ABSTRACT
Two oxylipins 12-OPDA (12-Oxo-10(Z),15(Z)-phytodienoic acid) and an ᵧ-ketol, 9,10-KODA (10-oxo-9-hydroxy-12(Z), 15(Z)-octadecadienoic acid) were recently identified as important long-distance-induced systemic resistance (ISR) signals in Trichoderma virens-treated maize. On the other hand, jasmonic acid (JA), long believed to be a major signal of ISR, was not involved, as the JA-deficient mutant, opr7 opr8, retained the capacity for T. virens-triggered ISR. In order to further understand the biochemical basis for ISR priming in maize leaves, diverse oxylipins and phytohormones in the leaves of wild-type maize or ISR-deficient lox10-3 mutants treated with T. virens were quantified. This analysis revealed that 12-OPDA and two novel ᵧ-ketols, 9,12-KOMA (12-Oxo-9-hydroxy-10(E)-octadecenoic acid) and 9,12-KODA (12-Oxo-9-hydroxy-10(E),15(Z)-octadecadienoic acid), accumulated at high levels in ISR-positive plants. In support of the notion that 12-OPDA serves as a priming agent for ISR in addition to being a xylem-mobile signal, leaf pretreatment with this JA precursor resulted in increased resistance to Colletotrichum graminicola. Furthermore, the injection of 9,12-KODA or 9,12-KOMA in wild-type plants enhanced resistance against C. graminicola infection, suggesting that they play roles in ISR priming.
KEYWORDS: Oxylipins, lipoxygenase, symbiont, defense priming, Colletotrichum graminicola
Aside from promoting plant growth and development, the colonization of plant roots by beneficial microorganisms triggers induced systemic resistance (ISR) to pathogen infection in aboveground tissues.1–3ISR involves the movement of long-distance, root-derived signals that travel systemically through the vasculature to prime defenses against pathogen attack.1,4,5 In general, ISR signaling has been postulated to involve jasmonic acid (JA) and ethylene (ET) in a salicylic acid (SA)-independent manner.6 The beneficial fungus, T. virens, induces systemic resistance in several host plants.7,8 In T. virens interactions with maize (Zea mays), the small-secreted proteins Sm1 (protein ID 110852) and Sir1 (protein ID 77560) have been identified as positive and negative regulators of ISR, respectively.9–11 Specifically, the knockout mutant Δsm1 is unable to trigger ISR in cotton or maize,9,10 while Δsir1 enhanced resistance in maize against the pathogens, Cochliobolus heterostrophus and Colletotrichum graminicola.11,12 Previously, we showed that Sm1 suppressed expression of a 9-lipoxygenase (LOX) gene, LOX3, in maize roots that acts as a negative regulator of ISR.13Maize lox3-4 mutants over-accumulated the defense hormones SA, JA, and ET in the roots and displayed strong constitutive resistance against several pathogens.14 The subsequent search for a positive ISR regulator in lox3-4 mutant resulted in the identification of a 13-LOX gene, LOX10,12 that is responsible for the biosynthesis of green leaf volatiles and an array of wound-induced oxylipins, including jasmonates and 12-OPDA (12-Oxo-10(Z),15(Z)-phytodienoic acid), a precursor of JA biosynthesis.15,16LOX10 is overexpressed in lox3-4 roots14 and acts as a positive regulator of ISR, as lox10-3 mutants displayed induced systemic susceptibility (ISS) when colonized by T. virens.12 By metabolite screening of xylem sap from T. virens-treated wild-type, B73 inbred line, and near-isogenic lox3-4 and lox10-3 mutants, we identified 12-OPDA and 9-hydroxy-10-oxo-12(Z),15(Z)-octadecadienoic (9,10-KODA, formerly named as KODA), an ⍺-ketol of linoleic acid, as major novel ISR long-distance signals.12 Surprisingly, JA-deficient opr7 opr8 mutants of maize displayed normal ISR, suggesting that JA is not a major long-distance ISR signal induced by T. virens. This current study extends the previously published metabolite profiling of xylem sap and roots with metabolite profiling of early leaf responses of maize to T. virens. These analyses uncover new potential priming compounds responsible for ISR.
To better understand the biochemical basis for ISR priming in maize leaves, we quantified diverse metabolites in the leaves of B73 or lox10-3 mutants treated with wild-type T. virens (TvWT). The plants were treated with ISR-positive or ISR-negative strains of T. virens, and shoot tissue was collected at 6 (fungal recognition) and 54 hours (advanced colonization) post-treatment for metabolite quantification.12,17 The results are presented in Figure 1, where the samples and individual metabolites (full names listed in Table 1) are grouped based on genotype/treatment and similarity in accumulation patterns (dendrogram not shown), respectively. The metabolites were separated into three groups. The most striking observation was that lox10-3 plants had a lower concentration of almost all the measured metabolites compared to B73, regardless of T. virens treatments, with few exceptions. Two of these exceptions were SA and benzoic acid, a precursor of SA,18,19 which was moderately induced in TvWT-treated lox10-3 at 54 h. This is not surprising, as lox10-3 has been demonstrated to accumulate elevated levels of SA when infected by C. graminicola20 and is deficient in many 13-oxylipins compared to B73.16 While SA is the major hormone for defense against this hemibiotrophic pathogen,20 the induction of SA in lox10-3 mutants was not sufficient to induce ISR, suggesting a combination with other defense metabolites, including those in Group III, may be necessary. Interestingly, Δsir1-treated B73 at 54 h accumulated the highest levels of SA, providing one mechanistic explanation for the superior ISR triggered by this strain. Surprisingly, 9,10-KODA (formerly designated as KODA)12 also showed increased accumulation in TvWT-treated lox10-3 at 6 h. Overall, these results did not provide any additional clues that explain ISS observed in TvWT-treated lox10-3. Interestingly, TvWT-treated B73 at 6 h accumulated higher amounts of metabolites in Groups II and III compared to untreated B73, though those subside to near control levels at 54 h. Most of the compounds in Group III that were upregulated in TvWT-treated B73 were not induced in Δsm1- or Δsir1-treated B73, suggesting that these compounds, while dependent on both functional Sm1 and Sir1 peptides, may not be necessary for the establishment of ISR. Elevated levels of several 9-LOX and α-dioxygenase (a-DOX) products in TvWT-treated B73 at 6 h suggest that these oxylipins may play defensive roles against C. graminicola, as was previously demonstrated with other pathogens.21,22
Figure 1.
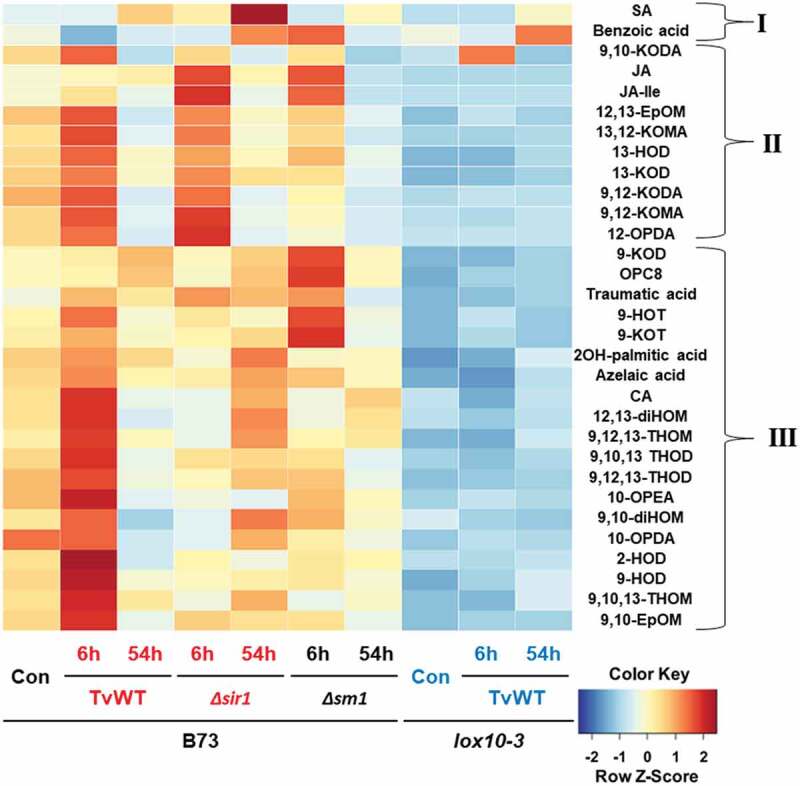
Metabolite and phytohormone levels in the shoot tissue of B73 (control or treated with TvWT, Δsm1, or Δsir1) and lox10-3 (control or treated with TvWT) are displayed as a heatmap with Z-score transformed concentrations. Controls (Con) are plants that were not treated by T. virens. The treatments were separated by maize genotype, T. virens strain, and time, while the metabolites were separated into Groups I, II, and III based on similarity in the accumulation patterns (dendrogram not shown). Treatment designations in black font indicate lack of ISR, while red font indicates ISR-positive genotype combinations and blue font indicates ISS-positive genotype combinations.
Table 1.
Common and formal names of oxylipins and phytohormones measured in this study.
| Class | Pathway | Lipid | Compound | Full Name |
|---|---|---|---|---|
| 9-LOX | REDUCTASE/PEROXYGENASE | 18:2 | 9-HOD | 9(S)-Hydroxy-10(E),12(Z)-octadecadienoic acid |
| 9-LOX | REDUCTASE/PEROXYGENASE | 18:3 | 9-HOT | 9(S)-Hydroxy-10(E),12(Z),15(Z)-octadecatrienoic acid |
| 9-LOX | PEROXYGENASE | 18:2 | 9,10-EpOM | 9(R),10(S)-Epoxy-12(Z)-octadecenoic acid |
| 9-LOX | PEROXYGENASE | 18:2 | 9,10-diHOM | 9(R),10(S)-Dihydroxy-9(Z)-octadecenoic acid |
| 9-LOX | LIPOXYGENASE | 18:2 | 9-KOD | 9-Oxo-10(E),12(Z)-octadecadienoic acid |
| 9-LOX | LIPOXYGENASE | 18:3 | 9-KOT | 9-Oxo-10(E),12(Z),15(Z)-octadecatrienoic acid |
| 9-LOX | ALLENE OXIDE SYNTHASE | 18:3 | 9,10-KODA | 10-Oxo-9-hydroxy-12(Z), 15(Z)-octadecadienoic acid |
| 9-LOX | ALLENE OXIDE SYNTHASE | 18:3 | 10-OPDA | 10-Oxo-11(Z),15(Z)-phytodienoic acid |
| 9-LOX | ALLENE OXIDE SYNTHASE | 18:2 | 10-OPEA | 10-Oxo-11(Z)-phytoenoic acid |
| 9-LOX | EPOXY ALCOHOL SYNTHASE | 18:2 | 9,12,13-THOM | 9(S),12(S),13(S)-Trihydroxy-10(E)-octadecenoic acid |
| 9-LOX | EPOXY ALCOHOL SYNTHASE | 18:3 | 9,12,13-THOD | 9(S),12(S),13(S)-Trihydroxy-10(E),15(Z)-octadecadienoic acid |
| 9-LOX | EPOXY ALCOHOL SYNTHASE | 18:2 | 9,10,13-THOM | 9(S),10(S),13(S)-Trihydroxy-11(E)-octadecenoic acid |
| 9-LOX | EPOXY ALCOHOL SYNTHASE | 18:3 | 9,10,13-THOD | 9(S),10(S),13(S)-Trihydroxy-11(E),15(Z)-octadecadienoic acid |
| 9-LOX | HYDROPEROXIDE LYASE | 18:2 | Azelaic acid | 1,9-Nonanedioic acid |
| 13-LOX | REDUCTASE/PEROXYGENASE | 18:2 | 13-HOD | 13(S)-Hydroxy-9(Z),11(E)-octadecadienoic acid |
| 13-LOX | PEROXYGENASE | 18:2 | 12,13-EpOM | 12(R),13(S)-Epoxy-9(Z)-octadecadienoic acid |
| 13-LOX | PEROXYGENASE | 18:2 | 12,13-diHOM | 12,13-Dihydroxy-9(Z)-octadecenoic acid |
| 13-LOX | LIPOXYGENASE | 18:2 | 13-KOD | 13-Oxo-9(Z),11(E)-octadecadienoic acid |
| 13-LOX | ALLENE OXIDE SYNTHASE | 18:2 | 9,12-KOMA | 12-Oxo-9-hydroxy-10(E)-octadecenoic acid |
| 13-LOX | ALLENE OXIDE SYNTHASE | 18:3 | 9,12-KODA | 12-Oxo-9-hydroxy-10(E),15(Z)-octadecadienoic acid |
| 13-LOX | ALLENE OXIDE SYNTHASE | 18:2 | 12,13-KOMA | 12-Oxo-13-hydroxy-9(Z)-octadecenoic acid |
| 13-LOX | ALLENE OXIDE SYNTHASE | 18:3 | 12-OPDA | 12-Oxo-10(Z),15(Z)-phytodienoic acid |
| 13-LOX | ALLENE OXIDE SYNTHASE | 18:3 | OPC-8 | 3-Oxo-2-(2-Pentenyl)-Cyclopentane-1-Octanoic Acid |
| 13-LOX | ALLENE OXIDE SYNTHASE | 18:3 | JA | 7-Iso-Jasmonic acid |
| 13-LOX | ALLENE OXIDE SYNTHASE | 18:3 | JA-Ile | 7-Iso-Jasmonoyl-L-isoleucine |
| 13/9-LOX | HYDROPEROXIDE LYASE | 18:2/3 | Traumatic acid | 2(E)-Dodecenedioic acid |
| Other | α-DIOXYGENASE | 16:3 | 2OH-palmitic acid | 2-Hydroxy-hexadecanoic acid |
| Other | α-DIOXYGENASE | 18:2/3 | 2-HOD | 2(R)-Hydroxy-9(Z),12(Z)-octadecadienoic acid |
| Other | PHENYLALANINE AMMONIA LYASE | NA | CA | Cinnamic acid |
| Other | PHENYLALANINE AMMONIA LYASE | NA | BA | Benzoic acid |
| Other | PHENYLALANINE AMMONIA LYASE/ISOCHORISMATE SYNTHASE | NA | SA | Salicylic acid |
Of special relevance are several compounds in Group II that accumulated to the highest levels only in TvWT- or Δsir1-treated B73, suggesting that these compounds may be involved in ISR priming. This group of metabolites included 12-OPDA (Figure 2a), which was high only at 6 h, consistent with our previous findings.12 Two other interesting compounds identified in Group II are the two poorly characterized ᵧ-ketols, 9,12-KODA and 9,12-KOMA (Figure 2b and c). These oxylipins are formed by hydrolysis of epoxides produced by reactions of 13-LOX and allene oxide synthase (AOS) on linoleic and linolenic acids, respectively (Figure 3). This is similar to the synthesis of the xylem-mobile ISR signal 9,10-KODA,12 except that this α-ketol is synthesized by 9-LOX and 9-AOS branch of the LOX pathway.
Figure 2.
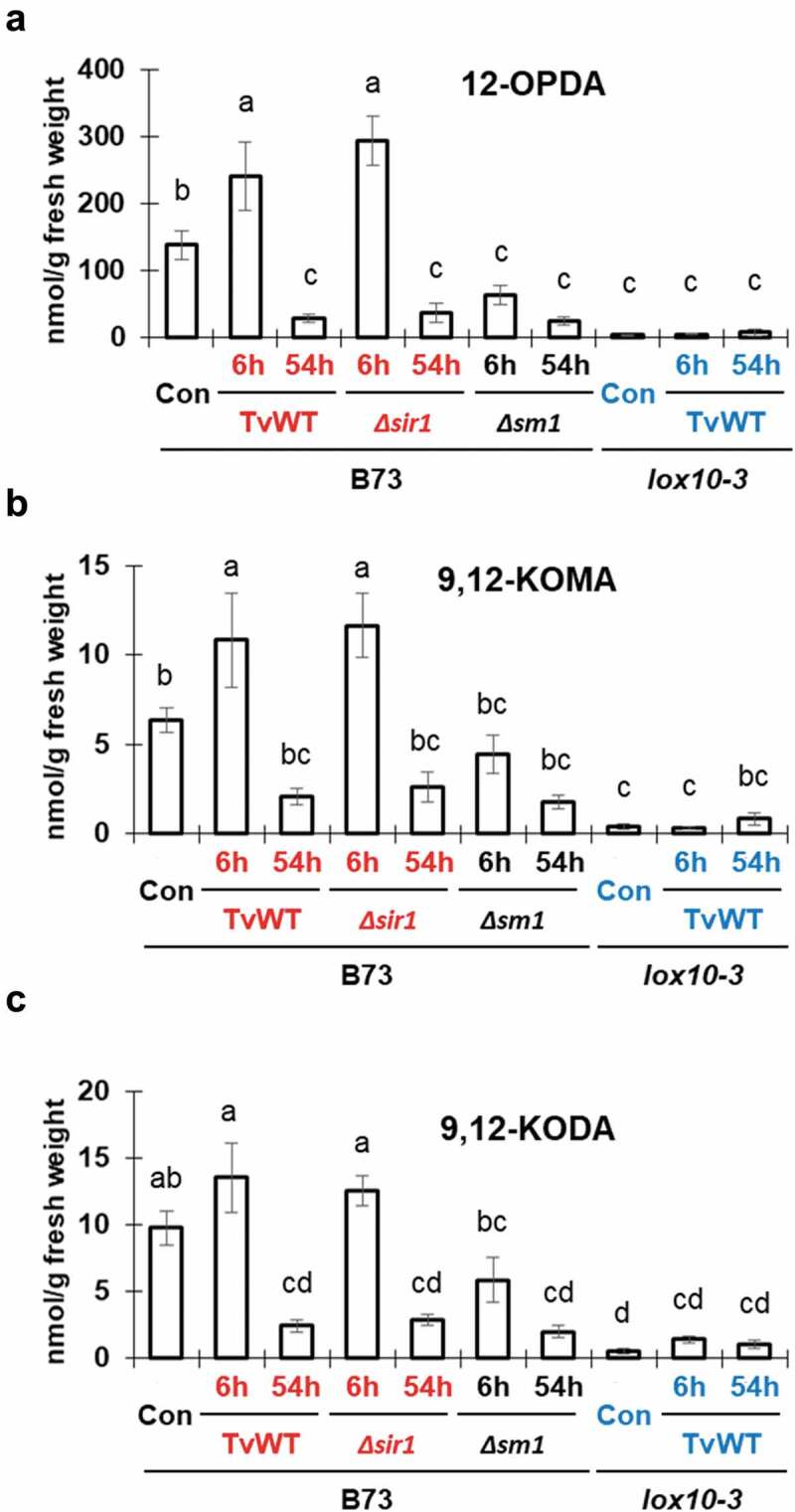
Accumulation of 12-OPDA, 9,12-KOMA, and 9,12-KODA in shoot tissues of B73 and lox10-3 treated with T. virens. Bar graphs for (a) 12-OPDA, (b) 9,12-KOMA, and (c) 9,12-KODA, represent means, with error bars representing standard error. Letters indicate significant differences among all treatments (Tukey’s HSD test, p < .05). ISR-negative genotype combinations of T. virens-treated maize are represented in black font, while ISR-positive genotype combinations are in red font and ISS-positive combinations are in blue font.
Figure 3.
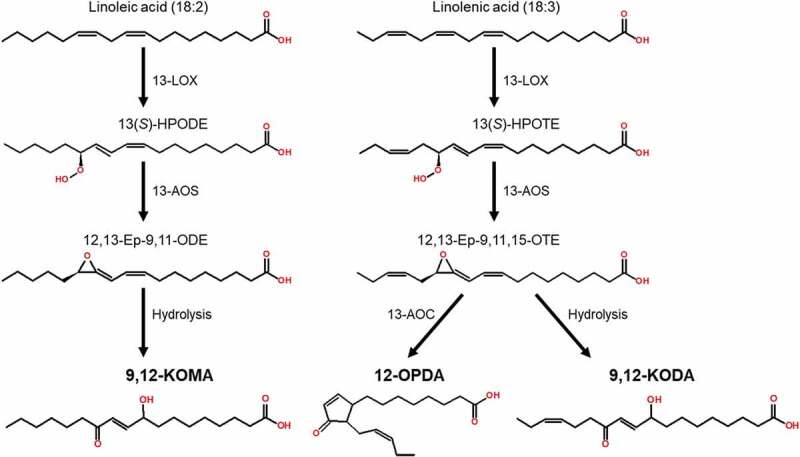
The biosynthesis pathways and chemical structures of 9,12-KOMA, 12-OPDA, and 9,12-KODA.
The early and strong induction of 12-OPDA, 9,12-KODA, and 9,12-KOMA in leaves prompted a hypothesis that these (and other metabolites in group II) are ISR priming agents. We tested this hypothesis by foliar application of 20 μM 12-OPDA, which significantly reduced lesion development on the leaves of W438 maize inbred line, while lesions of near-isogenic lox10-3 in W438 background remained unchanged (Figure 4a and b). Interestingly, this increased resistance is opposite to that of exogenous application of JA, which increased susceptibility to C. graminicola.20 These findings further support the role of 12-OPDA as a major long-distance signal and potent priming agent that is integral to T. virens-triggered ISR. The early induction of 9,12-KODA and 9,12-KOMA suggested roles in ISR priming of shoots. To test the relevance of these two α-ketols, we injected B73 plants with 10 µL of 10 nM or 100 nM concentrations of 9,12-KODA or 9,12-KOMA before infecting with C. graminicola. The plants pre-treated with 10 nM of either compound exhibited no change in resistance against infection (Figure 4c). On the other hand, treatment with 100 nM 9,12-KODA or 9,12-KOMA significantly increased resistance, suggesting that they act as important signals in T. virens-triggered ISR in maize and shed new light on the functional relevance of these oxylipins toward defense against pathogen infection. As other compounds in Group II, such as 12,13-EpOM, 12,13-KOMA, 13-HOD, and 13-KOD, also accumulated highly in ISR-positive plants, we plan to expand our testing of these molecules to determine if they may play any roles in regulating ISR. Two other compounds in Group II, JA and JA-Ile, were not induced as strongly by Tv-WT as other metabolites. Importantly, our previous studies provided strong genetic and pharmacological evidence that these compounds promote virulence of C. graminicola,12,20and therefore, cannot be considered as priming agents of ISR.
Figure 4.
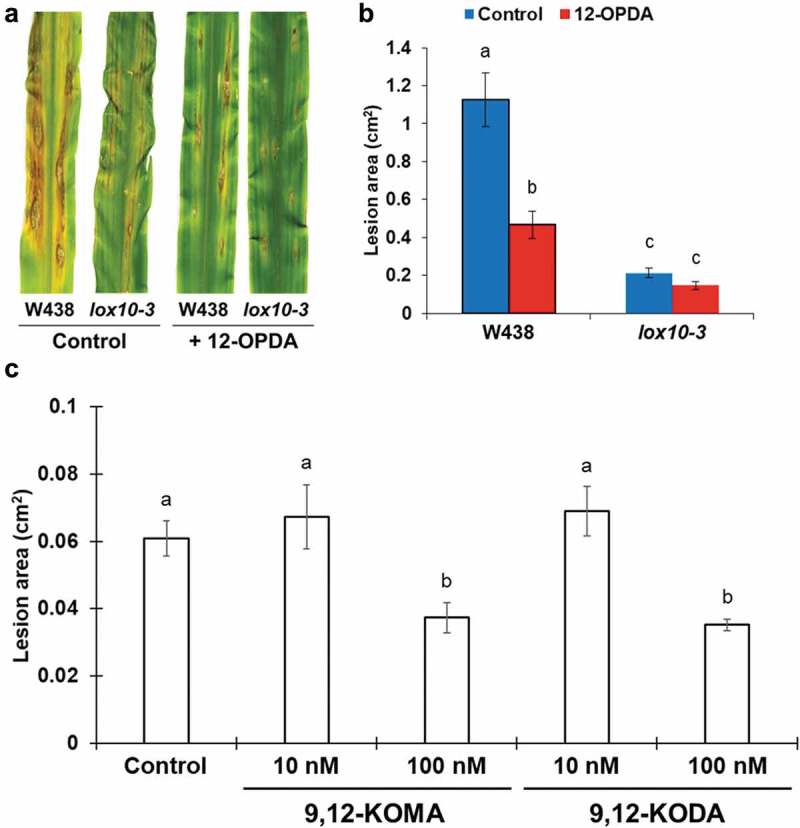
Exogenous application of 12-OPDA, 9,12-KOMA, and 9,12-KODA increases maize resistance to C. graminicola. (a) Lesions caused by C. graminicola infection of leaves of untreated by T. virens W438 inbred line and lox10-3 mutants in W438 background sprayed with 1 mL of 20 µM 12-OPDA (dissolved in ethanol) or ethanol (control). Spores of C. graminicola (approximately 1 × 106 spores/mL) were drop inoculated on leaves 6 h after spray treatment, and leaves were scanned 5 d after infection. (b) Bar graph represents average lesion area of W438 inbred maize and lox10-3 mutants in the W438 background sprayed with 20 µM 12-OPDA (dissolved in ethanol) or ethanol (control) and infected with C. graminicola. Error bars represent standard error. Letters indicate significant differences among all treatments (Tukey’s HSD test, p < .05). (c) Bar graph represents average lesion area of B73 plants transfused with sap supplemented with 100 nM or 10 nM 9,12-KODA or 9,12-KOMA and infected by C. graminicola, with error bars representing standard error. Letters indicate significant differences among all treatments (Tukey’s HSD test, p < .05).
In summary, this study was carried out to better understand the phytohormones and metabolites associated with regulating T. virens-triggered ISR in maize. Through metabolite screening, we determined that lox10-3 responded to T. virens very differently than B73, with a notable lack of accumulation of most detectable oxylipins. We showed the relevance of 12-OPDA as an inducer of resistance in maize leaves against C. graminicola infection. Additionally, we further demonstrated that treatment with 9,12-KODA and 9,12-KOMA enhanced maize resistance against pathogen infection, suggesting previously unknown roles for both oxylipins as potential ISR priming agents.
Acknowledgments
Support for this work is from the United States Department of Agriculture, National Institute of Food and Agriculture grants # 2016-67013-24730 and 2017-67013-26524. We thank Eli Borrego, Nasie Constantino, Ramadhika Damarwinasis, John Bennet, James Taylor, and Robert Dorosky for their help in preparing the hydroponic growth of maize and tissue harvesting. We would like to thank past undergraduate students Brianna Hankinson, Joseph Vasselli, and Andrew Horgan for their contributions in harvesting and preparing the tissue samples for hormone analysis.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Funding Statement
This work was supported by the United States Department of Agriculture National Institute of Food and Agriculture grant numbers 2017-67013-26524 and 2016-67013-24730.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Pieterse CM, Zamioudis C, Berendsen RL, Weller DM, Van Wees SC, Bakker PA.. Induced systemic resistance by beneficial microbes. Annu Rev Phytopathol. 2014;52:1–5. doi: 10.1146/annurev-phyto-082712-102340. [DOI] [PubMed] [Google Scholar]
- 2.Katagiri F, Tsuda K. Understanding the plant immune system. Mol Plant Microbe Interact. 2010;23:1531–1536. doi: 10.1094/MPMI-04-10-0099. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien PA. Biological control of plant diseases. Australas Plant Path. 2017;46:293–304. doi: 10.1007/s13313-017-0481-4. [DOI] [Google Scholar]
- 4.Enebe MC, Babalola OO. The impact of microbes in the orchestration of plants’ resistance to biotic stress: a disease management approach. Appl Microbiol Biotechnol. 2019;103:9–25. doi: 10.1007/s00253-018-9433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mauch-Mani B, Baccelli I, Luna E, Flors V. Defense priming: an adaptive part of induced resistance. Annu Rev Plant Biol. 2017;68:485–512. doi: 10.1146/annurev-arplant-042916-041132. [DOI] [PubMed] [Google Scholar]
- 6.Pieterse CM, Zamioudis C, Does D, Van Wees SC. Signalling networks involved in induced resistance. Induced resistance for plant defence: A sustainable approach to crop protection. 2nd ed. New York (NY): John Wiley and Sons; 2014. p. 58–80. [Google Scholar]
- 7.Contreras-Cornejo HA, Macías-Rodríguez L, Beltrán-Peña E, Herrera-Estrella A, López-Bucio J. Trichoderma-induced plant immunity likely involves both hormonal-and camalexin-dependent mechanisms in Arabidopsis thaliana and confers resistance against necrotrophic fungi Botrytis cinerea. Plant Signal Behav. 2011;6:1554–1563. doi: 10.4161/psb.6.10.17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzmán-Guzmán P, Porras-Troncoso MD, Olmedo-Monfil V, Herrera-Estrella A. Trichoderma species: versatile plant symbionts. Phytopathology. 2019;109:6–16. doi: 10.1094/PHYTO-07-18-0218-RVW. [DOI] [PubMed] [Google Scholar]
- 9.Djonovic S, Pozo MJ, Dangott LJ, Howell CR, Kenerley CM. Sm1, a proteinaceous elicitor secreted by the biocontrol fungus Trichoderma virens induces plant defense responses and systemic resistance. Mol Plant Microbe Interact. 2006;19:838–853. doi: 10.1094/MPMI-19-0838. [DOI] [PubMed] [Google Scholar]
- 10.Djonovic S, Vargas WA, Kolomiets MV, Horndeski M, Wiest A, Kenerley CM. A proteinaceous elicitor Sm1 from the beneficial fungus Trichoderma virens is required for induced systemic resistance in maize. Plant Physiol. 2007;145:875–889. doi: 10.1104/pp.107.103689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamdan NL, Shalaby S, Ziv T, Kenerley CM, Horwitz BA. Secretome of Trichoderma interacting with maize roots: role in induced systemic resistance. Mol Cell Proteomics. 2015;14:1054–1063. doi: 10.1074/mcp.M114.046607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang K-D, Borrego EJ, Kenerley CM, Kolomiets MV. Oxylipins Other Than Jasmonic Acid Are Xylem-Resident Signals Regulating Systemic Resistance Induced by Trichoderma virens in Maize. Plant Cell. 2020;32(1):166–185. doi: 10.1105/tpc.19.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Constantino NN, Mastouri F, Damarwinasis R, Borrego EJ, Moran-Diez ME, Kenerley CM, Gao X, Kolomiets MV. Root-expressed maize lipoxygenase 3 negatively regulates induced systemic resistance to Colletotrichum graminicola in shoots. Front Plant Sci. 2013;4:510. doi: 10.3389/fpls.2013.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao X, Starr J, Gobel C, Engelberth J, Feussner I, Tumlinson J, Kolomiets M. Maize 9-lipoxygenase ZmLOX3 controls development, root-specific expression of defense genes, and resistance to root-knot nematodes. Mol Plant Microbe Interact. 2008;21:98–109. doi: 10.1094/MPMI-21-1-0098. [DOI] [PubMed] [Google Scholar]
- 15.Christensen SA, Nemchenko A, Borrego E, Murray I, Sobhy IS, Bosak L, DeBlasio S, Erb M, Robert CAM, Vaughn KA, et al. The maize lipoxygenase, ZmLOX10, mediates green leaf volatile, jasmonate and herbivore-induced plant volatile production for defense against insect attack. Plant J. 2013;74:59–73. doi: 10.1111/tpj.12101. [DOI] [PubMed] [Google Scholar]
- 16.He Y, Borrego EJ, Gorman Z, Huang P-C, Kolomiets MV. Relative contribution of LOX10, green leaf volatiles and JA to wound-induced local and systemic oxylipin and hormone signature in Zea mays (maize). Phytochemistry. 2020;174:112334. doi: 10.1016/j.phytochem.2020.112334. [DOI] [PubMed] [Google Scholar]
- 17.Malinich EA, Wang K, Mukherjee PK, Kolomiets M, Kenerley CM. Differential expression analysis of Trichoderma virens RNA reveals a dynamic transcriptome during colonization of Zea mays roots. BMC Genomics. 2019;20:280. doi: 10.1186/s12864-019-5651-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ribnicky DM, Shulaev V, Raskin I. Intermediates of salicylic acid biosynthesis in tobacco. Plant Physiol. 1998;118:565–572. doi: 10.1104/pp.118.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mauch-Mani B, Slusarenko AJ. Production of salicylic acid precursors is a major function of phenylalanine ammonia-lyase in the resistance of Arabidopsis to Peronospora parasitica. Plant Cell. 1996;8:203–212. doi: 10.2307/3870265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorman Z, Christensen SA, Yan Y, He Y, Borrego E, Kolomiets MV. Green leaf volatiles and jasmonic acid enhance susceptibility to anthracnose diseases caused by Colletotrichum graminicola in maize. Mol Plant Pathol. 2020;21(5):702–715. doi: 10.1111/mpp.12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vicente J, Cascón T, Vicedo B, García-Agustín P, Hamberg M, Castresana C. Role of 9-lipoxygenase and α-dioxygenase oxylipin pathways as modulators of local and systemic defense. Mol Plant. 2012;5:914–928. doi: 10.1093/mp/ssr105. [DOI] [PubMed] [Google Scholar]
- 22.De León IP, Sanz A, Hamberg M, Castresana C. Involvement of the Arabidopsis α‐DOX1 fatty acid dioxygenase in protection against oxidative stress and cell death. Plant J. 2002;29:61–72. doi: 10.1046/j.1365-313x.2002.01195.x. [DOI] [PubMed] [Google Scholar]


