Ex-situ Arrhenius analyses and calculations reveal the activation mechanism of halide salts for molybdenum disulfide growth.
Abstract
Achieving large-size two-dimensional (2D) crystals is key to fully exploiting their remarkable functionalities and application potentials. Chemical vapor deposition growth of 2D semiconductors such as monolayer MoS2 has been reported to be activated by halide salts, for which various investigations have been conducted to understand the underlying mechanism from different aspects. Here, we provide experimental evidence showing that the MoS2 growth dynamics are halogen dependent through the Brønsted-Evans-Polanyi relation, based on which we build a growth model by considering MoS2 edge passivation by halogens, and theoretically reproduce the trend of our experimental observations. These mechanistic understandings enable us to further optimize the fast growth of MoS2 and reach record-large domain sizes that should facilitate practical applications.
INTRODUCTION
Two-dimensional (2D) semiconductors such as monolayer MoS2 are essential building blocks for next-generation ultrathin flexible and low-power electronics (1, 2). A recent study using semimetal Bi as the electrical contact to monolayer MoS2 has improved the on-current and contact resistance of the monolayer transistor to be on par with traditional Si-based transistors (3). As a premise for large-scale electronics, batch production of the 2D semiconductors requires large domain size (4, 5), large-area continuity, and thickness uniformity (6, 7). These are difficult tasks in particular for synthetic MoS2 and other transition metal dichalcogenides (TMDs), considering the less controllable mass flux from solid metal precursors (8) in chemical vapor deposition (CVD), as compared to the case of graphene growth with gaseous hydrocarbons (9). One recent advance to mitigate this is the use of alkali metal halide salts [e.g., NaCl, KI, and NaBr (10–12)], which, in conjunction with transition metal or metal oxide powders, could increase the mass flux of metal precursors and accelerate the 2D growth of TMDs. Various research efforts have been made to explain the mechanism, in terms of these metal halides as a molten salt to facilitate the evaporation of the metal oxide precursor (11) or as a surfactant to modify the substrate surface and the MoS2 edge (12). Both the alkali metal ions (13) and the halogens (11, 14) have been proposed to play an important role in the promoted growth. Continued endeavors are being devoted to shedding further light on understanding the detailed mechanism.
In this work, we provide unambiguous experimental evidence that halogens are closely related to MoS2 growth dynamics. To achieve this, we implement postgrowth Arrhenius analysis without any involvement of in situ characterizations. We find that within the same reaction family, the halide-assisted growths conform to Brønsted-Evans-Polanyi (BEP) relation, where their reaction barriers are linearly correlated to the Mo─X (X = I, Br, Cl, F, and O) bond dissociation energies (Eb), suggesting the substitution of Mo─X bonds by the Mo─S bonds to be the rate-limiting step for the CVD growth. On the basis of this, we propose a theoretical growth model that not only reproduces the BEP relation but also explains the sulfur concentration–dependent growth dynamics observed in our experiments. By harnessing the synergistic effect of the KI promoter and the sulfur supply, we can reproducibly and rapidly synthesize near millimeter-sized 2D MoS2 crystals dispersed over the entire SiO2/Si substrates. These results not only shed light on the detailed mechanism of TMD growth activated by the halide salts but also guide the designer growth toward larger domain sizes that should enable practical applications.
RESULTS
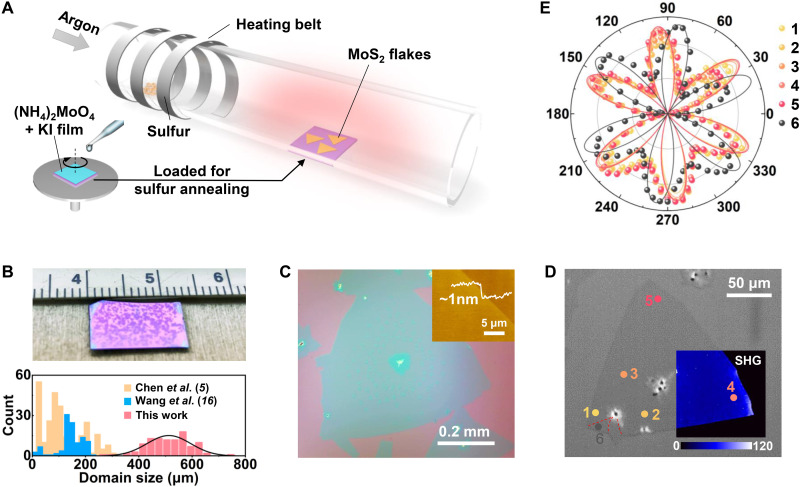
Figure 1A schematically illustrates our method to produce large-size atomically thin MoS2 crystals. Briefly, MoO3 dissolved in ammonia is spin-coated on the SiO2/Si substrates (15) and loaded subsequently into a tube furnace for high-temperature annealing under a sulfur atmosphere (see Materials and Methods for more experimental details). The MoO3/ammonia solution provides an advantage that soluble salts such as KI can be incorporated into the solution and mix uniformly with the Mo source in the spin-coated film. Moreover, the heating of the sulfur powder is controlled individually in terms of the start time and the target temperature to enable steady sulfur supply during the growth period (fig. S1). Under optimized sulfur heating temperature (TS = 165°C), large-size 2D MoS2 crystals are synthesized and distributed over the entire substrate, directly visualizable even with the naked eye (Fig. 1B). The flake sizes follow a Gaussian distribution centered at ~0.5 mm, which is among the largest synthetic 2D MoS2 domains reported (5, 16).
Fig. 1. Halide-enabled growth of large 2D MoS2 crystals.
(A) Schematic illustration of the growth process. (B) Photograph of near millimeter-sized 2D MoS2 crystals over the entire SiO2/Si substrate (top; photo credit: Qingqing Ji, Massachusetts Institute of Technology) and the corresponding domain size distribution (bottom). The domain size statistics are compared with state-of-the-art achievements on large-domain MoS2 growth (5, 16). (C) Optical image of a typical 2D MoS2 crystal with inset atomic force microscopy height image on the flake edge showing the monolayer thickness. (D) SHG mapping of a 2D MoS2 crystal overlaid on the optical image. Dashed red lines mark the grain boundaries. (E) Plot of the SHG patterns under parallel configuration on the six locations marked in (D). Solid lines are fitted curves.
Figure 1C is an optical image of a typical MoS2 crystal produced using the above method. The bulged triangle has a domain size of ~0.62 mm measured vertex to vertex, with atomic force microscopy confirming its monolayer thickness of ~1 nm (inset of Fig. 1C). Corresponding Raman and photoluminescence mapping images of this 2D crystal are provided in fig. S2, demonstrating its microscopic uniformity as a monolayer semiconductor. These flakes are proven to be mostly single crystals through second-harmonic generation (SHG) imaging. As shown in Fig. 1D, SHG mapping suggests the absence of any grain boundaries with uniform intensity over one-third of the flake region (17). Polarized SHG patterns under parallel configuration (Fig. 1E) are subsequently used to further verify the identical crystal orientation (18) for the remaining flake area (locations 1 to 5). The single crystallinity is found to extend over the entire flake until another small domain is encountered in the lower left part (location 6). In addition, SHG imaging of another 2D MoS2 flake (~0.4 mm in size) by stitching several mapping images is presented in fig. S3 and directly verifies its single crystallinity. We note that the scanning step of 0.8 μm used here is sufficient to visualize any embedded grain boundaries, as indicated by the arrows in fig. S3.
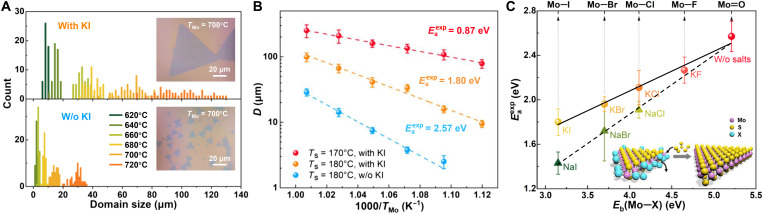
We further demonstrate experimentally that the domain size enhancement achieved here is due to the lowering of the reaction barrier in the presence of KI promoter, by comparing the MoS2 growth results with and without KI in the spin-coated films. Figure 2A presents domain size statistics for the two cases under varied growth temperature (TMo). Detailed growth results can be found in figs. S4 and S5. The effect of KI incorporation is evident, with consistently much larger crystal size than for specimens grown without KI (insets of Fig. 2A). Because all the other growth parameters in these experiments are kept identical except TMo, the average domain size D, being TMo-dependent and proportional to the crystal growth rate, can be plotted versus 1000/TMo for direct Arrhenius fitting (Fig. 2B)
| (1) |
where D0 = 1 μm is for normalization, C is a constant, is the experimentally derived reaction barrier, and kB is the Boltzmann’s constant. We find that the at TS = 180°C decreases substantially from 2.57 to 1.80 eV after KI incorporation. This indicates that the iodide salt modifies the detailed reaction pathway of the CVD process, in addition to increasing the vapor pressure of the Mo-containing species. With the presence of KI and TS lowering from 180° to 170°C, the further reduces to 0.87 eV along with the domain sizes increasing to more than 0.2 mm (Fig. 2B; see fig. S6 for the domain size statistics at TS = 170°C). Optimizing TS at 165°C results in even larger 2D MoS2 crystals (Fig. 1C), but the sulfurization reaction cuts off at TS ≤ 160°C, producing only unsulfurized species (fig. S7) (19). We hence conclude that the MoS2 growth dynamics are sensitive to the presence of KI and the sulfur concentration at TS > 160°C.
Fig. 2. Halide-dependent reaction barriers.
(A) Statistics of MoS2 domain size under varied growth temperature (TMo), for the growth with and without KI promoters (top and bottom, respectively). Insets are the corresponding optical images of MoS2 crystals grown at TMo = 700°C for direct comparison. Sulfur heating temperature (TS) was kept at 180°C. (B) Arrhenius plots of average domain size (D) versus 1000/TMo under varied growth conditions. (C) Plot of the experimentally extracted reaction barriers () versus Mo─X bond dissociation energies, Eb(Mo─X) (X = I, Br, Cl, F, and O), for the MoS2 growth assisted by various potassium and sodium halides. Inset: Schematic model of the bond substitution process that dominates MoS2 growth.
Following the strategy presented above, we have also been able to extract the CVD reaction barriers incorporating KBr, KCl, and KF promoters (fig. S8). All of these values are found to correlate linearly with the Mo─X bonding energies, Eb(Mo─X) (solid line in Fig. 2C) (20), which indicates that halogens play a vital role in tuning the MoS2 growth dynamics. The linear correlation can be understood in the context of the BEP principle (21) that predicts, for chemical reactions of the same class, Ea = E0 + αΔH, where ΔH is the enthalpy of reaction and E0 and α are linear fitting parameters. In our case, the replacement of ΔH with Eb(Mo─X) holds only if we consider MoXy + 2S → MoS2 + yX, where ΔH = yEb(Mo─X) − 2Eb(Mo─S), as the relevant reaction. Such a BEP relation is frequently observed in surface-catalyzed chemical processes (22, 23) and radical reactions dominated by bond substitution (24), in analog to which the above CVD reaction can also be inferred as bond substitution dominated. The process concerning this analysis is shown in the inset of Fig. 2C, schematically illustrating the substitution of Mo─X bonds by Mo─S bonds during MoS2 growth.
Furthermore, the above analysis framework has also been applied for sodium halide–assisted MoS2 growths, revealing and validating a BEP relation as well (dashed line in Fig. 2C). The difference of the two BEP lines suggest that alkali metal ions also play a role in tuning MoS2 growth dynamics, the details of which have been explored in a previous work that considers MoS2 lattice strain relaxation by sodium attachment on the edge (12). Our analysis method can thus provide a route to experimentally differentiate the roles of metal and halogen ions on promoting MoS2 growth.
DISCUSSION
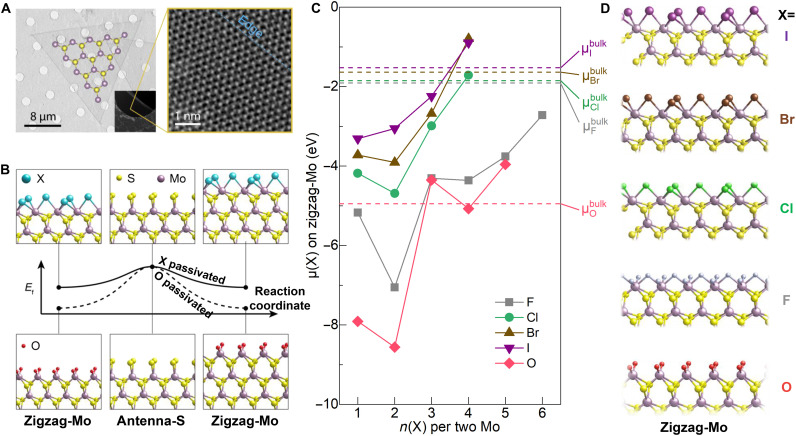
On the basis of the above understandings from experiments, we build a theoretical growth model termed edge passivation-substitution (EPS) to further rationalize the reaction barrier tuning by halide salts. From both selected-area electron diffraction (fig. S9) (25) and atomic-resolution scanning transmission electron microscopy (STEM) (Fig. 3A), the edge of the MoS2 crystals produced by halide-assisted growth has been identified to be along the crystallographic zigzag-Mo orientation, instead of the zigzag-S orientation. Knowing the edge type is useful to narrow down the possible range of the chemical potential of sulfur (μS): Lower (higher) μS favors zigzag-Mo (zigzag-S) edge type formation. We note that the edge type here indicates the crystal orientation rather than the detailed atomic structure of the edge (e.g., “zigzag-Mo orientation” corresponds to both zigzag-Mo and antenna-S structures at the edge, the latter being the sulfur-passivated zigzag-Mo edge).
Fig. 3. EPS model of the halide-assisted MoS2 growth.
(A) TEM and STEM images of the grown MoS2 flakes. The STEM image is taken close to an edge with the dashed blue line marking the edge orientation. (B) Energy landscapes and intermediate edge structures of MoS2 growth with (top) and without (bottom) halide promoters. (C) Plot of the calculated incremental chemical potentials of halogens and oxygen versus their coordination numbers on the edge. n(X) per two Mo is used to accommodate bridge bonding structures. Dashed lines mark the corresponding chemical potentials in the bulk phases. (D) Thermodynamically allowed zigzag-Mo edge structures passivated by maximum halogen and oxygen atoms.
Figure 3B is an illustration of the EPS model that exhibits the alternate attachment of S and MoXy clusters on a zigzag-Mo edge to imitate the MoS2 growth process (26). The involvement of MoXy is based on previous observation that molybdenum halides/oxyhalides are generated spontaneously and related to the salt-assisted MoS2 growth (11). The growth without halide promoters is plotted as a reference, where the zigzag-Mo edges are passivated by oxygen atoms (lower part of Fig. 3B), as confirmed in the BEP relation (Fig. 2C) that the reaction barrier in this case correlates with Eb(Mo═O). The thermodynamically allowed edge configurations passivated by halogens and oxygen are determined by comparing their incremental chemical potentials on the zigzag-Mo edge to that in the bulk phases, μbulk, which represent the upper limit of the chemical potentials of corresponding elements (Fig. 3C). If the energy gain of attaching an atom to the MoS2 edge is lower than its |μbulk|, then the edge structure with that additional atom is thermodynamically forbidden. Because the halogen/oxygen atoms are not consumed by the growth reaction, they can be rationally assumed to saturate the edge to its maximum extent, ignoring the kinetic effects. Coordination numbers can thus be derived with the detailed edge structures shown in Fig. 3D.
In our EPS model, the reaction barrier () of zigzag-Mo edge growth is approximated as the formation energy (Ef) difference between the sulfur-bonded intermediate state (antenna-S edge) and the halogen/oxygen-passivated zigzag-Mo edge. Likewise, in the case of zigzag-S edge growth, is the difference of Ef between the halogen/oxygen-passivated Mo-bonded intermediate state (antenna-Mo edge) and zigzag-S edge. More rigorously, we define
| (2) |
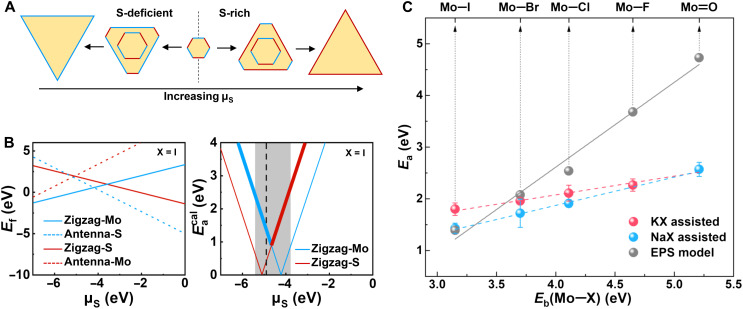
which takes into account the competitive formation of zigzag-Mo and zigzag-S edges governed by Wulff construction theory (27), because the edge growth with smaller activation energy diminishes quickly and the final growth is determined by the slower edge formation process (Fig. 4A). Detailed discussion of the growth model and the density functional theory calculations can be found in Materials and Methods, fig. S10, and tables S1 to S3.
Fig. 4. Theoretically derived reaction barrier and BEP relation using the EPS model.
(A) Schematic shape evolution of MoS2 crystals under S-deficient (Mo-rich) and S-rich conditions. Blue and red lines represent zigzag-Mo and zigzag-S terminated edges, respectively. (B) Edge formation energy (Ef) and derived reaction barrier () as a function of the chemical potential of sulfur (μS) in the case of iodine passivation. In the right panel, the shaded region indicates accessible μS, and the vertical dashed line marks where μS = −4.9 eV. (C) Halide-dependent (in red) versus Eb(Mo─X) calculated with the above growth model (in gray). μS = −4.9 eV is adopted to derive the . The experimental data (in red and blue) from Fig. 2C are also provided here for direct comparison.
Figure 4B exemplifies the calculated results for the growth with iodide promoter (calculations of the growth with passivation of oxygen and other halogens are presented in fig. S11). The overall μS-dependent according to Eq. 2 is highlighted in bold in the right panel, exhibiting a V-shape curve minimized at μS = −4.6 eV. Adopting μS = −4.9 eV, which is on the left branches of all the five curves that associates with zigzag-Mo edge formation, as confirmed by the STEM image above, we theoretically reproduce the BEP relation (Fig. 4C) and verify the halogen-dependent MoS2 growth dynamics. Notably, the calculated BEP slope is higher than the experimental counterparts, which possibly associates with the fact that we do not consider the synergistic Mo─X and Mo─S bond dissociation/formation process in the EPS model for simplicity (see Materials and Methods for more detailed discussion). Regarding the linearly correlated Ea and Eb(Mo─X), the qualitative consistency between experimental and theoretical results justifies our hypothetical EPS model, hence providing useful insights on further engineering the growth of MoS2 and other TMDs.
Our results suggest that edge passivation by oxygen or other additives, being overlooked in most previous studies, should be considered to better understand the MoS2 growth dynamics. In addition, while the role of alkali metal ions in the salts is not the primary focus of this work, we anticipate that our growth model could be further extended to incorporate these metal ions by, for example, placing them on the zigzag-S and antenna-S edges to modify the corresponding formation energies and the overall reaction barriers.
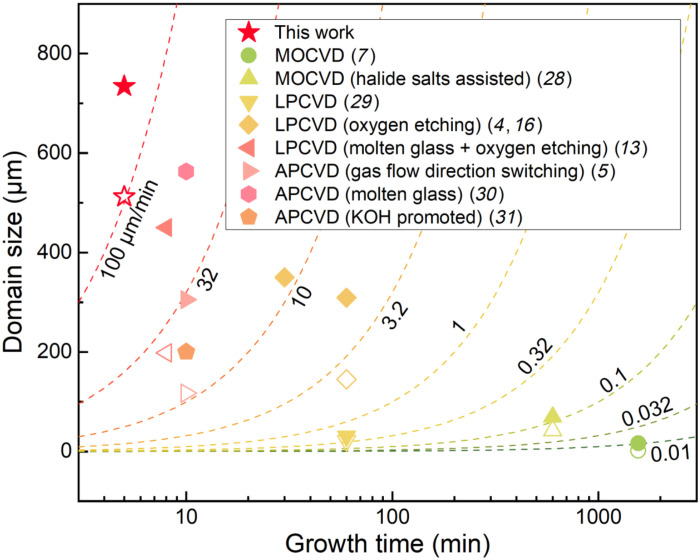
Comparing with previous efforts on large-domain MoS2 growth (4, 5, 7, 13, 16, 28–31), our method markedly improves the achievable domain size and shortens the required growth time (Fig. 5). It is worth noting that, while crystal growth does not precisely start (end) at the beginning (ending) of max{TMo}, the time span of max{TMo} is a good approximation of the growth time and a fair gauge for comparison across different methods. As a result, we enhance the growth velocity of MoS2 by three- to fivefold from the previous record (30). Reaction dynamics engineering mediated by the halide salts has been proven to be the key to achieve this, which differs from other works that focus on improving the nucleation dynamics [e.g., introducing oxygen for nuclei etching (4, 13)] or diffusion dynamics [e.g., using molten glass substrates to facilitate precursor diffusion (13, 30)]. Using a combination of these growth techniques, further improvement of the MoS2 domain size up to millimeter or even larger scales can be rationally anticipated, which represents a long-term pursuit among the 2D semiconductor community.
Fig. 5. Benchmark of the optimized halide-assisted MoS2 growth method.
The filled symbols describe the largest achievable domain sizes in these reports, while the corresponding hollow symbols mark the average domain sizes achieved. The “growth time” on the x axis indicates the time span of TMo. The dashed contour lines mark various growth velocities estimated by the domain size over the growth time. MOCVD, metal-organic CVD; LPCVD, low-pressure CVD; APCVD, ambient-pressure CVD.
In summary, we have explored large-domain MoS2 growth activated by halide salts and provided direct evidence that halogens associate closely with the reaction dynamics through the BEP relation. This mechanistic understanding has enabled us to establish a theoretical growth model that rationalizes all the experimental observations, as well as to guide the designer CVD growth toward millimeter-sized 2D MoS2 crystals with shortened growth time. We postulate that the uncovered activation mechanism is broadly applicable to the growth of diverse 2D metal chalcogenides and will be of great utility to further integrated functionalities.
MATERIALS AND METHODS
Halide-assisted growth of 2D MoS2 crystals
We take the KI-assisted MoS2 growth as an example to describe the experimental details. MoO3 (100 mg; ≥99.9%; Alfa Aesar) and KI (100 mg; ≥99%; Alfa Aesar) were dissolved in 20 ml of ammonia (weight, ~30%; Alfa Aesar) by 5-min sonication. The precursor solution was diluted four times and then spin-coated onto SiO2/Si at 2500 rpm for 1 min. The obtained precursor film was loaded into a single-zone tube furnace (Thermo Fisher Scientific–Lindberg) for high-temperature annealing under a sulfur atmosphere conveyed downstream by Ar gas flow. A detailed growth program is shown in fig. S1. The first 3 min under 1000-sccm (standard cubic centimeter per minute) Ar gas flow was for purging the tube system (2.54 cm in diameter) and assuring a favorable atmosphere for MoS2 growth. Under 30-sccm Ar, the furnace was heated to TMo = 600° to 720°C within 15 min and held at that temperature for 3 to 5 min to allow the growth of 2D MoS2 crystals. Sulfur powder of ~30 mg in a quartz boat was placed 6 cm away from the furnace edge and evaporated by a heating belt at TS = 165° to 180°C. The separate control of TMo and TS allows for more elaborate tuning of the growth process.
We note that the start time of sulfur heating plays an important role in realizing the desirable 2D growth. Too early introduction of sulfur vapor would lead to the sulfurization reaction occurring at low temperature (<550°C) and result in nanoparticulate products, while delayed sulfur introduction would yield unsulfurized species. In our case, the start point was optimized at t = 12.5 min so that TS was reached at ~3 min before TMo was reached, allowing for a nearly constant-rate supply of sulfur vapor during the growth period. Such growth program enabled us to achieve reliable Arrhenius fitting results and to better investigate the TMo- and TS-dependent MoS2 growth processes.
The synthesis of near millimeter-sized 2D MoS2 crystals (Fig. 1, B and C) can be achieved at high TMo (720°C) and low TS (165°C) with prolonged growth time (5 min). Small bilayer islands are found to be inevitable for large-domain MoS2 growth. If we reduce the amount of spin-coated Mo precursors using a lower-concentration solution, then multilayer nucleation can be greatly suppressed yet at the expense of lower MoS2 coverage and smaller domain size. To study the halide-dependent MoS2 growth dynamics, solutions containing 100 mg of MoO3 in mixture with 100 mg of KI, 72 mg of KBr, 45 mg of KCl, and 35 mg of KF, respectively, were prepared and spin-coated for the high-temperature annealing with sulfur vapors. The Mo/X molar ratio (X = I, Br, Cl, and F) was kept almost identical in these solutions for more rational comparison.
Details of the growth model and theoretical calculation of the reaction barriers
The first-principles calculations for geometric optimization and the electronic properties of crystal structure are carried out using density functional theory using projector augmented wave (PAW) method implemented in the Vienna Ab-initio Simulation Package (32, 33). The semilocal generalized gradient approximation in the form of Perdew-Burke-Ernzerhof and the PAW pseudopotentials are adopted. During the ionic optimization steps, the Hellman-Feynman forces of single atoms are optimized to be less than 0.01 eV/Å, where the energy cutoff is set to be 400 eV and only gamma point is used for k-space sampling. The k points are set to be 19 × 1 × 1 for calculating the formation energies.
The energy barrier of the edge growth is deduced from the formation energy of different edges. For example, in zigzag-Mo edge growth, the energy barrier during the growth procedure is the difference of Ef between zigzag-Mo and antenna-S edges (26). The same rule applies to the growth procedure of zigzag-S edge growth, where the barrier is the difference between the Ef of zigzag-S edge and antenna-Mo edge. The role of halogen atoms is to adjust the formation energies of zigzag-Mo and antenna-Mo edges and therefore tune the energy barriers of edge growth.
We begin by considering the energetically possible configurations of the halogen/oxygen-passivated zigzag-Mo and antenna-Mo edges, under the assumption that there are plenty of atomic sources for such edge passivation. Detailed edge structures are listed in tables S1 and S2. We note that according to our calculations, F atoms could passivate both Mo-terminated (zigzag-Mo and antenna-Mo) and S-terminated (zigzag-S and antenna-S) edges because of its distinct electronegativity, while I, Br, Cl, and O can only passivate Mo-terminated edges. Moreover, our calculation results indicate that it is energetically unfavorable to connect Na or K to antenna-S edge of MoS2 in an open system (grand canonical ensemble): The maximum ratio between K and S atoms on the edge is only 1:2, while no Na can connect to the antenna-S edge. On the other hand, our calculations indeed show that the preferred configuration for zigzag-S edge is to passivate 3 K or 3 Na for every 2 S atoms. Therefore, it is possible to incorporate the alkali metal ions in our EPS model to understand their roles in promoting MoS2 growth. Nevertheless, we note that the alkali metals can also affect the MoS2 growth dynamics though other mechanisms. For example, potassium molybdate salts derived from the MoO3/KI mixture as the liquid Mo source can have completely different surface transport and reaction kinetics (34) from that of gaseous molybdenum oxyhalides.
Given these edge passivation structures, their formation energies can be calculated by considering four types of nanostructures (35) in fig. S10. We denote the formation energies per unit length (the defined unit lengths are marked in fig. S10 by black rectangles) of zigzag-Mo, zigzag-S, antenna-S, and antenna-Mo edges as γI, γII, γIII, and γIV, respectively. The calculated energies of the three nanobelts and the nano-island are listed as E1, E2, E3, and E4. The chemical potentials of Mo, S, O, and the halogen atom X (X = F, Cl, Br, and I) are denoted as μMo, μS, μO, and μX, and the chemical potential of the unit cell of MoS2 is μMoS2. At the final equilibrium state, μMoS2 = μMo + 2μS.
As an example, for F-assisted growth process, the expressions connecting the above calculated energies are
| (3) |
where N1, N2, N3, and N4 are the total number of halogen atoms in the corresponding nanobelts and nano-islands in unit length. In the case of F-assisted growth process, N1 = 6, N2 = 6, N3 = 6, and N4 = 45. Solving the above equation set for formation energies of different edges, we have
| (4) |
From here, we can plot four lines indicating the formation energies of the four edges with respect to the change of sulfur chemical potential. Note that the growth process of MoS2 edge has merely two edge growth sequences: (i) zigzag-Mo → antenna-S → zigzag-Mo → antenna-S → … and (ii) zigzag-S → antenna-Mo → zigzag-S → antenna-Mo → ….
The formation energies of these edges are different, making one of the edges in the thread have higher energy than the other, therefore creating an energy barrier to cross over. Here, we assume that only these four edge states are related to the energy barriers. The four states are local minimums in the energy landscape, which are connected by many saddle points in between that truly accounts for the reaction barrier. Nevertheless, we are focusing on the qualitative trend instead of a quantitative description of the reaction path, and our simulation results have shown that this assumption is a good approximation for reproducing the trend with respect to different halogen atoms. Therefore, the energy barriers of the two edges under F passivation are
| (5) |
| (6) |
Calculating the formation energies of the edges with Cl, Br, I, and O passivation follows the same procedure as that of F except that we use different groups of N1, N2, N3, and N4 numbers listed in table S3. The calculated reaction barriers are all plotted along with the formation energies in fig. S11. Note that when X = Cl, Br, I, and O, there is also chemical potential of X, μX, in the final expression of formation energies and energy barriers.
In our experiment, we have calibrated the amount of sulfur needed for minimizing the growth barrier when using KI. Therefore, a good estimated value of sulfur chemical potential, μS, should be close to the minimum of the energy barrier, where we estimate to be −4.9 eV on the basis of three facts: (i) The sulfur concentration is kept at a lowest possible level where the growth speed is optimized. (ii) TEM characterization proves that the edge of MoS2 is Mo-zigzag type, so μS falls into the left side (blue color) of the “V” shape in Fig. 4B. (iii) The value of μS is sampled between −5.4 and −4.6 eV to best fit the trend of experiment results in Fig. 4C. Therefore, μS = −4.9 eV matches well with all the above criteria. Because the sulfur is kept in the same condition for different halogen-assisted growth, the μS of these growth processes should be very close to each other. By taking μS = − 4.9 eV shown as the vertical dashed line in fig. S11, we can estimate the energy barrier for each specific halogen-assisted process and use them for the BEP plot.
The EPS model considers thermodynamic properties of the edge structures to approximate the reaction dynamics, which, by nature, is less effective to account for a growth process involving notable kinetic effects. Regarding the nonconcave shape of the monolayer crystals (Fig. 1C) and the fact that MoS2 growth accelerates with reduced sulfur supply (Fig. 2B), both Mo and S sources are deduced to be sufficient for the reaction and contribute to negligible kinetic effects (36). This explains the validity of the thermodynamic EPS model for our growth results. Nevertheless, to include kinetic effects, a growth model that has inputs of empirical parameters should be developed, for which the vapor-liquid-solid model (34) and the phase-field model (37) are the most promising ones.
Our EPS model can, in principle, be applied for MoS2 growth with externally supplied Mo sources, on condition of inappreciable kinetic effects. This is however hardly achieved for volatile and reactive molybdenum halides (38), although the EPS model predicts them to be good precursors for fast and large-domain MoS2 growth. A local feeding strategy reported for graphene growth (39) could possibly avoid premixing and reaction of MoXy with sulfur in the gas phase and overcome the Mo-associated kinetic effects, lastly enabling the fast growth of ultralarge 2D MoS2 crystals.
Last, we explain the slope discrepancy for the BEP relationships obtained from both experiments and theoretical calculations (Fig. 4C). The BEP relationship in our case has the form of Ea = E0 + αEb(Mo─X), where Ea is the reaction barrier, Eb(Mo─X) is the Mo─X bond energy, and E0 and α are the fitting parameters. The slope α characterizes how strongly the reaction rate is limited by the dissociation of the Mo─X bonds (sufficiently large α indicates that Mo─X bond dissociation is a prerequisite to proceed the reaction). In the practical growth process, the Mo─X bond dissociation is more likely accompanied by the Mo─S bond formation in a synergistic way, which leads to the Ea having mitigated dependence on Eb(Mo─X) and hence a smaller α value. Our model, to provide a more concise picture, has simplified the growth process without considering the synergistic bond dissociation/formation states and thus overestimated the dependence on Eb(Mo─X). Nevertheless, the essential chemistry conveyed in this model is still valid that the reaction is dominated by the substitution of Mo─X bonds by the Mo─S bonds.
Acknowledgments
Funding: Q.J. and J.K. acknowledge support by the STC Center for Integrated Quantum Materials, NSF grant no. DMR-1231319. C.S. and J.K. acknowledge the support from the U.S. Army Research Office (ARO) under grant no. W911NF-18-1-0431. N.M. and J.K. acknowledge support by the U.S. Department of Energy, Office of Science, Basic Energy Sciences under award DE-SC0020042. C.S. is currently supported by the Kavli Energy NanoScience Institute/Heising-Simons Fellowship, Berkeley, CA, USA. X.T. and J.M. acknowledge support by the US Department of Energy (DOE), Office of Science, Basic Energy Sciences, Division of Materials Sciences and Engineering under award number DE-SC0010378 and the U.S. Army Research Office (ARO) under grant no. W911NF-18-1-0431. J.L. acknowledges support by an Office of Naval Research MURI through grant no. N00014-17-1-2661 and support by DTRA (Award No. HDTRA1-20-2-0002) Interaction of Ionizing Radiation with Matter (IIRM) University Research Alliance (URA). The work was also supported by the Director, Office of Science, Office of Basic Energy Sciences, Materials Sciences and Engineering Division of the U.S. Department of Energy under contract no. DE-AC02-05-CH11231, within the sp2-Bonded Materials Program (KC2207), which supported modeling calculations. Additional support was provided by the NSF under grant no. DMR-1807233 for TEM characterization. The STEM imaging is conducted at the Center for Nanophase Materials Sciences, which is a DOE Office of Science User Facility. Publication made possible in part by support from the Berkeley Research Impact Initiative (BRII) sponsored by the UC Berkeley Library.
Author contributions: Q.J., C.S., and J.K. conceived the project. J.K. supervised the whole project. Q.J. designed and performed the CVD growth of MoS2. C.S. conceived and performed the theoretical calculations. Q.J. and C.S. analyzed the experimental and theoretical data. N.M. performed the SHG experiment, supervised by W.A.T. X.T. performed the atomic-resolution STEM experiment, supervised by J.-C.I. and J.M. Q.J. and C.S. composed the paper. All authors were involved in the paper revisions.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S11
Tables S1 to S3
REFERENCES AND NOTES
- 1.Radisavljevic B., Radenovic A., Brivio J., Giacometti V., Kis A., Single-layer MoS2 transistors. Nat. Nanotechnol. 6, 147–150 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Akinwande D., Petrone N., Hone J., Two-dimensional flexible nanoelectronics. Nat. Commun. 5, 5678 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Shen P.-C., Su C., Lin Y., Chou A.-S., Cheng C.-C., Park J.-H., Chiu M.-H., Lu A.-Y., Tang H.-L., Tavakoli M. M., Pitner G., Ji X., Cai Z., Mao N., Wang J., Tung V., Li J., Bokor J., Zettl A., Wu C.-I., Palacios T., Li L.-J., Kong J., Ultralow contact resistance between semimetal and monolayer semiconductors. Nature 593, 211–217 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Chen W., Zhao J., Zhang J., Gu L., Yang Z., Li X., Yu H., Zhu X., Yang R., Shi D., Lin X., Guo J., Bai X., Zhang G., Oxygen-assisted chemical vapor deposition growth of large single-crystal and high-quality monolayer MoS2. J. Am. Chem. Soc. 137, 15632–15635 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Chen J., Tang W., Tian B., Liu B., Zhao X., Liu Y., Ren T., Liu W., Geng D., Jeong H. Y., Shin H. S., Zhou W., Loh K. P., Chemical vapor deposition of high-quality large-sized MoS2 crystals on silicon dioxide substrates. Adv. Sci. 3, 1500033 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji Q., Zhang Y., Gao T., Zhang Y., Ma D., Liu M., Chen Y., Qiao X., Tan P.-H., Kan M., Feng J., Sun Q., Liu Z., Epitaxial monolayer MoS2 on mica with novel photoluminescence. Nano Lett. 13, 3870–3877 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Kang K., Xie S., Huang L., Han Y., Huang P. Y., Mak K. F., Kim C.-J., Muller D., Park J., High-mobility three-atom-thick semiconducting films with wafer-scale homogeneity. Nature 520, 656–660 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Lee Y.-H., Zhang X.-Q., Zhang W., Chang M.-T., Lin C.-T., Chang K.-D., Yu Y.-C., Wang J. T.-W., Chang C.-S., Li L.-J., Lin T.-W., Synthesis of large-area MoS2 atomic layers with chemical vapor deposition. Adv. Mater. 24, 2320–2325 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Li X., Cai W., An J., Kim S., Nah J., Yang D., Piner R., Velamakanni A., Jung I., Tutuc E., Banerjee S. K., Colombo L., Ruoff R. S., Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 324, 1312–1314 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Li S., Wang S., Tang D.-M., Zhao W., Xu H., Chu L., Bando Y., Golberg D., Eda G., Halide-assisted atmospheric pressure growth of large WSe2 and WS2 monolayer crystals. Appl. Mater. Today 1, 60–66 (2015). [Google Scholar]
- 11.Zhou J., Lin J., Huang X., Zhou Y., Chen Y., Xia J., Wang H., Xie Y., Yu H., Lei J., Wu D., Liu F., Fu Q., Zeng Q., Hsu C.-H., Yang C., Lu L., Yu T., Shen Z., Lin H., Yakobson B. I., Liu Q., Suenaga K., Liu G., Liu Z., A library of atomically thin metal chalcogenides. Nature 556, 355–359 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Li X., Kahn E., Chen G., Sang X., Lei J., Passarello D., Oyedele A. D., Zakhidov D., Chen K.-W., Chen Y.-X., Hsieh S.-H., Fujisawa K., Unocic R. R., Xiao K., Salleo A., Toney M. F., Chen C.-H., Kaxiras E., Terrones M., Yakobson B. I., Harutyunyan A. R., Surfactant-mediated growth and patterning of atomically thin transition metal dichalcogenides. ACS Nano 14, 6570–6581 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Yang P., Zou X., Zhang Z., Hong M., Shi J., Chen S., Shu J., Zhao L., Jiang S., Zhou X., Huan Y., Xie C., Gao P., Chen Q., Zhang Q., Liu Z., Zhang Y., Batch production of 6-inch uniform monolayer molybdenum disulfide catalyzed by sodium in glass. Nat. Commun. 9, 979 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu C., Xu X., Qiu L., Wu M., Qiao R., Wang L., Wang J., Niu J., Liang J., Zhou X., Zhang Z., Peng M., Gao P., Wang W., Bai X., Ma D., Jiang Y., Wu X., Yu D., Wang E., Xiong J., Ding F., Liu K., Kinetic modulation of graphene growth by fluorine through spatially confined decomposition of metal fluorides. Nat. Chem. 11, 730–736 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Lee J., Pak S., Giraud P., Lee Y.-W., Cho Y., Hong J., Jang A.-R., Chung H.-S., Hong W.-K., Jeong H. Y., Shin H. S., Occhipinti L. G., Morris S. M., Cha S., Sohn J. I., Kim J. M., Thermodynamically stable synthesis of large-scale and highly crystalline transition metal dichalcogenide monolayers and their unipolar n–n heterojunction devices. Adv. Mater. 29, 1702206 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Wang Q., Li N., Tang J., Zhu J., Zhang Q., Jia Q., Lu Y., Wei Z., Yu H., Zhao Y., Guo Y., Gu L., Sun G., Yang W., Yang R., Shi D., Zhang G., Wafer-scale highly oriented monolayer MoS2 with large domain sizes. Nano Lett. 20, 7193–7199 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Yin X., Ye Z., Chenet D. A., Ye Y., O’Brien K., Hone J. C., Zhang X., Edge nonlinear optics on a MoS2 atomic monolayer. Science 344, 488–490 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Li Y., Rao Y., Mak K. F., You Y., Wang S., Dean C. R., Heinz T. F., Probing symmetry properties of few-layer MoS2 and h-BN by optical second-harmonic generation. Nano Lett. 13, 3329–3333 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Wang X., Feng H., Wu Y., Jiao L., Controlled synthesis of highly crystalline MoS2 flakes by chemical vapor deposition. J. Am. Chem. Soc. 135, 5304–5307 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Scheer M. D., Klein R., McKinley J. D., Halogens adsorbed on molybdenum: Their surface lifetimes and desorption kinetics. Surf. Sci. 30, 251–262 (1972). [Google Scholar]
- 21.Evans M. G., Polanyi M., Further considerations on the thermodynamics of chemical equilibria and reaction rates. Trans. Faraday Soc. 32, 1333 (1936). [Google Scholar]
- 22.Logadottir A., Rod T. H., Nørskov J. K., Hammer B., Dahl S., Jacobsen C. J. H., The Brønsted-Evans-Polanyi relation and the volcano plot for ammonia synthesis over transition metal catalysts. J. Catal. 197, 229–231 (2001). [Google Scholar]
- 23.Bligaard T., Nørskov J. K., Dahl S., Matthiesen J., Christensen C. H., Sehested J., The Brønsted-Evans-Polanyi relation and the volcano curve in heterogeneous catalysis. J. Catal. 224, 206–217 (2004). [Google Scholar]
- 24.Kerr J. A., Bond dissociation energies by kinetic methods. Chem. Rev. 66, 465–500 (1966). [Google Scholar]
- 25.van der Zande A. M., Huang P. Y., Chenet D. A., Berkelbach T. C., You Y., Lee G.-H., Heinz T. F., Reichman D. R., Muller D. A., Hone J. C., Grains and grain boundaries in highly crystalline monolayer molybdenum disulphide. Nat. Mater. 12, 554–561 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Chen J., Zhao X., Grinblat G., Chen Z., Tan S. J. R., Fu W., Ding Z., Abdelwahab I., Li Y., Geng D., Liu Y., Leng K., Liu B., Liu W., Tang W., Maier S. A., Pennycook S. J., Loh K. P., Homoepitaxial growth of large-scale highly organized transition metal dichalcogenide patterns. Adv. Mater. 30, 1704674 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Cao D., Shen T., Liang P., Chen X., Shu H., Role of chemical potential in flake shape and edge properties of monolayer MoS2. J. Phys. Chem. C 119, 4294–4301 (2015). [Google Scholar]
- 28.Kim H., Ovchinnikov D., Deiana D., Unuchek D., Kis A., Suppressing nucleation in metal–organic chemical vapor deposition of MoS2 monolayers by alkali metal halides. Nano Lett. 17, 5056–5063 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Ji Q., Kan M., Zhang Y., Guo Y., Ma D., Shi J., Sun Q., Chen Q., Zhang Y., Liu Z., Unravelling orientation distribution and merging behavior of monolayer MoS2 domains on sapphire. Nano Lett. 15, 198–205 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z., Xu X., Song J., Gao Q., Li S., Hu Q., Li X., Wu Y., High-performance transistors based on monolayer CVD MoS2 grown on molten glass. Appl. Phys. Lett. 113, 202103 (2018). [Google Scholar]
- 31.Zhu J., Xu H., Zou G., Zhang W., Chai R., Choi J., Wu J., Liu H., Shen G., Fan H., MoS2–OH bilayer-mediated growth of inch-sized monolayer MoS2 on arbitrary substrates. J. Am. Chem. Soc. 141, 5392–5401 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Kresse G., Furthmüller J., Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996). [DOI] [PubMed] [Google Scholar]
- 33.Kresse G., Joubert D., From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999). [Google Scholar]
- 34.Huang L., Thi Q. H., Zheng F., Chen X., Chu Y. W., Lee C.-S., Zhao J., Ly T. H., Catalyzed kinetic growth in two-dimensional MoS2. J. Am. Chem. Soc. 142, 13130–13135 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Zhu S., Wang Q., A simple method for understanding the triangular growth patterns of transition metal dichalcogenide sheets. AIP Adv. 5, 107105 (2015). [Google Scholar]
- 36.Zhang J., Wang F., Shenoy V. B., Tang M., Lou J., Towards controlled synthesis of 2D crystals by chemical vapor deposition (CVD). Mater. Today 40, 132–139 (2020). [Google Scholar]
- 37.Xu J., Srolovitz D. J., Ho D., The adatom concentration profile: A paradigm for understanding two-dimensional MoS2 morphological evolution in chemical vapor deposition growth. ACS Nano 15, 6839–6848 (2021). [DOI] [PubMed] [Google Scholar]
- 38.Yu Y., Li C., Liu Y., Su L., Zhang Y., Cao L., Controlled scalable synthesis of uniform, high-quality monolayer and few-layer MoS2 films. Sci. Rep. 3, 1866 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu T., Zhang X., Yuan Q., Xue J., Lu G., Liu Z., Wang H., Wang H., Ding F., Yu Q., Xie X., Jiang M., Fast growth of inch-sized single-crystalline graphene from a controlled single nucleus on Cu–Ni alloys. Nat. Mater. 15, 43–47 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S11
Tables S1 to S3