Abstract
The cyc1-512 mutation in Saccharomyces cerevisiae causes a 90% reduction in the level of iso-1-cytochrome c because of the lack of a proper 3′-end-forming signal, resulting in low levels of eight aberrantly long cyc1-512 mRNAs which differ in length at their 3′ termini. cyc1-512 can be suppressed by deletion of either of the nonessential genes CBC1 and CBC2, which encode the CBP80 and CBP20 subunits of the nuclear cap binding complex, respectively, or by deletion of the nonessential gene UPF1, which encodes a major component of the mRNA surveillance complex. The upf1-Δ deletion suppressed the cyc1-512 defect by diminishing degradation of the longer subset of cyc1-512 mRNAs, suggesting that downstream elements or structures occurred in the extended 3′ region, similar to the downstream elements exposed by transcripts bearing premature nonsense mutations. On the other hand, suppression of cyc1-512 defects by cbc1-Δ occurred by two different mechanisms. The levels of the shorter cyc1-512 transcripts were enhanced in the cbc1-Δ mutants by promoting 3′-end formation at otherwise-weak sites, whereas the levels of the longer cyc1-512 transcripts, as well as of all mRNAs, were slightly enhanced by diminishing degradation. Furthermore, cbc1-Δ greatly suppressed the degradation of mRNAs and other phenotypes of a rat7-1 strain which is defective in mRNA export. We suggest that Cbc1p defines a novel degradation pathway that acts on mRNAs partially retained in nuclei.
The lack of normal transcription termination or 3′-end processing of mRNAs can lead to drastic reductions in gene expression, as exemplified by the cyc1-512 mutation of the CYC1 gene, which encodes iso-1-cytochrome c in Saccharomyces cerevisiae. The cyc1-512 mutation, consisting of a 38-bp deletion, 8 nucleotides (nt) upstream from the normal poly(A) site, was found to cause an approximately 90% diminution in the CYC1 mRNA and in the corresponding iso-1-cytochrome c protein (88). The cyc1-512 mRNAs were aberrantly long, with many discrete 3′ termini ranging from the wild-type poly(A) site to endpoints greater than 2,000 nucleotide (nt) downstream. Apparently, the lack of the normal 3′-end-forming signals resulted in partial termination or processing at various sites beyond the normal poly(A) site. The abnormally long mRNAs were suggested to be rapidly degraded (88).
Genetic analysis revealed the following three types of cyc1-512 revertants that were isolated on lactate medium, a medium that requires increased levels of iso-1-cytochrome c for growth: (i) single- or multiple-base pair changes, resulting in intragenic revertants that contained new 3′-end-forming signals at various sites in the 3′ untranslated region of CYC1; (ii) gross chromosomal aberrations that resulted in the formation of abnormal 3′ regions; and (iii) extragenic suppressors that enhanced the levels of the cyc1-512 mRNA and of iso-1-cytochrome c by up to approximately fourfold (39, 89). The cyc1-512 suppressors constituted recessive mutations that could be assigned to at least two loci, which were designated SUT1 and SUT2 (39, 89).
As shown in this study, complementation of the suppressors sut1-2 (now designated cbc1-2) and sut2-2 (now designated upf1-102) and DNA sequencing of the appropriate cloned fragments revealed that SUT1 and SUT2 correspond to the previously identified genes CBC1 and UPF1, respectively. CBC1 encodes a protein corresponding to the orthologous nuclear cap binding protein, CBP80, in animal cells (16, 28, 29), whereas UPF1 encodes one of the major components of the nonsense-mediated mRNA decay (NMD) pathway (9, 12, 32, 41, 62, 84). Furthermore, the examination of the abundance and half-lives of cyc1-512 mRNAs and other normal mRNAs in cbc1-Δ and upf1-Δ strains revealed that Cbc1p may be a component of a novel nuclear mRNA decay pathway and that mRNAs with extended 3′ ends can be degraded by the NMD pathway. Also, studies with cbc1-Δ strains demonstrated that Cbc1p apparently is required to prevent promiscuous 3′-end formation.
MATERIALS AND METHODS
Genetic nomenclature.
The wild-type allele encoding iso-1-cytochrome c is designated CYC1 or CYC1+. Mutant alleles that produce either normal or decreased levels of iso-1-cytochrome c are designated cyc1 followed by the allele number, e.g., cyc1-512. CBC1 (or CBC1+), for example, denote the wild-type alleles. The cbc1-2, sut2-2, etc., gene symbols designate recessive mutant alleles, whereas cbc1-Δ or cbc1::URA3, for example, denotes a disruptant of CBC1.
CBC1 was previously designated SUT1 (39, 89), GCR3 (77, 78), and STO1 (11), whereas CBC2 has also been designated MUD13 (11). UPF1 was previously designated SUT2 (39, 89) before its identification. As mentioned above, the suppressors sut1-1 and sut1-2, etc., are now designated cbc1-1 and cbc1-2, etc., whereas the suppressors sut2-1 and sut2-2, etc., are now designated upf1-101 and upf1-102, etc.
Strains, media, and yeast genetics.
The strains of S. cerevisiae used in this study are listed in Table 1. λDE3 lysogens of Escherichia coli strain BL21 (F− ompT rB− mB−) (76) was used for the expression of a portion of Cbc1p. Standard YPD, YPG, SC-Ura (uracil omission), SC-Leu (leucine omission), and other omission media were used for yeast propagation and testing (70); chlorolactate medium was used for the cloning of CBC1 and UPF1 genes (71). Yeast genetic analysis was carried out by standard procedures described by Sherman (70).
TABLE 1.
Yeast strains
| Strain | Genotypea |
|---|---|
| D311-3A | MATa CYC1+ his1-1 lys2-1 trp2-1 |
| B-4060 | MATa cyc1-512 his1-1 lys2-1 trp2-1 |
| B-5419 | MATa cyc1-512 his1-1 lys2-1 cbc1-2 (sut1-2) trp2-1 |
| B-9037 | MATa cyc1-512 trp2-1 ura3-52 |
| B-9038 | MATa cyc1-512 trp2-1 ura3-52 cbc1::URA3 |
| B-9848 | MATa cyc1-512 trp2-1 ura3-52 upf1::URA3 |
| B-11559 | MATa cyc1-512 trp2-1 ura3-52 cbc1::hisG upf1::URA3 |
| B-12463 | MATa cyc1-512 trp2-1 ura3-52 cbc2::URA3 |
| B-9036 | MATa cyc1-512 cbc1-2 ura3-52 |
| B-9045 | MATa cyc1-512 cbc1-2::URA3 ura3-52 |
| B-9849 | MATa cyc1-512 cbc1-2 upf1::URA3 ura3-52 |
| B-9962 | MATa cyc1-512 upf1-102 (sut2-2) ura3-52 |
| B-6304 | MATα cyc1-512 leu2-1 |
| B-9042 | MATα cyc1-512 leu2-1 pAB1135 |
| B-7999 | MATα cyc1-947 cyc7-67 leu2-3 leu2-112 ura3-52 cyhR2 |
| B-9039 | MATα cyc1-947 cyc7-67 leu2-1 leu2-112 cbc1::URA3 ura3-52 cyhR2 |
| B-6978 | MATa CYC1+ cyc7-67 can1-100 ilv3 leu2-3 leu2-112 ura3-52 |
| B-9040 | MATa CYC1+ cyc7-67 can1-100 ilv3 leu2-3 leu2-112 cbc1::URA3 ura3-52 |
| B-11623 | MATa cyc1-72 aro7-1 his4-166 leu2-1 lys2-187 met8-1 ura3-52 |
| B-9198 | MATa cyc1-72 aro7-1 his4-166 leu2-1 lys2-187 met8-1 ura3-52 cbc1::URA3 |
| B-9199 | MATa cyc1-72 aro7-1 his4-166 leu2-1 lys2-187 met8-1 ura3-52 upf1::URA3 |
| B-10603 | MATa his3-Δ200 ura3-52 leu2-Δ1 |
| B-10099 | MATa his3-Δ200 ura3-52 leu2-Δ1 cbc1::URA3 |
| B-10095 | MATa his3-Δ200 ura3-52 leu2-Δ1 rat7-1 |
| B-10096 | MATa his3-Δ200 ura3-52 leu2-Δ1 rat7-1 cbc1::URA3 |
| B-10097 | MATa his3-Δ200 ura3-52 leu2-Δ1 rat7-1 upf1::URA3 |
| D-890 | MATa/MATα cyc1-1/cyc1-1 CYC7-H2/CYC7-H2 ARO7/aro7-1 HIS1/his1 LEU1/leu1-12 LYS2/lys2 TRP2/trp2 ura3/ura3 |
Gene symbols in parentheses designate earlier synonyms (see Materials and Methods).
Determination of cytochrome c content.
Total amounts of cytochrome c were determined by spectroscopic examination of intact cells at −196°C (71) and by comparing the intensities of the c Cα bands at 547 nm to strains having known amounts of cytochrome c. More accurate determinations of cytochrome c content in intact cells were made by low-temperature (−196°C)-spectrum recordings using a modified Cary 14 spectrophotometer (25).
Oligonucleotides.
Oligonucleotides used for oligonucleotide-directed mutagenesis, sequencing, primer extension, and PCR were synthesized on an Applied Biosystems 380A DNA synthesizer.
Plasmid construction.
Plasmids designated pAB1158, pAB1159, pAB1160, pAB1733, and pAB1734 were independently isolated from an S. cerevisiae genomic library constructed in plasmid YCp50 (59) using the complementation strategy outlined below. pAB1101 was derived from pAB1160 by deleting a 6-kb SalI fragment and was used to sequence the CBC1 gene. A 4.2-kb ClaI fragment from pAB1101, containing the CBC1 coding region and flanking sequences, was inserted into pAB625, resulting in the plasmid pAB1137. The pAB1137 plasmid was used to make mutations in the CBC1 gene by site-directed mutagenesis. pAB1135 was constructed by ligating a 4.5-kb SalI-HindIII fragment from pAB1101 into YEp51. The plasmid used for disrupting the CBC1 gene, pAB1100, was constructed by inserting a 2.1-kb SalI-BamHI fragment, which contained the CBC1 gene, from pAB1101 into the Bluescript SK vector (Stratagene) and subsequently inserting a 1.1-kb HindIII fragment, which contained the URA3+ gene from pYc5, into the HindIII site within the CBC1 gene. pAB81 was derived by inserting a 2.5-kb EcoRI fragment, containing the cyc1-512 allele, into pBR322. pAA1735 corresponds to the plasmid YCpPL63 described by Leeds et al. (42).
The blaster pAB2207 plasmid, used to disrupt CBC1+, was constructed in three steps. Initially, a 4.5-kb SalI-HindIII fragment containing the CBC1+ coding region and the flanking sequence from pAB1101 was inserted into a pUC19 vector. In the next step, a 1.25-kb BamHI-BglII fragment was deleted from the CBC1+ coding region and subsequently replaced by 3.8-kb BamHI-BglII blaster fragment containing a functional yeast URA3+ gene flanked by 1.1-kb direct repeats of Salmonella enterica serovar Typhimurium hisG DNA from pNKY51 (1).
Transformation, nucleic acid isolation, and manipulation.
S. cerevisiae cultures were transformed with plasmid DNA for complementation analysis and with linear DNA for gene disruption (60) by using the lithium acetate method (27) followed by selection on SC-Ura (uracil omission) or SC-Leu (leucine omission) medium. Plasmid DNA was isolated from transformed S. cerevisiae strains as described previously (72). The yeast chromosomal gene CBC1 was disrupted by transforming the appropriate yeast strains with DNA fragments that were prepared either by digesting the plasmid pAB1100 with SalI and BamHI or by digesting the blaster plasmid pAB2207 with PvuII and XbaI. Similarly, the UPF1 gene on the chromosome was disrupted by transforming the suitable yeast strains with DNA fragments that were prepared by digesting the plasmid YCpPL51 (42), with BamHI and EcoRI. The Escherichia coli strains HB101 and XL1-Blue were transformed by the protocol of Hanahan (22). Standard techniques of DNA manipulation, such as cloning, subcloning, and sequencing, used in this study are described by Sambrook et al. (67). Nucleic acid and protein sequences were analyzed by using the computer programs FASTA, TFASTA, BESTFIT, and BLAST from the University of Wisconsin Genetics Computer Group software version 7.0.
Cloning the CBC1+ and UPF1+ genes.
The cbc1-2 mutation was originally isolated as a suppressor of cyc1-512 (39, 89). The wild-type CBC1+ gene was cloned by complementing the cbc1-2 suppressor in strain B-9036 (Table 1) with a YCp50-based yeast genomic library. Briefly, approximately 105 Ura+ transformants were collected and plated on chlorolactate medium. Generally, strains with diminished levels of iso-1-cytochrome c form larger colonies on chlorolactate medium because of decreased utilization of chlorolactate, which is toxic (71). Approximately 50 large chlorolactate colonies were tested for levels of iso-1-cytochrome c by low-temperature (−196°C) spectroscopic examination of intact cells. Those strains having diminished levels of iso-1-cytochrome c were further tested for the levels after loss of the plasmid by isolating Ura− strains on 5-fluoroorotic acid medium. This screen resulted in the identification of three strains with lower levels of iso-1-cytochrome c in a plasmid-dependent manner. Plasmid DNA was extracted from these three yeast strains, amplified in E. coli, and shown by restriction endonuclease analysis to encompass a common region. Furthermore, Northern blot analysis showed that yeast strains harboring any one of these three plasmids contained the expected 10% of the CYC1 mRNA, similar to cyc1-512 strains (data not presented).
DNA sequencing and subsequent computer analysis of the relevant segment of the complementing plasmid revealed a 2,574-bp open reading frame (ORF) and the TACTAAC motif upstream of the ORF, which is indicative of an intron (data not shown). In addition, the putative 5′ and 3′ splicing sites could be theoretically assigned by considering the conserved sequences (86), and these assignments were confirmed by sequencing the appropriate region of the CBC1 cDNA. The presence of a 322-nt-long intron near the 5′ end was also confirmed by others (73, 78).
The 5′ ends of the CBC1 transcripts were mapped by primer extension to a 60-bp region from −36 to −96 nt. The major transcription initiation site was located at position −73, flanked by several minor sites (data not presented). Interestingly, all the 5′ ends aligned at purine residues, and usually at A residues; only the −36 position was at a G residue. The predicted translation product for CBC1 is 861 amino acids long, a size that would correspond to a protein of 100,017 Da. Comparison of Cbc1p protein with database sequences by using the BLAST method revealed that CBC1 is identical to GCR3, a gene involved in expression of the glycolytic genes of yeast (77). However, the initial published amino acid sequence of GCR3 is not identical to that of our deduced amino-terminal region because the intron of GCR3 was not considered by the authors and was included as part of the ORF.
UPF1 was cloned similarly by the method used to clone CBC1, except that strain B-9962 (cyc1-512 upf1-102 [sut2-2] ura3-52) (Table 1) was transformed with the yeast genomic library. Approximately 5 × 105 Ura+ transformants were transferred to chlorolactate plates, and the 87 largest colonies were chosen for spectral examination. Four of them were found to have diminished levels of cytochrome c compared to the parental strain. Plasmids from these four transformants were isolated and used to transform the strain B-9962. Two of these four transformants again contained reduced levels of cytochrome c. Restriction mapping demonstrated that these two plasmids contained the same insert, which was approximately 10 kb in size. Approximately 200-nt sequences at both ends of one insert were determined and compared to the GenBank database. The results revealed that the insert corresponded to a segment in chromosome XIII extending from nt 4354 to 13464 of cosmid 9582, accession number Z492559. This region of chromosome XIII contains the following genes: CHL12 (4522 to 6747), SEC14 (7008 to 8078), UPF1 (8731 to 11646), and MBR3 (12098 to 13114). Because UPF1 is required for the rapid degradation of certain abnormal mRNAs (see below), strain B-9962 was transformed with plasmid pAA1735 (YCpPL63) (42), which contains the UPF1 gene. Since the level of iso-1-cytochrome c diminished from 30 to 10% in the sut2-2 transformants bearing the wild-type UPF1+ gene in plasmid pAA1735, sut2-2 was considered to be complemented by UPF1+, and consequently, SUT2 is considered to correspond to UPF1. In addition, as described below, disruption of UPF1 leads to suppression of cyc1-512.
Analysis of mRNA steady-state levels and stability.
The stabilities of the various mRNAs and the pre-mRNA were determined by the inhibition of transcription with 4 μg of thiolutin/ml at 30°C, as described by Herrick et al. (24). Total RNA was isolated as described by Russo et al. (65) from approximately 108 cells, and enriched poly(A) RNA was isolated from a total of 1 mg of total RNA with the Oligotex mRNA kit (Qiagen Inc., Valentia, Calif.) as recommended by the vendor. Northern blot analyses of different mRNAs were conducted as outlined by Russo et al. (65). Messenger RNA levels were quantified by storage PhosphorImager analysis (model 425E; Molecular Dynamics) and normalized against the 18S rRNA signals when total RNA was used. Signals were normalized against ACT1 mRNA when poly(A) mRNA was used.
The decay rates and half-lives were estimated with the SigmaPlot (version 4.0) regression analysis program, using either a single-exponential decay formula, y = 100 e−bx, or a four-parameter double-exponential decay formula, y = ae−bx + ce−dx (a + c = 100). The variance was estimated by a nonlinear least-squares regression analysis using the S-Plus software package, version 3.4 (Mathsoft Inc., Cambridge, Mass.).
Antibody production.
An XhoI restriction site was created 19 bp upstream from the CBC1 3′ splicing site in pAB1137 by site-directed mutagenesis (40). The resulting XhoI-BamHI fragment, containing 175 codons of exon 2 of CBC1, was inserted in frame into the expression vector pET15b (Novagene), resulting in the plasmid pAB1133. The E. coli expression host BL21 (DE3) was transformed with pAB1133, and the expression of the target protein was induced by adding IPTG (isopropyl-β-d-thiogalactopyranoside) to the medium (76). The 25-kDa Cbc1p fragment, constituting approximately 90% of the inclusion body proteins, was washed twice with washing buffer (2% Triton X-100, 50 mM Tris-HCl, and 2 mM EDTA, pH 7.5) and used by Pocono Rabbit Farm (Canadensis, Pa.) to generate rabbit polyclonal antibodies.
Indirect immunofluorescence and Western blot analysis.
Cells grown in SC-Leu medium were fixed with 4% formaldehyde at room temperature for 2 h. Spheroplasts were prepared with zymolase 100T (ICN Immunobiologicals) and immobilized on polylysine-coated slides (79). Primary-antibody incubation was performed at 4°C overnight with 10,000-fold-diluted anti-Cbc1p antiserum or preimmune serum. The secondary antibody, Texas red-conjugated goat anti-rabbit immunoglobulin G, was incubated for 30 min at room temperature at a dilution of 1:100. Nuclei were stained with 1 μg of 4′,6-diamidino-2-phenylindole (DAPI)/ml.
Total yeast proteins (14) were separated by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis and transferred electrophoretically to nitrocellulose filters. To detect Cbc1p, the blotted filters were first blocked with phosphate-buffered saline (100 mM NaCl and 100 mM Na2HPO4, pH 7.5) containing 5% nonfat dry milk. After incubation with the primary anti-Cbc1p antiserum at a 1:5,000 dilution for 1 h at room temperature, the blots were washed with a solution of phosphate-buffered saline and 0.1% Tween 20 and incubated with the secondary antibody, horseradish peroxidase-linked donkey anti-rabbit antibody (Amersham), at a dilution of 1:3,000. The Cbc1p band was subsequently detected with an enhanced chemiluminescence kit (Amersham).
RESULTS
Suppression of cyc1-512 by cbc1-Δ, cbc2-Δ, and upf1-Δ.
The mechanism of suppression of the cyc1-512 mutation was investigated by identifying the genes corresponding to the sut1 and sut2 suppressors. As described in Materials and Methods, cloning of these two suppressors by complementation and subsequent sequencing revealed that these suppressors corresponded to the previously known nonessential genes CBC1 and UPF1, respectively.
CBC1 encodes the large subunit of the nuclear cap binding complex (CBC), CBP80, which influences a number of important functions in the maturation and export of capped RNAs (16, 28, 29). CBC1 encodes an 861-amino-acid protein and contains a 322-nt-long intron in the 5′ end of the translated region (data not shown). The yeast Cbc1p is 16.7% identical and 33% similar to the mammalian CBP80. The other suppressor, UPF1, plays a major role in the NMD decay pathway (9, 12, 32, 41, 61, 83).
It was originally suggested that the cbc1-2 suppressor coded for a protein with an altered function which might enhance the 3′-end formation of the aberrant cyc1-512 transcripts (89). This hypothesis was tested by disrupting CBC1 in the haploid strains B-9037 (cyc1-512 CBC1+) and B-9036 (cyc1-512 cbc1-2) (Table 2), which produced 10 and 45% of the normal level of iso-1-cytochrome c, respectively. cbc1-2 appeared to be equivalent to a null mutation, since the phenotypes of the cyc1-512 strains with a disruption of the CBC1+ gene or with the cbc1-2 allele were identical, having the same amount of iso-1-cytochrome c (Table 2). Furthermore, cbc1-Δ did not affect the expression of CYC1+, a finding which was consistent with the observation that cbc1-2 did not cause an increase in the level of iso-1-cytochrome c in a CYC1+ background (89). In addition, cbc1-Δ did not affect the expression of the cyc1-947 allele that contained a normal 3′ untranslated region but that was greatly reduced in transcription due to the lack of TATA elements (Table 2) (44), suggesting that neither cbc1-2 nor cbc1-Δ affects the rate of transcription.
TABLE 2.
Disruption of CBC1 in haploid strains
| Strain | Relevant genotype | % Normal iso-1-cytochrome c |
|---|---|---|
| B-9037 | cyc1-512 CBC1+ | 10 |
| B-9038 | cyc1-512 cbc1::URA3 | 45 |
| B-9848 | cyc1-512 upf1::URA3 | 30 |
| B-11559 | cyc1-512 cbc1::hisG upf1::URA3 | 45 |
| B-12463 | cyc1-512 cbc2::URA3 | 45 |
| B-9036 | cyc1-512 cbc1-2 | 45 |
| B-9045 | cyc1-512 cbc1-2::URA3 | 45 |
| B-7999 | cyc1-947 CBC1+ | 5 |
| B-9039 | cyc1-947 cbc1::URA3 | 5 |
| B-6978 | CYC1+ CBC1+ | 100 |
| B-9040 | CYC1+ cbc1::URA3 | 100 |
The finding that disruption of CBC1+ had the same phenotype as cbc1-2, along with the previous observation that cbc1-2 caused an increase in the cyc1-512 mRNA level (89), raised the possibility that cbc1-2 or cbc1-Δ could act by increasing the stability of cyc1-512 mRNA. Therefore, we investigated the stabilities and decay rates of a number of mRNAs in an isogenic series of strains to test this hypothesis (see below).
In order to examine whether mutation of either of the components of CBC could suppress the cyc1-512, we also disrupted the CBC2+ gene (11) in the haploid strain B-9037 (cyc1-512 CBC2+). Determination of the level of iso-1-cytochrome c in the resulting B-12463 strain (cyc1-512 cbc2-Δ) showed that disruption of CBC2+ has the same phenotype as that obtained with the disruption of the CBC1+ gene (Table 2).
Examination of the levels of iso-1-cytochrome c in the isogenic series of strains listed in Tables 2 and 3 revealed that upf1-Δ suppresses cyc1-512 to approximately the same extent as cbc1-Δ and that the double mutant, cbc1-Δ upf1-Δ, was not more effective than either of the single mutants (see Discussion).
TABLE 3.
Half-lives and relative steady-state amounts of mRNA and pre-mRNA and steady-state amounts of iso-1-cytochrome c in cyc1-512 strains having single and double cbc1-Δ and upf1-Δ mutations
| mRNA | Size (nt) | Half-life (min)
|
Relative steady-state amt
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| B-9037 (CBC1+ UPF1+) | B-9038 (cbc1-Δ UPF1+) | B-9848 (CBC1+ upf1-Δ) | B-11559 (cbc1-Δ upf1-Δ) | B-9037 (CBC1+ UPF1+) | B-9038 (cbc1-Δ UPF1+) | B-9848 (CBC1+ upf1-Δ) | B-11559 (cbc1-Δ upf1-Δ) | ||
| cyc1-512 mRNAs | |||||||||
| 1 | 630a | 33.1 ± 0.03 | 35.6 ± 0.6 | 29.6 ± 1.08 | 35.5 ± 0.72 | 45 | 158 | 40 | 125 |
| 630b | 33.8 ± 0.09 | 36.1 ± 0.03 | 21.5 ± 0.9 | 16.7 ± 0.3 | 43.4 | 128 | 37 | 118 | |
| 2 | 850b | 9.6 ± 0.1 | 8.88 ± 0.06 | 8.8 ± 0.3 | 6.9 ± 0.5 | 19.3 | 73 | 26 | 100 |
| 3 | 1,450b | 4.4 ± 0.003 | 12.8 ± 0.7 | 13.8 ± 1.5 | 12.4 ± 1.3 | 5.5 | 14.0 | 5.6 | 15.8 |
| 4 | 1,650b | 6.1 ± 0.05 | 17.4 ± 0.05 | 16.2 ± 2.0 | 12.1 ± 0.5 | 5.4 | 13 | 11.3 | 14.1 |
| 5 | 1,850b | 6.6 ± 0.05 | 20.2 ± 0.2 | 17.3 ± 0.9 | 14.2 ± 1.0 | 6.5 | 12 | 16.6 | 20 |
| 6 | 2,000b | ||||||||
| 7 | 2,400b | 6.07 ± 0.09 | 12.3 ± 1.5 | 18.4 ± 1.6 | 22.5 ± 1.3 | 7.7 | 20 | 19 | 26 |
| 8 | 3,500b | 5.5 ± 0.2 | 18.7 ± 1.4 | 12.5 ± 0.1 | 15.9 ± 3.6 | 5.9 | 21.6 | 24.5 | 21 |
| CYH2 mRNAa | 36.5 ± 1.3 | 46 ± 2.0 | 42 ± 3.06 | 41.5 ± 2.2 | 9.2 | 7.9 | 9.4 | 8.56 | |
| CYH2 pre-mRNAa | 3.0 ± 0.006 | 4.3 ± 0.007 | 12.7 ± 0.02 | 14.0 ± 0.03 | 3.0 | 2.9 | 76.0 | 73.2 | |
| ACT1 mRNAa | 41 ± 1.6 | 55 ± 1.4 | 33 ± 0.3 | 57 ± 1.6 | 7.9 | 6.8 | 7.2 | 7.1 | |
| Iso-1-cytochrome c | 10% | 45% | 30% | 45% | |||||
Determined from total RNA.
Determined from mRNA.
Disruption of CBC1 or CBC2 diminished cell growth on glucose medium.
To determine the phenotype of a null mutant at the CBC1 locus, one copy of CBC1 in a diploid strain, D-890 (Table 1), was disrupted. Analysis of 11 complete tetrads from a Ura+ transformant showed that all spores were viable and that the Ura+ marker segregated 2:2. Disruption of CBC1 by integration of the URA3 gene was confirmed by PCR amplification of chromosomal DNA isolated from the Ura+ segregants. Thus, CBC1 is not essential for cell viability.
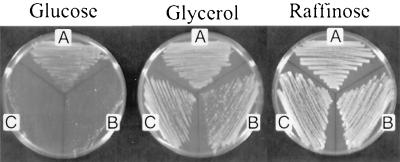
Although it was not lethal, disruption of CBC1 or CBC2 greatly diminished cell growth on glucose medium (YPD), indicating an important role for Cbc1p in normal cell growth. In contrast, CBC1+, cbc1-Δ, and cbc2-Δ strains grew nearly the same on nonfermentable glycerol medium (YPG) and fermentable raffinose medium (YPR) (Fig. 1), which extends the early results of Uemura and Jigmi (77), who reported similar findings with YPD and YPG.
FIG. 1.
Comparison of the growth of CBC1+, cbc1-Δ, and cbc2-Δ strains. The three isogenic strains were grown at 30°C on YPD glucose medium for 2 days, YPG glycerol medium for 4 days, or YPR raffinose medium for 2 days. (A) B-9037 (CBC1+); (B) B-9038 (cbc1-Δ); (C) B-12463 (cbc2-Δ).
Cbc1p is located in the nucleus.
The amino-terminal region of the deduced Cbc1 protein included two basic sequences, NRKRR (residues 3 to 7) and RPRMPKRQR (residues 20 to 28), which could serve as nuclear localization signals. Although the actual roles of the two basic regions have not been determined experimentally, their presence at the amino-terminal region strongly suggests that Cbc1p is localized in the nucleus. Because of its low abundance in normal cells, Cbc1p could not easily be detected in strain B-6304 by either Western blot or indirect immunofluorescence analysis. However, Cbc1p became detectable by overexpression of CBC1 with a 2μm plasmid, pAB1135. Cbc1p, detected by Western blot analysis, was approximately 100 kDa, agreeing with its predicted molecular mass (data not shown). Furthermore, strain B-9042, containing pAB1135, was prepared for immunofluorescence microscopic analysis. Experiments with highly diluted anti-Cbc1p antisera demonstrated that Cbc1p was located in the nuclei of the cells, as was evident from the coincident Texas red and DAPI stains (data not shown).
To rule out the possibility that Cbc1p could be mislocated to the nucleus by overexpression, an extract prepared from the normal strain B-6304 was fractionated into subcellular components (69). The location of Cbc1p was monitored by protein blot analysis of the proteins in subcellular fractions. Cbc1p could be readily detected in the nuclear fraction of strain B-6304 but not in the cytosol fraction or the total extract (data not shown). Similarly, Görlich et al. (16) used immunoelectron microscopy to demonstrate that Cbc1p was located primarily in the nucleus of yeast.
The cyc1-512 mRNAs.
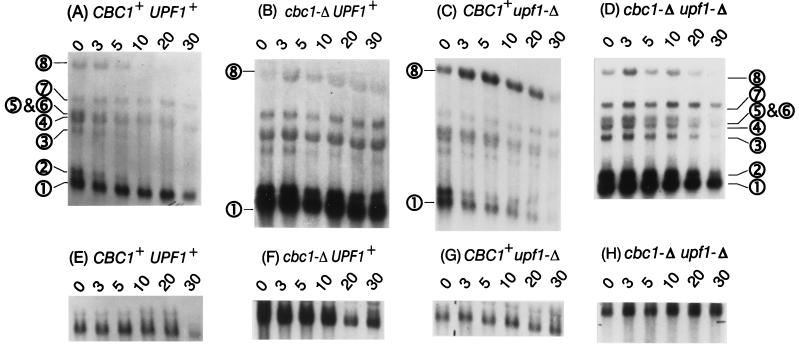
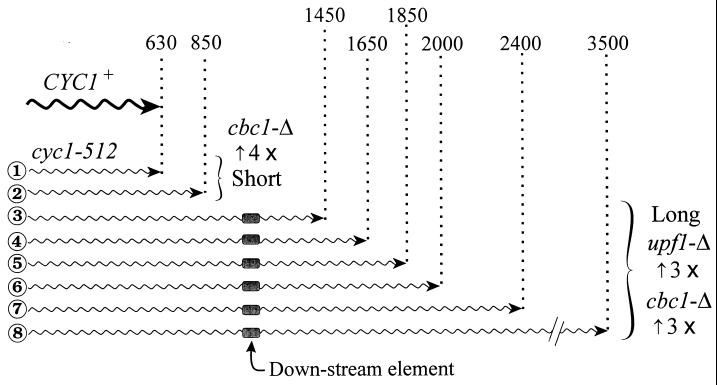
Northern blot analysis previously revealed a number of cyc1-512 mRNAs, including 630-, 850-, 1,350-, and 1,450-nt and longer transcripts (88, 89), whereas 3′-end mapping and sequencing further defined numerous transcripts between 630 and 700 nt (66). We have refined the Northern blot analysis and detected the following eight transcripts, as shown in Fig. 2 and schematically in Fig. 3: 630, 850, 1,450, 1,650, 1,850, 2,000, 2,400, and 3,500 nt. We have designated the 630- and 850-nt mRNAs the short cyc1-512 transcripts and designated the 1,450- through 3,500-nt mRNAs the long cyc1-512 transcripts.
FIG. 2.
Northern blot analyses of the eight cyc1-512 mRNAs listed in Table 3. Poly(A) RNA was extracted from four strains treated with 4 μg of thiolutin/ml for 0 to 30 min. (A and E) B-9037 (CBC1+ UPF1+); (B and F) B-9038 (cbc1-Δ UPF1+); (C and G) B-9848 (CBC1+ upf1-Δ); (D and H) B-11559 (cbc1-Δ upf1-Δ). (A to D) cyc1-512 mRNAs; (E to H) ACT1 mRNA. The values presented in Table 3 were estimated from these blots by normalizing them to ACT1 mRNA values, whose rates of degradation for these strains are known.
FIG. 3.
Schematic representation of the cyc1-512 mRNAs, showing their lengths in nucleotides and the steady-state increases due to suppression by cbc1-Δ or upf1-Δ.
Levels and degradation of mRNAs in cbc1-Δ and upf1-Δ strains.
The following series of isogenic strains was used for examining the possibility that cbc1-Δ and upf1-Δ could act by increasing the stability of cyc1-512 mRNAs and some other specific mRNAs: B-9037 (cyc1-512), B-9038 (cyc1-512 cbc1-Δ), B-9848 (cyc1-512 upf1-Δ), and B-11559 (cyc1-512 cbc1-Δ upf1-Δ) (Table 1). The steady-state levels and half-lives of the following RNAs from these strains were determined by comparing Northern blots after blocking transcription with the drug thiolutin (24): the eight cyc1-512 mRNAs, ACT1 mRNA, CYH2 mRNA, and CYH2 pre-mRNA. 18S rRNA was used as an internal standard for Northern blots with total-RNA preparations. In one experiment, where enriched poly(A) mRNA was used to detect the low levels of the longer cyc1-512 transcripts, ACT1 mRNA was used as an internal loading control, adjusted for the known rate of decay of ACT1 mRNA in these strains (Table 3). Examples of Northern blots (Fig. 2 and 4), the decay curves (Fig. 5), and the steady-state levels and half-lives (Table 3) are presented.
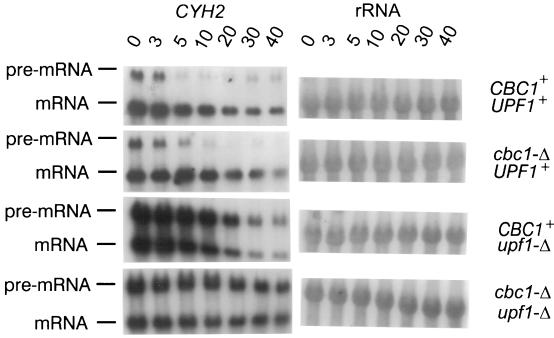
FIG. 4.
Northern blot analysis of CYH2 mRNA and pre-mRNA (left) from strains B-9037 (CBC1+ UPF1+), B-9038 (cbc1-Δ UPF1+), B-9848 (CBC1+ upf1-Δ), and B-11559 (cbc1-Δ upf1-Δ) treated with 4 μg of thiolutin/ml for 0 to 40 min as indicated above the blots. 18S rRNA is shown on the right-hand side.
FIG. 5.
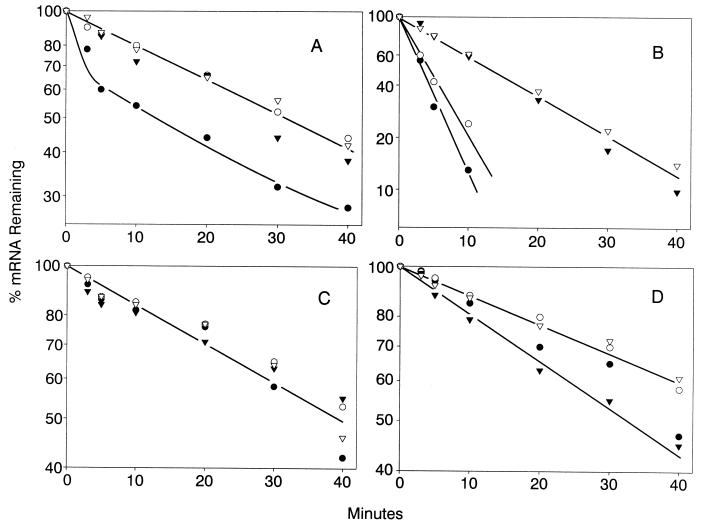
Decay in thiolutin-treated cells of cyc1-512 630-nt mRNA (A), CYH2 pre-mRNA (B), CYH2 mRNA (C), and ACT1 mRNA (D) from the following strains: B-9037 (normal; cyc1-512) (●); B-9038 (cyc1-512 cbc1-Δ) (○); B-9848 (cyc1-512 upf1-Δ) (▾); and B-11559 (cyc1-512 cbc1-Δ upf1-Δ) (▿). The decay was determined by Northern blot analysis of RNA extracted from cells treated with 4 μg of thiolutin/ml for 0 to 40 min. The results are presented as the percentage of remaining RNA versus time of incubation in the presence of thiolutin.
The half-lives clearly revealed that the degradation of certain mRNAs is greatly diminished by the upf1-Δ mutation. As expected and as previously observed, the degradation of CYH2 pre-mRNA was greatly diminished in the upf1-Δ strain because of the nonsense codon in the CYH2 intron (Fig. 4 and Table 3) (23), whereas that of CYH2 and ACT1 mRNAs was not affected (Table 3). Most importantly, the degradation and steady-state levels of the long cyc1-512 mRNAs were also diminished by two- to threefold, but not that of the short forms (Fig. 2 and 3 and Table 3). As discussed below, we suggest that the transcripts longer than 850 nt contain elements or structures that mimic the signals in mRNAs having premature terminating codons.
The effect of the cbc1-Δ mutation was more complex, not causing diminished degradation of the short (630- and 850-nt) cyc1-512 mRNAs but causing a pronounced increase in their steady-state levels (Fig. 3 and Table 3). The stabilities of the short cyc1-512 mRNAs were the same in all four strains, but their level are much higher in all cbc1-Δ strains (Fig. 2 and 3 and Table 3). This increase is unlikely to be the result of an enhanced rate of transcription for the following two reasons. (i) The relative levels of the short and long cyc1-512 mRNAs were substantially different in the cyc1-512 and cyc1-512 cbc1-Δ strains, which would not have been expected if this increase were due to enhanced transcription. (ii) As discussed above, cbc1-Δ did not have any enhancing effect on other CYC1 alleles. These observations suggest that the increased levels of short (630- and 850-nt) cyc1-512 mRNAs in all cbc1-Δ strains are due to a phenomenon designated promiscuous 3′-end formation (see Discussion).
In contrast, the longer cyc1-512 transcripts decayed more rapidly in the CBC1+ UPF1+ strain than in the cbc1-Δ strains, suggesting that degradation of these longer transcripts was affected by the cbc1-Δ mutation, similar to the results with the upf1-Δ mutation (see above) (Figs. 2A, B, and D). The stabilities, as well as the steady-state levels, of the longer transcripts increased by at least two- to threefold in all cbc1-Δ strains (Fig. 3 and Table 3). Furthermore, cbc1-Δ also slightly affected the degradation of other RNAs tested, including ACT1 and CYH2 mRNA and CYH2 pre-mRNA (Table 3). Thus, Cbc1p may play a role in the general degradation of all mRNAs.
Upf1-Δ, but not cbc1-Δ, suppresses nonsense mutations.
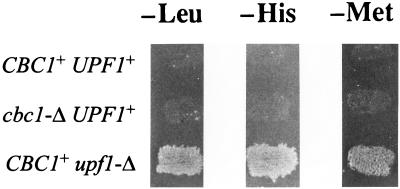
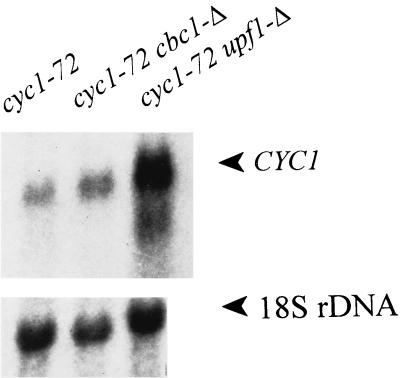
Both cbc1-Δ and upf1-Δ suppress cyc1-512 by increasing the levels of one or another of the cyc1-512 mRNAs, but these suppressors presumably act by different mechanisms. Although both cbc1-Δ and upf1-Δ diminished the degradation of long cyc1-512 mRNAs, only upf1-Δ, but not cbc1-Δ, suppressed nonsense mutations. This distinction was clearly revealed by testing the leu2-1 (UAA), met8-1 (UAG), and his4-166 (UGA) markers (Fig. 6), previously shown to be suppressed by upf1-Δ (42). Furthermore, the steady-state level of the cyc1-72 mRNA, containing a premature UAA mutation, was greatly enhanced in the upf1-Δ strain but not in the cbc1-Δ strain (Fig. 7).
FIG. 6.
cbc1-Δ does not suppress the nonsense mutations. Three strains, B-11623 (UPF1+ CBC1+), B-9198 (UPF1+ cbc1-Δ), and B-9199 (upf1-Δ CBC1+), were grown on YPD plates for 2 days, replicated onto omission media lacking either leucine (−Leu), histidine (−His), or methionine (−Met) and subsequently incubated for 3 days to test suppression of leu2-1 (UAA), his4-166 (UGA), and met8-1 (UAG).
FIG. 7.
Northern blot analysis of B-11623 (cyc1-72), B-9198 (cyc1-72 cbc1-Δ), and B-9199 (cyc1-72 upf1-Δ) showing the CYC1 steady-state mRNA levels compared to the level of 18S ribosomal DNA (rDNA).
Suppression of rat7-1 by cbc1-Δ.
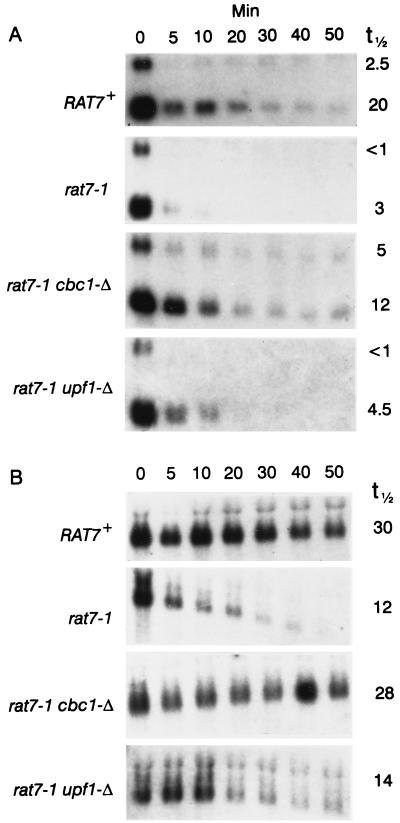
Because Cbc1p is primarily located in the nucleus (16), we considered the possibility that the abnormally long cyc1-512 mRNAs are partially retained in the nucleus and are subjected to a nuclear Cbc1p-dependent degradation system. We have tested this hypothesis by investigating the decay of specific mRNAs at the restrictive temperature of 37°C in rat7-1 strains, which do not grow or export mRNAs under the restricted conditions (17). All of the rat7-1 strains used in this study were derived from the same strain previously used by Gorsch et al. (17). ACT1 and CYH2 mRNAs, as well as CYH2 pre-mRNA, were more rapidly degraded in the rat7-1 strain than in the isogenic RAT7+ strain when shifted to 37°C (Fig. 8 and Table 4), although the values of the half-lives of the CYH2 pre-mRNA in the rat7-1 and rat7-1 upf1-Δ strains could not be accurately estimated because of rapid degradation. Furthermore, degradation of all of the mRNAs tested in rat7-1 strains was suppressed by cbc1-Δ but not upf1-Δ (Fig. 8 and Table 4). Cbc1-Δ also suppressed the growth defect inflicted by rat7-1 at 37°C (Fig. 9). As discussed below, we suggest that Cbc1p could be required for degrading mRNAs arrested in the nucleus.
FIG. 8.
Decay of ACT1 and CYH2 mRNAs and pre-mRNA at 37°C in B-10603 (RAT7+), B-10095 (rat7-1), B-10096 (rat7-1 cbc1-Δ), and B-10097 (rat7-1 upf1-Δ). The strains were grown at 23°C to the mid-log phase, shifted to the restricted temperature of 37°C for 15 min, and subsequently treated with 4 μg of thiolutin/ml to inhibit transcription, and the decay was followed for 0 to 50 min. Northern blots were probed for CYH2 mRNA (bottom band in each blot) and pre-mRNA (top band in each blot) (A) and for ACT1 mRNA (B). The numbers at the right denote the half-lives (t1/2) of the mRNAs in minutes.
TABLE 4.
Rates of decay of ACT1 and CYH2 mRNAs and pre-mRNA in RAT7+, rat7-1, rat7-1 cbc1-Δ, and rat7-1 upf1-Δ strains as described in Fig. 8
| Strain | Relevant genotype |
t1/2 (min)
|
||
|---|---|---|---|---|
| ACT1 mRNA | CYH2 mRNA | CYH2 pre-mRNA | ||
| B-10603 | RAT7+ | 30 | 20 | 2.5 |
| B-10095 | rat7-1 | 12 | 3 | <1 |
| B-10096 | rat7-1 cbc1-Δ | 28 | 12 | 5 |
| B-10097 | rat7-1 upf1-Δ | 14 | 4.5 | <1 |
FIG. 9.
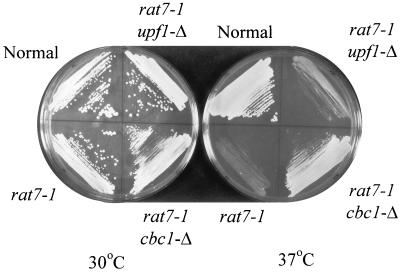
Growth of B-10603 (RAT7+), B-10095 (rat7-1), B-10096 (rat7-1 cbc1-Δ), and B-10097 (rat7-1 upf1-Δ) strains on YPD medium at 30 and 37°C for 3 days, showing the suppression of growth in rat7-1 strains by cbc1-Δ.
DISCUSSION
We uncovered three phenomena by systematically investigating two suppressors that partially restore the diminished levels of iso-1-cytochrome c and mRNAs in the cyc1-512 mutant that lacks the normal 3′-end-forming signal. First, we observed that degradation of the cyc1-512 mRNAs longer than 850 nt was suppressed in upf1-Δ mutants, which lack one of the main components of the NMD pathway. Second, we found that the long cyc1-512 mRNAs, and to some extent all mRNAs, are degraded, presumably in the nucleus, in a Cbc1p-dependent manner. This degradation is at least partially suppressed by cbc1-Δ mutations and presumably is more pronounced with mRNAs that are retained in the nucleus, either because of such mutations as rat7-1 or because of structures of the mRNA, such as abnormal extensions at the 3′ end. Finally, transcription termination and 3′-end formation, which are diminished because of incomplete or weak signals in the cyc1-512 allele, were found to be enhanced by cbc1-Δ mutations, a phenomenon we have designated promiscuous 3′-end formation.
NMD of the long cyc1-512 mRNAs.
The NMD pathway has evolved as a surveillance mechanism to degrade transcripts containing premature nonsense codons, thus preventing synthesis of incomplete and potentially deleterious proteins. In particular, NMD protects cells from the potentially deleterious effects of inefficient or inaccurate splicing, which are common and which could result in the translation of mRNAs with introns. Most introns contain nonsense codons and are therefore subjected to NMD. Also, certain normal mRNAs are subjected to NMD, presumably as a means to control steady-state levels (82). Upf1p is a key component of the NMD pathway, which exists in all eukaryotes that have been examined (9, 12, 32, 41, 46, 61, 85).
Muhlrad and Parker (48) and Hagen et al. (21) demonstrated that NMD proceeds by decapping and subsequent degradation by the action of the Xrn1p 5′→3′ exoribonuclease. Furthermore, studies of initiation of translation (30, 50) and the presence of circular polyribosomes in electron micrographs (31) have suggested that the 5′ cap (or 5′ untranslated region) and the poly(A) tail are associated. This interaction between the 5′ cap and the poly(A) tail may prevent decapping (48). It has been proposed that in-frame premature nonsense codons near the 5′ region cause release of the 80S ribosomes and expose an element, designated the downstream element (55, 62, 63, 93) or the sensitive element (87), which is situated downstream of the termination codon. One intriguing model that has been proposed is that this element may mediate an important interaction between a scanning ribosome or 40S ribosomal subunit, a consequence of which would be rapid decapping and degradation by Xrn1p, the major cytoplasmic 5′→3′ exoribonuclease (56, 93). As discussed by Yun and Sherman (87), 40S ribosomal subunits and RNPs, as well as 80S ribosomes, may serve to prevent interactions with these hypothetical downstream elements.
The positions of the termination codons determine whether the NMD will be triggered, and all of the yeast genes, URA3 (45), URA1 (54), PGK1 (55), HIS4 (21), and CYC1 (87), that have been investigated showed very low abundance of mRNA when the nonsense mutations were situated at or near the 5′ regions and normal or nearly normal levels when the nonsense mutations were in the 3′ regions of the ORF. This position-dependent triggering suggests that premature termination before the downstream elements is required for NMD. It has been suggested that a surveillance complex, which is composed of Upf1p and other proteins, scans 3′ of the termination codon for these elements. Peltz, Ruiz-Echevarría, Zhang, and colleagues (55, 62, 63, 93) suggested that rapid decapping and 5′→3′ decay of the mRNA occurs when the surveillance complex interacts with the downstream element. The sequence of the downstream element is degenerate and is present numerous times in virtually all mRNAs (93).
The NMD of cyc1-512 mRNAs can be simply explained by the presence of downstream elements, or structures in the extended 3′ region, specifically between 850 and 1,450 nt, as schematically presented in Fig. 3. In fact, the lack of a downstream element in such a large segment would be highly unlikely, in view of the fact that all or most mRNAs with premature nonsense mutations are subjected to the NMD pathway. Thus, it is reasonable to suggest that the same mechanism is used for degrading long cyc1-512 mRNAs and wild-type mRNAs that are targets of NMD. Our discovery of the involvement of the UPF1 gene in the degradation of long cyc1-512 mRNAs defines a novel class of substrates of the NMD pathway, which confirmed similar observations by Muhlrad and Parker (49). The cyc1-512 type of mRNA with extended 3′ sequences differs from the mRNAs subjected to NMD by leaky scanning within ORFs (82).
The reduction in mRNA level caused by a mutation or deletion of the poly(A) signals has also been observed in the r293 mutation of the unc-54 gene in Caenorhabditis elegans, which involves deletion of the poly(A) signal in the myosin heavy chain B mRNA (58), and in human thalassemias resulting from mutations in the conserved AAUAAA polyadenylation signal (26, 52, 64). In C. elegans, several smg mutations were shown to suppress the unc-54 (r293) mutation, resulting in near-normal amounts of the unc-54 (r293) mRNA (8, 58). Two other deletion mutations that removed the polyadenylation site, unc-17 (p1156) and unc-17 (md1447), were also suppressed by the smg suppressors (J. M. Rand, personal communication). One of the smg genes, smg-2, is homologous to the yeast UPF1 gene (53), suggesting conservation of a mechanism which restricts the levels of abnormal mRNAs.
Cbc1p-dependent mRNA degradation.
The identity of SUT1 and CBC1 established a role of the nuclear cap binding protein, Cbc1p, in mRNA degradation. CBC is composed of two proteins designated CBP80 and CBP20 in higher eukaryotes (28, 29, 34, 35, 51) and Cbc1p and Cbc2p, respectively, in yeast. Mutant forms of CBC1 and CBC2 have been uncovered in other genetic screens, and CBC1 has been previously designated not only SUT1 but also GCR3 (77, 78) and STO1 (11), whereas CBC2 has also been designated MUD13 (11). CBC binds to nuclear RNAs containing the capped 7-methyl-guanosine structure and, at least in mammalian cells, is involved in pre-RNA splicing, poly(A) site cleavage, and snRNA export (15, 28, 29, 34, 35, 51). Although CBC translocates through nuclear pore complexes attached to the 5′ ends of mRNPs in higher eukaryotes (81), it does not appear to play a direct role in their export. Similar to higher-eukaryotic CBC, yeast CBC is primarily located in the nucleus and functions in pre-RNA splicing (11, 43).
The requirement for CBC in pre-RNA splicing was revealed from the findings that immunodepletion of CBP80 greatly diminishes the activity in vitro of splicing extracts from higher-eukaryotic cells (29) and yeast (43). In addition, cbc2-Δ strains have diminished pre-mRNA splicing efficiency in vitro and the cbc2-Δ mutation is synthetically lethal with a U1 snRNA mutation (11). Similarly, evidence for the role of CBC in 3′-end formation was obtained by Flaherty et al. (15) using HeLa extracts with CBP80 immunodepleted. Cleavage of capped pre-RNA was inhibited to approximately 80%, whereas polyadenylation of a precleaved substrate was not significantly affected.
Although the primary sequence and the function in splicing of CBC are conserved from yeast to humans, CBC is not essential for growth in yeast. Growth of cbc1-Δ strains is severely retarded on glucose medium but only mildly diminished on glycerol and raffinose media. The dispensability of CBC in vivo can be explained by only a partial diminution of splicing and near-normal export of the bulk of polyadenylated RNAs in cbc1-Δ strains. Also, the role of CBC in the export of U snRNAs has not been demonstrated in yeast.
We have presented evidence that Cbc1p is involved in the rapid decay of long cyc1-512 mRNAs without affecting the decay rates of shorter 630- and 850-nt transcripts. Interestingly, the degradation of short cyc1-512 mRNAs followed at least two-component decay kinetics in a CBC1+ UPF1+ strain, indicative of heterogeneous mRNA molecules having different half-lives (Fig. 5A). We suggest that the long cyc1-512 mRNAs are undergoing rapid degradation in the CBC1+ UPF1+ strains by Cbc1p- and Upf1p-dependent degradation systems, and these partially degraded products of the long forms are situated at the same positions as the 630- and 850-nt bands, thus contributing to the heterogeneous mRNA molecules having different half-lives.
The major question resulting from this work is the nature of the mRNA degradation that is dependent on Cbc1p, or presumably on CBC, since both cbc1-Δ and cbc2-Δ equally suppressed cyc1-512. The decay of longer labile cyc1-512 mRNAs was diminished in both the cbc1-Δ and upf1-Δ strains, whereas the degradation of the transcripts containing premature nonsense codons, such as cyc1-72 mRNA and CYH2 pre-mRNA, was diminished in only the upf1-Δ strain. On the other hand, only the cbc1-Δ mutation stabilized all mRNAs arrested in the nucleus in the rat7-1 strain and slightly stabilized all mRNAs in RAT7+ normal strains.
The similarity in the degree of suppression of decay of the long cyc1-512 mRNAs by single upf1-Δ and cbc1-Δ strains and by the double upf1-Δ cbc1-Δ strains (Table 2) could suggest that Cbc1p and Upf1p are part of the same degradation pathway. In this regard it should be noted that the mRNA decay function can be separated from the activity modulating translation termination at nonsense codons (83, 84). For example, a subset of upf1 mutations which altered the Upf1p helicase region inactivated the decay activity but did not cause nonsense suppression (83), while another, different subset of upf1 mutations with alterations in an amino-terminal region of Upf1p suppressed certain nonsense mutations without preventing the NMD. Thus, it is reasonable to assume that deletion of certain components of the NMD pathway would diminish the decay properties without acting as suppressors. Hence, one could suggest that cbc1-2 or cbc1-Δ represents such a mutation that affects decay but not suppression of nonsense mutation and Cbc1p is part of the NMD pathway. One could further suggest that NMD takes place in both the nucleus and the cytoplasm but Cbc1p functions in this pathway solely in the nucleus. The finding that Upf3p is a nucleus- and cytosol-shuttling protein in yeast (74) and that NMD of newly synthesized mRNA copurifies with nuclei of mammalian cells (90–92) is consistent with this view.
However, the following lines of evidence indicate that NMD occurs in yeast with transcripts translated on cytoplasmic polysomes: Upf1p, Upf2p, and Upf3p are associated with polysomes (3, 4, 55, 56); NMD can be suppressed with tRNA nonsense suppressors (5, 18, 45); a cycle of initiation and termination is required for NMD, and translation reinitiation can prevent activation of NMD (61, 63, 87, 90); and nonsense codon-containing RNAs lose and regain their decay properties by the addition and removal, respectively, of the translational inhibitor cycloheximide (90). We favor the view that Cbc1p-dependent degradation is independent of NMD, because there is no evidence that CBC is associated with mRNAs during translation on polysomes and because CBC is primarily situated in the nucleus with only a brief exposure in the cytoplasm as part of the nuclear transport cycle. Furthermore, the decay of CYH2 pre-mRNA in rat7-1 strains was not specifically diminished in cbc1-Δ strains, which is one of the natural substrates for NMD (Fig. 8A and Table 4), indicating that Cbc1p is not involved in the degradation of substrates containing premature nonsense codons.
Thus, we suggest that Cbc1p-dependent degradation takes place in the nucleus and may be related to the stimulatory effect of CBC on pre-mRNA processing, including splicing and cleavage during 3′-end formation. We also argue that all mRNAs located in the nucleus are degraded by the Cbc1p-dependent pathway and that the net destruction of a particular mRNA is dependent on the length of time spent in the nucleus. Even though there is no direct evidence, we hypothesize that the long cyc1-512 mRNAs are preferentially retained in the nucleus and we further suggest that this retention in the nucleus is responsible for their high susceptibility to the Cbc1p-dependent degradation system.
The hypothesis that Cbc1p is part of a degradation system acting on mRNAs retained in the nucleus is also supported by the suppression of rat7-1 by cbc1-Δ. Rat7p is an essential nucleoporin 1,450 amino acids long that apparently plays a direct role in the nucleocytoplasmic export of mRNA (13, 17). The rat7-1 mutation is a single-base pair change introducing a premature stop codon approximately 100 amino acids upstream from the C terminus (13). After rat7-1 cells are shifted to 37°C, the restrictive temperature, there is a cessation of growth and of mRNA export, resulting in a diminished level of cytoplasmic mRNAs. We have observed that the stabilities of CYH2 mRNA and pre-mRNA, ACT1 mRNA, and presumably other mRNAs were greatly diminished in the rat7-1 mutants grown at 37°C compared to those in a corresponding isogenic RAT7+ strain (Fig. 8), a condition that allows growth of the RAT7+ strain. Most importantly, cbc1-Δ rescues both the rat7-1 phenotypes, lack of growth (Fig. 9) and instability of ACT1 and CYH2 mRNAs and CYH2 pre-mRNA at 37°C (Fig. 8).
While the cbc1-Δ suppression of rat7-1 at 37°C can be interpreted in several ways, including the suppression of the diminished export, we favor the view that nuclear retention of mRNAs in rat7-1 strains results in increased degradation by the action of the Cbc1p-dependent degradation system. This nuclear degradation leads to diminished levels of mRNAs that are available for the already abnormally reduced export to the cytoplasm. In other words, the suppression of rat7-1 by cbc1-Δ could be envisioned as enhancing the levels of nuclear mRNAs and thus allowing the defective transport system to produce a higher level of cytoplasmic mRNAs required for viability. Recently, Uemura et al. (78) reported that a cbc1 mutation suppressed the temperature-sensitive growth of hpr1 mutants, which are conditionally defective in the nuclear export of poly(A) RNA (68). We believe that cbc1 suppression of both hpr1 and rat7-1 may be operating by the same mechanism. It remains to be seen if cbc1 suppression of hpr1 and rat7-1 is due to diminished degradation of mRNAs retained in the nucleus or to reversal of the mRNA transport defects.
There are a few known ribonucleases that possibly could be components of the Cbc1p-dependent nuclear degradation system. Two possible candidates are Rrp6p, which is a nuclear 3′→5′ riboexonuclease (6, 7), and Rat1p, which is a nuclear 5′→3′ riboexonuclease (2, 33, 37, 57, 75). Burkard and Butler (7) suggested that Rrp6p plays a role in a nuclear RNA decay pathway that destroys slowly processed mRNAs. Furthermore, two-hybrid analyses and coimmunoprecipitation experiments demonstrated that Rrp6p interacts with Npl3p and Cbc1p (7; K. T. Burkard and J. S. Butler, personal communication). Further studies, which are in progress, would reveal the role of Cbc1p and other components in the nuclear degradation system.
Promiscuous 3′-end formation.
The finding of higher levels of 630- and 850-nt cyc1-512 mRNAs in cbc1-Δ mutants, without a corresponding increase in the half-lives (Table 3), suggested that the enhanced levels were due to more efficient 3′-end formation at these normally weak sites. Extensive work (reviewed in references 19 and 20) has established that 3′-end-forming signals in yeast consist of three elements: (i) the efficiency element, which enhances the efficiency of downstream positioning elements; (ii) the positioning element, which positions the poly(A) site; and (iii) the actual poly(A) site. These three elements are not only necessary but also sufficient for mRNA 3′-end formation in yeast. However, when the efficiency element is absent or lacks the optimum sequence, low levels of mRNA having the corresponding poly(A) site are still observed. It is believed that a portion of the mRNA molecules terminate at this site, whereas the bulk of the mRNA bypasses the site but can terminate downstream if a stronger signal is present. We used the term “preferred sites” to denote these discrete sites of mRNA 3′ ends in the cyc1-512 mutant that are used by the revertants and the normal CYC1+ strain (65). Apparently, the cbc1-Δ mutation enhances 3′-end formation at the 630- and 850-nt preferred sites, thereby acting as a suppressor of cyc1-512 without affecting the half-lives of these short mRNAs.
Numerous trans-acting factors have been shown to play roles in 3′-end formation in yeast, including approximately 20 gene products that have direct roles in the processing of pre-mRNAs in vitro (36). In particular, Hrp1p appears to stabilize the assembly of the cleavage complex at an authentic poly(A) site (47). Furthermore, Hrp1p directly interacts with the UAUAUA efficiency element (10, 38, 80). Our results with enhanced 3′-end formation at the 630- and 850-nt preferred sites in the cyc1-512 mRNAs lacking the efficiency elements suggest that CBC plays a role in the fidelity of this interaction and that promiscuous 3′-end formation can occur with mRNAs lacking the nuclear cap binding proteins.
Thus, studies with the upf1-Δ, cbc1-Δ, and cbc2-Δ mutants led to the following major conclusions: transcripts with extended 3′ untranslated regions could be recognized as substrates for NMD, even though the mRNAs lack a premature termination codon in the ORF; Cbc1p and Cbc2p are components of a novel mRNA decay pathway, which presumably acts in the nucleus; and Cbc1p and Cbc2p are required for high-fidelity 3′-end formation at certain sites. It is reasonable to suggest that either the upf1-Δ or cbc1-Δ mutations preferentially affect the levels of certain normal mRNAs. For example, cbc1-Δ could cause the overproduction of certain normal genes that are usually subjected to degradation by a Cbc1p-dependent pathway. Abnormal mRNA levels could account for diversed and unrelated phenotypes, such as inhibition of growth by glucose-repressing conditions (see above) (77, 78) and increase in the negative superhelicity of plasmids (78).
ACKNOWLEDGMENTS
We thank Michael R. Culbertson (University of Wisconsin) for the UPF1 disrupter and other UPF1 plasmids; Charles N. Cole (Dartmouth Medical School) for the rat7-1 and RAT7+ strains; V. Raju (Department of Cardiology, University of Rochester) for advice on Northern blot analysis; Edward Pagani (Pfizer Inc., Groton, Conn.) for a generous gift of thiolutin; Jay R. Greenberg (Department of Biochemistry and Biophysics, University of Rochester), Ding-Fang Yun (Cadus Pharmaceutical Corp., Tarrytown, N.Y.), Alan Sachs (University of California, Berkeley), Roy Parker (University of Arizona), Scott Butler (University of Rochester), Stuart Peltz (University of Medicine and Dentistry of New Jersey), and Allan Jacobson (University of Massachusetts Medical School) for helpful discussions; and Satarupa Das for assistance in the preparation of the cbc2-Δ disruptant.
This investigation was supported by NIH research grant GM12702 (to F.S.) and by an FCAR Canadian Postdoctoral Fellowship (to P.C.).
REFERENCES
- 1.Alani E, Cao L, Kleckner N. Method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics. 1987;116:541–545. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amberg D C, Goldstein L A, Cole C N. Isolation and characterization of RAT1: an essential gene of Saccharomyces cerevisiae required for the efficient nucleocytoplasmic trafficking of mRNA. Genes Dev. 1992;6:1173–1189. doi: 10.1101/gad.6.7.1173. [DOI] [PubMed] [Google Scholar]
- 3.Atkin A L, Schenkman L R, Eastham M, Dahlseid J N, Lelivelt M J, Culbertson M R. Relationship between yeast polyribosomes and Upf proteins required for nonsense mRNA decay. J Biol Chem. 1997;272:22163–22172. doi: 10.1074/jbc.272.35.22163. [DOI] [PubMed] [Google Scholar]
- 4.Atkin A L, Altamura N, Leeds P, Culbertson M R. The majority of yeast UPF1 co-localizes with polyribosomes in the cytoplasm. Mol Biol Cell. 1995;6:611–625. doi: 10.1091/mbc.6.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belgrader P, Cheng J, Maquat L E. Evidence to implicate translation by ribosomes in the mechanism by which nonsense codons reduce the nuclear level of human triosephosphate isomerase mRNA. Proc Natl Acad Sci USA. 1993;90:482–486. doi: 10.1073/pnas.90.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briggs M W, Burkard K T, Butler J S. Rrp6p, the yeast homologue of the human PM-Scl 100-kDa autoantigen, is essential for efficient 5.8S rRNA 3′ end formation. J Biol Chem. 1998;273:13255–13263. doi: 10.1074/jbc.273.21.13255. [DOI] [PubMed] [Google Scholar]
- 7.Burkard K T, Butler J S. A nuclear 3′-5′ exonuclease involved in mRNA degradation interacts with poly(A)-polymerase and the hnRNA protein Npl3p. Mol Cell Biol. 2000;20:604–616. doi: 10.1128/mcb.20.2.604-616.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cali B M, Kuchma S L, Latham J, Anderson P. smg-7 is required for mRNA surveillance in C. elegans. Genetics. 1999;151:605–616. doi: 10.1093/genetics/151.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caponigro G, Parker R. Mechanisms and control of mRNA turnover in Saccharomyces cerevisiae. Microbiol Rev. 1996;60:233–249. doi: 10.1128/mr.60.1.233-249.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S, Hyman L E. A specific RNA-protein interaction at yeast polyadenylation efficiency elements. Nucleic Acids Res. 1998;26:4965–4974. doi: 10.1093/nar/26.21.4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colot H V, Stutz F, Rosbash M. The yeast splicing factor Mud13p is a commitment complex component and corresponds to CBP20, the small subunit of the nuclear cap-binding complex. Genes Dev. 1996;10:1699–1708. doi: 10.1101/gad.10.13.1699. [DOI] [PubMed] [Google Scholar]
- 12.Culbertson M R. RNA surveillance, unforeseen consequences for gene expression, inherited genetic disorders and cancer. Trends Genet. 1999;15:74–80. doi: 10.1016/s0168-9525(98)01658-8. [DOI] [PubMed] [Google Scholar]
- 13.Del Priore V, Heath C, Snay C, MacMillan A, Gorsch L, Dagher S, Cole C. A structure/function analysis of Rat7p/Nup159p, an essential nucleoporin of Saccharomyces cerevisiae. J Cell Sci. 1997;110:2987–2999. doi: 10.1242/jcs.110.23.2987. [DOI] [PubMed] [Google Scholar]
- 14.Dumont M E, Mathews A J, Nall B T, Eustice D C, Sherman F. Differential stability of two apo-isocytochrome c in the yeast Saccharomyces cerevisiae. J Biol Chem. 1990;256:2733–2739. [PubMed] [Google Scholar]
- 15.Flaherty S M, Fortes P C E, Mattaj I W, Gilmartin G M. Participation of the nuclear cap binding complex in pre-mRNA 3′ processing. Proc Natl Acad Sci USA. 1997;94:11893–11898. doi: 10.1073/pnas.94.22.11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Görlich D, Kraft R, Kostka S, Vogel F, Hartmann E, Laskey R A, Mattaj I W, Izaurralde E. Importin provides a link between nuclear protein import and U snRNA export. Cell. 1996;87:21–32. doi: 10.1016/s0092-8674(00)81319-7. [DOI] [PubMed] [Google Scholar]
- 17.Gorsch L C, Dockerdorff T C, Cole C N. A conditional allele of the novel repeat-containing yeast nucleoporin RAT7/NUP159 causes both rapid cessation of mRNA export and reversible clustering of nuclear pore complexes. J Cell Biol. 1995;129:939–955. doi: 10.1083/jcb.129.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gozalbo D, Hohmann S. Nonsense suppression partially reverts the decrease of mRNA levels of a nonsense mutant allele in yeast. Curr Genet. 1990;17:77–79. doi: 10.1007/BF00313252. [DOI] [PubMed] [Google Scholar]
- 19.Guo Z, Sherman F. Signals sufficient for 3′ end formation of yeast mRNA. Mol Cell Biol. 1996;16:2772–2776. doi: 10.1128/mcb.16.6.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo Z, Sherman F. 3′ End forming signals of yeast mRNA. Trends Biochem Sci. 1996;21:477–481. doi: 10.1016/s0968-0004(96)10057-8. [DOI] [PubMed] [Google Scholar]
- 21.Hagan K W, Ruiz-Echevarria M J, Quan Y, Peltz S W. Characterization of cis-acting sequences and decay intermediates involved in nonsense-mediated mRNA turnover. Mol Cell Biol. 1995;15:809–823. doi: 10.1128/mcb.15.2.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 23.He F, Peltz S W, Donahue J L, Rosbash M, Jacobson A. Stabilization and ribosome association of unspliced pre-mRNAs in a yeast upf1− mutant. Proc Natl Acad Sci USA. 1993;90:7034–7038. doi: 10.1073/pnas.90.15.7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrick D, Parker R, Jacobson A. Identification and comparison of stable and unstable mRNAs in Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:2269–2284. doi: 10.1128/mcb.10.5.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hickey D R, Jayaraman K, Goodhue C T, Shah J, Clements J M, Tsunasawa S, Sherman F. Synthesis and expression of genes encoding tuna, pigeon, and horse cytochromes c in the yeast Saccharomyces cerevisiae. Gene. 1991;105:73–81. doi: 10.1016/0378-1119(91)90515-d. [DOI] [PubMed] [Google Scholar]
- 26.Higgs D R, Goodbourn S E Y, Lamb J, Clegg J B, Weatherall D J. α-Thalassemia caused by a polyadenylation signal mutation. Nature. 1983;306:398–400. doi: 10.1038/306398a0. [DOI] [PubMed] [Google Scholar]
- 27.Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izaurralde E, Stepinski J, Darzynkiewicz E, Mattaj I W. A cap binding protein that may mediate nuclear export of RNA polymerase II-transcribed RNAs. J Cell Biol. 1992;118:1287–1295. doi: 10.1083/jcb.118.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Izaurralde E, Lewis J, McGuigan C, Jankowska M, Darzynkiewicz E, Mattaj I W. A nuclear cap binding protein complex involved in pre-mRNA splicing. Cell. 1994;78:657–668. doi: 10.1016/0092-8674(94)90530-4. [DOI] [PubMed] [Google Scholar]
- 30.Jackson R J, Standart N. Do the poly(A) tail and 3′ untranslated region control mRNA translation? Cell. 1990;62:15–24. doi: 10.1016/0092-8674(90)90235-7. [DOI] [PubMed] [Google Scholar]
- 31.Jacobson A. Poly(A) metabolism and translation: the closed loop model. In: Hershey J, Mathews M, Sonenberg N, editors. Translational control. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1995. pp. 451–480. [Google Scholar]
- 32.Jacobson A, Peltz S W. Interrelationships of the pathways of mRNA decay and translation in eukaryotic cells. Annu Rev Biochem. 1996;65:693–739. doi: 10.1146/annurev.bi.65.070196.003401. [DOI] [PubMed] [Google Scholar]
- 33.Johnson A W. Rat1p and Xrn1p are functionally interchangeable exoribonucleases that are restricted to and required in the nucleus and cytoplasm, respectively. Mol Cell Biol. 1997;17:6122–6130. doi: 10.1128/mcb.17.10.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kataoka N, Ohno M, Moda I, Shimura Y. Identification of the factors that interact with NCBP, an 80 kDa nuclear cap binding protein. Nucleic Acids Res. 1995;23:3638–3641. doi: 10.1093/nar/23.18.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kataoka N, Ohno M, Kangawa K, Tokoro Y, Shimura Y. Cloning of a complementary DNA encoding an 80 kilodalton nuclear cap binding protein. Nucleic Acids Res. 1994;22:3861–3865. doi: 10.1093/nar/22.19.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keller W, Minvielle-Sebastia L. A comparison of mammalian and yeast pre-mRNA 3′-end processing. Curr Opin Cell Biol. 1997;9:329–336. doi: 10.1016/s0955-0674(97)80004-x. [DOI] [PubMed] [Google Scholar]
- 37.Kenna M, Stevens A, McCaammon M, Douglas M G. An essential yeast gene with homology to the exonuclease-encoding XRN1/KEM1 gene also encodes a protein with exoribonuclease activity. Mol Cell Biol. 1993;13:341–350. doi: 10.1128/mcb.13.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kessler M M, Henry M F, Shen E, Zhao J, Gross S, Silver P A, Moore C L. Hrp1, a sequence-specific RNA-binding protein that shuttles between the nucleus and the cytoplasm, is required for mRNA 3′-end formation in yeast. Genes Dev. 1997;11:2545–2556. doi: 10.1101/gad.11.19.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kotval J, Zaret K S, Consaul S, Sherman F. Revertants of a transcription termination mutant of yeast contain diverse genetic alterations. Genetics. 1983;103:367–388. doi: 10.1093/genetics/103.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kunkel T A, Roberts J D, Zakour R A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 41.Leeds P, Peltz S W, Jacobson A, Culbertson M R. The product of the yeast UPF1 gene is required for rapid turnover of mRNAs containing a premature translational termination codon. Genes Dev. 1991;5:2303–2314. doi: 10.1101/gad.5.12a.2303. [DOI] [PubMed] [Google Scholar]
- 42.Leeds P, Wood J M, Lee B S, Culbertson M R. Gene products that promote mRNA turnover in Saccharomyces cerevisiae. Mol Cell Biol. 1992;12:2165–2177. doi: 10.1128/mcb.12.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewis J, Görlich D, Mattaj I W. A yeast cap binding protein complex (yCBC) acts at an early step in pre-mRNA splicing. Nucleic Acids Res. 1996;24:3332–3336. doi: 10.1093/nar/24.17.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li W-Z, Sherman F. Two types of TATA elements for the CYC1 gene of the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:666–676. doi: 10.1128/mcb.11.2.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Losson R, Lacroute F. Interference of nonsense mutations with eukaryotic messenger RNA stability. Proc Natl Acad Sci USA. 1979;76:5134–5137. doi: 10.1073/pnas.76.10.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maquat L E. When cells stop making sense: effects of nonsense codons on RNA metabolism in vertebrate cells. RNA. 1995;1:453–465. [PMC free article] [PubMed] [Google Scholar]
- 47.Minvielle-Sebastia L, Beyer K, Krecic A M, Hector R E, Swanson M S, Keller W. Control of cleavage site selection during mRNA 3′ end formation by a yeast hnRNP. EMBO J. 1998;17:7454–7468. doi: 10.1093/emboj/17.24.7454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muhlrad D, Parker R. Premature translation termination triggers mRNA decapping. Nature. 1994;340:578–581. doi: 10.1038/370578a0. [DOI] [PubMed] [Google Scholar]
- 49.Muhlrad D, Parker R. Aberrant mRNAs with extended 3′ UTRs are substrates for rapid degradation by mRNA surveillance. RNA. 1999;5:1299–1307. doi: 10.1017/s1355838299990829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Munroe D, Jacobson A. mRNA poly(A) tail, a 3′ enhancer of translational initiation. Mol Cell Biol. 1990;10:3441–3455. doi: 10.1128/mcb.10.7.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohno M, Kataoka N, Shimura Y. A nuclear cap binding protein from HeLa cells. Nucleic Acids Res. 1990;18:6989–6995. doi: 10.1093/nar/18.23.6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Orkin S H, Cheng T C, Antnarakis S E, Kazazian H H. Thalassemia due to a mutation in the cleavage-polyadenylation signal of the human β-globin gene. EMBO J. 1985;4:453–456. doi: 10.1002/j.1460-2075.1985.tb03650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Page M F, Carr B, Anders K R, Grimson A, Anderson P. SMG-2 is a phosphorylated protein required for mRNA surveillance in Caenorhabditis elegans and related to Upf1p of yeast. Mol Cell Biol. 1999;19:5943–5951. doi: 10.1128/mcb.19.9.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pelsy F, Lacroute F. Effect of ochre nonsense mutations on yeast URA1 mRNA stability. Curr Genet. 1984;8:277–282. doi: 10.1007/BF00419725. [DOI] [PubMed] [Google Scholar]
- 55.Peltz S W, Brown A H, Jacobson A. mRNA destabilization triggered by premature translational termination depends on at least three cis-acting sequence elements and one trans-acting factor. Genes Dev. 1993;7:1737–1754. doi: 10.1101/gad.7.9.1737. [DOI] [PubMed] [Google Scholar]
- 56.Peltz S W, He F, Welch E, Jacobson A. Nonsense-mediated mRNA decay in yeast. Prog Nucleic Acid Res Mol Biol. 1994;47:271–298. doi: 10.1016/s0079-6603(08)60254-8. [DOI] [PubMed] [Google Scholar]
- 57.Poole T L, Stevens A. Comparison of features of the RNase activity of 5′-exonuclease 1 and 5′-exonuclease 2 of Saccharomyces cerevisiae. Nucleic Acid Symp Ser. 1995;33:79–81. [PubMed] [Google Scholar]
- 58.Pulak R, Anderson P. mRNA surveillance by the Caenorhabditis elegans smg genes. Genes Dev. 1993;7:1885–1897. doi: 10.1101/gad.7.10.1885. [DOI] [PubMed] [Google Scholar]
- 59.Rose M D, Novick P, Thomas J H, Botstein D, Fink G R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60:237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- 60.Rothstein R J. One step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- 61.Ruiz-Echevarría M J, Peltz S W. Utilizing the GCN4 leader region to investigate the role of the sequence determinants in nonsense-mediated mRNA decay. EMBO J. 1996;15:2810–2819. [PMC free article] [PubMed] [Google Scholar]
- 62.Ruiz-Echevarría M J, Czaplinski K, Peltz S W. Making sense of nonsense in yeast. Trends Biochem Sci. 1996;21:433–438. doi: 10.1016/s0968-0004(96)10055-4. [DOI] [PubMed] [Google Scholar]
- 63.Ruiz-Echevarría M J, Yasenchak J M, Han X, Dinman J D, Peltz S W. The upf3 protein is a component of the surveillance complex that monitors both translation and mRNA turnover and affects viral propagation. Proc Natl Acad Sci USA. 1998;95:8721–8726. doi: 10.1073/pnas.95.15.8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rund D, Dowling C, Najjar K, Rachmilewitz E A, Kazazian H H, Oppenheim A. Two mutations in the β-globin polyadenylation signal reveal extended transcripts and new RNA polyadenylation sites. Proc Natl Acad Sci USA. 1992;89:4324–4328. doi: 10.1073/pnas.89.10.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Russo P, Li W-Z, Hampsey D M, Zaret K S, Sherman F. Distinct cis-acting signals enhance 3′ endpoint formation of CYC1 mRNA in the yeast Saccharomyces cerevisiae. EMBO J. 1991;10:563–571. doi: 10.1002/j.1460-2075.1991.tb07983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Russo P, Li W-Z, Guo Z, Sherman F. Signals that produce 3′ termini in CYC1 mRNA of the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:7836–7849. doi: 10.1128/mcb.13.12.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 68.Schneiter R, Guerra C E, Lampl M, Gogg G, Kohlwein S D, Klein H. The Saccharomyces cerevisiae hyperrecombination mutant hpr1Δ is synthetically lethal with two conditional alleles of the acetyl coenzyme A carboxylase gene and causes a defect in nuclear export of polyadenylated mRNA. Mol Cell Biol. 1999;19:3415–3422. doi: 10.1128/mcb.19.5.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shen W-C, Selvakumar D, Stanford D R, Hopper A K. The Saccharomyces cerevisiae LOS1 gene involved in pre-tRNA splicing encodes a nuclear protein that behaves as a component of the nuclear matrix. J Biol Chem. 1993;268:19436–19444. [PubMed] [Google Scholar]
- 70.Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- 71.Sherman F, Stewart J W, Jackson M, Gilmore R A, Parker J K. Mutants of yeast defective in iso-1-cytochrome c. Genetics. 1974;77:255–284. doi: 10.1093/genetics/77.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sherman F, Fink G R, Hicks J B. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1986. [Google Scholar]
- 73.Spingola M, Grate L, Haussler D, Ares M., Jr Genome-wide bioinformatic and molecular analysis of introns in Saccharomyces cerevisiae. RNA. 1999;5:221–234. doi: 10.1017/s1355838299981682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shirley R L, Lelivelt M J, Schenkman L R, Dahlseid J N, Culbertson M R. A factor required for nonsense-mediated mRNA decay in yeast is exported from the nucleus to the cytoplasm by a nuclear export signal sequence. J Cell Sci. 1998;111:3129–3143. doi: 10.1242/jcs.111.21.3129. [DOI] [PubMed] [Google Scholar]
- 75.Stevens A, Poole T L. 5′-exonuclease-2 of Saccharomyces cerevisiae. Purification and features of ribonuclease activity with comparison to 5′-exonuclease-1. J Biol Chem. 1995;270:16063–16069. doi: 10.1074/jbc.270.27.16063. [DOI] [PubMed] [Google Scholar]
- 76.Studier F W, Moffatt R A. Use of bacteriophage T7 polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 77.Uemura H, Jigmi Y. GCR3 encodes an acidic protein that is required for expression of glycolytic genes in Saccharomyces cerevisiae. J Bacteriol. 1992;174:5526–5532. doi: 10.1128/jb.174.17.5526-5532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Uemura H, Pandit S, Jigmi Y, Sternglanz R. Mutations in GCR3, a gene involved in the expression of glycolytic genes in Saccharomyces cerevisiae, suppress the temperature growth of hpr1 mutants. Genetics. 1996;142:1095–1103. doi: 10.1093/genetics/142.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Underwood M R, Fried H M. Characterization of nuclear localizing sequences derived from yeast ribosomal protein L29. EMBO J. 1990;9:91–99. doi: 10.1002/j.1460-2075.1990.tb08084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Valentini S, Weiss V, Silver P. Arginine methylation and binding of Hrp1p to the efficiency element for 3′-end formation. RNA. 1999;5:272–280. doi: 10.1017/s1355838299981633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Visa N, Izaurralde E, Ferreira J, Daneholt B, Mattaj I W. A nuclear cap-binding complex binds Balbiani ring pre-mRNA cotranscriptionally and accompanies the ribonucleoprotein particle during nuclear export. J Cell Biol. 1996;133:5–14. doi: 10.1083/jcb.133.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Welch E M, Jacobson A. An internal open reading frame triggers nonsense-mediated decay of the yeast SPT10 mRNA. EMBO J. 1999;18:6134–6145. doi: 10.1093/emboj/18.21.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weng Y, Czaplinski K, Peltz S W. Genetic and biochemical characterization of mutations in the ATPase and helicase regions of the Upf1 protein. Mol Cell Biol. 1996;16:5477–5490. doi: 10.1128/mcb.16.10.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weng Y, Czaplinski K, Peltz S W. Identification and characterization of mutations in the UPF1 gene that affect nonsense suppression and the formation of the Upf protein complex but not mRNA turnover. Mol Cell Biol. 1996;16:5491–5506. doi: 10.1128/mcb.16.10.5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weng Y, Ruiz-Echevarría M J, Zhang S, Chu Y, Czaplinski K, Dinman J D, Peltz S W. Characterization of the nonsense-mediated mRNA decay pathway and its effect on modulating translation termination and programmed frameshifting. Mol Cell Biol. 1997;17:241–263. [Google Scholar]
- 86.Woolford J L., Jr Nuclear pre-mRNA splicing in yeast. Yeast. 1989;5:439–457. doi: 10.1002/yea.320050604. [DOI] [PubMed] [Google Scholar]
- 87.Yun D-F, Sherman F. Initiation of translation can occur only in a restricted region of the CYC1 mRNA of Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:1021–1033. doi: 10.1128/mcb.15.2.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zaret K S, Sherman F. DNA sequence required for efficient transcription termination in yeast. Cell. 1982;28:563–573. doi: 10.1016/0092-8674(82)90211-2. [DOI] [PubMed] [Google Scholar]
- 89.Zaret K S, Sherman F. Mutationally altered 3′ ends of yeast CYC1 mRNA affects transcript stability and translational efficiency. J Mol Biol. 1984;177:107–135. doi: 10.1016/0022-2836(84)90060-3. [DOI] [PubMed] [Google Scholar]
- 90.Zhang J, Maquat L E. Evidence that translation reinitiation abrogates nonsense-mediated mRNA decay in mammalian cells. EMBO J. 1997;16:826–833. doi: 10.1093/emboj/16.4.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang J, Sun X, Qian Y, LaDuca J P, Maquat L E. At least one intron is required for the nonsense-mediated decay of triosephosphate isomerase mRNA: a possible link between nuclear splicing and cytoplasmic translation. Mol Cell Biol. 1998;18:5272–5283. doi: 10.1128/mcb.18.9.5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang J, Sun X, Qian Y, Maquat L E. Intron function in the nonsense-mediated decay of β-globin mRNA: indications that pre-mRNA splicing in the nucleus can influence mRNA translation in the cytoplasm. RNA. 1998;4:801–815. doi: 10.1017/s1355838298971849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang S, Ruiz-Echevarría M J, Quan Y, Peltz S W. Identification and characterization of a sequence motif involved in nonsense-mediated mRNA decay. Mol Cell Biol. 1995;15:2231–2244. doi: 10.1128/mcb.15.4.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]