Abstract
The aim of this study was to characterize the phenotype and to identify the genetic etiology of a syndromic form of ichthyosis congenita (IC) observed in Italian Chianina cattle and to estimate the prevalence of the deleterious allele in the population. Sporadic occurrence of different forms of ichthyosis including IC have been previously reported in cattle. However, so far, no causative genetic variant has been found for bovine IC. Nine affected cattle presenting congenital xerosis, hyperkeratosis and scaling of the skin as well as urolithiasis and cystitis associated with retarded growth were examined. Skin histopathology revealed a severe, diffuse orthokeratotic hyperkeratosis with mild to moderate epidermal hyperplasia. The pedigree records indicated a monogenic recessive trait. Homozygosity mapping and whole-genome sequencing allowed the identification of a homozygous frameshift 1 bp insertion in the FA2H gene (c.9dupC; p.Ala4ArgfsTer142) located in a 1.92 Mb shared identical-by-descent region on chromosome 18 present in all cases, while the parents were heterozygous as expected for obligate carriers. These findings enable the selection against this sub-lethal allele showing an estimated frequency of ~ 7.5% in Chianina top sires. A sporadic incidence of mild clinical signs in the skin of heterozygous carriers was observed. So far, pathogenic variants affecting the encoded fatty acid 2-hydroxylase catalyzing the synthesis of 2-hydroxysphingolipids have been associated with myelin disorders. In conclusion, this study represents the first report of an FA2H-related autosomal recessive inherited skin disorder in a mammalian species and adds FA2H to the list of candidate genes for ichthyosis in humans and animals. Furthermore, this study provides a DNA-based diagnostic test that enables selection against the identified pathogenic variant in the Chianina cattle population. However, functional studies are needed to better understand the expression of FA2H in IC-affected Chianina cattle.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00438-021-01824-8.
Keywords: Bovine, Genodermatoses, Fatty acid 2-hydroxylase, Precision medicine, Skin, Urolithiasis
Introduction
The aim of this study was to report a series of nine cases of IC in Chianina cattle, to characterize the clinicopathological phenotype and finally to present the results of the genetic analysis that evidenced a homozygous frameshift variant in bovine FA2H gene. Moreover, the prevalence of the deleterious allele in a selected population of Chianina sires is also estimated.
Genodermatoses are sporadic inherited disorders of the skin that both in humans and in livestock animals mostly follow a monogenic mode of inheritance (Leeb et al. 2017; Jacinto et al. 2020; Pope 2020).
In human medicine, the concept of ‘genodermatosis with skin fragility’ was recently introduced by adding other genetic disorders with skin fragility such erosive or hyperkeratotic disorders (Pope 2020). Inherited ichthyosis characterized by an abnormal terminal keratinocyte differentiation belongs to this group of skin fragility disorders encompassing a clinically, pathologically and heritably heterogeneous presentation with a thickened stratum corneum resulting in localized or generalized scaling (Marukian and Choate 2016).
In human medicine, the classification of the different forms of ichthyosis is based on clinicopathological manifestations and mode of inheritance, being divided in two main types: non-syndromic forms when clinical findings are limited to the skin, and syndromic forms in case additional organs are involved (Oji et al. 2010). In this respect, ichthyosis has been associated to pathogenic variants in more than 30 genes that are involved in several cellular functions, such as DNA repair, lipid biosynthesis, adhesion and desquamation (Oji et al. 2010). In particular, recent advances have reinforced the causative role of mutations in genes encoding proteins essential to the formation of the hydrophobic barrier (Marukian and Choate 2016).
In domestic animals, ichthyosis has been described in dogs (Credille et al. 2009; Grall et al. 2012; Metzger et al. 2015; Bauer et al. 2017; Casal et al. 2017), pigs (Wang et al. 2019), sheep (Câmara et al. 2017) and cattle (Charlier et al. 2008; Woolley et al. 2019; Eager et al. 2020). Furthermore, it has also been reported in greater kudu calves (Chittick et al. 2002). While in sheep, pigs and greater kudu the underlying genetic cause of this condition has not been determined, in dogs pathogenic variants have been identified in five different candidate genes associated with the phenotype, four breed specific recessive inherited forms (TGM1, SLC27A4, PNPLA1, NIPAL4) (OMIA 000546-9615; OMIA 001973-9615; OMIA 001588-9615; OMIA 001980-9615) as well as a single dominant inherited form in a single affected dog (ASPRV1) (OMIA 002099-9615).
In Chianina, Shorthorn and Polled Hereford cattle, a form of ichthyosis named ichthyosis fetalis, which resembles the Harlequin-type ichthyosis described in human medicine, has been associated with recessively inherited mutations in ABCA12 (OMIA 002238-9913) (Charlier et al. 2008; Woolley et al. 2019; Eager et al. 2020). Affected calves are stillborn or die within the first days after birth and the skin is diffusely covered with large horny plates separated by deep fissures and resembling a ‘leather cuirass’. Furthermore, eversion at mucocutaneous junctions provokes eclabium and ectropion (Chittick et al. 2002; Molteni et al. 2006).
In Chianina cattle, a second less severe form of ichthyosis, named ichthyosis congenita (IC), has also been described (Testoni et al. 2006) and in subsequent time repeatedly presented to the authors. Animals with IC show milder but comparable lesions to those of ichthyosis fetalis. It is clinically characterized by a more or less extended scale-like hyperkeratosis and multifocal alopecic areas, and histopathologically by a diffuse lamellar orthokeratotic hyperkeratosis. The underlying genetic cause of this form of syndromic form of ichthyosis associated with retarded growth is unknown.
Methods
Animals
This study did not require official or institutional ethical approval as it was not experimental, but rather part of clinical and pathological veterinary diagnostics. All animals in this study were examined with the consent of their owners and handled according to good ethical standards. It deals with a total of 129 Chianina cattle, including 9 IC-affected animals, 4 dams, 6 sires and 110 artificial insemination (AI) top sires. The tenth affected animal included in the study (case 10) was the one previously reported by Testoni et al. (2006), whose blood had at that time been frozen and therefore had remained available for genetic studies.
Clinical and pathological investigations
Eight calves (cases 1–8) and one heifer (case 9) presenting cutaneous hyperkeratosis and retarded growth were recorded by the teaching hospital of the Department of Veterinary Medical Sciences, University of Bologna between 2005 and 2020 (Online Resource 1). The mean age of record of the calves was 2.6 months (minimum–maximum: 2 days–7 months), whereas the heifer was 18 months. The mean age at death was 11.2 months (natural death, euthanasia or slaughtering). All affected animals and one dam (case 8’s dam) were thoroughly clinically examined. Information related to the skin condition of the other dams as well as of the sires were obtained by interviewing the owners or the breeders’ association, respectively.
A parasitological test for detection of ectoparasites and fungi infection was performed on case 8’s dam.
Skin biopsies using an 8 mm punch were obtained from seven affected animals (cases 1–3 and cases 6, 8, 9) and from case 8’s dam. The collected samples were fixed in 10% neutral buffered formalin, trimmed, processed, embedded in paraffin wax, sectioned at 4 µm, and stained with hematoxylin and eosin (H&E) for further histological evaluation. Two affected animals (cases 2 and 8) were submitted to necropsy.
Pedigree design
Pedigree analysis was performed using Pedigraph version 2.4 software (Department of Animal Science, University of Minnesota, USA).
DNA extractions
Genomic DNA was extracted from the IC-affected animals (EDTA blood samples), four related dams (EDTA blood samples) and six related sires (semen) using Promega Maxwell RSC DNA system (Promega, Dübendorf, Switzerland). Furthermore, genomic DNA was also obtained from semen of 110 Chianina AI top sires with the same methodology.
SNP array genotyping and homozygosity mapping
High‐density SNP genotyping was carried out for seven cases (cases 1–7) and eight obligate carriers (three dams and five sires) (Online Resource 1) on the Illumina BovineHD BeadChip array including 777.962 SNPs. All given SNP positions correspond to the bovine ARS-UCD1.2 genome assembly. The PLINK v1.9 software (Chang et al. 2015) was used to perform basic quality filtering of the dataset. Even though no sample was excluded, a total of 146.440 variants were removed owing to minor allele thresholds. The total genotyping rate was approximately 0.98. With a total of 631.522 remaining markers, homozygosity mapping was performed for the 7 IC-affected animals using the software PLINK v1.9 (Purcell et al. 2007) with the commands ‐‐homozyg‐kb 100 (considering homozygous segments of at least 100 kb), ‐‐homozyg‐match 0.95 (for allelic matching between both cases) and ‐‐homozyg‐group (for generating an overlap‐file), resulting in shared runs of homozygosity (ROH) indicating chromosomal region of identity-by-descent (IBD).
Whole-genome sequencing and variant calling
WGS using the Illumina NovaSeq6000 (Illumina Inc., San Diego, CA, USA) was performed on the genomic DNA of two affected calves (cases 1 and 6). The sequenced reads were mapped to the ARS‐UCD1.2 reference genome, resulting in an average read depth of approximately 18.2× in case 1 and 17.9× in case 6, and single-nucleotide variants (SNVs) and small indel variants were called (Rosen et al. 2020). The applied software and steps to process fastq files into binary alignment map (BAM) and genomic variant call format files were in accordance with the 1000 Bull Genomes Project processing guidelines of run 7 (Hayes and Daetwyler 2019), except for the trimming, which was performed using fastp (Chen et al. 2018). Further preparation of the genomic data was done according to Häfliger et al. 2020 (Häfliger et al. 2020). To find private variants, we compared the genotypes of the two calves with 597 cattle genomes of various breeds that had been sequenced in the course of other ongoing studies and that are publicly available (Online Resource 2) in the European Nucleotide Archive (SAMEA7690197 and SAMEA7690198 are the samples accession number of case 1 and case 6, respectively; http://www.ebi.ac.uk/en). The filtered list of remaining variants were further checked for their occurrence in a global control cohort of 4110 genomes of a variety of breeds (Hayes and Daetwyler 2019). Integrative Genomics Viewer (IGV) (Robinson et al. 2017) software was used for visual inspection of genome regions containing possible candidate genes.
Variant validation and genotyping via Sanger sequencing
PCR and Sanger sequencing were used to confirm the WGS results and to perform targeted genotyping for the identified FA2H frameshift insertion variant (18:2205625C>CG). All IC-affected animals, four available dams and six sires, as well as 113 AI top sires that included three fathers of the studied cases, were genotyped for the identified variant. Also the case reported by Testoni in 2006 (case 10) (Testoni et al. 2006) was genotyped. Primers were designed using the Primer-BLAST tool (Ye et al. 2012). After amplification with AmpliTaqGold360Mastermix (Thermo Fisher Scientific) the purified PCR products were directly sequenced on an ABI3730 capillary sequencer (Thermo Fisher Scientific). The primer sequences used were the following: 5′-AAATTCCTGGTT-GGGGAGCC-3′ (forward primer) and 5′-CTCGACAACGAGACGCACC-3′ (reverse primer). The sequence data were analyzed using Sequencher 5.1 software (GeneCodes).
Results
Clinical phenotype
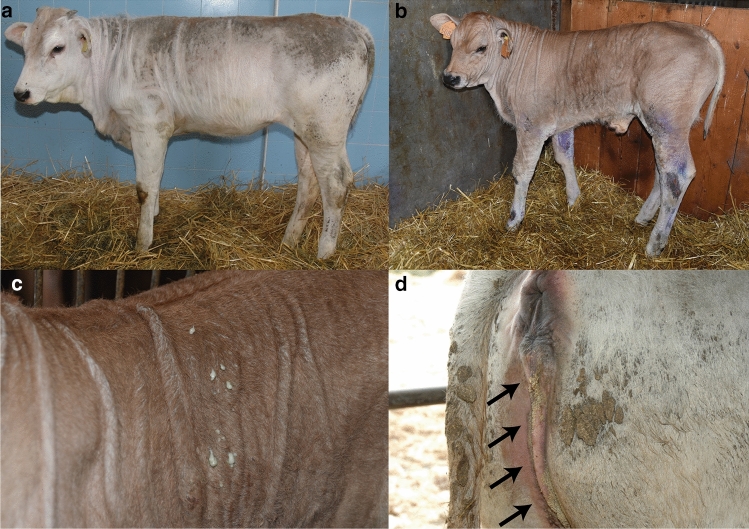
All patients (case 1–9) showed a more or less extended skin xerosis, hyperkeratosis and scaling besides a retarded growth. In the affected area the skin was dry and greyish with scale-like hyper-keratosis, and the most severe lesions were present at the level of trunk and neck (Fig. 1a). The coat was dull and bristly. Moreover, multifocal alopecic lesions were noticed, mostly affecting the muzzle, eyelids, ears, and inner region of limbs. The youngest calves displayed multiple wrinkles and folds (≤ 1 month of age) (Fig. 1b). No abnormalities were observed at the level of the mucocutaneous junctions. One of the animals (case 8) also showed secondary wounds and pyodermitis (Fig. 1c). Urolithiasis evidenced by the presence of small stones and crystals in the perigenital region (Fig. 1d) accompanied the cutaneous disease in cases 1, 7, 8 and 10. A hypoglycemic and hypothermic crisis that provoked the death of case 8 during the winter season was interpreted as a secondary phenomenon of imbalanced thermoregulation capacity. No abnormalities were registered at the level of the cardiovascular, respiratory, musculoskeletal, and nervous systems in any animals.
Fig. 1.
Clinical characterization of Chianina cattle affected by ichthyosis congenita. a Note the dry, greyish skin with scale-like hyperkeratosis over most of the body surface (case 1). b Note the multiple wrinkles, folds and wounds secondary to the hyperkeratosis (case 8). c Higher magnification of b from the skin of the thoracic region. Note the pyoderma. d Note the urolithiasis characterized by the presence of small stones and crystals (arrows) on the peri-genital region (case 7)
Interestingly, case 8's dam showed mild localized xerosis, hyperkeratosis and scaling in the region of the rump. Unfortunately, since in most cases the parents of the affected animals had already been slaughtered, we could not evaluate the phenotype in more of these obligate carriers. However, we did see a total of three other confirmed FA2H heterozygous Chianina cattle and they were clinically normal.
Based on the clinical observations, the affected animals were consequently suspected to suffer from IC as described in this breed in 2006 (Testoni et al. 2006). A similar diagnosis was advanced also for the dam of case 8, although in a very mild form. For this animal the differential diagnosis of ectoparasitosis and fungi infection were excluded on the base of a parasitological test.
Pathological phenotype
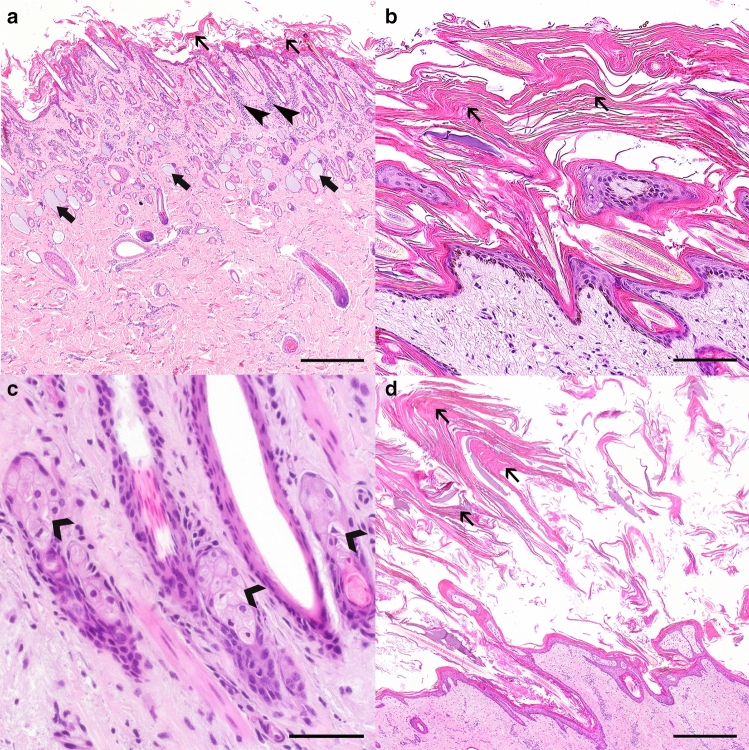
Histological analysis of the biopsies from the cutaneous lesions revealed a severe, diffuse orthokeratotic hyperkeratosis with mild to moderate epidermal hyperplasia (Fig. 2a, b). Serocellular crusts, serum lakes, and plant material were occasionally present among the abundant keratin scales. The superficial dermis displayed multifocal, moderate eosinophilic infiltrates, as well as a mostly perivascular, moderate infiltration with plasma cells and lymphocytes (Fig. 2a). Also, it was possible to observe the presence of intracytoplasmic, spindle-shaped, optically empty clefts within the sebocytes (Fig. 2c) in several of the affected animals, while the remaining adnexal structures were unremarkable. Similar findings were observed histologically in the punch biopsies taken from the dam of case 8 (Fig. 2d). These findings were consistent with the clinical diagnosis of IC.
Fig. 2.
Histology of the skin lesions displayed by a IC-affected Chianina calf (a–c) and its dam (d). a The epidermis of the calf (case 8) is irregular and mildly hyperplastic, with a thick overlying stratum corneum composed of abundant orthokeratotic, lamellar keratin scales (thin arrows). The sebaceous glands are not noticeable at this magnification, and the sweat glands are often dilated and filled with basophilic, homogeneous material (large arrows). Occasional interstitial inflammatory infiltrates can be observed in the superficial dermis (thin arrowheads). H&E staining, 500 µm. b Detail of the abundant orthokeratotic, lamellar keratin scales (thin arrows) (case 8). H&E staining, 100 µm. c Detail of the sebaceous glands. Some sebocytes display intracytoplasmic, spindle-shaped, optically empty clefts (large arrowheads) (case 8). H&E staining, 50 µm. d A severe orthokeratotic hyperkeratosis (thin arrows) could also be observed in the dam from case 8. H&E staining, 500 µm
Moreover, post-mortem examination of three cases (cases 1, 8 and 10) revealed inflammation of the urinary bladder (cystitis).
Genetic analysis
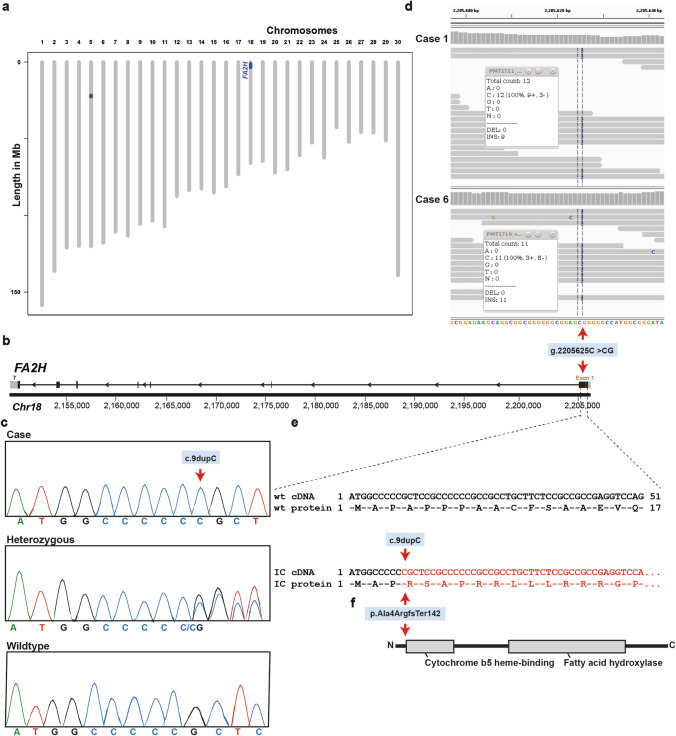
Pedigree records allowed the identification of a common ancestor as all IC-affected Chianina cattle were inbred from a sire born in 1976 (Online Resource 3). Pedigree analysis was consistent with monogenic autosomal recessive inheritance, and therefore carried out homozygosity mapping as all cases would likely be homozygous for a common chromosome segment flanking the causal mutation. This revealed a total of two identical-by-descent (IBD) segments shared by all seven cases with available SNP data (case 1–7): one 548 kb-sized region on chromosome 5 from 21.75 to 22.298 Mb and a second 1.92 Mb-sized region on chromosome 18 from 1.37 to 3.29 Mb (Fig. 3a).
Fig. 3.
Ichthyosis congenita (IC) FA2H frameshift variant in Chianina cattle. a Genetic mapping of the IC locus in the cattle genome. The two regions of shared homozygosity of seven cases are displayed in blue. Note that the largest segment of 1.92 Mb on chromosome 18 containing the, FA2H gene. b FA2H gene structure showing the variant located in exon 1. c Electropherograms of a case, heterozygous and wild-type genotypes. d Integrative Genomics Viewer (IGV) screenshot presenting the g.2205625C>CG variant in the two whole-genome sequenced cases. e Predicted wild-type (wt) and IC cDNA e protein. f Schematic representation of the bovine FA2H protein and its two domains and the identified pathogenic frameshift variant (p.Ala4fsTer142; red arrow)
Filtering of WGS for private shared homozygous variants present in sequenced genomes of cases 1 and 6 and absent in 597 available control genomes identified ten private protein-changing variants with a predicted moderate or high impact. Analyzing the occurrence of these variants in the global control cohort of 4110 genomes of a variety of breeds (Hayes and Daetwyler 2019), a single frameshift variant in FA2H with a predicted high impact on the encoded protein exclusively present in the genome of the case 1 and 6 remained. The homozygous variant in FA2H exon 1 on chromosome 18 (chr18:g.2205625C>CG; c.9dupC) was confirmed using IGV software (Fig. 3b, d). The deleterious FA2H variant (NM_001192455.1: c.9dupC) was predicted to result in a frameshift in the beginning of the protein after alanine 4 with a stop codon after aspartate 142 (NP_001179384.1: p.Ala4ArgfsTer142) resulting in a completely different amino acid sequence, if expressed, when compared with the wild type protein (Fig. 3e, f).
FA2H genotyping
To confirm and evaluate the presence of the FA2H variant, the affected genomic region was amplified by PCR and Sanger sequenced (Fig. 3c) in a total of ten cases, and presumable dams and sires when available. Analyzing the sequencing data, we observed that all cases were homozygous for the variants, whereas the available parents were heterozygous as expected for obligate carriers (Table 1). Furthermore, genotyping of 113 Chianina sires representing the active breeding population revealed a carrier ratio of 15% whereas the variant was absent in a global cohort of more than 4700 cattle of various breeds (Table 1).
Table 1.
Association of the 1 bp duplication (c.9dupC) variant in FA2H with ichthyosis congenita (IC) in Chianina cattle
| Genotype | |||
|---|---|---|---|
| wt/wt | wt/dup | dup/dup | |
| IC-affected cattle | 0 | 0 | 10 |
| Obligate carriersa | 0 | 10 | 0 |
| Chianina top sires | 96 | 17 | 0 |
| Normal control cattle from various breeds | 4707 | 0 | 0 |
aParents of the affected animal
Discussion
Here we describe the clinicopathological phenotype displayed by nine Italian Chianina cattle with IC and present the results of the genetic analysis that identified a recessively inherited frameshift mutation in FA2H, providing a novel candidate gene for skin disorders in both humans and animals. Furthermore, we provide a DNA-based diagnostic test that enables the selection against this sub-lethal allele that show an estimated frequency of ~ 7.5% in Chianina top sires.
Clinicopathological resemblances between familial forms of ichthyosis in humans and animals and the keratinization defect observed histologically in IC-affected Italian Chianina cattle led to the hypothesis that genetic variants in candidate genes for ichthyosis could be responsible for this disease in cattle. However, protein-changing variants within these more than 30 known candidate genes (Oji et al. 2010) were not found within the two mapped IBD regions, thereby excluding these as likely candidates. We then performed whole-genome sequencing of two IC-affected cattle that led to the identification of a frameshift mutation in FA2H (c.9dupC; p.Ala4ArgfsTer142). The genetic association of this variant with the bovine familial IC phenotype was confirmed by the homozygous genotype in eight additionally affected cattle, including an older case presented in 2006, and by its absence in all other sequenced genomes. Furthermore, reported expression of FA2H transcripts in the urinary tract supports the associated urolithiasis and cystitis seen in some of the IC-affected cattle. Finally, the predicted consequence of the frameshift variant demonstrating a loss-of-function supports causality. This variant is therefore the first in any domestic animal species to be associated with IC, and FA2H should be considered an additional candidate gene for syndromic forms of ichthyosis in humans.
While genetic analysis strongly suggested the association of p.Ala4ArgfsTer142 allele with IC in affected Italian Chianina cattle, the frameshift variant lies very near the N-terminal end of the protein. Therefore, the impact of such a significant truncation on protein function is probably high, thus the variant represents a most likely pathogenic loss-of-function mutation. Within a representative cohort of the current Italian Chianina population, a moderate allele frequency and the absence of the homozygous genotype for the deleterious allele was noticed.
In humans, mutations in FA2H (OMIM611026) are associated with recessively inherited spastic paraplegia type 35 (Dick et al. 2010), leukodystrophy with spasticity and dystonia (Edvardson et al. 2008), and fatty acid hydroxylase-associated neurodegeneration, a rare subtype neurodegeneration with brain iron accumulation (Kruer et al. 2010). So far, more than 40 different mutations have been associated with these neurological phenotypes (Rattay et al. 2019; Kawaguchi et al. 2020). However, to the best of our knowledge, no pathogenic variant in the FA2H associated to a form of ichthyosis has been reported in both animal and human species. Therefore, our study in cattle provides the first large-animal model of an FA2H-related congenital skin disorder.
FA2H encodes the endoplasmic reticulum enzyme fatty acid 2-hydroxylase, which plays a major role in the de novo synthesis of sphingolipids containing 2-hydroxy fatty acids (Alderson et al. 2004, 2005; Maldonado et al. 2008). 2-Hydroxy sphingolipids are very plentiful in neural tissue since the major components of myelin are galactolipids (galactosylceramide and sulfatide) with 2-hydroxy fatty acids (Alderson et al. 2005; Maldonado et al. 2008). However, the function of 2-hydroxyl modification of sphingolipids is still poorly known, although several studies evidently demonstrated that these compounds (including ceramides) play important roles in signal transduction (Hannun and Obeid 2008). Particularly, a study demonstrated that absence of FA2H lead to the impairment of cAMP-dependent cell cycle exit of Schwannoma cells, suggesting that FA2H sphingolipids may negatively regulate the cell cycle (Alderson and Hama 2009). Moreover, FA2H is highly expressed in the epidermis (Uchida et al. 2007). Notably, mammalian skin contains reasonably large amounts of 2-hydroxylated sphingolipids, which are involved in cell–cell recognition, signal transduction, and intercellular adhesion (Hakomori 2002; Uchida et al. 2007). The sphingolipids' ceramide backbone also plays a role as an intracellular signal of cell arrest, cellular senescence, and apoptosis in several types of cell, including keratinocytes (Hannun and Luberto 2000). Besides these ubiquitous bioregulatory functions, ceramide are abundant components of the extracellular lamellar membranes in the outermost layers of the epidermis, such as the stratum corneum, where they play an important role in the epidermal permeability barrier function (Holleran et al. 2006). Also, a notable increase in ceramide is noticed during epidermal differentiation (Holleran et al. 2006). In humans, it is known that differentiation-dependent up-regulation of ceramide synthesis and fatty acid elongation is accompanied by up-regulation of FA2H. Furthermore, the 2-hydroxylation of fatty acid by FA2H occurs prior to generation of ceramides/glucosylceramides, and 2-hydroxyceramides/2-hydroxyglucosylceramides are essential for epidermal lamellar membrane formation (Uchida et al. 2007). Such findings suggest that the expression of FA2H is essential for epidermal permeability barrier homeostasis and responsible for synthesis of 2-hydroxylated sphingolipids in keratinocytes of mammalian skin. Therefore, the severe orthokeratotic hyperkeratosis observed in the Chianina cattle with IC could be a consequence of the frameshift insertion in the FA2H gene. Notably, lack of FA2H in Fa2h−/− mice leads to hyperproliferation of sebocytes and enlarged sebaceous glands during hair follicle morphogenesis and anagen (active growth phase) in adult mice (Maier et al. 2011). Interestingly, the IC-affected animals included in this study often displayed intracytoplasmic, spindle-shaped, optically empty clefts within the sebocytes, although the sebaceous glands were similar in size to the ones observed in control animals. Sebaceous glands are holocrine glands that secrete a viscous, lipid-rich fluid rich in cholesterol and wax esters, triglycerides, squalene and cholesterol playing an important role in thermoregulation (Porter 2001). The rate of sebum is associated with the number and size of glands, and low production of sebum might lead to sebatosis or xerosis (Porter 2001; Shamloul and Khachemoune 2020). The major functions of sebum are to lubricate the skin and hair conferring impermeability to water, and in thermoregulation (Shamloul and Khachemoune 2020). Moreover, sebaceous glands play a role in immunity since sebum is thought to have antibacterial and antifungal properties (Strauss et al. 1983). Consequently, our findings may suggest that cattle affected by IC might be predisposed to develop skin secondary infections and present thermoregulation deficits due to this genetic defect. These situations were evidently suspected for two of our patients. Moreover, in Fa2h−/− mice, deficiency in Fa2h caused a delay in emergence of the fur during morphogenesis and depilation-induced anagen and a cyclic alopecia (Maier et al. 2011). Herein, the cases revealed a localized alopecia, but the hair follicles present in the biopsies taken from the IC cutaneous lesions were unremarkable.
In mice, depletion of FA2H decreases the protein levels of GLUT4 leading to reduced glucose uptake and lipogenesis under basal and insulin-stimulated conditions (Guo et al. 2010). GLUT4 deficiency in mice (Slc2a4tm1Mch/Slc2a4tm1Mch) leads to retarded growth, decreased expected longevity and abnormal cellular glucose and fat metabolism (Katz et al. 1995). Intriguingly, all Chianina cattle homozygous for the FA2H mutation showed retarded growth and decreased expected longevity. Unfortunately, metabolic analysis to access the glucose metabolism was not performed.
Beside the skin lesions that were displayed in all the cases, four out of ten cases showed urolithiasis and three out of ten revealed cystitis. It is worth to highlight that in the six animals where these findings were not recorded: one was euthanized 2 days after birth, and consequently, the absence of these findings might be explain by the young age of the calf; the remaining five were clinically examined only at the farms and, therefore, it was not possible to have a follow-up of the clinical status. On the contrary, the cases where we observed urolithiasis and cystitis were recovered at the clinic allowing the performance of a more detailed examination. Urolithiasis is a multifactorial disease resulting from complex interactions between environmental and genetic factors. Interestingly, FA2H in humans is also expressed in the urinary bladder and kidney (Fagerberg et al. 2014). However, physiological functions of FA2H in these organs are largely unknown.
Conclusions
Rare disorders like IC in livestock are usually not reported or are mis-diagnosed. Based on the known function of FA2H and its role in the skin, the predicted impact of the identified variant and its perfect co-segregation with the disease phenotype in the studied pedigree, we conclude that inherited IC in Chianina cattle is caused by a homozygous loss-of-function variant in FA2H. Thereby, this study represents an outstanding animal model for the understanding of similar conditions in different species and adds FA2H to the list of candidate genes for ichthyosis in humans. This example highlights the utility of precision diagnostics including genomics, for understanding rare disorders and the value of surveillance of cattle breeding populations for harmful genetic disorders.
Moreover, this study provides a DNA-based diagnostic test that allows selection against the identified pathogenic variant in the Chianina cattle population. Due to the high economic value of many Chianina cattle, including their skin for leather production, genetic testing should be pursued to prevent breeding of carriers that produce affected calves.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The Next Generation Sequencing Platform and the Interfaculty Bioinformatics Unit of the University of Bern are acknowledged for performing the WGS experiments and providing high-performance computing infrastructure. The technical assistance of Nathalie Besuchet Schmutz, Manuela Bozzo, Erika Bürgi, and Francesca Caravello is acknowledged.
Author contributions
JGPJ performed all clinical and genetic analyses and drafted the manuscript. IMH carried out bioinformatics. AL assisted in the genetic analyses. AG collected samples and drafted parts of the manuscript. IMBV performed histopathology and drafted parts of the manuscript. AG and CD designed the study, supervised the project and finalized the manuscript. All authors participated in writing the manuscript and have read and approved the final version.
Funding
Open Access funding provided by Universität Bern. This study was partially funded by the Swiss National Science Foundation.
Data availability
The whole-genome data of our group have been made freely available under study accession number PRJEB28191in the European Nucleotide Archive (http://www.ebi.ac.uk/ena). All accession numbers of the WGS are available in the Online Resource 2. SAMEA7690197 and SAMEA7690198 are the samples accession number of case 1 and case 6, respectively. All references to the bovine FA2H gene correspond to the NCBI accessions NC_037345.1 (chromosome 18, ARS-UCD1.2), NM_001192455.1 (FA2H gene), and NP_001179384.1 (FA2H protein). For the protein structure of FA2H, the UniProt database accession number E1BGC2 was used.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study did not require official or institutional ethical approval as ‘non-experimental clinical veterinary practices’ are specifically excluded from being considered regulated procedures under The Animals (Scientific Procedures) Act (ASPA), 1986, Section 2(8) (https://www.rcvs.org.uk/setting-standards/advice-and-guidance/code-of-professional-conduct-for-veterinary-surgeons/supporting-guidance/recognised-veterinary-practice/). The cattle were handled according to good ethical standards and all live animals were blood sampled by a veterinary for diagnostic purposes on the farm to determine the cause of the disease. All other sampling was carried out postmortem on affected animals after euthanasia on humane grounds. The aim was to identify the cause of the congenital disorder and thereby improve the animal welfare situation on the Chianina cattle population by identifying the underlying genetic cause and preventing breeding of further cases.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Joana G. P. Jacinto, Email: joana.goncalves2@studio.unibo.it
Irene M. Häfliger, Email: irene.haefliger@vetsuisse.unibe.ch
Inês M. B. Veiga, Email: ines.veiga@vetsuisse.unibe.ch
Anna Letko, Email: anna.letko@vetsuisse.unibe.ch.
Arcangelo Gentile, Email: arcangelo.gentile@unibo.it.
Cord Drögemüller, Email: cord.droegemueller@vetsuisse.unibe.ch.
References
- Alderson NL, Hama H. Fatty acid 2-hydroxylase regulates cAMP-induced cell cycle exit in D6P2T Schwannoma cells. J Lipid Res. 2009;50:1203–1208. doi: 10.1194/jlr.M800666-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderson NL, Rembiesa BM, Walla MD, et al. The human FA2H gene encodes a fatty acid 2-hydroxylase. J Biol Chem. 2004;279:48562–48568. doi: 10.1074/jbc.M406649200. [DOI] [PubMed] [Google Scholar]
- Alderson NL, Walla MD, Hama H. A novel method for the measurement of in vitro fatty acid 2-hydroxylase activity by gas chromatography–mass spectrometry. J Lipid Res. 2005;46:1569–1575. doi: 10.1194/jlr.D500013-JLR200. [DOI] [PubMed] [Google Scholar]
- Bauer A, Waluk DP, Galichet A, et al. A de novo variant in the ASPRV1 gene in a dog with ichthyosis. PLoS Genet. 2017;13:1–13. doi: 10.1371/journal.pgen.1006651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Câmara ACL, Borges PAC, Paiva SA, et al. Ichthyosis fetalis in a cross-bred lamb. Vet Dermatol. 2017;28:125–516. doi: 10.1111/vde.12459. [DOI] [PubMed] [Google Scholar]
- Casal ML, Wang P, Mauldin EA, et al. A Defect in NIPAL4 is associated with autosomal recessive congenital ichthyosis in American bulldogs. PLoS ONE. 2017;12:1–9. doi: 10.1371/journal.pone.0170708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Chow CC, Tellier LCAM, et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:1–16. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier C, Coppieters W, Rollin F, et al. Highly effective SNP-based association mapping and management of recessive defects in livestock. Nat Genet. 2008;40:449–454. doi: 10.1038/ng.96. [DOI] [PubMed] [Google Scholar]
- Chen S, Zhou Y, Chen Y, Gu J. Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittick EJ, Olivry T, Dalldorf F, et al. Harlequin ichthyosis in two greater Kudu (Tragelaphus strepsiceros) Vet Pathol. 2002;39:751–756. doi: 10.1354/vp.39-6-751. [DOI] [PubMed] [Google Scholar]
- Credille KM, Minor JS, Barnhart KF, et al. Transglutaminase 1-deficient recessive lamellar ichthyosis associated with a LINE-1 insertion in Jack Russell terrier dogs. Br J Dermatol. 2009;161:265–272. doi: 10.1111/j.1365-2133.2009.09161.x. [DOI] [PubMed] [Google Scholar]
- Dick KJ, Eckhardt M, Paisán-Ruiz C, et al. Mutation of FA2H underlies a complicated form of hereditary spastic paraplegia (SPG35) Hum Mutat. 2010;31:1251–1260. doi: 10.1002/humu.21205. [DOI] [PubMed] [Google Scholar]
- Eager KLM, Conyers LE, Woolley SA, et al. A novel ABCA12 frameshift mutation segregates with ichthyosis fetalis in a Polled Hereford calf. Anim Genet. 2020;51:837–838. doi: 10.1111/age.12973. [DOI] [PubMed] [Google Scholar]
- Edvardson S, Hama H, Shaag A, et al. Mutations in the fatty acid 2-hydroxylase gene are associated with leukodystrophy with spastic paraparesis and dystonia. Am J Hum Genet. 2008;83:643–648. doi: 10.1016/j.ajhg.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerberg L, Hallström BM, Oksvold P, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteom. 2014;13:397–406. doi: 10.1074/mcp.M113.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grall A, Guaguère E, Planchais S, et al. PNPLA1 mutations cause autosomal recessive congenital ichthyosis in golden retriever dogs and humans. Nat Genet. 2012;44:140–147. doi: 10.1038/ng.1056. [DOI] [PubMed] [Google Scholar]
- Guo L, Zhou D, Pryse KM, et al. Fatty acid 2-hydroxylase mediates diffusional mobility of raft-associated lipids, GLUT4 level, and lipogenesis in 3T3-L1 adipocytes. J Biol Chem. 2010;285:25438–25447. doi: 10.1074/jbc.M110.119933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häfliger IM, Wiedemar N, Švara T, et al. Identification of small and large genomic candidate variants in bovine pulmonary hypoplasia and anasarca syndrome. Anim Genet. 2020;51:382–390. doi: 10.1111/age.12923. [DOI] [PubMed] [Google Scholar]
- Hakomori SI. The glycosynapse. Proc Natl Acad Sci USA. 2002;99:225–232. doi: 10.1073/pnas.012540899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun YA, Luberto C. Ceramide in the eukaryotic stress response. Trends Cell Biol. 2000;10:73–80. doi: 10.1080/00397911.2014.916302. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- Hayes BJ, Daetwyler HD. 1000 bull genomes project to map simple and complex genetic traits in cattle: applications and outcomes. Annu Rev Anim Biosci. 2019;7:89–102. doi: 10.1146/annurev-animal-020518-115024. [DOI] [PubMed] [Google Scholar]
- Holleran WM, Takagi Y, Uchida Y. Epidermal sphingolipids: metabolism, function, and roles in skin disorders. FEBS Lett. 2006;580:5456–5466. doi: 10.1016/j.febslet.2006.08.039. [DOI] [PubMed] [Google Scholar]
- Jacinto JGP, Häfliger IM, Veiga IMB, et al. A heterozygous missense variant in the COL5A2 in Holstein cattle resembling the classical Ehlers–Danlos syndrome. Animals. 2020;10:1–14. doi: 10.3390/ani10112002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz EB, Stenbit AE, Hatton K, DePinho R, Charron MJ (1995) Cardiac and adipose tissue abnormalities but not diabetes in mice deficient in GLUT4. Nature 377(6545):151–155 [DOI] [PubMed]
- Kawaguchi M, Sassa T, Kidokoro H, et al. Novel biallelic FA2H mutations in a Japanese boy with fatty acid hydroxylase-associated neurodegeneration. Brain Dev. 2020;42:217–221. doi: 10.1016/j.braindev.2019.11.006. [DOI] [PubMed] [Google Scholar]
- Kruer MC, Paisán-Ruiz C, Boddaert N, et al. Defective FA2H leads to a novel form of neurodegeneration with brain iron accumulation (NBIA) Ann Neurol. 2010;68:611–618. doi: 10.1002/ana.22122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeb T, Müller EJ, Roosje P, Welle M. Genetic testing in veterinary dermatology. Vet Dermatol. 2017;28:4–e1. doi: 10.1111/vde.12309. [DOI] [PubMed] [Google Scholar]
- Maier H, Meixner M, Hartmann D, et al. Normal fur development and sebum production depends on fatty acid 2-hydroxylase expression in sebaceous glands. J Biol Chem. 2011;286:25922–25934. doi: 10.1074/jbc.M111.231977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado EN, Alderson NL, Monje PV, et al. FA2H is responsible for the formation of 2-hydroxy galactolipidsin peripheral nervous system myelin. J Lipid Res. 2008;49:153–161. doi: 10.1194/jlr.M700400-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marukian NV, Choate KA. Recent advances in understanding ichthyosis pathogenesis [version 1; referees: 2 approved] F1000Research. 2016;5:1–9. doi: 10.12688/f1000research.8584.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J, Wöhlke A, Mischke R, et al. A novel SLC27A4 splice acceptor site mutation in Great Danes with ichthyosis. PLoS ONE. 2015;10:1–13. doi: 10.1371/journal.pone.0141514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni L, Dardano S, Parma P, et al. Ichthyosis in Chianina cattle. Vet Rec. 2006;158:412–414. doi: 10.1136/vr.158.12.412. [DOI] [PubMed] [Google Scholar]
- Oji V, Tadini G, Akiyama M, et al. Revised nomenclature and classification of inherited ichthyoses: results of the First Ichthyosis Consensus Conference in Sorze 2009. J Am Acad Dermatol. 2010;63:607–641. doi: 10.1016/j.jaad.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Pope E. Epidermolysis bullosa: a 2020 perspective. Br J Dermatol. 2020;183:603. doi: 10.1111/bjd.19125. [DOI] [PubMed] [Google Scholar]
- Porter AMW. Why do we have apocrine and sebaceous glands? J R Soc Med. 2001;94:236–237. doi: 10.1177/014107680109400509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattay TW, Lindig T, Baets J, et al. FAHN/SPG35: a narrow phenotypic spectrum across disease classifications. Brain. 2019;142:1561–1572. doi: 10.1093/brain/awz102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JT, Thorvaldsdóttir H, Wenger AM, et al. Variant review with the integrative genomics viewer. Cancer Res. 2017;77:e31–e34. doi: 10.1158/0008-5472.CAN-17-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen BD, Bickhart DM, Schnabel RD, et al. De novo assembly of the cattle reference genome with single-molecule sequencing. Gigascience. 2020;9:1–9. doi: 10.1093/gigascience/giaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamloul G, Khachemoune A. An updated review of the sebaceous gland and its role in health and diseases Part 1: embryology, evolution, structure, and function of sebaceous glands. Dermatol Ther. 2020 doi: 10.1111/dth.14695. [DOI] [PubMed] [Google Scholar]
- Strauss J, Downing D, Ebling F. Sebaceous glands. In: Goldsmith L, editor. Biochemistry and physiology of the skin. New York: Oxford University Press; 1983. pp. 569–595. [Google Scholar]
- Testoni S, Zappulli V, Gentile A. Ichthyosis in two Chianina calves. Dtsch Tierarztl Wochenschr. 2006;113:351–354. [PubMed] [Google Scholar]
- Uchida Y, Hama H, Alderson NL, et al. Fatty acid 2-hydroxylase, encoded by FA2H, accounts for differentiation-associated increase in 2-OH ceramides during keratinocyte differentiation. J Biol Chem. 2007;282:13211–13219. doi: 10.1074/jbc.M611562200. [DOI] [PubMed] [Google Scholar]
- Wang X, Cao C, Li Y, et al. A harlequin ichthyosis pig model with a novel ABCA12 mutation can be rescued by acitretin treatment. J Mol Cell Biol. 2019;11:1029–1041. doi: 10.1093/jmcb/mjz021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley SA, Eager KLM, Häfliger IM, et al. An ABCA12 missense variant in a Shorthorn calf with ichthyosis fetalis. Anim Genet. 2019;50:749–752. doi: 10.1111/age.12856. [DOI] [PubMed] [Google Scholar]
- Ye J, Coulouris G, Zaretskaya I et al (2012). BMC Bioinform. 10.1186/1471-2105-13-134 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The whole-genome data of our group have been made freely available under study accession number PRJEB28191in the European Nucleotide Archive (http://www.ebi.ac.uk/ena). All accession numbers of the WGS are available in the Online Resource 2. SAMEA7690197 and SAMEA7690198 are the samples accession number of case 1 and case 6, respectively. All references to the bovine FA2H gene correspond to the NCBI accessions NC_037345.1 (chromosome 18, ARS-UCD1.2), NM_001192455.1 (FA2H gene), and NP_001179384.1 (FA2H protein). For the protein structure of FA2H, the UniProt database accession number E1BGC2 was used.
Not applicable.