ABSTRACT
Plant extracellular vesicles (EVs) are cell-secreted membrane structures enclosing cytosolic components, including pathogenesis-related proteins, tiny RNAs, and microRNAs et al. Their roles are shown to be involved in plant-microbe interactions. Albeit several marker proteins were developed for EVs labeling for Arabidopsis thaliana and other plant species, we lack similar knowledge on EVs isolated from model plant Nicotiana benthamiana, which serves as an excellent host for plant pathogen studies. Here, we transiently expressed two arabidopsis EV markers AtPEN1 and AtTET8 and one ESCRT protein VPS4 in Nicotiana benthamiana leaves and tested for their ability in EV labeling. We found that GFP tagged AtPEN1 expression in Nicotiana benthamiana leaves is more stable than other proteins tested, and GFP-AtPEN1 accumulated in Nicotiana benthamiana EVs. Furthermore, we showed that EVs isolated from Nicotiana benthamiana leaf apoplast have typical EV density and vesicle-like morphology. Our finding demonstrates that GFP-AtPEN1 can be used as an excellent marker protein to label Nicotiana benthamiana EVs.
KEYWORDS: Extracellular vesicles, Nicotiana benthamiana, apoplastic fluid, PEN1
Introduction
Plant extracellular vesicles (EVs) are membranous vesicles released from plant cells into neighboring spaces. In the 1960s, the carrot Multivesicular Bodies (MVB) origin EVs, or sometimes referred to as exosomes, were identified under the transmission electron microscope (TEM).1 In recent years, plant EVs or EV-like nanoparticles (ELN) were isolated from a variety of sources, like grape juice,2 ginger blend,3,4 olive pollen grains,5 apoplastic fluid of sunflower,6 and in coconut milk.7 In these cases, no direct evidence was provided for the purity of these preparations, albeit some of the EV preparation methods clearly can not avoid contamination from intracellular vesicles.
Studies on mammalian EVs presented the fact that different kinds of proteins can serve as EV markers, including 1) transmembrane or GPI-anchored proteins, like Tetraspanins, Rab GTPase; 2) cytosolic proteins, like GAPDH, Chaperons, and endosomal sorting complex required for transport (ESCRT) proteins.8 Rab11 was found to be associated with sunflower EVs and was proposed as a potential EV marker protein.6 Olive pollen allergens were confirmed to be in pollen tube secreted EVs.5 In a study revealing the existence of biotic and abiotic stress-related proteins in arabidopsis EVs, a plasma membrane syntaxin PEN1 was chosen as an EV marker protein.9 The purity of arabidopsis PEN1-positive EVs was supported by the evidence that only PEN1, but not other intracellular vesicle markers, was detected in those EV preparations. The proteomics analysis of EV fractions prepared from apoplastic fluids of Helianthus annuus seedlings revealed the existence of proteins usually found in mammalian EVs, cell wall remodeling enzymes, as well as plant defense proteins.10 Like human tetraspanins, the plant tetraspanins can also label EV fractions prepared from arabidopsis apoplastic fluid.11
Although still promiscuous in its function in plant development, shreds of evidence suggested that plant EVs are involved in plant defense against pathogens. During the early stages of powdery mildew infection on arabidopsis caused by Golovinomyces orontii, GFP-PEN1-positive EVs are incrementally deposited to the haustorial encasements to restrict fungal entry.12 TET8-GFP-positive EVs were shown to accumulate at the site of pathogenic fungi Botrytis cinerea infection in Arabidopsis leaves.11 These EVs contain endogenous plant sRNAs that are transferred into fungal cells to restrict fungi growth. A recent study revealed that the majority of non-infected arabidopsis EVs are 10–17 nucleotide long tiny RNAs, of which functions are not yet understood.13 Besides host sRNA, proteomics analyses of arabidopsis EVs revealed enrichment of pathogenesis-related proteins, suggestive of the involvement of EV in plant defense mechanisms.9
Nicotiana benthamiana has long been used as a model to study plant-microbe interactions,14 but there is limited information regarding its EVs compared with those isolated from other plants. Here, we report that N. benthamiana EVs have typical density and vesicle-like morphology comparing to the arabidopsis counterpart. We also established AtPEN1 as an excellent marker protein for Nicotiana benthamiana EVs.
Materials and methods
Plant materials
Nicotiana benthamiana plants are grown in 14 h light/10 h dark cycles at 22°C with ~50% relative humidity. 4–5 weeks old plants are used for experiments.
Expression plasmids
DNA fragments of the coding regions of AtPEN1 (AT3G11820), AtTET8 (AT2G23810), and AtVPS4 (AT2G27600) were PCR amplified and inserted into BamHI/SalI digested binary vector pGD-NEGFP,15 resulting in pGD-EGFP-AtPEN1, pGD-EGFP-AtTET8, and pGD-EGFP-AtVPS4, for the expression of N-terminally EGFP-tagged proteins; the coding region of AtTET8 were amplified via PCR and cloned into the BamHI/PstI digested binary vector pGD-CEGFP,15 generating pGD-AtTET8-EGFP for the expression of AtTET8-EGFP in plants. The GFP-tagged fusion proteins are under the control of 2 × 35 S promoter of Cauliflower mosaic virus (CaMV). In both pGD-NEGFP and pGD-CEGFP constructs, there is a FLAG-tag to distant the GFP from the tagged proteins. The recombinant fusion proteins were transiently expressed in N. benthamiana leaves via agroinfiltration. Agrobacterium strain EHA105 was used for the infiltration of plant leaves at the OD600 of 1.0.
Isolation of extracellular vesicles
Methods for plant EV isolation from apoplastic fluids is according to the previous report with minor modifications.9 Briefly, plant leaves were cut and washed with sterile water to remove the impurities attached. Leaves were collected and placed into a 250 ml beaker and evacuated in the Vesicle Isolation Buffer (VIB, 20 mM MES, 2 mM CaCl2, 0.1 M NaCl, pH 6.0). After vacuum-infiltrated with VIB buffer, leaves were taken out of the buffer and wiped with a paper towel. Then the leaves were spun in a home-made centrifuge tube at 700 x g for 20 min at 4°C to collect the apoplastic fluids. The collected apoplastic fluids were first centrifuged at 10,000 x g for 30 min at 4°C, filtered through a 0.45 nm polyethersulfone syringe filter, again centrifuged at 10,000 x g for 30 min at 4°C, and then spun at 100,000 x g for 3 h at 4°C in an ultracentrifuge. The pellets were then either directly suspended in 1x SDS-loading buffer for western blotting analysis or suspended in VIB for further analyses.
TEM observation of purified EVs
A piece of 200-mesh copper grids was used to stick purified EV droplets for 5–10 min. Grids with EV samples were washed with sterile water, dry wiped with a tissue paper, stained with 2% uranyl acetate (UA) for 1 min. The stained grids were again washed once with sterile water and dried wiped, followed by imaging using a transmission electron microscope (model H7650, HITACHI).
TEM observation of plant tissue samples
Leaf samples of N. benthamiana were cut and immediately fixed in a 2.5% glutaraldehyde solution at 4°C o/n. Fixed leaf samples were rinsed with phosphate buffer (0.1 M, pH 7.0) for 15 mins. The rinsing step was repeated for an additional two times. Samples were then fixed with 1% osmic acid for 1–2 h and rinsed with phosphate buffer (0.1 M, pH7.0) three times for 15 min each time. The fixed leaf samples were dehydrated with ethanol solutions with gradient concentration (30%, 50%, 70%, 80%, 90%, and 95%) for 15 min each concentration, and treated with 100% ethanol once for 20 min, followed by immersing into pure acetone solution for 20 min. The samples were then treated with a mixed solution of Spurr embedding media and acetone (V/V = 1/1) for 1 h, then with a mixed solution of Spurr embedding media and acetone (V/V = 3/1) for 3 h, and then with embedding media overnight. The overnight osmotically treated samples were then embedded and heated at 70 °C o/n. The embedded samples were sliced into 70–90 nm thin slices in a LEICA EM UC7 ultra-thin microtome. Leaf sections were subjected to lead citrate solution treatment for 5–10 min and then stained with 50% ethanol saturated solution of uranyl acetate for 5–10 min. The processed samples were imaged with a transmission electron microscope (model H7650, HITACHI).
Density gradient flotation of EVs
The 100,000 x g pellet of apoplastic fluid was resuspended in MES buffer (20 mM, pH 6.0) and mixed with 1.8 ml 60% iodixanol solution (Sigma-Aldrich, Cat. #D1556). The suspended EV fraction was placed on the bottom of an ultracentrifugation tube and then layered with 2 ml ea. lower density iodixanol solutions adjusted by MES buffer (from the bottom to top: 45%, 40%, 30%, 20%, 15%). The gradient was subjected to ultracentrifugation at 100,000 x g for 12 h. Total 13 fractions (~0.92 ml ea.) were withdrawn from the gradient, diluted with 2 x volume of VIB, and then centrifuged at 40, 000 x g for 3 h to collect the EVs for western blotting analysis.
Results
Nicotiana benthamiana produces EVs in the apoplast
We choose five weeks old seedlings of Nicotiana benthamiana for analysis of EVs production under the electron microscope. We occasionally observed vesicle-like structures in the apoplastic area of analyzed leaf sections (Figure 1a).
Figure 1.
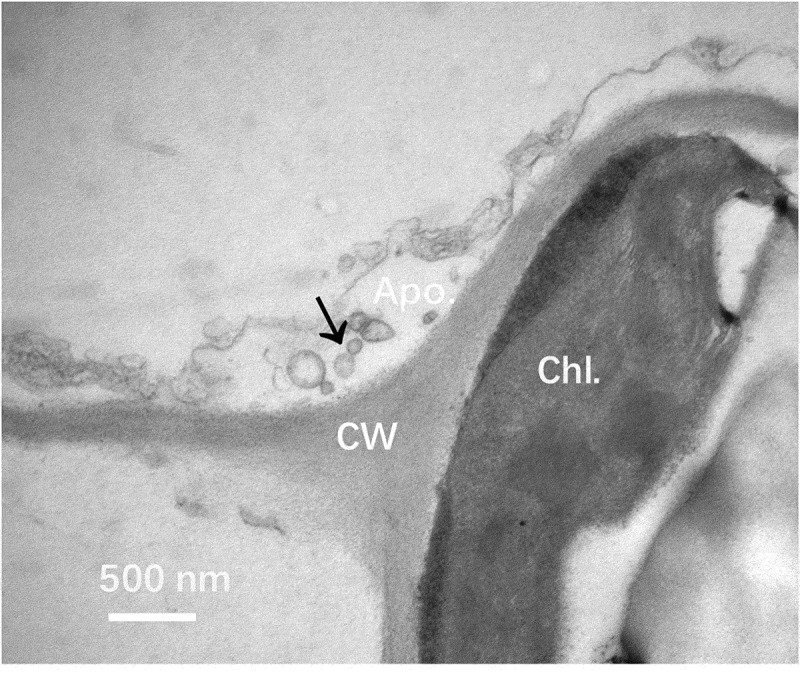
Electron microscope analysis of Nicotiana benthamiana EVs in leaf tissue. Leaves Nicotiana benthamiana plants were analyzed under a transmission electron microscope. A representative image from three independent repeats is shown. Vesicle-like structures were observed in the apoplastic area (arrow). Apo.: Apoplast; CW: Cell wall; Chl.: Chloroplast. Bar = 500 nm.
GFP tagged EV marker AtPEN1 labels Nicotiana benthamiana EVs
We then aim to test which EV marker can label Nicotiana benthamiana EVs. Three candidates were chosen based on previous reports: AtPEN1 and AtTET8 were shown to be useful markers to label Arabidopsis EVs;9,11 ATPase VPS4 of the ESCRT machinery was reported to be involved in EVs biogenesis in animal cells16 and localized in EVs .17 Four GFP-tagged protein candidates (GFP-AtPEN1, TET8-GFP, GFP-TET8, GFP-AtVPS4) were transiently expressed via agroinfiltration and monitored for their stability in Nicotiana benthamiana leaves at 1 to 6 days post infiltration (dpi) (Figure 2a). GFP-AtPEN1 has the highest stability and can be detected at 6 dpi. AtTET8-GFP accumulated to the highest at 2 dpi and can not be detected after 3 dpi. GFP-AtTET8 can only be detected on the second day after infiltration, while GFP-AtVPS4 was stable till 4 dpi. Notably, a higher molecular weight band (asterisked) was detected in GFP-AtTET8 sample which accumulated less comparing to AtTET8-GFP. This may due to the blockage of TET8 secretion by the N-terminally tagged GFP, leading to additional posttranslational modifications and protein degradation. Nevertheless, GFP-AtPEN1 was the most stable among the four when transiently expressed.
Figure 2.
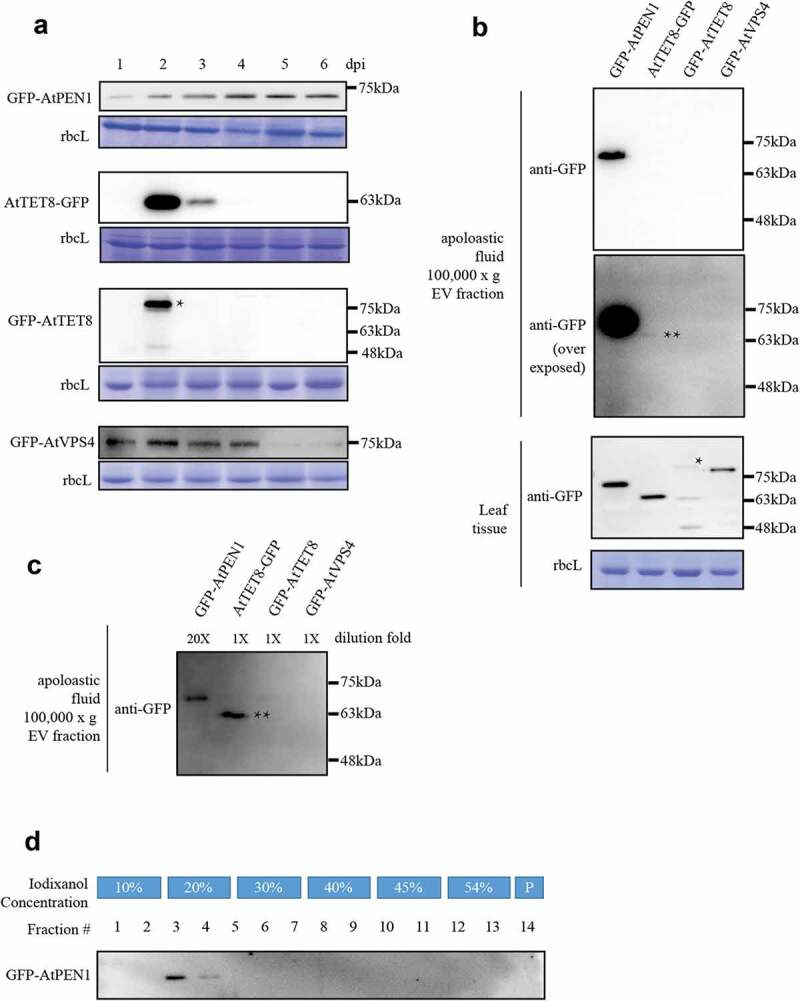
Comparative analysis of four candidate EV marker proteins. (a). Protein accumulation of four EV marker candidates transiently expressed in Nicotiana benthamiana leaves via agroinfiltration. Samples were taken from 1–6 days post infiltration (dpi) and blotted for the accumulation of GFP-fusion proteins using anti-GFP antibody (Abcam, Cat. #ab190584). Rubisco large subunit (rbcL) stained by coomassie blue was used as a control for protein loading. A higher molecular band of the GFP-AtTET8 sample was asterisked. (b). GFP-AtPEN1 can be detected in the 100,000 x g pellet of apoplastic fluid. Protein samples from the EV preparations or leaf tissue samples were analyzed by western blotting using an anti-GFP antibody. Overexposed blot showed the presence of AtTET8-GFP (double-asterisked). Coomassie blue staining of rbcL was used to control protein loading. (c). GFP-AtPEN1 EV preparation was diluted 20 times and blotted with other preparations. The band of AtTET8-GFP is double-asterisked. (d). Density gradient isolation of PEN1-positive EVs. Samples were tested for the presence of GFP-AtPEN1 by western blotting using an anti-GFP antibody. Pellet from the iodixanol density gradient was directly suspended into protein sample buffer (fraction #14) for western blotting analysis. All experiments are repeated at least 3 times.
Next, EVs were isolated from apoplastic fluids of N. benthamiana leaves expressing these GFP-tagged proteins at 2dpi, and analyzed for the presence of potential EV markers. Among these proteins tested, we detected GFP-AtPEN1 and AtTET8-GFP in the EV preparations (Figure 2b). However, the accumulation of AtTET8-GFP is about 20 times less than that of GFP-AtPEN1 (Figure 2c). The western blotting analysis showed that GFP-AtPEN1-positive EVs could be recovered from fractions #3 to #4 (Figure 2d), representing a density between 1.078 g/ml to 1.086 g/ml. This density of N. benthamiana EVs fell into the range of 1.056 g/ml to 1.103 g/ml, which was reported for arabidopsis AtPEN1-positive EVs.9
AtPEN1-positive EVs have a similar size to those observed in leaf tissue
To demonstrate that the fractions #3 – #4 indeed contains EVs, we then treated these samples using negative staining and observed them under the transmission electron microscope (Figure 3a). The cup-shaped structures and round vesicle-like structures were identified in these fractions, similar to previously reported plant and mammalian EV .9,10,17 Next, we measured the diameter of EVs from the apoplastic fluids and leaf tissue samples in the TEM images. We found that the diameters of EVs prepared from Nicotiana benthamiana apoplastic fluids ranged from 30 to 220 nm with a mean of 117 ± 9 nm (Mean ± SEM). These sizes are comparable to those observed in leaf tissue samples, which are from 50 to 370 nm in diameter with a mean of 138 ± 13 nm (Mean ± SEM) (Figure 3b).
Figure 3.

Negative staining and TEM analysis of fractions #3 – #4. A. EV preparations were observed with a transmission electron microscope. B. The diameter of vesicle-like structures was compared between those observed in Nicotiana benthamiana leaf tissue (n = 32) and those from fraction #3 – #4 enriched for GFP-AtPEN1 (n = 27). No significant difference (n.s.) was detected between these samples (p = .22).
Conclusion and future perspective
Based on these experiments, we concluded that GFP-AtPEN1 could serve as a marker for EVs prepared from Nicotiana benthamiana apoplastic fluid. PEN1 is a plasma membrane-localized Soluble N-ethylmaleimide-sensitive factor activating protein receptor (SNARE) protein. Its function is to regulate membrane fusion between the plasma membrane and other secretory trafficking vesicles.18 It was proposed that GFP-PEN1 is endocytosed from the PM, sorted through the intraluminal vesicles of MVB, and secreted into extracellular space in association with EVs.12 This sorting pathway of GFP-PEN1-EVs is similar to the MVB-origin aminal exosomes. In mammals, EVs can be generated by other pathways, including the budding of the PM as microvesicles19 or fusion of amphisomes with the PM.20 Whether plant undergoes similar EV secretion pathways as mammals do is still pending investigation. Future studies on secretion pathways of different EV markers in model plants, including Nicotiana benthamiana, will help understand those unanswered questions.
Funding Statement
This research was supported by the National Natural Science Foundation of China (31770164), Natural Science Foundation of Jiangsu Province (BK20180039), the Program for Jiangsu Excellent Scientific and Technological Innovation Team (17CXTD00014), and the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Halperin W, Jensen WA.. Ultrastructural changes during growth and embryogenesis in carrot cell cultures. J Ultrastruct Res. 1967;18:1–5. doi: 10.1016/S0022-5320(67)80128-X. [DOI] [PubMed] [Google Scholar]
- 2.Ju S, Mu J, Dokland T, Zhuang X, Wang Q, Jiang H, Xiang X, Deng ZB, Wang B, Zhang L, et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol Ther. 2013;21:1345–1357. doi: 10.1038/mt.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teng Y, Ren Y, Sayed M, Hu X, Lei C, Kumar A, Hutchins E, Mu J, Deng Z, Luo C, et al. Plant-derived exosomal MicroRNAs shape the gut microbiota. Cell Host Microbe. 2018;24:637–652 e8. doi: 10.1016/j.chom.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z, Wang H, Yin H, Bennett C, Zhang HG, Guo P.. Arrowtail RNA for ligand display on ginger exosome-like nanovesicles to systemic deliver siRNA for cancer suppression. Sci Rep. 2018;8:14644. doi: 10.1038/s41598-018-32953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prado N, Alche Jde D, Casado-Vela J, Mas S, Villalba M, Rodriguez R, Batanero E.. Nanovesicles are secreted during pollen germination and pollen tube growth: a possible role in fertilization. Mol Plant. 2014;7:573–577. doi: 10.1093/mp/sst153. [DOI] [PubMed] [Google Scholar]
- 6.Regente M, Corti-Monzon G, Maldonado AM, Pinedo M, Jorrin J, de la Canal L. Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Lett. 2009;583:3363–3366. doi: 10.1016/j.febslet.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Z, Yu S, Li M, Gui X, Li P. Isolation of exosome-like nanoparticles and analysis of MicroRNAs derived from coconut water based on small RNA high-throughput sequencing. J Agric Food Chem. 2018;66:2749–2757. doi: 10.1021/acs.jafc.7b05614. [DOI] [PubMed] [Google Scholar]
- 8.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 9.Rutter BD, Innes RW. Extracellular vesicles isolated from the leaf apoplast carry stress-response proteins. Plant Physiol. 2017;173:728–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regente M, Pinedo M, San Clemente H, Balliau T, Jamet E, de la Canal L. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J Exp Bot. 2017;68:5485–5495. doi: 10.1093/jxb/erx355. [DOI] [PubMed] [Google Scholar]
- 11.Cai Q, Qiao L, Wang M, He B, Lin FM, Palmquist J, Huang SD, Jin H. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science. 2018;360:1126–1129. doi: 10.1126/science.aar4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer D, Pajonk S, Micali C, O’Connell R, Schulze-Lefert P. Extracellular transport and integration of plant secretory proteins into pathogen-induced cell wall compartments. Plant J. 2009;57:986–999. doi: 10.1111/j.1365-313X.2008.03743.x. [DOI] [PubMed] [Google Scholar]
- 13.Baldrich P, Rutter BD, Karimi HZ, Podicheti R, Meyers BC, Innes RW. Plant extracellular vesicles contain diverse small RNA species and are enriched in 10- to 17-nucleotide “Tiny” RNAs. Plant Cell. 2019;31:315–324. doi: 10.1105/tpc.18.00872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bally J, Jung H, Mortimer C, Naim F, Philips JG, Hellens R, Bombarely A, Goodin MM, Waterhouse PM. The rise and rise of nicotiana benthamiana: A plant for all reasons. Annu Rev Phytopathol. 2018;56:405–426. doi: 10.1146/annurev-phyto-080417-050141. [DOI] [PubMed] [Google Scholar]
- 15.Xu K, Nagy PD. Enrichment of phosphatidylethanolamine in viral replication compartments via co-opting the endosomal Rab5 small GTPase by a positive-strand RNA virus. PLoS Biol. 2016;14:e2000128. doi: 10.1371/journal.pbio.2000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson CE, Scruggs BS, Schaffer JE, Hanson PI. Effects of inhibiting VPS4 support a general role for ESCRTs in extracellular vesicle biogenesis. Biophys J. 2017;113:1342–1352. doi: 10.1016/j.bpj.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA. 2004;101:13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waghmare S, Lileikyte E, Karnik R, Goodman JK, Blatt MR, Jones AME. SNAREs SYP121 and SYP122 mediate the secretion of distinct cargo subsets. Plant Physiol. 2018;178:1679–1688. doi: 10.1104/pp.18.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Q, Lu Q. Plasma membrane-derived extracellular microvesicles mediate non-canonical intercellular NOTCH signaling. Nat Commun. 2017;8:709. doi: 10.1038/s41467-017-00767-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, et al. Reassessment of exosome composition. Cell. 2019;177:428–445 e18. doi: 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]


