Abstract
Anhedonia is a transdiagnostic construct that can occur independent of other symptoms of depression; its role in neuropsychiatric disorders that are not primarily affective, such as obsessive compulsive disorder (OCD), hoarding disorder (HD), and post-traumatic stress disorder (PTSD) has received limited attention. This paper addresses this gap. First, the data revealed a positive contribution of anhedonia, beyond the effects of general depression, to symptom severity in OCD but not in HD or PTSD. Second, anhedonia was operationalized as a reduced sensitivity to rewards, which allowed employing the value based decision making framework to investigate effects of anhedonia on reward-related behavioral outcomes, such as increased risk aversion and increased difficulty of making value-based choices. Both self-report and behavior-based measures were used to characterize individual risk aversion: risk perception and risk-taking propensities (measured using the Domain Specific Risk Taking scale) and risk attitudes evaluated using a gambling task. Data revealed the positive theoretically predicted correlation between anhedonia and risk perception in OCD; effects on self-reported risk taking and behavior-based risk aversion were non-significant. The same relations were weaker in HD and absent in PTSD. Response time during a gambling task, an index of difficulty of making value-based choices, significantly correlated with anhedonia in individuals with OCD and individuals with HD, even after controlling for general depression, but not in individuals with PTSD. The results suggest a unique contribution of one aspect of anhedonia in obsessive-compulsive disorder and confirm the importance of investigating the role of anhedonia transdiagnostically beyond affective and psychotic disorders.
1. Introduction
Anhedonia is defined as a loss of interest in activities that an individual enjoyed previously and a decreased ability to pursue, experience, and learn about pleasure. It has been linked to diminished reward processing (Whitton et al., 2015). Anhedonia is a hallmark symptom of major depressive disorder (APA, 2013) and is associated with impaired functioning and worse treatment outcomes (Davidson et al., 2010; Dunlop and Nemeroff, 2007; Kouros et al., 2016; McCabe et al., 2009, 2010; Nutt et al., 2007; Price et al., 2009; Spijker et al., 2001). Recent studies suggest that anhedonia is a transdiagnostic construct that can occur independent of other depressive symptoms (Abramovitch et al., 2014; Insel et al., 2010; Insel and Cuthbert, 2015; Weinberg et al., 2015). For instance, it is a core negative feature of psychotic disorders (Anticevic et al., 2015; Barch et al., 2017a, 2017b; Dowd et al., 2016). It is also frequently seen in other neuropsychiatric disorders with which depression is commonly comorbid, such as obsessive compulsive (OCD, (Overbeek et al., 2002)), hoarding (HD, (Frost et al., 2011)), and post-traumatic stress disorders (PTSD, (Campbell et al., 2007)). However, the cross-diagnostic contribution of anhedonia to this latter group of psychopathologies remains unclear (Abramovitch et al., 2014; Nawijn et al., 2015). This study aims to address that gap.
Several lines of evidence suggest that anhedonia contributes to OCD, independent of comorbid depression. Individuals with OCD exhibit anhedonia, and it correlates with symptom severity even after controlling for comorbid depression (Abramovitch et al., 2014). Neuroimaging reveals abnormal activation and functional connectivity within reward processing circuitry, including ventral striatum and medial prefrontal cortex, in OCD (Anticevic et al., 2014b; Harrison et al., 2009, 2013). Our recent behavioral studies reveal increased inconsistency of value-based choices in OCD; this may be linked to aberrant value encoding, such as increased noisiness in or a blunting of the value signal (Pushkarskaya et al., 2015, 2017), which is related to anhedonia, as detailed below. The relationship of anhedonia to other conditions outside the primary affective and psychotic disorders is less clear. In post-traumatic stress disorder (PTSD), anhedonia may correlate with emotional numbing but has little relationship to other symptoms (Kashdan et al., 2006). Decreased reward processing may be more prominent in men than in women with PTSD (Nawijn et al., 2015). No studies have examined the association of anhedonia with hoarding disorder (HD).
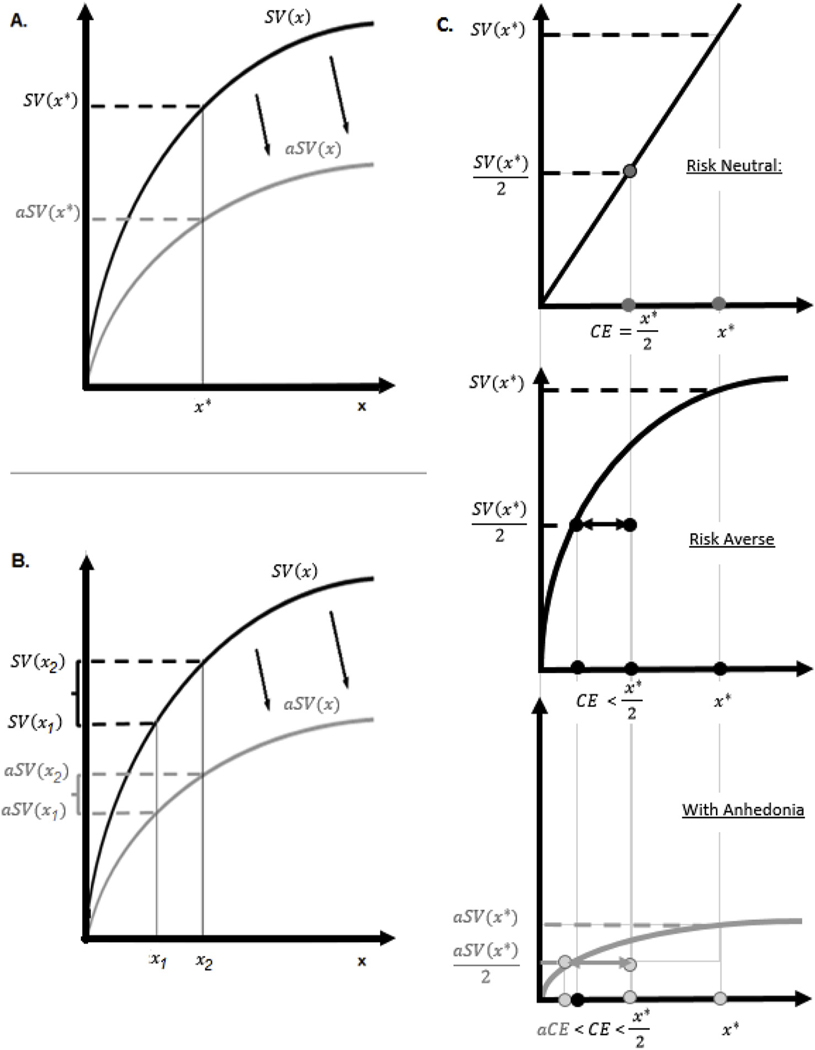
Anhedonia is a complex construct (Argyropoulos and Nutt, 2013; Treadway and Zald, 2013). Computational models of behavior propose several hypotheses as to how parameters of decision-making and learning models (such as feedback sensitivity, noise in valuation, and outcome magnitude sensitivity) may be linked to individual variation in anhedonia (Chung et al., 2017; Huys et al., 2013; Mueller et al., 2007). In major depression, this approach has produced mixed, often negative results (Robinson and Chase, 2017). Here, we employ a robust theoretical framework from behavioral economics, the value-based decision making framework (Rangel et al., 2008), to investigate how anhedonia may be linked to one parameter of the subjective value model (Kahneman and Tversky, 1979), reduced steepness of the subjective value (SV) function, cross-diagnostically, in individuals with OCD, HD, and PTSD. Reduced steepness of the SV function has several empirically testable implications for reward-related behavioral outcomes, such as increased risk aversion and increased difficulty of making value-based choices (Fig. 1). We used self-report and behavior-based data to test these predictions across the three diagnoses. Studying effects of anhedonia cross-diagnostically may help to elucidate whether different aspects of anhedonia manifest differentially in different psychopathologies.
Fig. 1.
Hypothesized effects of Anhedonia on Subjective Value. A. Anhedonia can be operationalized as reduced sensitivity to rewards, which implies flatter subjective value function (aSV). B. For an individual with anhedonia (flatter aSV) the choice between two alternatives, x1 and x2, is more difficult than it is for an individual without anhedonia (steeper SV). C. Risk aversion can be operationalized as willingness to pay extra money to avoid dealing with risk. For instance, a risk averse individual may agree to receive $4 with certainty, in preference to a lottery in which there are even odds of receiving $10 or nothing (i.e. with an expected value of $5). The subjective value of such a lottery ($4 in this case) is termed its ‘certainty equivalence’ (CE); the difference between the CE of $4 and the expected value (EV) of $5 is termed the ‘risk premium’ (RP). A risk neutral individual would have a RP of zero, such that the CE = EV; such an individual would not be willing to accept anything less than $5 in exchange for a lottery with an EV of $5. A risk-neutral individual would necessarily have a linear subjective value function (top panel). On the other hand, a concave subjective value function will always yield CE < EV and thus RP > 0, implying risk aversion (middle panel). Flatter subjective value function of an individual with anhedonia implies stronger risk aversion, aCE < CE and thus aRP > RP (bottom panel).
2. Methodology
2.1. Theoretical framework
The value-based decision framework (Rangel et al., 2008) suggests that, during decision making, individuals assign a subjective value (SV) to available options (valuation) and then choose the option with the largest SV (value-based choice). The relationship between objective and subjective values is typically positive but nonlinear: individuals tend to obtain diminishing levels of satisfaction, or marginal SV, from additional units of a valued outcome (Kahneman and Tversky, 1979). For instance, the difference between $0 and $10 is experienced as larger than the difference between $1000 and $1010. This is reflected in the concave SV function in the domain of gains (Fig. 1A).
Within this framework, anhedonia can be operationalized as a reduced subjective sensitivity to magnitude of objective rewards. This implies that the SV function of an anhedonic individual (aSV) is flattened (Fig. 1A): a given increment in objective reward leads to a smaller change in subjective reward (along the y-axis). Operationalizing anhedonia as a flattening of the individual subjective value function has several implications for reward-related processes, as detailed below. Another way to operationalize anhedonia within this framework is as increased noisiness in subjective valuation, often modeled by inverse temperature parameters (Robinson and Chase, 2017). Prior studies have investigated the latter; it is beyond the scope of this paper, although we control for inverse temperature in analyses of behavioral data, as detailed below.
Increased difficulty of making choices.
Choice between alternatives with clearly distinct SVs is straightforward. Choices may become difficult, however, when options are of similar SV. Fig. 1B illustrates that for an anhedonic individual (flatter aSV) the choice between two alternatives, x1 and x2, is more difficult. Choice difficulty is commonly associated with increased response time (Dodonov and Dodonova, 2012; Gilbert et al., 2012). Thus, we predict anhedonia severity to correlate positively with response time during difficult value-based choices.
Increased risk aversion.
The concave subjective value function implies risk aversion (see Fig. 1C). Individuals vary in how much money they are willing to give up to avoid dealing with risk (i.e. their risk premium, or RP). Fig. 1C illustrates that for an anhedonic individual, the flattened SV curve (bottom) implies higher RP. Thus, anhedonia is predicted to correlate positively with risk aversion.
Measuring risk aversion.
A variety of measures has been developed to evaluate risk aversion (Harrison et al., 2005). Two types of measures are typically used: self-report (evaluated via questionnaires) and behavior-based (derived from choice data during a laboratory experiment). While both are designed to measure the same theoretical construct, they do not necessarily correlate (Dislich et al., 2010). Evidence as to which has better ecological validity is mixed (Dohmen et al., 2005, 2011). We choose to remain agnostic and use both types of measure to test theoretically-predicted effects of anhedonia on risk aversion (see Measures).
2.2. Participants (Table 1)
Table 1.
Sample description: demographics.
| OCD | HD | PTSD | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Dx (N = 27) | GPC (N = 29) | p | Dx (N = 18) | GPC (N = 26) | p | Dx (N = 28) | VCC (N = 28) | p | |
| Age | 31.0 ± 2.0 | 29.6 ± 1.7 | 0.44 | 51.5 ± 2.1 | 47.8 ± 3.0 | 0.36 | 31.5 ± 1.4 | 35.0 ± 1.6 | 0.12 |
| Male | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.99 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.75 | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.71 |
| IQ | 104.0 ± 2.3 | 109.4 ± 2.2 | 0.13 | 110.0 ± 3.7 | 112.2 ± 2.4 | 0.99 | 105.6 ± 2.0 | 105.4 ± 2.1 | 0.95 |
Note: OCD – individuals with Obsessive Compulsive Disorder; HD – individuals with Hoarding disorder; PTSD – combat veterans with post-traumatic stress disorder; Dx – individuals from a corresponding clinical group; GPC – Controls from general population; VCC – controls combat veterans. Significance of the between-group difference, p-value, for Age and IQ is based on the one-way ANOVA; significance of the between -group difference, p-value, for Male is based on the Pearson’s chi-squared test (χ2).
All procedures were approved by the Yale University Human Investigation Committee, the VA Central Institutional Review Board (IRB), and the Hartford Hospital Institutional Review Board. All participants provided written informed consent and completed a demographic questionnaire and the Kaufman Brief Intelligence Test (Kaufman, 1979). All diagnoses were established by doctoral-level clinicians; PTSD diagnosis was confirmed using the Structural Clinical Interview for DSM-IV Disorders (SCID-IV; (First et al., 2012)), OCD and HD diagnoses were confirmed or excluded using a structured diagnostic interview for DSM-5 anxiety, mood, and obsessive-compulsive and related disorders (DIAMOND; (Tolin et al., 2016)). Only unmedicated or stably medicated individuals (SSRI monotherapy for ≥ 8 weeks) were included. Comorbid MDD was diagnosed in 8 OCD participants, 6 HD participants, and 15 PTSD participants. Other comorbid conditions included Generalized Anxiety Disorder, Social Anxiety, Bipolar Disorder, and Tic Disorder. For the full list of comorbid conditions see SM1.
These data were collected as part of a larger attitude study, focused primarily on how behavioral measures of risk and ambiguity are affected by psychopathologies (Pushkarskaya et al., 2015, 2017; Ruderman et al., 2016). Twenty-seven individuals with OCD without significant HD symptoms and 18 individuals with HD lacking significant OCD symptoms were recruited through the Yale OCD Research Clinic and the Anxiety Disorders Center at the Institute of Living, Hartford Hospital. Fifty-five control participants from the general population (GPC), matched on demographic and cognitive characteristics with the OCD and HD samples, were recruited in the New Haven, CT area using flyers. Twenty-eight combat veterans with PTSD and 28 control combat veterans without PTSD (VCC), matched on demographic and cognitive characteristics, were recruited through the VA National Center for PTSD, West Haven, CT.
Data from control groups were included in the analyses to evaluate general effects of psychiatric diagnoses on risk aversion and response time. Since HD individuals were older (51.5 ± 2.1 years) than OCD individuals (31.0 ± 2.0; t = 7.05, p < 0.001), and age can potentially affect risk aversion (Tymula et al., 2013), we generated two independent control subsamples that matched OCD and HD on age, gender, and IQ (Table 1), as in Pushkarskaya et al. (2017).
Approximately 40% of OCD and HD participants, as well as GPC, were males, allowing examination of potential gender effects. Combat veteran participants were mostly male (22 out of 26 with PTSD, and 24 out of 27 without PTSD), which does not allow for evaluation of the gender effects in this sample.
2.3. Measures (Table 2)
Table 2.
Key concepts and measures.
| Concept | Measure | Definition/Description | References |
|---|---|---|---|
| Sensitivity to rewards | |||
| Steepness of SV | Expresses relationship between objective payoff and subjective assessment this payoff. Flatter subjective value function reflects reduced subjective sensitivity to objective rewards, may imply reduced incentive motivation. | Hsee et al. (2005); McClure et al. (2003). | |
|
| |||
| Severity of Clinical Symptoms | |||
| Anhedonia and depression | BDI-II | Well-validated self-report measure of depression, including both anhedonia (3 items) and other aspects of depression (18 items). Confirmatory factor analysis of this scale has shown that a two-factor CFA model separating anhedonia from other items outperforms a one-factor model. | Joiner et al. (2003); Leventhal et al. (2006); Kashdan et al. (2006). |
| aBDI | Anhedonia Subscale of BDI-II, consisting of the 3 anhedonia-related items. It has been shown to correlate with individual hedonic capacity. | ||
| gBDI | Non-anhedonic (general) subscale of BDI-II, consisting of the other 18 items that capture other symptoms of depression. | ||
|
|
|||
| Disorder-specific symptom severity | Y-BOCS | Well-validated clinician-administered measure of OCD severity; includes obsession and compulsion subscales. | Goodman et al. (1989)a, b |
| SIR | Well-validated self-report measure of hoarding symptomatology. Can be used to access hoarding tendencies in general population; includes 3 validated subscales: Clutter, Difficulty Discarding, Excessive Acquisition | Frost et al. (2004); Tolin et al. (2011) | |
| CAPS | Well-validated clinician-administered measure of PTSD symptomatology. CAPS assesses Cluster B re-experiencing symptoms, Cluster C avoidance symptoms, Cluster C emotional numbing symptoms, and Cluster D hyperarousal symptoms. | Aker et al. (1999). | |
|
|
|||
| Risk Attitudes | |||
| Self-Reported | DOSPERT | Well-validated and broadly used by behavioral economics studies self-reported measure of risk attitudes in five domains: during ethical, financial, health/safety, social, and recreational decisions. The factor structure replicated in a wide range of settings and populations. Risk perception and risk taking measure different constructs; they are negatively correlated in the general population. | Weber et al. (2002); Blais and Weber (2006); Wu and Cheung (2014); Figner and Weber (2011) |
| RiskT | Self-reported Risk Taking, assessed by DOSPERT scale. Evaluates level of risk taking. E.g. evaluate “How likely you are to invest 10% of your income in a new business venture,” using a 7-point rating scale ranging from 1 (Extremely Unlikely) to 7 (Extremely Likely). | ||
| RiskP | Self-reported Risk Perception, assessed using the DOSPERT scale. Evaluates willingness to engage in a risky activity as a function of its perceived riskiness. E.g. indicate “How risky you think it is to invest 10% of your income in a new business venture,” using a 7-point rating scale ranging from 1 (Not at all) to 7 (Extremely Risky). | ||
| Behavior based | RiskB | Choice based measure of risk averse behavior during a laboratory gambling task. This task includes choices under risk and ambiguity, between gains and between losses separately (Risk and Ambiguity task). The measure of risk aversion is derived using choices during risk trials under gains only. During these trials, participants make 80 choices between a sure payoff of $5 and a lottery that offers a nonzero chance of a positive payoff. Both probabilities (0.13, 0.25, 0.38, 0.5, 0.75) and payoff amounts ($8, $20, $50, $125) vary systematically; each trial repeated 4 times. Choices of each participant are compared to choices of a hypothetical risk-neutral decision maker, which in this task would choose risky lotteries over the sure payoff 72.5% of the time. ; it is positive for a risk-averse participant, and negative for a risk-seeking participant. | Levy et al. (2010); Tymula et al. (2013); Pushkarskaya et al. (2015); Pushkarskaya et al. (2017). |
|
| |||
| Task Difficulty | |||
| RT | Response time during risky choices under gains of the Risk and Ambiguity task. Response time during each of these choices, excluding omissions, was log-transformed and averaged across trials. Response time has been demonstrated to increase with task difficulty in general population. | Wright and Ayton (1988); Konovalov and Krajbich (2017) | |
|
| |||
| Noise in Valuation | γ | Inverse temperature parameter, from the model used in the analysis of choice data ; where Pv is the probability that the participant chose a lottery, SVF and SVV are the SVs of the fixed sure payoff and a variable options (lottery), respectively, and γ is the slope of the logistic function, or equivalently a noise parameter. Along with steepness of a subjective value function, it has been shown to correlate with proportion of risky choices of a participant. | Gilboa and Schmeidler (1989); Levy et al. (2010). |
Clinical measures.
All participants from three clinical populations, as well as combat veteran controls (VCC), were assessed on the Beck Depression Inventory-II (BDI-II, (Beck et al., 1996)). For the BDI-II, we utilized two subscales, following the procedures of Joiner and colleagues (Joiner et al., 2003). An Anhedonic subscale was created by summing responses on BDI-II anhedonia-associated items (aBDI): loss of pleasure (item #4), loss of interest (item #12), and loss of interest in sex (item #21). A General Depression subscale (gBDI) consisted of the sum of the remaining 18 items. Prior studies have demonstrated that a two-factor CFA distinguishing anhedonic and nonanhedonic items outperformed a model with one latent variable defined by all 21 items (Joiner et al., 2003; Kashdan et al., 2006).
Symptom severity in the clinical groups was assessed using the Yale-Brown Obsessive Compulsive Scale (Y-BOCS; (Goodman et al., 1989a; Goodman et al., 1989b)) for OCD, the Saving Inventory Revised (SI-R; (Frost et al., 2004)) for HD, and the Clinician-Administered PTSD Scale for DSM-IV (CAPS-IV) for PTSD.
Self-report measures.
Twenty-three OCD participants, sixteen HD participants, all PTSD, and all control participants completed the Domain-Specific Risk-Taking (DOSPERT) Scale (Blais and Weber, 2006). This scale allows assessing both conventional risk attitudes (defined as the reported level of risk taking) and perceived risk attitudes (defined as the willingness to engage in a risky activity as a function of its perceived riskiness) in five commonly encountered domains: ethical, financial, health/safety, social, and recreational decisions (SM2). This scale has been broadly used and validated by behavioral economics studies, and its factor structure replicated in a wide range of settings and populations (Blais and Weber, 2006, 2009; Highhouse et al., 2017; Weber et al., 2002; Wu and Cheung, 2014). First, respondents rated the likelihood that they would engage in risky activities (Risk Taking, RiskT), and then they reported their perceptions of how risky these activities actually are (Risk Perception, RiskP). We calculated total scores on both RiskT and RiskP for each participant. In healthy individuals, risk perception and risk taking are highly negatively correlated: individuals are less likely to engage in activities that they perceive as more risky (Blais and Weber, 2006; Johnson et al., 2004; Weber and Hsee, 1998). The subjective value model predicts that severity of anhedonia correlates positively with risk aversion (i.e. negatively with risk taking).
Behavior-Based Measures.
Twenty-five OCD participants and all HD, PTSD, and control participants completed the Risk & Ambiguity Task, detailed in SM3 (Levy et al., 2010; Pushkarskaya et al., 2015, 2017). Briefly, participants made a series of choices between a sure payoff and a lottery; probabilities and magnitudes of payoffs varied systematically. To calculate behavior-based index of risk aversion (RiskB), we compared the proportion of risky choices of each participant during risky trials under gains to that of a hypothetical risk-neutral decision maker ((Pushkarskaya et al., 2017; Pushkarskaya et al., 2015); SM1). A positive score implies risk aversion (lower proportion of risky choices); a negative score implies risk seeking (higher proportion of risky choices). Note that RiskB reflects risk aversion, while RiskT reflects risk taking; thus, to the extent that these measures are tapping into the same underlying construct, RiskB may negatively correlate with RiskT.
A higher proportion of risky choices may also result from higher choice variability (Robinson and Chase, 2017), commonly modeled by the inverse temperature parameter (γ, estimated by fitting a theoretical model to the choice data as detailed in SM4; more negative scores imply less random choices, γ = 0 implies fully random choices), as well as on interaction between the steepness of SV and inverse temperature. Therefore, in our planned tests of relationship between RiskB and other variables of interest we control for γ. We also calculated the average log-transformed response time from each risky trial during gain blocks for each participant (van der Linden, 2006), excluding omissions (RT, an index of choice difficulty (Dodonov and Dodonova, 2012; Gilbert et al., 2012). The subjective value model predicts that anhedonia severity should correlate positively with response time (RT, Fig. 1B) and behavior-based risk aversion score (RiskB, Fig. 1C).
2.4. Data analysis
All variables of interest were tested for normality using the ShapiroWilk test. For between-group comparisons we employed one-way ANOVAs for normally distributed variables and nonparametric tests (Kruskal Wallis test or Mann-Whitney U test) for non-normally distributed variables. To examine correlations, we employed regression analyses (nonparametric, if variables were not normally distributed). Most statistical analyses were performed using SPSS v.24. Nonparametric multivariate regressions were performed using R 3.3.3 (using the command “gam”).
3. Results
3.1. Descriptive analyses
Anhedonia (aBDI) was not normally distributed OCD (Shapiro-Wilk p = 0.01) or HD (Shapiro-Wilk p < 0.01) subjects but was normally distributed in PTSD (Shapiro-Wilk p > 0.10). General depression (gBDI), DOSPERT, other clinical, and behavior-based measures were normally distributed in all groups. Inverse temperature (γ), which measures randomness in decision-making, was normally distributed in all groups once 5 extreme outliers (> 3 SD from subsample means) were removed (1 OCD, 1 HD Controls, 1 PTSD, and 2 Veteran Controls); these participants were removed from analyses that included inverse temperature parameter.
Anhedonia and general depression (Table 3) were similar in OCD and HD (Mann-Whitney U: p = 0.76 and p = 0.60 respectively) but higher in individuals with PTSD than in either OCD or HD (Kruskal Wallis: p = 0.005 for aBDI and p < 0.001 for gBDI).
Table 3.
Descriptive of measures.
| OCD | HD | PTSD | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Dx | GPC | p | Dx | GPC | p | Dx | VCC | p | |
| Clinical measures | N = 27 | N = 18 | N = 28 | N = 28 | |||||
| Symptom Severitya | 25.6 ± 1.0 | - | - | 53.3 ± 3.3 | - | - | 62.5 ± 4.1 | 6.9 ± 2.1 | < 0.001 |
|
| |||||||||
| Severity of depression | N = 27 | N = 18 | N = 28 | N = 28 | |||||
| Minimal (BDI:0–13) | 17 | - | - | 13 | - | - | 6 | 25 | - |
| Mild (BDI: 14–19) | 8 | - | - | 2 | - | - | 4 | 0 | - |
| Moderate (BDI: 20–28) | 1 | - | - | 2 | - | - | 9 | 3 | - |
| Severe (BDI: 29–63) | 1 | - | - | 1 | - | - | 9 | 0 | - |
|
| |||||||||
| Anhedonia (aBDI) | 1.4 ± 0.3 | - | - | 1.8 ± 0.5 | - | - | 3.7 ± 0.5 | 0.8 ± 0.2 | < 0.001 |
| Global Depression (gBDI) | 9.2 ± 1.4 | - | - | 8.8 ± 1.7 | - | - | 19.6 ± 1.7 | 4.5 ± 1.2 | < 0.001 |
|
| |||||||||
| Self-Report measures | N = 23 | N = 29 | N = 16 | N = 26 | N = 28 | N = 27 | |||
| Did not complete | N = 4 | 0 | 2 | 0 | 0 | 0 | |||
| Risk Perception (RiskP) | 24.1 ± 0.9 | 22.1 ± 0.7 | 0.04 | 27.1 ± 0.7 | 23.3 ± 0.9 | 0.004 | 22.5 ± 1.0 | 21.9 ± 0.8 | 0.65 |
| Risk Taking (RiskT) | 14.3 ± 1.0 | 16.0 ± 0.7 | 0.36 | 12.6 ± 0.8 | 14.6 ± 0.8 | 0.08 | 19.7 ± 0.8 | 16.9 ± 0.7 | 0.01 |
|
| |||||||||
| Behavior Based measures | N = 25 | N = 29 | N = 18 | N = 26 | N = 28 | N = 28 | |||
| Did not complete | N = 2 | N = 0 | N = 0 | N = 0 | N = 0 | N = 0 | |||
| Outliers with highly random choicesb | N = 1 | N = 0 | N = 1 | N = 0 | N=1 | N = 2 | |||
| Risk-aversion (RiskB) | 0.24 ± 0.03 | 0.18 ± 0.03 | 0.12 | 0.23 ± 0.03 | 0.27 ± 0.03 | 0.37 | 0.29 ± 0.04 | 0.28 ± 0.03 | 0.77 |
| Response time, msec, log-transformed (RT) | 7.8 ± 0.1 | 7.8 ± 0.1 | 0.77 | 8.0 ± 0.1 | 7.6 ± 0.04 | < 0.001 | 7.7 ± 0.1 | 7.7 ± 0.1 | 0.95 |
| Inverse temperature (γ)c | −2.2 ± 0.24 | −1.7 ± 0.25 | 0.21 | −2.2 ± 0.29 | −2.3 ± 0.20 | 0.79 | −2.7 ± 0.33 | −2.3 ± 0.23 | 0.38 |
Note: OCD – individuals with Obsessive Compulsive Disorder; HD – individuals with Hoarding disorder; PTSD – combat veterans with post-traumatic stress disorder; Dx – individuals from a corresponding clinical group; GPC – Controls from general population; VCC – controls combat veterans.
Bold indicates effects significant at p = 0.05 level.
Severity of OCD was measured by YBOCS (N = 27), severity of HD was measured by SIR (N = 15), and severity of PTSD was measured by CAPS.
Extreme outliers with inverse temperature greater than 3 SD above the subsample means; excluded from analyses that involved inverse temperature parameter.
Extreme outliers were excluded.
In all participants, anhedonia and general depression were positively correlated (OCD: Spearman’s r = 0.41, p = 0.03; HD: Spearman’s r = 0.69, p = 0.002; PTSD: r = 0.81, p < 0.001); in OCD this correlation was significantly weaker than in PTSD (Fisher z = −2.42, p = 0.008) but did not differ significantly from that in HD (Fisher z = −1.25, p = 0.21) (Siegel, 1956). Of note, inverse temperature, γ, did not differ significantly across groups, and correlated significantly with neither anhedonia nor general depression (SM5).
Correlations among three measures of risk aversion (regression models are detailed in SM6).
As expected (Blais and Weber, 2006; Johnson et al., 2004; Weber et al., 2002; Weber and Hsee, 1998), RiskP correlated negatively with RiskT in all groups. As in some prior studies (Dislich et al., 2010), self-report measures (RiskP and RiskT) did not correlate with RiskB in general population controls, nor in OCD or HD individuals. In PTSD and Veteran Controls, RiskB correlated positively with the γ × RiskT interaction term (γ × RiskTstandartized = 1.3, p = 0.006). This suggests that in individuals who made choices less randomly, self-reported risk taking negatively and more strongly correlated with behavior-based risk aversion.
Between-group differences in three measures of risk aversion (SM7).
RiskP was higher in OCD and HD than in matched controls (OCD: p = 0.04, Cohen’s d = 0.62; HD: p = 0.004, Cohen’s d = 0.78); but RiskT and RiskB did not differ between groups. In contrast, RiskT was higher in PTSD than in matched controls (p = 0.01, Cohen’s d = 0.68); but RiskP and RiskB did not differ between groups.
Between-group differences in response time.
RT was higher in HD than in matched controls (p < 0.001, Cohen’s d = 1.38); OCD and PTSD did not differ in RT from matched controls.
3.2. Primary analyses: effects of anhedonia severity
Symptom severity (Table 4, Fig. 2).
Table 4.
Anhedonia and symptom severity.
| Dependent variable | OCD, N = 27 | HD, N = 15 | PTSD, N = 27 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| β | SE | t | p | β | SE | t | p | β | SE | t | p | |
|
| ||||||||||||
| YBOCS | SIR | Emotional numbing (CAPS, C2) | ||||||||||
| Intercept | 23.11 | 1.28 | 18.03 | < 0.001 | 48.15 | 4.54 | 10.61 | < 0.001 | 12.16 | 2.43 | 5.00 | < 0.001 |
| Anhedonia | 1.84 | 0.67 | 2.74 | 0.01 | 2.47 | 1.57 | 1.57 | 0.14 | 1.20 | 0.55 | 2.17 | 0.04 |
| R2 | 0.2 | 0.1 | 0.39 | |||||||||
|
| ||||||||||||
| Intercept | 23.83 | 1.55 | 15.39 | < 0.001 | 46.08 | 5.14 | 8.96 | < 0.001 | 13.33 | 3.55 | 3.75 | 0.00 |
| Anhedonia | 2.13 | 0.76 | 2.80 | 0.01 | 0.78 | 2.49 | 0.31 | 0.76 | 1.55 | 0.96 | 1.62 | 0.12 |
| General Depression | −0.12 | 0.15 | −0.84 | 0.41 | 0.60 | 0.68 | 0.88 | 0.39 | −0.13 | 0.28 | −0.46 | 0.65 |
| R2 | 0.19 | 0.08 | 0.40 | |||||||||
|
| ||||||||||||
| Likelihood ratio test | ||||||||||||
| X2(1) | 0.77 | 0.947 | 0.21 | |||||||||
| p-value | 0.38 | 0.33 | 0.65 | |||||||||
Note: Relations between Anhedonia and YBOCS in OCD and SIR in HD were analyzed using nonparametric regressions; relation between anhedonia and Emotional Numbing was analyzed using heuristic regression.
Fig. 2.
Anhedonia vs. Symptom severity, scatterplots. A. In individuals with OCD, total Y-BOCS was normally distributed (Shapiro-Wilks p = 0.64); anhedonia was not normally distributed (Shapiro-Wilk p = 0.01); Y-BOCS significantly correlated with severity of anhedonia (Spearman’s ρ = 0.51, p = 0.006). B. In individuals with HD, total Saving Inventory Revised (SI-R) was normally distributed (Shapiro-Wilks p = 0.61); anhedonia was not normally distributed (Shapiro-Wilk p < 0.01); SI-R significantly correlated with severity of anhedonia (Spearman’s ρ = 0.56, p = 0.03). C. In individuals with PTSD, emotional numbing subscale of CAPS (C2) was normally distributed (Shapiro-Wilks p = 0.33); anhedonia was normally distributed (Shapiro-Wilk p > 0.10); it significantly correlated with severity of anhedonia in individuals with PTSD (r = 0.39, p = 0.04).
We evaluated effects of anhedonia on symptom severity using stepwise regression, with symptom severity as the dependent variable and severity of anhedonia (aBDI) and general depression (gBDI) as independent measures.
Total Y-BOCS score correlated positively with anhedonia in OCD, even when controlling for global depression severity (β = 2.13, p = 0.01). A numerically similar correlation in HD between total SI-R score and anhedonia was not statistically significant (β = 2.47, p = 0.14), and was dramatically reduced when controlling for general depression (β = 0.78, p = 0.76). In PTSD, the emotional numbing subscale of the CAPS-IV correlated significantly with anhedonia (β = 1.20, p = 0.04); however, this became non-significant when controlling for general depression (β = 1.55, p = 0.12).
Risk Aversion and Task Difficulty.
(Table 5, Fig. 3). To conserve statistical power, we performed regression analyses on a pooled sample of all clinical groups with dependent (DV) RiskP, RiskT, RiskB, and RT, and independent variables aBDI and gBDI. Since prior studies provide stronger evidence for anhedonia effects in OCD (Abramovitch et al., 2014), we used OCD as a reference group, testing whether effects in HD and PTSD are different from those expected in OCD. The models for RiskP, RiskT, and RT were:
| (1) |
| (2) |
Table 5.
Anhedonia versus Self-Report and Behavior-based measures.
| A. Self-Report measures | ||||
|
| ||||
| OCD = 25, HD = 16, PTSD = 27 | ||||
|
| ||||
| β | SE | t | p | |
|
| ||||
| Dependent variable: Risk Perception | ||||
| Intercept | 22.15 | 1.03 | 21.61 | < 0.001 |
| HD | 4.00 | 1.75 | 2.28 | 0.03 |
| PTSD | −1.70 | 1.72 | −0.99 | 0.33 |
| Anhedonia | 1.46 | 0.34 | 4.35 | < 0.001 |
| Anhedonia × HD | −0.97 | 0.61 | −1.58 | 0.12 |
| Anhedonia × PTSD | −0.92 | 0.46 | −1.99 | 0.05 |
| R2 | 0.31 | |||
|
|
|
|
|
|
| Intercept | 22.60 | 1.22 | 18.60 | < 0.001 |
| HD | 3.81 | 1.78 | 2.14 | 0.04 |
| PTSD | −2.15 | 1.85 | −1.16 | 0.25 |
| Anhedonia | 1.69 | 0.47 | 3.60 | < 0.001 |
| Anhedonia × HD | −0.94 | 0.61 | −1.54 | 0.13 |
| Anhedonia × PTSD | −1.06 | 0.50 | −2.10 | 0.04 |
| General Depression | −0.08 | 0.12 | −0.70 | 0.49 |
| R2 | 0.31 | |||
|
|
|
|
|
|
| Likelihood ratio test | ||||
| X2(1) | 0.53 | |||
| p | 0.47 | |||
| Dependent variable: Risk Taking | ||||
| Intercept | 13.82 | 1.24 | 11.16 | < 0.001 |
| HD | 0.05 | 2.12 | 0.03 | 0.98 |
| PTSD | 6.13 | 2.08 | 2.95 | 0.001 |
| Anhedonia | 0.76 | 0.41 | 1.86 | 0.07 |
| Anhedonia × HD | −1.41 | 0.74 | −1.91 | 0.06 |
| Anhedonia × PTSD | −0.83 | 0.55 | −1.50 | 0.14 |
| R2 | 0.25 | |||
|
|
|
|
|
|
| Intercept | 13.32 | 1.47 | 9.07 | < 0.001 |
| HD | 0.27 | 2.15 | 0.12 | 0.90 |
| PTSD | 6.63 | 2.23 | 2.97 | 0.001 |
| Anhedonia | 0.51 | 0.57 | 0.89 | 0.37 |
| Anhedonia × HD | −1.43 | 0.74 | −1.93 | 0.06 |
| Anhedonia × PTSD | −0.67 | 0.61 | −1.11 | 0.27 |
| General Depression | 0.09 | 0.15 | 0.64 | 0.53 |
| R2 | 0.24 | |||
|
|
|
|
|
|
| Likelihood ratio test | ||||
| X2(1) | 0.45 | |||
| p | 0.50 | |||
|
|
|
|
|
|
| B. Behavior-based measures | ||||
| OCD = 24, HD = 17, PTSD = 27 | ||||
| β | SE | t | p | |
|
|
|
|
|
|
| Dependent variable: Risk Aversion | ||||
| Intercept | 0.11 | 0.05 | 2.34 | 0.02 |
| HD | −0.04 | 0.05 | −0.84 | 0.40 |
| PTSD | −0.01 | 0.05 | −0.15 | 0.88 |
| Anhedonia | 0.00 | 0.01 | 0.03 | 0.98 |
| Anhedonia × HD | −0.01 | 0.03 | −0.51 | 0.62 |
| Anhedonia × PTSD | 0.00 | 0.01 | −0.20 | 0.85 |
| γ | −0.08 | 0.02 | −4.54 | < 0.001 |
| γ × Anhedonia | 0.00 | 0.01 | 0.81 | 0.42 |
| γ × Anhedonia × HD | −0.01 | 0.01 | −0.85 | 0.40 |
| γ × Anhedonia × PTSD | 0.00 | 0.00 | −0.29 | 0.78 |
| R2 | 0.57 | |||
|
|
|
|
|
|
| Intercept | 0.12 | 0.05 | 2.52 | 0.01 |
| HD | −0.05 | 0.05 | −0.92 | 0.36 |
| PTSD | −0.03 | 0.06 | −0.47 | 0.64 |
| Anhedonia | 0.01 | 0.02 | 0.64 | 0.52 |
|
| ||||
| A. Self-Report measures | ||||
|
| ||||
| OCD = 25, HD = 16, PTSD = 27 | ||||
|
| ||||
| β | SE | t | p | |
|
| ||||
| Anhedonia × HD | −0.02 | 0.03 | −0.68 | 0.50 |
| Anhedonia × PTSD | −0.01 | 0.02 | −0.55 | 0.59 |
| γ | −0.08 | 0.02 | −4.61 | < 0.001 |
| γ × Anhedonia | 0.00 | 0.01 | 0.74 | 0.46 |
| γ × Anhedonia × HD | −0.01 | 0.01 | −1.03 | 0.31 |
| γ × Anhedonia × PTSD | 0.00 | 0.00 | −0.12 | 0.91 |
| General Depression | 0.00 | 0.00 | −0.94 | 0.35 |
| R2 | 0.57 | |||
|
|
|
|
|
|
| Likelihood ratio test | ||||
| X2(1) | 1.04 | |||
| p | 0.31 | |||
|
|
|
|
|
|
| Dependent variable: Response Time | ||||
| Intercept | 7.70 | 0.07 | 106.29 | < 0.001 |
| HD | 0.19 | 0.12 | 1.65 | 0.10 |
| PTSD | 0.05 | 0.12 | 0.39 | 0.70 |
| Anhedonia | 0.06 | 0.02 | 2.73 | 0.01 |
| Anhedonia × HD | −0.03 | 0.04 | −0.64 | 0.52 |
| Anhedonia × PTSD | −0.07 | 0.03 | −2.30 | 0.02 |
| R2 | 0.15 | |||
|
|
|
|
|
|
| Intercept | 7.75 | 0.08 | 92.32 | < 0.001 |
| HD | 0.18 | 0.12 | 1.56 | 0.12 |
| PTSD | 0.00 | 0.13 | −0.03 | 0.97 |
| Anhedonia | 0.09 | 0.03 | 2.77 | 0.01 |
| Anhedonia × HD | −0.03 | 0.04 | −0.65 | 0.52 |
| Anhedonia × PTSD | −0.09 | 0.04 | −2.60 | 0.01 |
| General Depression | −0.01 | 0.01 | −1.19 | 0.24 |
| R2 | 0.15 | |||
|
|
|
|
|
|
| Likelihood ratio test | ||||
| X2(1) | 1.57 | |||
| p | 0.21 | |||
Note: Effects significant at p = 0.05 level are in bold.
Fig. 3.
Anhedonia vs. Self-Report and Behavior-based measures. A. Scatter plots of self-reported DOSPERT Risk Taking and DOSPERT Risk Perception versus severity of anhedonia in OCD, HD, and PTSD (top panels). Marginal effects of anhedonia (i.e. parameter estimates from nonparametric regressions, β) on self-reported DOSPERT Risk Taking and DOSPERT Risk Perception in OCD, HD, and PTSD (bottom panels); *** - significance at p = 0.01 level, ** - significance at p = 0.01 level, * - significance at p = 0.10 level. B. Scatter plots of behavior-based risk aversion and response time versus severity of anhedonia in OCD, HD, and PTSD (top panels). Marginal effects of anhedonia (i.e. parameter estimates from nonparametric regressions, β) on behavior-based risk aversion and response time in OCD, HD, and PTSD (bottom panels); *** - significance at p = 0.01 level, ** - significance at p = 0.01 level, * - significance at p = 0.10 level.
The models for RiskB, which take into account randomness in choice, were:
| (3) |
| (4) |
DOSPERT risk perception.
We observed a significant positive effect of anhedonia in OCD, even when controlling for general depression (β = 1.69, p < 0.001). This correlation, when controlled for general depression, was nominally weaker in HD (Anhedonia × HD = −0.94, p = 0.13) and significantly weaker in PTSD (Anhedonia × PTSD = −1.06, p = 0.04). The lack of an anhedonia effect in PTSD was confirmed when regression analysis was performed on PTSD alone (β = −0.06, p = 0.93).
DOSPERT risk taking.
No significant effect of anhedonia on RiskT was observed in any clinical group, whether or not we controlled for general depression.
Behavior-based risk attitude.
No effect of anhedonia on RiskB was observed in any clinical group, whether or not we controlled for general depression. As expected, RiskB correlated negatively with inverse temperature (γ = −0.08, p < 0.001).
Task difficulty.
We observed a positive effect of anhedonia on RT in OCD, even controlling for general depression (β = 0.09, p = 0.01). This effect was not significantly different in HD (Anhedonia × HD = −0.03, p = 0.52), but was weaker in PTSD (Anhedonia × PTSD = −0.09, p = 0.01). The lack of effect of anhedonia in PTSD was confirmed when regression was performed on PTSD alone (β = 0.04, p = 0.20).
3.3. Secondary analyses: gender effects (Table 6, Fig. 4)
Table 6.
Modulating effect of gender on relations between anhedonia and measures of risk attitudes.
| A. Self-Report measures | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| OCD = 25, HD = 16, PTSD = 27 | ||||||||
|
| ||||||||
| Risk Perception | Risk Taking | |||||||
|
|
||||||||
| β | SE | t | p | β | SE | t | p | |
| Intercept | 22.34 | 0.87 | 25.81 | < 0.001 | 13.01 | 1.56 | 8.33 | < 0.001 |
| Anhedonia | 2.20 | 0.37 | 5.89 | < 0.001 | 1.22 | 0.67 | 1.82 | 0.08 |
| Anhedonia × Gender | −1.20 | 0.38 | −3.17 | 0.003 | −1.63 | 0.69 | −2.38 | 0.02 |
| HD | 3.87 | 1.26 | 3.08 | 0.004 | 0.39 | 2.27 | 0.17 | 0.86 |
| Anhedonia × HD | −1.50 | 0.52 | −2.90 | 0.01 | −2.30 | 0.93 | −2.47 | 0.02 |
| Anhedonia × Gender × HD | 0.91 | 0.68 | 1.33 | 0.19 | 1.58 | 1.23 | 1.28 | 0.21 |
| General Depression | −0.03 | 0.09 | −0.39 | 0.70 | 0.15 | 0.16 | 0.94 | 0.35 |
|
| ||||||||
| R2-adjusted | 0.55 | 0.14 | ||||||
| n | 42 | 42 | ||||||
|
| ||||||||
| Likelihood ratio test | ||||||||
| X2(6) | 40.35 | 12.96 | ||||||
| p-value | < 0.001 | 0.04 | ||||||
| B. Behavior-based measures | ||||||||
|
| ||||||||
| OCD = 24, HD = 17, PTSD = 27 | ||||||||
| Risk Aversion |
Response Time |
|||||||
| β | SE | t | p | β | SE | t | p | |
|
| ||||||||
| Intercept | 0.26 | 0.05 | 5.51 | < 0.001 | 7.76 | 0.08 | 92.79 | < 0.001 |
| Anhedonia | −0.03 | 0.02 | −1.34 | 0.19 | 0.05 | 0.04 | 1.40 | 0.17 |
| Anhedonia × Gender | −0.01 | 0.02 | −0.35 | 0.73 | 0.09 | 0.04 | 2.54 | 0.02 |
| HD | −0.02 | 0.07 | −0.36 | 0.72 | 0.18 | 0.11 | 1.57 | 0.13 |
| Anhedonia × HD | −0.01 | 0.03 | −0.28 | 0.78 | 0.02 | 0.05 | 0.45 | 0.66 |
| Anhedonia × Gender × HD | 0.04 | 0.04 | 1.20 | 0.24 | −0.09 | 0.07 | −1.41 | 0.17 |
| γ | −0.05 | 0.03 | −1.74 | 0.09 | - | - | - | - |
| γ × Anhedonia | 0.00 | 0.01 | −0.19 | 0.85 | - | - | - | - |
| γ × Anhedonia × Gender | −0.02 | 0.01 | −1.23 | 0.23 | - | - | - | - |
| γ × HD | 0.00 | 0.06 | 0.04 | 0.97 | - | - | - | - |
| γ × Anhedonia × HD | −0.02 | 0.03 | −0.48 | 0.64 | - | - | - | - |
| γ × Anhedonia × Gender × HD | −0.02 | 0.09 | −0.27 | 0.79 | - | - | - | - |
| General Depression | 0.00 | 0.00 | 0.83 | 0.41 | −0.01 | 0.01 | −1.53 | 0.14 |
|
| ||||||||
| R2-adjusted | 0.43 | 0.24 | ||||||
| n | 43 | 43 | ||||||
|
| ||||||||
| Likelihood ratio test | ||||||||
| X2 | 38.67 | 18.41 | ||||||
| df | 12 | 6 | ||||||
| p-value | < 0.001 | 0.01 | ||||||
Bold indicates effects significant at p = 0.05 level.
Fig. 4.
Gender effects. A. Marginal effects of anhedonia (i.e. parameter estimates from nonparametric regressions, β) on self-reported DOSPERT Risk Taking and DOSPERT Risk Perception in OCD and HD, across genders (top panels); *** - significance at p = 0.01 level. B. Marginal effects of anhedonia (i.e. parameter estimates from nonparametric regressions, β) on behavior-based risk aversion and response time in OCD and HD, across genders (bottom panels); *** - significance at p = 0.01 level.
We anticipated stronger effects of anhedonia among females (Nawijn et al., 2015). PTSD was excluded from this analysis, as PTSD subjects and combat-exposed controls were predominantly male. OCD/females were a reference group. The regression model for RiskP, RiskT, and RT was:
| (5) |
The regression model for RiskB also included g and interaction with γ terms as in equations (3) and (4).
Gender modulated effects of anhedonia in OCD, but not in HD. In OCD, the effect on RiskP was stronger in females (Anhedonia = 2.20, p < 0.001; Anhedonia × Gender = −1.20, p = 0.003), but the effect on RT was stronger in males (Anhedonia × Gender = 0.09, p = 0.02). In HD, the effect on RiskP was significantly reduced relative to the OCD reference group (Anhedonia × HD = −1.50, p = 0.01), becoming nonsignificant (t(15) = 1.03, p = 0.68), and was not modulated by gender (Anhedonia × HD × Gender = 0.91, p = 0.19). The effect of anhedonia on RT in HD was nonsignificant in both males and females. Effects of anhedonia on RiskT and RiskB were non-significant in all groups.
4. Discussion
A broad literature has examined the role of anhedonia in symptoms of affective and psychotic disorders (Anticevic et al., 2012, 2014a, 2015; Barch et al., 2017a, 2017b; Dowd et al., 2016). Other psychiatric conditions have received significantly less attention. To address this gap, we investigated the relationship of anhedonia with symptoms and reward-related behavioral outcomes in individuals with OCD, HD, and PTSD. Several results of these analyses are notable.
First, we found a relationship between anhedonia and OCD symptoms, even after controlling for effects of general depression. This replicates and extends a previous observation (Abramovitch et al., 2014) in a better-characterized sample. We found no similar relationship in HD or PTSD. Most OCD and HD participants had only minimal depression, strengthening our results. A unique contribution of anhedonia to OCD symptoms is also supported by the finding that the correlation between severity of anhedonia and of global depression is reduced (though still significant) in OCD relative to the other two conditions. In PTSD, we found a relationship between anhedonia and the CAPS emotional numbing subscale, replicating previous work (Nawijn et al., 2015). However, this is accounted for when general depression is included in the model, indicating a broader relationship with depression and not a unique contribution of anhedonia. The range of both anhedonia and general depression in PTSD subsample was larger than in OCD and HD, also strengthening our results.
Second, we employed the value-based decision framework to investigate links between anhedonia and reduced steepness of the SV function; this allows deeper characterization of anhedonia’s effects, beyond correlations with symptom severity. Flatter SV predicts increased risk aversion and longer decision times during value-based choices, as does increased noisiness in valuation (reduced inverse temperature); thus, we controlled for inverse temperature in our analyses of behavior-based indices. Inverse temperature correlated with neither anhedonia nor general depression, thus including it in the analyses did not lead to multicollinearity. Importantly, we also used two self-report measures of risk attitudes: self-reported risk perception and risk-taking propensities (measured using the DOSPERT (Blais and Weber, 2006)). A limited literature has used the DOSPERT scale to characterize risk-taking in clinical populations (Lorian and Grisham, 2011); this is the first time, to our knowledge, that risk perception has also been evaluated in these populations. Our results suggest that theoretically-predicted correlations between anhedonia and risk aversion in OCD are driven by effects of anhedonia on risk perception; effects on risk taking (both self-reported and behavior-based) were non-significant. Also, we find a theoretically-predicted relation between anhedonia and response time during risky decisions in OCD and HD, even after controlling for general depression.
Our results are consistent with prior examinations of behavior-based indices that failed to reject the null hypothesis that MDD affects value sensitivity (Robinson and Chase, 2017). However, they help to reconcile prior findings that reported clear evidence for risk avoidance in OCD using self-report measures (Tolin et al., 2003) but negative or inconsistent results when employing behavior-based measures (Pushkarskaya et al., 2015, 2017). Dissociation between risk perception and risk-averse behaviors may complicate animal studies of the effects of anhedonia in OCD, as animal risk perception cannot readily be assessed. Such studies may benefit from incorporating other predictions of the subjective value model, such as effects of anhedonia on task difficulty, as measured by response time.
Our results indicate that anhedonia effects (on symptom severity, risk attitudes, and response time) are not uniform across disorders. The fact that we see the predicted effects in OCD suggests that formalizing anhedonia as reduced curvature of the value function captures aspects of anhedonia that may uniquely contribute to OCD, independent of general depression. Some prior studies argued that in MDD anhedonia may be better operationalized as the degree of choice randomness (Robinson and Chase, 2017). Neither anhedonia nor general depression significantly correlated with inverse temperature in OCD, HD, or PTSD. Operationalizing anhedonia as reduced curvature of the value function may reflect only one aspect of the heterogeneity of the concept as it is measured and used clinically; how anhedonia may best be parsed into sub-constructs is not yet clear (Argyropoulos and Nutt, 2013; Treadway and Zald, 2011).
Previous work suggests that anhedonia effects may be modulated by gender (Nawijn et al., 2015); exploratory analyses of our data uncovered such an effect in OCD. Anhedonia was more related to risk perception among females and to task difficulty (as indexed by response time) among males. This reinforces the importance of equal representation of both genders in clinical samples.
This work has several limitations to be addressed in future studies. First, we looked across only three DSM diagnoses; it will be valuable to examine these measures in a broader population of dimensionally assessed patients. Second, the HD group was smaller than the other two groups, and our PTSD subjects were predominantly male, limiting some conclusions. Third, several different depression-related processes may account for slower reaction time in anhedonic individuals, such as slower processing. Even though we controlled for effects of general depression, which are non-significant, future studies may examine whether including more targeted measures of psychomotor slowing would change our findings. Fourth, we did not incorporate direct measures of brain function in the current study. Structural and functional alterations within the brain’s reward circuitry are associated with impaired reward processing, across psychopathologies (Russo and Nestler, 2013). It will be important to investigate these effects of anhedonia, both in OCD and across traditional diagnoses. Finally, clinical anhedonia is a complex construct and may be dissociable into different underlying components; as optimal means to dissociate and measure such components becomes clearer, it will be important to investigate them independently and cross-diagnostically.
Our results, together with previous data (Abramovitch et al., 2014), suggest a unique contribution of one aspect of anhedonia, blunted reward sensitivity, in obsessive-compulsive disorder. This matches neuroimaging evidence suggesting abnormalities in the reward-related ventral striatal-mPFC circuitry (Anticevic et al., 2014b; Harrison et al., 2009, 2013), and previous behavioral data revealing imprecision in reward-related decision making in this population (Pushkarskaya et al., 2015, 2017). A focus on reward representation and clinical anhedonia may represent an important new perspective on OCD phenomenology and pathophysiology.
Supplementary Material
Acknowledgments
Financial disclosures
Dr. Tolin receives research support from Palo Alto Health Sciences and Pfizer. Dr. Pittenger receives research support from Hoffman F. La Roche, Ltd., and has received unrestricted educational grants from F. Hoffman La Roche, Ltd., and Medtronic, Inc. Drs. Pushkarskaya, Levy, Harpaz-Rotem, and Sobowale and Mr. Henick reported no biomedical financial interests or potential conflicts of interest.
This research was supported by National Institutes of Mental Health Grants K01 MH101326-01 (to H.P.), R01MH095790 (to C.P.), and R21MH102634 (to I.L. and I.H-R.).
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpsychires.2018.11.029.
References
- Abramovitch A, Pizzagalli DA, Reuman L, Wilhelm S, 2014. Anhedonia in obsessive-compulsive disorder: beyond comorbid depression. Psychiatr. Res 216 (2), 223–229. [DOI] [PubMed] [Google Scholar]
- Aker AT, Ozeren M, Basoglu M, Kaptanoglu C, Erol A, Buran B, 1999. Clinician administered post traumatic stress disorder scale (CAPS) reliability and validity study. Turk J Psychiatry 10, 286–293. [Google Scholar]
- Anticevic A, Cole MW, Repovs G, Murray JD, Brumbaugh MS, Winkler AM, Savic A, Krystal JH, Pearlson GD, Glahn DC, 2014a. Characterizing thalamocortical disturbances in schizophrenia and bipolar illness. Cerebr. Cortex 24 (12), 3116–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Hu S, Zhang S, Savic A, Billingslea E, Wasylink S, Repovs G, Cole MW, Bednarski S, Krystal JH, 2014b. Global resting-state functional magnetic resonance imaging analysis identifies frontal cortex, striatal, and cerebellar dysconnectivity in obsessive-compulsive disorder. Biol. Psychiatry 75 (8), 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Barch DM, 2012. Emotioneffects on attention, amygdala activation, and functional connectivity in schizophrenia. Schizophr. Bull 38 (5), 967–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Schleifer C, Youngsun TC, 2015. Emotional and cognitive dysregulation in schizophrenia and depression: understanding common and distinct behavioral and neural mechanisms. Dialogues Clin. Neurosci 17 (4), 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA, 2013. DSM-5 Task Force. Diagnostic and Statistical Manual of Mental Disorders: DSM-5™ American Psychiatric Publishing, Inc, Arlington, VA, US. [Google Scholar]
- Argyropoulos SV, Nutt DJ, 2013. Anhedonia revisited: is there a role for dopamine-targeting drugs for depression? J. Psychopharmacol 27 (10), 869–877. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Gold JM, Johnson SL, Kring AM, MacDonald III AW, Pizzagalli DA, Ragland JD, Silverstein SM, Strauss ME, 2017a. Explicit and implicit reinforcement learning across the psychosis spectrum. J. Abnorm. Psychol 126 (5), 694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Gold JM, Kring AM, 2017b. Paradigms for assessing hedonic processing and motivation in humans: relevance to understanding negative symptoms in psychopathology. Schizophr. Bull 43 (4), 701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK, 1996. Beck Depression Inventory-II. Psychological Corporation, San Antonio, TX b9.
- Blais A-R, Weber EU, 2006. A Domain-specific Risk-taking (DOSPERT) Scale for Adult Populations.
- Blais A, Weber E, 2009. The Domain-specific Risk Taking (DOSPERT) Scale for Adult Populations: Item Selection and Preliminary Psychometric Properties. Defence R & D, Toronto, Ontario. [Google Scholar]
- Campbell DG, Felker BL, Liu C-F, Yano EM, Kirchner JE, Chan D, Rubenstein LV, Chaney EF, 2007. Prevalence of depression–PTSD comorbidity: implications for clinical practice guidelines and primary care-based interventions. J. Gen. Intern. Med 22 (6), 711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung D, Kadlec K, Aimone JA, McCurry K, King-Casas B, Chiu PH, 2017. Valuation in major depression is intact and stable in a non-learning environment. Sci. Rep 7, 44374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson KW, Burg MM, Kronish IM, Shimbo D, Dettenborn L, Mehran R, Vorchheimer D, Clemow L, Schwartz JE, Rieckmann N, 2010. Association of anhedonia with recurrent major adverse cardiac events and mortality 1 year after acute coronary syndrome. Arch. Gen. Psychiatr 67 (5), 480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dislich FX, Zinkernagel A, Ortner TM, Schmitt M, 2010. Convergence of direct, indirect, and objective risk-taking measures in gambling. Z. Psychol. J. Psychol 218 (1), 20–27. [Google Scholar]
- Dodonov YS, Dodonova YA, 2012. Response time analysis in cognitive tasks with increasing difficulty. Intelligence 40 (5), 379–394. [Google Scholar]
- Dohmen T, Falk A, Huffman D, Sunde U, Schupp J, Wagner GG, 2005. Individual Risk Attitudes: New Evidence from a Large, Representative, Experimental-validated Survey. IZA Discussion Paper.
- Dohmen T, Falk A, Huffman D, Sunde U, Schupp J, Wagner GG, 2011. Individual risk attitudes: measurement, determinants, and behavioral consequences. J. Eur. Econ. Assoc 9 (3), 522–550. [Google Scholar]
- Dowd EC, Frank MJ, Collins A, Gold JM, Barch DM, 2016. Probabilistic reinforcement learning in patients with schizophrenia: relationships to anhedonia and avolition. Biol. Psychiatry: Cognit. Neurosci. Neuroimag 1 (5), 460–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB, 2007. The role of dopamine in the pathophysiology of depression. Arch. Gen. Psychiatr 64 (3), 327–337. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB, 2012. Structured Clinical Interview for DSM-IV®Axis I Disorders (SCID-I), Clinician Version, Administration Booklet. American Psychiatric Pub. [Google Scholar]
- Figner B, Weber E, 2011. Who takes risks when and why? Determinants of risk taking. Curr. Directions in Psychol.l Sci 20 (4), 211–216. [Google Scholar]
- Frost RO, Steketee G, Grisham J, 2004. Measurement of compulsive hoarding: saving inventory-revised. Behav. Res. Ther 42 (10), 1163–1182. [DOI] [PubMed] [Google Scholar]
- Frost RO, Steketee G, Tolin DF, 2011. Comorbidity in hoarding disorder. Depress. Anxiety 28 (10), 876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert S,Bird G, Frith C, Burgess P, 2012. Does“task difficulty”explain“task-induced deactivation?”. Front. Psychol 3, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa I, Schmeidler D, 1994. Additive representations of non-additive measures and the Choquet integral. Ann. Oper. Res 52 (1), 43–65. [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, Charney DS, 1989a. The Yale-Brown obsessive-compulsive scale. II. Validity. Arch. Gen. Psychiatr 46, 1012–1016. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS, 1989b. The Yale-Brown obsessive compulsive scale. I. Development, use, and reliability. Arch. Gen. Psychiatr 46, 1006–1011. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Cardoner N, Deus J, Alonso P, López-Solà M, Contreras-Rodríguez O, Real E, Segalàs C, Blanco-Hinojo L, 2013. Brain corticostriatal systems and the major clinical symptom dimensions of obsessive-compulsive disorder. Biol. Psychiatry 73 (4), 321–328. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Soriano-Mas C, Pujol J, Ortiz H, López-Solà M, Hernández-Ribas R, Deus J, Alonso P, Yücel M, Pantelis C, 2009. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch. Gen. Psychiatr 66 (11), 1189–1200. [DOI] [PubMed] [Google Scholar]
- Harrison JD, Young JM, Butow P, Salkeld G, Solomon MJ, 2005. Is it worth the risk? A systematic review of instruments that measure risk propensity for use in the health setting. Soc. Sci. Med 60 (6), 1385–1396. [DOI] [PubMed] [Google Scholar]
- Highhouse S, Nye CD, Zhang DC, Rada TB, 2017. Structure of the Dospert: is there evidence for a general risk factor? J. Behav. Decis. Making 30 (2), 400–406. [Google Scholar]
- Hsee CK, Rottenstreich Y, Xiao Z, 2005. When is more better? On the relationship between magnitude and subjective value. Cur. Direct. in Psychol. Sci 14 (5), 234–237. [Google Scholar]
- Huys QJ, Pizzagalli DA, Bogdan R, Dayan P, 2013. Mapping anhedonia onto reinforcement learning: a behavioural meta-analysis. Biol. Mood Anxiety Disord 3 (1), 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P, 2010. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry 167 (7), 748–751. [DOI] [PubMed] [Google Scholar]
- Insel TR, Cuthbert BN, 2015. Brain disorders? Precisely. Science 348 (6234), 499–500. [DOI] [PubMed] [Google Scholar]
- Johnson J, Wilke A, Weber EU, 2004. Beyond a Trait View of Risk Taking: a Domain-specific Scale Measuring Risk Perceptions, Expected Benefits, and Perceived-risk Attitudes in German-speaking Populations.
- Joiner TE, Brown JS, Metalsky GI, 2003. A test of the tripartite model’s prediction of anhedonia’s specificity to depression: patients with major depression versus patients with schizophrenia. Psychiatr. Res 119 (3), 243–250. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Tversky A, 1979. Prospect theory: an analysis of decision under risk. Econometrica: J. Econ. Soc 263–291.
- Kashdan TB,Elhai JD, Frueh BC, 2006. Anhedonia and emotional numbing in combat veterans with PTSD. Behav. Res. Ther 44 (3), 457–467. [DOI] [PubMed] [Google Scholar]
- Kaufman A, 1979. Intelligent Testing, W/SC-R. Wiley-Interscience, New York. [Google Scholar]
- Konovalov A, Krajbich I, April 23, 2017. Revealed Indifference: Using Response Times to Infer Preferences. Available at SSRN.
- Kouros CD, Morris MC, Garber J, 2016. Within-person changes in individual symptoms of depression predict subsequent depressive episodes in adolescents:a prospective study. J. Abnorm. Child Psychol 44 (3), 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Chasson GS, Tapia E, Miller EK, Pettit JW, 2006. Measuring hedonic capacity in depression: a psychometric analysis of three anhedonia scales. J. Clin. Psychol 62 (12), 1545–1558. [DOI] [PubMed] [Google Scholar]
- Levy I, Snell J, Nelson AJ, Rustichini A, Glimcher PW, 2010. Neural representation of subjective value under risk and ambiguity. J. Neurophysiol 103 (2), 1036–1047. [DOI] [PubMed] [Google Scholar]
- Lorian CN, Grisham JR, 2011. Clinical implications of risk aversion: an online study of risk-avoidance and treatment utilization in pathological anxiety. J. Anxiety Disord 25 (6), 840–848. [DOI] [PubMed] [Google Scholar]
- McCabe C, Cowen PJ, Harmer CJ, 2009. Neural representation of reward in recovered depressed patients. Psychopharmacology 205 (4), 667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C, Mishor Z, Cowen PJ, Harmer CJ, 2010. Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biol. Psychiatry 67 (5), 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Daw ND, Montague PR, 2003. A computational substrate for incentive salience. Trends in Neurosci 26 (8), 423–428. [DOI] [PubMed] [Google Scholar]
- Mueller A, Mueller U, Albert P, Mertens C, Silbermann A, Mitchell JE, De Zwaan M, 2007. Hoarding in a compulsive buying sample. Behav. Res. Ther 45 (11), 2754–2763. [DOI] [PubMed] [Google Scholar]
- Nawijn L, van Zuiden M, Frijling JL, Koch SB, Veltman DJ, Olff M, 2015. Reward functioning in PTSD: a systematic review exploring the mechanisms underlying anhedonia. Neurosci. Biobehav. Rev 51, 189–204. [DOI] [PubMed] [Google Scholar]
- Nutt D, Demyttenaere K, Janka Z, Aarre T, Bourin M, Canonico PL, Carrasco JL, Stahl S, 2007. The other face of depression, reduced positive affect: the role of catecholamines in causation and cure. J. Psychopharmacol 21 (5), 461–471. [DOI] [PubMed] [Google Scholar]
- Overbeek T, Schruers K, Vermetten E, Griez E, 2002. Comorbidity of obsessive-compulsive disorder and depression: prevalence, symptom severity, and treatment effect. J. Clin. Psychiatr 63 (12), 1106–1112. [DOI] [PubMed] [Google Scholar]
- Price J, Cole V, Goodwin GM, 2009. Emotional side-effects of selective serotonin reuptake inhibitors: qualitative study. Br. J. Psychiatr 195 (3), 211–217. [DOI] [PubMed] [Google Scholar]
- Pushkarskaya H, Tolin D, Ruderman L, Henick D, Kelly JM, Pittenger C, Levy I, 2017. Value-based decision making under uncertainty in hoarding and obsessive-compulsive disorders. Psychiatr. Res [DOI] [PMC free article] [PubMed]
- Pushkarskaya H, Tolin D, Ruderman L, Kirshenbaum A, Kelly JM, Pittenger C, Levy I, 2015. Decision-making under uncertainty in obsessive–compulsive disorder. J. Psychiatr. Res 69, 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel A, Camerer C, Montague PR, 2008. A framework for studying the neurobiology of value-based decision making. Nat. Rev. Neurosci 9 (7), 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Chase HW, 2017. Learning and choice in mood disorders: searching for the computational parameters of anhedonia. Comput. Psychiat 1, 208–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman L, Ehrlich DB, Roy A, Pietrzak RH, Harpaz‐Rotem I, Levy I, 2016. Posttraumatic stress symptoms and aversion to ambiguous losses in combat veterans. Depress. Anxiety 33 (7), 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ, 2013. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci 14 (9), 609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S, 1956. Onparametric Statistics: For the Behavioral Sciences. McGraw-Hill, New York, NY. [Google Scholar]
- Spijker J, Bijl R, De Graaf R, Nolen W, 2001. Determinants of poor1‐year outcome of DSM‐III‐R major depression in the general population: results of The Netherlands Mental Health Survey and Incidence Study (NEMESIS). Acta Psychiatr. Scand 103 (2), 122–130. [DOI] [PubMed] [Google Scholar]
- Tolin DF, Abramowitz JS, Brigidi BD, Foa EB, 2003. Intolerance of uncertainty in obsessive-compulsive disorder. J. Anxiety Disord 17 (2), 233–242. [DOI] [PubMed] [Google Scholar]
- Tolin DF, Meunier SA, Frost RO, Steketee G, 2011. Hoarding among patients seeking treatment for anxiety disorders. J. Anxiety Disord 25 (1), 43–48. [DOI] [PubMed] [Google Scholar]
- Tolin DF, Gilliam C, Wootton BM, Bowe W, Bragdon LB, Davis E, Hannan SE, Steinman SA, Worden B, Hallion LS, 2016. Psychometric Properties ofa Structured Diagnostic Interview for DSM-5 Anxiety, Mood, and Obsessive-compulsive and Related Disorders. Assessment. 1073191116638410. [DOI] [PubMed]
- Treadway MT, Zald DH, 2011. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci. Biobehav. Rev 35 (3), 537–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH, 2013. Parsing anhedonia: translational models of rewardprocessing deficits in psychopathology. Curr. Dir. Psychol. Sci 22 (3), 244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymula A, Belmaker LAR, Ruderman L, Glimcher PW, Levy I, 2013. Like cognitive function, decision making across the life span shows profound age-related changes. Proc. Natl. Acad. Sci. Unit. States Am 110 (42), 17143–17148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linden WJ, 2006. A lognormal model for response times on test items. J. Educ. Behav. Stat 31 (2), 181–204. [Google Scholar]
- Weber EU, Blais AR, Betz NE, 2002. A domain‐specific risk‐attitude scale: measuring risk perceptions and risk behaviors. J. Behav. Decis. Making 15 (4), 263–290. [Google Scholar]
- Weber EU, Hsee C, 1998. Cross-cultural differences in risk perception, but crosscultural similarities in attitudes towards perceived risk. Manag. Sci 44 (9), 1205–1217. [Google Scholar]
- Weinberg A, Liu H, Hajcak G, Shankman SA, 2015. Blunted neural response to rewards as a vulnerability factor for depression: results from a family study. J. Abnorm. Psychol 124 (4), 878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton AE, Treadway MT, Pizzagalli DA, 2015. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr. Opin. Psychiatr 28 (1),7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright G, Ayton P, 1988. Decision time, subjective probability, and task difficulty. Memory and Cognition. 16 (2), 176–185. [DOI] [PubMed] [Google Scholar]
- Wu J, Cheung HY, 2014. Confirmatory factor analysis of Dospert scale with Chinese university students. Psychol. Rep 114 (1), 185–197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.