequence of the antisense BTEB oligonucleotide was 5′-ATG TCC GCG GCC GCC TAC ATG-3′, and that of the sense BTEB oligonucleotide was 5′-CAT GTA GGC GGC CGC GGA CAT-3′.
The optimal concentration of antisense BTEB oligonucleotides to block BTEB translation was determined by Western blotting using a polyclonal anti-BTEB serum produced by Research Genetics (Huntsville, Ala.).
Transfection and CAT assay.
Sixty- to eighty-percent confluent HSC were transfected using the Lipofectamine reagent (Life Technologies). Cell extraction, quantitation, and a chloramphenicol acetyltransferase (CAT) assay were performed as previously described (8). Transfection efficiency was determined by cotransfection of a β-galactosidase reporter, pSV-β gal (Promega). The level of β-galactosidase activity was measured by a chemiluminescence assay kit (Tropix, Bedford, Mass.) according to the manufacturer's instructions.
Plasmid construction.
The dominant-negative JNK expression plasmid (dn-JNK) was a gift from R. J. Davis (University of Massachusetts). The dominant-negative Jun expression plasmid (dn-Jun) was kindly provided by John Kokondis (University of Chicago). To make dn-Jun, the cDNA fragment coding for the N-terminal portion of Jun, including the phosphorylation sites Ser-63 and Ser-73, was deleted. The AP-1 reporter plasmid, 3x-TRE-CAT, contains three AP-1 binding sites (TRE) upstream of a CAT reporter gene, and the empty control plasmid, pBL-CAT, has no AP-1 binding sites. Both plasmids were kindly provided by E. Fuchs (University of Chicago). The colCAT reporter plasmid p1.7/1.6 contains 1.7 kb of the 5′ promoter region of the rat αI(I) collagen gene, 1.6 kb of the first exon, and part of the first intron linked to a CAT reporter plasmid vector. Plasmid p1.7(GC box mut.)/1.6 was derived from plasmid p1.7/1.6 by overlap extension of site-directed mutagenesis (8). The site (−1494 to −1468) was mutated from 5′-GGTTTGGAGGAGGCGGGACTCCTTGC-3′ to 5′-GGTTTGGAGGAAATAAGACTCCTTGC-3′. The GC box of interest is underlined. Plasmid p1.7/1.6 (del. 516-786) was created by digestion of p1.7/1.6 with BstB1 and Afl2. After the digestion, the DNA fragment generated was blunted by mung bean nuclease and ligated by T4 DNA ligase (both from NEB BioLab, Beverly, Mass.). The deleted DNA fragment from +516 to +786 contains a putative AP-1 binding motif (5′-TGATTCAT-3′) at positions +557 to +564.
EMSA.
Nuclear protein extracts were prepared and electrophoretic mobility shift assays (EMSA) were performed as previously described (8). Briefly, 5 μg of nuclear proteins was incubated for 20 min at room temperature (RT) with 0.1 ng of [32P]-labeled double-stranded GC-box oligonucleotides (5′-TTGGAGGCGGGACTCCTTG-3′) from −1489 to −1470 of the 5′ UPS of the rat αI(I) collagen gene, synthesized by GIBCO, Life Technologies (Grand Island, N.Y.). Oligonucleotide-protein complexes were separated by electrophoresis on a 6% nondenaturing polyacrylamide gel in 0.5× Tris-borate-EDTA (TBE) buffer. In competition assays, up to a 50-fold excess of unlabeled GC-box oligonucleotides were preincubated with nuclear proteins for 10 min at RT before addition of the [32P]-labeled double-stranded GC-box oligonucleotides. Supershift assays began with incubation of nuclear extracts with the radiolabeled oligonucleotides. Then 2 μl of anti-BTEB serum or normal rabbit serum (NRS) was added to the mixture and incubated for an additional 20 min at RT. The integrity of the extracts was tested in a gel shift assay with a [32P]-labeled SP-1 consensus probe (5′-ATTCGATCGGGGCGGGGCGAGC-3′), resulting in distinct SP-1 shifts from all extracts (data not shown).
RNA isolation and RPA.
Total RNAs were isolated by the TRI-Reagent (Sigma) following the protocol provided by the manufacturer. The first exon (1 to 206) of the rat αI(I) collagen gene was subcloned in pGEM-3Zf(+) (Promega) (8). The T7 promoter in the plasmid was used to generate a single-stranded antisense RNA probe. The template for rat cyclophilin was obtained from Ambion and yields a 103-bp protected fragment. The antisense RNA probes were synthesized and labeled by in vitro Transcription Kits MAXIscript (Ambion). The synthesized probes were gel purified. RNase protection assays (RPA) were carried out with RPA II (Ambion) following the protocol provided by the manufacturer. The dried gels were exposed to a phosphorimaging system (Phosphor Image SI; Molecular Dynamics, Sunnyvale, Calif.). The radioactivities in each band were measured by computer-aided densitometry of the phosphorimage using IPLab Gel (Signal Analytics Corp.) as described previously (8). Cyclophilin was used as an internal control to normalize the loading of RNA in each sample.
Western blot analysis.
Using standard techniques, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with a 10% resolving gel was used to separate nuclear proteins (20 μg/lane). The separated proteins were electroblotted and detected by using either the anti-ACTIVE JNK polyclonal antibody (pAb) (Promega), an anti-total JNK antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.), or a polyclonal anti-BTEB antibody produced by Research Genetics and horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG; Santa Cruz Biotechnology). Protein bands were visualized by utilizing a chemiluminescence reagent (Kirkegaard & Perry Laboratories, Gaithersburg, Md.). The anti-ACTIVE JNK pAb preferentially recognizes the dual phosphorylated active forms of JNK1,2.
RESULTS AND DISCUSSION
The distal GC box is the acetaldehyde response element.
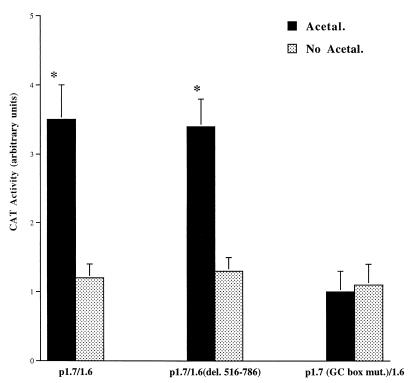
To localize the acetaldehyde response element in the 5′ promoter region of the rat αI(I) collagen gene, rat HSC were transfected with a collagen CAT reporter plasmid (p1.7/1.6) as a surrogate of the αI(I) collagen gene and treated with or without acetaldehyde (Fig. 1). Plasmid p1.7/1.6 contains 1.7 kb of the 5′ UPS of the αI(I) collagen gene and 1.6 kb of the intact first exon and part of the first intron. It was found that acetaldehyde caused an increase of approximately threefold in CAT activity in cells transfected with plasmid p1.7/1.6 (Fig. 1). The 1.7-kb promoter region contains numerous potential response elements, such as transforming growth factor β activation element (TAE), NF-1, SP-1, and a distal GC box (7, 8, 11). In order to locate the major acetaldehyde response element, several plasmids with site-directed mutagenesis in the promoter region or a DNA fragment deletion in the first intron were generated. It was found that plasmids containing site-directed mutations within sites of TAE, NF-1, or SP-1 did not change the acetaldehyde stimulatory patterns in transfected HSC (data not shown), which suggested that these sites were not required for acetaldehyde stimulation of αI(I) collagen gene transcription. Since previous study demonstrated that acetaldehyde increased the steady state levels of c-fos and c-jun mRNA transcripts in HSC (5), it is important to determine whether AP-1 is directly involved in acetaldehyde-induced αI(I) collagen gene transcription. Plasmid p1.7/1.6(del. 516-786) contains a full-length 5′ promoter region of 1.7 kb and a DNA fragment deletion from +516 to +786 in the first intron. The deleted DNA fragment contains a putative AP-1 binding motif at the site of +557 to +564. Transfections with this plasmid showed a similar stimulation by acetaldehyde (Fig. 1). This observation suggested that the putative AP-1 binding motif might not be required in the acetaldehyde induction of αI(I) collagen gene transcription in rat HSC. The AP-1 motif present in the first intron of the αI(I) collagen gene has been suggested to promote stimulatory activity in human and avian fibroblasts (1, 23). However, the role of the first intron in αI(I) collagen gene transcription has been somewhat controversial. When Houglum et al. examined the response to CCl4 stimulation in transgenic animals, they found that much of the first intron, including the putative AP-1 binding motif, was not required for the induction of the αI(I) collagen transgene (14). Our previous study had also found that the AP-1 binding motif in the first intron was not required for the UV-induced αI(I) collagen gene transcription in cultured HSC (8). Our experiments have previously located the UV response element in the distal GC box in the 5′ promoter region of the αI(I) collagen gene. Plasmid p1.7 (GC box mut)/1.6 is a mutant containing a site-directed mutagenized distal GC box in the 5′ promoter region of the gene. HSC transfected with this plasmid completely lost the stimulatory response to acetaldehyde (Fig. 1). This result indicated that the GC box in the 5′ promoter region of the αI(I) collagen gene was the acetaldehyde response element, which is required for the acetaldehyde induction of the αI(I) collagen gene transcription in rat HSC.
FIG. 1.
The distal GC box is required for acetaldehyde-induced αI(I) collagen gene transcription. Serum-starved HSC were transfected with 2 μg of one of the collagen CAT reporter plasmids [wild-type plasmid p1.7/1.6, p1.7/1.6(del. 516-786), which contains a deletion of a fragment containing a putative AP-1 binding motif in the first intron, or p1.7 (GC box mut.)/1.6, which contains a site-directed mutation in the distal GC box] as detailed in Materials and Methods. After transfection and recovery, cells were left untreated or treated with acetaldehyde for an additional 36 hr. CAT assays were performed as described in Materials and Methods. The transfection efficiency was normalized by β-galactosidase activity as described in Materials and Methods. Values presented here reflect the means ± standard deviations (n = 6). ∗, P < 0.05 compared with the control (No Acetal.).
Acetaldehyde induces BTEB protein production and DNA binding activity.
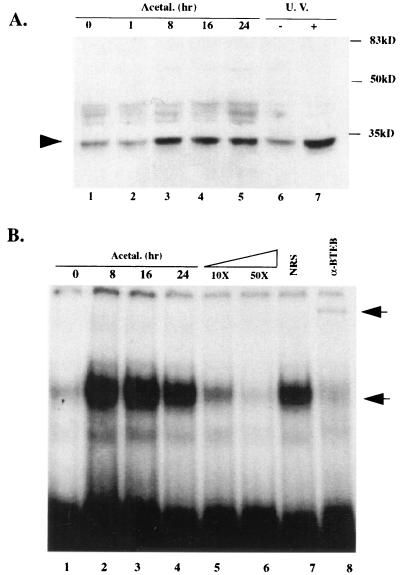
Further studies were focused on the protein which binds to the GC box in the 5′ UPS of the αI(I) collagen gene. Our previous study had demonstrated that BTEB bound to the distal GC box in the αI(I) collagen gene promoter in HSC (8). Protein extracts from serum-starved HSC treated with acetaldehyde were analyzed for the 32-kDa BTEB protein expression by Western blotting using a polyclonal anti-BTEB serum (Fig. 2A). It was found that acetaldehyde induced BTEB protein production in serum-starved HSC (Fig. 2A, lanes 1 to 5). BTEB was also found to be UV inducible (Fig. 2A, lanes 6 and 7). The presence of the faint BTEB band prior to the exposure to either acetaldehyde or UV irradiation is not surprising because HSC were culture activated before serum starvation.
FIG. 2.
Acetaldehyde induces BTEB protein abundance and enhances BTEB DNA binding activity. Sixty-eight-percent confluent HSC were preincubated in DMEM with 0.4% serum for 48 h before the acetaldehyde treatment (100 μM) for the indicated times (hours). Nuclear protein extracts were prepared as described in Materials and Methods. A whole-cell extract from HSC treated with UV irradiation (10 J/m2) as previously described (8) was used to study whether BTEB is UV inducible. (A) Twenty micrograms of nuclear extract proteins or 30 μg of whole-cell extracts of each sample were analyzed by Western blotting using a polyclonal anti-BTEB serum. A representative Western blot assay is shown here (repeated three times with similar results). (B) Ten micrograms of nuclear extract proteins from HSC exposed to acetaldehyde for the indicated times were analyzed by EMSA. [32P]-labeled double-stranded oligonucleotides containing a GC box identical to the sequence in the 5′ promoter of the αI(I) collagen gene were used as a probe (see Materials and Methods). The lower arrow indicates the oligonucleotide-BTEB complex. Ten- to 50-fold excesses of the unlabeled double-stranded oligonucleotides were used in the competition assays. Two microliters of the anti-BTEB serum (α-BTEB) or NRS was used in the supershift assays. Incubation with the anti-BTEB serum caused a supershift band, as indicated by the upper arrow, and a significant reduction in the oligo-BTEB complex band. A representative gel is shown here.
EMSA was used to study BTEB DNA binding activity in HSC after the acetaldehyde treatment with indicated time courses (Fig. 2B). EMSA was performed with a probe of [32P]-labeled double-stranded oligonucleotides which was identical to the distal GC box sequence in the 5′ UPS of the αI(I) collagen gene. After exposure of HSC to acetaldehyde for 8 h, a prominent gel shift band appeared, indicated by the lower arrow on the right (Fig. 2B, lanes 2 to 4). Excess unlabeled oligonucleotides abolished the shift band (Fig. 2B, lanes 5 and 6), indicating that the binding protein of the shift band was GC-box specific. Incubation with the polyclonal anti-BTEB serum generated a supershift band, indicated by the upper arrow on the right, and significantly reduced the abundance of the lower shift band (Fig. 2B, lane 8). In contrast, an equal amount of preimmune NRS had no effect on the GC-box gel shift band (Fig. 2B, lane 7). Taken together, these results demonstrated that BTEB protein production and BTEB GC-box binding activity were acetaldehyde inducible in HSC.
It has been known that a GC-box DNA motif in a gene promoter region could be bound by several different DNA binding proteins, such as SP-1 and BTEB (16, 21). A very recent study described another novel GC-box binding protein, Zf9, which is increased during HSC activation (30). We have previously shown that the BTEB mobility shift completely differed from that of SP-1, which clearly indicated that SP-1 did not bind to the distal GC box in the 5′ UPS of the αI(I) collagen gene (8). The molecular weight of Zf9 was 42 kDa, while that of BTEB was 32 kDa (16, 21, 30). The amino acid sequence of Zf9 is considerably different from that of BTEB (16, 21, 30). Our results do not suggest the direct binding of Zf9 to this GC box in the rat αI(I) collagen gene. We, however, cannot exclude a possible contribution of the novel GC box binding protein Zf9 in acetaldehyde-induced αI(I) collagen gene expression in HSC. The study by Imataka et al. suggested that flanking nucleotides, copy numbers, and the position of a GC box sequence in the promoter region of a gene, might play key roles in determining which protein would bind to the GC-box and its effects on gene transcription (16). Our observations suggest that BTEB is likely to be an unappreciated important protein binding to the distal GC box in the 5′ UPS of the αI(I) collagen gene and, in turn, regulating the gene expression in HSC (see below).
BTEB mediates acetaldehyde-induced αI(I) collagen gene expression.
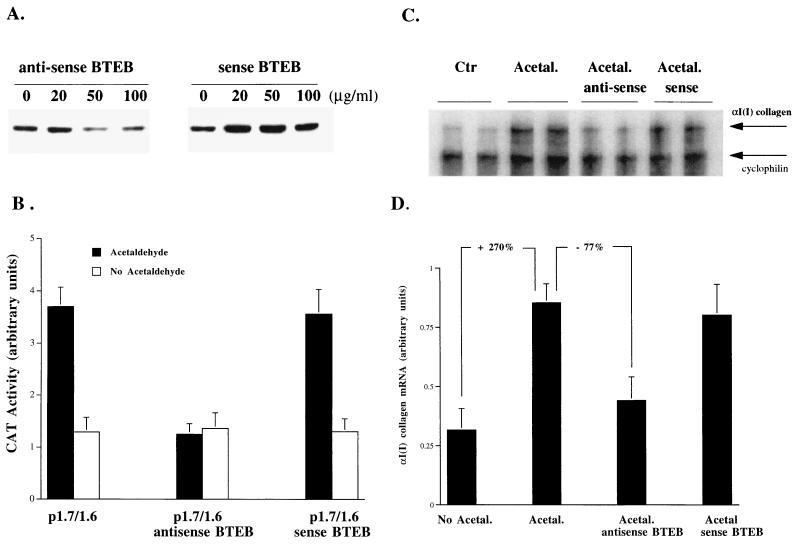
Antisense c-jun oligonucleotides have been successfully used to specifically block c-Jun protein translation in order to study the role of AP-1 activation in cell differentiation (9, 38). Antisense BTEB oligonucleotides were used to block BTEB protein production to determine the role of BTEB in the acetaldehyde-induced αI(I) collagen gene expression in HSC (Fig. 3). To determine the optimal concentration of antisense BTEB oligonucleotides to block BTEB protein translation, HSC were treated with acetaldehyde plus various concentrations of either antisense or sense c-jun oligonucleotides as a control. Western blots indicated that BTEB protein abundance was markedly reduced by antisense BTEB oligonucleotides at 50 μg/ml (Fig. 3A). As expected, the same concentration of sense BTEB oligonucleotides had no effect on BTEB protein abundance (Fig. 3A). Compared to serum-starved control HSC, which were not exposed to any treatments, antisense BTEB oligonucleotides at 50 μg/ml blocked most of the acetaldehyde-induced BTEB protein production (data not shown). The effectiveness of the BTEB antisense oligonucleotides at 50 μg/ml in blocking acetaldehyde-induced αI(I) collagen gene expression was confirmed in HSC transfected with the αI(I) collagen reporter P1.7/1.6 (Fig. 3B). It was found that BTEB antisense oligonucleotides at this dose abolished the acetaldehyde-induced increase in CAT activities in HSC transfected with the αI(I) collagen reporter plasmid P1.7/1.6. The role of BTEB in acetaldehyde-induced αI(I) collagen gene expression was studied by RPA (Fig. 3C). The total RNA was obtained from serum-starved HSC treated with acetaldehyde plus antisense or sense BTEB oligonucleotides at 50 μg/ml. The result of this experiment demonstrated that the steady-state levels of endogenous αI(I) collagen mRNA were significantly reduced by anti-BTEB oligonucleotides (∼77%) (Fig. 3C and D). In contrast, sense BTEB oligonucleotides at the same concentration had no detectable effect on the steady-state levels of endogenous αI(I) collagen mRNA (Fig. 3C and D). Since antisense BTEB oligonucleotides at 50 μg/ml did not completely block BTEB protein production, it is not surprising that the antisense BTEB oligonucleotides could not completely abolish the acetaldehyde-induced increase in αI(I) collagen mRNA (Fig. 3C and D). Taken together, these studies demonstrated that BTEB, as a regulator, mediated the acetaldehyde-induced αI(I) collagen gene transcription in HSC.
FIG. 3.
Inhibition of BTEB by antisense BTEB oligonucleotides significantly reduces acetaldehyde-induced αI(I) collagen mRNA levels. Serum-starved HSC were treated with or without acetaldehyde (100 μM) plus sense or antisense BTEB oligonucleotides at the indicated concentrations for 48 h. The medium was replaced once with fresh DMEM containing acetaldehyde and antisense or sense BTEB oligonucleotides. (A) To determine the optimal concentration of antisense BTEB oligonucleotides, whole-cell protein extracts (30 μg) were analyzed by Western blotting using a polyclonal anti-BTEB serum. (B) Compared to the BTEB sense oligonucleotides, the effectiveness of the BTEB antisense oligonucleotides at 50 μg/ml in blocking acetaldehyde-induced αI(I) collagen gene expression was confirmed in HSC transfected with the αI(I) collagen reporter P1.7/1.6 by CAT assays. Values presented here reflect the means ± standard deviations (n = 6). (C) A representative αI(I) collagen RPA gel is shown. Ten micrograms of total RNA from HSC treated with or without acetaldehyde (100 μM) plus sense or antisense BTEB oligonucleotides at 50 μg/ml were used. Upper arrow, αI(I) collagen mRNA; lower arrow, cyclophilin mRNA, as a control. (D) Quantitation of αI(I) collagen mRNA in an RPA (Fig. 3B) by computer-aided phosphorimaging densitometry. Loading variation was normalized by cyclophilin mRNA. Representative gels are shown.
Acetaldehyde activates JNK and AP-1 in HSC.
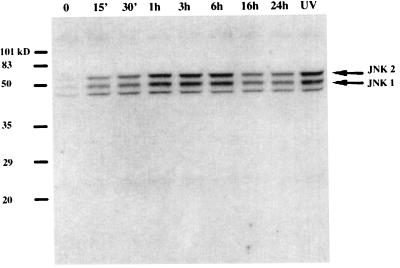
Additional studies were focused on signal transduction pathways to determine how the acetaldehyde signal was transduced to stimulate the activation of the αI(I) collagen gene promoter in HSC. It has been shown that fibronectin and inflammatory cytokines, such as TNF-α and IL-1α, activated JNK and AP-1 in rat HSC (29). HNE, an aldehydic product of lipid peroxidation, was found to interact directly with JNK in human HSC (27). We have recently observed that exposure of HSC to UV irradiation induced JNK activation and AP-1 gene transactivity as well as an increase in αI(I) collagen gene expression (8). The UV response element, as well as the acetaldehyde response element, were located in the same distal GC box in the 5′ promoter of the αI(I) collagen gene (8). It was, therefore, decided to determine whether acetaldehyde activated JNK in rat HSC. Whole-cell extracts were prepared from serum-starved HSC treated with acetaldehyde for Western blot analyses utilizing an anti-ACTIVE JNK antibody, which preferentially recognizes the dual phosphorylated active forms of JNK1,2 (Fig. 4). This study clearly indicated that acetaldehyde rapidly activated JNK1,2 within 15 min and reached its peak within 3 h (Fig. 4).
FIG. 4.
Acetaldehyde induces rapid JNK activation. Whole-cell extracts were prepared from serum-starved HSC exposed to acetaldehyde (100 μM) for the indicated times. A whole-cell extract from HSC treated with UV irradiation (10 J/m2) was used as a positive control for active forms of JNK as previously described (8). Twenty micrograms of proteins of each sample was used for Western blot analysis. Active forms of JNK were probed with a polyclonal antibody specific to the dual phosphorylated and activated JNK1 and JNK2 (Promega), as indicated by the arrows on the right of the gel. A representative gel is shown here.
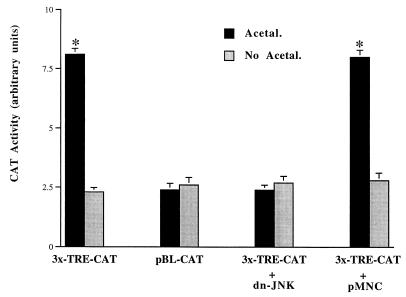
JNK is a major kinase for c-Jun/AP-1 activation. AP-1 activation is generally correlated with changes in the phosphorylation of c-Jun protein (28, 31). AP-1 consists of either Jun-Jun homodimers or Fos-Jun heterodimeric complexes, which bind to TRE sites found in the promoter regions of many genes and regulate the expression of these genes (18–20). To study the role of acetaldehyde in the induction of AP-1 gene transcriptional activity, HSC were transfected with either an AP-1 reporter plasmid, 3x-TRE-CAT, which has three AP-1 binding sites (TRE) linked to a CAT reporter gene, or with a control empty plasmid, pBL-CAT, which has no AP-1 binding site upstream of the CAT gene (Fig. 5). This study demonstrated that acetaldehyde significantly increased CAT activities in cells transfected with 3x-TRE-CAT and that there was no stimulation, as expected, in HSC transfected with the empty vector pBL-CAT. This result suggested that acetaldehyde induced AP-1 activation in HSC. To explore the role of JNK in AP-1 activation induced by acetaldehyde, HSC were cotransfected with 3x-TRE-CAT and either a dominant-negative JNK expression plasmid (dn-JNK) or an empty plasmid, pMNC (Fig. 5). The results indicated that CAT activities induced by acetaldehyde in cells transfected with 3x-TRE-CAT was completely blocked by cotransfected dn-JNK plasmid, suggesting that the activation of JNK was required for the AP-1 activation induced by acetaldehyde (Fig. 5). Taken together, these studies demonstrate that acetaldehyde activates JNK and AP-1 in HSC. These results are in basic agreement with previous findings that acetaldehyde increased the steady-state levels of c-fos and c-jun mRNA transcripts in HSC (5). It is known that activated AP-1 could bind to the TRE in the promoter of the c-jun gene, which would result in the up-regulation of the c-jun gene expression (18–20).
FIG. 5.
Acetaldehyde induces AP-1 activation via a JNK-dependent pathway. Sixty- to eighty-percent confluent HSC were transfected with either an AP-1 reporter plasmid, 3x-TRE-CAT, or an empty control plasmid, pBL-CAT. The AP-1 reporter plasmid 3x-TRE-CAT contains three AP-1 binding sites upstream of a CAT reporter gene. In cotransfection experiments, HSC were cotransfected with 3x-TRE-CAT and a dominant-negative JNK expression plasmid (dn-JNK) or an empty control plasmid, pMNC. After recovery, the transfected cells were treated with or without acetaldehyde (100 μM) for an additional 36 h in DMEM containing 0.4% fetal bovine serum. Transfection efficiency was normalized by measurement of β-galactosidase activity (see Materials and Methods). Values are expressed as means ± standard deviations (n = 6). ∗, P < 0.05 compared with the control (No Acetal).
Acetaldehyde stimulates αI(I) collagen gene promoter activation via a JNK-dependent pathway.
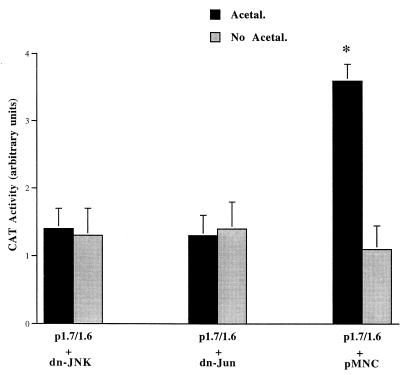
Previous studies have found that acetaldehyde increased αI(I) collagen accumulation in activated HSC (2, 6, 24, 26, 35, 36). Our previous studies discovered that UV irradiation activated JNK and AP-1 gene transactivity and stimulated αI(I) collagen gene expression in HSC via a JNK-dependent pathway (8). Since the intact distal GC box is required for UV as well as acetaldehyde stimulation of αI(I) collagen gene expression in HSC, it was hypothesized that acetaldehyde may be JNK dependent in stimulating αI(I) collagen gene expression in HSC. To explore this hypothesis, HSC were cotransfected with plasmid p1.7/1.6 and dn-JNK or dn-Jun and then treated with acetaldehyde (Fig. 6). It was found that dn-JNK or dn-Jun completely blocked the acetaldehyde-induced increase in CAT activities in cells transfected with p1.7/1.6. In contrast, the control plasmid pMNC had no effect (Fig. 6). These studies demonstrate that acetaldehyde induces αI(I) collagen gene promoter activation in HSC by a JNK- and c-Jun-dependent mechanism.
FIG. 6.
Acetaldehyde induces αI(I) collagen gene promoter activation by a JNK-dependent mechanism. Serum-starved HSC were cotransfected with the wild-type collagen CAT reporter plasmid p1.7/1.6 and either a dominant-negative JNK expression plasmid (dn-JNK), a dominant-negative c-Jun expression plasmid (dn-Jun), or an empty control plasmid, pMNC. After transfection and recovery, cells were treated with or without acetaldehyde for an additional 36 h as described for Fig. 1 and in Materials and Methods. Cotransfected β-galactosidase activity was used for normalization of transfection efficiency (n ± 6). ∗, P < 0.05 compared with the control (No Acetal.).
JNK activation is required for the endogenous αI(I) collagen gene expression induced by acetaldehyde.
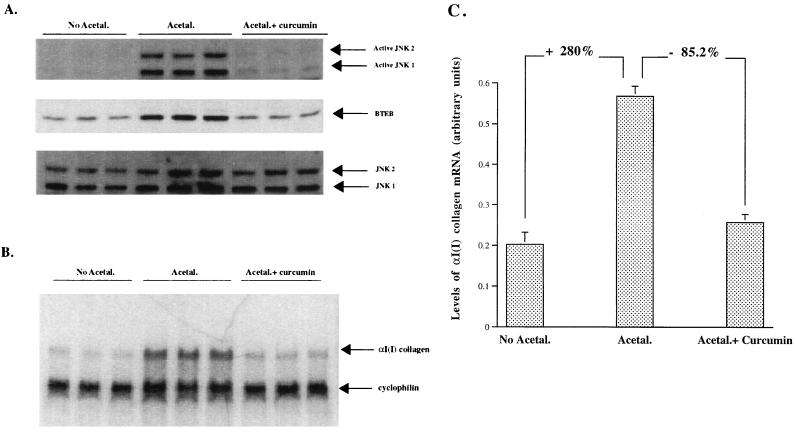
To further evaluate the effects of acetaldehyde-induced JNK activation on endogenous BTEB production and endogenous αI(I) collagen gene expression in HSC, JNK activation was specifically inhibited by curcumin. Curcumin, a dietary pigment responsible for the yellow color of curry, was reported to be a JNK inhibitor at a low dose in many cell lines (10, 15, 22, 33). Determination of the optimal concentration of curcumin in HSC by Western blot analyses found that curcumin at 10 to 20 μM inhibited JNK activation but not ERK activation (data not shown). To confirm that curcumin at this dose blocks JNK activation, further trial experiments were conducted in UV-irradiated HSC transfected with an AP-1 reporter plasmid. The results demonstrated that curcumin at 15 μM completely blocked UV-induced AP-1 activation in HSC (data not shown). These observations confirmed the previous observations in other cell lines (11, 15, 22, 33). Our further studies demonstrated that acetaldehyde specifically induced an increase in the phosphorylated JNK (the active forms of JNK), and that there is no significant change in the total JNK proteins detected by an antibody to JNK1,2 (Fig. 7A). Furthermore, curcumin at 15 μM inhibited JNK activation and subsequently reduced BTEB protein abundance induced by acetaldehyde in HSC (Fig. 7A). As expected, curcumin at 15 μM had no effect on the protein abundance of total JNK1,2. Without acetaldehyde or any other treatments, curcumin at 15 μM did not change the basic level of BTEB protein abundance in HSC (data not shown). Additional studies found that acetaldehyde increased the steady-state levels of αI(I) collagen mRNA (>2.8-fold) and that inhibition of JNK activation by curcumin at 15 μM resulted in a significant reduction in the endogenous steady state levels of αI(I) collagen mRNA (>85%) (Fig. 7B and C). However, curcumin itself at 15 μM had no effect on the endogenous steady-state levels of αI(I) collagen mRNA in HSC without any other treatments (data not shown). These studies provided direct evidence that JNK activation is required for acetaldehyde-induced αI(I) collagen gene expression in HSC. In addition, these studies also support our previous observations in this report that BTEB mediates acetaldehyde-induced JNK activation and acetaldehyde-induced αI(I) collagen gene expression in HSC. Furthermore, this result also explains why the BTEB antisense oligonucleotides had no detectable effects on the basal CAT activities in the transfected HSC incubated in DMEM with 0.4% serum without acetaldehyde treatment (Fig. 3B). The explanation is that because BTEB protein production requires JNK activation (Fig. 7A) and there is a negligible amount of JNK activator in DMEM with 0.4% serum, the BTEB antisense oligonucleotides cannot play any role in blocking the basal expression of the αI(I) collagen gene. It was found that basal expression of the gene requires only the 220 bp in the 5′ promoter region of the αI(I) collagen gene, which does not contain the BTEB-binding GC box (3, 25). A future project is to dissect the promoter of the BTEB gene and study the effects of overexpression of the BTEB gene in HSC on αI(I) collagen gene expression in the absence of acetaldehyde. The results of these studies will contribute to our understanding of the significance of acetaldehyde-induced JNK/AP-1 activation and BTEB production in mediating acetaldehyde stimulation and the increase in αI(I) collagen gene expression in HSC.
FIG. 7.
Inhibition of JNK by curcumin reduced BTEB protein abundance and decreased the endogenous αI(I) collagen mRNA levels. Serum-starved HSC were pretreated with (Acetal.+ curcumin) or without (Acetal.) curcumin at 15 μM for 3 h before being treated with acetaldehyde for 24 h. Cells treated with 0.2% ethyl alcohol (EtOH) only were used as a control (No Acetal.), as curcumin was dissolved in EtOH. Each treatment was performed in triplicate. (A) Nuclear proteins (20 μg/sample) were used for Western blot analyses and detected by anti-ACTIVE JNK pAb, a polyclonal antibody specific to the dual phosphorylated and activated JNK1,2, by anti-BTEB, or by anti-JNK1,2 total proteins as a control for the normalization of loading. (B) Total RNA from the cells (10 μg/lane) was analyzed for the endogenous αI(I) collagen mRNA by RPA. Cyclophilin was used as an internal control to normalize the loading of the total RNA in each lane. (C) The radioactivity in each band in panel B was quantified and normalized by computer-aided phosphorimaging densitometry. Representative gels are shown.
The increase in αI(I) collagen production in activated HSC results from stimulation by extracellular stimuli during hepatic injury. It is, therefore, of interest to identify the involved signal transduction pathways and to determine the required components in the induction of αI(I) collagen gene expression in HSC. Previous studies have demonstrated that the ERK and JNK cascades were involved in the induction of αI(I) collagen gene expression during the activation of HSC (5–7). It was discovered in the present study that acetaldehyde-induced αI(I) collagen gene expression occurs via a JNK-dependent pathway. The acetaldehyde response element was located in the distal GC box (−1484 to −1476) in the 5′ UPS of the αI(I) collagen gene. The GC box, also previously described as the UV response element, was exclusively required for the induction by acetaldehyde of αI(I) collagen gene promoter activation in HSC. In addition, the GC box was bound predominantly by the DNA binding protein, BTEB, which was acetaldehyde inducible as well. BTEB was required for acetaldehyde-induced αI(I) collagen gene expression in HSC. Taken together, these results support the contention that acetaldehyde induces BTEB expression, which then binds to the GC box in the 5′ UPS of αI(I) collagen gene and up-regulates the gene expression in HSC during alcohol-induced hepatic fibrogenesis. At this time, it remains unclear how acetaldehyde-induced JNK and AP-1 activations are involved in regulating BTEB and αI(I) collagen gene expression in HSC. It was not suggested from our results that the putative AP-1 binding motif in the first intron of the gene was a cis-acting factor and was required for the acetaldehyde stimulation in HSC. There is little information available concerning BTEB gene regulation and expression. At least two potential AP-1 binding sites could be found in the 5′ promoter region of the BTEB gene (16).
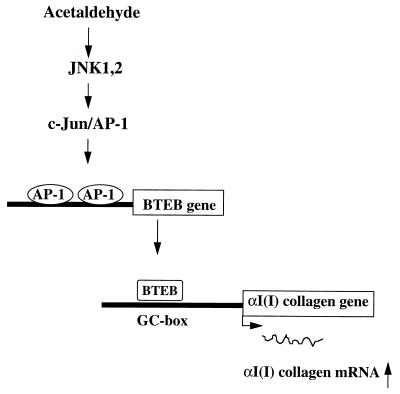
Based on the present and prior observations, a model is proposed to explain how acetaldehyde induces αI(I) collagen gene expression in HSC (Fig. 8). In this model, treatment of HSC with acetaldehyde rapidly activates JNK1,2, which, in turn, phosphorylates c-Jun and enhances AP-1 transactivating ability. The activated AP-1 subsequently binds to the potential AP-1 binding sites in the promoter of the BTEB gene and stimulates BTEB gene expression. The acetaldehyde-induced BTEB, in turn, binds to the distal GC box in the 5′ UPS of the αI(I) collagen gene and stimulates the expression of this gene in HSC.
FIG. 8.
Schema of acetaldehyde-induced αI(I) collagen gene expression in HSC. Exposure of HSC to acetaldehyde rapidly activates JNK1,2, though it remains unclear how JNKs are activated. Activated JNKs phosphorylate and activate c-Jun/AP-1, which, in turn, up-regulates BTEB gene expression by binding to the putative AP-1 binding sites in the promoter of BTEB gene. The acetaldehyde-induced BTEB acts as a mediator, binding to the distal GC box in the promoter and stimulating αI(I) collagen gene expression in HSC.
In summary, the present studies demonstrated that a cis-acting acetaldehyde response element was located in the distal GC box in the 5′ UPS of the αI(I) collagen gene. The GC box was bound by the DNA binding transcription factor BTEB, whose abundance and DNA binding activity were acetaldehyde inducible in HSC. Inhibition of BTEB protein expression by antisense BTEB oligonucleotides indicated the requirement of this protein in the acetaldehyde induction of αI(I) collagen gene expression. Further experiments found that acetaldehyde stimulated αI(I) collagen gene expression via a JNK- and AP-1-dependent pathway. This study identified a previously unappreciated link between the ubiquitous JNK, AP-1, BTEB, and the GC box. The relationship among them suggested in this report may not be unique to the αI(I) collagen gene but may involve other genes as well. These studies have practical importance for the understanding of the mechanisms of alcohol-induced cirrhosis because acetaldehyde is the chief metabolite of ethanol with major pathobiological significance during hepatic fibrogenesis.
ACKNOWLEDGMENTS
We thank J. J. Vande Vusse for technical assistance and D. Rowe, D. Breault, and R. Davis for plasmids used in transfection.
This work was supported by National Institute of Health grants DK 02022, DK40223, DK 42086, DK 07074-18, and DK 47995-01A2 and by the Liver Research Fund, University of Chicago.
REFERENCES
- 1.Bornstein P, McKay J. The first intron of the alpha 1(I) collagen gene contains several transcriptional regulatory elements. J Biol Chem. 1988;263:1603–1606. [PubMed] [Google Scholar]
- 2.Brenner D A, Chojkier M. Acetaldehyde increases collagen gene transcription in cultured human fibroblasts. J Biol Chem. 1987;262:17690–17695. [PubMed] [Google Scholar]
- 3.Brenner D A, Rippe R A, Veloz L. Analysis of the collagen alpha 1(I) promoter. Nucleic Acids Res. 1989;17:6055–6064. doi: 10.1093/nar/17.15.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casini A, Cunningham M, Rojkind M, Lieber C S. Acetaldehyde increases procollagen type I and fibronectin gene transcription in cultured rat fat-storing cells through a protein synthesis-dependent mechanism. Hepatology. 1991;13:758–765. [PubMed] [Google Scholar]
- 5.Casini A, Galli G, Salzano R, Ceni E, Franceschelli F, Rotella C M, Surrenti C. Acetaldehyde induces c-fos and c-jun proto-oncogenes in fat-storing cell cultures through protein kinase C activation. Alcohol Alcohol. 1994;29:303–314. [PubMed] [Google Scholar]
- 6.Casini A, Galli G, Salzano R, Rotella C M, Surrenti C. Acetaldehyde-protein adducts, but not lactate and pyruvate, stimulate gene transcription of collagen and fibronectin in hepatic fat-storing cells. J Hepatol. 1993;19:385–392. doi: 10.1016/s0168-8278(05)80547-1. [DOI] [PubMed] [Google Scholar]
- 7.Chen A, Beno D W A, Davis B H. Suppression of stellate cell type I collagen gene expression involves AP-2 transmodulation of nuclear factor-1-dependent gene transcription. J Biol Chem. 1996;271:25994–25998. doi: 10.1074/jbc.271.42.25994. [DOI] [PubMed] [Google Scholar]
- 8.Chen A, Davis B H. UV irradiation activates JNK and increases alpha I(I) collagen gene expression in rat hepatic stellate cells. J Biol Chem. 1999;274:158–164. doi: 10.1074/jbc.274.1.158. [DOI] [PubMed] [Google Scholar]
- 9.Chen A, Davis B H, Bissonnette M, Scaglione-Sewell B, Brasitus T A. 1,25-Dihydroxyvitamin D(3) stimulates activator protein-1-dependent Caco-2 cell differentiation. J Biol Chem. 1999;274:35505–35513. doi: 10.1074/jbc.274.50.35505. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y R, Tan T H. Inhibition of the c-Jun N-terminal kinase (JNK) signaling pathway by curcumin. Oncogene. 1998;17:173–178. doi: 10.1038/sj.onc.1201941. [DOI] [PubMed] [Google Scholar]
- 11.Davis B H, Chen A, Beno D W. Raf and mitogen-activated protein kinase regulate stellate cell type 1 collagen expression. J Biol Chem. 1996;271:11039–11042. doi: 10.1074/jbc.271.19.11039. [DOI] [PubMed] [Google Scholar]
- 12.Ham J, Babij C, Whitfield J, Pfarr C M, Lallemand D, Yaniv M, Rubin L L. A c-Jun dominant negative mutant protects sympathetic neurons against programmed cell death. Neuron. 1995;14:927–939. doi: 10.1016/0896-6273(95)90331-3. [DOI] [PubMed] [Google Scholar]
- 13.Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 14.Houglum K, Buck M, Alcorn J, Contreras S, Bornstein P, Chojkier M. Two different cis-acting regulatory regions direct cell-specific transcription of the collagen alpha 1(I) gene in hepatic stellate cells and in skin and tendon fibroblasts. J Clin Investig. 1995;96:2269–2276. doi: 10.1172/JCI118282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang T S, Lee S C, Lin J K. Suppression of c-Jun/AP-1 activation by an inhibitor of tumor promotion in mouse fibroblast cells. Proc Natl Acad Sci USA. 1991;88:5292–5296. doi: 10.1073/pnas.88.12.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imataka H, Sogawa K, Yasumoto K, Kikuchi Y, Sasano K, Kobayashi A, Hayami M, Fujii-Kuriyama Y. Two regulatory proteins that bind to the basic transcription element (BTE), a GC box sequence in the promoter region of the rat P-4501A1 gene. EMBO J. 1992;11:3663–3671. doi: 10.1002/j.1460-2075.1992.tb05451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson R S, van Lingen B, Papaioannou V E, Spiegelman B M. A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes Dev. 1993;7:1309–1317. doi: 10.1101/gad.7.7b.1309. [DOI] [PubMed] [Google Scholar]
- 18.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. Phil Trans R Soc Lond B. 1996;351:127–134. doi: 10.1098/rstb.1996.0008. [DOI] [PubMed] [Google Scholar]
- 19.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 20.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi A, Sogawa K, Imataka H, Fujii-Kuriyama Y. Analysis of functional domains of a GC box-binding protein, BTEB. J Biochem (Tokyo) 1995;117:91–95. doi: 10.1093/oxfordjournals.jbchem.a124727. [DOI] [PubMed] [Google Scholar]
- 22.Lin J K, Chen Y C, Huang Y T, Lin-Shiau S Y. Suppression of protein kinase C and nuclear oncogene expression as possible molecular mechanisms of cancer chemoprevention by apigenin and curcumin. J Cell Biochem Suppl. 1997;29:39–48. [PubMed] [Google Scholar]
- 23.Liska D J, Slack J L, Bornstein P. A highly conserved intronic sequence is involved in transcriptional regulation of the alpha 1(I) collagen gene. Cell Regul. 1990;1:487–498. doi: 10.1091/mbc.1.6.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McClain C J, Marsano L, Burk R F, Bacon B. Trace metals in liver disease. Semin Liver Dis. 1991;11:321–339. doi: 10.1055/s-2008-1040450. [DOI] [PubMed] [Google Scholar]
- 25.Nehls M C, Rippe R A, Veloz L, Brenner D A. Transcription factors nuclear factor I and Sp1 interact with the murine collagen α1(I) promoter. Mol Cell Biol. 1991;11:4065–4073. doi: 10.1128/mcb.11.8.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pares A, Potter J J, Rennie L, Mezey E. Acetaldehyde activates the promoter of the mouse alpha 2(I) collagen gene. Hepatology. 1994;19:498–503. doi: 10.1002/hep.1840190231. [DOI] [PubMed] [Google Scholar]
- 27.Parola M, Robino G, Marra F, Pinzani M, Bellomo G, Leonarduzzi G, Chiarugi P, Camandola S, Poli G, Waeg G, Gentilini P, Dianzani M U. HNE interacts directly with JNK isoforms in human hepatic stellate cells. J Clin Investig. 1998;102:1942–1950. doi: 10.1172/JCI1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piechaczyk M, Blanchard J M. c-fos proto-oncogene regulation and function. Crit Rev Oncol Hematol. 1994;17:93–131. doi: 10.1016/1040-8428(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 29.Poulos J E, Weber J D, Bellezzo J M, Di Bisceglie A M, Britton R S, Bacon B R, Baldassare J J. Fibronectin and cytokines increase JNK, ERK, AP-1 activity, and transin gene expression in rat hepatic stellate cells. Am J Physiol. 1997;273:G804–G811. doi: 10.1152/ajpgi.1997.273.4.G804. [DOI] [PubMed] [Google Scholar]
- 30.Ratziu V, Lalazar A, Wong L, Dang Q, Collins C, Shaulian E, Jensen S, Friedman S L. Zf9, a Kruppel-like transcription factor up-regulated in vivo during early hepatic fibrosis. Proc Natl Acad Sci USA. 1998;95:9500–9505. doi: 10.1073/pnas.95.16.9500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonnenberg J L, Mitchelmore C, Macgregor-Leon P F, Hempstead J, Morgan J I, Curran T. Glutamate receptor agonists increase the expression of Fos, Fra, and AP-1 DNA binding activity in the mammalian brain. J Neurosci Res. 1989;24:72–80. doi: 10.1002/jnr.490240111. [DOI] [PubMed] [Google Scholar]
- 32.Stefanovic B, Hellerbrand C, Holcik M, Briendl M, Liebhaber S A, Brenner D A. Posttranscriptional regulation of collagen α1(I) mRNA in hepatic stellate cells. Mol Cell Biol. 1997;17:5201–5209. doi: 10.1128/mcb.17.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeshita A, Chen Y, Watanabe A, Kitano S, Hanazawa S. TGF-beta induces expression of monocyte chemoattractant JE/monocyte chemoattractant protein 1 via transcriptional factor AP-1 induced by protein kinase in osteoblastic cells. J Immunol. 1995;155:419–426. [PubMed] [Google Scholar]
- 34.Treisman R. Regulation of transcription by MAP kinase cascades. Curr Opin Cell Biol. 1996;8:205–215. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- 35.Tsukamoto H. Oxidative stress, antioxidants, and alcoholic liver fibrogenesis. Alcohol. 1993;10:465–467. doi: 10.1016/0741-8329(93)90066-w. [DOI] [PubMed] [Google Scholar]
- 36.Tsukamoto H, Matsuoka M, French S W. Experimental models of hepatic fibrosis: a review. Semin Liver Dis. 1990;10:56–65. doi: 10.1055/s-2008-1040457. [DOI] [PubMed] [Google Scholar]
- 37.van Dam H, Duyndam M, Rottier R, Bosch A, de Vries-Smits L, Herrlich P, Zantema A, Angel P, van der Eb A J. Heterodimer formation of c-Jun and ATF-2 is responsible for induction of c-jun by the 243 amino acid adenovirus E1A protein. EMBO J. 1993;12:479–487. doi: 10.1002/j.1460-2075.1993.tb05680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H, Xie Z, Scott R E. Differentiation modulates the balance of positive and negative Jun/AP-1 DNA binding activities to regulate cellular proliferative potential: different effects in nontransformed and transformed cells. J Cell Biol. 1996;135:1151–1162. doi: 10.1083/jcb.135.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zorzano A, Herrera E. Disposition of ethanol and acetaldehyde in late pregnant rats and their fetuses. Pediatr Res. 1989;25:102–106. doi: 10.1203/00006450-198901000-00022. [DOI] [PubMed] [Google Scholar]