Summary
Magnetic resonance imaging has been used for evaluating of a brain edema in experimental animals to assess cytotoxic and vasogenic edema by the apparent diffusion coefficient (ADC) and T2 imaging. This paper brings information about the effectiveness of methylprednisolone (MP) on experimental brain edema. A total of 24 rats were divided into three groups of 8 animals each. Rats with cytotoxic/intracellular brain edema induced by water intoxication were assigned to the group WI. These rats also served as the additional control group CG when measured before the induction of edema. A third group (WIMP) was intraperitoneally administered with methylprednisolone 100 mg/kg during water intoxication treatment. The group WI+MP was injected with methylprednisolone 50 mg/kg into the carotid artery within two hours after the water intoxication treatment. We evaluated the results in four groups. Two control groups (CG, WI) and two experimental groups (WIMP, WI+MP). Rats were subjected to MR scanning 24 h after edema induction. We observed significantly increased ADC values in group WI in both evaluated areas – cortex and hippocampus, which proved the occurrence of experimental vasogenic edema, while ADC values in groups WIMP and WI+MP were not increased, indicating that the experimental edema was not developed and thus confirming the protective effect of MP.
Keywords: Magnetic resonance imaging, Water intoxication, Cytotoxic edema, Vasogenic edema, Methylprednisolone
Introduction
Brain edema is a fatal pathological state in which brain volume increases as a result of abnormal accumulation of fluid within the cerebral parenchyma. The abnormal accumulation of fluid causes an increase in brain volume and an intracranial pressure elevation because of an enclosed rigid skull. Two main types of brain edema are classified: vasogenic edema, and cytotoxic edema. Vasogenic edema is characterized by extravasation and extracellular accumulation of fluid into the cerebral parenchyma caused by disruption of the blood-brain barrier (BBB). In contrast, cytotoxic edema is characterized by intracellular accumulation of fluid and Na+ resulting in cell swelling. While cytotoxic edema is characterized by intracellular accumulation of fluid, it makes BBB more permeable with water accumulation in extracellular space – the main feature of vasogenic edema (Ayata and Ropper 2002, Liang et al. 2007, Marmarou 2007, Michinaga and Koyama 2015, Sorby-Adams et al. 2017).
To induce the cytotoxic/intracellular edema, we used the water intoxication model. This model best reflects simulation of hyponatremia because it induces a relative decrease of extracellular Na+ concentration. The water intoxication model is produced by intraperitoneal loading of large volume of distilled water corresponding to 10–40 % of the body weight of experimental animals. This excessive loading of water induces an increase of water content in the central nerve tissue and an excessive influx of water into cells resulting in brain swelling (Manley et al. 2000, Kozler and Pokorný 2003, Yang et al. 2008, Kozler et al. 2013, Kozler and Pokorný 2014, Kozler et al. 2018).
To model a vasogenic/extracellular edema, we use the method of BBB disruption by intracarotid injection of hyperosmolar solution (Kozler and Pokorný 2003, Kozler et al. 2014, Kozler et al. 2015). The disruption of the BBB by the application of an osmotic insult was first accomplished by Rapoport (1970). Administration of a hyperosmolar solution into one carotid causes shrinkage of endothelial cells, which leads to expansion of tight junctions to an average of 20 times their normal diameter. Owing to the distension of the tight junctions, BBB becomes permeable to high-molecular compounds, which should be followed by influx of water in the direction of osmotic gradient (Rapoport 2000). We used a slightly modified method implemented by Kroll and Neuwelt (1998) and Rapoport (2000): a solution of 20 % mannitol was used to achieve the osmotic BBB disruption.
For many years, the objective of experimental studies was to find a method to prevent the induction or the development of brain edema in its initial stages (Bullock et al. 1999, Michinaga and Koyama 2015). From this perspective, it appears promising the use of neuroprotective effect of certain substances that modulate permeability of cytoplasmic membranes, e.g. methylprednisolone (Faden and Salzman 1992, Hall 1992, Hall 1993, Park 1998, Slivka and Murphy 2001).
We documented the effect of MP on experimental brain edema in our previous neurohistological studies (Kozler and Pokorný 2004, Kozler et al. 2011, Kozler and Pokorný 2012) and in an intracranial pressure study (Kozler et al. 2017). For evaluating brain edema in experimental animals, MRI was also used. Both the T2 relaxation time and the apparent diffusion coefficient are sensitive to the presence of extra- and intracellular fluids (Loubinoux et al. 1997, Badaut et al. 2007, Michinaga and Koyama 2015).
The present publication brings information about the effect of methylprednisolone on experimental brain edema and its manifestation in quantitative magnetic resonance imaging.
Methods
All experiments were approved by the Ethical Committee of the First Faculty of Medicine (Charles University in Prague) and were in agreement with the Guidelines of the Animal Protection Law of the Czech Republic and Guidelines for the treatment of laboratory animals EU Guidelines 86/609/EEC. For experiments, male rats of the Wistar strain weighing 400–410 g of our own breed were used.
Groups of animals
A total of 24 experimental animals, eight in each group, were used. In the first group (CG), intact animals were scanned. The same animals were used later for induction of cytotoxic/intracellular brain edema by water intoxication (WI). In the group WIMP, methylpred-nisolone 100 mg/kg (Solu-Medrol®, Pfizer) was applied intraperitoneally at the same time as the injection of water. In the group WI+MP, methylprednisolone 50 mg/kg was injected into the carotid artery within 2 h after finishing the water intoxication. The doses were derived from their proven neuroprotective effect in other studies (Hall 1992, Park 1998, Slivka and Murphy 2001, Kozler et al. 2011, Kozler and Pokorný 2012). We evaluated the results of those four groups: two control (CG, WI) and two experimental (WIMP, WI+MP) groups.
Water intoxication and MP administration into carotid artery
The method of cytotoxic/intracellular brain edema induction by water intoxication as well as the method of MP application into carotid artery is described in our previous papers (Kozler and Pokorny 2003, Kozler et al. 2013, Kozler et al. 2018).
MR scanning
The animals were subjected to MR scanning before water application (group CG), one day after edema induction (24 h after the last water dosage application – WI Day 1), and 8 days after (WI Day 8). Experimental groups WIMP and WI+MP had the same schedule as the group WI, thus the interval of 24 h excluded the effect of the MP pharmacokinetics.
MR imaging was performed using a 1 T MR scanner ICON (Bruker, Ettlingen, Germany). The imaging protocol the imaging protocol included basic T2-weighted turbo-spin echo sequence in sagittal and/or coronal directions (effective echo time TEeff=60 ms, repetition time TR=2500 ms, turbo factor TF=16, number of acquisitions NA=16, 14 slices, slice thickness 1 mm, field of view FOV=30×30 mm2, matrix 128×128), axial T2-weighted turbo-spin echo sequence with higher resolution (TEeff=60 ms, TR=2500 ms, TF=16, NA=16, 14 slices, slice thickness 1 mm, FOV=30×30 mm2, matrix 256×256), CPMG sequence for T2-mapping (echo spacing TE=7.79 ms, TR=3600 ms, 7 slices, slice thickness 2 mm, FOV=30×30 mm2, matrix 128×128, NA=2) and diffusion-weighted sequences (echo planar imaging sequence, TE=28 ms, TR=3000 ms, NA=8, diffusion gradient in read (left-right) direction, five b-values: 35, 225, 425, 82, 1625 s/mm2, 9 slices, slice thickness 2 mm, FOV=30×30 mm2, matrix 64×64) for evaluation of ADC maps.
T2 and ADC maps were calculated using an in-house VIDI program (Herynek et al. 2012) written in Matlab (MathWorks, Natick, MA, USA). Relaxation times and diffusion coefficients were evaluated in the cortex and hippocampus in both hemispheres.
Anesthesia
Animals were anesthetized by spontaneous inhalation of isoflurane (Forane ®, AbbVie Ltd.) in air (3 % for induction, 1.5–2 % for maintenance) during all invasive procedures (water intoxication, methylpredniso-lone application) and MR examinations.
Statistical analysis
Differences were analyzed using an unpaired two-tailed t-test. p<0.05 was considered as statistically significant.
Results
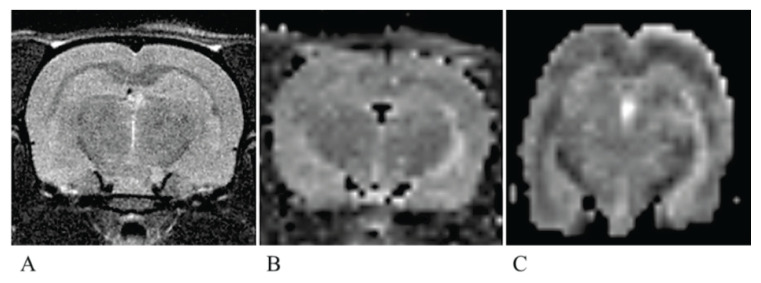
Typical T2W MR image and calculated T2 and ADC maps are shown in Figure 1.
Fig. 1.
A typical T2-weighted image, T2 map and ADC map. A typical T2-weighted image (A) of the rat brain showing the evaluated slice. (B) T2 map (interpolated), (C) ADC map (interpolated). Note geometrical distortions of the ADC map caused by magnetic field inhomogeneities in combination with EPI k-space acquisition.
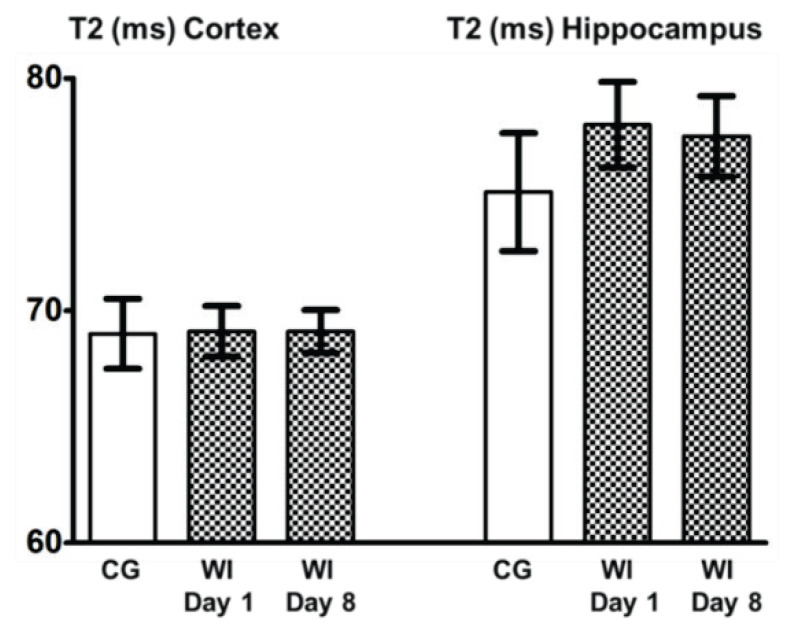
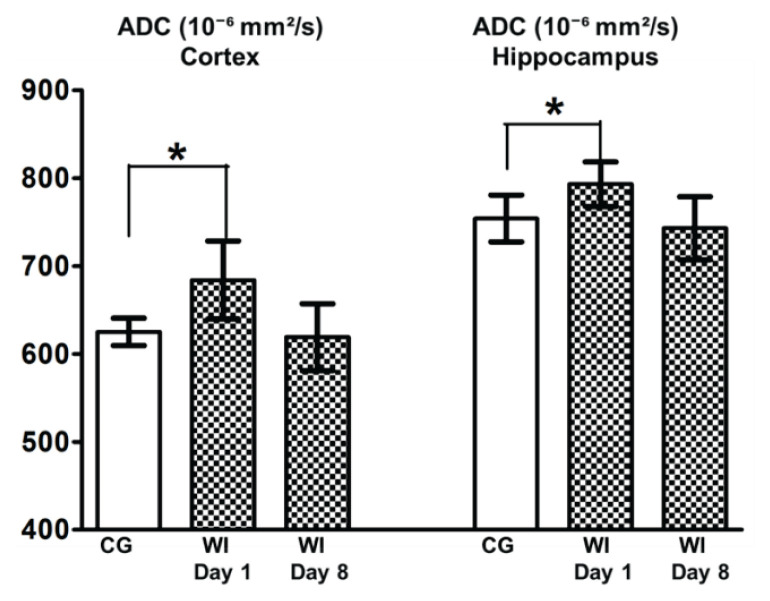
Changes in T2 and ADC values in the cortex and hippocampus before and after WI are summarized in Figure 2 and Figure 3.
Fig. 2.
T2 values after water intoxication. Columns with average value ± SEM, white columns: control group (intact animals, no WI), checkered columns: groups after edema induction by WI – WI Day 1, WI Day 8.
Fig. 3.
ADC values after water intoxication. Columns with average value ± SEM, white columns: control group (intact animals, no WI), checkered columns: groups after edema induction by WI – WI Day 1, WI Day 8, *=p<0.05.
T2 values in the cortex and hippocampus before edema induction (CG), 1 day after induction (WI Day 1), 8 days after induction (WI Day 8) as well as in experimental groups WIMP and WI+MP show no significant differences.
The increase of ADC was significantly higher (p<0.05) in Day 1 (24 h after finishing the edema induction by water intoxication – WI Day 1) both in the cortex and hippocampus. The animals reached normal values of ADC 8 days after edema induction (WI Day 8).
While T2 values in the monitored structures did not significantly change after edema induction (Fig. 2), ADC maps revealed a significantly higher apparent diffusion coefficient 24 h after edema induction in untreated animals (group WI, Fig. 3).
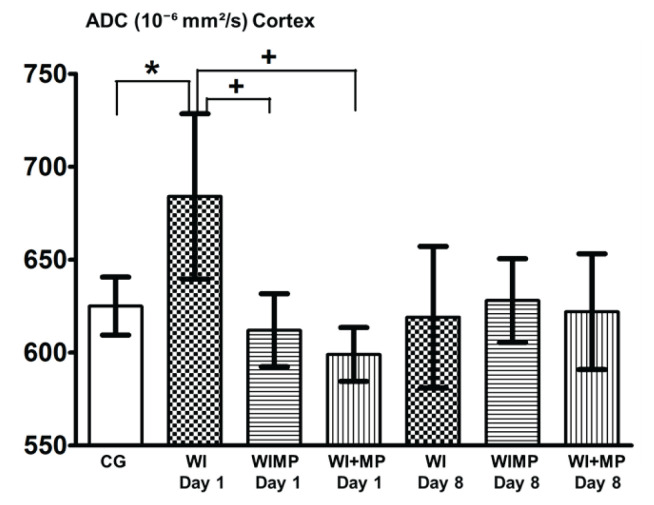
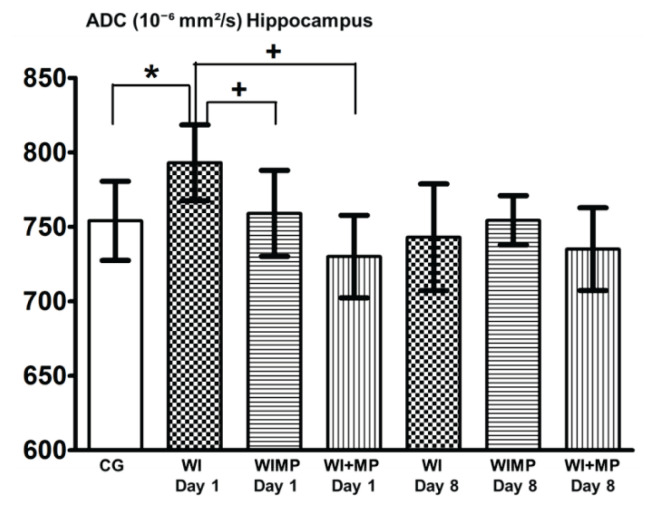
Figures 4 and 5 show significant differences between the group WI Day 1 (24 h after finishing edema induction) and groups WIMP and WI+MP (the differences vs. CG are marked with *, differences vs. WI group with +). No significant changes were found 8 days after edema induction between any experimental groups.
Fig. 4.
ADC values in cortex. Legend: columns with average value ± SD, white columns: control group (intact animals, no WI), checkered columns: groups after edema induction by WI – WI Day 1, WI Day 8, horizontally striped columns: groups WIMP Day 1, Day 8 – edema induction by WI and MP administration intraperitoneally, vertically striped columns: groups WI+MP Day 1, Day 8 – edema induction by WI and MP administration into carotid artery. (*) denotes statistically significant difference (p<0.05) from CG group, (+) denotes statistically significant difference (p<0.05) from WI group at the same time interval.
Fig. 5.
ADC values in hippocampus. Columns with average value ± SD, white columns: control group (intact animals, no WI), checkered columns: groups after edema induction by WI – WI Day 1, WI Day 8, horizontally striped columns: groups WIMP Day 1, Day 8 – edema induction by WI and MP administration intraperitoneally, vertically striped columns: groups WI+MP Day 1, Day 8 – edema induction by WI and MP administration into carotid artery. (*) denotes statistically significant difference (p<0.05) from CG group, (+) denotes statistically significant difference (p<0.05) from WI group at the same time interval.
Discussion
Each experimental model of the brain edema needs to be reliably verified by a suitable method. We showed (Kozler et al. 2013, Kozler et al. 2018) the significant increase of brain water content in an experimental model of cytotoxic/intracellular edema induced by water intoxication or after the osmotic BBB disruption.
We showed (Kozler and Pokorný 2003) the distribution of high-molecular Evans Blue (EB) tracer (MW 68500 Da) injected into carotid artery after osmotic BBB disruption in the extracellular brain compartment using fluorescence microscopy. This means that owing to the distension of the tight junctions, BBB becomes permeable to high-molecular substances, which should be followed by influx of water in the direction of the osmotic gradient (Rapoport 2000).
We also studied the tissue density quantified by computer tomography in a group of intact rats and in a group of rats after WI (Kozler and Pokorný 2014, Kozler et al. 2018) and showed the development of the brain edema after WI.
In addition to these conditions, fluorescence microscopy confirmed that EB injected into carotid artery was distributed in the brain after the induction of cytotoxic/intracellular edema by WI. This clearly showed that the BBB disruption is related to occurrence of a cytotoxic edema which in the case of WI is of the hypoosmotic origin with evidence of plasma hyponatremia (plasma natremia was 20 mmol/l lower, compared to intact animals) (Kozler and Pokorný 2003).
Methylprednisolone (MP) in the damaged central nervous system can induce its positive effect by several mechanisms depicted by Park (1998). The primary neuroprotective effect of MP is attributed to its antioxidative ability, which protects membrane lipids from peroxidation and against all subsequent adverse effects like changes in membrane fluidity or changes in the activity of membrane proteins (ion channels, transporters, enzymes). The reactive oxygen species affect also other cellular systems (mitochondria, intracellular enzymes and co-factors, systems of transcription and translation), thus altering various parameters of cell activity or inducing the cell death by apoptosis. Hence, the antioxidative ability of MP could interfere with the pathogenic process in nerve cells at different levels (Faden and Salzman 1992, Hall 1992, Hall 1993).
Being a steroid, MP has a lipophilic nature and is only weakly soluble in water. For being distributed in the body fluids it has to be in the form of ester methylprednisolone sodium succinate (MPSS). MPSS is not stable and due to activity of hepatic esterases, MP is released and subsequently bound to plasma proteins in the ratio of 40 to 60 %. Contrary to the free liposoluble MP, the high-molecular complexes cannot cross the blood-brain barrier. Only about one-half of the total MPSS administered intravenously or intraperitoneally can cross the BBB (Hall 1992). High molecular weight substances such as MP bound to the albumin (MP>50 kDa) can be incorporated into the brain either by osmotic BBB disruption or during the induction of cellular edema by WI – condition that increases the permeability of BBB.
MP is metabolized in the liver with a mean elimination half-life in the range from 1.8 to 5.2 h (Kozler et al. 2017).
We tested the effect of MP on experimental brain edema in four previous studies. The effect of MP on the permeability of cytoplasmatic membranes of neuronal populations was studied using an experimental model of cytotoxic/intracellular edema induced by WI followed by the application of MP selectively into the carotid artery (Kozler and Pokorný 2004). The results were assessed using fluorescence microscopy, which confirmed significantly reduced distribution of Evans Blue (EB) in the intracellular compartment of cortex and hippocampus. This experiment provided morphological evidence of the possibility of restoring the cell membrane integrity by means of MP administration.
In two other neurohistological experiments (Kozler et al. 2011, Kozler and Pokorný 2012) we studied MP for its effects on axonal impairment caused by induced vasogenic and cytotoxic edema. Both conditions led to axonal impairment accompanied with myelin disintegration. In the case of vasogenic edema, MP reduced the degree of myelin disintegration. The effect of MP on axonal impairment by a cytotoxic edema was different due to impairment of cellular metabolism resulting in myelin damage.
In an intracranial pressure (ICP) study (Kozler et al. 2017), a cytotoxic edema was induced by WI. MP was administered intraperitoneally before the start of edema induction, during induction and after induction. When MP was administered during or at the end of edema induction, it penetrated into the brain due to increased permeability of BBB. It could therefore affect the permeability of the cytoplasmic membrane, block the intracellular water accumulation, and prevent development of an edema already at the initial stage. ICP values during the 60-minute monitoring showed this MP effect.
Specific information may provide MRI. Quantitation of apparent diffusion coefficient (ADC) or T2 relaxation time has been proposed as a supplemental measurement for more specific differential diagnostics in various diseases (Wagnerová et al. 2012), and is suitable for investigation of an experimental brain edema (Michinaga and Koyama 2015). Cytotoxic and vasogenic edema after brain injury can be differentiated by a combination of ADC and T2 imaging. ADC is an indicator of the magnitude of the diffusion of water molecules within the tissue, and diffusion imaging provides information about the cellular architecture such as cellular size, membranes and volume fraction. ADC increases with higher extracellular volume and amount of fluids, and it is reduced when cell swelling is observed due to narrowing of the extracellular space within the cerebral parenchyma. T2 (transverse relaxation time of excited protons) is related to water content and vascular permeability. In general, reduced ADC values correlate with a cytotoxic edema (and vice versa increased ADC values correlate with an experimental extracellular/vasogenic edema) whereas increased T2 values reflect the development of a vasogenic edema. A cytotoxic edema represents mainly redistribution of water from extracellular to intracellular compartments; therefore, changes in T2 may be ambiguous (Ito et al. 1996, Badaut et al. 2007). Moreover, ADC imaging has proved to be more sensitive than T2 imaging (Loubinoux et al. 1997).
We observed significantly increased ADC values in group WI on Day 1 in both areas under study – cortex and hippocampus – which proved the occurrence of an experimental vasogenic edema 24 h after completing the induction of an experimental cytotoxic edema. This finding correlates with the result of a previous neurohistological study (Kozler and Pokorný 2003). EB was injected into the carotid artery after induction of a cytotoxic/intracellular edema by WI. Fluorescence microscopy showed that EB was distributed in the brain, which clearly proved that the BBB is affected by occurrence of a cytotoxic edema. Furthermore, this finding confirms the observations (Lythgoe et al. 2000, Sorby-Adams et al. 2017) that while the cytotoxic edema is characterized by intracellular accumulation of fluid, it makes the BBB more permeable and water accumulates in the extracellular space – the main feature of vasogenic edema. A cytotoxic edema induced by an experimental model of WI occurs immediately following the osmotic insult to the brain and is characterized by the intracellular accumulation of fluid (Ayata and Ropper 2002).
A vasogenic edema occurs in the setting of BBB disruption due to cytotoxic edema and in time course follows this primary condition (Sorby-Adams et al. 2017). Endothelial dysfunction, loss of tight junction integrity and enhanced transcellular transport are implicated in the driving the abnormal extravasation of large molecules and plasma proteins from the intravascular compartment to the extracellular space altering the osmotic pressure, and providing a driving force for the movement of water into the cerebral parenchyma (Battey et al. 2014).
ADC values in the group WIMP Day 1 (MP was given at the same time as the injection of water i.p.) and in the group WI+MP Day 1 (MP was injected into carotid artery within 2 h after finishing the water intoxication) were not increased. This indicates that due to the effect of MP in these groups the edema did not evolve. This finding correlates with the results of neurohistological studies and ICP study (Kozler and Pokorný 2004, Kozler et al. 2011, Kozler and Pokorný 2012, Kozler et al. 2017), where the effect of MP was observed after inducing both vasogenic and cytotoxic edema.
All animals (groups WI, WIMP and WI+MP) reached normal values of ADC at Day 8 after completing edema induction. This phenomenon is intimately interconnected with the resolution of the edema. Xu et al. (2010) assert in their paper on a traumatic brain edema that in all brain edema types, excess fluid leaves the brain parenchyma along three different routes: across the blood-brain barrier into the bloodstream, across the ependyma into the ventricles, and across the glia limiting membrane into CSF in the subarachnoid space. A vasogenic edema is generally believed to be cleared primarily by bulk flow of fluid through the extracellular space and glia limitans into the ventricles and subarachnoid space very quickly (Stummer 2007, Reullen 2010, Xu et al. 2010).
T2 values changed significantly after edema induction neither in the cortex nor in the hippocampus in any of the animal groups. T2 measurement is obviously less sensitive to detection of tissue swelling during the acute phase (Day 1) than ADC (Loubinoux et al. 1997). As the values did not change in the acute phase, it cannot confirm or rule out formation of a cytotoxic edema, which would involve only a compartmental shift of water from the extracellular to the intracellular space with no contribution to an increase in overall brain volume, or a specific T2 change (Simard et al. 2007). The volume increase is always due to a vasogenic edema (Sorby-Adams et al. 2017).
In this study, we showed a positive effect of methylprednisolone – no vasogenic edema developed after its administration. It should be stressed that we used purely experimental models of brain edema (WI, BBBd) without the occurrence of any pathological condition unlike the cited papers where various types and degrees of tissue damage were always present (ischemic or traumatic brain injury, and brain tumor). We therefore approached more closely to the interpretation of MP effect at the cellular level.
Acknowledgements
Supported by grant Progres Q35/LF1, and by Ministry of Education, Youth and Sports of the Czech Republic – Czech-BioImaging LM2015062.
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- AYATA C, ROPPER AH. Ischaemic brain oedema. J Clin Neurosci. 2002;9:113–124. doi: 10.1054/jocn.2001.1031. [DOI] [PubMed] [Google Scholar]
- BADAUT J, ASHWAL S, TONE B, REGLI L, TIAN HR, OBENAUS A. Temporal and regional evolution of aquaporin-4 expression and magnetic resonance imaging in a rat pup model of neonatal stroke. Pediatr Res. 2007;62:248–254. doi: 10.1203/pdr.0b013e3180db291b. [DOI] [PubMed] [Google Scholar]
- BATTEY TW, KARKI M, SINGHAL AB, WU O, SADAGHIANI S, CAMPBELL BC, DAVIS SM, DONNAN GA, SHETH KN, KIMBERLY WT. Brain edema predicts outcome after nonlacunar ischemic stroke. Stroke. 2014;45:3643–3648. doi: 10.1161/strokeaha.114.006884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULLOCK MR, LYETH BG, MUIZELAAR JP. Current status of neuroprotection trials for traumatic brain injury: lessons from animal models and clinical studies. Neurosurgery. 1999;45:207–220. doi: 10.1097/00006123-199908000-00001. [DOI] [PubMed] [Google Scholar]
- FADEN AI, SALZMAN S. Pharmacological strategies in CNS trauma. Trends Pharmacol Sci. 1992;13:29–35. doi: 10.1016/0165-6147(92)90013-v. [DOI] [PubMed] [Google Scholar]
- HALL ED. The neuroprotective pharmacology of methylprednisolone. J Neurosurg. 1992;76:13–22. doi: 10.3171/jns.1992.76.1.0013. [DOI] [PubMed] [Google Scholar]
- HALL ED. The role of oxygen radicals in traumatic injury: clinical implications. J Emerg Med. 1993;11(Suppl 1):31–36. [PubMed] [Google Scholar]
- HERYNEK V, WAGNEROVÁ D, HEJLOVÁ I, DEZORTOVÁ M, HÁJEK M. Changes in the brain during long-term follow-up after liver transplantation. J Magn Reson Imaging. 2012;35:1332–1337. doi: 10.1002/jmri.23599. [DOI] [PubMed] [Google Scholar]
- ITO J, MARMAROU A, BARZÓ P, FATOUROS P, CROWIN F. Characterization of edema by diffusion-weighted imaging in experimental traumatic brain injury. J Neurosurg. 1996;84:97–103. doi: 10.3171/jns.1996.84.1.0097. [DOI] [PubMed] [Google Scholar]
- KOZLER P, POKORNÝ J. Altered blood-brain barrier permeability and its effect on the distribution of Evans blue and sodium fluorescein in the rat brain applied by intracarotid injection. Physiol Res. 2003;52:607–614. [PubMed] [Google Scholar]
- KOZLER P, POKORNÝ J. Effects of intracarotid injection of methylprednisolone on cellular oedema after osmotic opening of the blood-brain barrier in rats. Prague Med Rep. 2004;105:279–290. [PubMed] [Google Scholar]
- KOZLER P, RILJAK V, POKORNÝ J. Methylprednisolone reduces axonal impairment in the experimental model of brain oedema. Neuro Endocrinol Lett. 2011;32:831–835. [PubMed] [Google Scholar]
- KOZLER P, POKORNY J. Effect of methylprednisolone on the axonal impairment accompanying cellular brain oedema induced by water intoxication in rats. Neuro Endocrinol Lett. 2012;33:782–786. [PubMed] [Google Scholar]
- KOZLER P, RILJAK V, POKORNÝ J. Both water intoxication and osmotic BBB disruption increase brain water content in rats. Physiol Res. 2013;62(Suppl 1):S75–S80. doi: 10.33549/physiolres.932566. [DOI] [PubMed] [Google Scholar]
- KOZLER P, RILJAK V, JANDOVÁ K, POKORNÝ J. CT imaging and spontaneous behavior analysis after osmotic blood-brain barrier opening in Wistar rat. Physiol Res. 2014;63(Suppl 4):S529–S534. doi: 10.33549/physiolres.932935. [DOI] [PubMed] [Google Scholar]
- KOZLER P, POKORNY J. CT density decrease in water intoxication rat model of brain oedema. Neuro Endocrinol Lett. 2014;35:608–612. [PubMed] [Google Scholar]
- KOZLER P, SOBEK O, POKORNÝ J. Signs of myelin impairment in cerebrospinal fluid after osmotic opening of the blood-brain barrier in rats. Physiol Res. 2015;64(Suppl 5):S603–S608. doi: 10.33549/physiolres.933220. [DOI] [PubMed] [Google Scholar]
- KOZLER P, MAREŠOVÁ D, POKORNÝ J. Methylprednisolone modulates intracranial pressure in the brain cellular edema induced by water intoxication. Physiol Res. 2017;66(Suppl 4):S511–S516. doi: 10.33549/physiolres.933797. [DOI] [PubMed] [Google Scholar]
- KOZLER P, MARESOVA D, POKORNY J. Cellular brain edema induced by water intoxication in rat experimental model. Neuro Endocrinol Lett. 2018;39:209–218. [PubMed] [Google Scholar]
- KROLL RA, NEUWELT EA. Outwitting the blood-brain barrier for therapeutic purposes: osmotic opening and other means. Neurosurgery. 1998;42:1083–1100. doi: 10.1097/00006123-199805000-00086. [DOI] [PubMed] [Google Scholar]
- LIANG D, BHATTA S, GERZANICH V, SIMARD JM. Cytotoxic edema: mechanisms of pathological cell swelling. Neurosurg Focus. 2007;22:E2. doi: 10.3171/foc.2007.22.5.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOUBINOUX I, VOLK A, BORREDON J, GUIRIMAND S, TIFFON B, SEYLAZ J, MÉRIC P. Spreading of vasogenic edema and cytotoxic edema assessed by quantitative diffusion and T2 magnetic resonance imaging. Stroke. 1997;28:419–426. doi: 10.1161/01.str.28.2.419. [DOI] [PubMed] [Google Scholar]
- LYTHGOE MF, THOMAS DL, CALAMANTE F, PELL GS, KING MD, BUSZA AL, SOTAK CH, WILLIAMS SR, ORDIDGE RJ, GADIAN DG. Acute changes in MRI diffusion, perfusion, T(1), and T(2) in a rat model of oligemia produced by partial occlusion of the middle cerebral artery. Magn Reson Med. 2000;44:706–712. doi: 10.1002/1522-2594(200011)44:5<706::aid-mrm8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- MANLEY GT, FUJIMURA M, MA T, NOSHITA N, FILIZ F, BOLLEN AW, CHAN P, VERKMAN AS. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- MARMAROU A. A review of progress in understanding the pathophysiology and treatment of brain edema. Neurosurg Focus. 2007;22:E1. doi: 10.3171/foc.2007.22.5.2. [DOI] [PubMed] [Google Scholar]
- MICHINAGA S, KOYAMA Y. Pathogenesis of brain edema and investigation into anti-edema drugs. Int J Mol Sci. 2015;16:9949–9975. doi: 10.3390/ijms16059949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK CO. The effects of methylprednisolone on prevention of brain edema after experimental moderate diffuse brain injury in rats: comparison between dosage, injection time, and treatment methods. Yonsei Med J. 1998;39:395–403. doi: 10.3349/ymj.1998.39.5.395. [DOI] [PubMed] [Google Scholar]
- RAPOPORT SI. Effect of concentrated solutions on blood-brain barrier. Am J Physiol. 1970;219:270–274. doi: 10.1152/ajplegacy.1970.219.1.270. [DOI] [PubMed] [Google Scholar]
- RAPOPORT SI. Osmotic opening of the blood-brain barrier: principles, mechanism, and therapeutic applications. Cell Mol Neurobiol. 2000;20:217–230. doi: 10.1023/a:1007049806660. [DOI] [PubMed] [Google Scholar]
- REULEN HJ. Bulk flow and diffusion revisited, and clinical applications. Acta Neurochir (Suppl) 2010;106:3–13. doi: 10.1007/978-3-211-98811-4_1. [DOI] [PubMed] [Google Scholar]
- SIMARD JM, KENT TA, CHEN M, TARASOV KV, GERZANICH V. Brain oedema in focal ischaemia: Molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6:258–268. doi: 10.1016/s1474-4422(07)70055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLIVKA PA, MURPHY EJ. High-dose methylprednisolone treatment in experimental focal cerebral ischemia. Exp Neurol. 2001;167:166–172. doi: 10.1006/exnr.2000.7532. [DOI] [PubMed] [Google Scholar]
- SORBY-ADAMS AJ, MARCOIONNI AM, DEMPSEY ER, WOENIG JA, TURNER RJ. The role of neurogenic inflammation in blood-brain barrier disruption and development of cerebral oedema following acute central nervous system (CNS) injury. Int J Mol Sci. 2017;18:E1788. doi: 10.3390/ijms18081788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUMMER W. Mechanisms of tumor-related brain edema. Neurosurg Focus. 2007;22:E8. doi: 10.3171/foc.2007.22.5.9. [DOI] [PubMed] [Google Scholar]
- WAGNEROVÁ D, HERYNEK V, MALUCELLI A, DEZORTOVÁ M, VYMAZAL J, URGOŠÍK D, SYRŮČEK M, JIRU F, SKOCH A, BARTOŠ R, SAMEŠ M, HAJEK M. Quantitative MR imaging and spectroscopy of brain tumours: a step forward? Eur Radiol. 2012;22:2307–2318. doi: 10.1007/s00330-012-2502-6. [DOI] [PubMed] [Google Scholar]
- XU M, SU W, XU QP. Aquaporin-4 and traumatic brain edema. Chin J Traumatol. 2010;13:103–110. [PubMed] [Google Scholar]
- YANG B, ZADOR Z, VERKMAN AS. Glial cell aquaporin-4 overexpression in transgenic mice accelerates cytotoxic brain swelling. J Biol Chem. 2008;22:15280–15286. doi: 10.1074/jbc.m801425200. [DOI] [PMC free article] [PubMed] [Google Scholar]