Abstract
Venous thromboembolism (VTE) is the third most common cause of cardiovascular disease. Connection between high level of physical activity (PA) and the onset of VTE is unknown. We searched the literature on the possible association between PA level, especially high levels, and the risk of VTE. A systematic review was carried out to identify relevant articles on the relation between PA level and VTE. The initial search was conducted together with the Karolinska Institutet University Library in February 2018, with follow-up searches after that. In total, 4383 records were found and then screened for exclusion of duplicates and articles outside the area of interest. In total, 16 articles with data on 3 or more levels of PA were included. Of these, 12 were cohort and 4 were case-control studies. Totally 13 studies aimed at investigating VTE cases primarily, while three studies had other primary outcomes. Of the 16 studies, five found a U-shaped association between PA level and VTE risk, although non-significant in three of them. Two articles described an association between a more intense physical activity and a higher risk of VTE, which was significant in one. Nine studies found associations between increasing PA levels and a decreasing VTE risk. Available literature provides diverging results as to the association between high levels of PA and the risk of venous thromboembolism, but with several studies showing an association. Further research is warranted to clarify the relationship between high level PA and VTE.
Supplementary Information
The online version of this article (10.1007/s11239-020-02372-5) contains supplementary material, which is available to authorized users.
Keywords: Physical activity, Venous thromboembolism, Deep venous thrombosis, Pulmonary embolism, Upper extremity venous thrombosis, Gender
Highlights
Low physical inactivity (PA) is a risk factor for venous thromboembolism (VTE).
Several studies but not all showed an association between high levels of PA and increased risk of VTE.
More studies on association between PA and VTE risk with proper methods are needed.
Introduction
Venous thromboembolism (VTE) is the third most common cause of cardiovascular disease, after coronary artery disease and stroke [1]. The annual incidence of VTE in Sweden is estimated at 150–200 per 100,000 person-years and the risk increases with age [2, 3]. In Sweden, over 11,000 patients are nursed annually in hospitals because of VTE, and around 40,000 medical visits within outpatient care are due to VTE [4].
VTE can express itself in various ways, from a completely asymptomatic thrombosis to a massive lung embolization with fatal outcome. Anticoagulant treatment is effective in preventing recurrence but can cause bleeding complications [5]. There are also models to predict recurrent VTE, above all the Vienna model [6], with a higher recurrent risk among men, patients with proximal DVT or PE, and higher D-dimer levels.
Physical activity and its positive effects on good health and well-being are well established. A total of 150 min of physical activity of at least moderate intensity per week, or at least 75 min of high intensity, is recommended by the Public Health Agency of Sweden which is consistent with that from the World Health Organisation (WHO) [7]. Low levels of physical activity are associated with an increased risk of cardiovascular morbidity [8]. As regards VTE, bed rest is a known risk factor for VTE, and a sedentary lifestyle also seems to be associated with an increased VTE risk [9–11].
Notwithstanding the positive health effects, there are also health risks associated with PA, in particular when strenuous exercise is concerned. The occurrence of cardiovascular events and also sudden cardiac death in, for example, long-distance running is documented [12]. In a Danish study on jogging, a U-shaped association between dose of jogging and all-cause mortality was described [13]. However, it is unclear whether this type of association also applies to PA and the risk of VTE. In particular, it is unclear whether highly intense exercise, as practiced today by a growing number of recreational athletes up to high age, is a protecting factor or a risk. Furthermore, it is unclear whether VTE contributes to the momentarily increased risk of cardiovascular events during highly intense exercise, and whether such risk is related to specific sports/modes of exercise. Two earlier reviews on the association between high and low levels of PA and VTE risk concluded, that higher PA level showed lower VTE risk [9, 11], but did explore the possible higher risk of VTE with highest PA levels.
The aim of this review was to assess current knowledge on the risk of venous thromboembolism in association with high levels of physical activity as described in the literature.
Method
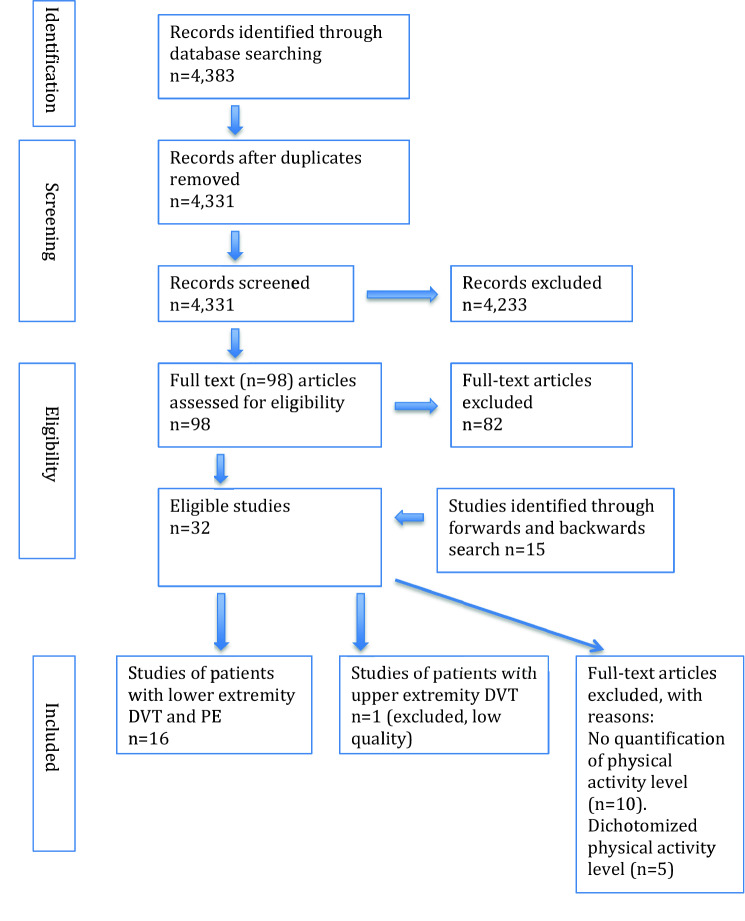
Figure 1 describes the process of article inclusion. We searched without restrictions in terms of year, or publication type in the following databases: Medline (Ovid), Embase (embase.com), and Web of Science (Clarivate Analytics), to identify relevant articles and references. The searches were conducted by two librarians at the Karolinska Institutet University Library in February 2018. The complete search strategies are available as a supplementary file. The extensive search strategy included both free-text and MeSH terms and was initially created in Medline and later adapted to the other databases with corresponding vocabularies. Reference lists of included articles were also searched for finding relevant papers, and articles citing the already included studies were identified in further Google Scholar searches. In the end it remained 32 possible articles, which we have read carefully and finally included 16 articles in our systematic review. In additional search the last four articles were found [9, 14–16]. If in doubt whether an article would be included, HDM and PW discussed how to judge it.
Fig. 1.
PRISMA 2009 flow diagram
An inclusion criterion was that physical activity (PA) should be categorized at least into three levels, in order to be able to quantify risk of VTE in strenuous activity level. Thus, 5 articles with only dichotomization of PA level were excluded, and besides another 11 not quantifying PA level at all were also excluded, leaving 16 articles left. A Flow chart on inclusion and exclusion of articles is shown in supplementary files.
We also assessed the quality of the review [17], as well as the included articles. In general, the included articles were of good quality according to both reviewers. As these were observational studies, both cohort and case-control studies were found and included. We excluded one article on venous thrombosis in the upper extremity owing to low quality, as it only showed the frequency of strenuous activity among men and associated incident thrombosis [18]. In the review by Evensen et al. original follow-up data from the Tromsø study was presented [9], i.e. 1994–2013, which extends results presented in an earlier article (1994–2007) [19]. We decided to include both these papers although review articles were otherwise excluded.
Results
This systematic review included 16 articles (Table 1). Out of these, 12 were cohort and 4 were case-control studies. A total of 13 studies aimed at study VTE cases primarily, while in three other studies this was a secondary aim [20–22].
Table 1.
Overview of included articles on the possible risk between high level of physical activity and venous thromboembolism
| Main author | Country | Study design | Year performed | Study population (N) | VTE cases (n) | Follow-up | Ages | Men/women | Adjustments | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Tsai 2002 [23] | US | Cohort (Atherosclerosis Risk in Communities study, ARIC; and Cardiovascular Health Study, CHS) | 1987–1998 | 19,293 | 215 (1.45 per 1000 person-y) | Median follow-up 7.8y; 148,054 person-y | 45–64 y (ARIC), ≥65 y (CHS) Mean age 59y at baseline | 8660/10,633 (cases 113/102) | Sex, age, race, BMI, blood pressure, smoking, alcohol intake, education | Low physical activity (5 categories, high level > =2317(=ref), 1080- < 2317, 390- < 1080, 135- < 390,<135) CHS 1.00 no 95%CI. PA kcal/w. |
| Sidney 2003 [26] | US | Case-control | 1998–2000 | 942 (196 cases/ 746 controls) | 196 | 15–44 y Mean age cases: 35.3y, mean age controls 36.2y | Women | Age, race and ethnic groups, BMI, income, and VTE in the past | Vigorous physical activity (3 categories, no reg PA = ref) OR 0.50 (95% CI 0.35–0.72) | |
| Glynn 2005 [1] | US | Cohort (Physician’s Health Study) | 1982–2003 | 18,662 | 358 (Incident rate 10.9 per 10,000 person-y) | Median follow-up 20.1y; 329,526 person-y | 40–84 y | Men | BMI, height, hypertension, elevated cholesterol, diabetes, current or former smoking, exercise, alcohol intake | HR 1.09 (95% CI 1.01–1.18) per one category increase (6 categories) |
| van Stralen 2007 [27] | The Netherlands | Population-based case-control (Multiple Environmental and Genetic Assessment of risk factors for venous Thrombosis, MEGA study) | 1999–2004 | 7862 (3608 cases/ 4254 controls) | 3608 (DVT 2093, PE 1044, DVT + PE 471) | 18-70y | 45.6%/54.4% of patients 46.7%/53.3% of controls | Sex, age, BMI, lifestyle factors and for matched/unmatched | Very strenuous sports activity (4 categories, no = ref) OR 0.66 (95% CI 0.56–0.76). | |
| van Stralen 2008 [21] | US | Cohort (Cardiovascular Health Study, CHS) | 1989–2001 | 5534 | 171 | Median follow-up time 11.6y; 52,308 person-y | ≥ 65y | 2376/3158 | Sex, age, race, self-reported health, BMI | U-shaped association. Strenuous exercise (4 categories, none = ref) HR 1.75 (95% CI 1.08–2.83) |
| Lindqvist 2009 [25] | Sweden | Cohort (Melanoma Inquiry of Southern Sweden, MISS) | 1990–2002 | 29,518 | 312 | Mean follow-up 11 y; 317,290 person-y | 25–64 y | Women | Age, diagnosis of cancer during the study period, parity, smoking, alcohol intake, combined oral contraceptives (COC), exercise, BMI | Strenuous activity every week (3 categories, no = ref) HR 0.5 (95% CI 0.3–0.9) |
| Borch 2010 [19] | Norway | Population-based cohort (Tromsø study) | 1994–2007 | 26,490 | 460 (DVT 295, PE 165) (1.61/1000 person-y) | Median follow-up 12.5 y; 286,467 person-y | 25–97 y | 12.598/ 13.892; cases 217/ 243 | Age, sex, BMI, smoking, diabetes, and hormone therapy (women) | U-shaped, non-significant association. Physical activity (4 categories, 0 h = ref) ≥ 3 h/wk. HR 1.13 (95% CI 0.80–1.61) |
| Lutsey 2010 [29] | US | Cohort (Iowa Women’s Health Study) | 1986–2004 | 40,377 | 2137 (DVT 1313, PE 824) (incidence rate 4.04 per 1000 person-years) | Median follow-up 13 y; 529,360 person-y | 55–69 y; mean age 61.8 y. | Women | Age, BMI, educational attainment, smoking status, physical activity level | High physical activity (3 categories, low = ref) HR 0.91 (95% CI 0.82–1.02) |
| Wattanakit 2012 [22] | US | Community-based cohort (Atherosclerosis Risk in Communities study, ARIC) | 1987–2005 | 15,340 | 468 | Mean follow-up time 15.5y; 237,375 person-y | 45-64y; mean age 54y | 45% /55% | Sex, age, race, BMI, ARIC field centres | High physical activity (quartiles, lowest level = ref) HR 0.81 (95% CI 0.62–1.06); lowest risk at Q2 for both unprovoked and provoked VTE. Slightly U-shaped. |
| Bergendal 2012 [24] | Sweden | Case-control (Thrombo Embolism Hormone Study, TEHS) | 2003–2009 | 2835 (1433 cases/ 1402 controls) | 1433 | 18-64y | Women | Age, BMI, smoking, use of hormones, bed rest/minor trauma, surgery, cast, surgery and cast, prothrombin mutation and/or factor V, contraceptives | Strenuous activity (4 categories, light = ref) premenopausal OR 0.55 (95% CI 0.37–0.80). Postmenopausal OR 0.64 (95% CI 0.42–0.99). | |
| Armstrong 2014 [20] | UK | Cohort (Million Women Study) | Scotland 1981–2008, England 1997–2012 | 1,119,239 | 14,550 (DVT 7712, PE ± DVT 7013) | Median follow-up 9y | 50-64y; mean age 55.9y. | Women | Socioeconomic status, region, the first 4 y of follow-up, BMI, smoking, alcohol intake | U-shaped association. Strenuous activity (5 categories, rarely/never = ref) all VTE 1.08 (95% CI 0.99–1.17); DVT 1.13 (95% CI 1.01–1.27) |
| Olson 2015 [15] | US | Cohort (REasons for Geographic And Racial Differences in Stroke, REGARDS) | 2003–2011 | 30,239 | 263 (DVT 153, PE ± DVT 122) | Median follow-up 5 y | ≥45 years | 45%/55%; case 153/110 | Age, sex, income, education, race, region, and race*region interaction | Ideal physical activity (3 levels, poor = ref) HR 0.59 (95% CI 0.43–0.81) |
| Ogunmoroti 2016 [28] | US | Cohort (Multi-Ethnic study of Atherosclerosis, MESA) | 2000–2015 | 6506 | 215 (3.3%) Event rates for poor, intermediate and ideal PA 4.0, 3.1 and 1.7 per 1000 person-y | Median follow-up 10.2 y | 45–84 years | 3074/3432; 215 cases | Age, sex, race/ethnicity, education, income | Ideal physical activity (3 levels, poor = ref) HR 0.70 (95% CI 0.52–0.95), intermediate 0.66 (95% CI 0.43–1.02) |
| Kim 2018 [16] | US | Case-control (NHS and NHSII, Nurses’ Health Study, HPFS, Health Professionals Follow-up Study) | 1976–2014, 1989–2011, 1986–2012 | 6024 (2134 cases/3890 controls) | 2134 | 30–55 y, 25–42 y, 40–75 y | Female, female, men F: 2450 (889 cases/1561 controls); +1766 (447 cases/1319 controls); M: 1808 (798 cases/1010 controls) | BMI, sitting time | ORs for poled data for MET quartiles in hr./wk.; Q1 (<5.6) ref., Q2 (5.6- < 15.4) 0.80 (0.69–0.94); Q3 (15.4- < 33.8) 0.84 (0.72–0.99); Q4 (33.8+) 0.71 (0.60–0.84) | |
| Evensen 2018 [9] | Norway | Population-based cohort (Tromsø study) | 1994–2013 | 26,490 | 754 | 19 y | 25–97 y | 12.598/ 13.892; cases | Age (as time scale), sex, BMI, and smoking | Slightly U-shaped, non-significant association. Hard physical activity (5 categories, 0 h = ref) > 3 h/wk. HR 1.11 (95% CI 0.83–1.49) |
| Johansson 2019 [14] | Sweden | Cohort (Venous thromboEmbolism In Northern Sweden, VEINS) | 1985–2014 | 108,025 | 2054 (1.37 per 1000 person-y) | Median follow-up 15.5 y (1,496,669 person-years) | 30–60 y; mean age 46.3 y | 53,393/54,632; cases 1110/944 | Age, BMI, hypertension, smoking, education level and cancer | Highest level ≥ 1–2 times/w (4 levels, never = ref) men 1.01 (95% CI 0.83–1.22), women 0.80 (95% CI 0.63–1.03) |
Of the studies, two found a statistically significant U-shaped association between physical activity level and VTE risk [20, 21], while three studies showed a non-significant U-shape, i.e. two from the Norwegian Tromsø study [9, 19], and another by Wattankit et al. [22]. One study found an association between increased level of PA and a greater risk of VTE [1].
In the article by Tsai et al. [23], two measures of physical activity were applied because of two pooled cohorts, the ARIC (Atherosclerosis Risk In Communities) study used a score and the CHS (Cardiovascular Health Study) assessing kcal/week. We choose the latter measure allowing for a better discrimination of different PA levels. In the CHS cohort (≥ 65 years) an increased level of physical activity was non-significantly associated with an increased VTE risk. However, in the article by van Stralen et al. [21], using the same CHS cohort, a significant U-shaped association was found. Different cut-offs for physical activity level were used in the analyses, and the definition of strenuous activity seemed stricter in the article by von Stralen et al. [21], than in the study by Tsai et al. [23].
Seven studies found that an increasing physical activity level was associated with a decreasing VTE risk [15, 16, 24–28], and one showed a non-significant association [29]. One study found divergent results for men and women, with a non-significant lower risk among women but a similar risk as the reference group among men [2].
A special topic is the possible association between strenuous arm activity and VTE. Two studies showed a slightly elevated risk of upper extremity thrombosis with strenuous muscular activity of the upper extremity [18, 30], although both studies were non-significant. However, these studies were later excluded from the review because of the insufficient graded levels of PA.
Discussion
The main finding of this systematic review is that the literature reports conflicting results regarding the association between different levels of PA and the associated risk of VTE, and especially regarding the potential risk of VTE with exercise on a high level.
While most of studies described a significantly decreasing VTE risk with increasing physical activity [15, 16, 24–28], also one with a non-significant association [29], another study found quite the opposite [1]. Some studies demonstrated or suggested a U-shaped association [9, 19–22]. One study found a different pattern between men and women [2], and one study found no association between PA level and VTE risk. However, as VTE may occur a long time after an exercise, a causal association and the identification of risk predictors may be difficult to establish.
Regarding levels of PA, this can be assessed in different ways, e.g. with the EPIC-PAQ suggested as suitable as a standardized measurement with 4 levels of PA [31]. For our concern a definition of strenuous levels of PA is warranted, and thus a more differentiated scale would be useful, e.g. the NOPAC with 10 categories [32], in order to be able to study the possible association between strenuous PA and incident VTE. In the study by Armstrong et al. [23], women reporting a strenuous exercise daily only comprised 3.2% of the population [33]. Categorizing samples into tertiles, quartiles or quintiles might not catch the group with the highest rate of strenuous physical activity.
There are possible mechanisms that could explain the association between strenuous PA and incident VTE. A review concluded, that long and vigorous extreme exertion causes hypercoagulability together with an augmented fibrinolysis [34], but the fibrinolytic parameters return to baseline quickly, whereas the procoagulant parameters remain elevated longer.
There are limitations with this study. Other reviews including a meta-analysis on the association between PA levels and VTE have been performed [11, 35]. However, these reviews compared low and high levels of PA in general regarding VTE risk, and not specifically very high PA levels in relation to VTE risk. We did not perform a meta-analysis, as the defined levels of PA differed largely across the studies, and especially the highest level of PA, with an obvious heterogeneity. The studies might not have identified a risk group with a very high level of physical activity and a higher VTE risk. The studies did measure physical activity level in different ways, why it could be hard to compare the different results. We decided to include different kind of studies, as our intention was to include all studies on the possible association between high levels of PA and incident VTE.
There are also several strengths. We performed a systematic search for relevant articles, and the findings in the earlier published review support that we have found relevant articles.
In conclusion, it is possible that high levels of PA could be a risk factor for VTE, but with the diverging results in the review the evidence for this is still unclear. More studies to analyse the association between high PA levels and VTE risk are needed, including an attempt to quantify possible risk level of high PA, and to try to use more standardized measurements of PA activity. Concerning the possible association between high level of PA and incident VTE a gap of evidence still remains.
Supplementary Information
Below is the link to the electronic supplementary material.
Electronic supplementary material 1 (DOCX 65 kb)
Funding
Open Access funding provided by Karolinska Institute.
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Glynn RJ, Rosner B. Comparison of risk factors for the competing risks of coronary heart disease, stroke, and venous thromboembolism. Am J Epidemiol. 2005;162(10):975–982. doi: 10.1093/aje/kwi309. [DOI] [PubMed] [Google Scholar]
- 2.Johansson M, Johansson L, Lind M. Incidence of venous thromboembolism in northern Sweden (VEINS): a population-based study. Thromb J. 2014;12(1):6. doi: 10.1186/1477-9560-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wandell P, Forslund T, Danin Mankowitz H, et al. Venous thromboembolism 2011-2018 in Stockholm: a demographic study. J Thromb Thrombolysis. 2019;48(4):668–673. doi: 10.1007/s11239-019-01966-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The National Board of Health and Welfare. The National Board of Health and Welfare guidelines for blood clot / venous thromboembolism 2004 [Socialstyrelsens riktlinjer för vård av blodpropp/venös tromboembolism 2004; in Swedish]. Stockholm: Socialstyrelsen; 2004
- 5.Konstantinides SV, Torbicki A, Agnelli G, et al. ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033–3069. doi: 10.1093/eurheartj/ehu283. [DOI] [PubMed] [Google Scholar]
- 6.Eichinger S, Heinze G, Kyrle PA. D-dimer levels over time and the risk of recurrent venous thromboembolism: an update of the Vienna prediction model. J Am Heart Assoc. 2014;3(1):e000467. doi: 10.1161/JAHA.113.000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yrkesföreningar för fysisk aktivitet (YFA). Physical Activity in the Prevention and Treatment of Disease (FYSS in Swedish). Third edition ed. Stockholm, Läkartidningen Förlag; 2017
- 8.Cheng W, Zhang Z, Yang C, et al. Associations of leisure-time physical activity with cardiovascular mortality: a systematic review and meta-analysis of 44 prospective cohort studies. Eur J Prev Cardiol. 2018;25(17):1864–1872. doi: 10.1177/2047487318795194. [DOI] [PubMed] [Google Scholar]
- 9.Evensen LH, Braekkan SK, Hansen JB. Regular physical activity and risk of venous thromboembolism. Semin Thromb Hemost. 2018;44(8):765–779. doi: 10.1055/s-0038-1673636. [DOI] [PubMed] [Google Scholar]
- 10.Douketis JD, Iorio A. The association between venous thromboembolism and physical inactivity in everyday life. BMJ. 2011;343:d3865. doi: 10.1136/bmj.d3865. [DOI] [PubMed] [Google Scholar]
- 11.Kunutsor SK, Makikallio TH, Seidu S, et al. Physical activity and risk of venous thromboembolism: systematic review and meta-analysis of prospective cohort studies. Eur J Epidemiol. 2020;35(5):431–442. doi: 10.1007/s10654-019-00579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JH, Malhotra R, Chiampas G, et al. Cardiac arrest during long-distance running races. N Engl J Med. 2012;366(2):130–140. doi: 10.1056/NEJMoa1106468. [DOI] [PubMed] [Google Scholar]
- 13.Schnohr P, O’Keefe JH, Marott JL, et al. Dose of jogging and long-term mortality: the Copenhagen City heart study. J Am Coll Cardiol. 2015;65(5):411–419. doi: 10.1016/j.jacc.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Johansson M, Johansson L, Wennberg P et al (2019) Physical activity and risk of first-time venous thromboembolism. Eur J Prev Cardiol 2047487319829310 [DOI] [PubMed]
- 15.Olson NC, Cushman M, Judd SE, et al. American Heart Association’s Life’s simple 7 and risk of venous thromboembolism: the Reasons for geographic and racial differences in stroke (REGARDS) study. J Am Heart Assoc. 2015;4(3):e001494. doi: 10.1161/JAHA.114.001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, Kraft P, Hagan KA, et al. Interaction of a genetic risk score with physical activity, physical inactivity, and body mass index in relation to venous thromboembolism risk. Genet Epidemiol. 2018;42(4):354–365. doi: 10.1002/gepi.22118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinelli I, Cattaneo M, Panzeri D, et al. Risk factors for deep venous thrombosis of the upper extremities. Ann Intern Med. 1997;126(9):707–711. doi: 10.7326/0003-4819-126-9-199705010-00006. [DOI] [PubMed] [Google Scholar]
- 19.Borch KH, Hansen-Krone I, Braekkan SK, et al. Physical activity and risk of venous thromboembolism. Tromso Study Haematol. 2010;95(12):2088–2094. doi: 10.3324/haematol.2009.020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armstrong ME, Green J, Reeves GK, et al. Frequent physical activity may not reduce vascular disease risk as much as moderate activity: large prospective study of women in the United Kingdom. Circulation. 2015;131(8):721–729. doi: 10.1161/CIRCULATIONAHA.114.010296. [DOI] [PubMed] [Google Scholar]
- 21.van Stralen KJ, Doggen CJ, Lumley T, et al. The relationship between exercise and risk of venous thrombosis in elderly people. J Am Geriatr Soc. 2008;56(3):517–522. doi: 10.1111/j.1532-5415.2007.01588.x. [DOI] [PubMed] [Google Scholar]
- 22.Wattanakit K, Lutsey PL, Bell EJ, et al. Association between cardiovascular disease risk factors and occurrence of venous thromboembolism. A time-dependent analysis. Thromb Haemost. 2012;108(3):508–515. doi: 10.1160/TH11-10-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai AW, Cushman M, Rosamond WD, et al. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Arch Intern Med. 2002;162(10):1182–1189. doi: 10.1001/archinte.162.10.1182. [DOI] [PubMed] [Google Scholar]
- 24.Bergendal A, Bremme K, Hedenmalm K, et al. Risk factors for venous thromboembolism in pre-and postmenopausal women. Thromb Res. 2012;130(4):596–601. doi: 10.1016/j.thromres.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 25.Lindqvist PG, Epstein E, Olsson H. The relationship between lifestyle factors and venous thromboembolism among women: a report from the MISS study. Br J Haematol. 2009;144(2):234–240. doi: 10.1111/j.1365-2141.2008.07460.x. [DOI] [PubMed] [Google Scholar]
- 26.Sidney S, Petitti DB, Soff GA, et al. Venous thromboembolic disease in users of low-estrogen combined estrogen-progestin oral contraceptives. Contraception. 2004;70(1):3–10. doi: 10.1016/j.contraception.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 27.van Stralen KJ, Le Cessie S, Rosendaal FR, et al. Regular sports activities decrease the risk of venous thrombosis. J Thromb Haemost. 2007;5(11):2186–2192. doi: 10.1111/j.1538-7836.2007.02732.x. [DOI] [PubMed] [Google Scholar]
- 28.Ogunmoroti O, Allen NB, Cushman M et al (2016) Association between Life’s simple 7 and noncardiovascular disease: the multi-ethnic study of atherosclerosis. J Am Heart Assoc 5(10) [DOI] [PMC free article] [PubMed]
- 29.Lutsey PL, Virnig BA, Durham SB, et al. Correlates and consequences of venous thromboembolism: the Iowa Women’s health study. Am J Public Health. 2010;100(8):1506–1513. doi: 10.2105/AJPH.2008.157776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blom JW, Doggen CJ, Osanto S, et al. Old and new risk factors for upper extremity deep venous thrombosis. J Thrombosis Haemostasis. 2005;3(11):2471–2478. doi: 10.1111/j.1538-7836.2005.01625.x. [DOI] [PubMed] [Google Scholar]
- 31.Peters T, Brage S, Westgate K, et al. Validity of a short questionnaire to assess physical activity in 10 European countries. Eur J Epidemiol. 2012;27(1):15–25. doi: 10.1007/s10654-011-9625-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borch KB, Ekelund U, Brage S, et al. Criterion validity of a 10-category scale for ranking physical activity in Norwegian women. Int J Behav Nutr Phys Act. 2012;9:2. doi: 10.1186/1479-5868-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huxley RR. Physical activity: can there be too much of a good thing? Circulation. 2015;131(8):692–694. doi: 10.1161/CIRCULATIONAHA.115.014721. [DOI] [PubMed] [Google Scholar]
- 34.Kicken CH, Miszta A, Kelchtermans H, et al. Hemostasis during extreme exertion. Semin Thromb Hemost. 2018;44(7):640–650. doi: 10.1055/s-0038-1639502. [DOI] [PubMed] [Google Scholar]
- 35.Evensen LH, Isaksen T, Braekkan SK, et al. Physical activity and risk of recurrence and mortality after incident venous thromboembolism. J Thromb Haemost. 2019;17(6):901–911. doi: 10.1111/jth.14449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic supplementary material 1 (DOCX 65 kb)